Abstract
Objectives
We assessed humoral responses and reactogenicity following the heterologous vaccination compared to the homologous vaccination groups.
Methods
We enrolled healthcare workers (HCWs) who were either vaccinated with ChAdOx1 followed by BNT162b2 (heterologous group) or 2 doses of ChAdOx1 (ChAdOx1 group) or BNT162b2 (BNT162b2 group). Immunogenicity was assessed by measuring antibody titers against receptor-binding domain (RBD) of SARS-CoV-2 spike protein in all participants and neutralizing antibody titer in 100 participants per group. Reactogenicity was evaluated by a questionnaire-based survey.
Results
We enrolled 499 HCWs (ChAdOx1, n = 199; BNT162b2, n = 200; heterologous ChAdOx1/BNT162b2, n = 100). The geometric mean titer of anti–receptor-binding domain antibody at 14 days after the booster dose was significantly higher in the heterologous group (11 780.55 binding antibody unit (BAU)/mL [95% CI, 10 891.52–12 742.14]) than in the ChAdOx1 (1561.51 [95% CI, 1415.03–1723.15]) or BNT162b2 (2895.90 [95% CI, 2664.01–3147.98]) groups (both p < 0.001). The neutralizing antibody titer of the heterologous group (geometric mean ND50, 2367.74 [95% CI, 1970.03–2845.74]) was comparable to that of the BNT162b2 group (2118.63 [95% CI, 1755.88–2556.32]; p > 0.05) but higher than that of the ChAdOx1 group (391.77 [95% CI, 326.16–470.59]; p < 0.001). Compared with those against wild-type SARS-CoV-2, the geometric mean neutralizing antibody titers against the Delta variant at 14 days after the boosting were reduced by 3.0-fold in the heterologous group (geometric mean ND50, 872.01 [95% CI, 685.33–1109.54]), 4.0-fold in the BNT162b2 group (337.93 [95% CI, 262.78–434.57]), and 3.2-fold in the ChAdOx1 group (206.61 [95% CI, 144.05–296.34]). The local or systemic reactogenicity after the booster dose in the heterologous group was higher than that of the ChAdOx1 group but comparable to that of the BNT162b2 group.
Discussion
Heterologous ChAdOx1 followed by BNT162b2 vaccination with a 12-week interval induced a robust humoral immune response against SARS-CoV-2, including the Delta variant, that was comparable to the homologous BNT162b2 vaccination and stronger than the homologous ChAdOx1 vaccination, with a tolerable reactogenicity profile.
Keywords: BNT162b2, ChAdOx1, COVID-19, Heterologous vaccination, SARS-CoV-2
Introduction
Effective vaccination regimens against COVID-19 are crucial for controlling the ongoing pandemic. In response to the instability of vaccine supply and vaccine-related adverse events such as thrombosis with thrombocytopenia syndrome, new immunization programs that combine vaccines from different platforms have been proposed [1]. Boosting with vaccines from the different platforms may become more prevalent if standard homologous vaccination fails to achieve long-lasting immunity or revised vaccines against SARS-CoV-2 variants are required [2,3]. Particularly, repeated vaccination with the same viral vector vaccines may be less effective due to the pre-existing vector-specific immunity [4,5]. In this regard, it is important to accrue empirical data on the safety and immunogenicity of heterologous prime-boost vaccination regimens in various settings. In this study, we evaluated the reactogenicity and immunogenicity of the heterologous prime-boost vaccination (ChAdOx1 followed by BNT162b2) in comparison to homologous 2-dose vaccinations of ChAdOx1 or BNT162b2.
Methods
Study design and participants
For this prospective observational cohort study, we recruited healthcare workers (HCWs) from ten domestic hospitals in South Korea who received either homologous prime-boost vaccination with the BNT162b2 mRNA vaccine or the ChAdOx1 adenoviral vector vaccine, or heterologous vaccination with ChAdOx1 followed by BNT162b2 booster. We planned to enroll a total of 200, 200, and 100 participants in the ChAdOx1/ChAdOx1, BNT162b2/BNT162b2, and ChAdOx1/BNT162b2 groups, respectively. HCWs with a history of SARS-CoV-2 infection were excluded from this study. Participants were recruited by placing leaflets for the recruitment at the vaccination sites and posting notice on the bulletin boards for HCWs at each hospital. Detailed information on participating hospitals is summarized in the Supplementary material (Table S1). This study was initiated under the leadership of the Korea Disease Control and Prevention Agency, and the study protocol was approved by the institutional review committee of each participating institution. All participants provided written informed consent before enrollment. Baseline data on demographics were collected by questionnaire (electronic case report form) at enrollment. Detailed schedule for vaccinations and blood sampling is described in the Appendix of the Supplementary material.
Reactogenicity
Data on the baseline demographic information and the local and systemic reactions during the 7 days post vaccination were obtained through a questionnaire-based survey. We assessed local and systemic reactions using a modified version of the Food and Drug Administration toxicity scale [6]. The reactogenicity is presented as a total symptom score, which was calculated as the sum of each symptom during the 7 days after vaccination by assigning a score according to the severity as follows: mild as 1 point, moderate as 2 points, severe as 3 points, and life-threatening as 4 points. Prophylactic use of antipyretics was not recommended but allowed depending on the health conditions. The English version of the questionnaire is provided in the Appendix in the Supplementary material.
Antibody response
Antigen-specific humoral immune response was analysed using the Elecsys Anti–SARS-CoV-2 S assay (Roche Diagnostics, Mannheim, Germany), a commercial electrochemiluminescence immunoassay that detects antibodies (including IgG) to the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein on the Cobas e module (Roche Diagnostics), with a measuring range from 0.4 U/mL to 250 U/mL (up to 2500 U/mL with onboard 1:10 dilution and up to 12 500 U/mL with onboard 1:50 dilution). Values higher than 0.8 U/mL were considered positive. We calibrated the Elecsys antibody test with serially diluted WHO International Standard (NIBSC Code 20/136) sera for anti–SARS-CoV-2 immunoglobulin. Then the results of Elecsys antibody test (U/mL) were converted to the WHO international unit, defined as binding antibody units per milliliter (BAU/mL) according to the correlation curve (see Supplementary material, Fig. S1).
Plaque-reduction neutralization assay
To evaluate the functionality of vaccine-induced antibody response, we performed the plaque-reduction neutralization (PRNT) assay by using sera from all 100 participants in the heterologous group and 100 randomly selected participants from each of the homologous groups. In addition, plasmas from ten participants of each vaccination group were randomly selected and neutralizing antibody titers were determined by PRNT against four SARS-CoV-2 variants of concern. Heat inactivated (56°C for 30 min) individual sera were serially two-fold diluted in culture medium with a starting dilution of 1/20. A diluted sera were incubated with the 50 plaque forming units (PFU) of wild-type SARS-CoV-2 virus (BetaCoV/Korea/KCDC03/2020) or SARS-CoV-2 variants (Alpha; B.1.1.7, Beta; B.1.351, Gamma; P.1, and Delta; B.1.617.2) for 1 hour at 37°C. Detailed procedures are described in the Appendix in the Supplementary materials.
Statistical analysis
SPSS Statistics for Windows v24.0 (IBM Corp, Armonk, NY, USA) was used for statistical analysis and GraphPad Prism v8.0 (GraphPad Software, San Diego, CA, USA) was used for graph plotting of the results. Humoral responses are presented by vaccination groups in geometric means with 95% CI. For comparisons between groups, independent Student's t-test or one-way analysis of variance was applied to log-transformed antibody data. Pearson's correlation was used for the analysis of the correlation. All tests of significance were two-tailed, and p < 0.05 were considered statistically significant.
Results
Characteristics of the study population
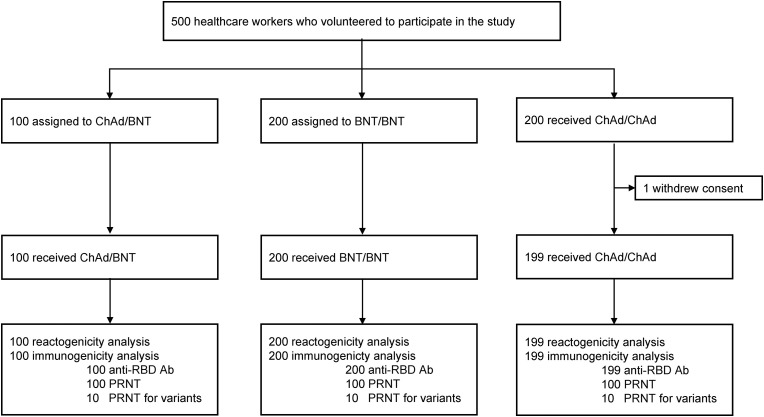
A total of 500 HCWs were enrolled in this study, including 200 in the ChAdOx1 homologous group, 200 in the BNT162b2 homologous group, and 100 in the ChAdOx1/BNT162b2 heterologous group (Fig. 1 ). After one participant in the ChAdOx1/ChAdOx1 group withdrew consent, a total of 499 people completed the prime-boost vaccination and blood collection schedule. The baseline characteristics of the study population are summarized in Table 1 . The mean age of the participants was 37.1 years, and there was no significant difference among the three groups (p > 0.99). The proportion of females was 80.4% in the total participants and did not show significant differences among the three groups (p = 0.90). The median interval between the prime and boost doses in the heterologous group was 12 weeks.
Fig. 1.
Study flowchart.
Table 1.
Baseline characteristics of the study population
| Total (n = 499) | Homologous ChAd/ChAd (n = 199) | Homologous BNT/BNT (n = 200) | Heterologous ChAd/BNT (n = 100) | p | |
|---|---|---|---|---|---|
| Sex, n (%) | 0.90 | ||||
| Female | 401 (80.4) | 159 (79.9) | 160 (80.0) | 82 (82.0) | |
| Male | 98 (19.6) | 40 (20.1) | 40 (20.0) | 18 (18.0) | |
| Age (y), mean ± SD | 37.1 ± 9.2 | 37.1 ± 9.0 | 37.0 ± 9.3 | 37.1 ± 9.1 | >0.99 |
| BMI (kg/m2), mean ± SD | 22.2 ± 2.9 | 22.2 ± 2.8 | 22.2 ± 2.9 | 22.0 ± 3.1 | 0.73 |
| Underlying disease, n (%) | |||||
| Hypertension | 12 (2.4) | 7 (3.5) | 3 (1.5) | 2 (2.0) | 0.40 |
| Diabetes | 6 (1.2) | 2 (1.0) | 1 (0.5) | 3 (3.0) | 0.16 |
| Solid tumor | 10 (2.0) | 4 (2.0) | 2 (1.0) | 4 (4.0) | 0.22 |
| Allergic diseasea | 9 (1.8) | 4 (2.0) | 1 (0.5) | 4 (4.0) | 0.10 |
| Asthma | 5 (1.0) | 3 (1.5) | 1 (0.5) | 1 (1.0) | 0.60 |
| Rheumatic disorder | 3 (0.6) | 2 (1.0) | 0 (0.0) | 1 (1.0) | 0.36 |
| Interval between the prime dose and the boost dose, days, median (IQR) | 77 (21–84) | 80 (78–84) | 21 (21–21) | 84 (79.5–87) | <.001 |
Abbreviations: BMI, body mass index; IQR, interquartile range; SD, standard deviation.
Atopic dermatitis and allergic rhinitis.
Reactogenicity
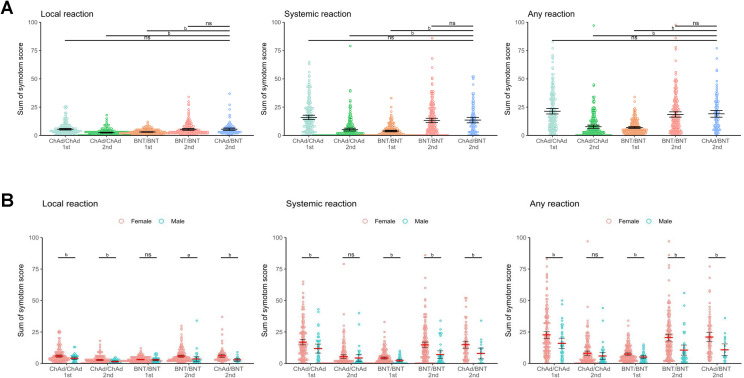
The data on reactogenicity were collected up to 7 days after the priming dose and the booster dose in each group, except for those following the priming dose in the heterologous group (see Supplementary material, Fig. S2). The most common reactions in the heterologous group were local pain (95%), fatigue (81%), headache (65%), and myalgia (64%). The frequencies and degrees of local and systemic reactions after the BNT162b2 booster dose in the heterologous group were largely comparable to those of the second dose in the BNT162b2 homologous group (see Supplementary material, Fig. S2). The reactivity of the BNT162b2 booster dose in the heterologous group was comparable to that of the first dose in the ChAdOx1 homologous group or the second dose in the BNT162b2 homologous group, and more frequent than that of the second dose in the ChAdOx1 homologous group or the first dose in BNT162b2 homologous group (Fig. 2 A). The reactivity was significantly higher in the female group after booster dose of heterologous vaccination in a stratified analysis according to sex group (Fig. 2B). Unsolicited adverse events after booster dose in each group are summarized in the Supplementary material (Table S2).
Fig. 2.
Local and systemic reactions. Reactogenicity of each vaccination regime was presented as the sum of symptom scores. (A) Reactogenicity of each vaccination group. (B) Reactogenicity according to sex group. Horizontal bars represent mean, and error bars represent 95% CI. ap <0.05, bp < 0.001. Abbreviations: ns, non-significant p value.
Immunogenicity
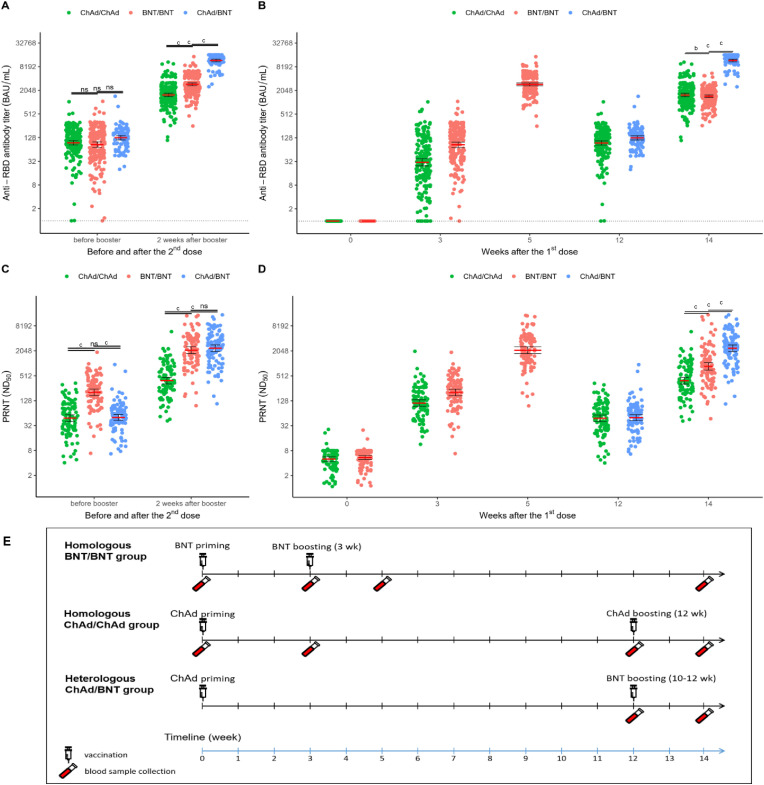
The humoral responses in the three groups were evaluated by measuring the RBD-binding antibody and 50% neutralizing dose by PRNT assay. The antibody titers for SARS-CoV-2 RBD were measured in the serial blood samples from all participants (n = 499). As shown in Fig. 3 A, the geometric mean titers of RBD antibodies at or within 1 week prior to the booster dose in the ChAdOx1 homologous group, BNT162b2 homologous group, and the heterologous group were 94.80 BAU/mL (95% CI, 83.61–107.48), 85.23 (95% CI, 72.70–99.92), and 127.27 (95% CI, 112.42–144.07), respectively (p = 0.14 by ANOVA). The geometric mean antibody titers at 14 days after the booster dose in the ChAdOx1 homologous group, BNT162b2 homologous group, and the heterologous group were 1561.51 BAU/mL (95% CI, 1415.03–1723.15), 2895.90 (95% CI, 2664.01–3147.98), and 11 780.55 (95% CI, 10 891.52–12 742.14), respectively, which were 16.5, 34.0, and 92.6-fold increases compared with those prior to boosting (all p < 0.001). The antibody titer at day 14 after the booster dose was significantly higher in the heterologous group than those in the ChAdOx1 homologous group and the BNT162b2 homologous group (both p < 0.001). The antibody titers by time point for each group are depicted in Fig. 3B.
Fig. 3.
Anti-RBD antibody titers and neutralizing antibody titers against wild-type SARS-CoV-2. (A) Anti-RBD antibody titers before and 2 weeks after booster dose. (B) Anti-RBD antibody titers by group related to time after first dose. (C) Neutralizing antibody titers before and 2 weeks after booster dose. (D) Neutralizing antibody titers by group related to time after first dose. (E) Schematic showing the dosing strategies and blood sampling time points. The red solid line indicates the mean titer, and the error bar depicts the 95% CI. The black dotted line in 3A and 3B indicates the limitation of detection (0.98 binding antibody unit /mL). ap < 0.05, bp < 0.001, cp < 0.0001. Abbreviations: ns, non-significant p value.
PRNT was performed on a total of 1000 blood samples obtained from 100 participants in each group. Baseline characteristics of participants by group in which PRNT was performed are summarized in the Supplementary material (Table S3). The titers of neutralizing antibodies against wild-type SARS-CoV-2 were determined from all sera obtained at 14 days after the booster dose. The geometric mean neutralizing antibody titer (ND50) of the heterologous group (2367.74 [95% CI, 1970.03–2845.74]) was comparable to that of the BNT162b2 group (2118.63 [95% CI, 1755.88–2556.32]; p > 0.05) but higher than that of the ChAdOx1 group (391.77 [95% CI, 326.16–470.59]; p < 0.001, Fig. 3C). The neutralizing antibody titers related to time after first dose are shown in Fig. 3D. Schematic showing the dosing strategies and blood sampling time points is shown in Fig. 3E. The significant correlation was observed between anti-RBD antibody titers and ND50; a strong positive correlation in all the tested samples (Pearson's r = 0.89; p < 0.001) and a fair positive correlation in post-boosting samples (Pearson's r = 0.61; p < 0.001; see Supplementary material, Fig. S3).
Neutralizing antibody titer against the four variants of concern
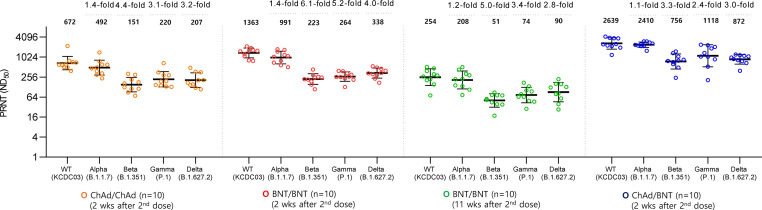
We randomly selected ten participants from each group to determine the serum level of neutralizing antibodies against the four SARS-CoV-2 variants of concern—B.1.1.7 (Alpha), B.1.351 (Beta), P.1 (Gamma), and B.1.617.2 (Delta). Baseline characteristics of selected participants are summarised in the Supplementary material (Table S4). All 2 doses of three vaccinated groups generated effectively neutralizing antibody activity against wild-type virus (KCDC03) (Fig. 4 ). At 2 weeks after 2 doses of each vaccination group, significantly lowered neutralizing antibody activities were shown against Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2) variants by comparing with that of wild-type virus. Of note, geometric mean ND50 against the Delta variant at 2 weeks after the booster dose was higher in the ChAdOx1/BNT162b2 group (872.01 [95% CI, 685.33–1109.54]) than the BNT162b2/BNT162b2 group (337.93 [95% CI, 262.78–434.57]; p < 0.001) and the ChAdOx1/ChAdOx1 group (206.61 [95% CI, 144.05–296.34]; p < 0.001).
Fig. 4.
Neutralizing antibody response against SARS-CoV-2 variants. Horizontal bars represent mean values. Error bars represent standard error of the mean. Neutralizing titers (ND50) and reduced folds compared to wild type are shown for each column.
Discussion
In this prospective observational study including 499 HCWs, we found that the heterologous vaccination with ChAdOx1 followed by BNT162b2 at 12-week intervals induced robust humoral responses against SARS-CoV-2. The humoral response at 2 weeks after booster dose in the heterologous vaccination was comparable to that of homologous BNT162b2 vaccination and more potent than homologous ChAdOx1 vaccination. Several studies have investigated the effectiveness of heterologous COVID-19 vaccination. The CombiVacS study showed that ChAdOx1 followed by BNT162b2 boosting elicited a strong humoral and cellular immune response compared with ChAdOx1 priming alone [7]. Likewise, three studies from Germany reported that the antibody responses after heterologous ChAdOx1/BNT162b2 vaccination were comparable to that after homologous BNT162b2 vaccination and superior to that after homologous ChAdOx1 vaccination [[8], [9], [10]]. Taken together, heterologous ChAdOx1/BNT162b2 vaccination elicited a stronger immune response against SARS-CoV-2 compared with the homologous challenge of ChAdOx1.
It should be noted that the robust humoral response induced by heterologous boosting in our study could be attributed to the extended interval between prime and booster dose. It has recently been reported that a stronger humoral response is induced at extended intervals compared to the standard 3–4 week interval in the homologous BNT162b2 vaccination [[11], [12], [13]]. Extended intervals of booster dose may result in higher neutralizing activity and wider breadth of humoral responses through germinal center responses, including somatic hypermutation and affinity maturation [14]. Comparison of immune responses in the heterologous vaccination to homologous BNT162b2 vaccination at similarly extended intervals may allow assessment of this issue.
We also found that the heterologous vaccination showed higher neutralizing activity than the homologous vaccination against various variants including delta. The neutralizing activity against the Delta variant decreased by 3- to 4-fold compared with those against the wild type in all vaccine regimen groups. However, compared with homologous vaccination of either ChAdOx1 or BNT162b2, heterologous vaccination resulted in a higher neutralizing activity against the Delta variant. The reason for the more favorable antibody response against various variants of concern after the heterologous challenge is not clear but may be due to the differences in the spike protein conformations between the priming (no proline mutation in ChAdOx1) and boosting (two proline mutation in BNT162b2) doses that elicit antibody responses against various portions of the spike protein, especially RBD. In addition, the prime-boosting strategy by different platforms might affect the magnitude and breadth of humoral immune responses [15,16].
We found that the degrees of solicited reactions were comparable among the first dose of ChAdOx1, second homologous challenge of BNT162b2, and heterologous challenge of BNT162b2 after ChAdOx1, while the second homologous challenge of ChAdOx1 and the first dose of BNT162b2 had relatively lower reactogenicity than their comparators. The Com-Cov study reported that both ChAdOx1/BNT126b2 and BNT126b2/ChAxOx1 heterologous vaccinations induced greater systemic reactogenicity than their homologous counterparts [17]; however, because the participants of the Com-Cov study had a median age of 57 years, their results could not be extrapolated to younger age groups. Considering that the reactogenicity is higher in the younger age groups after receiving the ChAdOx1 or BNT126b2 vaccines [18,19], there is still concern about whether the reactogenicity profile is tolerable in the heterologous regimen, especially in young individuals. Therefore, our data suggest that ChAdOx1 followed by BNT162b2 in young individuals may have some short-term disadvantage in terms of reactogenicity, but the reactogenicity would be largely tolerable without serious or life-threatening consequences.
This study has several limitations. First, this observational study may be subject to selection bias, particularly in that the heterologous vaccination group was recruited from those who experienced vaccine-related adverse reactions following the priming dose of ChAdOx1. However, this particular selection of participants may allow a different interpretation of our results in that heterologous vaccination can generate a robust antibody response without a significant safety concern in vaccine recipients who experience adverse events following the initial dose. Our cohort was younger and female predominant, which may affect vaccine-induced immune response [20]. However, given the similar age and sex distribution of the three vaccination groups, these factors are unlikely to introduce a significant bias to the results. Second, we did not assess the cellular immunity, which is as important as antibody response for virologic control [21]. Finally, at the time of conducting this study we were in the midst of the Delta variant era, so our data did not provide any direct information about the immunity against the Omicron variant. The recent studies consistently revealed that 2-dose COVID-19 mRNA vaccines elicited poor neutralization against Omicron, while 3-dose mRNA vaccines induced potent variant cross-neutralization, including Omicron [22,23]. However, there are limited data on whether 2-dose heterologous vaccination with ChAdOx1/BNT162b2 would induce poor neutralizing antibody against Omicron variant. One study reported that the 2-dose heterologous vaccination with ChAdOx1/BNT162b2 showed slightly higher neutralizing antibody titers than 2-dose homologous vaccination with BNT162b2, while these neutralizing titers against Omicron variant were substantially lower than those against other variants [24]. So, a booster shot should be recommended regardless of homologous or heterologous 2-shot series until further evidence on this issue is available.
In conclusion, we found that heterologous COVID-19 vaccination generated an antibody response to SARS-CoV-2 that was comparable to that of homologous BNT162b2 vaccination and more robust than homologous ChAdOx1 vaccination, while not resulting in serious adverse events. These findings provide evidence for the safety and immunogenicity of heterologous prime-boost COVID-19 vaccination using ChAdOx1 and BNT162b2.
Transparency declaration
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
This work was supported by Research Program funded by the Korea Disease Control and Prevention Agency (#2021-ER2601-00) and Samsung Medical Center Grant (#SMO1210321). Participant recruitment and sample collection were done by each institution. Immunologic assays and data analysis were performed in Samsung Medical Center and Korea Disease Control and Prevention Agency.
Author contributions
Writing—original draft: SB, JHK, SYL, SHK, and KRP; writing—review &editing: HCJ, JYC, SHK, and KRP; conceptualization: HCJ, SHK, and KRP; investigation: SB, JHK, JYA, JYC, WJP, SYL, JYA, KHS, KHL, YGS, YCK, YEO, WSC, HWJ, SWK, KTK, ARK, SJ, ESK, BK, and SSK; resources: SB, MHS and HJL; methodology: JHK, JYC, WJP, ESK, ARK, SJ, and SSK; data curation: SB, JHK, JYC, WJP, SYL, and SHK; formal analysis: SB, JHK, JYC, WJP, SYL, and SHK. All authors critically revised the manuscript for important intellectual content. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Acknowledgements
The authors thank all participants who volunteered for this study. We also thank Su-Hwan Kim, Hyeon nam Do, and June-Woo Lee for administrative support and experiment. We are grateful to Dr. Joon Seo Lim for his assistance in language editing. The results of this study were presented in a press release on July 26 by the Korea Centers for Disease Control and Prevention (KCDC).
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.04.019.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384:2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner J.S., O'Halloran J.A., Kalaidina E., Kim W., Schmitz A.J., Zhou J.Q., et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krause P.R., Fleming T.R., Longini I.M., Peto R., Briand S., Heymann D.L., et al. SARS-CoV-2 variants and vaccines. N Engl J Med. 2021;385:179–186. doi: 10.1056/NEJMsr2105280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20:615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ura T., Okuda K., Shimada M. Developments in viral vector-based vaccines. Vaccines (Basel) 2014;2:624–641. doi: 10.3390/vaccines2030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Center for Biologics Evaluation, Research Toxicity grading scale for volunteers in vaccine clinical trials; c2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical Available at:
- 7.Borobia A.M., Carcas A.J., Pérez-Olmeda M., Castano L., Bertran M.J., Garcia-Perez J., et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barros-Martins J., Hammerschmidt S.I., Cossmann A., Odak I., Stankov M.V., Ramos G.M., et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27:1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt T., Klemis V., Schub D., Mihm J., Hielscher F., Marx S., et al. Immunogenicity and reactogenicity of a heterologous COVID-19 prime-boost vaccination compared with homologous vaccine regimens. Nat Med. 2021;27:1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hillus D., Schwarz T., Tober-Lau P., Vanshylla K., Hastor H., Thibeault C., et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1-nCoV19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021;9:1255–1265. doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payne R.P., Longet S., Austin J.A., Skelly D.T., Dejnirattisai W., Adele S., et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell. 2021;184:5699–5714. doi: 10.1016/j.cell.2021.10.011. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tauzin A., Gong S.Y., Beaudoin-Bussières G., Marte-Laferriere V., Kaufmann D.E., Finzi A., et al. Strong humoral immune responses against SARS-CoV-2 Spike after BNT162b2 mRNA vaccination with a 16-week interval between doses. Cell Host Microbe. 2022;30:97–109. doi: 10.1016/j.chom.2021.12.004. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall V.G., Ferreira V.H., Wood H., Ierullo M., Majchrzak-Kita B., Manguiat K., et al. Delayed-interval BNT162b2 mRNA COVID-19 vaccination enhances humoral immunity and induces robust T cell responses. Nat Immunol. 2022;23:380–385. doi: 10.1038/s41590-021-01126-6. [DOI] [PubMed] [Google Scholar]
- 14.Laidlaw B.J., Ellebedy A.H. The germinal centre B cell response to SARS-CoV-2. Nat Rev Immunol. 2022;22:7–18. doi: 10.1038/s41577-021-00657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ledgerwood J.E., Wei C.J., Hu Z., Gordon I.J., Enama M.E., Hendel C.S., et al. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. Lancet Infect Dis. 2011;11:916–924. doi: 10.1016/S1473-3099(11)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein N., Bockstal V., Bart S., Luhn K., Robinson C., Gaddah A., et al. Safety and immunogenicity of heterologous and homologous two dose regimens of Ad26- and MVA-vectored ebola vaccines: a randomized, controlled phase 1 study. J Infect Dis. 2020:jiaa586. doi: 10.1093/infdis/jiaa586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw R.H., Stuart A., Greenland M., Liu X., Nguyen Van-Tam J.S., Snape M.D. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397:2043–2046. doi: 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bae S., Lee Y.W., Lim S.Y., Lee J.H., Lim J.S., Lee S., et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021;36:e115. doi: 10.3346/jkms.2021.36.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee Y.W., Lim S.Y., Lee J.H., Lim J.S., Kim M., Kwon S., et al. Adverse reactions of the second dose of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers in Korea. J Korean Med Sci. 2021;36:e153. doi: 10.3346/jkms.2021.36.e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demonbreun A.R., Sancilio A., Velez M.E., Ryan D.T., Pesce L., Saber R., et al. COVID-19 mRNA vaccination generates greater immunoglobulin g levels in women compared to men. J Infect Dis. 2021;224:793–797. doi: 10.1093/infdis/jiab314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457–466.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt F., Muecksch F., Weisblum Y., Da Silva J., Bednarski E., Cho A., et al. Plasma neutralization of the SARS-CoV-2 Omicron variant. N Engl J Med. 2022;386:599–601. doi: 10.1056/NEJMc2119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rössler A., Riepler L., Bante D., von Laer D., Kimpel J. SARS-CoV-2 Omicron variant neutralization in serum from vaccinated and convalescent persons. N Engl J Med. 2022;386:698–700. doi: 10.1056/NEJMc2119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.