Abstract
Background
The 2019 novel coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is currently a major challenge threatening the global healthcare system. Respiratory virus infection is the most common cause of asthma attacks, and thus COVID-19 may contribute to an increase in asthma exacerbations. However, the mechanisms of COVID-19/asthma comorbidity remain unclear.
Methods
The “Limma” package or “DESeq2” package was used to screen differentially expressed genes (DEGs). Alveolar lavage fluid datasets of COVID-19 and asthma were obtained from the GEO and GSV database. A series of analyses of common host factors for COVID-19 and asthma were conducted, including PPI network construction, module analysis, enrichment analysis, inference of the upstream pathway activity of host factors, tissue-specific analysis and drug candidate prediction. Finally, the key host factors were verified in the GSE152418 and GSE164805 datasets.
Results
192 overlapping host factors were obtained by analyzing the intersection of asthma and COVID-19. FN1, UBA52, EEF1A1, ITGB1, XPO1, NPM1, EGR1, EIF4E, SRSF1, CCR5, PXN, IRF8 and DDX5 as host factors were tightly connected in the PPI network. Module analysis identified five modules with different biological functions and pathways. According to the degree values ranking in the PPI network, EEF1A1, EGR1, UBA52, DDX5 and IRF8 were considered as the key cohost factors for COVID-19 and asthma. The H2O2, VEGF, IL-1 and Wnt signaling pathways had the strongest activities in the upstream pathways. Tissue-specific enrichment analysis revealed the different expression levels of the five critical host factors. LY294002, wortmannin, PD98059 and heparin might have great potential to evolve into therapeutic drugs for COVID-19 and asthma comorbidity. Finally, the validation dataset confirmed that the expression of five key host factors were statistically significant among COVID-19 groups with different severity and healthy control subjects.
Conclusions
This study constructed a network of common host factors between asthma and COVID-19 and predicted several drugs with therapeutic potential. Therefore, this study is likely to provide a reference for the management and treatment for COVID-19/asthma comorbidity.
Keywords: COVID-19, Asthma, Host factor interaction networks, Bioinformatics analysis, Comorbidity, Severity
1. Introduction
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has rapidly spread around the world, leading to damage to the global healthcare system and social economy [1,2]. Inflammation-mediated cytokine storm is a common factor for predisposing to severe COVID-19 complicated by acute respiratory distress syndrome (ARDS), especially in the elderly or severely ill patients [3]. The therapeutic strategies for COVID-19 mainly include immunomodulators, glucocorticoids, antibiotic and antiviral agents. However, there is no specific medicine for the treatment of COVID-19 so far.
Ending the COVID-19 pandemic takes more time than other pandemic diseases in this poor environment [2]. People with chronic respiratory diseases need to be more vigilant about contracting SARS-CoV-2 [4]. Asthma is characterized by recurrent and reversible bronchial limitation that results in variable respiratory symptoms, such as cough, wheezing and shortness of breath [5]. Maintenance treatment with inhaled corticosteroids (ICS) and long-acting β 2-agonists for asthma can relieve symptoms and reduce the risk of exacerbations [6]. In theory, patients with asthma should be more susceptible to SARS-CoV-2 infection due to a deficient antiviral immune response to respiratory viruses. Some researchers hold the idea that asthma morbidity is associated with the severity and mortality of COVID-19 [7], while others consider that asthma does not contribute to worse outcomes for severe COVID-19 patients [8]. The only consensus is that maintaining asthma control during the COVID-19 pandemic will be beneficial for asthma sufferers [9]. Allergic asthma characterized by blood eosinophilia does not significantly increase the risk of severe COVID-19 compared with nonallergic asthma [10]. Of note, eosinopenia is a biomarker of severe COVID-19 [11] and may be a protective target against excessive inflammatory response [12].
The overexpression of ACE2 (as a receptor of SARS-CoV-2) in lung cells contributes to the susceptibility to SARS-CoV-2 infection [13]. ICS is the cornerstone of asthma management, and asthma patients taking ICS have lower expression of ACE2 [14]. ICS use might alleviate the inflammatory response and lung damage to reduce the risk of infection [15]. Discontinuation of ICS treatment is not recommended for asthma patients due to the untoward effects [16]. However, Sarah L O'Beirne et al. show that the expression of ACE2 in the airway of asthma patients receiving long-term ICS treatment will increase, suggesting that patients with COVID-19/asthma comorbidity may suffer a more serious condition [17]. Moreover, Chloe I Bloom et al. find that the risk between asthma and COVID-19 is closely related to the asthma phenotype [18]. Therefore, it can be inferred that it is of significance to study the impact of COVID-19/asthma comorbidity on the prognosis of asthma patients complicated by COVID-19.
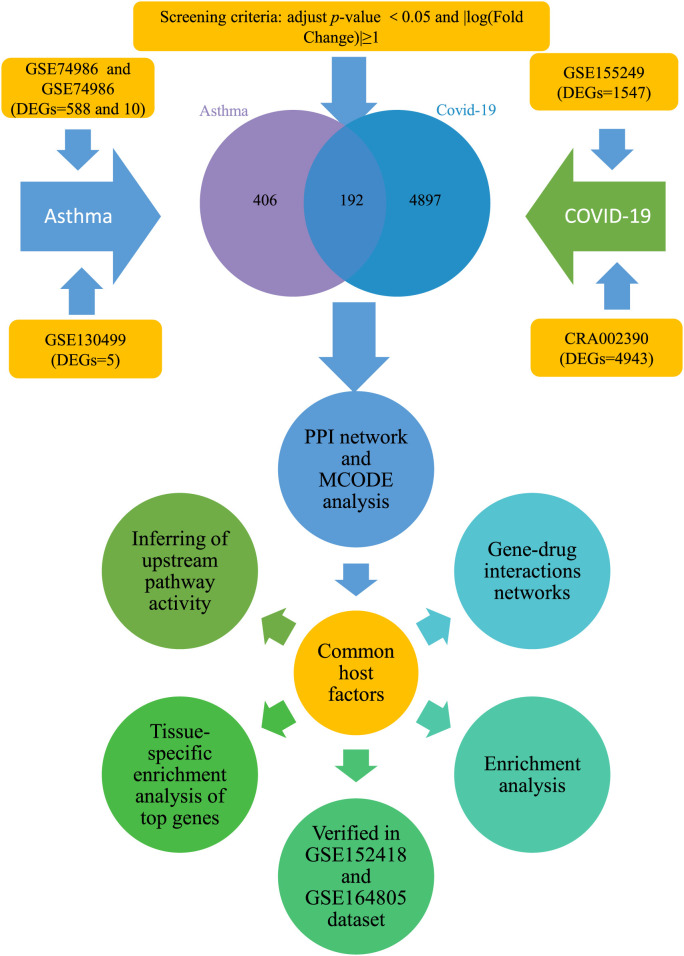
Viral infections as the main trigger of asthma are often associated with asthma exacerbations. Rhinovirus (RV) and respiratory syncytial virus (RSV), the most common viral infections, are also related to coronavirus infection [19]. Viruses must rely on host cellular factors to successfully promote the replication process. Therefore, virus-host interaction networks are potential targets for the in-depth study of viral infection and pathogenesis [20]. For patients with chronic respiratory diseases, the virus-host interaction relationship will be more complicated. The identification of host factors as drug targets is a potential treatment for patients who suffer from comorbidity with asthma and SARS-CoV-2 infection. To preliminarily explore the relationship between asthma and COVID-19 co-occurrence, we constructed the potential mechanism network of targeted host factors between COVID and asthma by using bioinformatics analysis. The identification of the host factor interaction network might clarify the molecular mechanisms and provide new insights into potential therapeutic targets for COVID-19 and asthma co-occurrence. The detailed flowchart is shown in Fig. 1 .
Fig. 1.
Flowchart of the procedure of exploring host factor interaction networks between COVID-19 and asthma.
DEGs: differentially expressed genes; MCODE: molecular complex detection; PPI: protein and protein interaction.
2. Materials and methods
2.1. Transcriptomic data acquisitions
To determine the molecular mechanisms and protein targets shared between SARS-CoV-2 and asthma, we obtained the asthma-related datasets according to the following filter criteria [1]: It originated from the GEO (https://www.ncbi.nlm.nih.gov/geo/) database of the National Center for Biotechnology Information (NCBI) [2]; The search keyword was “asthma” [3]; The research type was “expression profiling by array” [3]; The species was “Homo sapiens” [4]; The sample type was bronchoalveolar lavage fluid (BALF) [5]; The dataset must contain control group and asthma group. No suitable COVID-19 dataset was available in the GEO database based on the same search strategy. Thus the datasets recruited in the literature published in Pubmed (https://pubmed.ncbi. nlm.nih.gov/) were obtained following the same screening criteria.
The GSE155249 dataset included the BALF samples of 12 patients (10 COVID-19 positives and 2 COVID-19 negatives) [21]. CRA002390 contained the BALF samples of 2 patients (WHU01-2) from Zhongnan Hospital of Wuhan University [22] and 3 healthy BALF samples (Ctrl1-3) [23]. The “DESeq2 package” (v1.26.0) [24] in R was used to screen differential expressed genes (DEGs). The criteria were set as follows: adjusted P-value <0.05 and log|fold-change (FC)| ≥ 1. Genes were selected and normalized to counts per million (CPM) for further analysis. We obtained DEGs from the literature attachments of the data sources for further analysis. The GSE74986 dataset contained 74 BALF samples of asthma patients and 12 BALF samples of healthy controls [25]. The GSE130499 dataset comprised 118 BALF samples from individuals with asthma and 38 healthy samples, and the GSE67940 dataset included 31 control BALF samples and 83 asthma BALF samples [26]. The detailed information of the datasets was shown in Table 1 .
Table 1.
Overview of data sets with their geo-features and their quantitative measurements in this analysis.
| Disease name | GEO accession | GEO platform | Total DEGs count | Samples | Up regulated DEGs count | Down regulated DEGs count |
|---|---|---|---|---|---|---|
| Asthma | GSE74986 | GPL6480 | 588 | Asthma patients (N = 74); Healthy control (N = 14) | 81 | 507 |
| Asthma | GSE130499 | GPL4133 | 5 | Asthma patients (N = 116); Healthy control (N = 38) | 1 | 4 |
| Asthma | GSE67940 | GPL6480 | 10 | Asthma patients (N = 73); Healthy control (N = 31) | 1 | 9 |
| COVID-19 | CRA002390 | / | 3943 | COVID-19 patients (N = 3); Healthy control (N = 3) | 2249 | 1694 |
| COVID-19 | GSE155249 | GPL24676 | 1547 | COVID-19 patients (N = 10); Healthy control (N = 2) | 242 | 1305 |
DEGs: differentially expressed genes.
The “GEO2R” and the “limma” package in R [27] were used to obtain DEGs (adjusted P-value <0.05 and log| FC|≥1). Finally, the overlapping host factors between asthma and COVID-19 were received and displayed by the Evenn online website (http://www.ehbio. com/test/venn/#/).
2.2. Gene Ontology and pathway enrichment analyses
The DEGs shared between asthma and COVID-19 were biologically classified by enrichment analysis. The “clusterProfiler” package in R [28] (version: 4.1.0) was used for the annotation of common host factors in Gene Ontology (GO; http://www. geneontology.org/), including biological processes (BP), cellular component (CC) and molecular function (MF). Kyoto Encyclopedia of Genes and Genomes (KEGG; https://www.kegg.jp/) and WIKI pathways enrichment results were generated via the online platform of WEB-based GEne SeT AnaLysis Toolkit (version 0.61, WebGestalt, http://www.webgestalt.org/) by using the “ORA” algorithm [29].
2.3. Protein-protein interaction analysis and network construction
Common host factors were put into the STRING database (version 11.5, https://string-db.org/) for generating a protein-protein interaction (PPI) network. The standard of connectivity between protein targets was greater than 0.4 (medium confidence). The PPI network was analyzed and visualized through Cytoscape software (version: 3.6.1, https://cytoscape.org/). The “degree” criterion in cytoHubba (http://apps.cytoscape.org/apps/cytohubba) was used to perform network topology analysis [30]. Then, module analysis was performed by the Molecular Complex Detection (MCODE) plug in Cytoscape software. Subsequently, the “clusterProfiler” package in R was used for the annotation of modules. The hub common host factors between COVID-19 and asthma were finally obtained based on the above two methods.
2.4. Inferring of upstream pathway activity and tissue-specific enrichment analysis of hub genes
SPEED2 (https://speed2.sys-bio.net/) [31] was used to infer upstream pathway activity of the host factor interaction network between asthma and COVID-19. The Bates test was used to quantify the mean rank change in enrichment statistics. And the hub common genes were selected to perform tissue-specific enrichment analysis by using the “Multi Gene Query” function available on the GTEx (V8, https://www.gtexportal.org/home/). The heatmap was used to show the expression of the transcripts per million (TPM) values for multiple genes in various tissues.
2.5. Gene-drug interactions networks
STITCH (version: 5.0, http://stitch.embl.de/) is a database that uses phenotypic effects, text mining and similarity information of chemical structure to predict the interaction between chemical proteins and small molecules [32]. The STITCH database contains interaction information of more than 68000 different chemical substances and 2200 drugs. The STITCH database was used to predict drugs that have potential therapeutic effects on COVID-19/asthma comorbidity. The gene-drug interaction network was constructed and the importance of a drug was ranked by the value of false discovery rate.
2.6. Verification of the expression levels of key host factors in COVID-19 datasets
The GSE152418 dataset was an RNAseq analysis of PBMCs from 17 COVID-19 subjects and 17 healthy controls. GSM4614985 was a sample obtained from the recovery period of COVID-19 and excluded from the second analysis. GSE164805 was also an RNAseq analysis of the PBMCs that contained 5 healthy controls, 5 mild and 5 severe COVID-19 patients. The clinical information of the two datasets were shown in Table 2 and Table 3 . The “Differential expression analyses” function in the COVID19db (http://hpcc.siat.ac.cn/covid19db/home) database was used to obtain DEGs and displayed as a heatmap, boxplots and PCA diagram. The “Anova” method was used to analyze the expression differences of key targets in COVID-19 with varying severities and displayed as a boxplot.
Table 2.
The clinical information of GSE152418 dataset.
| Healthy (N = 17) | Moderate (N = 5) | Severe (N = 8) | ICU (N = 4) | F-value | p | ||
|---|---|---|---|---|---|---|---|
| Gender | Male | 8 | 0 | 5 | 2 | X2 = 4.322 | 0.229 |
| Female | 9 | 4 | 3 | 2 |
Table 3.
The clinical information of GSE164805 dataset.
| Healthy (N = 5) | Mild (N = 5) | Severe (N = 5) | F-value | p-value | ||
|---|---|---|---|---|---|---|
| Age (X ± M) | 59.80 ± 6.943 | 51.40 ± 4.393 | 58.00 ± 9.083 | 2 | 0.184 | |
| Gender | Male | 4 | 4 | 5 | X2 = 1.154 | 0.562 |
| Female | 1 | 1 | 0 | |||
2.7. Statistical analysis
All statistical analyses were calculated based on R (version: 4.1.0, https://www.r-project.org/). One-way ANOVA was used for the comparison of continuous variables among the three groups, and the chi-square test was used for the comparison of categorical variables among the three groups. Student t-test was used to judge whether there were differences between the two groups [27]. Benjamini & Hochberg method was used to calculate the adjusted P-value (adj.P.Value) and the adj.P.value < 0.05 was set as the filter criterion.
3. Results
3.1. Identification of common host factors between COVID-19 and asthma
BALF samples were selected for subsequent analysis to obtain the common host factors between COVID-19 and asthma. According to the screening criteria of adjusted P-value <0.05 and log |FC|>1, 588 (18327), 5 (19287) and 10 (19563) DEGs in asthma were obtained from GSE74986, GSE130499 and GSE67940 datasets, respectively (Supplementary Table 1-3). 1547 (56476) and 4943 (9610) DEGs between COVID-19 and healthy people were respectively obtained from GSE155249 and CRA002390 datasets (Supplementary Table 4-5). 192 common host factors of COVID-19/asthma comorbidity were received by taking the DEGs intersection between COVID-19 and asthma. These genes may be potential host factors for the SARS-CoV-2 virus to cause asthma and COVID-19 infection.
3.2. GO, KEGG and WIKI enrichment analyses highlighted the mechanisms for COVID-19/asthma comorbidity
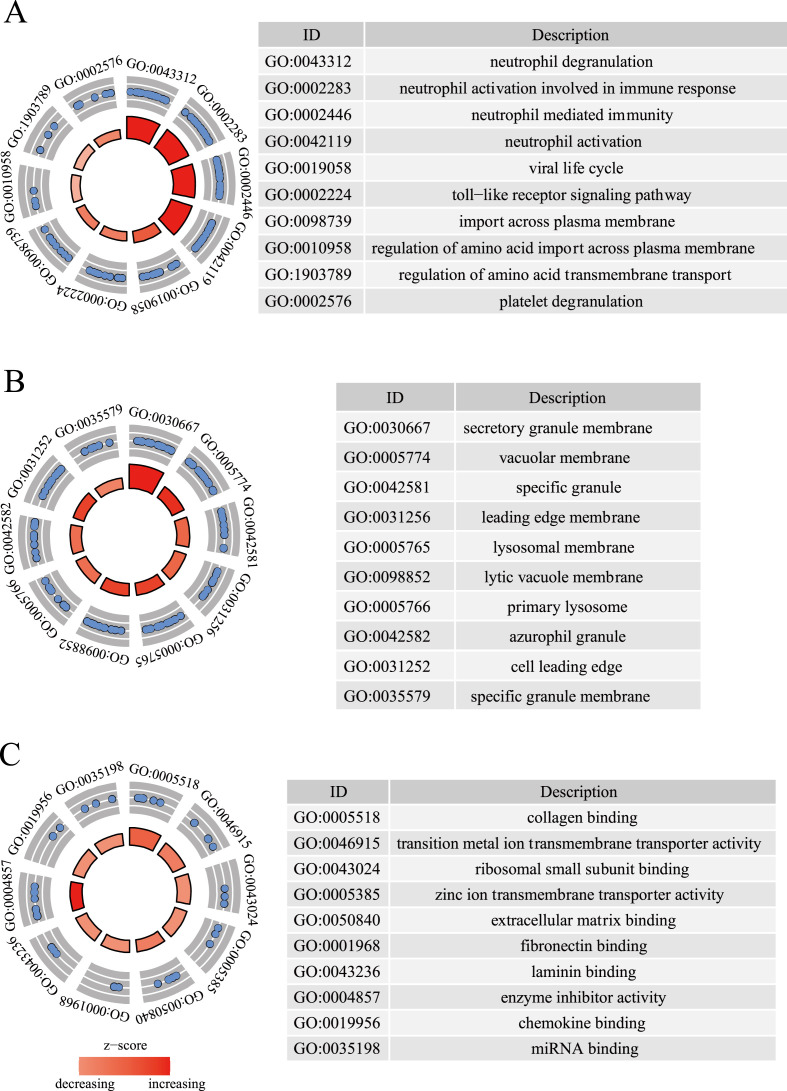
BPs results showed that common host genes were involved in the occurrence and development of two diseases, including neutrophil degranulation and neutrophil activation involved in immune response, neutrophil-mediated immunity, neutrophil activation, viral life cycle and other immune and virus-related signal pathways (Fig. 2 A and Supplementary Table 6). According to KEGG pathway enrichment analysis, metabolic pathways, PI3K/AKT signaling pathway, pathways in cancer, lysosome, human T-cell leukemia virus 1 infection, proteoglycans in cancer, cytokine-cytokine receptor interaction, chemokine signaling pathway, phagosome and human papillomavirus infection played important roles in COVID-19/asthma comorbidity (Table 4 ). The top 10 pathways in WIKI enrichment analysis were the VEGFA-VEGFR2 signaling pathway, nuclear receptors meta-pathway, insulin signaling, angiopoietin-like protein 8 regulatory pathway, focal adhesion-PI3K/AKT/mTOR-signaling pathway, PI3K/AKT signaling Pathway, Nrf2 pathway and circadian rhythm related genes (Table 5 ).
Fig. 2.
Enrichment analysis of common host factors. (A): biological process analysis. (B): cellular component analysis. (C): molecular function enrichment analysis.
Table 4.
KEGG enrichment analysis.
| Gene Set | Description | Size | Expect | Ratio | P-Value | FDR |
|---|---|---|---|---|---|---|
| hsa01100 | Metabolic pathways | 16 | 8 | 2 | 0.0000030122 | 0.000030122 |
| hsa04151 | PI3K-Akt signaling pathway | 8 | 4 | 2 | 0.0027808 | 0.013904 |
| hsa05200 | Pathways in cancer | 7 | 3.5 | 2 | 0.0060738 | 0.020246 |
| hsa04142 | Lysosome | 6 | 3 | 2 | 0.013082 | 0.021803 |
| hsa05166 | Human T-cell leukemia virus 1 infection | 6 | 3 | 2 | 0.013082 | 0.021803 |
| hsa05205 | Proteoglycans in cancer | 6 | 3 | 2 | 0.013082 | 0.021803 |
| hsa04060 | Cytokine-cytokine receptor interaction | 5 | 2.5 | 2 | 0.027799 | 0.027799 |
| hsa04062 | Chemokine signaling pathway | 5 | 2.5 | 2 | 0.027799 | 0.027799 |
| hsa04145 | Phagosome | 5 | 2.5 | 2 | 0.027799 | 0.027799 |
| hsa05165 | Human papillomavirus infection | 5 | 2.5 | 2 | 0.027799 | 0.027799 |
KEGG: Kyoto Encyclopedia of Genes and Genomes.
Table 5.
WIKI enrichment analysis.
| Gene Set | Description | Size | Expect | Ratio | P-Value | FDR |
|---|---|---|---|---|---|---|
| WP3888 | VEGFA-VEGFR2 Signaling Pathway | 12 | 4.6047 | 2.6061 | 0.0000023205 | 0.000018564 |
| WP2882 | Nuclear Receptors Meta-Pathway | 9 | 3.4535 | 2.6061 | 0.000083868 | 0.00022365 |
| WP481 | Insulin Signaling | 9 | 3.4535 | 2.6061 | 0.000083868 | 0.00022365 |
| WP3915 | Angiopoietin Like Protein 8 Regulatory Pathway | 7 | 2.6860 | 2.6061 | 0.00079507 | 0.0010601 |
| WP3932 | Focal Adhesion-PI3K-Akt-mTOR-signaling pathway | 7 | 2.6860 | 2.6061 | 0.00079507 | 0.0010601 |
| WP4172 | PI3K-Akt Signaling Pathway | 7 | 2.6860 | 2.6061 | 0.00079507 | 0.0010601 |
| WP2884 | NRF2 pathway | 5 | 1.9186 | 2.6061 | 0.0068148 | 0.0068148 |
| WP3594 | Circadian rhythm related genes | 5 | 1.9186 | 2.6061 | 0.0068148 | 0.0068148 |
3.3. Protein-protein interaction network construction
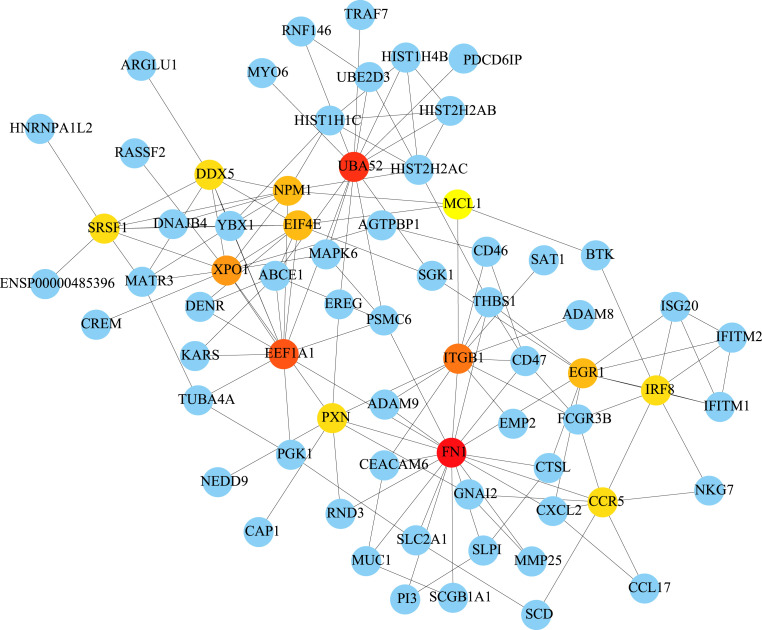
The interaction network of the 192 common host factors of COVID-19/asthma comorbidity was generated by the STRING database and visualized by the Cytoscape software. The connectivity of 192 genes were sorted according to the degree values. The top genes were FN1, UBA52, EEF1A1, ITGB1, XPO1, NPM1, EGR1, EIF4E, SRSF1, CCR5, PXN, IRF8 and DDX5 (Fig. 3 ).
Fig. 3.
Protein-protein interaction network of 192 common host factors between asthma and COVID-19. The dot represents the host factor, and the line represents the interaction of each host factor. The darker the color, the greater the connection.
3.4. MCODE analysis revealed the critical common host factors for COVID-19 and asthma
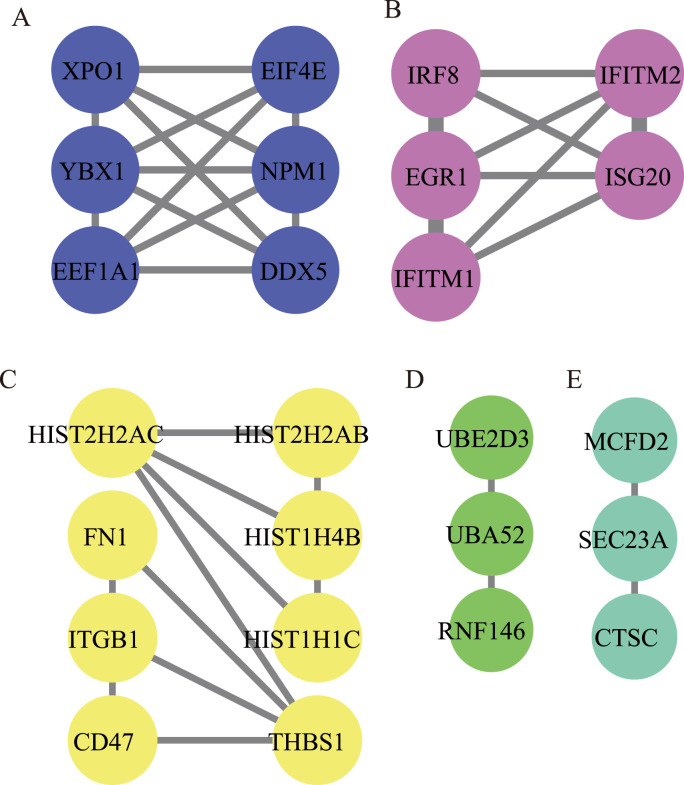
MCODE analysis of 192 genes were performed and the five modules with the closest connection were obtained. In module 1, EEF1A1 and DDX5 with the highest degree values were significantly enriched in “nucleic acid transport” and “nucleocytoplasmic transport”. In Module 2, EGR1, IFITM2, IRF8, IFITM1 and ISG20 with the same difference were mainly involved in the “type I interferon signaling pathway”. In Module 3, HIST2H2AC and THBS1 were the most critical host factors, and were significantly enriched in “extracellular matrix organization” and “ECM-receptor interaction”. In module 4, UBE2D3, UBA52 and RNF146 had the same degree of connectivity, and were most prominent in “protein polyubiquitination” and “ubiquitin mediated proteolysis”. In module 5, SEC23A, MCFD2 and CTSC had the same degree values, and were most prominent in “vesicle targeting, rough ER to cis-Golgi” and “apoptosis”. EEF1A1, EGR1, UBA52, DDX5 and IRF8 were finally identified as the key co-host factors in COVID-19 and asthma based on the degree values ranking in the PPI network and MCODE analysis (Fig. 4 and Table 6 ).
Fig. 4.
Module analysis (A–E) of 192 common host factors between asthma and COVID-19. The dot represents the host factor, and the line represents the interaction of each host factor. Different color represents different modules.
Table 6.
Top items of enrichment analysis of genes in five modules.
| Module | ONTOLOGY | ID | Description | P value | P.adjust | geneID | Count |
|---|---|---|---|---|---|---|---|
| 1 | BP | GO:0050657 | Nucleic acid transport | 1.74E-07 | 1.43E-05 | EIF4E/XPO1/YBX1/NPM1 | 4 |
| 1 | KEGG | hsa03013 | Nucleocytoplasmic transport | 1.04E-03 | 1.56E-02 | EEF1A1/XPO1 | 2 |
| 2 | BP | GO:0060337 | Type I interferon signaling pathway | 2.91E-12 | 2.43E-10 | EGR1/IFITM2/IRF8/IFITM1/ISG20 | 5 |
| 2 | KEGG | hsa05133 | Pertussis | 2.79E-02 | 6.19E-02 | IRF8 | 1 |
| 3 | BP | GO:0043062 | Extracellular structure organization | 1.88E-07 | 3.44E-05 | THBS1/ITGB1/FN1/CD47 | 4 |
| 3 | KEGG | hsa04512 | ECM-receptor interaction | 1.30E-08 | 4.02E-07 | THBS1/ITGB1/FN1/CD47 | 4 |
| 4 | BP | GO:0000209 | Protein polyubiquitination | 5.65E-06 | 4.40E-04 | UBE2D3/UBA52/RNF146 | 3 |
| 4 | KEGG | hsa04120 | Ubiquitin mediated proteolysis | 3.05 E−04 | 2.74E-03 | UBE2D3/UBA52 | 2 |
| 5 | BP | GO:0048207 | Vesicle targeting, rough ER to cis-Golgi | 4.09E-08 | 9.04E-07 | SEC23A/MCFD2/CTSC | 3 |
| 5 | KEGG | hsa04210 | Apoptosis | 3.33E-05 | 4.17E-02 | CTSC | 1 |
BP: biological process; KEGG: Kyoto Encyclopedia of Genes and Genomes.
3.5. SPEED2 inferred the upstream pathway activity of common host factors in COVID-19 and asthma
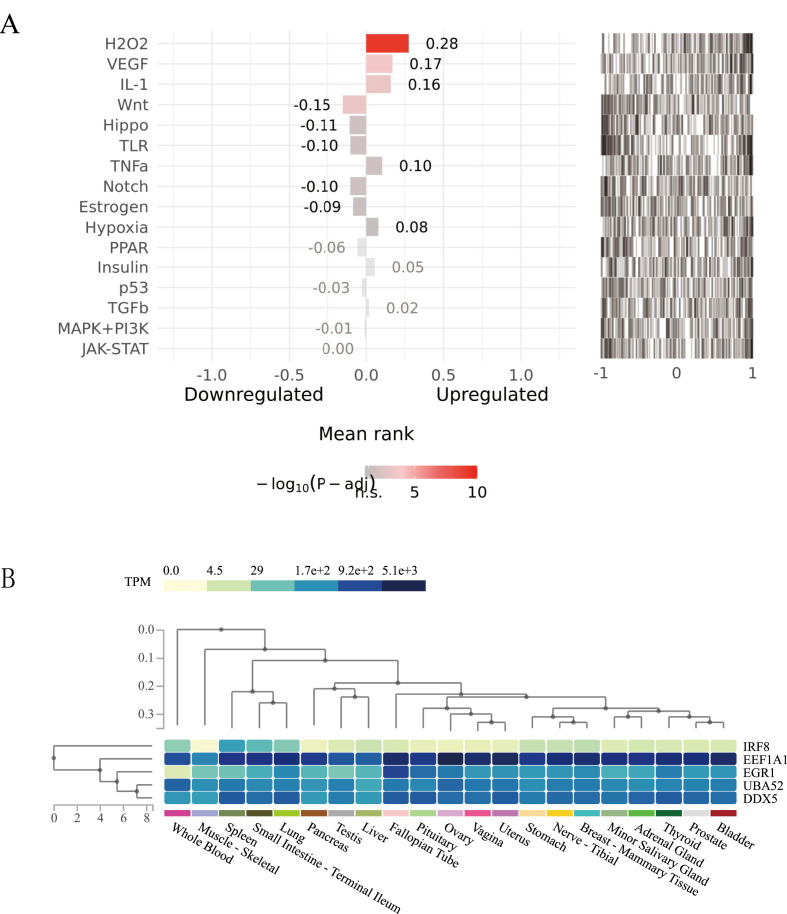
The activities of the upstream pathways of 192 host factors between COVID-19 and asthma were predicted by using the SPEED2 database. Fig. 5 A ranked the important pathways and showed the average rank of the genome provided in each entry. H2O2, VEGF, IL-1 and Wnt signaling pathways had the strongest activities in the upstream pathways.
Fig. 5.
(A): SPEED2 signaling pathway enrichment analysis of common targets between COVID-19 and asthma. (B): Tissue-specific expression analysis of the critical host factors (The vertical axis represents different tissues, and the horizontal axis represents the expression levels of 13 key host factors in each tissue. The expression levels of genes are measured by Transcripts Per Million (TPM). The darker the color, the higher the distribution of the corresponding host factor in a specific tissue).
3.6. Tissue-specific enrichment analysis revealed the expression of critical host factors in COVID-19 and asthma
The results of the tissue-specific analysis in the GETx database showed that EEF1A1 had a higher expression level in many tissues, and IRF8 had a lower expression level in many tissues. Most of the host factors between asthma and COVID-19 were distributed in tissues such as the lung, stomach, vagina, breast, liver, ovary, small intestine, spleen, whole blood and so on (Fig. 5B).
3.7. Gene-drug interactions networks indicated the potential drugs for COVID-19 and asthma
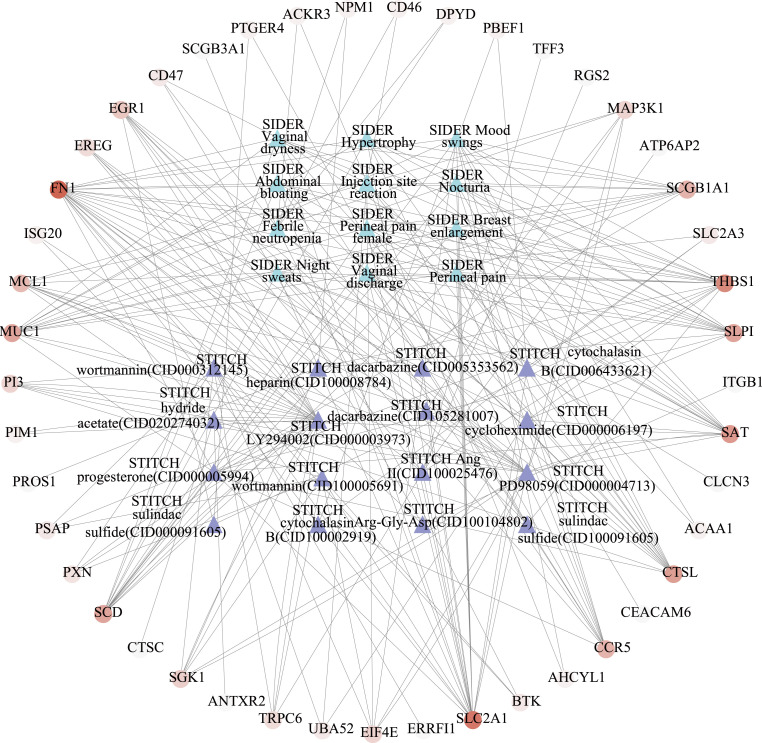
The 192 key host factors were searched in the STTICH database to identify known drugs that interact with host factors co-expressed between asthma and COVID-19. The host factor interaction networks were identified to probe candidate drugs that may be effective in the treatment of COVID-19/asthma comorbidity. Among all host factors, FN1 has the largest degree value. Ultimately, LY294002, wortmannin, PD98059 and heparin would be identified as the most potent drugs for COVID-19/asthma comorbidity (Table 7 and Fig. 6 ).
Table 7.
List of recommended medications for COVID-19 and asthma comorbidities.
| Enrichment FDR | Genes | Pathway Genes | Fold Enrichment | Pathway |
|---|---|---|---|---|
| 1.47E-05 | 13 | 200 | 8.1864088 | STITCH LY294002 (CID000003973) |
| 1.51E-04 | 11 | 175 | 7.9165272 | STITCH LY294002 (CID100003973) |
| 9.41E-04 | 10 | 176 | 7.1559518 | STITCH wortmannin (CID000312145) |
| 2.25E-03 | 13 | 350 | 4.6779479 | SIDER Mood swings |
| 2.41E-03 | 9 | 163 | 6.9540047 | STITCH wortmannin (CID100005691) |
| 3.85E-03 | 10 | 223 | 5.6477467 | STITCH PD98059 (CID000004713) |
| 8.81E-03 | 9 | 200 | 5.6675138 | STITCH PD98059 (CID100004713) |
| 1.43E-02 | 11 | 325 | 4.2627454 | SIDER Febrile neutropenia |
| 2.13E-02 | 10 | 313 | 4.0237940 | SIDER Abdominal bloating |
| 2.13E-02 | 10 | 314 | 4.0109793 | SIDER Breast enlargement |
| 2.13E-02 | 10 | 312 | 4.0366907 | SIDER Injection site reaction |
| 2.13E-02 | 10 | 316 | 3.9855934 | SIDER Nocturia |
| 2.13E-02 | 10 | 313 | 4.0237940 | SIDER Perineal pain |
| 2.13E-02 | 10 | 313 | 4.0237940 | SIDER Perineal pain female |
| 2.13E-02 | 12 | 412 | 3.6682937 | SIDER Vaginal discharge |
| 2.13E-02 | 10 | 313 | 4.0237940 | SIDER Vaginal dryness |
| 2.13E-02 | 8 | 188 | 5.3593511 | STITCH Ang II (CID100025476) |
| 2.13E-02 | 4 | 34 | 14.817030 | STITCH cytochalasin B (CID100002919) |
| 2.13E-02 | 10 | 310 | 4.0627339 | STITCH progesterone (CID000005994) |
| 2.13E-02 | 3 | 13 | 29.064173 | STITCH sulindac sulfide (CID100091605) |
| 2.45E-02 | 11 | 386 | 3.5890991 | SIDER Night sweats |
| 2.50E-02 | 4 | 41 | 12.287293 | STITCH cytochalasin B (CID006433621) |
| 2.50E-02 | 7 | 161 | 5.4758588 | STITCH heparin (CID100008784) |
| 2.50E-02 | 7 | 162 | 5.4420572 | STITCH hydride acetate (CID020274032) |
| 2.55E-02 | 11 | 396 | 3.4984653 | SIDER Hypertrophy |
| 2.55E-02 | 3 | 18 | 20.990792 | STITCH dacarbazine (CID005353562) |
| 2.55E-02 | 3 | 18 | 20.990792 | STITCH dacarbazine (CID105281007) |
| 2.90E-02 | 3 | 19 | 19.886013 | STITCH sulindac sulfide (CID000091605) |
| 3.66E-02 | 8 | 233 | 4.3242833 | STITCH cycloheximide (CID000006197) |
| 3.68E-02 | 3 | 21 | 17.992107 | STITCH Arg-Gly-Asp (CID100104802) |
Fig. 6.
Drug-gene interaction network. The circle represents the common host factor between asthma and COVID-19, the darker the color, the higher the degree value; the triangle represents the drug and side effect resource (SIDER) enriched by the host factors, blue represents SIDER and purple represents different drugs; the line represents the interaction between host factors and drugs or SIDER.
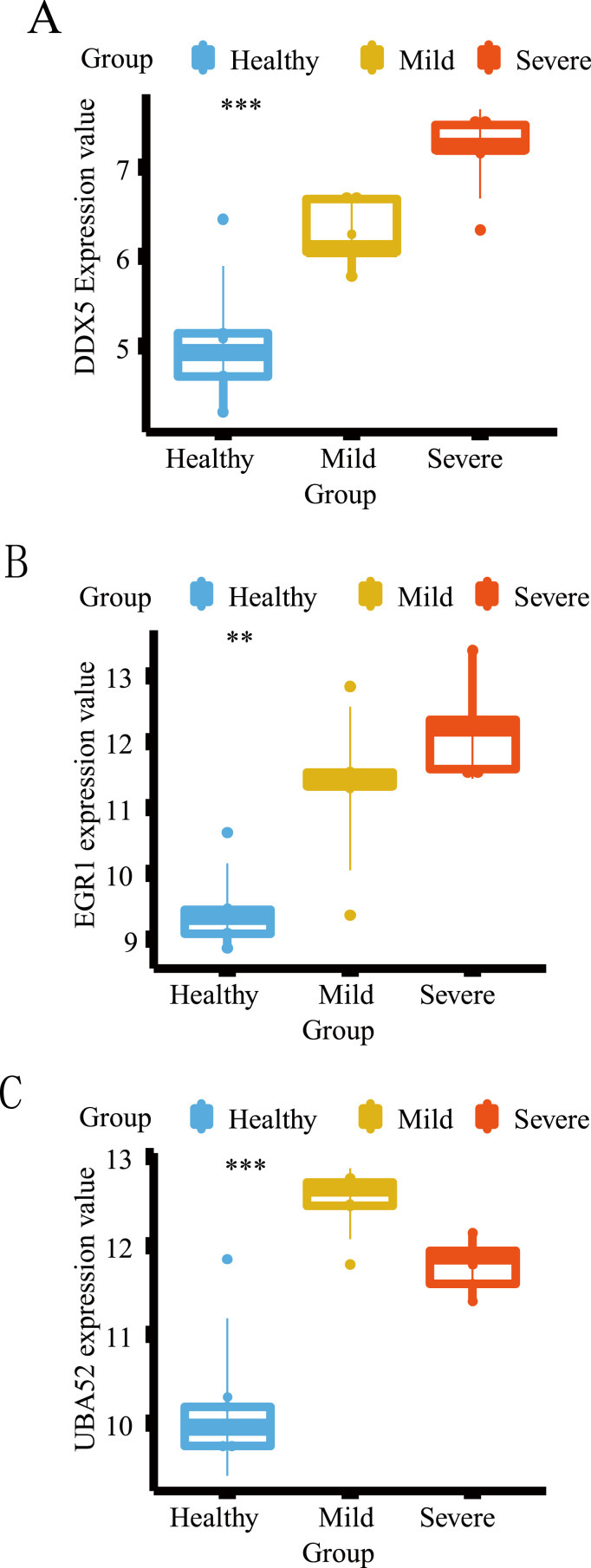
3.8. Verification of the expression levels of key host factors in COVID-19 related datasets
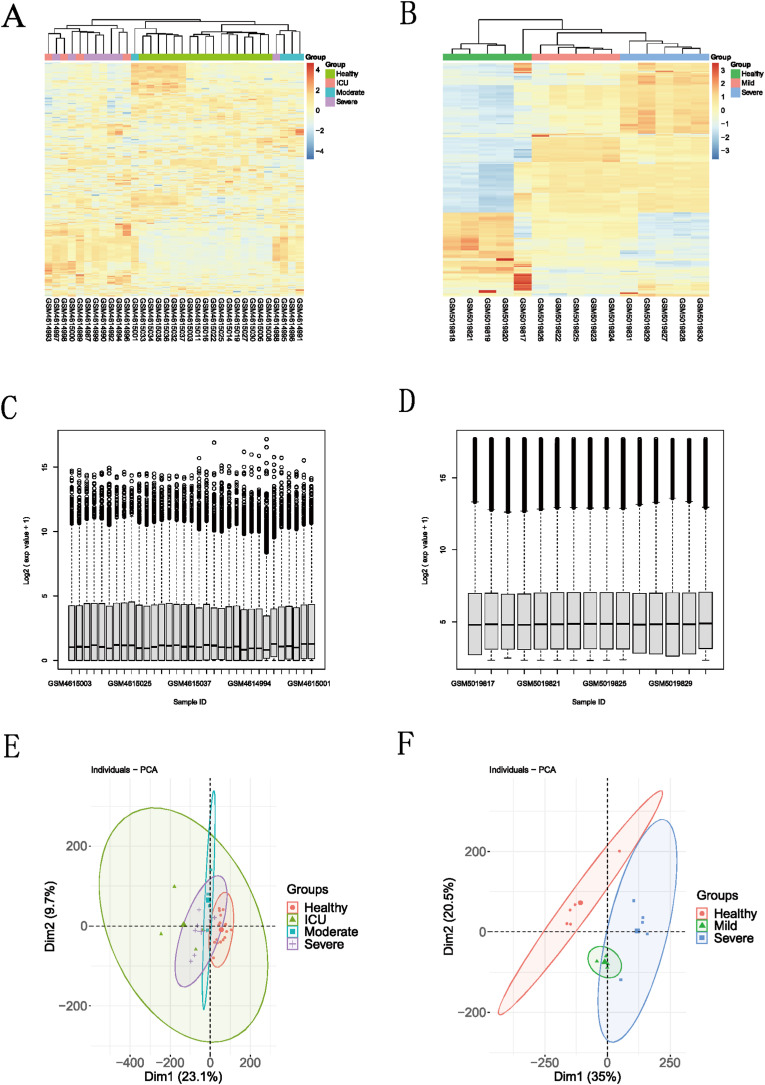
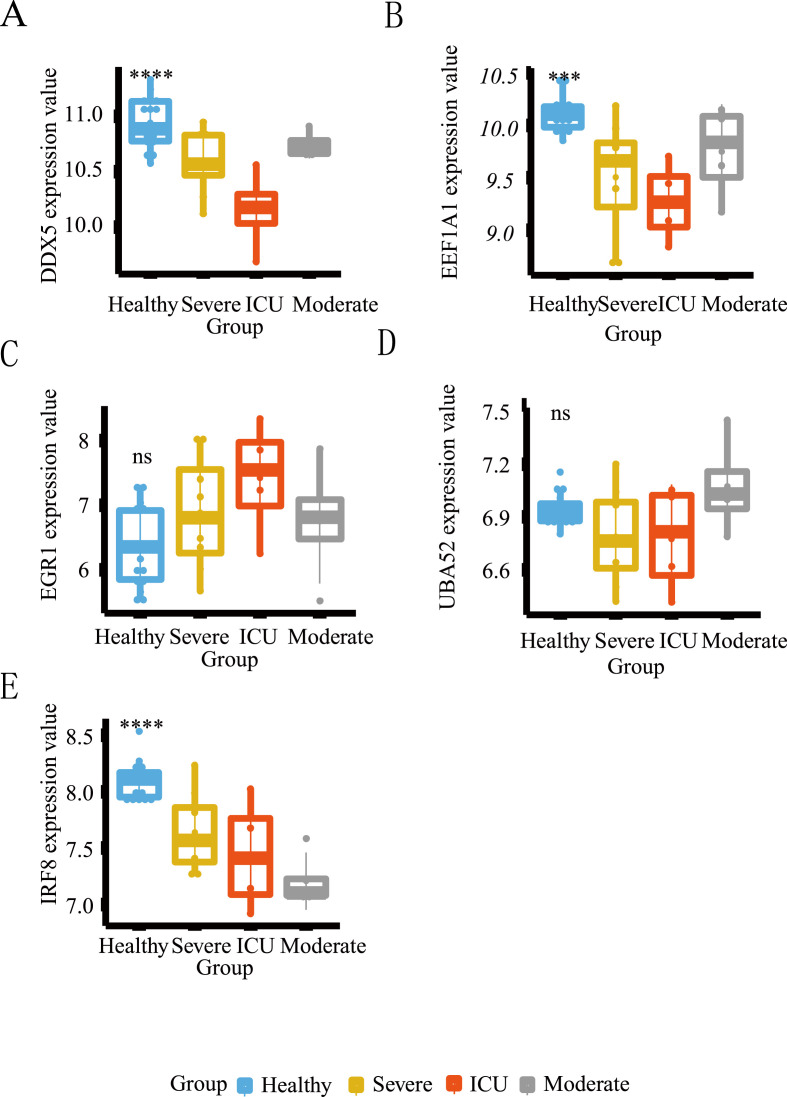
The heatmaps, boxplots and PCA diagrams in Fig. 7 showed the differences between COVID-19 and healthy groups in the GSE152418 and GSE164805 datasets. The expression levels of the hub host factors between asthma and COVID-19 were further verified in these two datasets (Fig. 8 and Fig. 9 ). Notably, DDX5 was lowly expressed in the COVID-19 group in the CRA002390 dataset. However, DDX5 showed an opposite trend against severity in both validation sets, which needs to be confirmed by further experiments. Although there was no statistical difference in the expression of EGR1 between the two groups in the GSE152418 dataset; the mRNA expression of EGR1 gradually increased with the severity of COVID-19 and showed the same trend in both validation datasets. UBA52 was lowly expressed in the healthy group, but there was no correlation between its increased expression and the severity of COVID-19. IRF8 and EEF1A1 were only validated in the GSE152418 dataset, and both were lowly expressed in COVID-19. As the severity of COVID-19 increased, the mRNA expressions of IRF8 and EEF1A1 showed a decreasing trend.
Fig. 7.
(A, B): Heatmap between different severity of COVID-19 and healthy group in the GSE152418 and GSE164805 dataset; the blue box represents the healthy group, the gray box represents the moderate COVID-19 group, the orange box represents the severe COVID-19 group, and the red box represents living in ICU of the COVID-19.group. (C, D) Boxplot for viewing the distribution of sample values in the GSE152418 and GSE164805 datasets. A value-centered on the median indicates that the data is normalized and cross-comparable; (E, F): Principal Component Analysis between different severity of COVID-19 and healthy group in the GSE152418 and GSE164805 dataset.
Fig. 8.
Differential expression analysis of the hub common host factors in the GSE152418 dataset. *: P < 0.05, **: P < 0.01, ***: P < 0.001, ****: P < 0.0001. The horizontal axis represents the different groups, and the vertical axis represents the gene expression level. The blue box represents the healthy group, the gray box represents the moderate COVID-19 group, the orange box represents the severe COVID-19 group, and the red box represents the living in ICU of the COVID-19 group.
Fig. 9.
Differential expression analysis of the hub common host factors in the GSE164805 dataset. *: P < 0.05, **: P < 0.01, ***: P < 0.001, ****: P < 0.0001. The horizontal axis represents the different groups, and the vertical axis represents the gene expression level. The blue box represents the healthy group, the gray box represents the moderate COVID-19 group, the orange box represents the severe COVID-19 group, and the red box represents the living in ICU of the COVID-19 group.
4. Discussion
The initial diagnosis and subsequent monitoring of asthma largely depend on lung function tests, and lung function tests will produce droplets or aerosols that may cause virus diffusion. Thus, the restrictions imposed by COVID‐19 pandemic have severely affected the management of asthma. According to the results of the two systematic reviews, the risk of SARS-CoV-2 infection and mortality after infection for asthma sufferers are reduced compared with non-asthma patients [33,34]. However, a Korean cohort study shows that the incidence of asthma is related to the severity and mortality of COVID-19 [7]. Research indicates that some asthma patients are at risk of severe COVID-19 [18]. Nevertheless, the risk of SARS-CoV-2 infection in asthma patients is still an objective matter. Compared with healthy people, the pathogenic process and virus-host interaction networks of asthma patients as the SARS-CoV-2-host have not yet been fully elucidated. Therefore, this study mainly studied mechanisms from the perspective of host factors between asthma and COVID-19 to illustrate the biological processes and explore possibly influential factors and therapeutic drugs for comorbidity.
In the first step, the common DEGs of COVID-19 and asthma were identified in BALF samples, and they were considered to be strongly correlated with the targets of host factor interaction. Then a series of differential analyses were conducted to identify the potential and effective host factors interaction networks for COVID-19 and asthma. Core host factors were considered as the potential targets for treating co-occurring comorbidity and several relevant drugs were found to be possible treatments for comorbidity. And the expression levels of core host factors were verified in COVID-19 related datasets.
4.1. Enrichment analysis highlights the bioinformatics mechanisms of host factors and significant shared signaling pathways
Several important terms of BP, CC and MF of COVID-19 in asthma patients' co-pathogenic mechanisms were obtained through GO analysis. The BP was mainly related to the immune response that neutrophils participate in. Neutrophilic asthma (NA) is a clinically important asthma phenotype and characterized by neutrophil infiltration, which is different from allergic asthma [35]. External stimulus induces the migration of neutrophils to inflamed sites and activates immune response [36]. In addition, the increased levels of neutrophils are associated with severe COVID-19 [37], indicating that neutrophil-mediated immuno-inflammatory response is crucial in the pathological process of asthma and COVID-19. The CC involved in the immune response mainly included secretory granule membrane, vacuolar membrane, cell leading edge, lysosomal membrane and lytic vacuole membrane. Enzyme inhibitor activity is the most important molecular function. p38 MAPK upregulation promotes the expression of various proinflammatory cytokines and chemokines leading to bronchial inflammation and airway remodeling, thus some enzyme inhibitors (such as MAPK inhibitors) are considered to be a protector for asthma patients [38]. Similarly, MAPK inhibitors can reduce COVID-19 infection by inhibiting inflammatory response and thus are regarded as a promising treatment [39].
KEGG analysis revealed that PI3K/AKT signaling pathway was one of the important pathways for comorbidity. PI3K/AKT pathway may play a key role in asthma by regulating airway remodeling [40]. PI3K/AKT pathway can promote T-helper 2 cytokine expression, eosinophil infiltration and mucus production, thus aggravating airway inflammation and hyperresponsiveness [41]. PI3K inhibitors can significantly reduce the number of eosinophils in the lungs, the level of eosinophil chemokine, and the expression of IL-5 and IL-13 in BALF of asthmatic mice [42]. Inhibition of PI3K/AKT/mTOR pathway with targeted molecules or natural compounds can attenuate peri-bronchial and peri-vascular inflammation to protect airways, such as LY294002 and Bixin [43,44]. On the other hand, the mTOR inhibitor rapamycin is identified as a potential medication for COVID-19 patients by constructing the drug-target network for the human coronavirus–host interactome via various bioinformatics approaches [45]. Mortality of diabetic patients hospitalized with COVID-19 is significantly reduced in the metformin-treated group, which may be related to the mTOR-inhibiting effect of metformin [46]. SARS-CoV-2 spikes can promote COVID-19-related inflammation and apoptosis through the PI3K/AKT/mTOR signaling pathway [47]. In the late stage of lung injury in mice, AKT inhibitors can promote the recovery of lung injury by increasing the number of regulatory T cells [48], which can inhibit lung fiber proliferation and inflammation [49,50]. Therefore, inhibition of AKT may increase the number of regulatory T cells in the lungs of COVID-19 patients, thereby reducing lung inflammation and promoting repair [51]. Thrombosis of microvessels and large vessels is one of the characteristics of COVID-19, which significantly increases the possibility of COVID-19 patients complicated by disseminated intravascular coagulation (DIC) [52]. Coincidentally, PI3K/AKT signaling pathway can activate platelets and promote coagulation [53]. The blocking of the PI3K/AKT signaling pathway is accompanied by a significant inhibitory with thrombosis [54]. Therefore, PI3K/AKT signaling pathway has great potential to reduce thrombosis and prevent DIC in COVID-19 patients [55]. The significance of PI3K/AKT in COVID/19 comorbidity was also determined in the WIKI enrichment analysis. Furthermore, MAPK and PI3K/AKT pathways are involved in inflammation. It is well known that COVID-19 is characterized by an overwhelming inflammation, and asthma is a chronic airway inflammatory disease. It is found that lung inflammation may promote the expression of ACE2 and TMPRSS2 and increase the risk of COVID-19 [56]. Our research revealed that the inflammatory process exists in COVID-19 and asthma. Therefore, it is of great value to find inflammatory pathways acting on COVID-19 and asthma comorbidity. And MAPK inhibitors that block inflammation are the drug candidate, which is consistent with the predicted results of pathway enrichment analysis.
Although human T-cell leukemia virus 1 infection does not increase the severity of COVID-19, it can cause immunosuppression [57]. A Genome-wide Association Study of Asthma shows that Wnt and cytokine-cytokine receptor interaction play key roles in the progress of asthma [58]. Immune effector cells can precipitate abnormal systemic inflammatory responses by releasing large amounts of pro-inflammatory cytokines and chemokines during SARS-CoV infection [59]. Besides, the common host factors between asthma and COVID-19 were significantly related to metabolic pathways, tumors, lysosome, phagosome and human papillomavirus infections.
In addition, WIKI enrichment analysis revealed that host-pathogen interaction of human coronaviruses-PI3K/AKT signaling pathway was closely related to the host factor interaction network. Nrf2 exerts an anti-inflammatory effect by inhibiting the expression of pro-inflammatory factors, such as IL-6 and IL-1β [60]. Nrf2 also induces the expression of several macrophage-specific genes that involve in the virus surveillance, including macrophage receptors, scavenger receptors for oxidized low-density lipoprotein, CD36, and receptors required for bacterial phagocytosis and IL-17D [[61], [62], [63]]. Nrf2 in macrophages and epithelial cells can effectively inhibit the pro-inflammatory and oxidative effects of particulate pollutants in allergic inflammation and asthma [64]. In fact, Nrf2 deficient mice are more likely to develop severe airway inflammation and asthma [65]. Abnormal proliferation and hypertrophy of airway smooth muscle cells are one of the pathological characteristics of asthma [66]. Nrf2 can activate antioxidant capacity and inhibit abnormal proliferation in airway smooth muscle cells [67]. In addition, Nrf2 can enhance the integrity of the airway epithelial barrier [68], which contributes to reducing air pollution and respiratory virus infection [69]. The above researches indicate that the Nrf2 pathway may be a potential and important target against asthma and COVID-19 comorbidity. SARS-CoV2 can interact with the Nrf2 pathway, indicating that targeting the Nrf2 pathway may help to inhibit the replication of SARS-CoV2 in cell lines [70].
4.2. PPIs and MCODE analyses reveal essential host genes for comorbidity
PPI network analysis was conducted to identify the key genes of COVID-19 and asthma, the top genes were FN1, UBA52, EEF1A1, ITGB1, XPO1, NPM1, EGR1, EIF4E, SRSF1, CCR5, PXN, IRF8 and DDX5. Cellular FN, a protein produced by human bronchial epithelial cells as a marker of vascular injury, is demonstrated to be positively associated with asthma severity and prothrombotic blood alterations [71]. FN drives airway remodeling and chronic inflammation when deposited into the asthmatic airways [72]. Research has shown that the high-resolution profile of immunoglobulin regions exhibits an upward trend of FN1 during COVID-19 [73]. Therefore, we can speculate that the expression of FN1 may increase in asthma patients infected with SARS-CoV-2. ITGB1 is involved in cytoskeletal pathways and increased in myofibroblasts by regulating the TGF-β signaling pathway [74]. ITGB1 is an essential host factor for selected adenoviruses-associated variants that facilitate cell attachment and entry [75]. These studies demonstrate that the high expression of ITGB1 in asthma patients may contribute to cell adsorption and invasion after SARS-CoV-2 infection. XPO1 is a fraction of the karyopherin-β superfamily of nuclear transport proteins. Selective XPO1 inhibitors can exert anti-inflammatory, anti-viral and anti-oxidant effects on COVID-19 patients [76]. Therefore, XPO1 may be a potential host factor for asthma and COVID-19 co-occurrence by mediating airway inflammation and virus replication. EIF4E is involved in the process of protein translation and its upstream molecule is MAPK-activated protein kinase (MNK)-1. Inhibition of MNK-1 can reduce inflammation and airway remodeling [77]. In addition, the phosphorylation and release of 4EBP (the corresponding downstream binding protein of EIF4E) are required for hypertrophy of human airway smooth muscle cells [78]. The SARS-CoV-2 replication depends on the interaction between viral mRNA and EIF4E, and blocking this interaction is one of the targets for the treatment of COVID-19 [79]. COVID-19 patients have been proved to be at increased risk of stroke, pulmonary embolism and other thromboembolism [80]. Elevated expression of CCL5, a chemokine that binds to CCR5, has been demonstrated in patients with COVID-19 [81,82]. Platelet activation leads to the initiation of the coagulation cascade, which can be triggered by the CCL5/CCR5 axis [83]. Moreover, CCR5 inhibitors can reduce inflammatory cytokines in critically ill COVID-19 patients, increase CD8 T cells and reduce SARS-CoV-2 in plasma [84]. Therefore, the blocking of the CCL5/CCR5 axis may reduce the risk of complications caused by thrombosis in COVID-19 patients. Other targets related to virus infection and replication mainly included NPM1, EGR1, DDX5, SRSF1 and HIST2H2AC.
PPI networks were divided into different areas by module analysis according to different biological functions and showed the core targets in each module. EEF1A1 and DDX5 were the core targets with higher degree values in module 1. EEF1A1, a translation regulator, is involved in the protein translation process of eukaryotes as a plasmid vector for cell transfection. DDX5, a prototypical member of the RNA helicases family, is known to participate in RNA metabolism involving transcription and translation. EEF1A1 and DDX5 are confirmed to interact with several viral proteins to promote virus replication [85,86]. The host factors in module 2 were mainly enriched in the “type I interferon signaling pathway”, of which EGR1 was the most significant. EGR1 directly binds to the IRAV promoter and upregulates the expression of IFN-regulated antiviral targets to suppress the replication of PEDV (a globally distributed alphacoronavirus) [87]. In Module 3, HIST2H2AC and THBS1 were the most critical host factors and significantly enriched in “extracellular matrix organization” and “ECM-receptor interaction”. In addition, UBE2D3, UBA52 and RNF146 had been regarded as the core targets in module 4. UBE2D3 plays an essential role in RIG-I-mediated virus infection as activators, and RIG-I senses viral RNA and initiates an innate immune response for type I interferon production [88]. UBA52 was identified as a host factor that interacts with the RNA polymerase acidic protein for virus replication in influenza [89] and relates to ubiquitination [90]. SEC23 has the function of transporting newly synthesized proteins and lipids from the endoplasmic reticulum to the Golgi apparatus for further processing and secretion. SEC23A, the most important target in module 5 involved in vitamin D synthesis, may affect the level of vitamin D synthesis [91]. Vitamin D deficiency increases the risk of COVID-19 and worsens the severity of asthma [92,93]. IRF8 was revealed as another key co-host factor for COVID-19 and asthma co-occurrence. The dysregulated immune response in COVID-19 may be related to decreased autophagy-related genes expression and the expansion of myeloid-derived suppressor cell subsets via suppressing IRF8-mediated mechanisms [94].
4.3. Inferring upstream pathway activity and tissue-specific enrichment analysis of hub genes
We tried to infer the upstream pathway activity via SPEED2 and upregulated genes were found, including H2O2, VEGF, IL-1 and so on. H2O2-mediated oxidative stress plays an important role in airway inflammation in asthma [95] and predicts COVID-19-associated mortality [96]. VEGF enhances disintegrin and metalloproteinase-33 expression and airway smooth muscle cell proliferation, which may be implicated in inflammation and airway vascular remodeling in asthma [97]. Inflammatory cytokines can activate the VEGF signaling pathway in COVID-19 patients [98], which may increase vascular permeability related to endothelial dysfunction to promote endothelial inflammation [98,99]. Severe vascular endothelial dysfunction predisposes to cause endodermatitis, vascular leakage and thrombosis [100,101]. Besides, VEGF participates in the cytokine storm exacerbation and thus anti-VEGF compounds are supposed to be helpful candidates for COVID-19 [102]. IL-1 is a potential therapeutic target in patients with neutrophilic asthma [103] and severe inflammatory ARDS complicated by COVID-19 [104].
Tissue-specific enrichment analysis was carried out to explore the novel association of tissue with genes in interaction networks, and we found that the expression of EEF1A1 was highly enriched in the lung. EEF1A1 is a translation regulator involved in protein synthesis in line with the result that EEF1A1 had a higher distribution density in other tissues and whole blood. Thus, EEF1A1 may be an important regulator in the pathophysiological response of patients with asthma and COVID-19.
4.4. Identification of candidate drugs for COVID-19/asthma comorbidity and corresponding biological functions
The host factors interaction network between COVID-19 and asthma was constructed to probe candidate drugs that may be effective for comorbidity. Ultimately, LY294002, wortmannin, PD98059 and heparin were identified as the most potent drugs.
LY294002, a PI3K/AKT pathway inhibitor, reduces PI3K and AKT expression and suppresses chronic inflammation [105]. Moreover, LY294002 ameliorates glucocorticoid insensitivity in severe asthma by restoring histone deacetylase 2 activity and inhibiting the phosphorylation of nuclear signaling transcription factors [106]. LY294002 can inhibit the expression of MCP-1, IL-6 and IL-8 released by bronchial epithelial cells, promote pulmonary neutrophil apoptosis and reduce the inflammatory response [107]. LY294002 can inhibit the proliferation, migration and secretion of proinflammatory factors of airway smooth muscle cells to reduce airway hyperresponsiveness and airway inflammation [108]. Wortmannin selectively inhibits PI3K and affects the signal pathway transduction, leading to reduced iNOS expression and NO production in bronchiole epithelial cells to alleviate airway inflammation and hyperresponsiveness in asthma patients [109]. The PI3K/AKT pathway-specific inhibitors are supposed to ameliorate COVID-19 by inhibiting excessive inflammation, protecting cells and antiviral effect [110]. As mentioned, KEGG analysis revealed that PI3K/AKT signaling pathway was one of the important pathways for comorbidity. Considering the context of important scientific literature, the specific PI3K/AKT pathway inhibitors are recommended as the treatment for COVID-19/asthma comorbidity. LY294002 and Wortmannin with a function of selectively inhibiting PI3K and affecting the signal pathway transduction are potential therapeutic drugs for COVID-19/asthma comorbidity predicted by the STITCH database. Therefore, LY294002 and wortmannin are both considered to be potential pharmacological targets for COVID-19/asthma comorbidity. EGR1, a transcription factor related to vascular dysfunction, was highly expressed in COVID-19 patients compared with healthy people in this study. The study has shown that EGR1 is inhibited by pharmacological blockade of the PI3K/AKT pathway via wortmannin [111].
PD98059, an inhibitor of ERK, has an effect on regulating the imbalance of Th1/Th2 and Treg/Th17 for neutrophilic asthma [112] and was predicted to be a druggable target. We also revealed that the MAPK signaling pathway was related to the host factor interaction network, but it was not clear which specific way will be affected.
Exogenous heparin can inhibit the adhesion of SARS-CoV-2 to target cells to reduce virus infection [113]. Heparin and its chemical derivatives have been proved to be effective heparanase (HPSE) inhibitors [114,115]. HPSE can cause vascular endothelial barrier dysfunction and vascular leakage, leading to pulmonary edema and proteinuria nephropathy [[116], [117], [118]]. Coincidentally, the expression of HPSE is increased in the plasma of COVID-19 patients [119]. Therefore, heparin may reduce the severe clinical manifestations of COVID-19 patients by inhibiting HPSE activity. Heparin has been shown to have a variety of anti-inflammatory properties [[120], [121], [122]]. In addition, heparin can also inhibit the NF-κB signaling pathway and reduce the production of TNF-α, IFN-γ, IL-6 and IL-8 [122,123]. The excessive inflammatory response in severe COVID-19 patients is closely related to cytokine storm [124], suggesting that heparin may contribute to inhibiting the cytokine storm in severe COVID-19. A randomized clinical trial finds that the nebulized low molecular weight heparin (LMWH) can be used as an adjunctive treatment for an acute mild-moderate asthma attack [125].In addition, LMWH administered by inhalation can significantly improve the symptoms of ARDS and dyspnea to treat hypoxemia caused by COVID-19 [126]. Therefore, aerosol inhalation of heparin may be effective in the treatment of COVID and asthma comorbidity.
4.5. The expressions of the five key host factors in COVID-19 patients
EGR1 and UBA52 were proved to be highly expressed in COVID-19 patients compared with healthy people, indicating the above core genes may play an important role in the pathogenesis of COVID-19. EEFEA1, DDX5 and IRF8 showed an upward trend in the healthy group, suggesting that these genes might be decreased in COVID-19 patients. The key host factors that were highly expressed in COVID-19 patients may be the major pathogenic factors, while those that were highly expressed in healthy people and reduced in COVID-19 patients may be protective factors.
EGR1, a transcription factor that regulates antiviral genes, is reported to be restricted at the post-transcriptional level by SARS-CoV-2 infection [127]. This existing research result seems to be inconsistent with ours. UBA52 is a conserved host factor that interacts with H5N1 avian influenza virus proteins and plays an important role in viral amplification [89]. According to the results of this study, we speculate that the increased expression of UBA52 promotes ubiquitination of modified proteins, participates in SARS-CoV-2 replication and inhibits the innate immune response of the host.
EEFEA1 regulates the enzymatic delivery of aminoacyl tRNAs to the ribosome. The EEF1A1-based vector is applied for the expression of receptor-binding domain protein of SARS-CoV-2 to regulate the correct exposure of antigenic determinants, which has a significant impact on the accuracy of serological tests [128]. Furthermore, siRNA silencing of EEFEA1 induces a decrease in viral N protein levels during SARS-CoV-2 infection, indicating that the reduced EEFEA1 is a drug target for the inhibition of SARS-CoV-2 replication [129]. DDX5 enhances virus replication via interaction with several viral proteins as mentioned earlier. Although EEFEA1 and DDX5 are related to SARS-CoV-2 replication, there is no definite evidence to show the changes in EEFEA1 and DDX5 during SARS-CoV-2 infection. IRF8 is a transcription factor that belongs to the interferon regulatory factor family. In line with the results of this study is that the mRNA levels of IRF8 in mild and severe COVID-19 patients are significantly reduced compared with the healthy population [94].
5. Conclusion
To explore the mechanisms of COVID-19/asthma comorbidity, we screened the COVID-19 and asthma datasets derived from BALF samples and strictly processed the core common host factors. Successively, we conducted a series of bioinformatics analyses, such as the PPI network, enrichment analysis, inferring of upstream pathway activity, tissue-specific enrichment analysis and the expressions of key host factors in COVID-19. Our research reveals the immune response that neutrophils participate in is the most important biological process of COVID-19/asthma comorbidity. The PI3K/AKT signaling pathway may involve in allergic reactions in asthma patients and enhance the SARS-CoV-2 infection through mediating inflammation and apoptosis. And the WIKI enrichment analysis revealed that the Nrf2 signaling pathway may be the key signaling pathway to mediate the host-pathogen interaction. We also identified EEF1A1, EGR1, UBA52, DDX5 and IRF8 as the key co-host factors for COVID-19/asthma comorbidity. Finally, we confirmed that LY294002, wortmannin, PD98059 and heparin may develop as druggable targets against COVID-19/asthma comorbidity.
6. Limitation
Datasets of this study were obtained from BALF samples, although we selected 2 PBMC-related datasets to verify the predicted targets, whole blood and lung tissue samples of COVID-19 patients were inevitably excluded to ensure the homogeneity resulting in the loss of some DEGs, which is a limitation in choosing asthma samples. In the future, further research needs to be carried out to compare the commonalities and differences by recruiting more samples of asthma patients complicated by COVID-19. It is worth noting that the combination of complete clinical information and transcriptome analysis will bring breakthroughs to the mechanism of COVID-19/asthma comorbidity. Finally, we have theoretically found some evidence to support the therapeutic values of potential drugs, but more clinical evidence is still needed.
Data availability statements
The data and R code of this article are available at Gene Expression Omnibus public repository and supplementary file.
Funding
This work was supported by the National Natural Science Foundation of Guangdong, China (Grant no. 2020A1515010589 and Grant no. 2021A1515010146) and supported by the Sanming Project of Medicine in Shenzhen (Grant No. SZZYSM202106006). This work was supported by Traditional Chinese Medicine Bureau of Guangdong Province, China (Grant no. 20221144). This work was also supported by the “Double First-Class” and High-level University Discipline Collaborative Innovation Team Project of Guangzhou University of Chinese Medicine (Grant No.2021XK16), Guangdong Provincial Department of Education Innovation Team Project (Grant No: 2018KCXTD007), the Key-Area Research and Development Program of Guangdong Province (Grant No. 2020B1111100002), the National Natural Science Foundation of China (Grant No. 81973814 and No. 81904132), and the Technology Research of COVID-19 Treatment and Prevention and Special Project of Traditional Chinese Medicine Application-Research on the platform construction for the prevention and treatment of viral infectious diseases with traditional Chinese medicine (Grant No. 2020KJCX-KTYJ-130).
Authors' contributions
Xiu-Fang Huang, Shao-Feng Zhan and Xiao-Hong Liu participated in the guidance and revision of the entire article. Yong Jiang, Qian Yan and Cheng-Xin Liu conducted the writing and data analysis. Chen-Wen Peng participated in data analysis in the revision process. Hong-Fa Zhuang, Qiong Liu, Hui-Li Liao and Wen-Jiang Zheng performed the literature searches and data analysis.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Liu Xiaohong reports article publishing charges was provided by Shenzhen Hospital of Integrated Traditional Chinese and Western Medicine.
Acknowledgments
We thank Lingnan Medical Research Center of Guangzhou University of Chinese Medicine and the Famous Traditional Chinese Medicine inheritance physician unit of Xiao-Hong Liu of Guangdong for their support. We thank Professor LingJun Wang for his support in carrying out this work. We thank Professor Xiaohong Liu for her kind help in the initiation of this research and her selfless assistance.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.compbiomed.2022.105601.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Sharma A., Ahmad Farouk I., Lal S.K. COVID-19: a review on the novel coronavirus disease evolution, transmission, detection, control and prevention. Viruses. 2021;13(2) doi: 10.3390/v13020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Lancet Infectious D. COVID-19: endgames. Lancet Infect. Dis. 2020;20(5):511. doi: 10.1016/S1473-3099(20)30298-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omarjee L., Perrot F., Meilhac O., Mahe G., Bousquet G., Janin A. Immunometabolism at the cornerstone of inflammaging, immunosenescence, and autoimmunity in COVID-19. Aging. 2020;12(24):26263–26278. doi: 10.18632/aging.202422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sockrider M., Tal-Singer R. Managing your chronic lung disease during the COVID-19 pandemic. Am. J. Respir. Crit. Care Med. 2020;202(2):P5–P6. doi: 10.1164/rccm.2020C8. [DOI] [PubMed] [Google Scholar]
- 5.Papi A., Brightling C., Pedersen S.E., Reddel H.K. Asthma. Lancet (London, England) 2018;391(10122):783–800. doi: 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- 6.Li X. Hot topic: precision medicine for asthma-has the time come? Curr. Allergy Asthma Rep. 2019;19(10):45. doi: 10.1007/s11882-019-0881-3. [DOI] [PubMed] [Google Scholar]
- 7.Kim S.H., Ji E., Won S.H., Cho J., Kim Y.H., Ahn S., et al. Association of asthma comorbidity with poor prognosis of coronavirus disease 2019. World Allergy Organ. J. 2021;14(8) doi: 10.1016/j.waojou.2021.100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovinsky-Desir S., Deshpande D.R., De A., Murray L., Stingone J.A., Chan A., et al. Asthma among hospitalized patients with COVID-19 and related outcomes. J. Allergy Clin. Immunol. 2020;146(5):1027–1034. doi: 10.1016/j.jaci.2020.07.026. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skene I.P., Pfeffer P.E. Improved asthma control during the COVID-19 pandemic: are there lessons to be learnt? Thorax. 2021;76(9):852–853. doi: 10.1136/thoraxjnl-2021-216930. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Z., Hasegawa K., Ma B., Fujiogi M., Camargo C.A., Jr., Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J. Allergy Clin. Immunol. 2020;146(2):327–329. doi: 10.1016/j.jaci.2020.06.001. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green I., Merzon E., Vinker S., Golan-Cohen A., Magen E. COVID-19 susceptibility in bronchial asthma. J. Allergy Clin. Immunol. Pract. 2021;9(2) doi: 10.1016/j.jaip.2020.11.020. 684-92.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carli G., Cecchi L., Stebbing J., Parronchi P., Farsi A. Is asthma protective against COVID-19? Allergy. 2021;76(3):866–868. doi: 10.1111/all.14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters M.C., Sajuthi S., Deford P., Christenson S., Rios C.L., Montgomery M.T., et al. COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids. Am. J. Respir. Crit. Care Med. 2020;202(1):83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S., Zhi Y., Ying S. COVID-19 and asthma: reflection during the pandemic. Clin. Rev. Allergy Immunol. 2020;59(1):78–88. doi: 10.1007/s12016-020-08797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halpin D.M.G., Singh D., Hadfield R.M. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur. Respir. J. 2020;55(5) doi: 10.1183/13993003.01009-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Beirne S.L., Salit J., Kaner R.J., Crystal R.G., Strulovici-Barel Y. Up-regulation of ACE2, the SARS-CoV-2 receptor, in asthmatics on maintenance inhaled corticosteroids. Respir. Res. 2021;22(1):200. doi: 10.1186/s12931-021-01782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloom C.I., Cullinan P., Wedzicha J.A. Asthma phenotypes and COVID-19 risk: a population-based observational study. Am. J. Respir. Crit. Care Med. 2022;205(1):36–45. doi: 10.1164/rccm.202107-1704OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mikhail I., Grayson M.H. Vol. 123. 2019. Asthma and viral infections: an intricate relationship; pp. 352–358. (Annals of Allergy, Asthma & Immunology). official publication of the American College of Allergy, Asthma, & Immunology. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dapat C., Oshitani H. Novel insights into human respiratory syncytial virus-host factor interactions through integrated proteomics and transcriptomics analysis. Expert Rev. Anti-infect. Ther. 2016;14(3):285–297. doi: 10.1586/14787210.2016.1141676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant R.A., Morales-Nebreda L., Markov N.S., Swaminathan S., Querrey M., Guzman E.R., et al. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature. 2021;590(7847):635–641. doi: 10.1038/s41586-020-03148-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microb. Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michalovich D., Rodriguez-Perez N., Smolinska S., Pirozynski M., Mayhew D., Uddin S., et al. Obesity and disease severity magnify disturbed microbiome-immune interactions in asthma patients. Nat. Commun. 2019;10(1):5711. doi: 10.1038/s41467-019-13751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y., Peng I., Webster J.D., Suto E., Lesch J., Wu X., et al. Inhibition of the kinase ITK in a mouse model of asthma reduces cell death and fails to inhibit the inflammatory response. Sci. Signal. 2015;8(405):ra122. doi: 10.1126/scisignal.aab0949. [DOI] [PubMed] [Google Scholar]
- 26.Li X., Hastie A.T., Hawkins G.A., Moore W.C., Ampleford E.J., Milosevic J., et al. eQTL of bronchial epithelial cells and bronchial alveolar lavage deciphers GWAS-identified asthma genes. Allergy. 2015;70(10):1309–1318. doi: 10.1111/all.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu G., Wang L.G., Han Y., He Q.Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao Y., Wang J., Jaehnig E.J., Shi Z., Zhang B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019;47(W1):W199–W205. doi: 10.1093/nar/gkz401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chin C.H., Chen S.H., Wu H.H., Ho C.W., Ko M.T., Lin C.Y. cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014;8(Suppl 4):S11. doi: 10.1186/1752-0509-8-S4-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rydenfelt M., Klinger B., Klunemann M., Bluthgen N. SPEED2: inferring upstream pathway activity from differential gene expression. Nucleic Acids Res. 2020;48(W1):W307–W312. doi: 10.1093/nar/gkaa236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhn M., von Mering C., Campillos M., Jensen L.J., Bork P. STITCH: interaction networks of chemicals and proteins. Nucleic Acids Res. 2008;36(Database issue):D684–D688. doi: 10.1093/nar/gkm795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sunjaya A.P., Allida S.M., Di Tanna G.L., Jenkins C.R. Asthma and coronavirus disease 2019 risk: a systematic review and meta-analysis. Eur. Respir. J. 2021;59(3):1–16. doi: 10.1183/13993003.01209-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hou H., Xu J., Li Y., Wang Y., Yang H. The association of asthma with COVID-19 mortality: an updated meta-analysis based on adjusted effect estimates. J. Allergy Clin. Immunol. Pract. 2021;9(11):3944–3968.e5. doi: 10.1016/j.jaip.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nair P., Surette M.G., Virchow J.C. Neutrophilic asthma: misconception or misnomer? Lancet Respir. Med. 2021;9(5):441–443. doi: 10.1016/S2213-2600(21)00023-0. [DOI] [PubMed] [Google Scholar]
- 36.Azim A., Green B., Lau L., Rupani H., Jayasekera N., Bruce K., et al. Peripheral airways type 2 inflammation, neutrophilia and microbial dysbiosis in severe asthma. Allergy. 2021;76(7):2070–2078. doi: 10.1111/all.14732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meizlish M.L., Pine A.B., Bishai J.D., Goshua G., Nadelmann E.R., Simonov M., et al. A neutrophil activation signature predicts critical illness and mortality in COVID-19. Blood Adv. 2021;5(5):1164–1177. doi: 10.1182/bloodadvances.2020003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelaia C., Vatrella A., Gallelli L., Lombardo N., Sciacqua A., Savino R., et al. Role of p38 mitogen-activated protein kinase in asthma and COPD: pathogenic aspects and potential targeted therapies. Drug Des. Dev. Ther. 2021;15:1275–1284. doi: 10.2147/DDDT.S300988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimes J.M., Grimes K.V. p38 MAPK inhibition: a promising therapeutic approach for COVID-19. J. Mol. Cell. Cardiol. 2020;144:63–65. doi: 10.1016/j.yjmcc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan J., Yang Q., Zhou Y., Deng H., Zhu Y., Zhao D., et al. MicroRNA-221 modulates airway remodeling via the PI3K/AKT pathway in OVA-induced chronic murine asthma. Front. Cell Dev. Biol. 2020;8:495. doi: 10.3389/fcell.2020.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medina-Tato D.A., Ward S.G., Watson M.L. Phosphoinositide 3-kinase signalling in lung disease: leucocytes and beyond. Immunology. 2007;121(4):448–461. doi: 10.1111/j.1365-2567.2007.02663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duan W., Aguinaldo Datiles A.M., Leung B.P., Vlahos C.J., Wong W.S. An anti-inflammatory role for a phosphoinositide 3-kinase inhibitor LY294002 in a mouse asthma model. Int. Immunopharm. 2005;5(3):495–502. doi: 10.1016/j.intimp.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Ma B., Athari S.S., Mehrabi Nasab E., Zhao L. PI3K/AKT/mTOR and TLR4/MyD88/NF-kappaB signaling inhibitors attenuate pathological mechanisms of allergic asthma. Inflammation. 2021;44(5):1895–1907. doi: 10.1007/s10753-021-01466-3. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Y., Sun D., Liu H., Sun L., Jie J., Luo J., et al. Bixin protects mice against bronchial asthma though modulating PI3K/Akt pathway. Int. Immunopharm. 2021;101(Pt B) doi: 10.1016/j.intimp.2021.108266. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo P., Qiu L., Liu Y., Liu X.L., Zheng J.L., Xue H.Y., et al. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am. J. Trop. Med. Hyg. 2020;103(1):69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li F., Li J., Wang P.H., Yang N., Huang J., Ou J., et al. SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2021;1867(12) doi: 10.1016/j.bbadis.2021.166260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Artham S., Verma A., Alwhaibi A., Adil M.S., Manicassamy S., Munn D.H., et al. Delayed Akt suppression in the lipopolysaccharide-induced acute lung injury promotes resolution that is associated with enhanced effector regulatory T cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2020;318(4):L750–L761. doi: 10.1152/ajplung.00251.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garibaldi B.T., D'Alessio F.R., Mock J.R., Files D.C., Chau E., Eto Y., et al. Regulatory T cells reduce acute lung injury fibroproliferation by decreasing fibrocyte recruitment. Am. J. Respir. Cell Mol. Biol. 2013;48(1):35–43. doi: 10.1165/rcmb.2012-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin S., Wu H., Wang C., Xiao Z., Xu F. Regulatory T cells and acute lung injury: cytokines, uncontrolled inflammation, and therapeutic implications. Front. Immunol. 2018;9:1545. doi: 10.3389/fimmu.2018.01545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Somanath P.R. Is targeting Akt a viable option to treat advanced-stage COVID-19 patients? Am. J. Physiol. Lung Cell Mol. Physiol. 2020;319(1):L45–L47. doi: 10.1152/ajplung.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemostasis. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su X.L., Su W., Wang Y., Wang Y.H., Ming X., Kong Y. The pyrrolidinoindoline alkaloid Psm2 inhibits platelet aggregation and thrombus formation by affecting PI3K/Akt signaling. Acta Pharmacol. Sin. 2016;37(9):1208–1217. doi: 10.1038/aps.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Z., Li T., Kareem K., Tran D., Griffith B.P., Wu Z.J. The role of PI3K/Akt signaling pathway in non-physiological shear stress-induced platelet activation. Artif. Organs. 2019;43(9):897–908. doi: 10.1111/aor.13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khezri M.R., Varzandeh R., Ghasemnejad-Berenji M. The probable role and therapeutic potential of the PI3K/AKT signaling pathway in SARS-CoV-2 induced coagulopathy. Cell. Mol. Biol. Lett. 2022;27(1):6. doi: 10.1186/s11658-022-00308-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saheb Sharif-Askari F., Goel S., Saheb Sharif-Askari N., Hafezi S., Al Heialy S., Hachim M.Y., et al. Asthma associated cytokines regulate the expression of SARS-CoV-2 receptor ACE2 in the lung tissue of asthmatic patients. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.796094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gessain A., Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 2012;3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barreto-Luis A., Corrales A., Acosta-Herrera M., Gonzalez-Colino C., Cumplido J., Martinez-Tadeo J., et al. A pathway-based association study reveals variants from Wnt signalling genes contributing to asthma susceptibility. Clin. Exp. Allergy. 2017;47(5):618–626. doi: 10.1111/cea.12883. [DOI] [PubMed] [Google Scholar]
- 59.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kobayashi E.H., Suzuki T., Funayama R., Nagashima T., Hayashi M., Sekine H., et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7 doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seelige R., Washington A., Jr., Bui J.D. The ancient cytokine IL-17D is regulated by Nrf2 and mediates tumor and virus surveillance. Cytokine. 2017;91:10–12. doi: 10.1016/j.cyto.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harvey C.J., Thimmulappa R.K., Sethi S., Kong X., Yarmus L., Brown R.H., et al. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci. Transl. Med. 2011;3(78) doi: 10.1126/scitranslmed.3002042. 78ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saddawi-Konefka R., Seelige R., Gross E.T., Levy E., Searles S.C., Washington A., Jr., et al. Nrf2 induces IL-17d to mediate tumor and virus surveillance. Cell Rep. 2016;16(9):2348–2358. doi: 10.1016/j.celrep.2016.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li N., Alam J., Venkatesan M.I., Eiguren-Fernandez A., Schmitz D., Di Stefano E., et al. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J. Immunol. 2004;173(5):3467–3481. doi: 10.4049/jimmunol.173.5.3467. [DOI] [PubMed] [Google Scholar]
- 65.Rangasamy T., Guo J., Mitzner W.A., Roman J., Singh A., Fryer A.D., et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 2005;202(1):47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munakata M. Airway remodeling and airway smooth muscle in asthma. Allergol. Int. 2006;55(3):235–243. doi: 10.2332/allergolint.55.235. [DOI] [PubMed] [Google Scholar]
- 67.Michaeloudes C., Chang P.J., Petrou M., Chung K.F. Transforming growth factor-β and nuclear factor E2–related factor 2 regulate antioxidant responses in airway smooth muscle cells: role in asthma. Am. J. Respir. Crit. Care Med. 2011;184(8):894–903. doi: 10.1164/rccm.201011-1780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shintani Y., Maruoka S., Gon Y., Koyama D., Yoshida A., Kozu Y., et al. Nuclear factor erythroid 2-related factor 2 (Nrf2) regulates airway epithelial barrier integrity. Allergol. Int. 2015;64(Suppl):S54–S63. doi: 10.1016/j.alit.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 69.Hellings P.W., Steelant B. Epithelial barriers in allergy and asthma. J. Allergy Clin. Immunol. 2020;145(6):1499–1509. doi: 10.1016/j.jaci.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olagnier D., Farahani E., Thyrsted J., Blay-Cadanet J., Herengt A., Idorn M., et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 2020;11(1):4938. doi: 10.1038/s41467-020-18764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bazan-Socha S., Kuczia P., Potaczek D.P., Mastalerz L., Cybulska A., Zareba L., et al. Increased blood levels of cellular fibronectin in asthma: relation to the asthma severity, inflammation, and prothrombotic blood alterations. Respir. Med. 2018;141:64–71. doi: 10.1016/j.rmed.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 72.Ge Q., Zeng Q., Tjin G., Lau E., Black J.L., Oliver B.G., et al. Differential deposition of fibronectin by asthmatic bronchial epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;309(10):L1093–L1102. doi: 10.1152/ajplung.00019.2015. [DOI] [PubMed] [Google Scholar]
- 73.Geyer P.E., Arend F.M., Doll S., Louiset M.L., Virreira Winter S., Müller-Reif J.B., et al. High-resolution serum proteome trajectories in COVID-19 reveal patient-specific seroconversion. EMBO Mol. Med. 2021;13(8) doi: 10.15252/emmm.202114167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walker E.J., Heydet D., Veldre T., Ghildyal R. Transcriptomic changes during TGF-β-mediated differentiation of airway fibroblasts to myofibroblasts. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-56955-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Havlik L.P., Das A., Mietzsch M., Oh D.K., Ark J., McKenna R., et al. Receptor switching in newly evolved adeno-associated viruses. J. Virol. 2021;95(19) doi: 10.1128/JVI.00587-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kashyap T., Murray J., Walker C.J., Chang H., Tamir S., Hou B., et al. Selinexor, a novel selective inhibitor of nuclear export, reduces SARS-CoV-2 infection and protects the respiratory system in vivo. Antivir. Res. 2021;192 doi: 10.1016/j.antiviral.2021.105115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seidel P., Sun Q., Costa L., Lardinois D., Tamm M., Roth M. The MNK-1/eIF4E pathway as a new therapeutic pathway to target inflammation and remodelling in asthma. Cell. Signal. 2016;28(10):1555–1562. doi: 10.1016/j.cellsig.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 78.Zhou L., Goldsmith A.M., Bentley J.K., Jia Y., Rodriguez M.L., Abe M.K., et al. 4E-binding protein phosphorylation and eukaryotic initiation factor-4E release are required for airway smooth muscle hypertrophy. Am. J. Respir. Cell Mol. Biol. 2005;33(2):195–202. doi: 10.1165/rcmb.2004-0411OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumar R., Afsar M., Khandelwal N., Chander Y., Riyesh T., Dedar R.K., et al. Emetine suppresses SARS-CoV-2 replication by inhibiting interaction of viral mRNA with eIF4E. Antivir. Res. 2021;189 doi: 10.1016/j.antiviral.2021.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020;296(3) doi: 10.1148/radiol.2020201544. E186-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li S., Jiang L., Li X., Lin F., Wang Y., Li B., et al. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight. 2020;5(12) doi: 10.1172/jci.insight.138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao Y., Qin L., Zhang P., Li K., Liang L., Sun J., et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight. 2020;5(13) doi: 10.1172/jci.insight.139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Machlus K.R., Johnson K.E., Kulenthirarajan R., Forward J.A., Tippy M.D., Soussou T.S., et al. CCL5 derived from platelets increases megakaryocyte proplatelet formation. Blood. 2016;127(7):921–926. doi: 10.1182/blood-2015-05-644583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Patterson B.K., Seethamraju H., Dhody K., Corley M.J., Kazempour K., Lalezari J., et al. CCR5 inhibition in critical COVID-19 patients decreases inflammatory cytokines, increases CD8 T-cells, and decreases SARS-CoV2 RNA in plasma by day 14. Int. J. Infect. Dis. 2021;103:25–32. doi: 10.1016/j.ijid.2020.10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abbas W., Kumar A., Herbein G. The eEF1A proteins: at the crossroads of oncogenesis, apoptosis, and viral infections. Front. Oncol. 2015;5:75. doi: 10.3389/fonc.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng W., Chen G., Jia H., He X., Jing Z. DDX5 RNA helicases: emerging roles in viral infection. Int. J. Mol. Sci. 2018;19(4) doi: 10.3390/ijms19041122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang H., Kong N., Jiao Y., Dong S., Sun D., Chen X., et al. EGR1 suppresses porcine epidemic diarrhea virus replication by regulating IRAV to degrade viral nucleocapsid protein. J. Virol. 2021;95(19) doi: 10.1128/JVI.00645-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi Y., Yuan B., Zhu W., Zhang R., Li L., Hao X., et al. Ube2D3 and Ube2N are essential for RIG-I-mediated MAVS aggregation in antiviral innate immunity. Nat. Commun. 2017;8 doi: 10.1038/ncomms15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Q., Li Q., Liu T., Chang G., Sun Z., Gao Z., et al. Host interaction analysis of PA-N155 and PA-N182 in chicken cells reveals an essential role of UBA52 for replication of H5N1 avian influenza virus. Front. Microbiol. 2018;9:936. doi: 10.3389/fmicb.2018.00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kobayashi M., Oshima S., Maeyashiki C., Nibe Y., Otsubo K., Matsuzawa Y., et al. The ubiquitin hybrid gene UBA52 regulates ubiquitination of ribosome and sustains embryonic development. Sci. Rep. 2016;6 doi: 10.1038/srep36780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang X., O'Reilly P.F., Aschard H., Hsu Y.H., Richards J.B., Dupuis J., et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat. Commun. 2018;9(1):260. doi: 10.1038/s41467-017-02662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Katz J. Increased risk for Covid-19 in patients with Vitamin D deficiency. Nutrition. 2021;90 doi: 10.1016/j.nut.2021.111361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Al-Thagfan S.S., Alolayan S.O., Ahmed S., Emara M.M., Awadallah M.F. Impacts of deficiency in vitamin D derivatives on disease severity in adult bronchial asthma patients. Pulm. Pharmacol. Therapeut. 2021;70 doi: 10.1016/j.pupt.2021.102073. [DOI] [PubMed] [Google Scholar]
- 94.Tomić S., Đokić J., Stevanović D., Ilić N., Gruden-Movsesijan A., Dinić M., et al. Reduced expression of autophagy markers and expansion of myeloid-derived suppressor cells correlate with poor T cell response in severe COVID-19 patients. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.614599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al-Harbi N.O., Nadeem A., Al-Harbi M.M., Imam F., Al-Shabanah O.A., Ahmad S.F., et al. Oxidative airway inflammation leads to systemic and vascular oxidative stress in a murine model of allergic asthma. Int. Immunopharm. 2015;26(1):237–245. doi: 10.1016/j.intimp.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 96.Badawy M.A., Yasseen B.A., El-Messiery R.M., Abdel-Rahman E.A., Elkhodiry A.A., Kamel A.G., et al. Neutrophil-mediated oxidative stress and albumin structural damage predict COVID-19-associated mortality. Elife. 2021:10. doi: 10.7554/eLife.69417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pei Q.M., Jiang P., Yang M., Qian X.J., Liu J.B., Zheng H., et al. Upregulation of a disintegrin and metalloproteinase-33 by VEGF in human airway smooth muscle cells: implications for asthma. Cell Cycle. 2016;15(20):2819–2826. doi: 10.1080/15384101.2016.1220462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Polidoro R.B., Hagan R.S., de Santis Santiago R., Schmidt N.W. Overview: systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in COVID-19. Front. Immunol. 2020;11:1626. doi: 10.3389/fimmu.2020.01626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smadja D.M., Mentzer S.J., Fontenay M., Laffan M.A., Ackermann M., Helms J., et al. COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects. Angiogenesis. 2021;24(4):755–788. doi: 10.1007/s10456-021-09805-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bonaventura A., Vecchié A., Dagna L., Martinod K., Dixon D.L., Van Tassell B.W., et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021;21(5):319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rajendran P., Rengarajan T., Thangavel J., Nishigaki Y., Sakthisekaran D., Sethi G., et al. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013;9(10):1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sahebnasagh A., Nabavi S.M., Kashani H.R.K., Abdollahian S., Habtemariam S., Rezabakhsh A. Anti-VEGF agents: as appealing targets in the setting of COVID-19 treatment in critically ill patients. Int. Immunopharm. 2021;101(Pt B) doi: 10.1016/j.intimp.2021.108257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Evans M.D., Esnault S., Denlinger L.C., Jarjour N.N. Sputum cell IL-1 receptor expression level is a marker of airway neutrophilia and airflow obstruction in asthmatic patients. J. Allergy Clin. Immunol. 2018;142(2):415–423. doi: 10.1016/j.jaci.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cauchois R., Koubi M., Delarbre D., Manet C., Carvelli J., Blasco V.B., et al. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc. Natl. Acad. Sci. U.S.A. 2020;117(32):18951–18953. doi: 10.1073/pnas.2009017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ma B., Athari S.S., Mehrabi Nasab E., Zhao L. PI3K/AKT/mTOR and TLR4/MyD88/NF-κB signaling inhibitors attenuate pathological mechanisms of allergic asthma. Inflammation. 2021;44(5):1895–1907. doi: 10.1007/s10753-021-01466-3. [DOI] [PubMed] [Google Scholar]
- 106.Bi J., Min Z., Yuan H., Jiang Z., Mao R., Zhu T., et al. PI3K inhibitor treatment ameliorates the glucocorticoid insensitivity of PBMCs in severe asthma. Clin. Transl. Med. 2020;9(1):22. doi: 10.1186/s40169-020-0262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim D.H., Gu A., Lee J.S., Yang E.J., Kashif A., Hong M.H., et al. Suppressive effects of S100A8 and S100A9 on neutrophil apoptosis by cytokine release of human bronchial epithelial cells in asthma. Int. J. Med. Sci. 2020;17(4):498–509. doi: 10.7150/ijms.37833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang H., Zhong B., Geng Y., Hao J., Jin Q., Zhang Y., et al. TIPE2 inhibits PDGF-BB-induced phenotype switching in airway smooth muscle cells through the PI3K/Akt signaling pathway. Respir. Res. 2021;22(1):238. doi: 10.1186/s12931-021-01826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xia X., Hu X., Xu H., Wu L., Dai Y., Yang L., et al. Phosphatidylinositol 3-kinase inhibitor suppresses inducible nitric oxide synthase expression in bronchiole epithelial cells in asthmatic rats. Mol. Cell. Biochem. 2012;359(1–2):293–299. doi: 10.1007/s11010-011-1023-y. [DOI] [PubMed] [Google Scholar]
- 110.Basile M.S., Cavalli E., McCubrey J., Hernández-Bello J., Muñoz-Valle J.F., Fagone P., et al. Drug discovery today; 2021. The PI3K/Akt/mTOR Pathway: A Potential Pharmacological Target in COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Truong V., Jain A., Anand-Srivastava M.B., Srivastava A.K. Angiotensin II-induced histone deacetylase 5 phosphorylation, nuclear export, and Egr-1 expression are mediated by Akt pathway in A10 vascular smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2021;320(4):H1543–H1554. doi: 10.1152/ajpheart.00683.2020. [DOI] [PubMed] [Google Scholar]
- 112.Zhou Q.L., Wang T.Y., Li M., Shang Y.X. Alleviating airway inflammation by inhibiting ERK-NF-κB signaling pathway by blocking Kv1.3 channels. Int. Immunopharm. 2018;63:110–118. doi: 10.1016/j.intimp.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 113.Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell. 2020;183(4):1043–1057. doi: 10.1016/j.cell.2020.09.033. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pala D., Rivara S., Mor M., Milazzo F.M., Roscilli G., Pavoni E., et al. Kinetic analysis and molecular modeling of the inhibition mechanism of roneparstat (SST0001) on human heparanase. Glycobiology. 2016;26(6):640–654. doi: 10.1093/glycob/cww003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bar-Ner M., Eldor A., Wasserman L., Matzner Y., Cohen I.R., Fuks Z., et al. Inhibition of heparanase-mediated degradation of extracellular matrix heparan sulfate by non-anticoagulant heparin species. Blood. 1987;70(2):551–557. [PubMed] [Google Scholar]
- 116.Haraldsson B., Nyström J., Deen W.M. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol. Rev. 2008;88(2):451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 117.Garsen M., Rops A.L., Rabelink T.J., Berden J.H., van der Vlag J. The role of heparanase and the endothelial glycocalyx in the development of proteinuria. Nephrol. Dial. Transplant. 2014;29(1):49–55. doi: 10.1093/ndt/gft410. [DOI] [PubMed] [Google Scholar]
- 118.LaRivière W.B., Schmidt E.P. The pulmonary endothelial glycocalyx in ARDS: a critical role for heparan sulfate. Curr. Top. Membr. 2018;82:33–52. doi: 10.1016/bs.ctm.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 119.Xiang J., Lu M., Shi M., Cheng X., Kwakwa K.A., Davis J.L., et al. Heparanase blockade as a novel dual-targeting therapy for COVID-19. J. Virol. 2022 doi: 10.1128/jvi.00057-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mousavi S., Moradi M., Khorshidahmad T., Motamedi M. Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci. 2015 doi: 10.1155/2015/507151. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Young E. The anti-inflammatory effects of heparin and related compounds. Thromb. Res. 2008;122(6):743–752. doi: 10.1016/j.thromres.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 122.Ludwig R.J. Therapeutic use of heparin beyond anticoagulation. Curr. Drug Discov. Technol. 2009;6(4):281–289. doi: 10.2174/157016309789869001. [DOI] [PubMed] [Google Scholar]
- 123.Shastri M.D., Stewart N., Horne J., Peterson G.M., Gueven N., Sohal S.S., et al. In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0126763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Motamed H., Verki M.M., Nematollahi A.V., Hesam S. Evaluation of efficacy of nebulized low molecular weight heparin as an adjunctive extra treatment for acute mild-moderate asthma attack; a randomized clinical trial study. Pulm. Pharmacol. Therapeut. 2021;68 doi: 10.1016/j.pupt.2021.102037. [DOI] [PubMed] [Google Scholar]
- 126.Erelel M., Kaskal M., Akbal-Dagistan O., Issever H., Dagistanli A.S., Balkanci H., et al. Early effects of low molecular weight heparin therapy with soft-mist inhaler for COVID-19-induced hypoxemia: a phase IIb trial. Pharmaceutics. 2021;13(11) doi: 10.3390/pharmaceutics13111768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Alexander M.R., Brice A.M., Jansen van Vuren P., Rootes C.L., Tribolet L., Cowled C., et al. Ribosome-profiling reveals restricted post transcriptional expression of antiviral cytokines and transcription factors during SARS-CoV-2 infection. Int. J. Mol. Sci. 2021;22(7) doi: 10.3390/ijms22073392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sinegubova M.V., Orlova N.A., Kovnir S.V., Dayanova L.K., Vorobiev High-level expression of the monomeric SARS-CoV-2 S protein RBD 320-537 in stably transfected CHO cells by the EEF1A1-based plasmid vector. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0242890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.White K.M., Rosales R., Yildiz S., Kehrer T., Miorin L., Moreno E., et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science. 2021;371(6532):926–931. doi: 10.1126/science.abf4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and R code of this article are available at Gene Expression Omnibus public repository and supplementary file.