Abstract
Though liver transplantation (LT) yields survival benefit for patients with acute-on-chronic liver failure grade 3 (ACLF-3), knowledge gaps remain regarding risk factors for post-LT mortality. We retrospectively reviewed data from 10 centers in the United States and Canada for patients transplanted between years 2018 – 2019 and who required care in the intensive care unit prior to LT. ACLF was identified using the EASL-CLIF criteria. A total of 318 patients were studied, of whom 106 patients (33.3%) had no ACLF, 61 (19.1%) had ACLF-1, 74 (23.2%) had ACLF-2, and 77 (24.2%) had ACLF-3 at transplantation. Survival probability one year after LT was significantly higher in patients without ACLF (94.3%) compared to patients with ACLF (87.3%) (p=0.015), but similar between ACLF-1 (88.5%), ACLF-2 (87.8%), and ACLF-3 (85.7%) (p=0.258). Recipients with ACLF-3 and circulatory failure (n=29) had similar 1-year post-LT survival (82.3%) compared ACLF-3 patients without circulatory failure (89.6%, p=0.317), including those requiring multiple vasopressors. For patients transplanted with ACLF-3 including respiratory failure (n=20), there was a trend towards significantly lower post-LT survival (p=0.069) among those with respiratory failure (74.1%) compared to those without (91.0%). The presence of portal vein thrombosis (PVT) at LT for ACLF-3 patients (n=15), however, yielded significantly lower survival (91.9% vs 57.1%, p<0.001). Multivariable logistic regression analysis revealed that PVT was significantly associated with post-LT mortality within 1 year on (HR=7.33, 95% CI 1.90–28.3). No correlation was found between survival after LT and the location or extent of PVT, presence of transjugular intrahepatic portosystemic shunt, or anticoagulation.
Conclusions:
LT in patients with ACLF-3 requiring vasopressors yields excellent 1-year survival. LT should be approached cautiously among candidates with ACLF-3 and PVT.
Keywords: Organ failure, post-transplant outcomes, respiratory failure, portal vein thrombosis
Introduction
Acute-on-chronic liver failure (ACLF) is a syndrome occurring in patients with decompensated cirrhosis, associated with severe systemic inflammation and organ system failures,(1) which is rising in prevalence both in the United States(2) and globally(3). The 28-day mortality associated with ACLF grade 3 (ACLF-3) may approach 90% and potentially exceed that of patients with acute liver failure.(1, 4, 5) As there is currently no available medical therapy for ACLF, liver transplantation (LT) represents the only life-saving intervention for this syndrome, particularly ACLF-3.(6–8) Additionally, a recent European study demonstrated that LT in patients with ACLF provides clear survival benefit, including among recipients with ACLF-3.(9)
Despite the established survival benefit, however, multiple studies have demonstrated that patients with ACLF-3 at LT have the lowest 1-year post-transplant survival.(6, 9–11) Furthermore, as the clinical presentation of ACLF-3 is heterogenous, a greater understanding of patient characteristics associated with post-LT mortality is necessary to refine patient selection for transplantation in ACLF-3. Though certain predictors of post-LT mortality among recipients with ACLF-3 have been identified, including older age, mechanical ventilation, and arterial lactate level, several variables still remain unexplored.(10, 11) For instance, with regards to the development of circulatory failure, wide variation in vasopressor requirements can exist and prior studies have not published data correlating post-transplant survival with specific vasopressor dosages. Subsequently, current recommendations regarding maximum vasopressors dosages to safely proceed with LT are based on expert opinion.(12, 13) An additional factor which remains uninvestigated is the impact of portal vein thrombosis (PVT) at LT on post-LT survival. As PVT is present in nearly 10% of patients undergoing LT(14) and may introduce challenges leading to greater post-LT mortality particularly among those who are critically ill, (15) an understanding of whether the presence of PVT portends reduced post-LT survival in high-grade ACLF patients is warranted.
To address these knowledge gaps, we analyzed primary multicenter data from a North American consortium to: (1) assess the success of transplantation in the setting of ACLF-3 and circulatory failure based on specific vasopressor requirements at LT and (2) determine the impact of PVT at transplantation on post-LT survival.
Methods
A total of 10 transplant centers in the United States and Canada participated in the study, known as the Multi-Organ Dysfunction and Evaluation for Liver transplantation (MODEL) consortium. The study protocol was approved by the institutional review board at Cedars-Sinai Medical Center, the primary center for the study, with subsequent approval obtained from the respective institutional review boards of the other participating institutions. After execution of data user agreements, de-identified patient information was entered from each center using the Research Electronic Data Capture (REDCap) system. Prior to data entry, a training session was conducted with the principal investigator (VS) and each site investigator and relevant study staff, to increase the accuracy of entered data. After data extraction from the central database, a query was then sent to institutions in the event of erroneous or missing data, followed by a second round of data entry as needed. The study design and analysis were performed consistent with STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines.
Patient population (MODEL consortium)
We evaluated patients age 18 years or older who were transplanted from January 1, 2018 through July 1, 2019, and who required admission to the intensive care unit (ICU) prior to transplantation. However, it was not a requirement for the patient to be in the ICU specifically at the time of LT. For instance, a patient who needed admission to the ICU during their transplant hospitalization but was then transferred to the ward prior to the time of LT could be included in the study. However, all enrolled patients needed to be in the ICU a minimum of 2 days prior to LT, in order to eliminate patients who were admitted from home directly to a reserved ICU bed for post-transplant care. The time period of years 2018–2019 was chosen to capture the current landscape of the epidemiology of liver disease, recent advances in critical care and transplant surgery, and to allow for 1 year of post-transplant follow up.
Patients listed as status-1a, were re-transplanted, or who underwent multi-organ transplantation were excluded. We did include patients who underwent simultaneous liver and kidney transplantation (SLKT) given the substantial rise in performance of this operation since 2002.(16) We collected data regarding recipient characteristics at the time of hospital admission, at the time of transfer to the ICU, and at the time of transplantation. We utilized a Braden score of <16 as a surrogate marker of pre-transplant disability.(17) Donor variables and post-transplant outcomes were also obtained.
Identification of ACLF
ACLF at the time of LT was identified based on the European Association for the Study of the Liver-Chronic Liver Failure (EASL-CLIF) criteria of having a single hepatic decompensation and the presence of one or more of the following organ failures: single renal failure, single non-renal OF with renal dysfunction or hepatic encephalopathy (HE), or two non-renal organ failures. Decompensating events included the presence of ascites, HE, variceal hemorrhage or bacterial infection. These were ascertained precisely in the MODEL consortium given availability of granular data. A patient was not considered to have renal failure if review of records indicated a need for dialysis due to an indication of chronic kidney disease or if their creatinine at LT was less than 1.5 times their baseline creatinine. A patient was designated as having respiratory failure if they had a Pa02/Fi02 ratio <200 or required mechanical ventilation specifically for respiratory support. Those who were mechanically ventilated for airway protection were not categorized as having respiratory failure. Finally, a patient was considered to have circulatory failure at LT if they required vasopressor support at the time of transplantation for either a mean arterial pressure < 70mm/Hg or an indication of hypotension. Circulatory failure was determined on the day of transplantation and not on the day that the donor organ was offered. Grade of ACLF was determined based on the number of organ failures at time of transplantation.
For patients with ACLF-3 who developed circulatory failure, we grouped patients according to whether they were receiving a moderate dosage or low dose of vasopressors at LT. A moderate dose of vasopressors was defined as a norepinephrine infusion at a dose greater than 0.10 mcg/kg/min, as based on expert opinion.(18) The diagnosis of PVT was made based on the most recent ultrasound or contrast enhanced imaging prior to LT. We also collected detailed information regarding the location and extent of the PVT, as well as treatment with anticoagulation.
Statistical analysis
All statistical analyses were performed using the Stata statistical package (version 16, Stata Corporation, College Station, TX). Comparisons in descriptive statistics, as stratified by ACLF grade in the MODEL consortium, were made utilizing Chi-square or Fisher’s exact testing for categorical variables and analysis of variance (ANOVA) or Kruskal-Wallis testing for continuous variables. Survival analysis methods were performed using Kaplan-Meier analysis, with log-rank testing to evaluate differences in survival probability, where the outcome of interest was one-year post-LT mortality with time zero beginning at the date of transplant. Univariable and multivariable models were created using logistic regression for the outcome of 1-year post-transplant mortality. Variables were selected for the models a priori. For all hypothesis tests, an alpha threshold of 5% was used to determine statistical significance.
Results
Patient characteristics at the time of LT
A total of 318 patients with cirrhosis admitted to the ICU prior to LT were included in the study, of which 106 (33.3%) had no ACLF and 212 (67.6%) had ACLF at transplantation. Regarding ACLF severity, 61 patients (19.1%) had ACLF-1, 74 patients (23.2%) had ACLF-2, and 77 patients (24.2%) had ACLF-3. (Figure S1) At the time of LT, there were no significant differences with respect to patient age, gender or ethnicity among the patient groups, though a trend was seen towards patients without ACLF or with ACLF-1 being older (Table 1). Alcohol-associated liver disease was the predominant etiology of cirrhosis. Among the patients with a defined precipitant of ACLF, bacterial infection was the most common in patients with ACLF-1 (23.9%), while alcoholic hepatitis was most common among those with ACLF-2 (31.0%) and ACLF-3 (27.3%). An ACLF precipitant could not be determined in 44.2% of ACLF-1 patients, 27.1% of ACLF-2 patients and 24.6% of ACLF-3 patients. Presence of portal vein thrombosis was also similar among all groups (p=0.393). Renal failure was the most prevalent organ failure among patients transplanted with ACLF-1 (29.5%). Patients with ACLF-3 were transplanted with significantly higher rates of organ failure including liver failure (81.8%), renal failure (58.1%), brain failure (54.6%), circulatory failure (38.9%), respiratory failure (25.9%), and coagulation failure (59.7%) (p<0.001 for all). There was no significant difference in median donor age or cold ischemia time between groups (p=0.697 and 0.890 respectively).
Table 1.
| No ACLF (n=106) | ACLF-1 (n=61) | ACLF-2 (n=74) | ACLF-3 (n=77) | p-value | |
|---|---|---|---|---|---|
| Age (years) | 59 (52–62) | 59 (50–64) | 55 (40–61) | 55 (48–62) | 0.058 |
| Male | 63 (59.4) | 41 (67.2) | 37 (50.0) | 48 (62.3) | 0.209 |
| Caucasian | 78 (73.6) | 37 (60.6) | 58 (78.4) | 55 (71.4) | 0.113 |
| Etiology of liver disease | 0.126 | ||||
| Alcohol | 29 (27.4) | 23 (37.7) | 26 (35.1) | 34 (44.2) | |
| NAFLD | 17 (16.0) | 14 (22.9) | 11 (14.9) | 12 (15.6) | |
| HCV | 24 (22.6) | 5 (8.2) | 3 (4.1) | 8 (10.4) | |
| Other | 36 (33.9) | 19 (31.1) | 34 (45.9) | 23 (29.8) | |
| Reason for admission | |||||
| Ascites/hydrothorax | 17 (16.0) | 7 (11.5) | 14 (18.9) | 11 (14.3) | 0.223 |
| SBP | 5 (4.7) | 5 (8.2) | 6 (8.1) | 5 (6.5) | 0.768 |
| Hepatic encephalopathy | 13 (12.3) | 18 (29.5) | 21 (28.4) | 22 (28.6) | 0.007 |
| Ascites w/ AKI | 15 (14.1) | 27 (44.3) | 12 (16.2) | 19 (24.7) | <0.001 |
| Variceal bleeding | 2 (1.9) | 2 (3.3) | 4 (5.4) | 4 (5.2) | 0.562 |
| Other | 54 (50.9) | 2 (3.3) | 17 (22.9) | 16 (20.8) | <0.001 |
| ACLF precipitant | 0.031 | ||||
| Alcoholic hepatitis | 13 (19.4) | 23 (31.0) | 21 (27.3) | ||
| Gastrointestinal bleed | 7 (11.5) | 13 (17.6) | 17 (22.1) | ||
| Bacterial Infection | 14 (23.9) | 18 (24.3) | 20 (25.9) | ||
| Unknown | 27 (44.2) | 20 (27.1) | 19 (24.6) | ||
| Comorbidities | |||||
| Admission Braden score <16 | 5 (4.7) | 12 (19.7) | 10 (13.5) | 16 (20.9) | <0.001 |
| History of TIPS insertion | 9 (8.5) | 6 (9.8) | 9 (12.3) | 6 (7.8) | 0.783 |
| Portal vein thrombosis | 12 (11.3) | 7 (11.7) | 10 (13.7) | 15 (19.7) | 0.393 |
| Diabetes mellitus | 37 (34.9) | 20 (32.8) | 17 (22.9) | 28 (36.4) | 0.276 |
| Chronic kidney disease | 20 (18.9) | 12 (19.7) | 16 (21.6) | 14 (18.4) | 0.287 |
| Hepatocellular carcinoma | 25 (23.6) | 8 (13.1) | 6 (8.2) | 9 (11.8) | 0.024 |
| Laboratory Data | |||||
| MELD-Na score | 18 (12–22) | 26 (20–31) | 28 (22–32) | 31 (25–37) | <0.001 |
| Total bilirubin | 4.2 (2.0–7.1) | 8.5 (3.8–16.3) | 10.7 (4.6–20.7) | 23.6 (9.9–39.0) | <0.001 |
| Creatinine | 1.07 (0.8–1.3) | 1.83 (1.5–2.6) | 1.39 (1.0–2.2) | 1.45 (0.9–2.6) | <0.001 |
| INR | 1.6 (1.3–2.0) | 1.9 (1.6–2.4) | 2.1 (1.4–2.6) | 2.5 (1.7–2.9) | <0.001 |
| Albumin | 3.2 (2.7–3.7) | 3.1 (2.6–3.6) | 2.8 (2.4–3.3) | 2.9 (2.5–3.3) | 0.009 |
| White blood cell count | 6.0 (4.2–9.7) | 7.3 (4.0–10.0) | 7.9 (4.5–13.6) | 8.9 (5.6–13.1) | 0.027 |
| Serum lactate (venous) | 2.8 (1.8–3.3) | 2.7 (1.8–3.6) | 2.5 (1.6–3.5) | 2.6 (1.3–4.0) | 0.483 |
| Missing (%) | 32.0 | 24.6 | 17.6 | 15.6 | |
| Bacterial infection pre-LT | 18 (16.9) | 17 (27.8) | 19 (25.7) | 22 (28.6) | 0.022 |
| In ICU at LT | 26 (24.5) | 20 (32.8) | 39 (52.7) | 54 (70.1) | 0.004 |
| Mechanical ventilation | 7 (6.6) | 15 (24.6) | 18 (24.3) | 29 (37.7) | 0.006 |
| Mean arterial pressure | 77 (72–85) | 73 (67–77) | 74 (68–81) | 73 (69–77) | 0.030 |
| PaO2/FiO2 | 395 (310–425) | 346 (300–462) | 238 (90–303) | 215 (76–360) | <0.001 |
| Organ failures | |||||
| Liver failure | 2 (1.9) | 17 (27.8) | 33 (44.6) | 63 (81.8) | <0.001 |
| Renal failure | 0 (0.0) | 18 (29.5) | 18 (26.9) | 43 (58.1) | <0.001 |
| Brain failure | 2 (1.9) | 12 (19.7) | 24 (32.4) | 42 (54.6) | <0.001 |
| Circulatory failure | 3 (2.8) | 3 (4.9) | 12 (16.2) | 29 (37.6) | <0.001 |
| Respiratory failure | 3 (2.8) | 2 (3.3) | 10 (13.5) | 20 (25.9) | <0.001 |
| Coagulation failure | 2 (1.9) | 9 (14.8) | 24 (32.4) | 46 (59.7) | <0.001 |
| 4–6 organ failures | 26 (33.8) | ||||
| Transplant characteristics | |||||
| Simultaneous liver-kidney | 4 (3.8) | 6 (9.8) | 12 (16.2) | 3 (3.9) | 0.021 |
| Donor age | 38 (27–51.5) | 39.5 (29–47) | 41 (29–55) | 36 (30–48) | 0.697 |
| Missing | 6 (5.6) | 7 (11.4) | 10 (13.5) | 8 (10.3) | |
| Cold ischemia time (minutes) | 320 (248–395) | 308 (258–379) | 330 (263–394.2) | 323.6 (249–389) | 0.890 |
| Missing | 36 (33.9) | 12 (19.7) | 27 (36.5) | 26 (33.8) |
Continuous variables presented as Median and IQR; categorical variables are presented as N (column %)
Data at the time of transplantation unless otherwise specified
Post-transplant survival
Survival at one year after LT was significantly higher in patients without ACLF (94.3%) compared to patients with ACLF (87.3%) (p=0.015). Across each ACLF grade, 1-year post-LT survival probability was similar between ACLF-1 (88.5%), ACLF-2 (87.8%), and ACLF-3 (85.7%) (p=0.258). (Figure 1) A total of 27 patients transplanted with ACLF died within the first year, of whom 7 patients had ACLF-1, 9 had ACLF-2, and 11 had ACLF-3. The primary causes of death included sepsis (n=12, 44.4%), respiratory failure (n=7, 25.9%), cardiac arrest (n=3, 11.1%), graft failure (n=2, 7.4%), and HCC recurrence (n=1, 3.7 %) and unknown (n=2, 7.4%).
Figure 1.
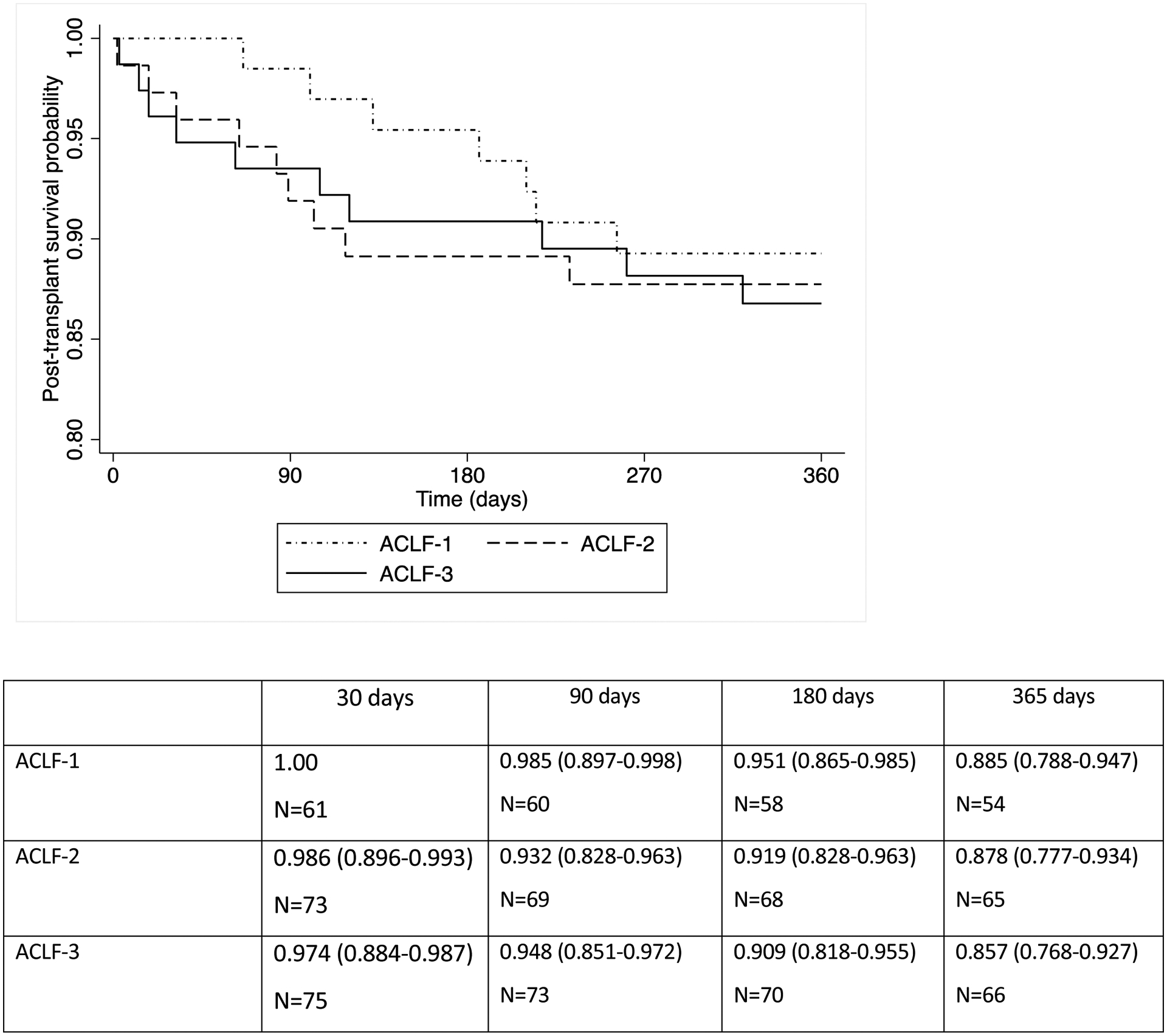
Post-transplant patient survival by ACLF status at time of transplantation (p=0.258)
Circulatory failure and post-LT survival in recipients with ACLF-3
We performed additional analysis regarding circulatory organ failure in the subgroup of ACLF-3 recipients. One year survival probability was similar (p=0.317) among ACLF-3 patients with circulatory failure (82.3%) compared to those without circulatory failure (89.6%). (Figure 2a) In table 2, we describe vasopressor requirements at time of transplantation, among patients with circulatory failure in the setting of ACLF-3. We identified 29 patients with ACLF-3 (37.6%) who also required vasopressors at transplantation, of whom 24 (82.8%) survived beyond 1 year. A total of 16 patients were taking a moderate dosage of vasopressors at LT and 13 recipients required a low dosage of vasopressors. Of the 5 patients (17.2%) who died within the first year after LT, 3 were maintained on a low dose of vasopressors and 2 were taking a moderate dosage of vasopressors at the time of transplant. Causes of death within 1 year in the recipients requiring a low dosage of vasopressors included respiratory failure/sepsis, hepatocellular carcinoma recurrence, and cardiac arrest. The two patients undergoing LT while on a moderate dosage of vasopressor therapy died from sepsis within 1 year of LT.
Figure 2a.
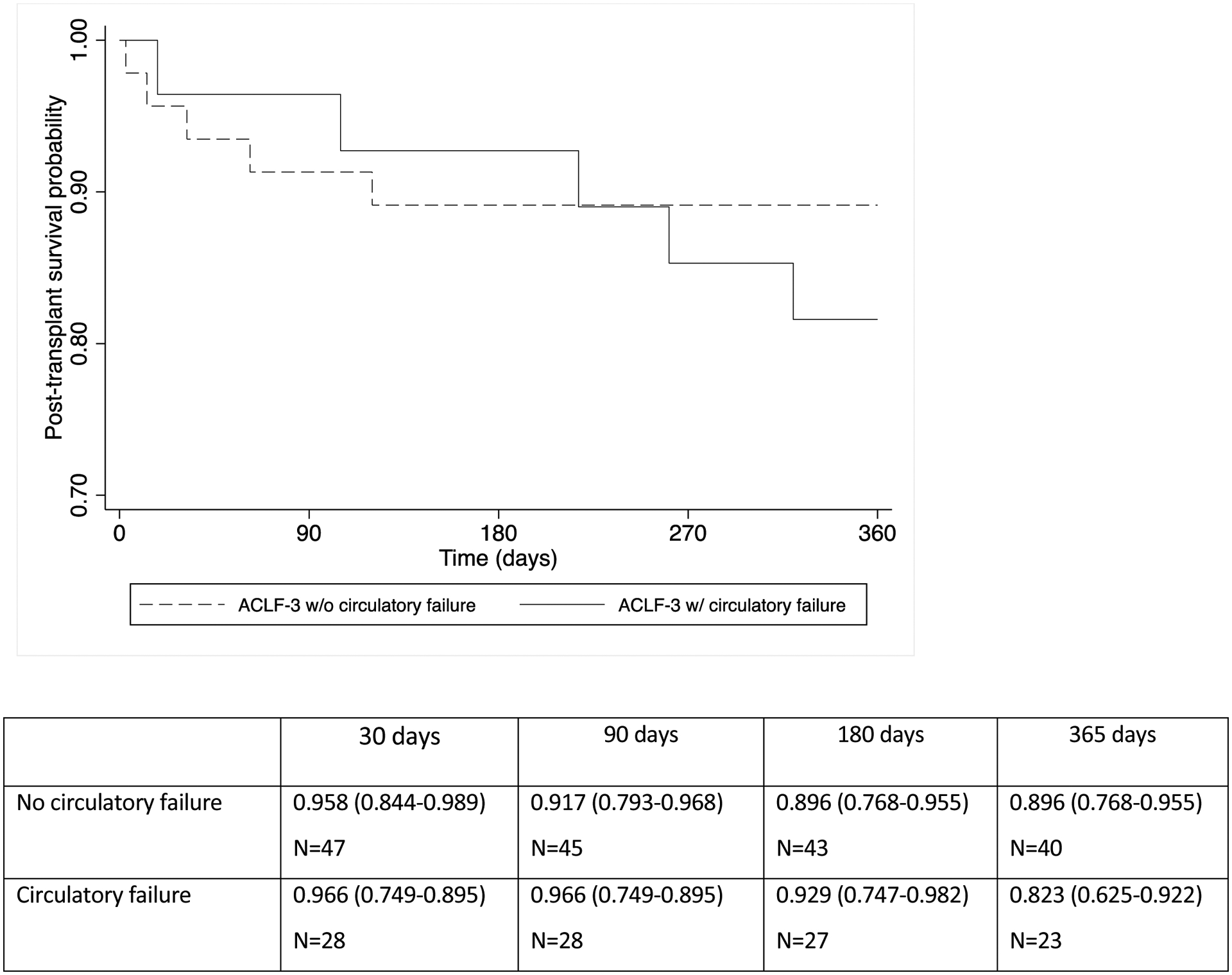
Post-transplant patient survival among recipients with ACLF-3 according to presence of circulatory failure at transplantation (p=0.317)
Table 2.
Vasopressor requirements at the time of transplantation, among recipients with ACLF-3
| Age (years) | Vasopressor requirements | Outcome | Cause of death | |
|---|---|---|---|---|
| Moderate dose | 63 | Norepinephrine 0.24 | Alive | |
| Vasopressin 0.3 | ||||
| Epinephrine 0.4 | ||||
| 66 | Norepinephrine 0.4 | Alive | ||
| Vasopressin 0.04 | ||||
| 57 | Norepinephrine 0.4 | Alive | ||
| Vasopressin 0.03 | ||||
| 55 | Norepinephrine 0.3 | Dead | Sepsis | |
| Vasopressin 0.04 | ||||
| 47 | Norepinephrine 0.3 | Alive | ||
| Vasopressin 0.04 | ||||
| 36 | Norepinephrine 0.3 | Alive | ||
| Phenylephrine 0.04 | ||||
| 45 | Norepinephrine 0.15 | Alive | ||
| Vasopressin 0.02 | ||||
| 69 | Epinephrine 0.4 | Alive | ||
| Vasopressin 0.03 | ||||
| 65 | Norepinephrine 0.19 | Alive | ||
| 39 | Norepinephrine 0.18 | Alive | ||
| 45 | Phenylephrine 0.15 | Alive | ||
| 55 | Norepinephrine 0.12 | Dead | Sepsis | |
| Vasopressin 0.02 | ||||
| 66 | Norepinephrine 0.12 | Alive | ||
| Vasopressin 0.02 | ||||
| 52 | Norepinephrine 0.15 | Alive | ||
| 55 | Norepinephrine 0.15 | Alive | ||
| 47 | Norepinephrine 0.12 | Alive | ||
| Low dose | 54 | Norepinephrine 0.07 | Dead | Sepsis |
| 29 | Norepinephrine 0.09 | Alive | ||
| 64 | Norepinephrine 0.07 | Alive | ||
| 61 | Norepinephrine 0.07 | Alive | ||
| 67 | Norepinephrine 0.06 | Alive | ||
| 69 | Norepinephrine 0.04 | Alive | ||
| 58 | Norepinephrine 0.03 | Dead | HCC recurrence | |
| 64 | Norepinephrine 0.03 | Dead | Cardiac arrest | |
| 50 | Vasopressin 0.04 | Alive | ||
| 65 | Vasopressin 0.04 | Alive | ||
| 51 | Vasopressin 0.04 | Alive | ||
| 65 | Vasopressin 0.03 | Alive | ||
| 50 | Vasopressin 0.03 | Alive |
Units: Norepinephrine, Epinephrine, Phenylephrine: mcg/kg/min; Vasopressin: units/min.
Portal vein thrombosis and post-LT survival in recipients with ACLF-3
We further explored the effects of PVT presence on post-LT outcomes. There were 15 patients with PVT (19.5%) among the 77 recipients who presented with ACLF-3 at LT. Characteristics at LT of ACLF-3 patients classified by PVT presence are described in table S1. The two groups were similar though there was a trend towards older donor age among patients with PVT. All PVT were chronic in nature and were bland thrombi as opposed to tumor thrombi. A total of six patients had findings of cavernous transformation. The presence of PVT at transplantation was associated with significantly lower first year post-transplant survival (91.9% vs 57.1%, p<0.001). (Figure 2b) Among the 6 patients with ACLF-3 and PVT who died within 1 year (Table 3), four were deceased from sepsis, one from respiratory failure, and one from recurrent HCC. No appreciable survival differences were noted based on PVT extent or location, use of pre-LT anticoagulation, history of transjugular intrahepatic portosystemic shunt (TIPS) insertion, or patient age.
Figure 2b.
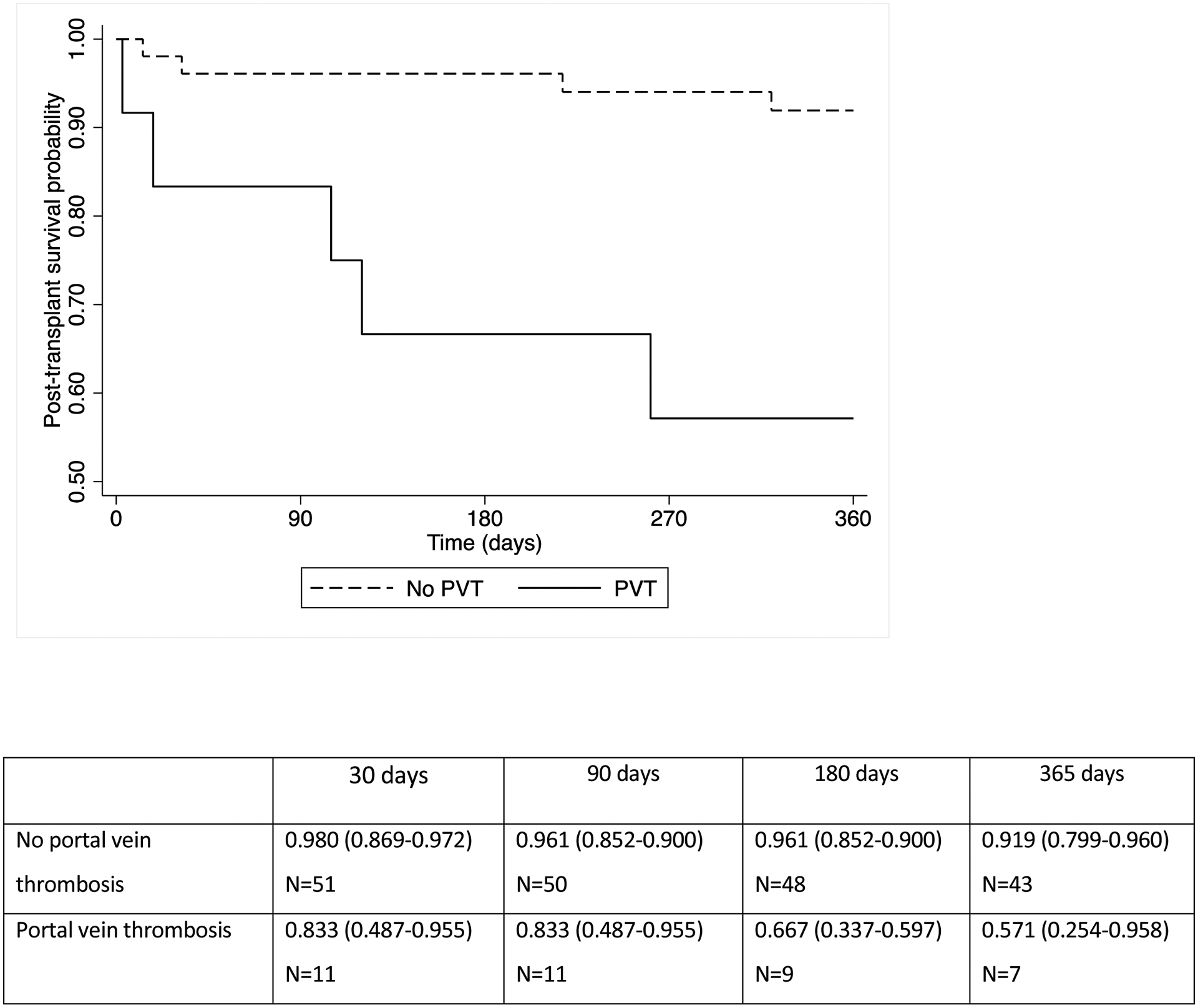
Post-transplant patient survival among recipients with ACLF-3 according to presence of portal vein thrombosis at transplantation (p=<0.001)
Table 3.
Characteristics of patients with ACLF-3 and portal vein thrombosis at LT
| Age | Occlusive | Location | Anticoagulated | TIPS | Portal vein reconstruction | Outcome |
|---|---|---|---|---|---|---|
| 24 | No | PVT to SMV confluence | No | Yes | Venous jump graft to SMV | Alive |
| 61 | No | Main and right portal vein | No | No | No | Died due to sepsis |
| 55 | Yes | Main portal vein to portosplenic confluence | No | No | Thrombectomy | Alive |
| 53 | No | Left, right, main portal veins to splenic vein and SMV | Yes | Yes | Portocaval hemitransposition | Died, due to sepsis |
| 50 | No | Main and right portal vein | Yes | No | No | Alive |
| 45 | No | Main portal vein | No | No | No | Died, due to sepsis |
| 73 | No | Right portal vein | Yes | No | No | Alive |
| 61 | No | Right portal vein | No | No | No | Alive |
| 68 | No | Main portal vein | Yes | No | No | Alive |
| 64 | No | Right and main portal vein, splenic confluence | Yes | No | Portocaval hemitransposition | Died, due to respiratory failure |
| 55 | No | Right portal vein | Yes | No | No | Died, due to recurrent HCC |
| 71 | No | Main portal vein | No | No | No | Alive |
| 50 | No | Left portal vein | No | No | No | Alive |
| 60 | Yes | Right and main portal vein | No | No | No | Died, due to sepsis |
| 44 | No | Left and main portal vein | No | No | No | Alive |
Respiratory failure and post-LT survival in recipients with ACLF-3
Although our primary focus was to evaluate in detail the impact of circulatory failure and PVT on post-LT survival, we performed additional analysis regarding the need for mechanical ventilation at transplantation, in consideration of prior findings which have demonstrated need for ventilation is associated with poorer survival after LT.(10, 19) Among the patients with ACLF-3 at LT, we identified 20 patients with respiratory failure, of whom 18 (90%) were mechanically ventilated. There were no patients who were transplanted with concurrent respiratory and circulatory failure. There was a trend towards significantly lower post-LT survival (p=0.069) among patients with ACLF-3 and respiratory failure (74.1%) compared to those without respiratory failure (91.0%), as displayed in figure 2c. Among the five patients who died, cause of death included respiratory failure (n=3), cardiac arrest (n=1), and sepsis (n=1). Four of these five patients died within 180 days of LT.
Figure 2c.
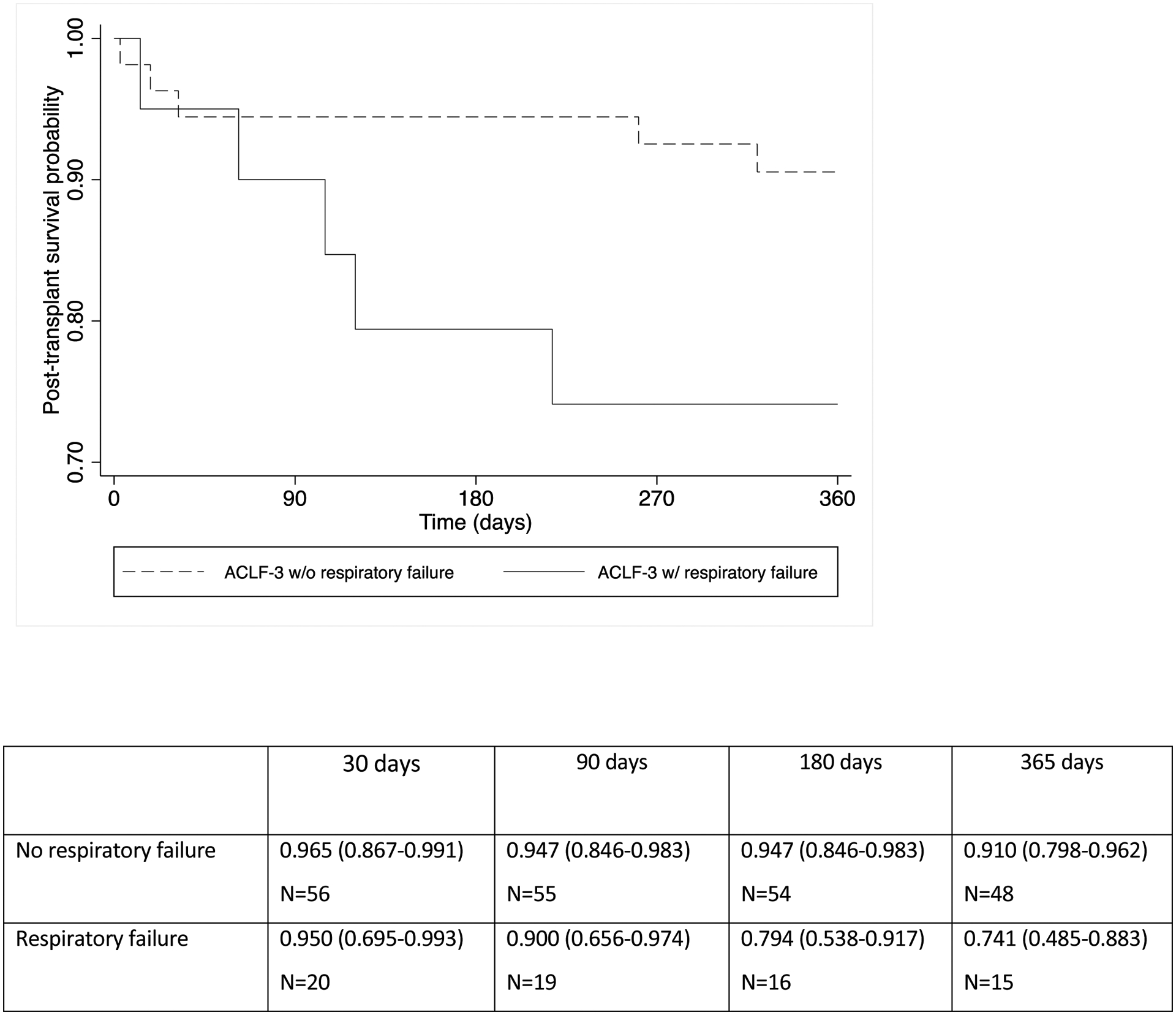
Post-transplant patient survival among recipients with ACLF-3 according to presence of respiratory failure at transplantation (p=0.069)
Predictors of mortality within the first year after LT
Univariable and multivariable logistic regression models for mortality within the first year after LT for patients with ACLF-3 are displayed in Table 4. The presence of PVT (OR=8.33, 95% CI: 2.33 – 29.7) and age (OR=1.06, 95% CI: 1.01–1.10) were the only significant predictors for mortality, among recipients with ACLF-3. Of note, the presence of PVT at transplantation was not associated with post-LT mortality among patients with ACLF grade s1 (OR=1.52, 95% CI 0.26–8.95) or 2 (OR=4.07, 95% CI 0.82–20.0). The presence of specific organ failures, donor age, cold ischemia time, Braden score, pre-transplant bacterial infection, and MELD-Na score at time of LT were not associated with first year post-transplant mortality. On multivariable analysis adjusting for age, the presence of PVT at LT remained significant (OR=7.33, 95% CI 1.90–28.3).
Table 4.
Univariable and multivariable analysis for predictors of mortality within 1-year post-LT among recipients with ACLF-3
| Univariable analysis OR (95% CI) | Multivariable analysis OR (95% CI) | |
|---|---|---|
| Portal vein thrombosis | 8.33 (2.33–29.7) | 7.33 (1.90 –28.3) |
| Age | 1.06 (1.01–1.10) | 1.01 (0.95–1.08) |
| Body mass index | 1.01 (0.90–1.12) | |
| MELD-Na score at LT | 1.09 (0.96–1.25) | |
| Respiratory failure at LT | 1.72 (0.43–6.88) | |
| Circulatory failure at LT | 1.52 (0.31–7.57) | |
| Brain failure at LT | 1.01 (0.24–4.22) | |
| Liver failure at LT | 0.30 (0.60–1.49) | |
| Renal failure at LT | 1.04 (0.21–5.36) | |
| Coagulation failure at LT | 1.25 (0.29–5.17) | |
| Braden score < 16 | 0.81 (0.22–11.4) | |
| Bacterial infection pre-LT | 4.33 (0.75–25.2) | |
| Donor age > 60 | 1.28 (0.33–4.83) | |
| Cold ischemia time | 1.01 (0.99–1.01) |
Discussion
Based on our study of 318 transplant recipients requiring ICU level care, of whom 212 developed ACLF prior to LT, we report two primary messages. The first is that certain patients with ACLF-3 and circulatory failure at LT can have excellent post-transplant survival above 80% at 1-year, even when requiring multiple vasopressors, thereby suggesting that vasopressor support does not preclude LT. The second is that the presence of PVT at transplantation is significantly associated with poorer 1-year post-LT outcomes in the setting of ACLF-3, regardless of the location, extent, prior TIPS placement, or treatment with anticoagulation. The current paper describes data from the ever first multi-center North American consortium to study post-LT outcomes in a patients with ACLF-3. We analyzed a large sample of ACLF-3 transplant recipients and had access to both recent and granular patient data, thereby ensuring accurate identification of organ failures and reflecting current ICU and post-transplant management, as well the existing landscape of liver disease since introduction of direct acting antiviral agents for hepatitis C virus.
Our investigation is the first to explore the impact of circulatory failure in detail. Although expert consensus suggests caution in proceeding with LT for patients with ACLF-3 requiring vasopressors, particularly at a dosage of norepinephrine > 0.1 ug/kg/min (13), there is currently no published data regarding the safety of LT in correlation with specific dosages of perioperative vasopressors. A prior study by Artru et al demonstrated a greater than 80% 1-year survival among recipients with ACLF-3 requiring a norepinephrine dosage of ≤ 3mg/hr (0.7 ug/kg/min for a 70 kg person), implying that this may be the maximum dosage of vasopressor support to proceed with LT, since patients requiring a higher dosage were excluded from the study.(6) However, our study indicates that a greater than 80% post-transplant 1-year survival is achievable even among ACLF-3 patients requiring higher dosages of vasopressors, as well as multiple vasopressors. We acknowledge that we were unable to specify a cutoff point at which transplantation would be successful or futile, and this can only be ascertained from prospectively obtained data accounting for factors including the duration of vasopressor requirement and control of infection. Therefore, while patient selection for ACLF-3 transplantation in the setting of circulatory requires further study, our preliminary findings suggest that moderate vasopressor requirements do not appear to represent an absolute contraindication to LT, and that transplantation may lead to recovery of circulatory failure. This is of particular importance, since ACLF is a highly dynamic syndrome which can worsen rapidly (4) and it is possible that delaying LT until a patient no longer requires vasopressors may lead to a missed opportunity for transplantation, either due to further clinical deterioration or not receiving suitable organ offers in regions with high median MELD scores at transplantation.
An additional novel message from our study is the profound association between PVT and reduced post-transplant survival in recipients with ACLF-3, leading to a greater than 30% survival difference at 1 year. Although prior investigations have established that PVT can decrease post-LT survival(14, 15), ours is the first to evaluate the association between PVT and post-LT outcomes specifically in ACLF-3 recipients. It should be noted that our cohort represents the largest sample of transplant recipients with ACLF-3 and PVT, whereas in previous multi-center studies examining outcomes after LT among those with severe ACLF, PVT was not included in the analysis.(6, 9, 10) Our findings indicate that the presence of PVT alone may negatively affect post-LT outcomes, regardless of location or extent. It is additionally interesting that the effect of PVT on post-LT mortality was only demonstrated among patients with ACLF-3, indicating that the medical complexity of patients with ACLF grade 3, in conjunction with concurrent PVT, may be challenging for surgeons to address and still achieve acceptable post-LT outcomes. These poorer clinical outcomes may be related to the complexity of the transplant surgery to achieve adequate portal blood inflow. (20, 21) Alternatively, PVT may be a surrogate for loss of adequate hepatic perfusion as a result of worsening intrahepatic resistance, combined with prothrombotic and inflammatory factors triggered during the systemic inflammatory syndrome of ACLF, particularly with increasing ACLF grade.(20, 22) Thus, the addition of PVT to complicate the transplant surgery in a critically ill patient with multi-organ failure, may be too challenging to overcome. Although further prospective research is needed to validate these associations, caution may be warranted in transplanting patients with ACLF-3 and PVT. Furthermore, since PVT may be difficult to treat in patients who have developed ACLF-3 and PVT due to severe dysregulation of thrombosis and hemostasis(23), we believe that treatment with anticoagulation prior to the development of severe ACLF should be considered.
Finally, although our study was focused on the impact of vasopressor requirements and PVT in post-LT outcomes, we also evaluated whether the presence of respiratory failure at LT portends reduced survival after transplant in recipients with ACLF-3. While not statistically significant, possibly due to small sample size, the observed association of reduced 1 year post-LT survival among patients with ACLF-3 and respiratory failure were consistent with prior observations.(10, 11) Although these findings are confirmatory, we believe it is important to report them since our data was collected over a recent period of time (years 2018–2019), thereby reflecting the current practice of critical care and post-transplant management. It is noteworthy that in our study, only a minority of ACLF-3 patients that underwent LT had respiratory failure (25.9%) at the time of transplant, suggesting that that while advances have been made in critical care management regarding which patients are supportable through transplant, respiratory failure is still deemed by transplant providers to create significant challenges in this population.
Interpretation of our study findings should also consider the context of its limitations. First, the study is subject to biases associated with its retrospective design, including misclassification. However, we attempted to minimize this concern by conducting a training session prior to data entry and collecting objective and easily obtainable clinical information. Secondly, it is difficult to account retrospectively for factors which are also incorporated in the decision to transplant such as deconditioning, malnutrition, length of time on vasopressors or antibiotics, and day-to-day changes in the clinical course of ACLF. Thirdly, we were not able to determine the chronicity of PVT. Finally, although we performed adjusted analysis, we acknowledge that our study is primarily exploratory in nature and therefore temper our conclusions accordingly. Subsequently, we emphasize that decision to proceed with LT should be ultimately based on the clinical judgement of the transplant team. Regardless, given the complexity of the patient with severe ACLF, along with the scarcity of data regarding the nuances of transplantation in this population, we believe our findings remain useful for clinical decision making and as a foundation for larger prospective investigations. We hope, therefore, that our data will provide further insight to help clinicians in making these complicated decisions.
In conclusion, our study revealed that LT across all grades of ACLF yielded 1-year post-transplant survival probability approaching 85%, including patients with ACLF-3. Furthermore, patients with ACLF-3 can potentially undergo safe transplantation even when requiring multiple vasopressors, though the decision to move forward with LT should ultimately be based on clinical judgment and experience. However, the presence of PVT is associated with a significantly reduced post-transplant survival among those transplanted with ACLF-3. We therefore suggest caution in proceeding with LT for patients who fit this clinical profile.
Supplementary Material
Grants and financial support:
none
Abbreviations:
- ACLF
Acute on chronic liver failure
- ALD
Alcoholic liver disease
- EASL-CLIF
European Association for the Study of the Liver-Chronic Liver Failure
- HCV
Hepatitis C virus
- HE
Hepatic Encephalopathy
- LT
Liver transplantation
- OF
Organ failure
- PVT
Portal Vein Thrombosis
- UNOS
United Network for Organ Sharing
Footnotes
Conflicts of interest: VS reports consulting with Saol therapeutics and speaker’s bureau for Gilead, Intercept, and Abbvie. The remaining authors have no disclosures to report.
References
- 1.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426–1437, 1437 e1421–1429. [DOI] [PubMed] [Google Scholar]
- 2.Sundaram V, Jalan R, Shah P, Singal AK, Patel AA, Wu T, Noureddin M, et al. Acute on chronic liver failure from nonalcoholic fatty liver disease: a growing and aging cohort with rising mortality. Hepatology 2020. [DOI] [PubMed] [Google Scholar]
- 3.Mezzano G, Juanola A, Cardenas A, Mezey E, Hamilton JP, Pose E, Graupera I, et al. Global burden of disease: acute-on-chronic liver failure, a systematic review and meta-analysis. Gut 2021. [DOI] [PubMed] [Google Scholar]
- 4.Gustot T, Fernandez J, Garcia E, Morando F, Caraceni P, Alessandria C, Laleman W, et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 2015;62:243–252. [DOI] [PubMed] [Google Scholar]
- 5.Sundaram V, Jalan R, Ahn JC, Charlton MR, Goldberg DS, Karvellas CJ, Noureddin M, et al. Class III obesity is a risk factor for the development of acute-on-chronic liver failure in patients with decompensated cirrhosis. J Hepatol 2018;69:617–625. [DOI] [PubMed] [Google Scholar]
- 6.Artru F, Louvet A, Ruiz I, Levesque E, Labreuche J, Ursic-Bedoya J, Lassailly G, et al. Liver transplantation in the most severely ill cirrhotic patients: A multicenter study in acute-on-chronic liver failure grade 3. J Hepatol 2017;67:708–715. [DOI] [PubMed] [Google Scholar]
- 7.Sundaram V, Shah P, Wong RJ, Karvellas CJ, Fortune BE, Mahmud N, Kuo A, et al. Patients With Acute on Chronic Liver Failure Grade 3 Have Greater 14-Day Waitlist Mortality Than Status-1a Patients. Hepatology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thuluvath PJ, Thuluvath AJ, Hanish S, Savva Y. Liver Transplantation in Patients with Multiple Organ Failures: Feasibility and Outcomes. J Hepatol 2018. [DOI] [PubMed] [Google Scholar]
- 9.Belli LS, Duvoux C, Artzner T, Bernal W, Conti S, Cortesi PA, Sacleux SC, et al. Liver transplantation for patients with acute-on-chronic liver failure (ACLF) in Europe: results of the ELITA/EF-CLIF collaborative study (ECLIS). J Hepatol 2021. [DOI] [PubMed] [Google Scholar]
- 10.Artzner T, Michard B, Weiss E, Barbier L, Noorah Z, Merle JC, Paugam-Burtz C, et al. Liver transplantation for critically ill cirrhotic patients: Stratifying utility based on pretransplant factors. Am J Transplant 2020. [DOI] [PubMed] [Google Scholar]
- 11.Sundaram V, Jalan R, Wu T, Volk ML, Asrani SK, Klein AS, Wong RJ. Factors Associated with Survival of Patients With Severe Acute on Chronic Liver Failure Before and After Liver Transplantation. Gastroenterology 2018. [DOI] [PubMed] [Google Scholar]
- 12.Jalan R, Gustot T, Fernandez J, Bernal W. ‘Equity’ and ‘Justice’ for patients with acute-on chronic liver failure: A call to action. J Hepatol 2021. [DOI] [PubMed] [Google Scholar]
- 13.Weiss E, Saner F, Asrani SK, Biancofiore G, Blasi A, Lerut J, Durand F, et al. When Is a Critically Ill Cirrhotic Patient Too Sick to Transplant? Development of Consensus Criteria by a Multidisciplinary Panel of 35 International Experts. Transplantation 2021;105:561–568. [DOI] [PubMed] [Google Scholar]
- 14.Zanetto A, Rodriguez-Kastro KI, Germani G, Ferrarese A, Cillo U, Burra P, Senzolo M. Mortality in liver transplant recipients with portal vein thrombosis - an updated meta-analysis. Transpl Int 2018;31:1318–1329. [DOI] [PubMed] [Google Scholar]
- 15.Ghabril M, Agarwal S, Lacerda M, Chalasani N, Kwo P, Tector AJ. Portal Vein Thrombosis Is a Risk Factor for Poor Early Outcomes After Liver Transplantation: Analysis of Risk Factors and Outcomes for Portal Vein Thrombosis in Waitlisted Patients. Transplantation 2016;100:126–133. [DOI] [PubMed] [Google Scholar]
- 16.Miles CD, Westphal S, Liapakis A, Formica R. Simultaneous Liver-Kidney Transplantation: Impact on Liver Transplant Patients and the Kidney Transplant Waiting List. Curr Transplant Rep 2018;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundaram V, Lim J, Tholey DM, Iriana S, Kim I, Manne V, Nissen NN, et al. The Braden Scale, A standard tool for assessing pressure ulcer risk, predicts early outcomes after liver transplantation. Liver Transpl 2017;23:1153–1160. [DOI] [PubMed] [Google Scholar]
- 18.Olson JC, Karvellas CJ. Critical care management of the patient with cirrhosis awaiting liver transplant in the intensive care unit. Liver Transpl 2017;23:1465–1476. [DOI] [PubMed] [Google Scholar]
- 19.Sundaram V, Kogachi S, Wong RJ, Karvellas CJ, Fortune BE, Mahmud N, Levitsky J, et al. Effect of the clinical course of acute on chronic liver failure prior to liver transplantation on post-transplant survival. J Hepatol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agbim U, Satapathy SK. PRO: Portal Vein Thrombosis Impacts Liver Transplantation Outcomes. Clin Liver Dis (Hoboken) 2020;16:127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rugivarodom M, Charatcharoenwitthaya P. Nontumoral Portal Vein Thrombosis: A Challenging Consequence of Liver Cirrhosis. J Clin Transl Hepatol 2020;8:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Premkumar M, Sarin SK. Current Concepts in Coagulation Profile in Cirrhosis and Acute-on-Chronic Liver Failure. Clin Liver Dis (Hoboken) 2020;16:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blasi A, Calvo A, Prado V, Reverter E, Reverter JC, Hernandez-Tejero M, Aziz F, et al. Coagulation Failure in Patients With Acute-on-Chronic Liver Failure and Decompensated Cirrhosis: Beyond the International Normalized Ratio. Hepatology 2018;68:2325–2337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


