Abstract
Background:
Aneurysmal subarachnoid hemorrhage (aSAH) patients with electroencephalographic epileptiform activity (seizures, periodic/rhythmic patterns, and sporadic discharges) are frequently treated with anti-seizure medications (ASMs). However, the safety and effectiveness of ASM treatment for epileptiform activity has not been established. We used observational data to investigate the effectiveness of ASM treatment in aSAH patients undergoing continuous electroencephalography (cEEG), to develop causal hypothesis for testing in prospective trials.
Methods:
Retrospective single-center cohort study of aSAH patients admitted between 2011–2016. Patients underwent ≥24-hrs of cEEG within four days of admission. All patients received primary ASM prophylaxis until aneurysm treatment (typically within 24 hours of admission). Treatment exposure was defined as re-initiation of ASMs after aneurysm treatment and cEEG initiation. We excluded patients with non-cEEG indications for ASMs (e.g. epilepsy, acute symptomatic seizures). Outcomes measures were 90-day mortality and good functional outcome (modified Rankin Scale 0–3). Propensity scores were used to adjust for baseline covariates and disease severity.
Results:
94 subjects were eligible (40 continued ASM treatment; 54 received prophylaxis only). ASM continuation was not significantly associated with higher 90-day mortality (propensity adjusted HR=2.01 [0.57–7.02]). ASM continuation was associated with lower likelihood for 90-day good functional outcome (propensity adjusted HR=0.39 [0.18–0.81]). In a secondary analysis, low intensity treatment (low-dose single ASM) was not significantly associated with mortality (propensity adjusted HR=0.60 [0.10–3.59]), though it was associated with a lower likelihood of good outcome (propensity adjusted HR=0.37 [0.15–0.91]), compared to prophylaxis. High intensity treatment (high-dose single ASM, multiple ASMs or anesthetics) was associated with higher mortality (propensity adjusted HR=6.80 [1.67–27.65]) and lower likelihood for good outcomes (propensity adjusted HR=0.30 [0.10–0.94]) compared to prophylaxis only.
Conclusion:
Our findings suggest the testable hypothesis that continuing ASMs in aSAH patients with cEEG abnormalities does not improve functional outcomes. This hypothesis should be tested in prospective randomized studies.
Keywords: subarachnoid hemorrhage, electroencephalopgraphy, anticonvulsants, seizures, outcome assessment
Introduction
Aneurysmal subarachnoid hemorrhage patients with electroencephalographic epileptiform activity (seizures, periodic and rhythmic patterns, and sporadic discharges) are frequently treated with anti-seizure medications (1,2). Despite mounting evidence that epileptiform activity is associated with worse outcomes, there is limited data to guide treatment (2,3). Primary prophylaxis with anti-seizure medications (ASMs) is associated with worse cognitive and functional outcomes in aneurysmal subarachnoid hemorrhage (aSAH) patients (4,5). Standardized guidelines, therefore, do not recommend primary prophylaxis beyond the immediate post-hemorrhage period (6). Nevertheless, ASMs are commonly prescribed in aSAH patients when epileptiform activity is detected, with the rationale of preventing seizures and secondary brain injury. However, the safety and effectiveness of prescribing ASMs in aSAH patients with epileptiform activity has not been established (2,3).
We hypothesized that use of ASMs to treat epileptiform activity in aSAH often causes net harm, increasing mortality and worsening functional outcomes. While a randomized clinical trial would be needed to test this hypothesis definitively, here we sought to develop preliminary support for the hypothesis through analysis of existing observational data, to evaluate the safety and effectiveness of ASM treatment in aSAH patients undergoing continuous electroencephalography (cEEG) monitoring. We used strict inclusion and exclusion criteria and propensity methodology to investigate the association of ASM treatment for epileptiform activity with survival and functional outcomes.
Methods
This is a retrospective cohort study of patients with aSAH admitted at our center between September 2011 and February 2016. The data that support the findings of this study are available from the corresponding author, (SFZ) upon reasonable request. The study was approved by the Institutional Review Board of Mass General Brigham. Informed consent was not required for this retrospective study. There were four main eligibility criteria: 1) admission for treatment of aSAH; 2) age >18 years; 3) cEEG monitoring initiated within 4 days of admission; 4) ≥24 hours of cEEG monitoring. We restricted inclusion to patients with cEEG beginning within 4 days of admission as the majority of patients develop epileptiform activity during this period (7). To increase homogeneity of the cohort, we excluded patients likely to receive ASMs regardless of EEG findings e.g., a history of epilepsy or acute symptomatic clinical seizures on admission.
All patients received primary ASM prophylaxis until the aneurysm was secured based on institutional protocol and consensus guidelines (6). The aneurysm is typically treated within the first 24 hours of admission, and primary prophylaxis discontinued immediately thereafter, unless the patient undergoes craniectomy or craniotomy in which case primary prophylaxis is continued at the treating team’s discretion. We excluded patients who were continued on primary ASM prophylaxis after the aneurysm was secured for the indication of craniectomy or craniotomy, regardless of EEG findings. Figure 1 displays the inclusion and exclusion flowchart.
Figure 1. Patient selection process.
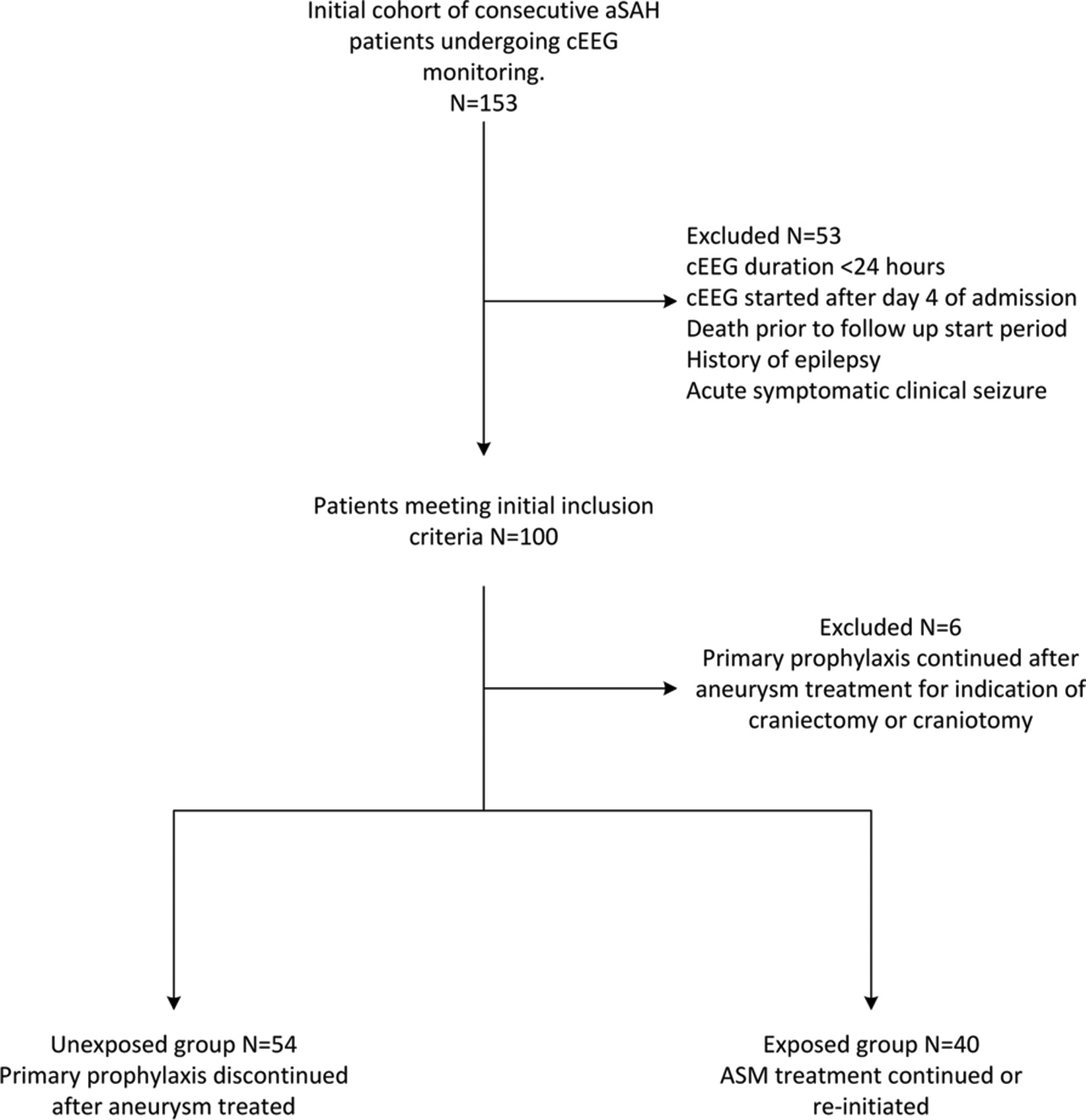
A graphical description of the inclusion and exclusion criteria and exposed and unexposed groups is provided.
aSAH: aneurysmal subarachnoid hemorrhage; ASM: anti-seizure medication; cEEG: continuous electroencephalopgraphy
Exposure definition
We compared subjects who received prophylactic versus continued ASM therapy. Levetiracetam at a dose of 1000mg/day is the standard prophylactic dose per institutional protocol. We defined ASM treatment exposure as continuation or re-initiation of ASMs for >48 hours after aneurysm treatment and 24 hours after initiation of cEEG through day 10 of admission (Figure 2). We used this exposure window because the likelihood of developing epileptiform activity is highest within the first 5 days of admission and decreases after day 10 of admission (7). In addition, the highest risk of delayed cerebral ischemia, which is closely associated with epileptiform activity, is within the first 10 days after aSAH (7–9). The unexposed group consisted of patients who only received primary ASM prophylaxis until the aneurysm was secured.
Figure 2. EEG and Treatment exposure windows.
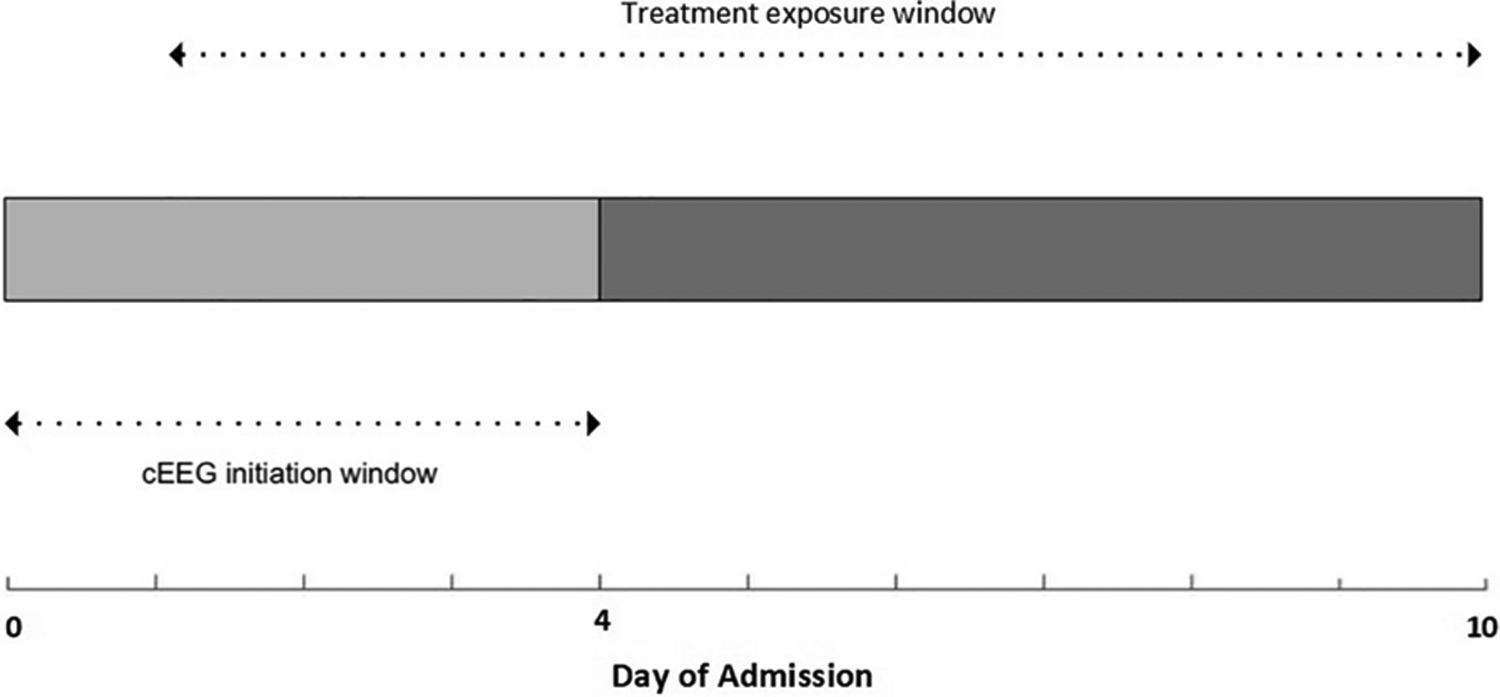
All cEEGs were performed within 4 days of admission and were at-least 24 hours in duration.
Treatment exposure window: 24-hrs post EEG to up to day 10 of admission
Unexposed group: ASM prophylaxis only
- Low intensity treatment: Monotherapy with: levetiracetam at a dose of <2000mg/day (<1000mg/day in impaired renal function), phenytoin at ≤ 300mg/day or mean level < 15mcg/mL, valproic acid at ≤ 15mg/kg/day or mean level < 75mcg/mL, or lacosamide at ≤ 200mg/day
- High intensity treatment: >48 hours of levetiracetam at doses of ≥ 2000mg/day (≥ 1000mg/day in impaired renal function), phenytoin at >300mg/day or mean level ≥ 15mcg/mL, Valproic acid at >15mg/kg/day or mean level ≥ 75mcg/mL, lacosamide > 200mg/day, use of 2 or more ASMs, or initiation of anesthetics for treatment of epileptiform activity.
ASM: anti-seizure medications; cEEG: continuous electroencephalography
In secondary analyses, we classified continued ASM therapy into two groups: 1) Low intensity treatment defined as continuation or re-initiation of monotherapy with: levetiracetam at a dose of <2000mg/day (<1000mg/day in patients with renal dysfunction); phenytoin with mean serum levels of <15 mcg/mL or dose of ≤300 mg/day if no serum levels available; valproic acid with mean serum levels <75 mcg/mL or dose of ≤ 15mg/kg/day if no serum levels available; lacosamide at ≤ 200mg/day; and 2) High intensity treatment defined as treatment with: levetiracetam at doses of ≥ 2000mg/day (≥1000mg/day in patients with renal dysfunction); phenytoin with mean serum levels ≥15 mcg/mL or dose >300mg/day if no levels available; Valproic acid with mean serum levels ≥75 mcg/mL or dose >15mg/kg/day if no levels available; lacosamide > 200mg/day; use of 2 or more ASMs; initiation of anesthetics for treatment of epileptiform activity.
Follow up for outcomes started after day 10 of admission and continued in an intention-to-treat scheme until 100 days post admission (or 90 days from start of follow up).
Clinical covariates
We collected demographic and clinical variables from the electronic health record. We calculated critical illness severity, i.e., Admission Acute Physiology and Chronic Health Evaluation II (APACHE II) scores (10), and aSAH severity scores, e.g., Hunt and Hess (HH), Fisher and FRESH scores (11–13). The FRESH score predicts long-term outcomes and is comprised of age, Hunt and Hess score, physiologic APACHE II score and presence of re-bleed (13). Charlson Comorbidity index (CCI) was calculated as an integrated measure of baseline chronic health conditions (14). Delayed complications including delayed cerebral ischemia (DCI) and hospital-acquired infections were recorded. Methods for DCI adjudication have been previously published (2,15).
EEG features
Methods for reviewing and reporting EEGs, classifying epileptiform activity, and quantifying burden of epileptiform activity have been previously published (2). At our center all patients with high-grade aSAH (≥HH3 or ≥Fisher3) under go cEEG monitoring for ischemia detection. Additional indications for cEEG monitoring include evaluation for subclinical seizures and monitoring the depth of sedation.
We defined epileptiform activity using the ACNS nomenclature (16). We included the following patterns in our definition of epileptiform activity: lateralized periodic discharges (LPDs), bilateral independent periodic discharges (BIPDs), generalized periodic discharges (GPDs), lateralized rhythmic delta activity (LRDA), and sporadic discharges. Epileptiform activity burden was quantified using the ACNS nomenclature: rare: <1%, occasional: 1–9%, frequent: 10–49%, abundant: 50–89%, continuous: ≥90% (16). Epileptiform activity burden was calculated for the first 24 hours of recording and the maximum burden (peak burden) within any 24-hour epoch as previously described (2).
Mortality and functional outcomes
Primary outcomes were 90-day mortality and 90-day modified Rankin Scale (mRS). The mRS is a 6-point scale: 0 - no symptoms; 1 - no significant disability; 2 - slight disability; 3 - moderate disability; 4 - moderately severe disability; 5 - severe disability; 6 – dead (17,18). mRS was abstracted from physician and physical therapy clinical examinations by three reviewers (MA, PS, SR) who were blinded to the EEG findings and ASM treatment. Good outcomes were defined as mRS 0–3, and poor outcome as mRS 4–6.
Additional outcomes
We also collected information on the occurrence of in-hospital ASM-specific adverse effects through the same electronic health record chart abstraction (see supplemental material Table 1). In addition, we measured the time to sustained EEG improvement, defined as time taken (in hours) for peak burden to decrease to a lower level as measured by American Clinical Neurophysiology Society (ACNS) criteria and be sustained at a lower level for >48 hours. We also examined time to late-onset (after post-bleed day 14) clinical seizures.
Statistical analysis
Univariate analysis was performed using Fisher’s exact test for dichotomized and categorical variables, and the Mann-Whitney-U-test for continuous variables. Significance was set at 0.05, and 2-sided P values are reported. Equality of survival functions was assessed using the log-rank test.
To adjust for potential differences between patients receiving prophylactic versus continued ASM therapy, we calculated propensity scores for continued therapy using logistic regression models. The propensity score regression models included variables likely to be associated with ASM treatment, predictors of poor functional outcomes and risk factors associated with DCI (2,3,11–13,19,20). Variables selected for the propensity score captured illness severity, EEG findings, and comorbidities and were measured prior to the exposure window. The following variables were included in the propensity score regression model for the primary analysis: CCI, FRESH score, Fisher score, first 24-hr epileptiform activity burden. We used the FRESH score in our propensity model instead of the individual components in order to avoid overfitting the model. The outcome/dependent variable in the propensity score logistic regression model was ASM treatment exposure. The area under the receiver operating curve (ROC) for the propensity score regression model for the primary analysis was 0.80.
In secondary analyses we performed pairwise comparisons assessing ASM treatment intensity. Three separate propensity scores were built to estimate the likelihood of 1) low intensity ASM treatment vs. prophylaxis only, 2) high intensity treatment vs. prophylaxis only, and 3) high intensity ASM treatment vs. low intensity treatment. Independent variables included in each of the models were: CCI, FRESH score, Fisher score and the first 24-hour epileptiform activity burden. Performance of the propensity score regression models was assessed using the area under the ROC: Low intensity treatment vs. prophylaxis only – 0.76; high intensity treatment vs. prophylaxis only – 0.87; high intensity vs. low intensity treatment – 0.76.
For each primary and secondary analysis, the corresponding estimated propensity score was included in a Cox-proportional hazard model to assess the association between ASM treatment and outcomes. The proportional hazards assumption was checked using Schoenfeld residuals and log-log survival plots. Hazard ratios are presented with 95% confidence intervals as HR [95% CI]. Patients lost to follow up were right censored in the analysis.
Results
Overall, 94 patients met inclusion criteria; 54 (57.4%) patients received prophylaxis only, and 40 (42.6%) either continued or re-initiated ASM treatment (combined low and high intensity). Patients in the prophylaxis only group received a median of 24 hours of ASMs (IQR 24–24). Patients continued on treatment received a median of 14 days of inpatient ASM treatment (IQR 11–20). Of the 31 patients continued on treatment that were alive at discharge, 20 (64.5%) were discharged with ASM prescriptions. Table 1 summarizes the clinical and demographic characteristics. There was no missing data. Patients continuing or re-initiating ASM treatment were likely to have higher APACHE II (16 [11–21] vs. 11[7–18], p=0.035) and FRESH scores (4.4[3.7–6.1] vs. 3[1.8–4.4], p=0.003), compared with patients receiving ASM prophylaxis only. Patients treated with ASMs were also more likely to have epileptiform activity, with higher first 24-hr and peak burdens. Peak burden was defined as the epileptiform activity burden in the 24-hr epoch with the highest burden.
Table 1.
Clinical and Demographic variables
| Prophylaxis only N= 54 | ASM treatment (low +high intensity) N=40 | P value | |
|---|---|---|---|
| Age, median (IQR) | 57 [49–68] | 60 [57–73] | 0.243 |
| Gender, female (%) | 42 (78%) | 29 (73%) | 0.556 |
| CCI, median (IQR) | 2 [1–3] | 2 [1–3] | 0.447 |
| APACHE II, median (IQR) | 11 [7–18] | 16 [11–21] | 0.035 |
| Hunt and Hess | 0.028 | ||
| 1 | 15 (28%) | 4 (10%) | |
| 2 | 9 (17%) | 11 (28%) | |
| 3 | 15 (28%) | 6 (15%) | |
| 4 | 12 (22%) | 11 (28%) | |
| 5 | 3 (6%) | 8 (20%) | |
| Fisher Score | 0.244 | ||
| 1 | 0 (0%) | 0 (0%) | |
| 2 | 6 (11%) | 2 (5%) | |
| 3 | 42 (78%) | 29 (73%) | |
| 4 | 6 (11%) | 9 (23%) | |
| FRESH Score | 3 [1.9–4.4] | 4.4 [3.0–6.1] | 0.003 |
| Treatment modality | 0.699 | ||
| Coil | 32 (59%) | 19 (48%) | |
| Clip | 19 (35%) | 19 (48%) | |
| Clip plus coil | 1 (2%) | 1 (3%) | |
| Flow diverter | 1 (2%) | 1 (3%) | |
| Flow diverter plus coil | 1 (2%) | 0 (0%) | |
| Re-bleed | 0 (0%) | 9 (23%) | <0.0001 |
| Delayed Cerebral Ischemic | 25 (46%) | 30 (75%) | 0.006 |
| Days to EEG start, median (IQR) | 2 [1–3] | 2 [1–2] | 0.820 |
| EEG duration in days, median (IQR) | 6.6 [4.8–8.8] | 8.7 [6.9–9.7] | 0.005 |
| First 24-hr epileptiform activity burden | 0.0001 | ||
| None | 46 (85%) | 20 (50%) | |
| Rare (<l%) | 5 (9%) | 3 (8%) | |
| Occasional (1–9%) | 1 (2%) | 4 (10%) | |
| Frequent (10–49%) | 0 (0%) | 3 (8%) | |
| Abundant (50–89%) | 2 (4%) | 9 (23%) | |
| Continuous (>90%) | 0 (0%) | 1 (3%) | |
| Peak epileptiform activity burden | 0.0001 | ||
| None | 38 (70%) | 8 (20%) | |
| Rare (<1%) | 6 (11%) | 3 (8%) | |
| Occasional (1–9%) | 5 (9%) | 4 (10%) | |
| Frequent (10–49%) | 2 (4%) | 9 (23%) | |
| Abundant (50–89%) | 3 (6%) | 9 (23%) | |
| Continuous (>90%) | 0 (0%) | 7 (18%) | |
| Hospital Acquired Pneumonia | 19 (35%) | 22 (55%) | 0.062 |
| Duration of Mechanical ventilation in days, median [IQR] | 0 [0–3] | 10.5 [4–15.5] | <0.0001 |
| ICU length of stay in days, median [IQR] | 14.5 [11–17] | 18 [15–22] | 0.0004 |
CCI: Charlson Comorbidity index; IQR: Inter-quartile range
Table 2 summarizes disease severity and epileptiform activity burden across the three levels of treatment intensity. All patients in the low intensity group received levetiracetam at <2000 mg/day, and had preserved renal function. In the high intensity group 16 (94.1%) received levetiracetam at >2000mg/day, 2 (11.8%) received phenytoin at >300mg/day (mean serum level of 15.4 mcg/mL, and peak levels of 17.4mcg/mL and 22.1mcg/mL), 1 (6.8%) received lacosamide at >200mg/day, 6 (37.5%) received multiple ASMs and 1 (6.8%) received anesthetics for treatment of cEEG findings (these percentages are not mutually exclusive).
Table 2.
Clinical variables across levels of treatment intensity
| Prophylaxis only vs. Low intensity treatment | |||
|---|---|---|---|
| Prophylaxis only N=54 | Low intensity treatment N=23 | P value | |
| Age | 57[49–68] | 65 [50–78] | 0.109 |
| CCI | 2 [1–3] | 3 [1–4] | 0.140 |
| Fisher Score | 0.225 | ||
| 1 | 0 (0%) | 0 (0%) | |
| 2 | 6 (11%) | 0 (0%) | |
| 3 | 42 (78%) | 19 (83%) | |
| 4 | 6 (11%) | 4 (17%) | |
| Fresh Score | 3 [2–4] | 5[4–6] | 0.008 |
| First 24-hr epileptiform activity burden | 0.078 | ||
| None | 46 (85%) | 15 (65%) | |
| Rare | 5 (9%) | 2 (9%) | |
| Occasional | 1 (2%) | 2 (9%) | |
| Frequent | 0 (0%) | 0 (0%) | |
| Abundant | 2 (4%) | 3 (13%) | |
| Continuous | 0 (0%) | 1 (4%) | |
| Prophylaxis only vs. High intensity treatment | |||
| Prophylaxis only N=54 | High intensity treatment N=17 | ||
| Age | 57[49–68] | 59 [51–64] | 0.909 |
| CCI | 2 [1–3] | 2 [1–2] | 0.654 |
| Fisher Score | 0.163 | ||
| 1 | 0 (0%) | 0 (0%) | |
| 2 | 6 (11%) | 2 (12%) | |
| 3 | 42 (78%) | 10 (56%) | |
| 4 | 6 (11%) | 5 (29%) | |
| Fresh Score | 3 [2–4] | 4[4–6] | 0.043 |
| First 24-hr epileptiform activity burden | |||
| None | 46 (85%) | 5 (29%) | <0.001 |
| Rare | 5 (9%) | 1 (6%) | |
| Occasional | 1 (2%) | 2 (12%) | |
| Frequent | 0 (0%) | 3 (18%) | |
| Abundant | 2 (4%) | 6 (35%) | |
| Continuous | 0 (0%) | 0 (0%) | |
| Low intensity vs. High intensity treatment | |||
| Low intensity treatment N=23 | High intensity treatment N=17 | p-value | |
| Age | 65 [50–78] | 59 [51–64] | 0.191 |
| CCI | 3 [1–4] | 2 [1–2] | 0.072 |
| Fisher Score | 0.119 | ||
| 1 | 0 (0%) | 0 (0%) | |
| 2 | 0 (0%) | 2 (12%) | |
| 3 | 19 (83%) | 10 (56%) | |
| 4 | 4 (17%) | 5 (29%) | |
| Fresh Score | 5[4–6] | 4[4–6] | 0.528 |
| First 24-hr epileptiform activity burden | 0.053 | ||
| None | 15 (65%) | 5 (29%) | |
| Rare | 2 (9%) | 1 (6%) | |
| Occasional | 2 (9%) | 2 (12%) | |
| Frequent | 0 (0%) | 3 (18%) | |
| Abundant | 3 (13%) | 6 (35%) | |
| Continuous | 1 (4%) | 0 (0%) | |
CCI: Charlson Comorbidity index
Although limited by retrospective chart review, we attempted to examine the indications for ASM treatment exposure. Among the ASM treatment exposure group, we found that in 26/32 (82%) patients with epileptiform activities, there was clear documentation of ASMs being continued or re-initiated in response to cEEG epileptiform activity. In the remaining 6 patients with epileptiform activity within the exposure group, the indication for ASM continuation or initiation was not clearly documented. Among the 8 patients in the ASM treatment exposure group with no epileptiform activity, 2 were treated for generalized rhythmic slowing and one for potential risk of alcohol withdrawal seizure. Indication for continuation and/or initiation of ASMs in the remaining 5 patients was not clearly documented.
Relative hazards of primary outcomes
Figures 3a and 3b show the unadjusted and adjusted survival curves. At 90 days, mortality was 9/40 (23%) in the ASM continuation group and 5/54 (9%) in the prophylaxis only group. All patients with mortality had withholding of life sustaining therapy or were discharged to hospice. The unadjusted HR for 90-day mortality in the ASM treatment group was 2.83 [0.95–8.45]. After adjusting for the propensity score, the HR remained elevated, though confidence intervals included the null hypothesis value (HR 2.01 [0.57–7.02]).
Figure 3. 90-day mortality and functional outcomes.
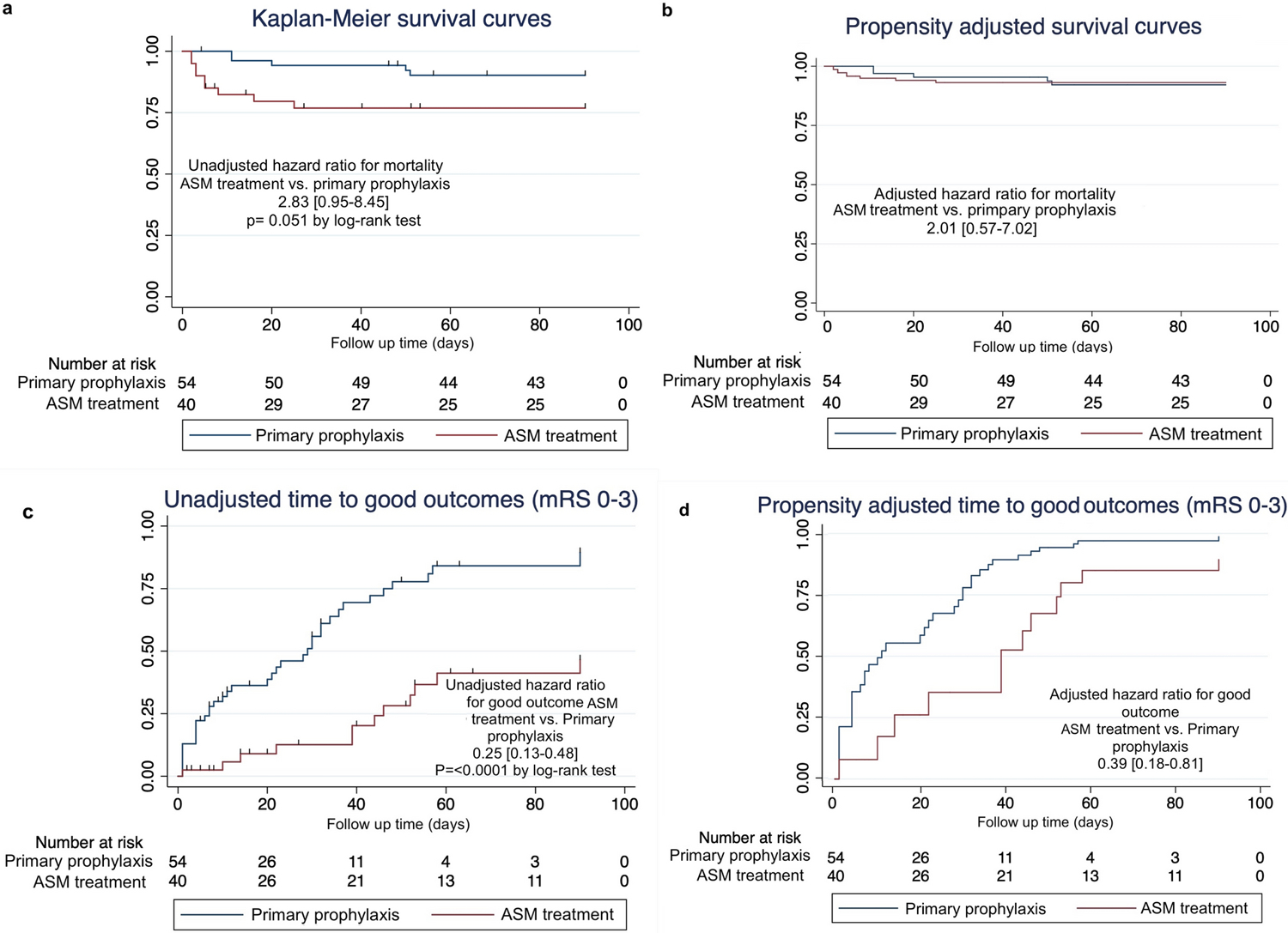
- Kaplan-Meier curves for time to death comparing ASM treatment (low + high intensity) vs. prophylaxis only.
- Propensity score adjusted survival curves for time to death comparing ASM treatment (low + high intensity) vs. prophylaxis only.
- Kaplan-Meier curves for time to good functional outcome (mRS 0–3) comparing ASM treatment (low+ high intensity) vs. prophylaxis only.
- Propensity score adjusted survival curves for time to good functional outcome (mRS 0–3) comparing ASM treatment (low+ high intensity) vs. prophylaxis only.
ASM: anti-seizure medications
Figures 3c and 3d show the unadjusted and adjusted survival curves for good outcomes defined as mRS 0–3. At 90 days 12 (30%) patients in the ASM continuation group had good functional outcomes vs. 38 (70%) in the prophylaxis only group. The unadjusted HR for good functional outcome in the ASM treatment group was 0.25 [0.13–0.48] compared to ASM prophylaxes only. After adjusting for the propensity score, ASM treatment continued to be associated with lower likelihood for good functional outcome at 90-days (HR 0.39 [0.18–0.81]). Survival data, including loss to follow up rates, are summarized in Supplemental Tables 2 and 3.
Within pairwise comparisons, there was no significant difference in 90-day survival comparing low intensity treatment to prophylaxis (adjusted HR: 0.60 [0.10–3.59]). High intensity treatment was associated with higher 90-day mortality compared to both prophylaxis (adjusted HR: 6.80 [1.67–27.65]) and low intensity treatment (adjusted HR:9.79 [1.75–54.7]), although the confidence intervals are wide.
Both low intensity treatment (adjusted HR: 0.37 [0.15–0.91]) and high intensity treatment (adjusted HR: 0.30 [0.10–0.94] were less likely to be associated with good 90-day functional outcomes, compared with prophylaxis only. Functional outcomes were not significantly different comparing high vs. low intensity treatment (adjusted HR 1.10 [0.31–3.88]).
Additional outcomes
The distribution of adverse effects and head imaging studies across patients receiving any ASM treatment (combined low and high intensity) vs. prophylaxis only is summarized in supplemental Table 4. Most adverse effects, including delirium, sedation, cardiac and gastrointestinal adverse outcomes, were more frequent in the ASM treatment group, though not significant. Patients receiving ASM treatment (combined low and high intensity) vs. prophylaxis only underwent a higher number of head imaging studies (p=0.004).
The median time to sustained EEG improvement was 48 hours in both prophylaxis-only and ASM treatment groups. The mean time to sustained EEG improvement in the ASM treatment (combined low and high intensity) group was 62.6 hours. As the largest observed analysis time in the ASM prophylaxis group was censored, we report both restricted and extended mean time to EEG improvement. The restricted mean time for sustained improvement in the ASM prophylaxis only group was 61.2 hours (largest observed analysis time was censored and mean underestimated). The extended mean time to EEG improvement (computed by exponentially extending the survival curve to zero) was 86.7 hours for the ASM prophylaxis group. Only one patient in our cohort had late seizures; these occurred in the ASM continuation group at 3-months.
Sensitivity analysis
We performed additional sensitivity analysis including DCI in our cox regression models. After adjusting for the propensity score and DCI, the HR for 90-day mortality was 1.82 [0.51–5.51]. After adjusting for the propensity score and DCI, ASM treatment continued to be associated with lower likelihood of good functional outcome at 90-days (HR 0.42 [0.20–0.88]).
Discussion
Our study adds support to the hypothesis that continuation and escalation of ASM treatments in aSAH patients with cEEG abnormalities contributes to worse outcomes. Our data show that aggressive treatment with high dose and multiple ASMs may be associated with increased mortality, even after adjusting for disease severity. We also found that, while low intensity ASM treatment was not significantly associated with mortality, its association with worse functional outcomes was similar to high intensity ASM treatment. Taken together, these findings suggest that the optimal approach to manage ASM treatment in aSAH patients with epileptiform cEEG abnormalities needs to consider both the risk of harmful epileptiform abnormalities and the risk of adverse ASM effects, in order to carefully balance risks and benefits.
In current practice, ASMs are often escalated in acutely ill patients in response to EEG findings, and are often continued long-term (21,22). Similarly, patients with aSAH undergoing EEG monitoring are frequently treated with ASMs (2,3). In the absence of clear treatment guidelines, this may result in overtreatment of patients and exposure to ASM related adverse effects. In our cohort, after propensity-score adjustment there was an increase in the hazards for worse survival in the high-intensity treatment group vs. the prophylaxis and the low-intensity groups. Although the confidence intervals were large, this suggests that aggressive treatment with multiple ASMs may yield net harm.
Low intensity treatment showed a trend towards better survival; however this effect was small and with confidence intervals that included the null hypothesis. At the same time, low intensity treatment was associated with worse functional outcomes. Future trials can help determine if aSAH patients with epileptiform abnormalities may benefit from a brief course of ASM treatment during the acute phase, followed by rapid weaning, to minimize adverse effects that may worsen functional outcomes.
ASM treatment (combined low and high intensity) was associated with worse functional outcomes compared with ASM prophylaxis only. Up to 80% of patients with epilepsy taking ASMs experience side effects including cognitive slowing, gait unsteadiness, mood symptoms, headaches and drowsiness (23–25). These adverse effects could explain the increased likelihood of worse functional outcomes observed in patients receiving prolonged high intensity ASM treatment. Although patients with ASM treatment had a higher frequency of adverse effects, our study was underpowered to examine significant differences in adverse effects, and larger studies will be needed to either confirm or refute our findings.
Lack of immediate EEG improvement with ASMs may result in further escalation of ASM treatment. However, EEG improvement in isolation should not be considered the only treatment endpoint (26). In addition, as demonstrated in our cohort, even in patients who do not receive ASM treatment, epileptiform activity burden often decreases with time. While clinical improvement is a more reliable target, this is often difficult to demonstrate in severe aSAH patients who are comatose. Epileptiform activity is associated with increased brain metabolism as demonstrated by PET studies, which has been interpreted to imply risk for secondary brain injury (27). In addition, epileptiform activity in aSAH patients has been shown to be associated with decreased brain tissue oxygenation, and in patients with traumatic brain injury with increased brain lactate/pyruvate ratios (28,29). Future studies examining such biomarkers of brain metabolism as treatment targets could provide further insight into appropriate ASM treatment strategies in this population.
New or worsening epileptiform activity in aSAH patients is also a harbinger for DCI (7,9). While DCI itself is also associated with worse outcomes, we did not include it in our regression analysis as it occurs downstream from both the development of epileptiform activity and exposure to ASM treatment. We did, however include initial clinical presentation, imaging findings and EEG findings in the propensity score, all of which are predictors of DCI (7,9,20). In our sensitivity analysis, after adjusting for DCI, we found ASM treatment exposure continued to have a significant association with functional outcomes. DCI pathophysiology is complex and multifactorial, including early arteriolar vasospasm, microthrombosis, spreading depolarizations, inflammatory responses and large-vessel vasospasm (30). Therefore a combination of ASM treatment with interventions geared towards increasing cerebral blood flow e.g. induced hypertension, may serve as a more effective treatment strategy. Figure 4 provides a conceptual diagram based on our findings and our suggested interpretation of them, contrasting current practice and our hypothesized optimal treatment of cEEG epileptiform activity in aSAH patients. We propose the optimal treatment of aSAH patients found to have epileptiform abnormalities (seizures, LPDs, GPDs and LRDA) on cEEG is a combination of brief duration low to moderate dose ASM treatment along with treatments targeting cerebral perfusion, and guided by biomarkers of brain metabolism. Future randomized studies are indicated to address the impact of this intervention strategy.
Figure 4. Conceptual diagram contrasting ASM treatment strategies in aSAH patients.
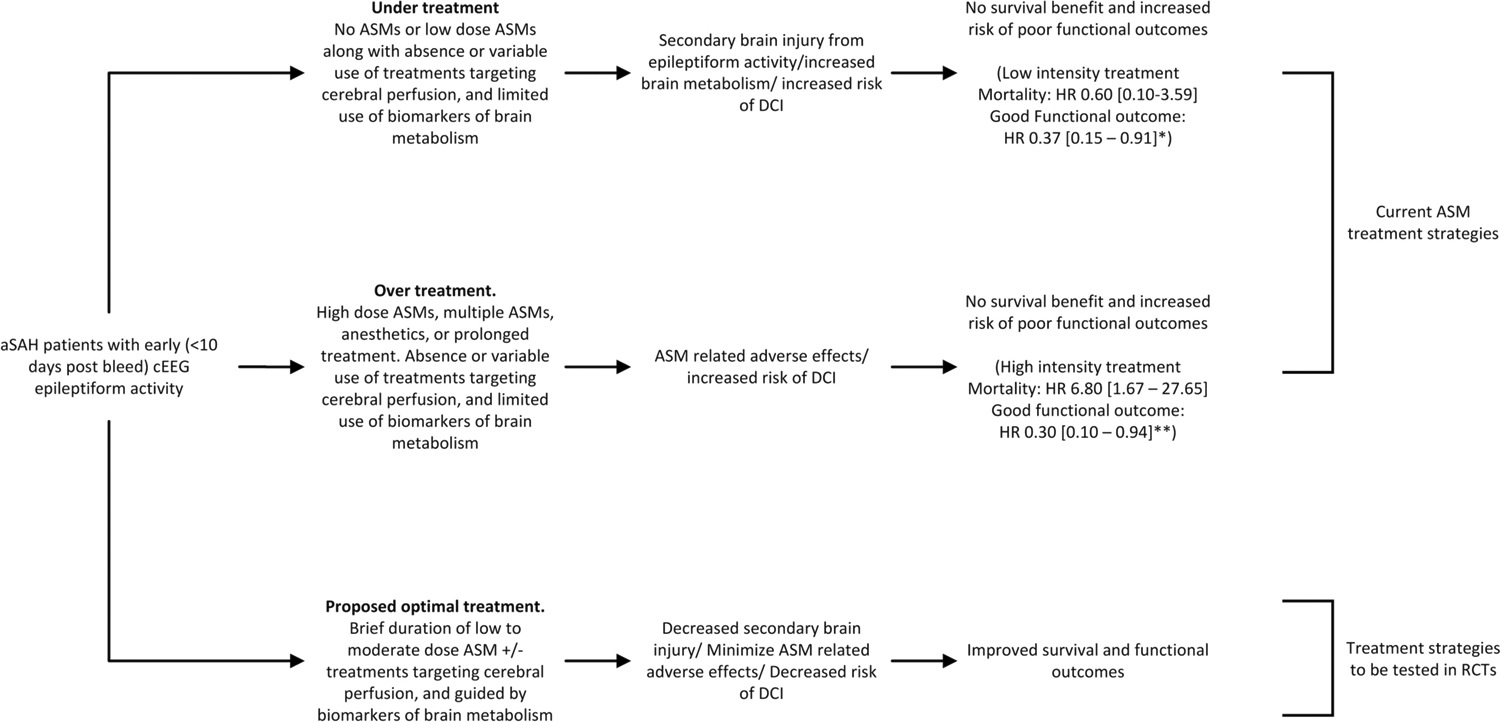
Comparison of risks and benefits of ASM treatment at different intensities, and in combination with treatment modalities aimed at improving cerebral perfusion are shown. We hypothesize that treatment of cEEG epileptiform activity with low to moderate dose ASMs in conjunction with treatments aimed at augmenting cerebral perfusion, and guided by biomarkers of brain metabolism and oxygenation, can improve survival and functional outcomes in aSAH patients. Future randomized studies utilizing cEEG are indicated to test this hypothesis.
* Hazard ratio and confidence interval for mortality and good functional outcomes comparing low intensity treatment with ASM prophylaxis only
** Hazard ratio and confidence interval for mortality and good functional outcomes comparing high intensity treatment with ASM prophylaxis only
aSAH: aneurysmal subarachnoid hemorrhage; ASM: anti-seizure medication; DCI: delayed cerebral ischemia; cEEG: continuous electroencephalography
Limitations of our study include the retrospective nature, the small sample size, and the potential for residual confounding, which limit the ability to draw causal conclusions. This is a single center study, limiting generalizability. Additionally, our institutional approach of primary ASM prophylaxis until the aneurysm is secured may not generalize. Finally, all patients with mortality were transitioned to hospice or had withholding of life sustaining therapies. Our strict inclusion and exclusion criteria were aimed at decreasing confounding by indication; however, this came at the expense of sample size. We used propensity methodology to address confounding by indication, and as demonstrated by the shift from crude to adjusted estimates, the scores performed well in adjusting for disease severity and propensity for treatment. Nevertheless, as a retrospective analysis, our results should be taken not as proof for our proposed optimal ASM management strategy for epileptiform abnormalities, but rather as supporting the need for a prospective clinical trial.
Conclusions
Our data suggest that current ASM treatment strategies in aSAH patients may be associated with worse functional outcomes. One possible interpretation is that increased protocolized cEEG monitoring in this population leads to overtreatment and worse functional outcomes. On the other hand, there is clear evidence that epileptiform abnormalities predict DCI and are associated with worse outcomes in aSAH patients, thus intervening might improve outcomes (1–3, 7,9). Overall, it is most likely we do not yet know which cases warrant treatment, and nor do we know how to match treatment intensity to the nature of the epileptiform abnormalities. Future prospective and randomized studies are needed to determine whether low to moderate dose treatment of epileptiform activity in the acute phase can improve survival and functional outcomes. Further work is needed to determine clear ASM treatment targets, including biomarkers for brain metabolism and cerebral blood flow. Studies using composite endpoints of EEG findings, clinical exam, and biomarker improvement, could identify which epileptiform activity patterns warrant treatment to improve long-term outcomes. Finally, larger studies are needed to determine ASM safety and overall risk-benefit ratio of treatment.
Supplementary Material
Conflicts of Interest and Disclosure
Dr. Zafar is supported by NIH grant K23NS114201. Dr. Zafar is a clinical neurophysiologist for Corticare, unrelated to this work. Dr. Rosenthal received consulting fees from UCB Pharma, Inc. and Ceribell, Inc., unrelated to this work. Dr. Postma reports no disclosures. Dr. Sanches reports no disclosures. Dr. Ayub reports no disclosures. Dr. R reports no disclosures. Dr. Kim reports no disclosure. Dr. Rubin has a pending patent for “System and Method for Determining Treatment Outcomes for Neurological Disorders Based on Functional Connectivity Parameters”. Dr. Lee reports no disclosures. Dr. Patel reports no disclosures. Dr. Hsu reports grants from the NIH (P01AG032952 and R01AG062282). Dr. Patorno is supported by K08AG055670 from the National Institute on Aging and is investigator of an investigator-initiated grant to the Brigham and Women’s Hospital from Boehringer Ingelheim, not related to this work.. Dr. Westover is cofounder of Beacon Biosignals unrelated to this work, and was supported by the Glenn Foundation for Medical Research and American Federation for Aging Research (Breakthroughs in Gerontology Grant); American Academy of Sleep Medicine (AASM Foundation Strategic Research Award); Football Players Health Study (FPHS) at Harvard University; Department of Defense through a subcontract from Moberg ICU Solutions, Inc; and NIH (1R01NS102190, 1R01NS102574, 1R01NS107291, 1RF1AG064312).
STROBE checklist was used for this study.
Funding
This study received research support from NIH K23NS114201 (SFZ).
Footnotes
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
Publisher's Disclaimer: The manuscript complies with all instructions to authors. Authorship requirements have been met and the final manuscript was approved by all authors. This manuscript has not been published elsewhere and is not under consideration by another journal. This manuscript adheres to ethical guidelines. The study was approved by the Institutional Review Board of Mass General Brigham. Informed consent was not required for this retrospective study.
References
- 1.Claassen J, Hirsch LJ, Frontera JA, Fernandez A, Schmidt M, Kapinos G, Wittman J, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2006;4:103–12. [DOI] [PubMed] [Google Scholar]
- 2.Zafar SF, Postma EN, Biswal S, Boyle EJ, Bechek S, O’Connor K, Shenoy A, et al. Effect of epileptiform abnormality burden on neurologic outcome and antiepileptic drug management after subarachnoid hemorrhage. Clin Neurophysiol. 2018;129:2219–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Marchis GM, Pugin D, Meyers E, Velasquez A, Suwatcharangkoon S, Park S, Falo MC, et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology. 2016;86:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naidech AM, Kreiter KT, Janjua N, Ostapkovich N, Parra A, Commichau C, Connolly ES, et al. Phenytoin exposure is associated with functional and cognitive disability after subarachnoid hemorrhage. Stroke. 2005;36:583–7. [DOI] [PubMed] [Google Scholar]
- 5.Yoon SJ, Joo JY, Kim YB, Hong CK, Chung J. Effects of prophylactic antiepileptic drugs on clinical outcomes in patients with a good clinical grade suffering from aneurysmal subarachnoid hemorrhage. J Cerebrovasc Endovasc Neurosurg. 2015;17(3):166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012;43:1711–37. [DOI] [PubMed] [Google Scholar]
- 7.Kim JA, Rosenthal ES, Biswal S, Zafar S, Shenoy AV, O’Connor KL, Bechek SC, et al. Epileptiform abnormalities predict delayed cerebral ischemia in subarachnoid hemorrhage. Clin Neurophysiol. 2017;128:1091–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vergouwen MD, Vermeulen M, van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, Mendelow AD, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–5. [DOI] [PubMed] [Google Scholar]
- 9.Rosenthal ES, Biswal S, Zafar SF, O’Connor KL, Bechek S, Shenoy AV, et al. Continuous electroencephalography predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective study of diagnostic accuracy. Ann Neurol. 2018;83:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit care med. 1985;13:818–29. [PubMed] [Google Scholar]
- 11.Hunt WE, Hess RM. “Surgical risk as related to time of intervention in the repair of intracranial aneurysms.” J Neurosurg 1968;28:14–20. [DOI] [PubMed] [Google Scholar]
- 12.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. [DOI] [PubMed] [Google Scholar]
- 13.Witsch J, Frey HP, Patel S, Park S, Lahiri S, Schmidt JM, Agarwal S, et al. Prognostication of long‐term outcomes after subarachnoid hemorrhage: The FRESH score. Ann Neurol. 2016;80:46–58. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 15.Zafar S, Westover MB, Gaspard N, Gilmore E, Foreman B, O’Connor K, Rosenthal ES. Inter-rater agreement for consensus definitions of delayed ischemic events following aneurysmal subarachnoid hemorrhage. J Clin Neurophysiol. 2016;33:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, Mani R, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. [DOI] [PubMed] [Google Scholar]
- 17.Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry. 1991;54:1044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel N, Rao VA, Heilman-Espinoza ER, Lai R, Quesada RA, Flint AC. Simple and reliable determination of the modified rankin scale score in neurosurgical and neurological patients: the mRS-9Q. Neurosurgery. 2012;71:971–5. [DOI] [PubMed] [Google Scholar]
- 19.Zafar SF, Postma EN, Biswal S, Fleuren L, Boyle EJ, Bechek S, O’Connor K, et al. Electronic health data predict outcomes after aneurysmal subarachnoid hemorrhage. Neurocrit care. 2018;28:184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams HP, Kassell NF, Torner JC, Haley EC. Predicting cerebral ischemia after aneurysmal subarachnoid hemorrhage: influences of clinical condition, CT results, and antifibrinolytic therapy. A report of the Cooperative Aneurysm Study. Neurology. 1987;37:1586–1591 [DOI] [PubMed] [Google Scholar]
- 21.Kilbride RD, Costello DJ, Chiappa KH. How seizure detection by continuous electroencephalographic monitoring affects the prescribing of antiepileptic medications. Arch Neurol. 2009;66:723–8. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez V, Ruiz AA, LaRoche S, Hirsch LJ, Parres C, Voinescu PE, Fernandez A, et al. The use and yield of continuous EEG in critically ill patients: A comparative study of three centers. Clin Neurophys. 2017;128:570–8. [DOI] [PubMed] [Google Scholar]
- 23.Baker GA, Jacoby A, Buck D, Stalgis C, Monnet D. Quality of life of people with epilepsy: a European study. Epilepsia 1997.38:353. [DOI] [PubMed] [Google Scholar]
- 24.Brodie MJ, Richens A, Yuen AW. Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. UK Lamotrigine/Carbamazepine Monotherapy Trial Group. Lancet 1995;345:476–479. [DOI] [PubMed] [Google Scholar]
- 25.Perucca P, Carter J, Vahle V, Gilliam FG. Adverse antiepileptic drug effects Toward a clinically and neurobiologically relevant taxonomy. Neurology. 2009;72:1223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubinos C, Reynolds AS, Claassen J. The ictal–interictal continuum: to treat or not to treat (and how)?. Neurocrit care. 2018;29:3–8. [DOI] [PubMed] [Google Scholar]
- 27.Struck AF, Westover MB, Hall LT, Deck GM, Cole AJ, Rosenthal ES. Metabolic correlates of the ictal-interictal continuum: FDG-PET during continuous EEG. Neurocrit care. 2016;24:324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vespa P, Tubi M, Claassen J, Buitrago-Blanco M, McArthur D, Velazquez AG, Tu B, et al. Metabolic crisis occurs with seizures and periodic discharges after brain trauma. Ann Neurol. 2016:79: 579–90. [DOI] [PubMed] [Google Scholar]
- 29.Witsch J, Frey H, Schmidt JM, Velazquez A, Falo CM, Reznik M, Roh D, et al. Electroencephalographic Periodic Discharges and Frequency-Dependent Brain Tissue Hypoxia in Acute Brain Injury. JAMA Neurol. 2017. 74, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nature Reviews Neurology. 2014;10:44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


