Abstract
Malnutrition is common in patients with heart failure with reduced ejection fraction (HFrEF) and may influence the long-term prognosis and allocation of combination medical therapy. We reviewed 1231 consecutive patient-level records from a multicenter Japanese registry of hospitalized HFrEF patients. Nutritional status was assessed using geriatric nutritional risk index (GNRI). Combination medical therapy were categorized based on the use of beta-blockers, renin-angiotensin system inhibitors, and mineralocorticoid receptor antagonists. The composite outcome of all-cause death and HF rehospitalization was assessed. The mean age was 72.0 ± 14.2 years and 42.6% patients were malnourished (GNRI < 92). At discharge, 43.6% and 33.4% of patients were receiving two and three agents, respectively. Malnourished patients had lower rates of combination medical therapy use. The standardized GNRI score was independently associated with the occurrence of adverse events (hazard ratio [HR]: 0.88, 95% confidence interval [CI] 0.79–0.98). Regardless of the GNRI score, referenced to patients receiving single agent, risk of adverse events were lower with those receiving three (HR: 0.70, 95% CI 0.55–0.91) or two agents (HR: 0.70, 95% CI 0.56–0.89). Malnutrition assessed by GNRI score predicts long-term adverse outcomes among hospitalized HFrEF patients. However, its prognosis may be modified with combination medical therapy.
Subject terms: Cardiology, Heart failure
Introduction
Heart failure (HF) with reduced ejection fraction (HFrEF; left ventricular ejection fraction [LVEF] of ≤ 40%) affects over 2.5 million adults in the United States alone and is associated with high economic costs and rates of impaired health status, morbidity, and mortality1–3. Similar trends are observed in Japan4, where more than 230,000 patients were hospitalized for HF in 2015. This number appears to be increasing by more than 10,000 cases each year, with one-half of these cases involving HFrEF5. High-quality evidence currently supports the treatment of HFrEF patients using combination medical therapy, which has traditionally included beta-blockers (BBs), angiotensin-converting enzyme inhibitors (ACEis)/angiotensin receptor blockers (ARBs), and mineralocorticoid receptor antagonists (MRAs)5–7. Novel disease-modifying therapies, such as angiotensin receptor-neprilysin inhibitors (ARNIs) and sodium-glucose cotransporter-2 inhibitors (SGLT2is), have also been introduced, and these agents are expected to further improve the outcomes of HFrEF patients incremental to the backbone of combination medical therapy listed above8.
Previous studies have identified important barriers to the full implementation of combination medical therapy, including renal dysfunction, hyperkalemia, hypotension, bradycardia, old age, and low functional status9–11. Malnutrition is also common among patients with HF and is independently associated with adverse outcomes, which are linked to both HF and mechanisms that are related to other chronic disease states12. In addition, malnutrition may reflect a state of abnormal drug metabolism, which may cause unfavorable treatment effects13,14. Therefore, malnutrition can also negatively affect clinical decision-making for patients with significant clinical conditions, such as cancer15,16. However, contemporary large-scale randomized controlled trials (RCTs) have generally excluded malnourished patients, and there is limited data regarding the interaction between nutritional status and real-world use of HF-related combination medical therapy17,18. Given the typically stringent entry criteria for clinical trials, observational studies may provide a more realistic assessment of malnutrition among the HF patients.
This study evaluated the prevalence of malnutrition among HFrEF patients, their long-term outcomes, and whether their nutritional status was associated with the use of combination medical therapy. The West Tokyo Heart Failure (WET-HF) registry in Japan has historically included HF patients who have lower body mass index or who are older (vs. Western HF studies19), thereby providing a unique opportunity to investigate this topic.
Methods
Study design
Patient-level data were extracted from the WET-HF registry20,21, which prospectively collected information on patients who were admitted to three university hospitals and three tertiary referral hospitals within the Tokyo metropolitan area (Japan) in 2006–2017. This multicenter registry was designed to collect data on the clinical characteristics and outcomes of patients hospitalized with a primary diagnosis of acute HF, which is defined as rapid-onset HF with changes in signs and symptoms that indicate an urgent need for hospitalization and therapy22. Clinical diagnoses were made by experienced cardiologists at each institution, who excluded patients who presented with acute coronary syndrome. A robust assessment of care and patient outcomes was facilitated by dedicated clinical research coordinators collecting baseline data and outcomes from medical records and querying attending physicians. Data were entered into an electronic data-capturing system that has a robust data query engine and system validations to ensure data quality. Exclusive on-site auditing by the investigators ensured proper registration of each patient. This study’s retrospective protocol was approved by the Institutional Review Board of Keio University School of Medicine and conducted in accordance with the Declaration of Helsinki. And all patients provided informed consent for their treatment and research use of their data.
Patient population
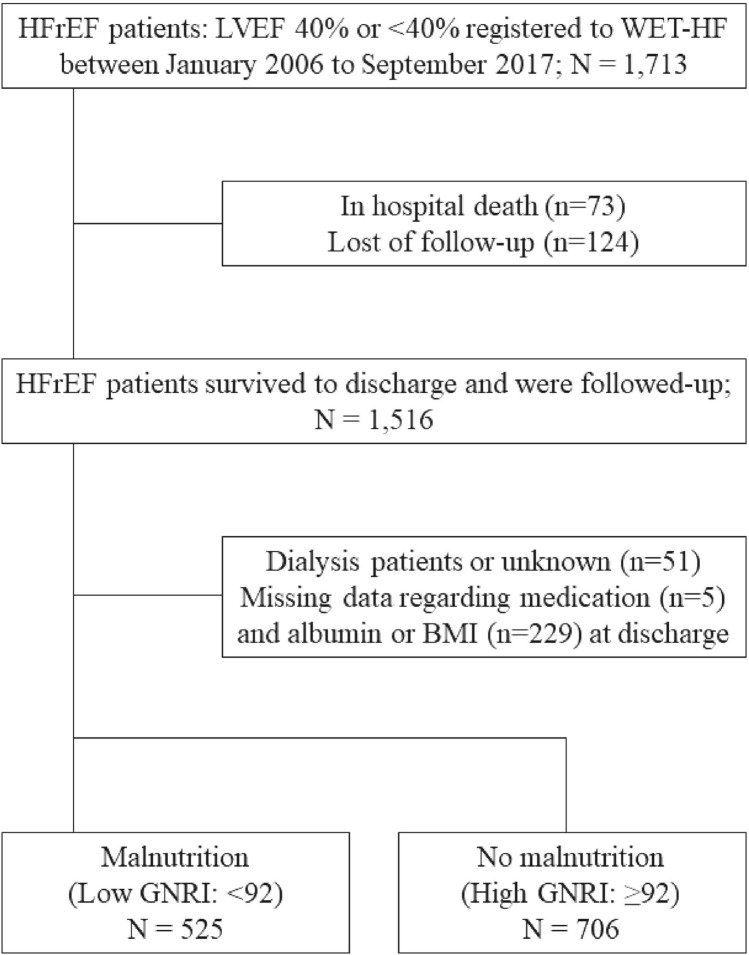
This study evaluated prospectively collected data from 1713 consecutive patients who were hospitalized for HFrEF. However, it excluded 73 patients who died during hospitalization, 51 dialysis patients, 124 patients who were lost to follow-up, 229 patients with missing albumin or body mass index data that prevented evaluation of nutritional status, and five patients with missing data regarding medication use at discharge. Thus, the study analyzed the complete data from 1231 patients (Fig. 1).
Figure 1.
Study flowchart. The HFrEF patients were categorized according to their GNRI score into groups with malnutrition (low GNRI) or normal nutritional status (high GNRI). HFrEF heart failure with reduced ejection fraction, WET-HF West Tokyo Heart Failure, GNRI geriatric nutritional risk index.
Definitions of variables and outcomes
Baseline characteristics included age, sex, causes of HF, medical history, previous procedures, vital signs, laboratory data, echocardiographic data, and medications used at discharge. The definition of a reduced ejection fraction (EF ≤ 40%) was based on the universal definition and classification of HF23.
Combination medical therapy, or optimal medical therapy for single agent use, was defined as the prescription of BBs, RASis (ACEis or ARBs), and MRAs at the time of discharge. During the study period, ARNI, SGLT2i, and ivabradine for HF patients were not available in Japan. Thus, based on the medication(s) at discharge, we classified the patients as having received none of the three agents, single therapy (one agent), double therapy (two agents), or triple therapy (all three agents).
Patients at risk of malnutrition can be identified using several risk indexes. The present study evaluated nutritional status based on the GNRI, which is a simple formula that has been demonstrated to be clinically useful among patients with various medical conditions24,25. The GNRI score was calculated at the time of discharge as follows:
Clinical characteristics and mortality were compared between the following two groups: low GNRI group (< 92) with moderate or severe nutritional risk and high GNRI group (≥ 92) with low or no nutritional risk according to a previous report26. In the present study, patients with low GNRI score were defined as “malnourished.”
Ischemic etiology was defined as left ventricular dysfunction (EF of ≤ 40%) with a history of myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, or at least one major epicardial coronary artery with ≥ 75% stenosis. As coronary angiography (CAG) is usually recommended for patients with a high pre-test probability of coronary artery disease, CAG was performed during index hospitalization for selected patients based on their clinical course, in this cohort. The eGFR was calculated using the Modification of Diet in Renal Disease Equation for Japanese Patients, which was proposed by the Japanese Society of Nephrology27. The EF was calculated using the modified Simpson’s method. Ultrasound cardiography was performed by highly experienced cardiologists or clinical technologists during the hospitalization. The NYHA functional class was evaluated at admission by the treating cardiologists at each institution.
Follow-up data were mainly collected via telephone contact or a chart review. The primary outcome of interest was defined as a composite of all-cause death and HF rehospitalization after discharge, with the decision to rehospitalize the patient made by the treating physicians based on the usual standard of care. Each of these events was assessed individually as secondary endpoints.
To ensure the accuracy of the adverse event ascertainment, the WET-HF registry is supported by a central study committee that adjudicates determination of the endpoint.
Statistical analysis
Data were reported as number and percentage for categorical variables and as median (interquartile range) for continuous variables. Continuous variables were compared using the Mann–Whitney U test and Kruskal–Wallis test, whereas categorical variables were compared using the Pearson’s chi-squared test.
A multivariable logistic regression model was used to evaluate whether medical therapy use was related to different clinical variables. Models were adjusted for age, sex, serum potassium concentration, total cholesterol level, eGFR level, LVEF, systolic blood pressure, heart rate, history of HF hospitalization, atrial fibrillation, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, ischemic cardiomyopathy, and GNRI status. Survival curves were compared using the Kaplan–Meier method and log-rank test.
Multivariable Cox proportional hazard models were also created to assess the association between combination medical therapy use (none, single, double, or triple therapy) and each endpoint, which was adjusted for age, sex, systolic blood pressure, and heart rate at discharge, renal dysfunction (eGFR < 60 mL/min/1.73 m2), LVEF, history of HF hospitalization, ischemic etiology, atrial fibrillation, chronic obstructive pulmonary disease, stroke, diabetes mellitus, use of loop diuretics, statin use, and standardized GNRI score. Results were reported as HRs, 95% CIs, and p-values. We also created a 4-knot restricted cubic spline model of the HRs according to the GNRI status (continuous value), which was adjusted for the same factors as the Cox hazards model.
A sensitivity analysis was also performed after excluding patients with advanced renal impairment (eGFR < 30 mL/min/1.73 m2) or higher mortality risk (an estimated mortality risk of > 10% based on a GWTG-HF risk score of > 57), considering the possibility of patients who may not benefit from or tolerate combination medical therapy21. To verify the GNRI cutoff points, we performed a sensitivity analysis using the median of the GNRI (< or ≥ 94.1) in the whole analysis cohort. All analyses were performed at a two-sided significance level of 0.05. There was no correction for multiple comparisons given the exploratory nature of this observational analysis. IBM SPSS software (version 26; IBM Corp., Armonk, NY, USA) and R software (version 3.6.3; Foundation for Statistical Computing, Vienna, Austria) were used for statistical analysis.
Ethics approval and participant’s consent
The study’s retrospective protocol was approved by the Institutional Review Board of Keio University School of Medicine and conducted in accordance with the Declaration of Helsinki. And all patients provided informed consent for their treatment and research use of their data.
Results
Baseline characteristics
The 1231 HFrEF patients were predominantly men (62%) and had a median age of 72.0 years (interquartile range: 61.0–81.0 years). The baseline characteristics of patients divided into two groups according to their GNRI values are listed in Table 1. The low GNRI group had a higher proportion of female patients, tended to be older, had more severe symptoms (New York Heart Association [NYHA] III or IV), had lower hemoglobin and albumin concentrations, and had higher creatinine and B-type natriuretic peptide concentrations. There were no significant inter-group differences in terms of systolic blood pressure and heart rate at admission. At the time of discharge, 20, (IQR 20–40) mg/day of loop diuretic (converted to furosemide dose) and 25% (IQR 12.5–50) of maximum dose of beta-blocker (converted to carvedilol dose) were prescribed.
Table 1.
Baseline characteristics of patients by nutritional status.
| Variables | Low GNRI (< 92) (n = 525) | High GNRI (GNRI ≥ 92) (n = 706) | p value |
|---|---|---|---|
| Age, years | 78 [69, 84] | 68 [57, 77] | < 0.001 |
| Female, n (%) | 200 (38.1) | 159 (22.5%) | < 0.001 |
| Systolic blood pressure, mmHg | 132 [110, 151] | 132 [112, 155] | 0.507 |
| Heart rate, beats/min | 94 [78, 114] | 96 [78, 112] | 0.814 |
| LVEF, % | 30 [25, 35] | 30 [23, 35] | 0.013 |
| Ischemic etiology, n (%) | 205 (39.0) | 246 (34.8) | 0.130 |
| NYHA (III–IV) | 432 (86.7) | 553 (81.4) | 0.015 |
| Comorbidities | |||
| Prior HF hospitalization, n (%) | 188 (36.4) | 246 (35.1) | 0.642 |
| Hypertension, n (%) | 342 (65.1) | 440 (62.3) | 0.309 |
| Diabetes mellitus, n (%) | 201 (38.3) | 270 (38.2) | 0.988 |
| Dyslipidemia, n (%) | 193 (37.3) | 319 (45.8) | 0.003 |
| Smoking, n (%) | 209 (41.4) | 357 (52.2) | < 0.001 |
| Atrial fibrillation, n (%) | 201 (38.4) | 290 (41.1) | 0.336 |
| Stroke, n (%) | 70 (13.3) | 89 (12.7) | 0.728 |
| COPD, n (%) | 26 (5.0) | 24 (3.4) | 0.173 |
| Laboratory findings at admission | |||
| Hemoglobin, g/dL | 11.9 [10.4, 13.5] | 13.4 [12.0, 15.0] | 0.001 |
| BUN, mg/dL | 25.1 [18.2, 36.3] | 20.3 [15.9, 27.4] | < 0.001 |
| Creatinine, mg/dL | 1.14 [0.81, 1.61] | 1.04 [0.86, 1.31] | 0.003 |
| eGFR, ml/min/1.73 m2 | 45.2 [29.8, 61.6] | 53.6 [40.2, 66.5] | < 0.001 |
| Sodium, mEq/L | 139 [137, 142] | 140 [138, 142] | < 0.001 |
| Potassium, mEq/L | 4.4 [3.9, 4.8] | 4.3 [4.0, 4.7] | 0.823 |
| BNP, pg/mL | 1155 [702, 1989] | 707 [383, 1082] | < 0.001 |
| Alb, mg/dL | 3.4 [3.1, 3.7] | 3.8 [3.6, 4.1] | < 0.001 |
Values are median [with interquartile ranges] or n (%).
GNRI geriatric nutritional risk index, NYHA New York Heart Association, LVEF left ventricular ejection fraction, HF heart failure, COPD chronic obstructive pulmonary disease, BUN blood urea nitrogen, eGFR estimated glomerular filtration rate, BNP B-type natriuretic peptide.
GNRI and outcomes
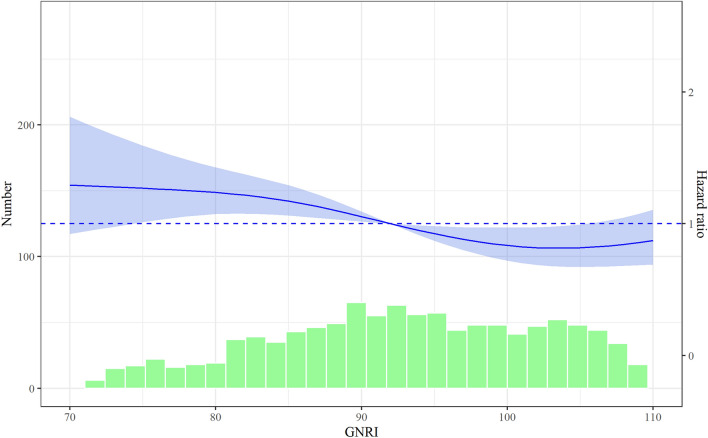
During a median follow-up period of 2.0 years (interquartile range: 0.8–3.1 years), the composite outcome was observed for 549 patients (44.6%), including 298 patients (24.3%) who died and 414 patients (33.6%) who were re-hospitalized because of HF. The unadjusted Kaplan–Meier curves revealed that the low GNRI group had a higher risk of the composite outcome (Supplementary Fig. S1). The restricted cubic spline analysis also revealed a positive correlation between the GNRI score and long-term outcomes, particularly among patients with a GNRI score of < 100 (Fig. 2).
Figure 2.
Hazard ratios for the composite outcome (all-cause death and heart failure rehospitalization) according to the Geriatric Nutritional Risk Index score. The blue line represents the continuous hazard ratio (HRs) and the light blue area represents the 95% confidence intervals. The green bars represent the numbers of patients with each Geriatric Nutritional Risk Index (GNRI) score.
Factors influencing the implementation of optimal medical therapy
At discharge, 49 patients (4.0%) were receiving none of the three agents, 234 patients (19.0%) were receiving a single agent, 537 patients (43.6%) were receiving double therapy, and 411 patients (33.4%) were receiving triple therapy. The overall usage rates were 87.4% for BBs, 71.7% for RASis, and 51.0% for MRAs (Supplementary Fig. S2). The triple therapy group was younger, had a higher frequency of non-ischemic dilated cardiomyopathy, and had better renal function. Furthermore, the combination medical therapy group had a significantly higher median GNRI score (triple therapy: 96.7 [interquartile range: 88.5–104.8], double therapy: 94.5 [interquartile range: 87.3–102.9], single therapy: 91.2 [interquartile range: 82.3–98.2], and no therapy: 88.6 [interquartile range: 82.1–94.8]; p < 0.001).
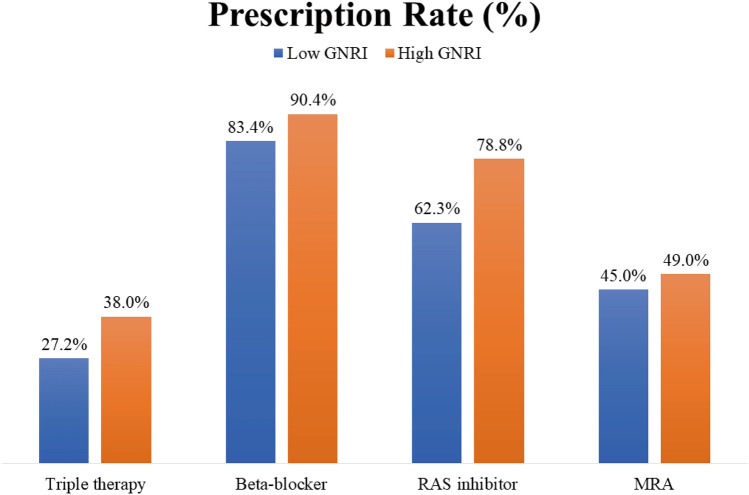
The triple therapy as well as specific therapy groups (i.e., the BB, RASi, and MRA groups) were compared according to their nutritional risk using GNRI values. Lower GNRI values tended to be associated with lower administration rates of triple therapy and each agent (Fig. 3). The associations between specific therapy groups and clinical factors are described in Table 2. After multivariable adjustment, age was significantly associated with BB use (OR: 0.95, 95% confidence interval [CI] 0.93–0.97; p < 0.001) and renal function was associated with RASi use (OR: 1.02, 95% CI 1.02–1.03; p < 0.001) and MRA use (OR: 1.01, 95% CI 1.00–1.02; p = 0.004). Furthermore, the GNRI score was significantly associated with BB use (OR: 1.02, 95% CI 1.00–1.04; p = 0.043) and RASi use (OR: 1.03, 95% CI 1.02–1.05; p < 0.001), but not with MRA use (OR: 1.00, 95% CI 0.99–1.01; p = 0.597).
Figure 3.
Prescription rate of each agent according to Geriatric Nutritional Risk Index: Low GNRI for moderate or severe nutritional risk with GNRI < 92, and high GNRI for low or no nutritional risk with GNRI ≥ 92. GNRI geriatric nutritional risk index, RASi renin-angiotensin-system inhibitor, MRA mineralocorticoid receptor antagonist, GNRI geriatric nutritional risk index, ACEi angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, MRA mineralocorticoid receptor antagonist.
Table 2.
Clinical variables contributing to optimal medial therapy administration.
| Variable | Beta-blockers | RAS-inhibitors | MRAs | |||
|---|---|---|---|---|---|---|
| OR [95% CI] | p value | OR [95% CI] | p value | OR [95% CI] | p value | |
| Male | 0.57 [0.37–0.90] | 0.016 | 0.84 [0.60–1.16] | 0.287 | 0.83 [0.62–1.11] | 0.209 |
| Age (per 1 year increase) | 0.95 [0.93–0.97] | < 0.001 | 0.99 [0.98–1.00] | 0.164 | 0.99 [0.98–1.01] | 0.234 |
| Prior HF hospitalization | 1.06 [0.69–1.62] | 0.805 | 1.12 [0.82–1.54] | 0.473 | 1.61 [1.22–2.13] | < 0.001 |
| Systolic blood pressure (per 1 mmHg increase) | 1.00 [1.00–1.01] | 0.512 | 1.00 [1.00–1.01] | 0.202 | 0.99 [0.99–1.00] | 0.310 |
| Heart rate (per 1 beat/min increase) | 1.01 [1.00–1.01] | 0.171 | 1.00 [0.99–1.00] | 0.149 | 1.00 [0.99–1.01] | 0.469 |
| eGFR (per 1 mL/min/1.72 m2 increase) | 1.00 [0.99–1.01] | 0.912 | 1.02 [1.02–1.03] | < 0.001 | 1.01 [1.00–1.02] | 0.004 |
| Potassium level (per 1 mEq/L increase) | 1.32 [0.88–1.98] | 0.188 | 1.15 [0.86–1.54] | 0.361 | 0.95 [0.73–1.24] | 0.721 |
| Total cholesterol level (per 1 mg/dL) | 1.00 [0.99–1.00] | 0.239 | 1.00 [1.00–1.01] | 0.544 | 1.00 [1.00–1.00] | 0.034 |
| Atrial fibrillation | 0.97 [0.65–1.46] | 0.882 | 1.20 [0.88–1.64] | 0.247 | 0.92 [0.70–1.20] | 0.528 |
| Ischemic cardiomyopathy | 1.17 [0.77–1.77] | 0.456 | 1.23 [0.90–1.68] | 0.200 | 1.15 [0.87–1.51] | 0.339 |
| Diabetes mellitus | 1.02 [0.67–1.54] | 0.946 | 0.78 [0.58–1.06] | 0.782 | 1.20 [0.92–1.57] | 0.178 |
| Hypertension | 1.43 [0.94–2.16] | 0.094 | 1.36 [0.99–1.88] | 0.060 | 0.99 [0.75–1.31] | 0.948 |
| COPD | 0.61 [0.28–1.29] | 0.228 | 1.41 [0.66–2.97] | 0.374 | 0.67 [0.35–1.28] | 0.228 |
| LVEF (per 1% increase) | 0.98 [0.95–1.01] | 0.103 | 1.00 [0.98–1.02] | 0.855 | 0.97 [0.95–0.99] | 0.002 |
| GNRI (continuous variable) | 1.02 [1.00–1.04] | 0.043 | 1.03 [1.02–1.05] | < 0.001 | 1.00 [0.99–1.01] | 0.597 |
RAS-I renin-angiotensin system inhibitor, MRA mineralocorticoid receptor antagonist, HF heart failure, eGFR estimated glomerular filtration rate, COPD chronic obstructive pulmonary disease, LVEF left ventricular ejection fraction, GNRI geriatric nutritional risk index.
Implementation of combination medical therapy and clinical outcomes
The unadjusted Kaplan–Meier curves revealed that patients who received more therapeutic agents had a lower risk of the composite outcomes (Supplementary Fig. S3). The multivariable Cox proportional hazards model revealed that, regardless of the GNRI status, combination medical therapy was significantly associated with a lower risk of the composite outcome after adjustment for other patient characteristics. Compared with patients who received single therapy, the risk of the composite outcome was higher among those receiving none of the three agents (adjusted hazard ratio [HR]: 1.62, 95% CI 1.07–2.47; p = 0.024) and lower among those receiving double therapy (adjusted HR: 0.70, 95% CI 0.56–0.89; p = 0.003) and triple therapy (adjusted hazard ratio [HR]: 0.70, 95% CI 0.55–0.91; p = 0.006) (Table 3). Furthermore, the effects of combination medical therapy appeared to be consistent in lowering the risks of all-cause death (triple therapy: HR: 0.67, 95% CI 0.46–0.96; p = 0.028 or double therapy: HR: 0.69, 95% CI 0.50–0.96; p = 0.028) (Supplementary Fig. S4), and hospitalization for HF (triple therapy: HR: 0.65, 95% CI 0.49–0.87; p = 0.003 or double therapy: HR: 0.67, 95% CI 0.52–0.88; p = 0.004) (Supplementary Fig. S5), respectively.
Table 3.
Multivariable Cox proportional hazards models of the primary outcome.
| Medication class | Outcome analysis | |
|---|---|---|
| HR [95%CI] | p value | |
| Triple therapy | 0.70 [0.55–0.91] | 0.006 |
| Double therapy | 0.70 [0.56–0.89] | 0.003 |
| Single therapy | Reference | |
| None medical therapy | 1.62 [1.07–2.47] | 0.024 |
These models were adjusted by the following variables: age, sex, systolic blood pressure, heart rate, renal dysfunction (eGFR < 60 ml/min/1.73m2), ejection fraction, history of heart failure hospitalization, ischemic etiology, atrial fibrillation, chronic obstructive pulmonary disease, stroke, diabetes mellitus, use of loop diuretics, use of statins and medical therapy (triple, double, single, and no optimal medical therapy), as well as geriatric nutritional risk index.
BB beta-blocker, RASi renin-angiotensin system inhibitor, MRA mineralocorticoid receptor antagonist.
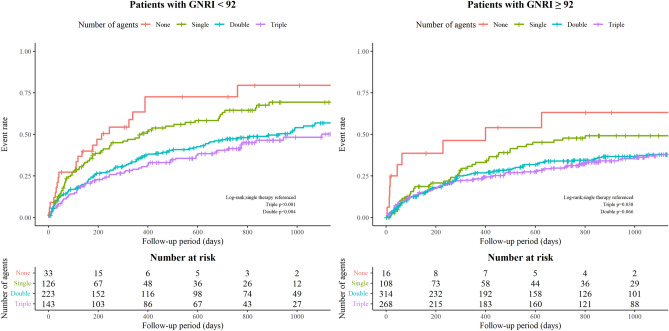
Moreover, among the malnourished (i.e. low GNRI group), the risk for subsequent adverse outcomes was lower for patients who received double, and triple therapies than for those receiving a single agent (Fig. 4). In the low GNRI (< 92) subgroup, triple therapy (HR 0.57, 95% CI 0.40–0.82; p = 0.003) and double therapy patients (HR 0.68, 95% CI 0.50–0.94; p = 0.019) had better outcome when referenced to single therapy patients. On the contrary, within the high GNRI group, triple therapy (HR 0.95, 95% CI 0.65–1.39; p = 0.780) and double therapy patients (HR 0.74, 95% CI 0.52–1.06; p = 0.104) had no significant relationship with the composite outcome (P value for interaction also was not significant; p = 0.550). These results indicate that the advantage of combination therapy may be particularly prominent among malnourished patients rather than among those with normal nutritional status.
Figure 4.
Unadjusted Kaplan–Meier curves for the composite outcome (all-cause death and heart failure rehospitalization) in each treatment group (no, single, double, and triple therapy) according to Geriatric Nutritional Risk Index score (GNRI: < 92 vs. ≥ 92).
Sensitivity analysis
The results of our analyses were also similar in sensitivity analyses that excluded patients with advanced renal impairment (estimated glomerular filtration rate [eGFR]: < 30 mL/min/1.73 m2) or higher mortality risk (Get With The Guideline-Heart Failure [GWTG-HF] risk score: > 57) (Supplementary Fig. S6). Compared with patients who received single therapy, the risk of the composite outcome was higher with those with not receiving any of the agents (adjusted HR: 1.81, 95% CI 1.10–2.96; p = 0.018) and lower in those receiving double therapy (adjusted HR: 0.66, 95% CI 0.50–0.88; p = 0.004) and triple therapy (adjusted HR: 0.69, 95% CI 0.51–0.93; p = 0.015). This tendency was consistent in the risk reduction of all-cause death (triple therapy: HR: 0.66, 95% CI 0.43–1.02; p = 0.061 or double therapy: HR: 0.55, 95% CI 0.37–0.84; p = 0.005) and HF rehospitalization (triple therapy: HR: 0.65, 95% CI 0.46–0.92; p = 0.014 or double therapy: HR: 0.67, 95% CI 0.49–0.93; p = 0.016).
Furthermore, the results of our analyses were also similar to that of the sensitivity analysis using the GNRI cutoff of 94.1 with the whole analytic cohort (Supplementary Fig. S7).
Discussion
This study evaluated data from a contemporary multicenter registry of acute HF cases and revealed three main findings. First, among patients with HFrEF, malnutrition (based on a low GNRI score) was associated with significantly lower rates of combination medical therapy initiation. Second, patients with malnutrition had an increased risk of the composite outcome that involved all-cause death and HF rehospitalization. Third, even among patients with malnutrition, increased use of combination medical therapy was independently associated with lower risks of the composite outcome of rehospitalization or all-cause mortality.
Treatment for HF aims to prevent morbidities and prolong morbidity-free survival. Combination therapy using RAASis (i.e., ACEis/ARBs/ARNIs and MRAs) with BBs is a guideline-recommended standard therapy for patients with HFrEF. However, despite increased awareness of this recommendation, a study of the CHAMP-HF registry demonstrated that prescription rates for these disease-modifying drugs remain lower than expected in the ambulatory setting and have not improved over 15 years since the Euro Heart Survey on Heart Failure9,28. Similarly, a study on acute HF patients hospitalized with HFrEF from the Japanese Cardiac Registry of Heart Failure (2004–2005) revealed low proportions of drug use at discharge (BBs: 57.9%, ACEis: 4.3%, ARBs: 45.6%, MRAs: 46.0%)29. Aside from the rate of BB use, our findings are generally comparable and suggest that there is room for improvement in the treatment rates of ACEi/ARB and MRA, similar to that observed in Western countries. Additionally, the rate of MRA administration was similar across nutritional status. The purpose of its use may have been different than that of the other two agents and it may be prescribed as a potassium-sparing diuretic more frequently than as a cardioprotective agent. This may have influenced the relationship between its administration and GNRI value.
The gaps in combination medical therapy use in this setting may be related to various factors, including physician aversion and inertia, patient intolerance, and side effects9. Frailty, a possible reason for physicians being hesitant to change therapy, is also strongly related to malnutrition, but has different features that require investigation and call for further evaluation of the interaction and its association with HF treatment and outcomes, since either of the two may lead to a vicious cycle of physical impairment. In previous studies, patients with low GNRI were more likely to have characteristics of frailty, which represents a state of increased vulnerability to stressors resulting from multisystem dysregulation and is associated with a higher risk of impaired physical function and mortality30. As our study showed that combination medical therapy use was associated with better outcomes even in patients with low GNRI, it may be possible to improve their prognoses.
Malnutrition may make physicians reluctant to alter a patient’s treatment considering the medicine’s side-effects during liver dysfunction16, as it decreases oxidative metabolism in the liver, which is performed by cytochrome P-450 isoenzymes, via depletion of nicotinamide adenine dinucleotide phosphate reserves. Other liver metabolic pathways can also be impaired, which can decrease drug clearance, thereby increasing drug exposure and potentially resulting in harmful side effects. There are also concerns regarding drug absorption changes due to poor nutritional status (e.g., because of vitamin and/or mineral deficiency), which may influence the physician’s decisions based on the medication’s effectiveness and potential side effects31 and partially explain the present study’s findings. Moreover, the existing RCTs have generally excluded malnourished patients, who are often older and have more comorbidities. Thus, although there is still limited evidence regarding whether nutritional status influences the effectiveness and side effects of optimal medical therapies particularly when they are combined, our results suggest that even malnourished patients with HFrEF can benefit from prescription of combination medical therapy.
Malnourished patients are often older and tend to have a greater number of comorbidities, including renal dysfunction, which may make physicians hesitant to add new medications32. We have previously reported that RASi prescriptions were more appropriate for patients with better renal function (eGFR: > 30 mL/min/1.73 m2), and RASi prescription was associated with lower risks of all-cause death and HF rehospitalization, although RASi use was not significantly associated with adverse outcomes among patients with a lower eGFR33. Similar results have been reported for MRA use, as data from the GWTG-HF registry revealed that spironolactone was less frequently prescribed for HFrEF patients with advanced renal impairment (serum creatinine: 1.5–2.0 mg/dL) than for patients with serum creatinine concentrations of < 1.5 mg/dL34. Another report based on the OPTIMIZE-HF registry revealed that MRA use was associated with good clinical outcomes, even among older patients, if their eGFR was > 30 mL/min/1.73 m235. Ferreira et al. also evaluated MRA prescription patterns in a sub-analysis of data from RCTs and reported that lower MRA doses were prescribed to patients with chronic kidney disease36. This may be because these patients experience more frequent episodes of worsening renal function during MRA treatment, which necessitates close surveillance and dose adaptations. Nevertheless, patients still benefited from lower MRA doses. In this clinical context, the latest European Cardiology Society Guidelines for the diagnosis and treatment of acute and chronic heart failure recommend initial combination therapies with low doses for HF with reduced ejection fraction followed by up-titration of each agent if possible37. Clinicians must consider the risks and benefits of any specific treatment strategy, with careful dose adjustment, and initiate combination therapy as much as possible.
Strengths and limitations
This study highlights the real-world challenges of HF management in malnourished patients, although it also has several inherent limitations. First, the retrospective observational nature is prone to bias and confounding by unmeasured or unidentified variables, although we adjusted the analyses for relevant clinical characteristics and tested different cut-offs as sensitivity analyses. Although well-designed RCTs would be needed to ensure the efficacy of combination medical therapy, especially triple therapy, given the strength of the evidence for combination medical therapy among non-malnourished patients, there may be ethical questions regarding testing these therapies against placebo in clinical trials. In addition, the registry did not contain data regarding the specific doses of each agent and their titration or withdrawal after discharge, which precluded dose-related analyses. In the treatment of HF, titration and discontinuation of medications are not uncommon38, thus future studies including this information is needed for patients with malnourishment. Finally, frailty can coexist with malnutrition, although we were unable to consider the relationship between these two conditions because of the lack of data regarding frailty.
Conclusion
Malnourished HFrEF patients, who are typically excluded from clinical trials, exhibited poorer prognoses and combination medical therapy prescription rates, when compared with patients with better nutritional status. However, our study revealed that combination medical therapy was independently associated with favorable outcomes regardless of nutritional status. Effective approaches to increase combination medical therapy utilization are needed.
Supplementary Information
Acknowledgements
The authors thank the investigators, clinical research coordinators, and institutions involved in the administration of the West Tokyo Heart Failure Registry.
Author contributions
The author contributions are started as follows; Y.K., Y.S., and S.K. drew the manuscript. Y.K. and N.N. prepared images. Y.S., S.K., T.K., A.G., Y.N., Y.N., M.S., M.T., Y.I., S.N., and T.Y. collected patient information. T.K., A.G., Y.N., Y.N., M.S., M.T., Y.I., N.N., A.T.S.S., S.N., T.Y. and K.F. provided a critical revision of the manuscript for the key intellectual content of supervision. All the authors have approved all aspects of our work, read, and approved the manuscript.
Funding
The West Tokyo Heart Failure Registry was supported by a grant from the Japan Agency for Medical Research and Development [S.K. 201439013C], Grants-in-Aid for Scientific Research [T.Y. 18K08056, 21K08142; T.K. 17K09526, 20K08408; S.K. 20H03915], a Grant-in-Aid for Young Scientists [Y.S. 18K15860], a Grant-in-Aid for Clinical Research from the Japanese Circulation Society [Y.S. 2019], JPSS KAKENHI [T.Y. 23591062, 26461088], and Sakakibara Clinical Research Grant for the Promotion of Science [T.Y. 2012-2020].
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Competing interests
Dr. Shiraishi is affiliated with an endowed department by Nippon Shinyaku Co., Ltd., Medtronic Japan Co., Ltd., and BIOTRONIK JAPAN Inc. and received research grants from the SECOM Science and Technology Foundation and the Uehara Memorial Foundation and honoraria from Otsuka Pharmaceutical Co., Ltd. and Ono Pharmaceutical Co., Ltd.. Dr. Kohsaka has received an unrestricted research grant from the Department of Cardiology, Keio University School of Medicine, Bayer Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., and Novartis Pharmaceutical Co., Ltd.. The remaining authors have no conflicts of interest to disclose. There are no patents, products in development, or marketed products to declare. Dr. Sandhu receives research support from the US National Heart, Lung, and Blood Institute (1K23HL151672-01).
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Shun Kohsaka, Email: sk@keio.jp.
West Tokyo Heart Failure Registry:
Yasuyuki Shiraishi, Shun Kohsaka, Takashi Kohno, Ayumi Goda, Yuji Nagatomo, Yosuke Nishihata, Mike Saji, Makoto Takei, Yukinori Ikegami, Shintaro Nakano, and Tsutomu Yoshikawa
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-12357-4.
References
- 1.Groenewegen, A., Rutten, F. H., Mosterd, A. & Hoes, A. W. Epidemiology of heart failure. Eur. J. Heart Fail. 7–9 (2020). 10.1002/ejhf.1858. [DOI] [PMC free article] [PubMed]
- 2.Virani SS, et al. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 3.Sakata Y, Shimokawa H. Epidemiology of heart failure in Asia. Circ. J. 2013;77:2209–2217. doi: 10.1253/circj.CJ-13-0971. [DOI] [PubMed] [Google Scholar]
- 4.Shiraishi Y, et al. 9-year trend in the management of acute heart failure in japan: A report from the national consortium of acute heart failure registries. J. Am. Heart Assoc. 2018;7:e008687. doi: 10.1161/JAHA.118.008687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsutsui H, et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure - digest version. Circ. J. 2019;83:2084–2184. doi: 10.1253/circj.CJ-19-0342. [DOI] [PubMed] [Google Scholar]
- 6.Ponikowski P, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016;37:2129–2200m. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 7.Yancy CW, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: An update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinic. Circulation. 2016;134:e282–e293. doi: 10.1161/CIR.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 8.Ouwerkerk W, et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: A prospective European study. Eur. Heart J. 2017;38:1883–1890. doi: 10.1093/eurheartj/ehx026. [DOI] [PubMed] [Google Scholar]
- 9.Bozkurt B. Reasons for lack of improvement in treatment with evidence-based therapies in heart failure. J. Am. Coll. Cardiol. 2019;73:2384–2387. doi: 10.1016/j.jacc.2019.03.464. [DOI] [PubMed] [Google Scholar]
- 10.Bhatt AS, DeVore AD, DeWald TA, Swedberg K, Mentz RJ. Achieving a maximally tolerated β-blocker dose in heart failure patients: Is there room for improvement? J. Am. Coll. Cardiol. 2017;69:2542–2550. doi: 10.1016/j.jacc.2017.03.563. [DOI] [PubMed] [Google Scholar]
- 11.Gilstrap, L. G. et al. Initiation, continuation, or withdrawal of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and outcomes in patients hospitalized with heart failure with reduced ejection fraction. J. Am. Heart Assoc.6, (2017). [DOI] [PMC free article] [PubMed]
- 12.Sze S, Pellicori P, Zhang J, Clark AL. Malnutrition, congestion and mortality in ambulatory patients with heart failure. Heart. 2019;105:297–306. doi: 10.1136/heartjnl-2018-313312. [DOI] [PubMed] [Google Scholar]
- 13.Krishnaswamy K. Drug metabolism and pharmacokinetics in malnourished children. Clin. Pharmacokinet. 1989;17(Suppl 1):68–88. doi: 10.2165/00003088-198900171-00006. [DOI] [PubMed] [Google Scholar]
- 14.Niederberger E, Parnham MJ. The impact of diet and exercise on drug responses. Int. J. Mol. Sci. 2021;22:7692. doi: 10.3390/ijms22147692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karabulut S, et al. Does nutritional status affect treatment tolarability, response and survival in metastatic colorectal cancer patients? Results of a prospective multicenter study. J. Oncol. Pharm. Pract. 2020 doi: 10.1177/1078155220959427. [DOI] [PubMed] [Google Scholar]
- 16.Santarpia L, Contaldo F, Pasanisi F. Nutritional screening and early treatment of malnutrition in cancer patients. J. Cachexia. Sarcopenia Muscle. 2011;2:27–35. doi: 10.1007/s13539-011-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dewan P, et al. The prevalence and importance of frailty in heart failure with reduced ejection fraction—an analysis of paradigm-HF and atmosphere. Eur. J. Heart Fail. 2020;22:2123–2133. doi: 10.1002/ejhf.1832. [DOI] [PubMed] [Google Scholar]
- 18.Hempenius L, et al. Inclusion of frail elderly patients in clinical trials: Solutions to the problems. J. Geriatr. Oncol. 2013;4:26–31. doi: 10.1016/j.jgo.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Greene SJ, et al. Medical therapy for heart failure with reduced ejection fraction: The CHAMP-HF registry. J. Am. Coll. Cardiol. 2018;72:351–366. doi: 10.1016/j.jacc.2018.04.070. [DOI] [PubMed] [Google Scholar]
- 20.Takei M, et al. Effect of estimated plasma volume reduction on renal function for acute heart failure differs between patients with preserved and reduced ejection fraction. Circ. Hear. Fail. 2015;8:527–532. doi: 10.1161/CIRCHEARTFAILURE.114.001734. [DOI] [PubMed] [Google Scholar]
- 21.Shiraishi Y, et al. Validation of the Get With The Guideline-Heart Failure risk score in Japanese patients and the potential improvement of its discrimination ability by the inclusion of B-type natriuretic peptide level. Am. Heart J. 2016;171:33–39. doi: 10.1016/j.ahj.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 22.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The Framingham study. N. Engl. J. Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 23.Bozkurt B, et al. Universal definition and classification of heart failure: A report of the heart failure society of america, heart failure association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition. J. Card. Fail. 2021;27:387–413. doi: 10.1016/j.cardfail.2021.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Sze S, et al. Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin. Res. Cardiol. 2017;106:533–541. doi: 10.1007/s00392-017-1082-5. [DOI] [PubMed] [Google Scholar]
- 25.Kaneko H, et al. Geriatric nutritional risk index in hospitalized heart failure patients. Int. J. Cardiol. 2015;181:213–215. doi: 10.1016/j.ijcard.2014.11.167. [DOI] [PubMed] [Google Scholar]
- 26.Bouillanne O, et al. Geriatric nutritional risk index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005;82:777–783. doi: 10.1093/ajcn/82.4.777. [DOI] [PubMed] [Google Scholar]
- 27.Matsuo S, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 28.Lenzen MJ, et al. Under-utilization of evidence-based drug treatment in patients with heart failure is only partially explained by dissimilarity to patients enrolled in landmark trials: A report from the Euro Heart Survey on Heart Failure. Eur. Heart J. 2005;26:2706–2713. doi: 10.1093/eurheartj/ehi499. [DOI] [PubMed] [Google Scholar]
- 29.Hamaguchi S, et al. Spironolactone use at discharge was associated with improved survival in hospitalized patients with systolic heart failure. Am. Heart J. 2010;160:1156–1162. doi: 10.1016/j.ahj.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 30.Minamisawa M, et al. Impact of malnutrition using geriatric nutritional risk index in heart failure with preserved ejection fraction. JACC. Heart Fail. 2019;7:664–675. doi: 10.1016/j.jchf.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Wright RM, et al. Underuse of indicated medications among physically frail older US veterans at the time of hospital discharge: Results of a cross-sectional analysis of data from the geriatric evaluation and management drug study. Am. J. Geriatr. Pharmacother. 2009;7:271–280. doi: 10.1016/j.amjopharm.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akita K, et al. Current use of guideline-based medical therapy in elderly patients admitted with acute heart failure with reduced ejection fraction and its impact on event-free survival. Int. J. Cardiol. 2017;235:162–168. doi: 10.1016/j.ijcard.2017.02.070. [DOI] [PubMed] [Google Scholar]
- 33.Higuchi S, et al. Association of renin-angiotensin system inhibitors with long-term outcomes in patients with systolic heart failure and moderate-to-severe kidney function impairment. Eur. J. Intern. Med. 2019;62:58–66. doi: 10.1016/j.ejim.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez AF, et al. Associations between aldosterone antagonist therapy and risks of mortality and readmission among patients with heart failure and reduced ejection fraction. JAMA. 2012;308:2097–2107. doi: 10.1001/jama.2012.14795. [DOI] [PubMed] [Google Scholar]
- 35.Bayoumi E, et al. Spironolactone and outcomes in older patients with heart failure and reduced ejection fraction. Am. J. Med. 2019;132:71–80.e1. doi: 10.1016/j.amjmed.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira JP, et al. MRAs in elderly HF patients: Individual patient-data meta-analysis of RALES, EMPAHSIS-HF, and TOPCAT. JACC Hear. Fail. 2019;7:1012–1021. doi: 10.1016/j.jchf.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 37.McDonagh TA, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 38.Vaduganathan M, et al. Treatment persistence of renin-angiotensin-aldosterone-system inhibitors over time in heart failure with reduced ejection fraction. J. Card. Fail. 2021;1:1–10. doi: 10.1016/j.cardfail.2021.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.