Abstract
New insights into mechanisms linking obesity to poor health outcomes suggest a role for cellular aging pathways, casting obesity as a disease of accelerated biological aging. Although obesity has been linked to accelerated epigenetic aging in middle-aged adults, the impact during childhood remains unclear. We tested the association between body mass index (BMI) and accelerated epigenetic aging in a cohort of high-risk children. Participants were children (N = 273, aged 8 to 14 years, 82% investigated for maltreatment) recruited to the Child Health Study, an ongoing prospective study of youth investigated for maltreatment and a comparison youth. BMI was measured as a continuous variable. Accelerated epigenetic aging of blood leukocytes was defined as the age-adjusted residuals of several established epigenetic aging clocks (Horvath, Hannum, GrimAge, PhenoAge) along with a newer algorithm, the DunedinPoAm, developed to quantify the pace-of-aging. Hypotheses were tested with generalized linear models. Higher age-and sex- adjusted z-scored BMI was significantly correlated with household income, blood cell counts, and three of the accelerated epigenetic aging measures: GrimAge (r = 0.31, P < .0001), PhenoAge (r = 0.24, P < .0001), and DunedinPoAm (r = 0.38, P < .0001). In fully adjusted models, GrimAge (β = 0.07; P = .0009) and DunedinPoAm (β = 0.0017; P < .0001) remained significantly associated with higher age- and sex-adjusted z-scored BMI. Maltreatment-status was not associated with accelerated epigenetic aging. In a high-risk cohort of children, higher BMI predicted epigenetic aging as assessed by two epigenetic aging clocks. These results suggest the association between obesity and accelerated epigenetic aging begins in early life, with implications for future morbidity and mortality risk.
Subject terms: Paediatric research, Obesity
Introduction
Obesity remains a pressing global health issue, despite decades of attention and research. Comorbidities associated with obesity overlap with aging and age-related phenotypes, and it is hypothesized that obesity may accelerate aging across a range of cellular and bodily systems, leading to increased risk of age-related diseases1–3. The health impacts of obesity begin in childhood, as children with obesity face increased risk of childhood onset psychological and physical morbidities (e.g., depression, systemic inflammation, type II diabetes, cardiovascular abnormalities)4. Understanding the early-life etiology of the association between obesity and accelerated biological aging is thus critical for the mitigation of future disease risk.
Several approaches for quantifying biological aging have been used to assess links with obesity, including telomere length and epigenetic aging clocks5–8. Regulation of methylation at many cytosine-phosphate-guanine dinucleotides (CpGs) is positively correlated with chronological age, a finding that led to the creation of first-generation epigenetic algorithms (i.e., the Horvath and Hannum clocks), which use methylation levels to predict chronological age9,10. A second generation of epigenetic clocks, built on physiological measures of health and disease risk, aimed to predict lifespan and healthspan (i.e., GrimAge and PhenoAge clocks)11,12. Most recently, a new generation epigenetic clock predicting the rate of biological aging, the Dunedin Pace of Aging methylation clock (DunedinPoAm) was introduced using longitudinal data on organ-system integrity across 12 years13. The DunedinPoAm aims to quantify the pace of an individual’s biological aging through a single-time-point measure of DNA methylation.
While some evidence exists linking accelerated epigenetic aging and obesity in adults7,8, prior work in children is scarce. We are aware of only one recent study looking at socioeconomic disadvantage in children which reported a correlation between higher BMI and faster salivary DunedinPoAm-measured pace of biological aging14.
Early-life adversity is a potent risk factor for childhood obesity. Longitudinal and cross-sectional studies demonstrate exposure to childhood maltreatment (CM; e.g., physical/sexual abuse and neglect) is associated with accelerated increases in BMI through adolescence and early adulthood, thereby elevating risk of obesity in adulthood15–17. Differences in epigenetic programming offer an intriguing possibility to further account for this association. Developing an understanding of the association between obesity and accelerated aging in early life, both in the moderating context of CM and more broadly in all children, is critical to prevention efforts of many obesity-related negative health outcomes. Our study is the first to comprehensively examine the association between accelerated epigenetic aging and BMI in children using multiple epigenetic clocks. Using data from the Child Health Study (CHS), a cohort study of children with a high prevalence of Child Protective Services investigations due to suspected CM exposure, we tested the association between age- and sex-adjusted z-scored BMI and accelerated aging in childhood using a diverse panel of first-, second-, and new-generation epigenetic clocks. We hypothesized that BMI z-scores would be associated with accelerated epigenetic aging. Given the high prevalence of reported maltreatment in our cohort, we additionally explored the role of CM in this association.
Results
Sample descriptives
No significant differences were detected in mean age, distribution of biological sex, or ethnicity between the maltreatment and comparison groups. For both the full cohort (N = 439) and our analytic sample (N = 273), the maltreatment group was lower income (maltreatment mean = $33,000 [SD $32,000], comparison mean = $54,000 [SD $39,000]; P = 0.009) and had a higher raw BMI (maltreatment mean = 22.1 [SD 6.1], comparison mean = 20.3 [SD 5.3]; P = 0.04) (see Additional File Table S1 for full cohort sample demographics). There were additional group differences in ‘Other’ racial identification (Table 1). Family income was the only demographic factor to remain significant after correction for multiple comparisons at . Correlations among study outcomes and covariates are depicted in Table 2.
Table 1.
Summary demographics for analytic sample.
| Variable, mean (SD) | Total (N = 273) | Maltreatment (N = 225) | Comparison (N = 48) | P-value |
|---|---|---|---|---|
| Body mass index (BMI) | 21.8 (6.0) | 22.1 (6.1) | 20.3 (5.3) | .04* |
| Age (at collection) | 11.4 (1.5) | 11.4 (1.5) | 11.1 (1.5) | .15 |
| Biological sex M(F)% | 49.5% (50.5) | 49.3% (50.7) | 50.0% (50.0) | .93 |
| Income $10,000/year | 3.7 (3.5) | 3.3 (3.2) | 5.7 (4.0) | .0003** |
| Race | ||||
| Black | 16.5% | 16.9% | 14.6% | .70 |
| White | 67.8% | 65.3% | 79.2% | .06 |
| Other | 15.8% | 17.8% | 6.3% | .04* |
| Ethnicity | ||||
| Hispanic | 11.4% | 12.9% | 4.2% | .08 |
†P < .10, *P < .05, **P < .01, ***P < .0001.
Table 2.
Correlations among outcomes and covariates.
| BMI z-score | Age | Sex (Male) | Maltreatment | Household income | Proportion lymphocytes | Proportion monocytes | Proportion granulocytes | HorvathAA | HannumAA | GrimAgeAA | PhenoAgeAA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.00 | – | – | – | – | – | – | – | – | – | – | |
| Sex (Male) | 0.00 | −0.07 | – | – | – | – | – | – | – | – | – | – |
| Maltreatment | 0.09 | 0.09 | −0.010 | – | – | – | – | – | – | – | – | – |
| Household income | −0.19** | −0.005 | 0.04 | −0.26*** | – | – | – | – | – | – | – | – |
| Proportion lymphocytes | −0.26*** | −0.06 | 0.14* | −0.01 | 0.10 | – | – | – | – | – | – | – |
| Proportion monocytes | 0.12* | 0.07 | 0.04 | −0.06 | −0.03 | −0.48*** | – | – | – | – | – | – |
| Proportion granulocytes | 0.26*** | 0.05 | −0.17** | −0.03 | −0.11† | −0.97*** | 0.27*** | – | – | – | – | – |
| HorvathAA | 0.05 | 0.01 | 0.06 | 0.02 | −0.04 | −0.01 | −0.07 | 0.02 | – | – | – | – |
| HannumAA | 0.11† | 0.01 | −0.08 | −0.03 | −0.05 | −0.58*** | 0.35*** | 0.53*** | 0.30*** | – | – | – |
| GrimAgeAA | 0.31*** | 0.002 | 0.12† | 0.14* | −0.32*** | −0.48*** | 0.28*** | 0.45*** | 0.08 | 0.40*** | – | – |
| PhenoAgeAA | 0.24*** | 0.01 | −0.22** | 0.03 | −0.09 | −0.71*** | 0.33*** | 0.69*** | 0.27*** | 0.71*** | 0.48*** | – |
| Dunedin PoAm | 0.38*** | −0.02 | −0.15* | 0.08 | −0.13* | −0.68*** | 0.31*** | 0.67*** | 0.09 | 0.54*** | 0.54*** | 0.67*** |
†P < .10, *P < .05, **P < .01, ***P < .0001.
BMI and epigenetic aging clocks
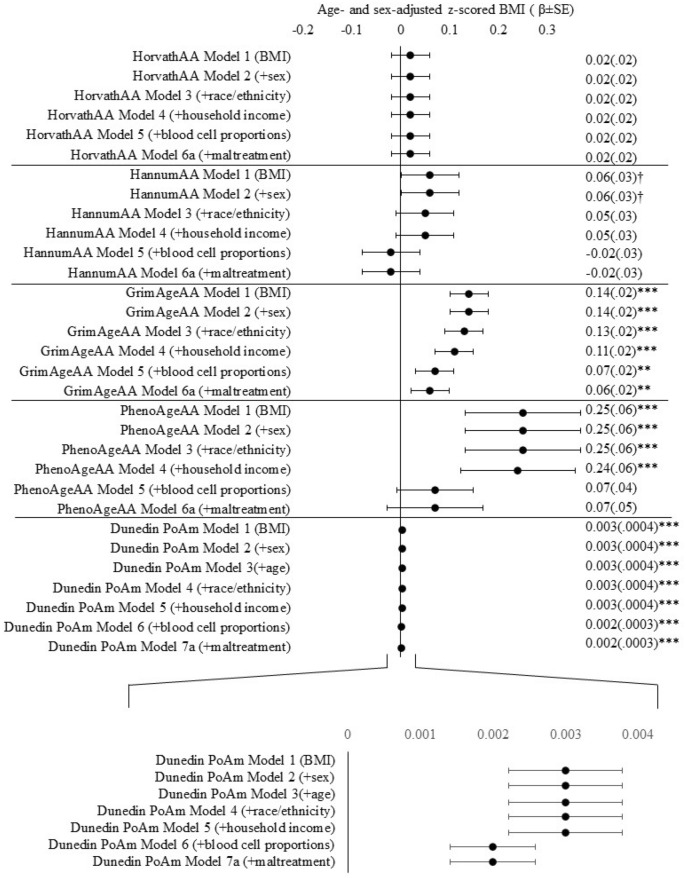
BMI z-score was moderately correlated with household income (r = −0.19, P = 0.004), proportion lymphocytes (r = −0.26, P < 0.0001), proportion granulocytes (r = 0.26, P < 0.0001), and three epigenetic age acceleration clocks: GrimAge (r = 0.31, P < 0.0001), PhenoAge (r = 0.24, P < 0.0001), and DunedinPoAm (r = 0.38, P < 0.0001). Excepting the correlation between BMI and income, these remained significant after correction for multiple comparisons at . The correlation between BMI z-scores and HannumAA did not reach statistical significance (r = 0.11, P = 0.051) and BMI was not correlated with HorvathAA (r = 0.05, P = 0.46).
In models adjusting for sex, race, ethnicity, and family income, higher BMI z-score was consistently associated with GrimAgeAA and PhenoAgeAA (see Additional File Tables S2-S6 for full model specifications). After accounting for blood cell proportions, BMI z-score remained significantly associated with accelerated aging measured by GrimAgeAA (β = 0.07; CI 0.02, 0.10; P = 0.0009) but was attenuated below α = 0.05 for PhenoAgeAA (β = 0.06; CI −0.02, 0.15; P = 0.13). An age- and sex-adjusted BMI increase of one standard deviation above the cohort mean was associated with an additional 0.07 years of accelerated aging as measured by GrimAge (Fig. 1). BMI z-score was not associated with epigenetic age acceleration derived from HorvathAA or HannumAA in any model tested (all P > 0.05).
Figure 1.
Estimates for BMI z-scores across all accelerated epigenetic aging and DunedinPoAm outcomes. Each row specifies the stepwise addition of a covariate to the base model of accelerated epigenetic aging outcome regressed onto age- and sex-adjusted BMI z-scores. Zoomed-in view of estimates for models for DunedinPoAm. †P < .10, *P < .05, **P < .01, ***P < .0001.
Higher BMI z-score was significantly associated with a faster pace-of-aging as measured by DunedinPoAm (b = 0.0017; CI 0.001, 0.002; P < 0.0001) adjusting for all covariates. A BMI increase of one standard deviation above the cohort mean was associated with an increase of 0.002 years in the pace of epigenetic aging. Associations between BMI and GrimAgeAA and DunedinPoAm remained significant after correcting for multiple comparisons at .
Maltreatment exposure, BMI, and epigenetic aging clocks
Given the opportunity to explore accelerated epigenetic aging in this cohort of children with a high prevalence of maltreatment exposure, we tested whether maltreatment was associated with accelerated epigenetic aging or moderated the association between BMI z-scores and accelerated epigenetic aging. Though raw BMI was significantly different between the maltreatment and comparison groups in initial demographic comparisons (maltreatment BMI mean = 22.1 (SD 6.1), comparison BMI mean = 20.3 (SD 5.3); P = 0.04), this difference was attenuated to non-significance with the inclusion of covariates (see Additional File Table S7 for full model specifications). Maltreatment was not independently associated with epigenetic age acceleration in our analytic sample and did not moderate the association between BMI z-scores and accelerated epigenetic aging measures after Bonferroni correction for multiple testing.
Discussion
In a cohort of high-risk children, we tested the association between age- and sex-adjusted z-scored BMI and accelerated aging using three generations of epigenetic aging measures. We further explored whether maltreatment status moderated this association. Higher BMI in childhood was associated with accelerated epigenetic aging and accelerated pace of epigenetic aging after accounting for covariates. Specifically, children with one standard deviation higher age- and sex-adjusted BMI had 0.07 years (approximately 25 days) age acceleration measured by GrimAge, an epigenetic aging measure designed to predict time to death that also associates with cardiovascular disease risk in adults12. Findings of accelerated GrimAge in children may or may not be clinically meaningful as childhood is a period of marked biological growth and development. Children with higher BMI also demonstrated an accelerated DunedinPoAm-measured pace of epigenetic aging by approximately 0.002 years per one standard deviation increase in age- and sex-adjusted BMI. DunedinPoAm is a DNA methylation clock built by measuring organ-system decline from young adulthood to midlife13. Recent work in a cohort of socioeconomically disadvantaged children observed a correlation between BMI and DunedinPoAm-measured pace of aging in saliva DNA14, a finding that is supported and extended here in leukocyte DNA though these findings are not surprising given the use of anthropometric traits in the construction of the DunedinPoAm13. Previous work has found an association between obesity and accelerated epigenetic aging using the Horvath clock in adult liver tissue7 and in whole blood of middle-aged adults8. Our results lend support for the development of this association in childhood, though we are unable to determine to what extent these relationships reflect the construct of “biological aging” typically associated with adverse outcomes in adulthood rather than elements of typical growth and development associated with childhood.
Exploration of the relation between maltreatment and accelerated epigenetic aging failed to replicate previous work in this domain, though important differences exist between study designs. For example, recent studies in children using salivary DNA to calculate HorvathAA found that threat-based, but not deprivation-based, maltreatment was related to accelerated epigenetic aging18, and that direct exposure to violence accelerated epigenetic aging19. Data collection for the CHS is ongoing, thus we are exploring maltreatment association in a subset of the final cohort using a dichotomous maltreatment-status variable (‘yes’, ‘no’). The currently restricted sample limits our power to detect differences between maltreatment and control groups, and the binary categorization of maltreatment limits our ability to dissect associations between specific types of maltreatment and accelerated epigenetic aging that may exist in this high-risk sample. It is plausible that experiences of violence and threat within the CHS cohort may have unique effects on epigenetic aging that may be revealed with future collection of detailed knowledge around the types of maltreatment exposure for participants.
Higher BMI across the lifespan has been associated with early onset of age-related diseases and mortality, generating interest in the link between obesity and aging20. Although we observed an association between obesity and accelerated epigenetic aging that is already present in childhood, the cross-sectional nature of our data prevents us from addressing mechanism and causality. Longitudinal work in the Avon Longitudinal Study of Parents and Children provides early support for obesity as a driver of variation in DNA methylation21. Mechanistic links connecting obesity and accelerated aging have yet to be fully elucidated. Research suggests, however, that cellular processes of fat storage, inflammation, oxidative stress, and energy homeostasis may be involved2,22,23. Dysregulation in these processes may drive epigenetic alterations resulting in an accelerated epigenetic aging profile. Methylation of CpG sites is a plastic process moderated by multifactorial intrinsic and extrinsic processes. Obesity has been linked to both hyper- and hypo-methylation of CpG sites across the genome, which in turn associate with the regulation of leptin, adiponectin, and other features of cellular energy balance21,24,25. Direct evidence of obesogenic factors altering methylation patterns related to aging is lacking and support for this framework derives mainly from evidence of the impact nutrition and caloric restriction may have in altering epigenetic aging26–28.
The prevalence of childhood obesity has increased dramatically over the past four decades29. Our findings of accelerated epigenetic aging and an accelerated pace of epigenetic aging in children with higher BMI have important implications for health and the mitigation of future disease risk. Children with obesity are at increased risk for childhood and early-onset adult diseases. Chronic conditions, such as type 2 diabetes, hypertension, atherosclerosis, and a range of other negative mental and physical health outcomes are more prevalent in individuals with childhood obesity30–33. Further, these diseases often co-occur, raising the cost of medical care and increasing the likelihood of early-onset disability and a reduced lifespan. Many of the diseases associated with childhood obesity are also diseases of aging. Our findings point to the possible role of accelerated aging at the cellular level in driving the early-onset disease phenotypes seen in children with obesity as they become adolescents and adults.
We acknowledge several limitations. Recruitment of participants, in particular demographically matched comparison children, is ongoing. Although the findings of accelerated epigenetic aging and accelerated pace of epigenetic aging do not hinge on additional recruitment within the CHS cohort, the exploratory analysis of maltreatment as a modifier of the obesity-epigenetic aging association should be understood as preliminary and used as a direction for future cross-sectional and longitudinal research with the final assembled cohort. Along these lines, future analyses of data from this cohort will be able to include more nuanced maltreatment variables including duration of abuse, age of abuse onset, type of abuse, and polyvictimization status. The addition of these variables will be critical to understanding the associations between both maltreatment and obesity, as well as maltreatment and accelerated aging within this cohort. For example, timing of abuse has been shown to be a critical factor in the association between CM and epigenetic aging34. Further, additional consideration should be given to the generalizability of these findings within low-risk cohorts of children.
The use of BMI as a variable to conceptualize body fat mass, although widely used, is also contended. Evidence exists that in both childhood and adulthood, the specific areas of fat mass accumulation (e.g., upper body, lower body, subcutaneous, visceral) may be critical to determining future disease risk35,36, an issue that may be relevant for the deposition of fat following maltreatment37. Using BMI instead of more nuanced measures of adiposity distribution may obscure associations among accelerated epigenetic aging, obesity, and maltreatment within our current sample of children. Finally, mechanistic understanding of the association between biological processes of aging and the three generations of epigenetic clocks examined here is lacking. These clocks are derived from regressing observed features of aging, morbidity, and mortality onto measured epigenetic changes in adulthood. These correlations do not necessarily yield direct insights into the processes of biological aging. Investigations into CpGs of some epigenetic clocks demonstrate enrichment for genes involved in organismal development and cell survival10, and a tendency to colocalize within glucocorticoid response elements38. However, it remains uncertain whether this is the case for all epigenetic clocks, which are constructed using a variety of CpGs. The relative contribution of individual CpGs is also obscured when tens or hundreds are collapsed into a single composite measure. Despite this limitation, these epigenetic aging measures may have important clinical and research utility in pediatric populations with obesity.
Conclusions
Our findings point to the possible utility of epigenetic aging measures for future clinical and research work in children with obesity. Though we found no evidence for moderation of the relationship between epigenetic aging and obesity by maltreatment, this relationship should be explored in more detail with larger, more balanced cohorts. Epigenetic aging and pace of epigenetic aging measures may serve as useful proxies when assessing the efficacy of obesity interventions for children39,40. The true endpoints for obesity interventions, risk of obesity-related diseases and reduced lifespan, can take years to decades to manifest. These timescales often preclude longitudinal intervention studies from using disease risk and lifespan as outcome measures due to cost and complexity of study design. Epigenetic aging and pace of epigenetic aging measures may be appropriate outcomes by which interventions can be judged as efficacious in ameliorating childhood obesity-related disease risks.
Materials and methods
Study design and sample recruitment
Participants for this study were drawn from the ongoing CHS, a large multidisciplinary study designed to provide prospective, longitudinal data on the health and development of children with and without a history of maltreatment. The CHS is recruiting a large state-wide cohort of children recently investigated for CM and non-maltreated comparison children. The goals of the CHS are to elucidate the multiple etiological processes, as well as mediators and moderators, believed to play a role in the onset and maintenance of adverse health outcomes among victims, and to better inform intervention opportunities to reverse the negative consequences of maltreatment.
Children with a recent (< 12 months) report of maltreatment exposure are identified in collaboration with Pennsylvania’s Statewide Child Welfare Information System (CWIS). Subjects with recent involvement in the CWIS are invited to participate in the study through home mailings and phone contact by study coordinators. Eligibility criteria includes: (1) aged 8 to 13 years, (2) subject of a CWIS maltreatment report (i.e., an allegation is made and investigated) and agreement for participation within 12 months of CWIS involvement, and (3) agreement of participation by a non-abusing caregiver. Non-maltreated comparison children are recruited via targeted advertisements from the same Pennsylvania counties as maltreated children with the goal of demographically matching at least one maltreated child based on age, race, ethnicity, sex, income level, and region within the State. Eligibility for participation includes: (1) no previous CWIS reports or contact, and (2) demographic match to a maltreatment participant. After recruitment, participating families are invited to visit the Center for Healthy Children at The Pennsylvania State University. The Center was established to serve as a dedicated on-campus facility for the CHS. The physical space houses a research lab with areas dedicated to biospecimen sample collection, measurement of physical health by trained nursing staff, participant and family interview rooms, and physical/hard-copy data storage41. The Pennsylvania State University Institutional Review Board approved the study, and informed assent (child) and consent (caregiver) was obtained from all participants.
Cross-sectional data reported here were drawn from the baseline (i.e., Time 1) assessment of currently enrolled CHS participants. Although recruitment for this cohort study is ongoing with a target enrollment of 900 children, an initial subset of 439 was available for the purposes of these analyses. Of the 439 participants who have completed Time 1, 435 consented to anthropometric measurements and 401 consented to and successfully completed blood draws (1 caregiver refusal, 33 participant refusals, 4 attempted but incomplete blood draws). The first 300 participant identification numbers were sent as the first batch for methylation analysis (constituting 286 total samples sent) and 273 samples survived methylation QC measures. These 273 participants are our final analytic sample. Summary statistics for participants included in our analyses are provided in Table 1 (see Additional File for comparative summary statistics of the 439 participants available at Time 1).
Body mass index
Anthropometric surveys, including height and weight, were conducted by trained CHS staff during participants’ visit to the research lab. BMI, defined as total body mass in kilograms divided by the squared body height, was age- and sex-adjusted to account for confounding effects of age and sex and z-scored to aid interpretation of differences in childhood BMI. As a sensitivity analysis, we tested a dichotomous obesity variable (children with obesity vs. children without obesity) as determined by age- and sex-specific BMI > 95th percentile cutoffs using the Center for Disease Control’s Childhood BMI Calculator42. Results were unchanged using a dichotomous obesity variable and we report results below using only the continuous BMI z-score variable.
Assessment of DNA methylation and calculation of accelerated aging variables
Genomic DNA was extracted from whole blood using a semi-automated approach (Qiasymphony, Qiagen). Genomic DNA purity and concentration was assessed using a nanophotometer (ImplenP300, Implen). Infinium methylation EPIC Beadchip (EPIC array, Illumina, San Diego CA, USA) was used to describe variation in DNA methylation across the genome. Genomic DNA (1ug) from whole blood was treated with sodium bisulfite using the Zymo EZ-96 DNA Methylation Kit™ (Zymo Research, Orange, CA, USA) with 200 ng of bisulfite-treated DNA amplified, fragmented, and hybridized on the EPIC array. Samples were randomized across plates to avoid potential confounding between sources of technical variation and phenotypes of interest (e.g., maltreatment status). The resulting raw intensity values (idat files) are directly loaded into R for quality control and normalization using the Meffil package43. We used normal-exponential out-of-band (noob) for background correction and dye-bias adjustment. Samples and probes with low signal intensity were removed. Concordance between predicted biological sex based on DNA methylation data and self-reported gender were verified for each sample with discordant samples removed. Finally, we used a Bayes method (ComBat) to correct for sources of technical variation (i.e., slide)44. Resulting methylation levels were used to calculate five separate measures of epigenetic age: the Horvath clock, Hannum clock, GrimAge, PhenoAge, and the DunedinPoAm. Measures of epigenetic age acceleration (HorvathAA, HannumAA, GrimAgeAA and PhenogeAA) were derived from beta values using a publicly available tool45. Likewise, the Dunedin methylation pace of aging (DunedinPoA) score was derived from the DNA methylation of 46 CpGs as described previously13.
Other measures
Income, biological sex, race, ethnicity, and predicted blood cell proportions (described using DNA methylation data and a reference-based approach)46 were included as covariates due to known associations with BMI and epigenetic aging measures. Income was assessed via caregiver self-report as current total household family income before taxes in increments of $10,000 (e.g., under $10,000 coded as ‘0’, $10,000-$19,999 coded as ‘1’, $20,000–$29,999 coded as ‘2’ and an income over $120,000 coded as ‘11’). Biological sex was determined via self-report as either ‘male’ or ‘female’ and cross-validated using the DNA methylation predicted sex. Two participants self-identified as ‘other/transgender’ but had not undergone any gender-reassignment treatments and were therefore coded as their cross-validated biological sex. Race and ethnicity were reported by caregivers for participating youth. Race was coded as ‘White/Caucasian’, ‘Black/African American’, or ‘Other’ (American Indian, Alaskan Native, Asian, Pacific Islander, Multiracial, or Other). Ethnicity was reported as either ‘Hispanic’ or ‘Non-Hispanic’. Proportions of lymphocytes (summed estimates for CD8, CD4, natural killer, and B cells), monocytes, and granulocytes were extracted from epigenetic estimates of blood cell counts on the same blood used for methylation measures using an established reference-based approach, and included as covariates as needed for certain robustness checks46.
Given the association between BMI and pubertal stage47, we additionally investigated pubertal stage as a covariate. Pubertal stage was assessed using Tanner staging, an index of physical ratings from 1 (prepubertal), 2 (pubertal onset, presence of breast buds and pubic hair) through 5 (fully mature) with each participant giving two separate ratings (breast/testis development and pubic hair development)48,49. A final pubertal stage was conceptualized as the average of these two measures of pubertal development. The correlation between BMI and pubertal stage (r = 0.34, P < 0.0001) was similar in size, direction, and strength to that of BMI and age, and the correlation between pubertal stage and age was moderate and positive (r = 0.65, P < 0.0001). Correlations between pubertal stage and accelerated epigenetic aging variables were non-significant with the exception of GrimAgeAA (r = 0.18, P = 0.002). Inclusion of pubertal stage did not modify findings and, given its correlation with age, it was not included in our final models.
Statistical analysis
Statistical analyses were performed with SAS V.9.4. Mean differences in demographic variables between maltreatment and comparison groups were assessed via two-tailed t-tests for continuous variables and two-way Chi-Square tests for dichotomous variables. There were no statistically significant differences in demographic measures between the current total Time 1 cohort and the sub-sample included in the current analyses. Three individuals were missing covariate data on family income. No significant differences in demographic characteristics were found for these individuals, thus missing data were addressed simultaneously using multiple imputation and complete case analysis. We created 20 imputed datasets using PROC MI and combined imputed results with PROC MIANALYZE. Data were analyzed both with and without imputation. Imputed and complete-case datasets produced similar results with no changes in direction or size of effects, and we report results with the imputed data.
We assessed outcome variables for skewness via Box Cox analyses that indicated the marginal utility of a Y-1 transformation to the BMI and accelerated epigenetic aging variables. After running all models using both transformed and non-transformed outcome variables, we found no differences in the direction or significance of any findings and thus retained the non-transformed outcome models for ease of interpretability. All covariates survived assessment for multicollinearity via variance inflation factor analysis in PROC REG (OPTIONS = VIF and COLLINOINT) and were thus retained.
Though the majority of children included in our models were from unique families, siblings were included in the study and for our analytic sample we included four families with three siblings, 38 families with two siblings, and 185 families with individual children. To account for the partial-nesting of children within families (e.g., the violation of independence of child-level observations), all models were estimated with family-level cluster-robust standard errors in PROC GENMOD, with family ID as the repeated subject. Statistical significance was set at two-tailed P < 0.05. Unless specified, all data are presented as estimate (SE) with 95% confidence intervals. Where appropriate, models were corrected for multiple comparisons using Bonferroni adjusted p-values.
Ethics approval and consent to participate
Approval for this study was provided by the institutional review board of The Pennsylvania State University (protocol STUDY00006550). Informed assent (child) and consent (caregiver) was obtained from all participants in the study. All methods were carried out in accordance with relevant guidelines and regulations (Declaration of Helsinki).
Supplementary information
Acknowledgements
We thank the children and caregivers for their participation in the study, and Child Health Study staff for their dedication, hard work and insights. Research reported in this manuscript was supported by grants from the National Institutes of Health, National Institute of Child Health and Human Development P50HD089922 (J.G.N), R01HD072468 (J.G.N), the National Institutes of Aging through R01AG04879 (J.G.N). L.E. was supported by National Institute on Aging T32 AG049676 to The Pennsylvania State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CM
Child maltreatment
- CWIS
Child welfare information system
- CHS
Child health study
- BMI
Body mass index
- AA
Accelerated aging
- DunedinPoAm
Dunedin pace of aging methylation
Author contributions
L.E. generated laboratory data, carried out data analyses, drafted, and revised the manuscript. W.J.H. generated laboratory data, reviewed, and revised the manuscript. M.A.H. reviewed the manuscript. J.G.N. founded and directs the Center for Healthy Children, coordinated and supervised data collection, and reviewed the manuscript. C.H., H.M.C.S. are MPIs of the Child Health Study (Project 1 of P50HD089922) from which data were drawn; provided overall coordination and supervision. M.J.M. coordinated and supervised sample processing and methylation analyses. E.J.R., C.S. coordinated and supervised data collection, and reviewed the manuscript. K.J.O. coordinated and supervised sample processing, carried out data analyses, reviewed and revised the manuscript. I.P. processed samples and reviewed and revised the manuscript. I.S. conceptualized the current paper, coordinated and supervised data collection, reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Data availability
The dataset analyzed during the current study is available through agreement with the Study investigators.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-11562-5.
References
- 1.Ahima RS. Connecting obesity, aging and diabetes. Nat. Med. 2009;15(9):996–997. doi: 10.1038/nm0909-996. [DOI] [PubMed] [Google Scholar]
- 2.Bentley, R.A., Ross, C.N., & O’Brien, M.J. Obesity, metabolism, and aging: a multiscalar approach. In Progress in Molecular Biology and Translational Science. Vol 155, pp 25–42. (Elsevier, Amsterdam, 2018). doi:10.1016/bs.pmbts.2017.11.016 [DOI] [PubMed]
- 3.Tam BT, Morais JA, Santosa S. Obesity and ageing: two sides of the same coin. Obes. Rev. 2020;21(4):e12991. doi: 10.1111/obr.12991. [DOI] [PubMed] [Google Scholar]
- 4.Di Cesare M, Sorić M, Bovet P, et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. 2019;17(1):212. doi: 10.1186/s12916-019-1449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buxton JL, Walters RG, Visvikis-Siest S, Meyre D, Froguel P, Blakemore AIF. Childhood obesity is associated with shorter leukocyte telomere length. J. Clin. Endocrinol. Metab. 2011;96(5):1500–1505. doi: 10.1210/jc.2010-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemente DBP, Maitre L, Bustamante M, et al. Obesity is associated with shorter telomeres in 8 year-old children. Sci. Rep. 2019;9(1):1–8. doi: 10.1038/s41598-019-55283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horvath S, Erhart W, Brosch M, et al. Obesity accelerates epigenetic aging of human liver. Proc. Natl. Acad. Sci. 2014;111(43):15538–15543. doi: 10.1073/pnas.1412759111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nevalainen T, Kananen L, Marttila S, et al. Obesity accelerates epigenetic aging in middle-aged but not in elderly individuals. Clin. Epigenet. 2017;9(1):20. doi: 10.1186/s13148-016-0301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell. 2013;49(2):359. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. doi: 10.1186/gb-2013-14-10-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. 2018;10(4):573. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging. 2019;11(2):303. doi: 10.18632/aging.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belsky DW, Caspi A, Arseneault L, et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife. 2020 doi: 10.7554/eLife.54870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raffington L, Belsky DW, Kothari M, Malanchini M, Tucker-Drob EM, Harden KP. Socioeconomic disadvantage and the pace of biological aging in children. Pediatrics. 2021;147(6):e2020024406. doi: 10.1542/peds.2020-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gooding HC, Milliren C, Austin SB, Sheridan MA, McLaughlin KA. Exposure to violence in childhood is associated with higher body mass index in adolescence. Child. Abuse Negl. 2015;50:151–158. doi: 10.1016/j.chiabu.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noll JG, Zeller MH, Trickett PK, Putnam FW. Obesity risk for female victims of childhood sexual abuse: a prospective study. Pediatrics. 2007;120(1):e61–e67. doi: 10.1542/peds.2006-3058. [DOI] [PubMed] [Google Scholar]
- 17.Shin SH, Miller DP. A longitudinal examination of childhood maltreatment and adolescent obesity: results from the national longitudinal study of adolescent health (AddHealth) study. Child. Abuse Negl. 2012;36(2):84–94. doi: 10.1016/j.chiabu.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Sumner JA, Colich NL, Uddin M, Armstrong D, McLaughlin KA. Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biol. Psych. 2019;85(3):268–278. doi: 10.1016/j.biopsych.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jovanovic T, Vance LA, Cross D, et al. Exposure to violence accelerates epigenetic aging in children. Sci. Rep. 2017;7(1):8962. doi: 10.1038/s41598-017-09235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collaboration PS. Body-mass index and cause-specific mortality in 900000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richmond RC, Sharp GC, Ward ME, et al. DNA methylation and BMI: investigating identified methylation sites at HIF3A in a causal framework. Diabetes. 2016;65(5):1231–1244. doi: 10.2337/db15-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbas G, Salman A, Rahman SU, et al. Aging mechanisms: linking oxidative stress, obesity and inflammation. Mat. Sci. Medica. 2017;1(1):30–33. doi: 10.26480/msm.01.2017.30.33. [DOI] [Google Scholar]
- 23.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006;83(2):461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 24.Houde AA, Légaré C, Biron S, et al. Leptin and adiponectin DNA methylation levels in adipose tissues and blood cells are associated with BMI, waist girth and LDL-cholesterol levels in severely obese men and women. BMC Med. Genet. 2015;16(1):1–10. doi: 10.1186/s12881-015-0174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Zhu H, Snieder H, et al. Obesity related methylation changes in DNA of peripheral blood leukocytes. BMC Med. 2010;8(1):1–8. doi: 10.1186/1741-7015-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouchard L, Rabasa-Lhoret R, Faraj M, et al. Differential epigenomic and transcriptomic responses in subcutaneous adipose tissue between low and high responders to caloric restriction. Am. J. Clin. Nutr. 2010;91(2):309–320. doi: 10.3945/ajcn.2009.28085. [DOI] [PubMed] [Google Scholar]
- 27.Milagro FI, Campión J, Cordero P, et al. A dual epigenomic approach for the search of obesity biomarkers: DNA methylation in relation to diet-induced weight loss. FASEB J. 2011;25(4):1378–1389. doi: 10.1096/fj.10-170365. [DOI] [PubMed] [Google Scholar]
- 28.Salvestrini V, Sell C, Lorenzini A. Obesity may accelerate the aging process. Front. Endocrinol. 2019;10:266. doi: 10.3389/fendo.2019.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. 2018 doi: 10.1542/peds.2017-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Africa JA, Newton KP, Schwimmer JB. Lifestyle interventions including nutrition, exercise, and supplements for nonalcoholic fatty liver disease in children. Dig Dis Sci. 2016;61(5):1375. doi: 10.1007/s10620-016-4126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halfon N, Larson K, Slusser W. Associations between obesity and comorbid mental health, developmental, and physical health conditions in a nationally representative sample of US children aged 10 to 17. Acad. Pediat. 2013;13(1):6–13. doi: 10.1016/j.acap.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Morrison KM, Shin S, Tarnopolsky M, Taylor VH. Association of depression and health related quality of life with body composition in children and youth with obesity. J. Affect. Disord. 2015;172:18–23. doi: 10.1016/j.jad.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Pollock NK. Childhood obesity, bone development, and cardiometabolic risk factors. Mol. Cell Endocrinol. 2015;410:52–63. doi: 10.1016/j.mce.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marini S, Davis KA, Soare TW, et al. Adversity exposure during sensitive periods predicts accelerated epigenetic aging in children. Psychoneuroendocrinology. 2020 doi: 10.1016/j.psyneuen.2019.104484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lloyd LJ, Langley-Evans SC, McMullen S. Childhood obesity and risk of the adult metabolic syndrome: a systematic review. Int. J. Obes. 2012;36(1):1–11. doi: 10.1038/ijo.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suliga E. Visceral adipose tissue in children and adolescents: a review. Nutr. Res. Rev. 2009;22(2):137–147. doi: 10.1017/S0954422409990096. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Chassan RA, Bruer EH, Gower BA, Shelton RC. Childhood maltreatment increases the risk for visceral obesity: childhood maltreatment and visceral obesity. Obesity. 2015;23(8):1625–1632. doi: 10.1002/oby.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zannas AS, Arloth J, Carrillo-Roa T, et al. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol. 2015;16(1):266. doi: 10.1186/s13059-015-0828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fitzgerald KN, Hodges R, Hanes D, et al. Potential reversal of epigenetic age using a diet and lifestyle intervention: a pilot randomized clinical trial. Aging. 2021;13(7):9419–9432. doi: 10.18632/aging.202913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sae-Lee C, Corsi S, Barrow TM, et al. Dietary intervention modifies DNA methylation age assessed by the epigenetic clock. Mol. Nutr. Food Res. 2018;62(23):1800092. doi: 10.1002/mnfr.201800092. [DOI] [PubMed] [Google Scholar]
- 41.Schreier HMC, Heim CM, Rose EJ, Shalev I, Shenk CE, Noll JG. Assembling a cohort for in-depth, longitudinal assessments of the biological embedding of child maltreatment: methods, complexities, and lessons learned. Dev. Psychopathol. 2021;33(2):394–408. doi: 10.1017/S0954579420001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Center for Disease Control. BMI Percentile Calculator for Child and Teen. Published June 7, 2021. Accessed June 21, 2021. https://www.cdc.gov/healthyweight/bmi/calculator.html
- 43.Min, J.L., Hemani, G., Davey Smith, G., Relton, C., & Suderman, M. Meffil: efficient normalization and analysis of very large DNA methylation datasets. In Bioinformatics (ed Hancock, J.) Published online June 21, 2018. doi:10.1093/bioinformatics/bty476 [DOI] [PMC free article] [PubMed]
- 44.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 45.Horvath, S. DNA Methylation Age of Human Tissues and Cell Types. 2013. https://dnamage.genetics.ucla.edu/home [DOI] [PMC free article] [PubMed]
- 46.Houseman EA, Accomando WP, Koestler DC, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinform. 2012;13(1):86. doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121(Supplement 3):S208–S217. doi: 10.1542/peds.2007-1813F. [DOI] [PubMed] [Google Scholar]
- 48.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 49.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analyzed during the current study is available through agreement with the Study investigators.