Abstract
Background and aim
Coronavirus disease 2019 (COVID-19) in people living with human immunodeficiency virus (HIV) who has a compromised immune system can be associated with more significant risks for severe complications. To date, no comprehensive study has been performed to evaluate HIV in patients with COVID-19. In the present study, we assessed the status of patients co-infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and HIV as a systematic review and meta-analysis.
Methods
A systematic literature search strategy was conducted via reviewing original research articles published in Medline, Web of Science, and Embase databases in 2019 and 2020. Statistical analysis was performed using STATA software, version 14.0 (Stata Corporation, College Station, Texas, USA), to report the prevalence of HIV among patients with COVID-19. Case reports/case series were also evaluated as a systematic review.
Results
Sixty-three studies (53 case reports/case series and ten prevalence studies) were included in our study. A meta-analysis of prevalence studies showed that HIV infection among patients with COVID-19 was reported in 6 countries (Uganda, China, Iran, USA, Italy, and Spain) with an overall frequency of 1.2% [(95% CI) 0.8–1.7] among 14,424 COVID-19 patients. According to the case reports and case series, 111 patients with HIV have been reported among 113 patients with COVID-19 from 19 countries. Most of the cases were in the USA, China, Italy, and Spain.
Conclusion
The small number of SARS-CoV-2-HIV co-infected patients reported in the literature makes it difficult to draw precise conclusions. However, since people with HIV are more likely to develop more severe complications of COVID-19, targeted policies to address this raised risk in the current pandemic should be considered. Our findings highlight the importance of identifying underlying diseases, co-infections, co-morbidities, laboratory findings, and beneficial treatment strategies for HIV patients during the COVID-19 pandemic.
Abbreviations: COVID-19, Coronavirus disease 2019; SARS-CoC-2, Severe acute respiratory syndrome coronavirus 2; WHO, World Health Organization; CDC, Centers for Disease Control and Prevention; HIV, Human immunodeficiency virus
Keywords: Co-infection, 2019 novel coronavirus, COVID-19, HIV, Meta-analysis
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the responsible agent of the current coronavirus disease 2019 (COVID-19) pandemic, emerged in China in December 2019 and has so far been reported from all around the world (Zhou et al., 2020). The World Health Organization (WHO) lists it as the sixth public health emergency worldwide (Wu et al., 2020). Although many people with the infection are asymptomatic or show mild symptoms, many patients develop the severe form of the disease, leading to severe complications or even death (Garg, 2020; Sameni et al., 2020). The proper response of the host to COVID-19 depends on the activation of both innate and acquired immunity. For this reason, there is a significant concern about the vulnerability of immunodeficient patients before or during the COVID-19 pandemic (Wang et al., 2020a; Yang et al., 2020). Immunodeficiency is a disorder of the immune system in which the host body is disabling to establish effective immune responses to various infections (Chaplin, 2010; Dropulic and Cohen, 2011). As a result, such people are prone to more intense forms of infection (Grabbe et al., 2020). Since the Centers for Disease Control and Prevention (CDC) has identified immunocompromised patients as a high-risk group for severe forms of new coronavirus infection, it is essential to conduct studies to determine the relationship between human immunodeficiency virus (HIV) infection and the severity of COVID-19 (Grabbe et al., 2020; Faust et al., 2020; Li et al., 2020a). However, there are limited data regarding the clinical impact of COVID-19 on people infected with HIV. In fact, since there is a significant relationship between immunodeficiency the and severity of COVID-19 (Hoffmann et al., 2021), it may be possible to reduce the severe complications and mortality due to the infection in this group of patients by using appropriate immunomodulators (Gaziano et al., 2021; Hall et al., 2022).
As far as we know, a comprehensive study has not yet evaluated the status of patients who suffer from HIV and COVID-19 at the same time. Hence, the purpose of the current study was to assess the status of patients who had concurrent COVID-19 and HIV as a systematic review and meta-analysis.
2. Methods
2.1. Search strategy
A comprehensive systematic literature search was conducted by reviewing original research papers published in Medline, Web of Science, and Embase databases in 2019 and 2020. The following phrases were used in the search strategy of this article: COVID OR COVID-19 OR novel coronavirus OR new coronavirus OR coronavirus 2019 OR 2019-nCoV OR nCoV OR CoV-2 OR SARS-2 OR SARS-CoV-2 OR severe acute respiratory syndrome coronavirus 2, HIV OR human immunodeficiency virus OR AIDS OR acquired immune deficiency syndrome.
2.2. Inclusion and exclusion criteria
All case reports/case series and prevalence studies about HIV among patients with COVID-19 were evaluated. These studies reported sufficient data for analysis, such as the number of patients with COVID-19, HIV and SARS-CoV-2 co-infection, clinical symptoms, and laboratory findings. In the next step, two authors independently evaluated the titles, abstracts, and full texts of the recorded papers based on the inclusion and exclusion criteria. The exclusion criteria were as follows: (1) animal research only, (2) studies considering HIV only, (3) studies considering patients with COVID-19 only, (4) review articles, (5) abstracts presented in conferences, and (6) duplicate studies. BH, MG, and NK selected appropriate papers after considering all studies based on inclusion and exclusion criteria.
2.3. Data extraction and definitions
In each study, the following items were considered: the first author's last name, time of the study, time of publication, region, number of COVID-19 patients, number of COVID-19 patients with HIV, clinical symptoms, laboratory findings, outcomes, diagnostic methods, and treatment. The data were obtained by two independent individuals and verified by another researcher.
2.4. Meta-analysis
Statistical analysis was performed using STATA software, version 14.0 (Stata Corporation, College Station, Texas, USA), to report the frequency of HIV among patients with COVID-19. Statistical heterogeneity was assessed using the Q-test and the I2 statistical methods. P-value < 0.05 was considered as statistically significant.
3. Results
3.1. Characteristics of included studies
Overall, 1930 citations were recorded in the initial database searches. Three databases were searched, and therefore, many duplicate studies were selected. After removing 785 duplicates, 1145 non-duplicate studies remained. After checking titles and abstracts, 735 non-relevant studies were removed from our review. In the step of full-text screening, 346 irrelevant articles were also excluded. Eventually, 63 publications were selected for the final analysis (Fig. 1 ).
Fig. 1.
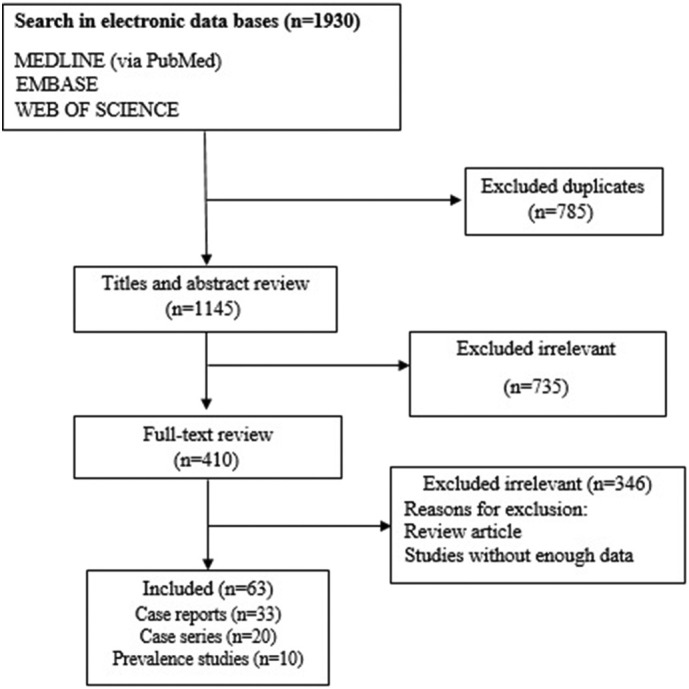
Flow chart of study selection for inclusion in systematic review.
3.2. The frequency of HIV among patients with COVID-19 based on evaluated studies
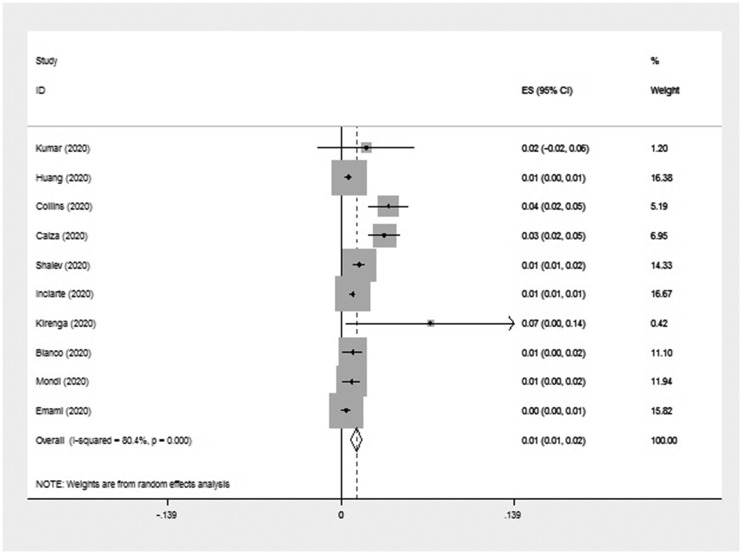
Out of 63 publications that reported the HIV among patients with COVID-19, ten articles (One from Africa, two from Asia, three from America, and four from Europe) were prevalence studies, and 53 articles (17 from Asia, 17 from Europe, 15 from America, and four from Africa) were case reports/case series (Table 1, Table 2 ). Meta-analyses of prevalence studies indicated that the frequency of HIV among patients with COVID-19 was reported in 6 countries (Uganda, China, Iran, USA, Italy, and Spain) with an overall frequency of 1.2% [(95% CI) 0.8–1.7] among 14,424 COVID-19 patients (Table 3 ). Fig. 2 summarizes almost all of the necessary data for a meta-analysis. The heterogeneity among assessed articles can be seen in Fig. 3, Fig. 4 . 167 patients with COVID-19 also have HIV.
Table 1.
Characteristics of included prevalence studies.
| First author | Country | Published time | Type of study | Number of patients with COVID-19 | Number of COVID-19 patients with HIV | Mean age | Male/female | Diagnostic method |
|---|---|---|---|---|---|---|---|---|
| Kumar et al. (2020) | USA | 2020 | Case series | 50 | 1 | 50 | M | Real-time PCR |
| Calza et al. (2020) | Italy | 2020 | Case series | 756 | 26 | 53.8 | 19M/7F | Real-time PCR, blood tests, CD4 count |
| Collins et al. (2020) | USA | 2020 | Case series | 530 | 20 | 57 | 13M/6F/1TSF | NR |
| Emami et al. (2020) | Iran | 2020 | Case series | 1239 | 5 | NR | NR | Molecular tests, HRCT |
| Huang et al. (2020) | China | 2020 | Case series | 2804 | 17 | NR | NR | serology, Real-time PCR |
| Inciarte et al. (2020) | Spain | 2020 | Case series | 5683 | 53 | 44 | 8F/45M | ELISA, Real time PCR |
| Kirenga et al. (2020) | Uganda | 2020 | Case series | 56 | 4 | NR | NR | Real-time PCR, CXR |
| Mondi et al. (2020) | Italy | 2020 | Case series | 604 | 5 | 49.6 | 4M/1TSF | Real-time PCR, serology, radiology imaging |
| Shalev et al. (2020) | USA | 2020 | Case series | 2159 | 31 | 60.7 | 24M/7F | Real-time PCR, CT scan, CXR, blood tests, HIV VL |
| Blanco et al. (2020) | Spain | 2020 | Case series | 543 | 5 | 37.8 | 3M/2TSF | Real-time PCR, CXR, blood tests, CD4+ count |
TSF; Trans Female, PCR; Polymerase Chain Reaction, HRCT; High-resolution computed tomography, VL; Viral Load, CXR; Chest X-Ray, ELISA; Enzyme-Linked Immunosorbent Assay, NR; not report.
Table 2.
Characteristics of case reports/case series studies.
| First author | Country | Published time | Type of study | Number of patients with COVID-19 | Number of COVID-19 patients with HIV | Mean age | Male/female | Diagnostic method |
|---|---|---|---|---|---|---|---|---|
| Parker et al. (2020) | South Africa | 2020 | Case report | 1 | 1 | 41 | M | Real-time PCR |
| Baluku et al. (2022) | Uganda | 2020 | Case report | 1 | 1 | 34 | F | Real-time PCR |
| Elhadi et al. (2020) | Libya | 2020 | Case report | 1 | 1 | 86 | F | CT, Real-time PCR |
| Khaba et al. (2020) | South Africa | 2020 | Case report | 1 | 1 | 19 | M | Real-time PCR, HIV VL, CD4+ count |
| Bertolini et al. (2020) | Argentina | 2020 | Case report | 1 | 1 | 43 | M | Real-time PCR, DH |
| Modi et al. (2019) | USA | 2020 | Case report | 1 | 1 | 32 | M | PCR, HIV VL, CD4+ count |
| Cipolat and Sprinz (2020) | Brazil | 2020 | Case report | 1 | 1 | 63 | F | Real-time PCR |
| Haddad et al. (2020) | USA | 2020 | Case report | 1 | 1 | 41 | M | Real-time PCR, blood test; CT scan |
| Rivas et al. (2020) | Panama | 2020 | Case series | 2 | 2 | 41 | 2M | Chest radiography, Real-time PCR, CD4 count, VL, CT scan, GeneXpert |
| Suwanwongse and Shabarek (2020) | USA | 2020 | Case series | 9 | 9 | 58 | 7M/2F | Real-time PCR, CXR, HIV VL, CD4+ count |
| Przydzial et al. (2020) | USA | 2020 | Case series | 2 | 2 | 57 | 2M | Real-time PCR, CXR, CT scan, blood test, HIV VL, CD4 count |
| Benkovic et al. (2020) | USA | 2020 | Case series | 4 | 4 | 59.75 | 4M | Real-time PCR, CXR, CD4 count |
| Pata et al. (2020) | USA | 2020 | Case series | 3 | 3 | 50.3 | 2M/F | Real-time PCR, CXR, CT scan, blood test |
| Farias et al. (2020) | Brazil | 2020 | Case series | 2 | 2 | 41 | 2M | CT-scan Real-time PCR, blood tests, PCR |
| Ridgway et al. (2020) | USA | 2020 | Case series | 5 | 5 | 48 | M/4F | PCR, CXR, CT-scan, CD4 count, blood testing |
| Chiappe Gonzalez et al. (2020) | Peru | 2020 | Case report | 1 | 1 | 38 | M | Real-time PCR, CT-scan; blood test; CD4 Count |
| Mahmood et al. (2020) | USA | 2020 | Case report | 1 | 1 | NR | M | CT |
| Byrd et al. (2020) | USA | 2020 | Case series | 9 | 9 | 55 | 7M/F/TSF | PCR, CT |
| Patel (2020) | USA | 2020 | Case report | 1 | 1 | 58 | M | Real-time PCR, CD4 count |
| Zhao et al. (2020) | China | 2020 | Case report | 1 | 1 | 38 | M | Serology, Real-time PCR |
| Zhang et al. (2020b) | China | 2020 | Case series | 2 | 2 | 30.5 | 2M | Blood test, CT-scan, Real-time PCR, serology |
| Rahbarnia et al. (2020) | Singapore | 2020 | Case series | 2 | 2 | 45 | 2M | Real-time PCR, CXR |
| Ruan et al. (2021) | China | 2020 | Case series | 4 | 4 | `40.75 | 4M | Real-time PCR, chest CT-scan, CD4 count, blood tests |
| Janakiram Marimuthu and Gandhi (n.d.) | South India | 2020 | Case series | 6 | 6 | 38 | 3M/2F/TSF | Real-time PCR, CT-scan; CXR, blood test |
| Adachi et al. (2020) | Japan | 2020 | Case series | 2 | 2 | 41.5 | 2TSF | Real-time PCR, CT-scan, blood test,CXR |
| Li et al. (2020b) | China | 2020 | Case report | 1 | 1 | 24 | M | CT, PCR, serology |
| Wu et al. (2020) | China | 2020 | Case report | 1 | 1 | 47 | M | CT |
| Nakamoto et al. (2020) | Japan | 2020 | Case report | 1 | 1 | 28 | M | Blood testing, CT-scan, Real-time PCR |
| Sun et al. (2020) | Singapore | 2020 | Case report | 1 | 1 | 37 | M | Chest radiogeraph, Real-time PCR, CD4+ count |
| Li et al. (2020b) | China | 2020 | Case report | 1 | 1 | 37 | M | CT, PCR, serology |
| Chen et al. (2020c) | China | 2020 | Case report | 1 | 1 | 24 | M | Real-time PCR, CT-scan |
| Zhu et al. (2020a) | China | 2020 | Case report | 1 | 1 | 61 | M | Real-time PCR, CT-scan, blood test |
| Wang et al. (2020b) | China | 2020 | Case report | 1 | 1 | 37 | M | Real-time PCR, CD4 count, CT-scan,serology,blood tests |
| Wu et al. (2020) | China | 2020 | Case report | 1 | 1 | 60 | M | CT, Real-time PCR |
| Zhu et al. (2020b) | China | 2020 | Case report | 1 | 1 | NR | NR | Real-time PCR |
| Tian et al. (2020) | China | 2020 | Case report | 1 | 1 | 24 | M | CT-scan, Real-time PCR, blood tests |
| Ciccullo et al. (2020) | Italy | 2020 | Case series | 4 | 2 | 27.5 | 2M | Real-time PCR, CT |
| Bartilotti Matos and Davies (2021) | UK | 2020 | Case report | 1 | 1 | 51 | M | CT, PCR |
| Di Giambenedetto et al. (2020) | Italy | 2020 | Case report | 1 | 1 | 75 | M | Real-time PCR, CXR |
| Riva et al. (2020) | Italy | 2020 | Case series | 3 | 3 | 60.6 | 2M/F | Real-time PCR, CXR |
| Aydin et al. (2020) | Turkey | 2020 | Case series | 4 | 4 | 37.25 | 4M | Real-time PCR, CT-scan, serology |
| Toombs et al. (2020) | UK | 2020 | Case series | 3 | 3 | 55 | 2M/F | CXR, blood tests, CD4 count, Real-time PCR |
| Sasset et al. (2020) | Italy | 2020 | Case series | 2 | 2 | 61.5 | 2M | Real-time PCR, chest radiograph, blood tests, HIV VL, CD4+ count |
| Bartilotti Matos and Davies (2021) | UK | 2020 | Case report | 1 | 1 | 38 | M | CT, PCR |
| d’Ettorre et al. (2020) | Italy | 2020 | Case report | 1 | 1 | 52 | F | Real-time PCR, CT-scan, blood test |
| Farinacci et al. (2020) | Italy | 2020 | Case report | 1 | 1 | 59 | M | PCR, CT |
| Coleman et al. (2020) | UK | 2020 | Case report | 1 | 1 | 55 | M | Multiplex PCR, Real-time PCR, CT-scan, |
| Iordanou et al. (2020) | Cyprus | 2020 | Case report | 1 | 1 | 58 | M | Real-time PCR |
| Molina-Iturritza et al. (2020) | Spain | 2020 | Case series | 8 | 8 | 47.5 | 7M/F | Real-time PCR |
| Pinnetti et al. (2020) | Italy | 2020 | Case report | 1 | 1 | 55 | NR | Real-time PCR, IFA, chest HRTC |
| Di Biagio et al. (2020) | Italy | 2020 | Case series | 4 | 4 | 65 | 3M/1F | CT |
| Joumaa et al. (2020) | France | 2020 | Case report | 1 | 1 | 65 | M | CT, Real-time PCR, VL, CD4 count |
| Pinnetti et al. (2020) | Italy | 2020 | Case report | 1 | 1 | 32 | NR | Chest HRTC, PCR, IFA |
TSF; Trans Female, PCR; Polymerase Chain Reaction, HRCT; High-resolution computed tomography, VL; Viral Load, CXR; Chest X-Ray, ELISA; Enzyme-Linked Immunosorbent Assay, IFA; Immunofluorescence assay, NR; not report.
Table 3.
Frequency of HIV among patients with COVID-19.
| COVID-19 patients with HIV | Prevalence% (95% CI) |
Number of studies | Number of patients | I-squared | ||
|---|---|---|---|---|---|---|
| Overall | 1.2 (0.8–1.7) | 10 | 167/14424 | 80.4% | ||
| Subgroup | Europe | Italy | 1.3 (0.6–2.1) | 4 | 89/7586 | 78.7% |
| Spain | ||||||
| America | USA | 2.4 (0.5–4.2) | 3 | 52/2739 | 72.6% | |
Fig. 2.
Forest plot of the meta-analysis on the prevalence of HIV among patients with COVID-19.
Fig. 3.
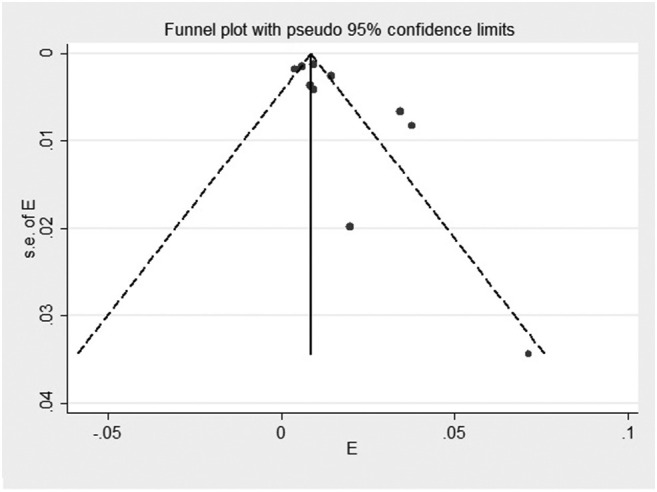
Funnel plot of the meta-analysis on the prevalence of HIV among patients with COVID-19.
Fig. 4.
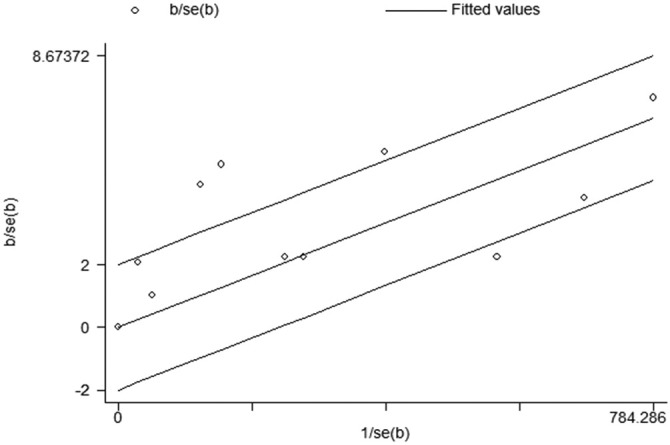
Galbraith of meta-analysis on the prevalence of HIV among patients with COVID-19.
3.3. The frequency of HIV among patients with COVID-19 among different continents based on prevalence studies
The meta-analysis of prevalence studies showed that the frequency of HIV among patients with COVID-19 was 2.4% (95% CI 0.5–4.2) among 2739 patients in America, 1.3% (95% CI 0.6–2.1) among 7586 patients in Europe, and 0.9% (95% CI 0.3–1.4) among 4043 patients in Asia (Table 3). There were no data on HIV infection prevalence among COVID-19 patients from Oceania. As shown in Table 1, the most COVID-19 patients with HIV were reported in Europe (Italy and Spain with 31 and 58 cases, respectively).
3.4. The frequency of HIV among patients with COVID-19 based on case reports/case series
We assessed the HIV infection among cases with COVID-19, which was reported in the mentioned electronic databases. Characteristics of case reports/case series studies (which were not taken into account during the analyses as mentioned above) are shown in Table 2. Conforming to the results of these studies, 111 HIV patients have been reported among 113 patients with COVID-19 from 19 countries (Table 4 ). Most of the cases were in the USA (36 cases), China (16 cases), Italy (16 cases), and Spain (8 cases) (Table 4). Among the patients whose gender was mentioned, 18 patients with HIV were women, 86 were men, 4 were trans females (TSF), and the rest (3 patients) were unknown. Evaluation of case reports/case series revealed that out of 111 patients with COVID-19, 78 patients had the underlying disease, most of them from Europe and America, respectively. The continents of Asia and Africa were also in the next ranks. At the time of this study, there were no reports of underlying disease in COVID-19 patients with HIV in Oceania. According to the results of the present study, the most common underlying diseases were hypertension (30/111), diabetes (18/111), cardiovascular diseases (12/111) and lung diseases (11/111), and obesity (9/111). In COVID-19 patients with HIV, the clinical symptoms were also considered. Thirty-eight forms of clinical symptoms have been identified in these patients, depending on the findings of the investigations (Table 4). Of these, fever (66/111), cough (56/111), dyspnea (50/111), diarrhea (11/111), and chest pain (10/111) were the most common clinical symptoms among COVID-19 patients with HIV infection. Of the 12 types of co-infections registered in the studies, infection with Hepatitis C virus (HCV) (Yang et al., 2020), Mycobacterium tuberculosis (Wang et al., 2020a), Pneumocystis jirovecii (Sameni et al., 2020), HBV/Candida albicans (3 studies for each), and Treponema pallidum/Cryptococcus neoformans (2 studies for each) were the most common infections in HIV-COVID-19 patients (Table 4). Accordingly, HCV (10.8%), M. tuberculosis (6.3%), and T. pallidum/P. jirovecii (3.6% for each) were the most common infections reported in COVID-19 patients with HIV. Computerized tomography (CT) scan was mentioned in 48 articles as one of the diagnostic methods used for COVID-19. The findings of patients' CT-scan were as follows: Ground-glass opacification (31.5%), bilateral abnormalities (16.2%), large cavitation (0.9%), pleural empyema (2.7%), reticular interstitial thickening (2.7%), high density patchy (2.7%), atypical bilateral pneumonia (14.4%), interstitial infiltrate (2.7%) and normal (8.1%). Ground-glass opacification was reported in 24 studies and 36 patients (Table 4), which was the most common finding of CT scans in the evaluated patients. Laboratory examination of patients showed that elevated C-reactive protein (CRP) (44.1%), low CD4 (38.7%), lymphocytopenia (29.7%), high LDH (24.3%), elevated ferritin (17.1%), and increased D-dimer (16.2%) were the most common findings (Table 4). Additionally, the study evaluated the patients' outcomes. The analyzed studies showed that 83 out of 111 patients survived, 19 died, and nine patients' outcomes were not reported (Table 4).
Table 4.
Summary of the case reports/case series findings.
| Overall | ||||
|---|---|---|---|---|
| Types of study | Number of studies | Total patients with COVID-19 | Total patients with COVID-19 and HIV | n/Na (%) |
| Case report | 33 | 36 | 36 | 36/36 (100.0) |
| Case series | 20 | 77 | 75 | 75/77 (97.4) |
| Variables | Number of studies | Number of patients with COVID-19 and HIV | n/Na (%) | |
|---|---|---|---|---|
| Underlying disease | Metabolic syndrome | 1 | 1 | 1/111 (0.9) |
| Dementia | 1 | 1 | 1/111 (0.9) | |
| Cancer | 4 | 4 | 4/111 (3.6) | |
| CVAb | 2 | 2 | 2/111 (1.8) | |
| COPDc | 4 | 7 | 7/111 (6.3) | |
| Obesity | 7 | 9 | 9/111 (8.1) | |
| Renal disease | 4 | 4 | 4/111 (3.6) | |
| Hyperlipidemia | 3 | 3 | 3/111 (2.7) | |
| Asthma | 1 | 1 | 1/111 (0.9) | |
| Hypertension | 18 | 30 | 30/111 (27) | |
| Obstructive sleep apnea | 1 | 1 | 1/111 (0.9) | |
| Coronary heart disease | 9 | 12 | 12/111 (10.8) | |
| Diabetes | 12 | 18 | 18/111 (16.2) | |
| Liver disease | 1 | 1 | 1/111 (0.9) | |
| Pulmonary disease | 5 | 11 | 11/111 (9.9) | |
| Clinical manifestation | Cough | 34 | 56 | 56/111 (50.4) |
| Diarrhea | 11 | 11 | 11/111 (9.9) | |
| Skin lesion | 2 | 2 | 2/111 (1.8) | |
| Hypoventilation | 1 | 1 | 1/111 (0.9) | |
| Fatigue | 8 | 8 | 8/111 (7.2) | |
| Myalgia | 9 | 9 | 9/111 (8.1) | |
| Poor appetite | 3 | 3 | 3/111 (2.7) | |
| Fever | 41 | 66 | 66/111 (59.4) | |
| Blood pressure | 5 | 7 | 7/111 (6.3) | |
| Anorexia | 4 | 4 | 4/111 (3.6) | |
| Headache | 7 | 9 | 9/111 (8.1) | |
| Hypoxemia | 4 | 4 | 4/111 (3.6) | |
| Malaise | 2 | 2 | 2/111 (1.8) | |
| Weight loos | 1 | 1 | 1/111 (0.9) | |
| Dysphagia | 1 | 1 | 1/111 (0.9) | |
| Dyspnoea | 30 | 50 | 50/111 (45.0) | |
| Nausea | 2 | 2 | 2/111 (1.8) | |
| Adynamia | 1 | 2 | 2/111 (1.8) | |
| Hyposmia | 1 | 1 | 1/111 (0.9) | |
| Sore throat | 8 | 8 | 8/111 (7.2) | |
| Asthenia | 2 | 5 | 5/111 (4.5) | |
| Weakness | 6 | 6 | 6/111 (5.4) | |
| Muscle aches | 1 | 1 | 1/111 (0.9) | |
| Chills | 1 | 1 | 1/111 (0.9) | |
| Chest pain | 10 | 10 | 10/111 (9.0) | |
| Tachycardia | 2 | 3 | 3/111 (2.7) | |
| Abdominal pain | 5 | 5 | 5/111 (4.5) | |
| Encephalopathy | 1 | 1 | 1/111 (0.9) | |
| Rhinorrhea | 1 | 1 | 1/111 (0.9) | |
| Wheezing | 1 | 1 | 1/111 (0.9) | |
| Dizziness | 2 | 2 | 2/111 (1.8) | |
| Confusion | 3 | 3 | 3/111 (2.7) | |
| Vomiting | 2 | 2 | 2/111 (1.8) | |
| Hypogeusia | 1 | 1 | 1/111 (0.9) | |
| Seizure | 1 | 1 | 1/111 (0.9) | |
| Pharyngitis | 1 | 2 | 2/111 (1.8) | |
| Concurrent infection | Hepatitis C virus | 6 | 12 | 12/111 (10.8) |
| M. tuberculosis | 5 | 7 | 7/111 (6.3) | |
| Histoplasma capsulatum | 1 | 1 | 1/111 (0.9) | |
| Hepatitis B virus | 3 | 3 | 3/111 (2.7) | |
| Herpes simplex virus | 1 | 1 | 1/111 (0.9) | |
| T. pallidum | 2 | 4 | 4/111 (3.6) | |
| S. pneumoniae | 1 | 1 | 1/111 (0.9) | |
| Influenza A virus | 1 | 1 | 1/111 (0.9) | |
| P. jirovecii | 4 | 4 | 4/111 (3.6) | |
| C. albicans | 3 | 3 | 3/111 (2.7) | |
| C. neoformans | 2 | 2 | 2/111 (1.8) | |
| CT Scan findings | Ground-glass opacification | 24 | 35 | 35/111 (31.5) |
| Bilateral abnormalities | 15 | 18 | 18/111 (16.2) | |
| Large cavitation | 1 | 1 | 1/111 (0.9) | |
| Pleural empyema | 3 | 3 | 3/111 (2.7) | |
| Reticular interstitial thickening | 1 | 3 | 3/111 (2.7) | |
| High-density patchy | 3 | 3 | 3/111 (2.7) | |
| Atypical bilateral pneumonia | 12 | 16 | 16/111 (14.4) | |
| Interstitial infiltrate | 2 | 3 | 3/111 (2.7) | |
| Normal | 4 | 9 | 9/111 (8.1) | |
| Laboratory findings | Lymphocytopenia | 20 | 33 | 33/111 (29.7) |
| Leukopenia | 6 | 7 | 7/111 (6.3) | |
| Thrombocytopenia | 3 | 3 | 3/111 (2.7) | |
| Elevated CRPd | 30 | 49 | 49/111 (44.1) | |
| Elevated platelet | 1 | 1 | 1/111 (0.9) | |
| Elevated IL-6 | 5 | 12 | 12/111 (10.8) | |
| Neutrophilia | 3 | 3 | 3/111 (2.7) | |
| High lymphocyte | 6 | 8 | 8/111 (7.2) | |
| High ferritin | 9 | 19 | 19/111 (17.1) | |
| High ESRe | 1 | 1 | 1/111 (0.9) | |
| High WBC | 7 | 9 | 9/111 (8.1) | |
| High LDHf | 16 | 27 | 27/111 (24.3) | |
| Increased ASTg | 7 | 12 | 12/111 (10.8) | |
| Increased ALTh | 7 | 11 | 11/111 (9.9) | |
| D-dimer elevated | 11 | 18 | 18/111 (16.2) | |
| Anemia | 1 | 2 | 2/111 (1.8) | |
| High CD4 | 4 | 5 | 5/111 (4.5) | |
| Low CD4 | 25 | 43 | 43/111 (38.7) | |
| Detection methods | Serology | 21 | 45 | 45/111 (40.5) |
| Real-time PCR | 42 | 85 | 85/111 (76.5) | |
| CTi Scan | 35 | 70 | 70/111 (63.0) | |
| CXRj | 10 | 35 | 35/111 (31.5) | |
| PCRk | 11 | 24 | 24/111 (21.6) | |
| Gender | Male | 46 | 86 | 86/111 (77.4) |
| Female | 13 | 18 | 18/111 (16.2) | |
| TSFl | 3 | 4 | 4/111 (3.6) | |
| Outcome | Live | 41 | 83 | 83/111 (74.7) |
| Death | 10 | 19 | 19/111 (17.1) |
n, number of patients with any variables; N, the total number of patients with COVID-19, nr; not reported.
Cerebrovascular accident.
Chronic obstructive pulmonary disease.
C-reactive protein.
Erythrocyte sedimentation rate.
Lactate dehydrogenase.
Aspartate aminotransferase.
Alanine aminotransferase.
Computerized tomography.
Chest X-rays.
Polymerase chain reaction.
Trans female.
In the case reports/case series articles, the drugs used to treat COVID-19 patients with HIV are summarized in Table 5 . Emtricitabine (47.7%), tenofovir (64.2%), lamivudine (26.6%), ritonavir (22.0%), and dolutegravir (21.1%) were the most widely used antiretroviral drugs for the treatment of these patients. According to the results, 18 studies reported the use of ritonavir for the treatment of patients; of these, five studies (27.7%) reported the use of a combination of atazanavir/ritonavir, and 13 studies (72.22%) reported the use of a combination of lopinavir/ritonavir for the effective treatment. Among the antibacterial agents, azithromycin (25.6%), ceftriaxone (13.7%), and moxifloxacin (8.2%) were the most widely used drugs reported in 16, 8, and 6 studies, respectively. Several treatment regimens, including hydroxychloroquine (HCQ) and azithromycin, have been used in some studies. Other used drug combinations are presented in Table 5 as “others”. As it turns out, HCQ (in 18 studies), enoxaparin (in 5 studies), methylprednisolone, atorvastatin, and prednisolone (3 studies for each) were other drug combinations that were used more than others in the treatment of patients.
Table 5.
Agents used in the treatment of COVID-19 patients.
| Agent | Number of studies | n/Na | % | |
|---|---|---|---|---|
| Antiviral drug | Oseltamivir | 6 | 10 | 10/111 (9.0) |
| Efavirenz | 13 | 17 | 17/111 (15.3) | |
| Lopinavir | 12 | 18 | 18/111 (16.2) | |
| Ritonavir | 18 | 24 | 24/111 (21.6) | |
| Emtricitabine | 27 | 52 | 52/111 (46.8) | |
| Tenofovir | 42 | 70 | 70/111 (63.0) | |
| Raltegravir | 8 | 10 | 10/111 (9.0) | |
| Lamivudine | 19 | 29 | 29/111 (26.1) | |
| Dolutegravir | 17 | 23 | 23/111 (20.7) | |
| Atazanavir | 4 | 5 | 5/111 (4.5) | |
| Zidovudine | 3 | 4 | 4/111 (3.6) | |
| Rilpivirine | 3 | 5 | 5/111 (4.5) | |
| Darunavir | 9 | 19 | 19/111 (17.1) | |
| Cobicistat | 11 | 18 | 18/111 (16.2) | |
| Acyclovir | 1 | 1 | 1/111 (0.9) | |
| Remdesivir | 2 | 2 | 2/111 (1.8) | |
| Elvitegravir | 6 | 10 | 10/111 (9.0) | |
| Abacavir | 6 | 8 | 8/111 (7.2) | |
| Nevirapine | 2 | 3 | 3/111 (2.7) | |
| Maraviroc | 1 | 1 | 1/111 (0.9) | |
| Etravirine | 1 | 1 | 1/111 (0.9) | |
| Bictegravir | 5 | 7 | 7/111 (6.3) | |
| Umifenovir | 6 | 8 | 8/111 (7.2) | |
| Ribavirin | 1 | 1 | 1/111 (0.9) | |
| Protease inhibitor | 1 | 3 | 3/111 (2.7) | |
| Nucleoside reverse transcriptase inhibitors | 1 | 7 | 7/111 (6.3) | |
| Integrase inhibitor | 1 | 4 | 4/111 (3.6) | |
| IFNb b | 1 | 2 | 2/111 (1.8) | |
| IFN αlb | 1 | 1 | 1/111 (0.9) | |
| Antibacterial drug | Azithromycin | 16 | 28 | 28/111 (25.9) |
| Clindamycin | 1 | 1 | 1/111 (0.9) | |
| Ampicillin | 1 | 1 | 1/111 (0.9) | |
| Cefoperazone-sulbactam | 2 | 2 | 2/111 (1.8) | |
| Cefepime | 1 | 1 | 1/111 (0.9) | |
| Amoxicillin–clavulanic acid | 3 | 3 | 3/111 (2.7) | |
| Sulfamethoxazole | 1 | 1 | 1/111 (0.9) | |
| Levofloxacin | 5 | 5 | 5/111 (4.5) | |
| Piperacillin/Tazobactam | 3 | 3 | 3/111 (2.7) | |
| Gentamicin | 1 | 1 | 1/111 (0.9) | |
| Spiramycin | 1 | 1 | 1/111 (0.9) | |
| Cefotaxime | 1 | 1 | 1/111 (0.9) | |
| Trimethoprim-sulfamethoxazole | 9 | 10 | 10/111 (9.0) | |
| Benzylpenicillin | 1 | 1 | 1/111 (0.9) | |
| Rifabutin | 2 | 2 | 2/111 (1.8) | |
| Tazocin | 2 | 2 | 2/111 (1.8) | |
| Doxycycline | 2 | 2 | 2/111 (1.8) | |
| Isoniazid | 3 | 4 | 4/111 (3.6) | |
| Meropenem | 3 | 4 | 4/111 (3.6) | |
| Ethambutol | 4 | 4 | 4/111 (3.6) | |
| Pyrazinamide | 2 | 2 | 2/111 (1.8) | |
| Rifampin | 2 | 2 | 2/111 (1.8) | |
| Ciprofloxacin | 2 | 2 | 2/111 (1.8) | |
| Cefdinir | 1 | 3 | 3/111 (2.7) | |
| Ceftriaxone | 8 | 15 | 15/111 (13.5) | |
| Vancomycin | 3 | 3 | 3/111 (2.7) | |
| Moxifloxacin | 7 | 9 | 9/111 (8.1) | |
| Others | Hydroxychloroquine | 18 | 28 | 28/111 (25.2) |
| Enoxaparin | 5 | 5 | 5/111 (4.5) | |
| Hormonal anti-inflammatory therapy | 1 | 1 | 1/111 (0.9) | |
| Ibuprofen | 1 | 1 | 1/111 (0.9) | |
| Heparin | 2 | 2 | 2/111(1.8) | |
| Methylprednisolone | 3 | 6 | 6/111 (5.4) | |
| Human serum albumin | 2 | 2 | 2/111 (1.8) | |
| Thymosin | 1 | 1 | 1/111(0.9) | |
| Ulinastatin | 1 | 1 | 1/111 (0.9) | |
| Aspirin | 1 | 1 | 1/111 (0.9) | |
| Atorvastatin | 3 | 3 | 3/111 (2.7) | |
| Ezetimibe | 1 | 1 | 1/111 (0.9) | |
| Bisoprolol | 1 | 1 | 1/111 (0.9) | |
| Trimetazidine | 1 | 1 | 1/111 (0.9) | |
| Alogliptin | 1 | 1 | 1/111 (0.9) | |
| Metformin | 2 | 2 | 2/111 (1.8) | |
| γ-globulin | 1 | 1 | 1/111 (0.9) | |
| Prednisolone | 3 | 4 | 4/111 (3.6) | |
| Mycophenolate mofetil | 1 | 1 | 1/111 (0.9) | |
| Tacrolimus | 1 | 1 | 1/111 (0.9) | |
| Anti-thymocyte globulin | 1 | 1 | 1/111 (0.9) | |
| Amphotericin B deoxycholate | 2 | 2 | 2/111 (1.8) | |
| Itraconazole | 1 | 1 | 1/111 (0.9) | |
| Acetaminophen | 2 | 2 | 2/111 (1.8) | |
| Zinc sulfate | 2 | 2 | 2/111 (1.8) | |
| Vitamin C | 1 | 1 | 1/111 (0.9) | |
| Hydrocortisone | 1 | 1 | 1/111 (0.9) | |
| Hydrochlorothiazide | 1 | 1 | 1/111 (0.9) | |
| Losartan | 1 | 1 | 1/111 (0.9) | |
| Alfuzosin | 1 | 1 | 1/111 (0.9) | |
| Doxazosin | 1 | 1 | 1/111 (0.9) | |
| Metoprolol | 1 | 1 | 1/111 (0.9) | |
| Amlodipine | 1 | 1 | 1/111 (0.9) | |
| Irbesartan | 1 | 1 | 1/111 (0.9) | |
| Nebivolol | 1 | 1 | 1/111 (0.9) | |
| Antipyretics | 1 | 2 | 2/111 (1.8) | |
| Lisinopril | 1 | 1 | 1/111 (0.9) | |
| Coumadin | 1 | 1 | 1/111 (0.9) | |
| Vitamin A | 1 | 1 | 1/111 (0.9) | |
| Vitamin D | 1 | 1 | 1/111 (0.9) | |
| Antihypertensive | 1 | 2 | 2/111 (1.8) | |
| Paracetamol | 2 | 6 | 6/111 (5.4) | |
| Antitussives | 1 | 2 | 2/111 (1.8) | |
| Iron-folic acid | 1 | 1 | 1/111 (0.9) | |
| Vitamin supplements | 1 | 5 | 5/111 (4.5) | |
| Fluconazole | 2 | 2 | 2/111 (1.8) | |
| Rehydration salts | 1 | 1 | 1/111 (0.9) | |
| Corticosteroids | 2 | 2 | 2/111 (1.8) | |
| Intravenous (IV) proton-pump inhibitors | 1 | 1 | 1/111 (0.9) | |
| Chloroquine | 1 | 1 | 1/111 (0.9) | |
| Caspofungin | 1 | 1 | 1/111 (0.9) | |
| Perindopril | 1 | 1 | 1/111 (0.9) | |
| Sarilumab | 1 | 1 | 1/111 (0.9) | |
| Liposomal Amphotericin B | 1 | 1 | 1/111 (0.9) | |
| Flucytosine | 1 | 1 | 1/111 (0.9) | |
| Insulin | 1 | 1 | 1/111 (0.9) | |
| Mucolytic syrup | 1 | 1 | 1/111 (0.9) |
n; the number of patients under treatment, N; the total number of patients with COVID-19.
Interferon.
4. Discussion
COVID-19, which emerged in late 2019, has infected more than 60 million people globally and has caused many deaths in various countries (Lee et al., 2020; Giannattasio et al., 2010). So, it can be assumed that this disease is a tremendous threat to human life worldwide. Although countless valuable studies have been carried out on the virus in the short term, there is still much ambiguity in fully identifying its pathogenic behavior in humans. The relationship between COVID-19 and other infections is among the most critical issues to consider. An elevated risk of COVID-19 and death appears to be associated with certain diseases, such as HIV. However, there is little evidence on how HIV infection can affect the risk of COVID-19. According to various studies conducted on the role of HIV in respiratory virus epidemics previously, HIV is associated with an increased risk of respiratory infections, including seasonal flu (Giannattasio et al., 2010).
In the present study, prevalence studies, as well as case reports and case series studies, were reviewed and analyzed. Accordingly, 14,424 patients with COVID-19 in prevalence studies and 113 similar patients in case reports and case series researches were screened for HIV infection. Meta-analysis of the prevalence studies showed that most COVID-19 patients infected with HIV were from continental America (mainly from the USA); the frequency of these patients was 2.4% (0.5–4.2). In this regard, the frequency of these patients in published studies from Europe was 1.3% (0.6–2.1) which was mainly reported in Italy and Spain. Accordingly, the prevalence of COVID-19 patients with concurrent HIV is higher in the United States than in Europe. Based on case reports and case series studies, the United States, with 36 reported cases, had the highest number of patients co-infected with SARS-CoV-2 and HIV. Genetics, age, lifestyle, diet, environmental parameters, and geographic area in which patients live are some of the reasons for this difference in the prevalence of COVID-19 and HIV infected patients. On the other hand, health facilities, the quality of patient care in hospitals, and the ability to accurately diagnose the disease also influence this difference.
One of the most critical issues in the current study was the evaluation of underlying diseases in people co-infected with SARS-CoV-2 and HIV. Evaluation of case reports and case series studies showed that 78 out of 111 COVID-19 patients co-infected with HIV had underlying diseases, mainly from continental Europe followed by continental America. The continents of Asia and Africa were also in the following ranks. It should be noted that at the time of this study, there were no reports of underlying disease in patients with COVID-19 and concurrent HIV infection from Oceania. According to the results of the present study, the most common underlying diseases in the subjects were hypertension, diabetes, cardiovascular, and lung diseases. The cohort study results revealed that some of the mentioned underlying diseases (predominantly hypertension and diabetes) in patients infected with SARS-CoV-2 and HIV are higher than in those with HIV without COVID-19 (Vizcarra et al., 2020). In a review study conducted by Mirzaei et al. (2020), hypertension, obesity, chronic obstructive pulmonary disease, and diabetes were the most common underlying diseases.
Consequently, high blood pressure, diabetes, and lung diseases can be regarded as frequent underlying diseases in COVID-19 patients with HIV. These underlying diseases were also reported in a study conducted by McMichael et al. (2020). In their study of the 81 COVID-19 patients admitted to the hospital, 69.1% had high blood pressure, 43.2% had kidney failure, and 37.0% had diabetes. Based on evaluated case reports and case series studies, the number of male patients was four times higher than female patients, and the mean age of all patients was 50 years.
As noted in the present study, the most common clinical symptoms reported in patients with SARS-CoV-2-HIV co-infection were fever, cough, respiratory and gastrointestinal problems. Also, hypoventilation, inappetence, weight loss, dysphagia, hyposmia, muscle aches, chills, encephalopathy, rhinorrhea, wheezing, hypogeusia, seizure, and pharyngitis were the least common. As mentioned above, Simultaneous infection between different microorganisms and SARS-COV-2 is a serious problem in the COVID-19 pandemic, especially in patients with underlying conditions. However, there are few reports about SARS-CoV-2 co-infection with other microorganisms. Obtaining clinical data about infections coinciding with SARS-CoV-2 is of great value and plays an important role in adopting more appropriate treatment regimens for patients with COVID-19. Different studies revealed that some infections are considerably higher in patients with severe forms of COVID-19 than those without COVID-19 (Cohen et al., 2022; Scott et al., 2022; Chen et al., 2020a). Therefore, extensive researches are needed to determine the types and prevalence of COVID-19 concomitant infections to adopt appropriate treatment and prevention strategies as well as to avoid the complications of these concomitant infections. The findings of such studies will help significantly reduce the mortality rate of coronavirus-infected patients who have other infections. In this regard, another parameter to consider in our study was the evaluation of co-infections with SARS-COV-2. According to the results of our study, infection with HCV, M. tuberculosis, Streptococcus pneumoniae, P. jirovecii, Hepatitis B virus (HVB), and C. albicans were the most common infections in COVID-19 patients with HIV. As reported by recent clinical studies, concomitant virus infection mainly includes respiratory viruses such as entero/rhinovirus, human metapneumovirus, respiratory syncytial virus (RSV), and coronavirus (other than SARS-CoV-2) (Lin et al., 2020). On the other hand, GU et al. (Jiang et al., 2020) have recently discovered that the relative abundance of opportunistic pathogens such as Streptococcus, Rothia, Veillonella, and Actinomyces is significantly higher in patients with COVID-19. Meanwhile, a descriptive study by Chen et al. (2020b) revealed that fungi such as Aspergillus spp. C. albicans and Candida glabrata are other prevalent microorganisms that can cause co-infections in patients with COVID-19. In a study by Lansbury et al. (2020), it was found that 7% of patients with COVID-19 had bacterial concomitant infections. In this research, M. pneumoniae, Pseudomonas aeruginosa, Haemophilus influenzae, and RSV and influenza A virus were the most common microorganisms causing infection in patients suffering from COVID-19. Another important issue in COVID-19 is the use of appropriate diagnostic methods. CT scan is one of the diagnostic methods with optimal sensitivity (Radpour et al., 2020). This procedure can be beneficial for the diagnosis of infection as well as for assessing lung involvement and disease progression in patients with COVID-19. However, this method may not detect lung involvement in the early stages of the disease and may not reliably confirm the presence of COVID-19 in the patients (Chate et al., 2020). Based on the present study results, CT-scan findings in most reported COVID-19 HIV-infected patients were ground-glass opacification, bilateral abnormalities, and atypical bilateral pneumonia, respectively. However, in some cases, patients had normal CT scans. Other manifestations such as adjacent pleural, paving patterns, mediastinal lymphadenopathies, and hilar lymphadenopathies have not been reported. Evaluating the laboratory results of patients with SARS-CoV-2–HIV co-infection in the current study showed that certain important factors, such as elevated CRP, lymphocytopenia, and increased D-dimer, have been more commonly identified in the patients' laboratory test results. Zhang et al.'s research (Zhang et al., 2020a) also indicates an increase in CRP in 92.14% of 140 COVID-19 patients. While T-cells levels can initially increase in COVID-19, these patients generally appear to have low lymphocyte counts. This condition is associated with an increase in the severity of COVID-19. Therefore, it was found that people who died of COVID-19 had lower lymphocyte counts (Jafarzadeh et al., 2021). In addition, recent studies have shown that about 85% of patients with severe forms of COVID-19 suffer from lymphopenia (Fathi and Rezaei, 2020). A study conducted by Zheng et al. (2020) revealed that NK and CTL cells were significantly reduced in patients with COVID-19. Furthermore, another study indicated that the total number of CD8+ and CD4+T cells in SARS-CoV-2 patients was significantly reduced, especially in patients over 60 years of age and in the intensive care unit (Diao et al., 2020). As noted by Zhang et al. (2020a), infection with SARS-CoV-2 increases D-dimer level and destroys the fibrinogen products that lead to acro ischemia, which is associated with cyanosis of the fingers. Several studies have also shown a rise in D-dimer in laboratory findings of COVID-19 patients (Léonard-Lorant et al., 2020; Shah et al., 2020; Levi et al., 2020). This laboratory evidence is probably related to the severity of the disease, although it is not possible to state with certainty the exact cause and origin of these laboratory changes in patients. In general, based on various studies, it is concluded that there is no significant difference between the main clinical symptoms of COVID-19 in people with and without HIV infection (Collins, 2020; Ssentongo et al., 2021; Cabello et al., 2021).
Despite numerous efforts to control the COVID-19 pandemic, the disease remains a significant challenge to the global community and a threat to public health. Although much research has been done to combat COVID-19 worldwide, there is no definitive cure. Nevertheless, supportive treatments such as fever-reducing steps, oxygen therapy to alleviate the respiratory problems of patients, rest at home, and drinking fluids in the early stages of the disease may be beneficial. According to the analysis performed in the present study, the most antiviral drugs prescribed for patients include tenofovir, emtricitabine, and ritonavir (as HIV protease inhibitors). A clinical trial performed on 199 patients showed that ritonavir-boosted lopinavir had no advantages compared to standard care for COVID-19 (Xu et al., 2017). Another study in HIV-infected people with COVID-19 reported from China found that reverse transcriptase nucleoside inhibitors (NRTIs) plus non-nucleoside reverse transcriptase (RT) inhibitors (NNRTIs) did not prevent COVID-19 infection (Livingston and Bucher, 2020). Studies have shown that in addition to antiviral drugs, azithromycin and HCQ are among the most widely used drugs in treating patients with COVID-19. In addition, medications such as tocilizumab, paracetamol, methylprednisolone as well as vitamin supplements are among the most widely used compounds in the treatment regimen of patients with HIV and COVID-19. Unfortunately, there is inadequate information about the effectiveness of the drugs listed in Table 5. So, the exact efficiency of these agents in the management of patients cannot be determined.
Another point to note is the death rate in patients with COVID19-HIV, which seems to be high according to the findings of the current report (17.1%). However, other confounding factors may influence this result, such as patients' old age or underlying diseases. Several underlying diseases and old age were common among patients who died because of COVID-19 whose personal data were recorded. It is also noteworthy that only patients referred to the hospital were assessed in selected studies, so this may be another factor that would have impacted the death rate, which is higher than in the general population. Some studies revealed no sufficient evidence showing a varying disease course or higher COVID-19 infection or even death rates in HIV-infected people compared to non-infected ones (Prabhu et al., 2020; Sarkar et al., 2021). Despite this finding, another study conducted by Mellor et al. found that people with HIV had a higher risk of COVID-19 mortality than the general population (Mellor et al., 2021). There is much debate about whether HIV plays a role in COVID-19 prognosis. Further research is needed to resolve this issue.
Current clinical findings suggest that old age, concomitant infections such as HIV, and underlying disorders such as hypertension, cardiovascular disease, diabetes, and chronic respiratory disease are the key risk factors for mortality due to this virus (World Health, 2020). In general, adequate infection control coupled with the development of effective drugs in the treatment of COVID-19 is crucial to substantial progress in combating the disease and reducing mortality. Therefore, it is essential to follow infection control protocols in health care. The lack of preventive health initiatives, inappropriate staff training, and deficient hospital infection control programs may play an important role in raising the number of patients with COVID-19 and likely increasing the morbidity and mortality in patients, especially those who suffer from HIV infection.
Eventually, it is essential to note the study's limitations. First of all, only patients referred to the hospital were assessed, and their information was registered and documented in the results of the papers. A variety of patients with SARS-CoV-2-HIV may not be admitted to hospitals, and most have been treated at home. It may also make it impossible for us to accurately measure the rate of HIV infection in people with COVID-19 worldwide. Second, some published reports lacked the criteria to enter our study. For example, some articles did not have enough data such as laboratory findings, co-morbidities, CT-scan results, and results to analyze the factors mentioned in the current study. Third, since there are inadequate data on the number of COVID19-HIV patients in many countries, it was challenging to determine a complete picture of the prevalence of COVID19-HIV patients worldwide. Fourth, in the present study, only patients with related symptoms were examined, while most COVID-19 patients were asymptomatic or had mild symptoms. Fifth, a further restriction of this research was that several papers did not publish adequate details to determine the side effects of medications or infections. As a result, we have not been able to analyze them and explore in-depth the exact mechanisms by which they work and their impact on the recovery process of patients with COVID19-HIV.
5. Conclusion
SARS-CoV-2 co-infection can be a further burden to people living with HIV. Due to the importance of assessing the status of patients suffering from simultaneous HIV and COVID-19, various studies have been conducted in this field that has provided different information. In the present study, we summarized the results presented in these articles. However, due to the small number of articles reviewed and the patients reported in them, we could not provide an accurate and general conclusion about the impact of HIV on the morbidity and mortality rate of COVID-19. Future researches should address a better understanding of the factors that may increase the mortality rate of COVID-19 among people living with HIV around the world. On the other hand, it should be noted that in the conducted studies, only the results of the evaluation of patients with clinical symptoms have been reported, HIV-infected patients may have no clinical symptoms, and no information is available regarding this population. This highlights the need for more comprehensive studies on different populations of patients to achieve more accurate conclusions about the association between HIV and COVID-19.
CRediT authorship contribution statement
MD and BH designed the study. BH, AD, and FS conducted the search strategy. BH, AD, FS, MG, and RA performed the data extraction. MD, MGH, and BH wrote and edited the manuscript. MD carried out the statistical analysis. MD, MJN, and SHY assumed overall responsibility for the accuracy and integrity of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
No funding.
Ethical approval
Not required.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank Dr. Ali Hashemi, Department of Microbiology at Shahid Beheshti University of Medical Sciences, Tehran, Iran, for his sincere assistance and efforts to make this project happen.
Edited by Jormay Lim
References
- Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- F. Wu S. Zhao B. Yu Y.-M. Chen W. Wang Z.-G. Song A new coronavirus associated with human respiratory disease in China Nature 579 7798 2020 265 9. [DOI] [PMC free article] [PubMed]
- Garg S. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69 doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sameni F., Hajikhani B., Yaslianifard S., Goudarzi M., Owlia P., Nasiri M.J., et al. COVID-19 and skin manifestations: an overview of case reports/case series and meta-analysis of prevalence studies. Front. Med. 2020;7 doi: 10.3389/fmed.2020.573188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhou J., Marshall B., Rekaya R., Ye K., Liu H.X. SARS-CoV-2 receptor ACE2 is enriched in a subpopulation of mouse tongue epithelial cells in nongustatory papillae but not in taste buds or embryonic oral epithelium. ACS Pharmacol. Transl. Sci. 2020;3(4):749–758. doi: 10.1021/acsptsci.0c00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;Mar 12;10(10.1016) doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010;125(2 Suppl 2):S3–S23. doi: 10.1016/j.jaci.2009.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dropulic L.K., Cohen J.I. Severe viral infections and primary immunodeficiencies. Clin. Infect. Dis. 2011;53(9):897–909. doi: 10.1093/cid/cir610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabbe S., Beissert S., Enk A. Systemic immunosuppression in times of COVID-19: do we need to rethink our standards? J. Dtsch. Dermatol. Ges. 2020;18(8):810–813. doi: 10.1111/ddg.14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust J.S., Lin Z., Del Rio C. Comparison of estimated excess deaths in New York City during the COVID-19 and 1918 influenza pandemics. JAMA Netw. Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.17527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., et al. Coronavirus infections and immune responses. J. Med. Virol. 2020;92(4):424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C., Casado J.L., Härter G., Vizcarra P., Moreno A., Cattaneo D., et al. Immune deficiency is a risk factor for severe COVID-19 in people living with HIV. HIV Med. 2021;22(5):372–378. doi: 10.1111/hiv.13037. [DOI] [PubMed] [Google Scholar]
- Gaziano R., Pistoia E., Campione E., Fontana C., Marino D., Favaro M., et al. Immunomodulatory agents as potential therapeutic or preventive strategies for COVID-19. Eur. Rev. Med. Pharmacol. Sci. 2021;25(11):4174–4184. doi: 10.26355/eurrev_202106_26061. [DOI] [PubMed] [Google Scholar]
- Hall M.W., Joshi I., Leal L., Ooi E.E. Immune immunomodulation in coronavirus disease 2019 (COVID-19): strategic considerations for personalized therapeutic intervention. Clin. Infect. Dis. 2022;74(1):144–148. doi: 10.1093/cid/ciaa904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.Y.-P., Lin R.T.P., Renia L., Ng L.F.P. Serological approaches for COVID-19: epidemiologic perspective on surveillance and control. Front. Immunol. 2020;11(879) doi: 10.3389/fimmu.2020.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannattasio A., Vecchio A.L., Russo M.T., Pirozzi M.R., Barbarino A., Ruberto E., et al. Pandemic flu: a comparative evaluation of clinical, laboratory, and radiographic findings in HIV-positive and negative children. AIDS. 2010;24(14):2292–2294. doi: 10.1097/QAD.0b013e32833d2096. [DOI] [PubMed] [Google Scholar]
- Vizcarra P., Pérez-Elías M.J., Quereda C., Moreno A., Vivancos M.J., Dronda F., et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;7(8):e554–e564. doi: 10.1016/S2352-3018(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei H., McFarland W., Karamouzian M., Sharifi H. COVID-19 among people living with HIV: a systematic review. AIDS Behav. 2020;1–8 doi: 10.1007/s10461-020-02983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael T.M., Clark S., Pogosjans S., Kay M., Lewis J., Baer A., et al. COVID-19 in a long-term care facility - King County, Washington, February 27-march 9, 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(12):339–342. doi: 10.15585/mmwr.mm6912e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R., Finn T., Babushkin F., Geller K., Alexander H., Shapiro M., et al. High rate of bacterial respiratory tract co-infections upon admission amongst moderate to severe COVID-19 patients. Infect. Dis. 2022;54(2):134–144. doi: 10.1080/23744235.2021.1985732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H., Zahra A., Fernandes R., Fries B.C., Thode H.C., Jr., Singer A.J. Bacterial infections and death among patients with Covid-19 versus non Covid-19 patients with pneumonia. Am. J. Emerg. Med. 2022;51:1–5. doi: 10.1016/j.ajem.2021.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Liao B., Cheng L., Peng X., Xu X., Li Y., et al. The microbial coinfection in COVID-19. Appl. Microbiol. Biotechnol. 2020;1–9 doi: 10.1007/s00253-020-10814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D., Liu L., Zhang M., Hu Y., Yang Q., Guo J., et al. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients. Sci. China Life Sci. 2020;63(4):606–609. doi: 10.1007/s11427-020-1668-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Yu Y., Zeng W., Guo J., Lv F., Wang X., et al. Whole-genome analysis of New Delhi metallo-beta-lactamase-1-producing Acinetobacter haemolyticus from China. J. Glob. Antimicrob. Resist. 2020;20:204–208. doi: 10.1016/j.jgar.2019.05.012. [DOI] [PubMed] [Google Scholar]
- Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet (London, England) 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chate R.C., Fonseca E., Passos R.B.D., Teles G., Shoji H., Szarf G. Presentation of pulmonary infection on CT in COVID-19: initial experience in Brazil. J. Bras. Pneumol. 2020;46(2) doi: 10.36416/1806-3756/e20200121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Cao W., Xiao M., Li Y.J., Yang Y., Zhao J., et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua xue ye xue za zhi. 2020;41:E006. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PubMed] [Google Scholar]
- Jafarzadeh A., Jafarzadeh S., Nozari P., Mokhtari P., Nemati M. Lymphopenia an important immunological abnormality in patients with COVID-19: possible mechanisms. Scand. J. Immunol. 2021;93(2) doi: 10.1111/sji.12967. [DOI] [PubMed] [Google Scholar]
- Fathi N., Rezaei N. Lymphopenia in COVID-19: therapeutic opportunities. Cell Biol. Int. 2020;44(9):1792–1797. doi: 10.1002/cbin.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cellular & molecular immunology. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19) medRxiv. 2020;11:827. doi: 10.3389/fimmu.2020.00827. 2020.02.18.20024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léonard-Lorant I., Delabranche X., Séverac F., Helms J., Pauzet C., Collange O., et al. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to d-dimer levels. Radiology. 2020;296(3):E189–E191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S., Shah K., Patel S.B., Patel F.S., Osman M., Velagapudi P., et al. medRxiv; 2020. Elevated D-Dimer Levels Are Associated With Increased Risk of Mortality in COVID-19: A Systematic Review and Meta-analysis. 2020.04.29.20085407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. COVID-19 symptoms in HIV positive people similar to general population in Wuhan. HIV Treat. Bull. 2020:10–11. [Google Scholar]
- Ssentongo P., Heilbrunn E.S., Ssentongo A.E., Advani S., Chinchilli V.M., Nunez J.J., et al. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Sci. Rep. 2021;11(1):1–12. doi: 10.1038/s41598-021-85359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello A., Zamarro B., Nistal S., Victor V., Hernández J., Prieto-Pérez L., et al. COVID-19 in people living with HIV: a multicenter case-series study. Int. J. Infect. Dis. 2021;102:310–315. doi: 10.1016/j.ijid.2020.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Chen X., Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. J. Am. Soc. Hypertens. 2017;11(8):530–540. doi: 10.1016/j.jash.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Livingston E., Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA. 2020;323(14):1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- Prabhu S., Poongulali S., Kumarasamy N. Impact of COVID-19 on people living with HIV: a review. J. Virus Erad. 2020;6(4) doi: 10.1016/j.jve.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Khanna P., Singh A.K. Impact of COVID-19 in patients with concurrent co-infections: a systematic review and meta-analyses. J. Med. Virol. 2021;93(4):2385–2395. doi: 10.1002/jmv.26740. [DOI] [PubMed] [Google Scholar]
- Mellor M.M., Bast A.C., Jones N.R., Roberts N.W., Ordóñez-Mena J.M., Reith A.J., et al. Risk of adverse coronavirus disease 2019 outcomes for people living with HIV. AIDS (London, England) 2021;35(4):F1. doi: 10.1097/QAD.0000000000002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health O. World Health Organization; Geneva: 2020. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-nCoV) Infection Is Suspected: Interim Guidance, 28 January 2020. 2020. Contract No.: WHO/nCoV/Clinical/2020.3. [Google Scholar]
- Kumar R.N., Tanna S.D., Shetty A.A., Stosor V. COVID-19 in an HIV-positive kidney transplant recipient. Transpl. Infect. Dis. 2020:e13338. doi: 10.1111/tid.13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calza L., Bon I., Tadolini M., Borderi M., Colangeli V., Badia L., et al. COVID-19 in patients with HIV-1 infection: a single-centre experience in northern Italy. Infection. 2020;1–5 doi: 10.1007/s15010-020-01492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L.F., Moran C.A., Oliver N.T., Moanna A., Lahiri C.D., Colasanti J.A., et al. Clinical characteristics, comorbidities and outcomes among persons with HIV hospitalized with coronavirus disease 2019 in Atlanta, Georgia. AIDS (London, England) 2020;34(12):1789. doi: 10.1097/QAD.0000000000002632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami A., Javanmardi F., Akbari A., Kojuri J., Bakhtiari H., Rezaei T., et al. Survival rate in hypertensive patients with COVID-19. Clin. Exp. Hypertens. 2020;1–4 doi: 10.1080/10641963.2020.1812624. [DOI] [PubMed] [Google Scholar]
- Huang Y., Chen Z., Wang Y., Han L., Qin K., Huang W., et al. Clinical characteristics of 17 patients with COVID-19 and systemic autoimmune diseases: a retrospective study. Ann. Rheum. Dis. 2020;79(9):1163–1169. doi: 10.1136/annrheumdis-2020-217425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciarte A., Gonzalez-Cordon A., Rojas J., Torres B., de Lazzari E., de la Mora L., et al. Clinical characteristics, risk factors, and incidence of symptomatic coronavirus disease 2019 in a large cohort of adults living with HIV: a single-center, prospective observational study. AIDS (London, England) 2020;34(12):1775. doi: 10.1097/QAD.0000000000002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirenga B., Muttamba W., Kayongo A., Nsereko C., Siddharthan T., Lusiba J., et al. Characteristics and outcomes of admitted patients infected with SARS-CoV-2 in Uganda. BMJ Open Respir. Res. 2020;7(1) doi: 10.1136/bmjresp-2020-000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondi A., Cimini E., Colavita F., Cicalini S., Pinnetti C., Matusali G., et al. COVID-19 in people living with HIV: clinical implications of dynamics of the immune response to SARS-CoV-2. J. Med. Virol. 2020;93(3):1796–1804. doi: 10.1002/jmv.26556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev N., Scherer M., LaSota E.D., Antoniou P., Yin M.T., Zucker J., et al. Clinical characteristics and outcomes in people living with human immunodeficiency virus hospitalized for coronavirus disease 2019. Clin. Infect. Dis. 2020;71(16):2294–2297. doi: 10.1093/cid/ciaa635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco J.L., Ambrosioni J., Garcia F., Martínez E., Soriano A., Mallolas J., et al. COVID-19 in patients with HIV: clinical case series. Lancet HIV. 2020;7(5):e314–e316. doi: 10.1016/S2352-3018(20)30111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A., Shaw J., Karamchand S., Lahri S., Schrueder N., Chothia M., et al. HIV and SARS-CoV-2 co-infection: the diagnostic challenges of dual pandemics. SAMJS. Afr. Med. J. 2020;110(6):1–3. doi: 10.7196/SAMJ.2020.v110i6.14825. [DOI] [PubMed] [Google Scholar]
- Elhadi M., Momen A., Abdulhadi O., Msherghi A. New Microbes and New Infections. Vol. 37. 2020. Multi-organ failure after acute kidney injury in patient with HIV and COVID-19; p. 100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaba M.C., Ngale T.C., Madala N. COVID-19 in an HIV-infected patient. Lessons learned from an autopsy case. Int. J. Infect. Dis. 2020;101:243–246. doi: 10.1016/j.ijid.2020.09.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini M., Mutti M.F., Barletta J.A., Falak A., Cuatz D., Sisto A., et al. COVID-19 associated with AIDS-related disseminated histoplasmosis: a case report. Int. J. STD AIDS. 2020;31(12):1222–1224. doi: 10.1177/0956462420957518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A.R., Koval C.E., Taege A.J., Modaresi Esfeh J., Eghtesad B., Menon K.N., Disease Coronavirus, et al. In an orthotopic liver transplant recipient living with human immunodeficiency virus. Transpl. Infect. Dis. 2019;2020 doi: 10.1111/tid.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat M.M., Sprinz E. COVID-19 pneumonia in an HIV-positive woman on antiretroviral therapy and undetectable viral load in Porto Alegre, Brazil. Braz. J. Infect. Dis. 2020;24(5):455–457. doi: 10.1016/j.bjid.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad S., Tayyar R., Risch L., Churchill G., Fares E., Choe M., et al. Encephalopathy and seizure activity in a COVID-19 well controlled HIV patient. IDCases. 2020:e00814. doi: 10.1016/j.idcr.2020.e00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas N., Espinoza M., Loban A., Luque O., Jurado J., Henry-Hurtado N., et al. Case report: COVID-19 recovery from triple infection with Mycobacterium tuberculosis, HIV, and SARS-CoV-2. Am. J. Trop. Med. Hyg. 2020;103(4):1597–1599. doi: 10.4269/ajtmh.20-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanwongse K., Shabarek N. Clinical features and outcome of HIV/SARS-CoV-2 co-infected patients in the bronx, New York City. J. Med. Virol. 2020;92(11):2387–2389. doi: 10.1002/jmv.26077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przydzial P., Tchomobe G., Amin K., Engell C.A., Okoh A.K. COVID-19 crossing paths with AIDS in the homeless. J. Med. Virol. 2020;93(1):155–157. doi: 10.1002/jmv.26255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkovic S., Kim M., Sin E. Four cases: human immunodeficiency virus and novel coronavirus 2019 co-infection in patients from Long Island, New York. J. Med. Virol. 2020;92(11):2338–2340. doi: 10.1002/jmv.26029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pata R.K., Ahmady A., Kiani R. Human immunodeficiency virus: a dark cloud with silver lining during the COVID-19 pandemic. Cureus. 2020;12(7) doi: 10.7759/cureus.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias L.A.B.G., Moreira A.L.G., Corrêa E.A., de Oliveira Lima C.A.L., Lopes I.M.P., de Holanda P.E.L., et al. Case report: coronavirus disease and pulmonary tuberculosis in patients with human immunodeficiency virus: report of two cases. Am. J. Trop. Med. Hyg. 2020;103(4):1593–1596. doi: 10.4269/ajtmh.20-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway J.P., Farley B., Benoit J.-L., Frohne C., Hazra A., Pettit N., et al. A case series of five people living with HIV hospitalized with COVID-19 in Chicago, Illinois. AIDS Patient Care STDs. 2020;34(8):331–335. doi: 10.1089/apc.2020.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappe Gonzalez A.J., Montenegro-Idrogo J.J., Vargas Vadillo A.R., Slee Torres M., Vargas Matos I., Resurrección Delgado C.P. Hospital-acquired SARS-CoV-2 pneumonia in a person living with HIV. Int. J. STD AIDS. 2020;31(13):1320–1322. doi: 10.1177/0956462420957528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood K., Rashed E.R., Oliveros E., Chau V.Q., Hermle T., Jacobs S., et al. Predisposition or protection? COVID-19 in a patient on LVAD support with HIV/AIDS. JACC: Case Reports. 2020;2(9):1337–1341. doi: 10.1016/j.jaccas.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd K.M., Beckwith C.G., Garland J.M., Johnson J.E., Aung S., Cu-Uvin S., et al. SARS-CoV-2 and HIV coinfection: clinical experience from Rhode Island, United States. J. Int. AIDS Soc. 2020;23(7) doi: 10.1002/jia2.25573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R.H. Clinical outcomes and prognosis of patients with HIV and SARS-CoV-2 coinfection. J. Med. Virol. 2020;93(1):105–106. doi: 10.1002/jmv.26177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Liao X., Wang H., Wei L., Xing M., Liu L., et al. Early virus clearance and delayed antibody response in a case of COVID-19 with a history of co-infection with HIV-1 and HCV. Clin. Infect. Dis. 2020;71(16):2233–2235. doi: 10.1093/cid/ciaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Chen X., Huang Y., Zhang Q., Cheng Y., Zhang N., et al. A study of two cases co-infected with SARS-CoV-2 and human immunodeficiency virus. Virol. Sin. 2020;1–4 doi: 10.1007/s12250-020-00280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radpour A., Bahrami-Motlagh H., Taaghi M.T., Sedaghat A., Karimi M.A., Hekmatnia A., et al. COVID-19 evaluation by low-dose high resolution CT scans protocol. Acad. Radiol. 2020;27(6):901. doi: 10.1016/j.acra.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbarnia L., Farajnia S., Khaneshi H., Farajnia H., Naghili B., Tanomand A. Detection of blaOXA-23 and blaNDM-1 carbapenemase among clinical isolates of A. baumannii in Tabriz, north-west of Iran. Gene Rep. 2020;18 [Google Scholar]
- Ruan L., Zhang Y., Luo Y., Yu X., Zeng Y., Peng H., et al. Clinical features and outcomes of four HIV patients with COVID-19 in Wuhan, China. J. Med. Virol. 2021;93(1):133–136. doi: 10.1002/jmv.26223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janakiram Marimuthu DBSK, Gandhi PA. HIV and SARS CoV‐2 co‐infection: a retrospective, record based, case series from South India. J. Med. Virol. [DOI] [PMC free article] [PubMed]
- Adachi E., Saito M., Ikeuchi K., Hoshina T., Yotsuyanagi H. Cases of coronavirus disease-2019 in HIV-infected transgender women. AIDS. 2020;34(9):1435–1436. doi: 10.1097/QAD.0000000000002573. [DOI] [PubMed] [Google Scholar]
- Li W., Ma Q., Wang X., Tang M., Lin J., Xiao B. The characteristics of two patients coinfected with SARS-CoV-2 and HIV in Wuhan, China. J. Med. Virol. 2020;93(1):85–88. doi: 10.1002/jmv.26155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Chen T., Zhang H. Recovery from COVID-19 in two patients with coexisted HIV infection. J. Med. Virol. 2020;92(11):2325–2327. doi: 10.1002/jmv.26006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto T., Kutsuna S., Yanagawa Y., Kanda K., Okuhama A., Akiyama Y., et al. A case of SARS-CoV-2 infection in an untreated HIV patient in Tokyo, Japan. J. Med. Virol. 2020;93(1):40–42. doi: 10.1002/jmv.26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.J., Wong S.X.L., Gollamudi S. A case of HIV and SARS-CoV-2 co-infection in Singapore. J. Acquir. Immune Defic. Syndr. 2020;84(4):e23–e24. doi: 10.1097/QAI.0000000000002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Cheng X., Wang R., Zeng X. Computed tomography imaging of an HIV-infected patient with coronavirus disease 2019 (COVID-19) J. Med. Virol. 2020;29(10):1174–1176. doi: 10.1002/jmv.25879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Cao Y., Xu S., Zhou M. Co-infection of SARS-CoV-2 and HIV in a patient in Wuhan city, China. J. Med. Virol. 2020;92(6):529–530. doi: 10.1002/jmv.25732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Luo L., Bu H., Xia H. Case report: one case of coronavirus desease 2019 (COVID-19) in patient co-nfected by HIV with a low CD4+ T cell count. Int. J. Infect. Dis. 2020;96:148–150. doi: 10.1016/j.ijid.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Cao Y., Xu S., Zhou M. Reply to comments on’co-infection of SARS-CoV-2 and HIV in a patient in Wuhan city, China’. J. Med. Virol. 2020;92(19):1417–1418. doi: 10.1002/jmv.25838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C., Tang L., Wu J., Li W., Ming X., Zhou H., et al. An HIV-infected patient with coronavirus disease 2019 has a favourable prognosis: a case report. Ann. Palliat. Med. 2020;9(6):3–6. doi: 10.21037/apm-20-576. [DOI] [PubMed] [Google Scholar]
- Ciccullo A., Borghetti A., Dusina A., Segala F., Visconti E., Tamburrini E., et al. The need to continue testing for HIV, even during the coronavirus disease 2019 (COVID-19) pandemic. HIV Med. 2020;1:3–4. doi: 10.1111/hiv.12936. [DOI] [PubMed] [Google Scholar]
- Baluku J.B., Mwebaza S., Ingabire G., Nsereko C., Muwanga M. HIV and SARS-CoV-2 co-infection: a case report from Uganda. J. Med. Virol. 2022;92(11):2351–2353. doi: 10.1002/jmv.26044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartilotti Matos F., Davies P. Pearls and pitfalls: two contrasting HIV diagnoses in the COVID-19 era and the case for screening. J. Med. Virol. 2021;93(2):652–654. doi: 10.1002/jmv.26428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giambenedetto S., Del Giacomo P., Ciccullo A., Porfidia A., De Matteis G., Cianci R., et al. SARS-CoV-2 infection in a highly experienced person living with HIV. AIDS. 2020;34(8):1257–1258. doi: 10.1097/QAD.0000000000002572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva A., Conti F., Bernacchia D., Pezzati L., Sollima S., Merli S., et al. Darunavir does not prevent SARS-CoV-2 infection in HIV patients. Pharmacol. Res. 2020;157 doi: 10.1016/j.phrs.2020.104826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin O.A., Karaosmanoglu H.K., Yasar K.K. HIV/SARS-CoV-2 coinfected patients in Istanbul, Turkey. J. Med. Virol. 2020;92(11):2288–2290. doi: 10.1002/jmv.25955. [DOI] [PubMed] [Google Scholar]
- Toombs J.M., Van den Abbeele K., Democratis J., Merricks R., Mandal A.K., Missouris C.G. COVID-19 in three people living with HIV in the United Kingdom. J. Med. Virol. 2020;93(1):107–109. doi: 10.1002/jmv.26178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasset L., Di Meco E., Cavinato S., Cattelan A.M. Coinfection of severe acute respiratory syndrome coronavirus 2 and HIV in a teaching hospital: still much to learn. AIDS. 2020;34(11):1694–1696. doi: 10.1097/QAD.0000000000002609. [DOI] [PubMed] [Google Scholar]
- d’Ettorre G., Recchia G., Ridolfi M., Siccardi G., Pinacchio C., Innocenti G.P., et al. Analysis of type I IFN response and T cell activation in severe COVID-19/HIV-1 coinfection: a case report. Medicine. 2020;99(36) doi: 10.1097/MD.0000000000021803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinacci D., Ciccullo A., Borghetti A., Visconti E., Tamburrini E., Izzi I.M., et al. People living with HIV in the COVID-19 era: a case report. AIDS Res. Hum. Retrovir. 2020;37(4):253–254. doi: 10.1089/AID.2020.0149. [DOI] [PubMed] [Google Scholar]
- Coleman H., Snell L.B., Simons R., Douthwaite S.T., Lee M.J. Coronavirus disease 2019 and pneumocystis jirovecii pneumonia: a diagnostic dilemma in HIV. AIDS. 2020;34(8):1258–1260. doi: 10.1097/QAD.0000000000002571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanou S., Koukios D., Matsentidou C.T., Markoulaki D., Raftopoulos V. Severe SARS-CoV-2 pneumonia in a 58-year-old patient with HIV: a clinical case report from the Republic of Cyprus. J. Med. Virol. 2020;92(11):2361–2365. doi: 10.1002/jmv.26053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Iturritza E., San-José-Muñiz I., Ganchegui-Aguirre M., Balerdi-Sarasola L., Ortiz-de-Zárate-Ibarra Z., Gainzarain-Arana J.C., et al. Coronavirus disease 2019 in patients with HIV in the province of Araba, Basque Country, Spain. Aids. 2020;34(11):1696–1697. doi: 10.1097/QAD.0000000000002608. [DOI] [PubMed] [Google Scholar]
- Pinnetti C., Vergori A., Agrati C., Castilletti C., Campioni P., Gagliardini R., et al. SARS-CoV-2 infection does not induce HIV viral escape in the central nervous system: a case series. Int. J. Infect. Dis. 2020;101:38–41. doi: 10.1016/j.ijid.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biagio A., Taramasso L., Dentone C., Vena A., Giacobbe D.R., De Maria A., et al. Is a step-down antiretroviral therapy necessary to fight severe acute respiratory syndrome coronavirus 2 in HIV-infected patients? AIDS. 2020;34(12):1865–1867. doi: 10.1097/QAD.0000000000002630. [DOI] [PubMed] [Google Scholar]
- Joumaa H., Regard L., Carlier N., Chassagnon G., Alabadan E., Canouï E., et al. A severe COVID-19 despite ongoing treatment with lopinavir-ritonavir. Respir. Med. Res. 2020;78 doi: 10.1016/j.resmer.2020.100780. [DOI] [PMC free article] [PubMed] [Google Scholar]