Abstract
Individual cyanobacterial cells are normally identified in environmental samples only on the basis of their pigmentation and morphology. However, these criteria are often insufficient for the differentiation of species. Here, a whole-cell hybridization technique is presented that uses horseradish peroxidase (HRP)-labeled, rRNA-targeted oligonucleotides for in situ identification of cyanobacteria. This indirect method, in which the probe-conferred enzyme has to be visualized in an additional step, was necessary since fluorescently monolabeled oligonucleotides were insufficient to overstain the autofluorescence of the target cells. Initially, a nonfluorescent detection assay was developed and successfully applied to cyanobacterial mats. Later, it was demonstrated that tyramide signal amplification (TSA) resulted in fluorescent signals far above the level of autofluorescence. Furthermore, TSA-based detection of HRP was more sensitive than that based on nonfluorescent substrates. Critical points of the assay, such as cell fixation and permeabilization, specificity, and sensitivity, were systematically investigated by using four oligonucleotides newly designed to target groups of cyanobacteria.
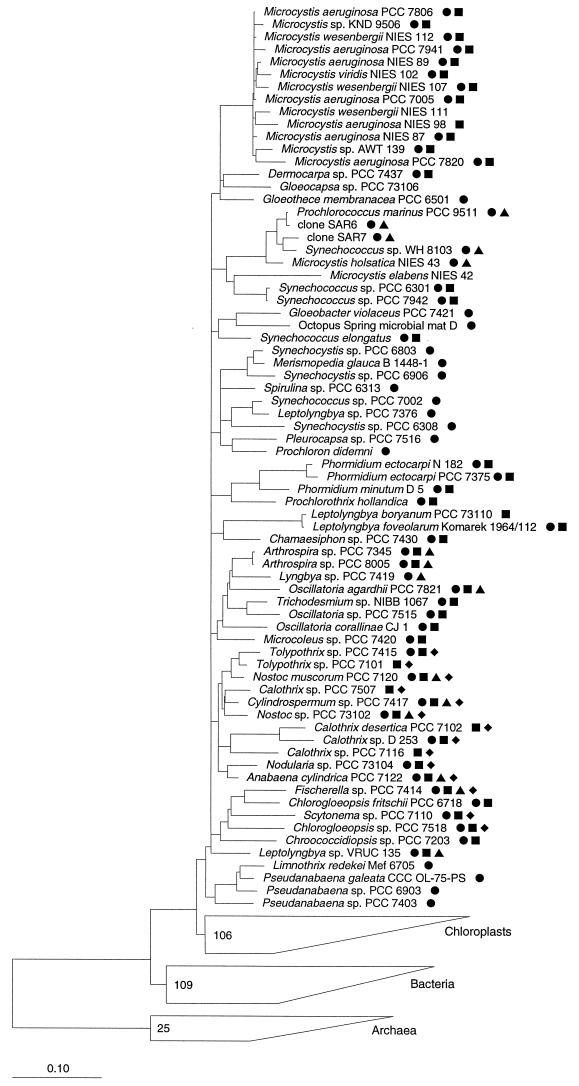
Cyanobacteria are a morphologically diverse group of photoautotrophic bacteria whose classification, in accordance with the botanical (4) and bacterial (9, 10, 11, 12, 30) codes, is almost entirely based on phenotypic traits. Molecular data, most importantly those arising from the comparative analysis of 16S rRNA sequences, show that this has resulted in an artificial classification not reflecting the phylogeny of many cyanobacteria (17, 25, 26, 29). The current phylogenetic tree of cyanobacteria (Fig. 1) demonstrates that cyanobacteria lacking conspicuous morphological details, such as those currently classified as Synechococcus, Synechocystis, and Leptolyngbya spp., are polyphyletic. The availability of nucleic acid sequence data from cyanobacteria is today forming the basis for new identification techniques such as restriction fragment length polymorphism (15, 19), PCR (24, 28, 32), or denaturing gradient gel electrophoresis (14, 28). All of these require extracted nucleic acids and do not allow identification of individual cells. One particular technique that has found many applications in molecular ecology is the identification of whole fixed cells by in situ hybridization with rRNA-targeted oligonucleotide probes (for a review see reference 2). The fluorescent labels that are used in the standard protocol of this technique can hardly be used for cyanobacteria due to the strong autofluorescence of the cells. Attempts to reduce the background by extraction of photosynthetic pigments were largely unsuccessful (21a; unpublished results). Consequently, we initially intended to develop a nonfluorescent assay based on directly horseradish peroxidase (HRP)-labeled oligonucleotides (3) for identification of individual cells of cyanobacteria. After hybridization, the enzyme marker can be detected inside cells by its ability to precipitate a colored, nonfluorescent substrate such as diaminobenzidine (DAB) by oxidative polymerization. In the course of the study, a fluorescent detection system based on enzymatic signal amplification became available (34, 39) and was also evaluated. The so-called tyramide signal amplification (TSA) system is supposed to be based on the covalent binding of radicalized fluorochrome-tyramide substrate molecules to electron-rich moieties, such as tyrosines or tryptophans (6), in the HRP-containing cells and results in a very bright fluorescent staining that could potentially overcome the intrinsic autofluorescence of the cyanobacteria.
FIG. 1.
Phylogenetic tree of cyanobacteria and probe specificities. Sequences with complementary target sites are identified by the symbols ● (CYA361), ■ (CYA762), ▴ (CYA664), and ⧫ (CIV/V1342). References for sequences not included in the public ARB datafile are as follows: Calothrix (Rivularia) sp. strain PCC7116, Calothrix sp. strain PCC7507, Prochlorococcus marinus PCC9511, Tolypothrix sp. strain PCC7101, and Tolypothrix sp. strain PCC7415 (all according to reference 12a), Limnothrix redekei MEF6705 (41), Pseudanabaena sp. strain PCC7403 (17a), Microcystis wesenbergii NIES111 (EBI accession no. D89034), M. aeruginosa NIES98 (accession no. D89032), M. aeruginosa NIES87 (accession no. D89031), Synechococcus sp. strain PCC7942 (accession no. D88288), M. holsatica NIES43 (accession no. D89036), Synechococcus elongatus (accession no. D83715), Merismopedia glauca (accession no. X94705), Synechocystis sp. strain PCC6803 (accession no. D90916), Synechococcus sp. strain PCC7002 (accession no. D88289), and Calothrix sp. strain D253 (accession no. X99213). Strain names refer to the database entries, in most cases regardless of taxonomic validity. The bar indicates 10% estimated sequence divergence.
MATERIALS AND METHODS
Tree reconstruction.
The phylogenetic tree in Fig. 1 is a consensus tree combining the results of three different tree construction methods, namely, the distance matrix, maximum-parsimony, and maximum-likelihood methods done with the ARB program (37). In cases where the branching order was not supported by all three methods, multifurcations were drawn (18). To the consensus tree reconstructed with almost full-length sequences, the partial sequences (with less than 1,400 bases) were added by the maximum-parsimony approach. For analyses of the sequences of cyanobacterial origin, a 50% conservation filter was used as described by Ludwig et al. (18).
Probes.
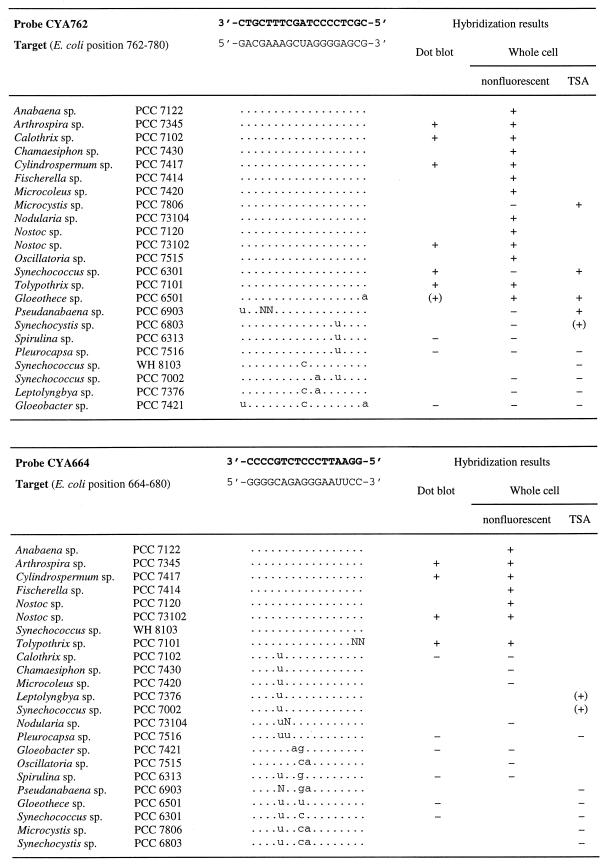
ARB (37) tools were used for probe design and probe matching. Probe sequences are given in Fig. 2. Oligonucleotides and probes labeled with HRP as described earlier (3, 38) were obtained from Interactiva (Ulm, Germany).
FIG. 2.
Difference alignments of the PCC reference strains, probe and target sequences, probe positions, and results of the different hybridization methods. Probe positions correspond to the E. coli 16S rRNA numbering of Brosius et al. (8). Dots in the difference alignments indicate bases that are identical to those in the target sequences. N stands for an unknown base in the respective sequence. Hybridization results are shown as follows: +, positive; −, negative; (+), positive signal but weaker than that obtained by hybridization of reference probe EUB338 with the same method.
Strains and cultivation.
The reference strains listed in Fig. 2 were from the Pasteur Culture Collection of Cyanobacteria (Institut Pasteur, Paris, France) and grown as described in the catalogue of strains (31).
16S rRNA gene (rDNA) amplification.
Reference DNA for evaluation of probe specificity by dot blot hybridizations was obtained by PCR amplification. Cells were collected from 7.5-ml samples of liquid cultures by centrifugation (4,500 × g, 10 min), washed with Tris-EDTA buffer (1 M Tris-HCl, 0.1 M EDTA, pH 8.0), centrifuged again, and finally resuspended in 100 μl of sterile, double-distilled H2O. The suspension was successively frozen in liquid nitrogen and thawed in a water bath at 40°C five times for 1 min each time. After centrifugation, 1 μl of the supernatant was used for PCR with primers GM3F and GM4R as described by Muyzer et al. (23). Aliquots (1 to 3 μl) of the PCR products were denatured by incubation with 200 μl of 0.4 M NaOH for 20 min at room temperature and blotted onto Hybond N+ membranes (Amersham, Braunschweig, Germany). Nucleic acids were immobilized by UV cross-linking for 2 min.
Cell fixation.
For whole-cell hybridization, 1-ml samples of liquid cultures were fixed by adding the same amount of absolute ethanol. Alternatively, cells were centrifuged (5,000 × g, 5 min), washed once in 1×PBS (130 mM sodium chloride, 10 mM sodium phosphate buffer, pH 8.4), centrifuged again, resuspended in an appropriate volume (50 to 200 μl) of 1×PBS, and fixed by addition of the same volume of absolute ethanol. Cells were stored at −20°C for up to several months. For fluorescence detection, cells were also fixed with paraformaldehyde as described by Manz et al. (21). For hybridization, the fixed cells were spotted onto microscope slides and immobilized by air drying. The slides were then subjected to an ethanol series (50, 80, and 96%, 3 min each) and air dried again. If necessary, cells were treated with lysozyme, followed by a second ethanol series. Different lysozyme concentrations and incubation times were tested. The best results were obtained by covering the immobilized cells with a drop of lysozyme solution (5 mg of lysozyme [109,000 U/mg; Fluka, Buchs, Switzerland] per ml in a buffer containing 0.1 M Tris-HCl and 0.05 M EDTA [pH 8.0]) and incubating them for 30 min at 37°C in a humid chamber.
Microbial mats were sampled in the outlet area of sulfurous sublacustrine springs in Lago di Cadagno (Switzerland) in August 1995, fixed immediately with 70% ethanol, and stored at −20°C until further use. Cautious rehydration and subsequent infiltration of the samples with embedding medium (Einbettmedium; Microm Laborgeräte GmbH, Walldorf, Germany) for several hours were necessary prior to cryosectioning of the samples. Sections about 10 μm thick were prepared on a cryomicrotome (HM 500 OM; Carl Zeiss, Jena, Germany) at a temperature of −20°C and placed on coated slides. The slides could be stored for up to several months at room temperature. Before hybridization, the embedding medium was removed by spotting H2O onto the sections, incubating them for a few min, draining off the water, and air drying them.
Hybridization.
For dot blot hybridization with oligonucleotide probes, the membrane filters were prehybridized for at least 1 h with 10 ml of a solution containing 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 4% blocking reagent (Boehringer Mannheim, Mannheim, Germany), 0.2% N-lauroylsarcosine, and 0.04% sodium dodecyl sulfate. Hybridization was then performed with 100 ng of an HRP-labeled probe in 2 ml of hybridization buffer (3) containing different concentrations of formamide (30, 40, 50, 55, 60, and 65%) for 30 min. Subsequently, the filters were washed twice for 15 min with 20 ml of washing buffer without EDTA (3) and containing NaCl at molarities equivalent to the stringency of the formamide concentrations used during hybridization (0.074, 0.037, 0.019, and 0.013 M NaCl and no NaCl for the latter two, respectively) and finally rinsed in 1×PBS. Prehybridization, hybridization, and washing steps were done at 35°C. Specifically bound nucleic acid probes were visualized via HRP-induced chemiluminescence (ECL; Amersham, Braunschweig, Germany). Emitted light was recorded by exposure of membranes to X-ray films (Hyperfilm; Amersham).
Whole-cell hybridization with HRP-labeled probes was done as described by Amann et al. (3). Formamide concentrations in the hybridization buffer for the respective probes were as follows: EUB338, 30 to 65%, CYA361 and CIV/V1342, 50%, CYA664, 55%, CYA762, 65%. The corresponding concentrations of NaCl in the washing buffer were as mentioned above.
For nonfluorescent detection of hybridization to whole cells with HRP-labeled probes, the slides were briefly rinsed with H2O and air dried after the stringent washing step. Fresh substrate for the enzyme was prepared by mixing 1 ml of incubation buffer (0.1 M Tris-HCl, 0.1 M NaCl, 0.05 M MgCl2, pH 8.0); 5 μl of BM TETON POD substrate, precipitating [4-(1,4,7,10-tetra-oxadecyl)-1-naphthol; Boehringer Mannheim]; and 0.6 μl of H2O2 (30% [vol/vol]; Merck, Darmstadt, Germany). After this solution was spotted onto the hybridized cells, the slides were incubated for 5 to 10 min at room temperature. The substrate solution was rinsed off with distilled H2O, and the slides were air dried. Alternatively, DAB substrate (metal enhanced, precipitating; Boehringer Mannheim) was used as the substrate as recommended by the supplier. For specific detection of two mixed cultures, hybridizations with appropriate probes were performed successively by using DAB as the primary substrate and TETON for detection of the second hybridization, since only the former is stable during hybridization. Glycerol was used as the mounting medium for microscopy with a Zeiss Axiophot2 (Carl Zeiss). Phase-contrast and bright-field photomicrographs were done on Kodak Ektachrome 100S film. For TSA-based detection (modified from reference 34), slides were briefly rinsed with H2O after the washing step and equilibrated in TNT buffer (0.1 M Tris-HCl [pH 7.5], 0.15 M NaCl, 0.05% Tween 20) for 15 min at room temperature. The fluorochrome-tyramide substrate solution was modified by addition of dextran sulfate (39) to reduce unspecific staining of nontarget cells during long-term (30 to 45 min) incubation. Van Gijlswijk et al. (39) assumed that this improved localization might be due in part to the higher viscosity of the substrate solution. The amplification diluent (part of the kit with the fluorochrome-tyramides) was mixed 1:1 with a 40% (wt/vol) solution of dextran sulfate (39) in H2O and 1 μl of fluorescein-tyramide or tetramethylrhodamine-tyramide (both from NEN Life Science Products, Boston, Mass.; each is sold in a kit), respectively, was added per 50 μl. For one slide, 100 μl of this solution was used to cover the wells after draining off of the TNT buffer. After a 30- to 45-min incubation at room temperature in the dark, the slides were rinsed in TNT buffer and washed for 15 min in the same buffer at 55°C. Again, double staining was possible by two successive hybridizations, using one substrate for the first detection and the other for the second. The same microscope mentioned above equipped for epifluorescence with a high-pressure mercury bulb (100 W) and Zeiss filter sets 09 and 15 was used. Epifluorescence micrographs and the corresponding phase-contrast images were captured with Kodak Panther 1600 film. For confocal microscopy, a Zeiss LSM 510 (Carl Zeiss) was used.
RESULTS AND DISCUSSION
Probe design.
For the development of an in situ identification method for cyanobacteria, the design of new probes was required. To evaluate the general applicability of the approach, it was important that the probes target the majority of cyanobacteria. Consequently, four 16S rRNA probes were designed and used together with EUB338 (1), a probe complementary to the 16S rRNA of most bacteria. Two of the probes, CYA361 and CYA762, target signature regions on the 16S rRNA that are shared by most of the sequenced cyanobacteria regardless of their phylogenetic clustering within this phylum (Fig. 1). Probe CYA664 was selected since it is targeted to a region within the 16S rRNA shown to be accessible in situ in other bacteria (2). It has a more restricted specificity but also binds to organisms from several cyanobacterial clusters. Additionally, these three probes are also fully complementary to the respective regions of the 16S rRNAs of several chloroplasts. These probes were not intended to identify phylogenetic groups within the cyanobacteria but were primarily tools for the establishment of a single-cell identification assay. They were used for the evaluation of hybridization conditions and specificity of hybridization to cyanobacteria with known differences in the numbers and distributions of mismatches in the target sites. In contrast, the fourth probe, CIV/V1342, was designed to be specific for the heterocystous strains of cyanobacteria (sections IV and V according to Rippka et al. [30]). The organisms having 16S rRNA sequences fully complementary to the respective probes are indicated by the symbols in Fig. 1.
Dot blot hybridizations.
Probes were first hybridized to membrane-bound nucleic acids of a set of reference organisms to determine binding characteristics without the influence of cell envelopes and possible secondary structures of the ribosomes. At low stringency, probes bound not only to almost full-length amplified 16S rDNA fragments containing fully complementary target sites but also to 16S rDNA fragments with few mismatches. An increase of stringency obtained by addition of formamide resulted in the expected discrimination in all cases but one. This was reached for probes CIV/V1342, CYA361, CYA664, and CYA762, at 50, 50, 55, and 65% formamide, respectively. In only one case, that of Gloeothece sp. strain PCC6501, could a single terminal mismatch not be fully discriminated. The signal was, however, already significantly reduced (Fig. 2). Unspecific binding was not observed for any of the probes, and for all of them, specific hybridization signals were fully abolished by a further increase in stringency. The optimal stringencies were subsequently used for whole-cell hybridization.
Permeabilization of whole fixed cyanobacteria for HRP-labeled probes.
Even though cyanobacteria are referred to as gram-negative bacteria, their peptidoglycan layer is, in most cases, thicker than in other gram-negative bacteria (35). HRP is a relatively large marker molecule which may not enter bacterial cells unless they have been well permeabilized (3, 34). Consequently, difficulties were expected with the free diffusion of the probe to the intracellular target molecules. To evaluate penetration independent of potential inaccessibility of probe target sites, cells were initially hybridized with probe EUB338, which is complementary to a signature found in most 16S rRNA molecules of bacteria. Following fixation with ethanol, most of the cyanobacterial strains examined could be detected with HRP-labeled probe EUB338 in combination with the substrate TETON. However, the staining was quite uneven, and only 40 to 95% of all of the cells of a pure culture or even those within a single filament could be detected. The detection rate for a particular strain was reproducible when hybridizations were either repeated with the same probe or done with the other probes used in this study. Pretreatment with lysozyme increased the proportion of stained cells, but not to 100%. An increase in substrate incubation time did not result in better staining. The two nonfluorescent substrates tested, TETON and DAB, had a maximum effect after an incubation time of 5 to 10 min. Some strains could not be detected with this method at all: Leptolyngbya sp. strain PCC7376, Microcystis sp. strain PCC7806, Pleurocapsa sp. strain PCC7516, Pseudanabaena sp. strain PCC6903, Synechococcus sp. strain PCC6301, Synechococcus sp. strain PCC7002, and Synechocystis sp. strain PCC6803. Other treatments intended to improve the permeability of the cell envelope, such as incubation with proteinase K (1 mg/ml in H2O; 10 min at 0°C; Boehringer Mannheim) and treatment with sodium dodecyl sulfate-dithiothreitol (27) or peracetic acid-hydrogen peroxide (13), resulted in cell lysis that was difficult to control or showed no effect at all. At this stage of our experiments, it remained open whether the failure of detection was caused by lack of assay sensitivity or permeabilization for the probe.
Whole-cell hybridization in combination with TSA.
Consequently, the highly sensitive TSA system was applied for the detection of HRP-labeled probes (34). Indeed, those strains with fully complementary target sites that were not detected with TETON under the same conditions of fixation and treatment were readily detected with TSA, proving their permeability for HRP-labeled probes (Fig. 2). Whereas for TSA, every conversion of a substrate molecule should result in covalent binding of a fluorochrome to the cell, for the nonfluorescent substrate, oxidized molecules have to react with each other to produce a polymer that precipitates. With a relatively low HRP concentration, diffusion is faster than formation of oxidation products and the probability of polymerization is therefore low. As for TETON, some of the pure cultures were unevenly stained with TSA.
The probe-conferred fluorescent signal achieved with TSA was sufficiently strong to fully overstain the autofluorescence (Fig. 3). As expected, fluorescein-tyramide is superior to tetramethylrhodamine-tyramide, since upon blue excitation its green fluorescence can be clearly distinguished from the yellowish red autofluorescence of the cyanobacterial pigments. However, the strong red signal of tetramethylrhodamine-tyramide with green excitation is still sufficient to be distinguished from the equally red but weaker autofluorescence. It might be possible to reduce autofluorescence by decolorizing the cells prior to hybridization by the protocol of Scanlan et al. (33) for marine phycoerythrin-containing cyanobacteria.
FIG. 3.
Micrographs of artificial mixtures of cyanobacteria and of a microbial mat after hybridization. (A) Artificial mixture of Nodularia sp. strain PCC73104 and Spirulina sp. strain PCC6313 hybridized with probe CIV/V1342, which was detected with fluorescein-tyramide. Shown are a phase-contrast image (left) and an epifluorescence image obtained with filter set 09 (right). Note that the heterocysts of Nodularia sp. were not stained, likely due to lack of probe permeability. (B) Artificial mixture of two Synechococcus sp. strains, WH8103 and PCC6301, hybridized successively with probes CYA664 and CYA762. Fluorescein-tyramide was used to detect the former probe, and tetramethylrhodamine was used to detect the latter probe. Shown are a phase-contrast image (left) and a double-exposure epifluorescence image obtained with filter sets 09 and 15, yielding green and red epifluorescence, respectively (right). Both mixtures were recorded at an original magnification of ×1,000. The bars represent 10 μm. (C) Left to right, autofluorescence of a section of a microbial mat as seen with filter set 09 and bright-field micrographs, respectively, the latter two obtained after hybridization with probes CYA762 and CYA664 and detection with TETON. The original magnification was ×100. (D) Part of the same mat after hybridization with probe CYA762 and detection with fluorescein-tyramide was recorded by confocal laser scanning microscopy and is shown as an all-in-focus image of selected optical sections. Both channels, the green for the fluorescein signal and the red for autofluorescence, were detected simultaneously. Yellow color results from the overlay of autofluorescence and signals within the same cells. The original magnification was ×630.
Evaluation of probe specificities.
Evaluation of probe specificities was started with the TETON method and later continued with TSA. In difficult cases, e.g., when not all of the cells were stained or when the staining was weak, the hybridization signal obtained on a reference strain with one of the four new cyanobacterial probes was always compared to the positive control, EUB338. This comparison was not always necessary, since in most cases the results were clear-cut and corresponded well to the predicted specificity (Fig. 2). The differentiation of Nodularia sp. strain PCC73104 and Spirulina sp. strain PCC6313 by probe CIV/V1342 using the TSA method is shown in Fig. 3A. Due to the distinct morphology of these two cyanobacterial species, the specificity of this hybridization, based on a single central mismatch in Spirulina sp., can be easily seen in the photomicrographs. The specificity of two other probes is demonstrated in Fig. 3B. An artificial mixture of two phylogenetically distant Synechococcus sp. strains, namely, WH8103 and PCC6301, with 16S rRNA target sequences that were each fully complementary to only one of the probes, was hybridized successively with CYA664 and CYA762. The HRP conferred by the former probe during the first hybridization was detected with fluorescein-tyramide. The HRP conferred by the latter probe to the larger rod-like cells of strain PCC6301 was detected with tetramethylrhodamine-tyramide after the second hybridization, resulting in clear green-red discrimination of the two strains.
Successful in situ hybridization with probe CYA361 could be obtained neither in the nonfluorescent nor in the fluorescent protocol. It is likely that this probe binding site (positions 361 to 378; Escherichia coli 16S rRNA numbering according to Brosius et al. [8]) is inaccessible in whole fixed cells of cyanobacteria due to higher-order structures of the ribosome such as RNA-protein or RNA-RNA interactions (2). CYA361 indeed bound readily to the denatured amplified 16S rDNAs of a selection of target strains during dot blot hybridization (Fig. 2). The accessibility of the other three target sites was as good as (CYA664 and CIV/V1342) or better than (CYA762) that of the positive control probe, EUB338.
Due to the increased sensitivity of TSA, weak but detectable signals were obtained on whole fixed cells of some of the reference strains with a single mismatch in the target site (Fig. 2), in contrast to detection with the nonfluorescent method using the substrate TETON. However, the signal was always much weaker than that obtained on the same cell preparation with a fully complementary probe. Consequently, those who wish to apply the TSA approach should always be aware that even at the highest possible stringency, and of course even more at lower stringency, the discrimination of one or a few mismatches might be difficult.
Environmental application.
Sections from a microbial mat of a high alpine lake in Switzerland were hybridized with probes EUB338, CYA762, and CYA664 to demonstrate the applicability of single-cell identification of cyanobacteria with HRP-labeled oligonucleotide probes. According to the theoretical specificities of the probes, the numbers of stained cells should decrease from the more general cyanobacterial probe CYA762 to the more specific probe CYA664. The expected effect can be seen in Fig. 3C. The mat contained numerous cyanobacteria, as evident from the strong autofluorescence, and probe CYA762 with nonfluorescent detection stained not only more cells than probe CYA664 but also a greater diversity of morphotypes. The nonfluorescent approach was appropriate for localization of low-density cell populations in thin sections (10 μm). In dense microbial communities or if high-resolution three-dimensional analysis of cell layers is the focus of the study, the fluorescent approach together with confocal laser scanning microscopy is the better choice. A confocal laser scanning image of a detail of the upper part of the same microbial mat is shown after hybridization with probe CYA762 and incubation with fluorescein-tyramide (Fig. 3D). Interestingly, the cyanobacteria close to the mat surface showed stronger signals. Since the total RNA content (20), as well as the rRNA content (5), increases with the growth rate and the cells that are at the surface and therefore closer to the light will be growing more rapidly than those in the deeper regions of the mat, this phenomenon is most likely due to a decrease in ribosome content with increased depth rather than reduced permeability of the cells for the probes.
Perspectives.
We have described here a method for the in situ identification of cyanobacterial cells based on 16S rRNA. It may be directly applied to environmental samples to quantitatively analyze the distribution of defined cyanobacterial populations on the single-cell level. We recognize that autofluorescence may vary considerably with environmental conditions and that, if it is excessive, it might mask the tetramethylrhodamine-tyramide signal. In this case, fluorescein-tyramide will become the method of choice in the field, since we have demonstrated that this compound completely overcomes the background noise. A second potential problem in application to environmental samples is that the low rRNA content of slow-growing cells may hinder their detection. However, the methods developed were sufficiently sensitive to detect cyanobacterial cells in the deeper layers of mats, where growth is expected to be limited, and they should therefore be generally applicable. This genotypic method will certainly complement the traditional method based on phenotypic traits such as morphology and promises to make in situ identification of cyanobacteria more reliable. Even though examples exist in which differing phenotypic traits, such as strong and weak autofluorescence, have allowed the differentiation of distinct cyanobacterial populations (22), Stockner (36) and, later on, Weisse (40) have stated in their reviews on autotrophic picoplankton in freshwater and marine systems that phenotypic traits are not always sufficient for discrimination. This in situ hybridization method is also ideally suited to link those cyanobacterial sequences so far retrieved only from environmental 16S rRNA gene libraries (7, 16) to defined populations with known morphology and ecological distribution. Furthermore, for unicellular cyanobacteria, the fluorescence detection protocol may be combined with flow cytometry, thereby allowing rapid analyses of samples by cell sorting, autofluorescence, and probe-specific identification.
ACKNOWLEDGMENTS
We thank Ramona Appel for her excellent technical assistance; Thérèse Coursin, Annick Wilmotte, and Filip Haes for providing unpublished sequences; Nyree West for the fruitful discussion on signal amplification; and David Scanlan for providing Synechococcus sp. strain WH8103.
This work was supported by EC grant BIO4-CT96-0256 and in part by the Institut Pasteur and CNRS (URA 1129).
REFERENCES
- 1.Amann R I, Binder B J, Olson R J, Chisholm S W, Devereux R, Stahl D A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann R I, Ludwig W, Schleifer K-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann R I, Zarda B, Stahl D A, Schleifer K-H. Identification of individual prokaryotic cells with enzyme-labeled, rRNA-targeted oligonucleotide probes. Appl Environ Microbiol. 1992;58:3007–3011. doi: 10.1128/aem.58.9.3007-3011.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anagnostidis K, Komárek J. Modern approach to the classification system of cyanophytes. 1. Introduction. Arch Hydrobiol Suppl. 1985;71:291–302. [Google Scholar]
- 5.Binder B J, Liu Y C. Growth rate regulation of rRNA content of a marine Synechococcus (cyanobacterium) strain. Appl Environ Microbiol. 1998;64:3346–3351. doi: 10.1128/aem.64.9.3346-3351.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobrow M N, Harris T D, Shaughnessy K J, Litt G J. Catalyzed reporter deposition, a novel method of signal amplification. Application to immunoassays. J Immunol Methods. 1989;125:279–285. doi: 10.1016/0022-1759(89)90104-x. [DOI] [PubMed] [Google Scholar]
- 7.Britschgi T B, Giovannoni S J. Phylogenetic analysis of a natural marine bacterioplankton population by rRNA gene cloning and sequencing. Appl Environ Microbiol. 1991;57:1707–1713. doi: 10.1128/aem.57.6.1707-1713.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brosius J, Dull T, Sleeter D D, Noller H F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981;148:107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- 9.Castenholz R W. Subsection III, order Oscillatoriales. In: Staley J T, Bryant M P, Pfennig N, Holt J G, editors. Bergey’s manual of systematic bacteriology. Baltimore, Md: The Williams & Wilkins Co.; 1989. pp. 1771–1780. [Google Scholar]
- 10.Castenholz R W. Subsection IV, order Nostocales. In: Staley J T, Bryant M P, Pfennig N, Holt J G, editors. Bergey’s manual of systematic bacteriology. Baltimore, Md: The Williams & Wilkins Co.; 1989. pp. 1780–1793. [Google Scholar]
- 11.Castenholz R W. Subsection V, order Stigonematales. In: Staley J T, Bryant M P, Pfennig N, Holt J G, editors. Bergey’s manual of systematic bacteriology. Baltimore, Md: The Williams & Wilkins Co.; 1989. pp. 1794–1799. [Google Scholar]
- 12.Castenholz R W, Waterbury J B. Oxygenic photosynthetic bacteria, group I. Cyanobacteria. In: Staley J T, Bryant M P, Pfennig N, Holt J G, editors. Bergey’s manual of systematic bacteriology. Baltimore, Md: The Williams & Wilkins Co.; 1989. pp. 1710–1728. [Google Scholar]
- 12a.Coursin, T. Personal communication.
- 13.El-Gammal S M A, Sadek M A. Enzymatic saccharification of some pretreated agricultural wastes. Zentralbl Mikrobiol. 1988;143:55–62. [Google Scholar]
- 14.Ferris M J, Muyzer G, Ward D M. Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring microbial mat community. Appl Environ Microbiol. 1996;62:340–346. doi: 10.1128/aem.62.2.340-346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gebhardt J S, Nierzwicki-Bauer S A. Identification of a common cyanobacterial symbiont associated with Azolla spp. through molecular and morphological characterization of free-living and symbiotic cyanobacteria. Appl Environ Microbiol. 1991;57:2141–2146. doi: 10.1128/aem.57.8.2141-2146.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giovannoni S J, Britschgi T B, Moyer C L, Field K G. Genetic diversity in Sargasso Sea bacterioplankton. Nature. 1990;345:60–63. doi: 10.1038/345060a0. [DOI] [PubMed] [Google Scholar]
- 17.Giovannoni S J, Turner S, Olsen G J, Barns S, Lane D J, Pace N R. Evolutionary relationships among cyanobacteria and green chloroplasts. J Bacteriol. 1988;170:3584–3592. doi: 10.1128/jb.170.8.3584-3592.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Haes, F. Personal communication.
- 18.Ludwig W, Strunk O, Klugbauer S, Klugbauer N, Weizenegger M, Neumaier J, Bachleitner M, Schleifer K H. Bacterial phylogeny based on comparative sequence analysis. Electrophoresis. 1998;19:554–568. doi: 10.1002/elps.1150190416. [DOI] [PubMed] [Google Scholar]
- 19.Lyra C, Hantula J, Vainio E, Rapala J, Rouhianinen L, Sivonen K. Characterization of cyanobacteria by SDS-PAGE of whole-cell proteins and PCR/RFLP of the 16S rRNA gene. Arch Microbiol. 1997;168:176–184. doi: 10.1007/s002030050485. [DOI] [PubMed] [Google Scholar]
- 20.Mann N, Carr N G. Control of macromolecular composition and cell division in the blue-green alga Anacystis nidulans. J Gen Microbiol. 1974;83:399–405. doi: 10.1099/00221287-83-2-399. [DOI] [PubMed] [Google Scholar]
- 21.Manz W, Amann R, Ludwig W, Wagner M, Schleifer K-H. Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria: problems and solutions. Syst Appl Microbiol. 1992;15:593–600. [Google Scholar]
- 21a.Minz, D. Personal communication.
- 22.Moore L R, Rocap G, Chisholm S W. Physiology and molecular phylogeny of coexisting Prochlorococcus ecotypes. Nature. 1998;393:464–467. doi: 10.1038/30965. [DOI] [PubMed] [Google Scholar]
- 23.Muyzer M, Teske A, Wirsen C O, Jannasch H W. Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch Microbiol. 1995;164:165–172. doi: 10.1007/BF02529967. [DOI] [PubMed] [Google Scholar]
- 24.Neilan B A. Identification and phylogenetic analysis of toxigenic cyanobacteria by multiplex randomly amplified polymorphic DNA PCR. Appl Environ Microbiol. 1995;61:2286–2291. doi: 10.1128/aem.61.6.2286-2291.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neilan B A, Jacobs D, del Dot T, Blackall L L, Hawkins P R, Cox P T, Goodman A E. rRNA sequences and evolutionary relationships among toxic and nontoxic cyanobacteria of the genus Microcystis. Int J Syst Bacteriol. 1997;47:693–697. doi: 10.1099/00207713-47-3-693. [DOI] [PubMed] [Google Scholar]
- 26.Nelissen B, De Baere R, Wilmotte A, De Wachter R. Phylogenetic relationships of nonaxenic filamentous cyanobacterial strains based on 16s rRNA sequence analysis. J Mol Evol. 1996;42:194–200. doi: 10.1007/BF02198845. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson W L, Setlow P. Sporulation, germination and outgrowth. In: Harwood C W, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, England: Wiley; 1990. pp. 391–450. [Google Scholar]
- 28.Nübel U, Garcia-Pichel F, Muyzer G. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl Environ Microbiol. 1997;63:3327–3332. doi: 10.1128/aem.63.8.3327-3332.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palinska K A, Liesack W, Riehl E, Krumbein W E. Phenotypic variability of identical genotypes: the need for a combined approach in cyanobacterial taxonomy demonstrated on Merismopedia-like isolates. Arch Microbiol. 1996;166:224–233. doi: 10.1007/s002030050378. [DOI] [PubMed] [Google Scholar]
- 30.Rippka R, Deruelles J, Waterbury J B, Herdman M, Stanier R Y. Generic assignments, strain histories, and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- 31.Rippka R, Herdman M. Pasteur Culture Collection of Cyanobacteria. Catalogue and taxonomic Handbook. Paris, France: Institut Pasteur; 1992. [Google Scholar]
- 32.Rudi K, Skulberg O M, Larsen F, Jakobsen K S. Strain characterization and classification of oxyphotobacteria in clone cultures on the basis of 16S rRNA sequences from the variable regions V6, V7, and V8. Appl Environ Microbiol. 1997;63:2593–2599. doi: 10.1128/aem.63.7.2593-2599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scanlan D J, Silman N J, Donald K M, Wilson W H, Carr N G, Joint I, Mann N H. An immunological approach to detect phosphate stress in populations and single cells of photosynthetic picoplankton. Appl Environ Microbiol. 1997;63:2411–2420. doi: 10.1128/aem.63.6.2411-2420.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schönhuber W, Fuchs B, Juretschko S, Amann R. Improved sensitivity of whole-cell hybridization by the combination of horseradish peroxidase-labeled oligonucleotides and tyramide signal amplification. Appl Environ Microbiol. 1997;63:3268–3273. doi: 10.1128/aem.63.8.3268-3273.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanier R Y, Cohen-Bazire G. Phototrophic prokaryotes: the Cyanobacteria. Annu Rev Microbiol. 1977;31:225–274. doi: 10.1146/annurev.mi.31.100177.001301. [DOI] [PubMed] [Google Scholar]
- 36.Stockner J G. Autotrophic picoplankton in freshwater ecosystems: the view from the summit. Int Rev Gesamten Hydrobiol. 1991;76:483–493. [Google Scholar]
- 37.Strunk, O., O. Gross, B. Reichel, M. May, S. Hermann, N. Stuckmann, B. Nonhoff, M. Lenke, A. Ginhart, A. Vilbig, T. Ludwig, A. Bode, K. H. Schleifer, and W. Ludwig. 2 October 1998, posting date. [Online.] ARB: a software environment for sequence data. http://www.mikro.biologie.tu-muenchen.de. Department of Microbiology, Technische Universität München, Munich, Germany. [2 October 1998, last date accessed.]
- 38.Urdea M S, Warner B D, Running J A, Stempien M, Clyne J, Horn T. A comparison of non-radioisotopic hybridization assay methods using fluorescent, chemiluminescent, and enzyme labeled synthetic oligodeoxyribonucleotide probes. Nucleic Acids Res. 1988;16:4937–4956. doi: 10.1093/nar/16.11.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Gijlswijk R P M, Wiegant J, Raap A K, Tanke H J. Improved localization of fluorescent tyramides for fluorescence in situ hybridization using dextran sulfate and polyvinyl alcohol. J Histochem Cytochem. 1996;44:389–392. doi: 10.1177/44.4.8601698. [DOI] [PubMed] [Google Scholar]
- 40.Weisse T. Dynamics of autotrophic picoplankton in marine and freshwater ecosystems. Adv Microb Ecol. 1993;13:327–370. [Google Scholar]
- 41.Wilmotte, A. Personal communication.