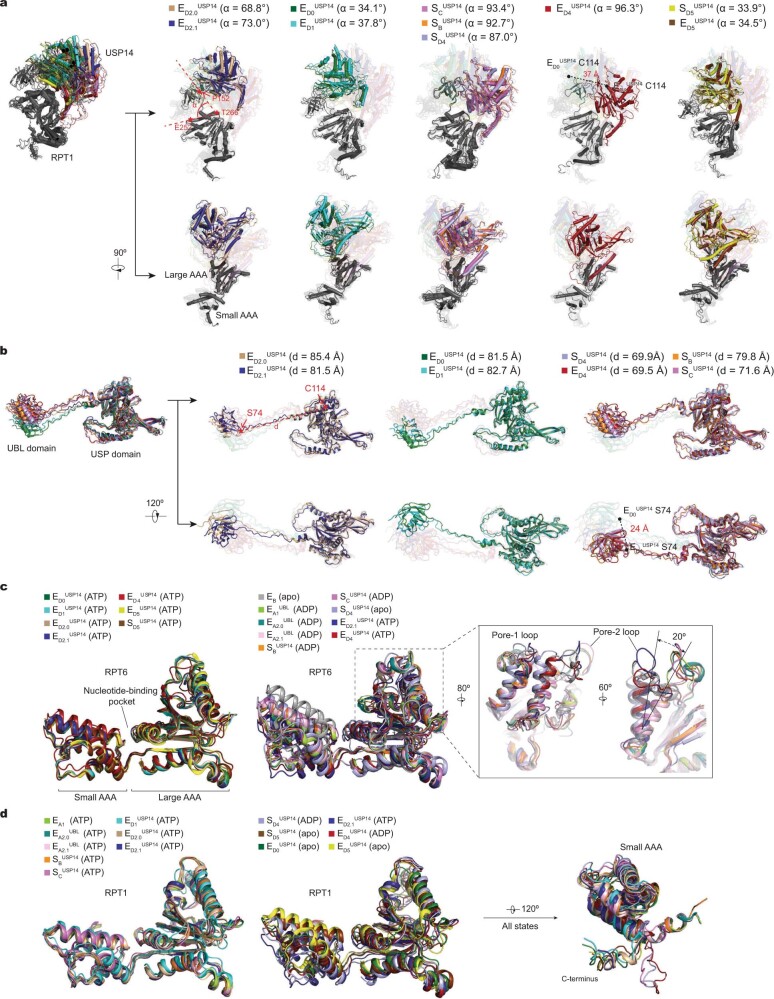
Extended Data Fig. 8. Dynamics of USP14 and key ATPase subunits in the proteasome.
a, Superposition of the USP14-RPT1 subcomplex structures from different states aligned against the RPT1 large AAA subdomain. USP14 rotates together with the RPT1 OB domain and moves up over 37 Å (from to ) relative to the RPT1 AAA domain. The angle between the OB domain and the AAA domain is measured and labelled for each state. b, Superposition of the USP14 structures from different states aligned against their USP domain. The UBL domain moves up over 24 Å (from to ) relative to the USP domain. The distance between Ser74 and Cys114 is measured and labelled for each state. c, Superposition of the RPT6 AAA domain structures from different states aligned against the large AAA subdomain. Left, comparison of the RPT6 AAA structures in the ATP-bound states. Middle, comparison of states , and , the ADP-bound states and state EB (PDB ID: 6MSE) shows conformational changes of the AAA domain driven by the ATP hydrolysis and nucleotide exchange. Right, the open-gate states , , and show different refolding of both the pore-1 and pore-2 loops. d, Superposition of the RPT1 AAA domain structures from different states aligned against the large AAA subdomain. Left, comparison of the structures in the ATP-bound states. Middle, comparison of the structures in different nucleotide-binding states. Right, the C-terminal tails of RPT6 exhibit three major orientations.