Abstract
Purpose:
Determine 1) if adults with facioscapulohumeral muscular dystrophy (FSHD) exhibit exercise intolerance and 2) potential contributing mechanisms to exercise intolerance, specific to FSHD.
Methods:
Eleven people with FSHD (47±13 years, 4 females) and eleven controls (46±13 years, 4 females) completed one visit, which included a volitional peak oxygen consumption (VO2peak) cycling test. Breath-by-breath gas exchange, ventilation, and cardiovascular responses were measured at rest and during exercise. The test featured three-minute stages (speed: 65–70 RPM) with incremental increases in intensity (FSHD: 20-watts/stage; control: 40 to 60-watts/stage). Body lean mass (LM (kg, %)) was collected via dual-energy x-ray absorptiometry.
Results:
VO2peak was 32% lower (24.5±9.7 vs, 36.2 ±9.3 mL/kg/min; p<0.01) and wattage was 55% lower in FSHD (112.7±56.1 vs. 252.7±67.7 watts; p<0.01). When working at a relative submaximal intensity (40% of VO2peak), wattage was 55% lower in FSHD (41.8±30.3 vs. 92.7±32.6 watts, p=0.01), though ratings of perceived exertion (RPE) (FSHD: 11±2 vs. control: 10±3, p=0.61), and dyspnea (FSHD: 3±1 vs. control: 3±2, p=0.78) were similar between groups. At an absolute intensity (60-watts), RPE was 63% higher (13±3 vs. 8±2, p<0.01) and dyspnea was 180% higher in FSHD (4±2 vs. 2±2, p<0.01). VO2peak was most strongly correlated with resting O2 pulse in controls (p<0.01, r=0.90) and %leg LM in FSHD (p<0.01; r=0.88). Among FSHD participants, VO2peak was associated with self-reported functionality (FSHD-HI score; activity limitation: p<0.01, r=−0.78), indicating a strong association between perceived and objective impairments.
Conclusions:
Disease-driven losses of LM contribute to exercise intolerance in FSHD, as evidenced by a lower VO2peak and elevated symptoms of dyspnea and fatigue during submaximal exercise. Regular exercise participation may preserve LM, thus providing some protection against exercise tolerance in FSHD.
Keywords: Functional capacity, VO2max, skeletal muscle mass, muscular dystrophy, exercise capacity
INTRODUCTION
Facioscapulohumeral muscular dystrophy (FSHD), a dominantly inherited genetic myopathy linked to an overexpression of the DUX4 gene, is the third most common type of muscular dystrophy. The expression of the DUX4 gene influences many common pathways, eventually leading to muscle cell death (1). FSHD is characterized by progressive, often asymmetrical muscular atrophy and weakness in the face, shoulder girdle, and upper-arm region (2, 3). Among people with FSHD, the loss of lean mass (LM) manifests physiologically in a reduced functional capacity (4), or “an individual’s ability to perform work (4).” Therefore, adults with FSHD have the potential for exercise intolerance, a condition which has been characterized by exertional fatigue, labored breathing (dyspnea) during exercise, and an inability to meet age- and sex-predicted values of physical performance (5). Exercise intolerance can be driven by a combination of individual factors, such as those which stem from neural, hemodynamic, and peripheral causes, and include low cardiac output (Q) and stroke volume (6, 7), impaired pulmonary function (7), alterations in the absolute and proportional volumes of adipose and muscle tissue (8–10), and skeletal muscle myopathies (11–13). Further, exercise intolerance is a strong indicator of mortality in certain clinical groups, including those with cardiovascular disease, kidney disease, or a history of childhood cancer (14–16), thus making its identification valuable in guiding medical care in a high-risk dystrophic population.
Exercise intolerance has already been demonstrated in the elderly (17) and among people with chronic disease (18, 19). Exercise intolerance has been observed in several muscular dystrophies to some degree especially among people with Duchenne (20, 21), Becker (21), and ANO5 dystrophy (22), a finding which is believed to be related in part to the inherently higher rates of sarcolemma instability, neurocellular signaling defects, and extracellular calcium influx among these clinical groups (20). Importantly, the well-defined alterations in LM (23), and the subsequent contribution of this marker on exercise tolerance (8–10), particularly among people with myopathic disease (24), means that the phenomenon is also likely present within the FSHD population. The purpose of this study, therefore, is to investigate whether exercise intolerance is more pronounced among people with FSHD, as compared with a sex- and age-matched control group; secondly, we also aim to determine whether markers of LM can predict exercise intolerance among people with FSHD. Based on the influence of body composition (23), we hypothesize that adults with FSHD will exhibit exercise intolerance as compared with age- and sex-matched controls. Furthermore, we believe that factors contributing to exercise intolerance will differ between adults with FSHD and controls. These findings will serve as an important foundation in developing rehabilitative strategies designed to improve functional performance and quality-of-life in the FSHD population.
METHODS
Subjects
Eleven people with genetically confirmed FSHD (47±13 years) and eleven age- and sex-matched control participants (46±13 years) (n=22 combined; males: 14, females: 8) completed the study. Inclusion criteria included an age of ≥18 years, and no prior history of cardiovascular, pulmonary, orthopedic, or neuromuscular disorders other than FSHD; female participants were excluded if they were currently pregnant or breastfeeding (25, 26). Physical activity level was calculated via the Minnesota Leisure Time Physical Activity Questionnaire (27), and reflected as an activity metabolic index score. The FSHD Health Index survey was used to compute the severity of disease burden, whereby a score of 100 reflects the highest disease, and 0 reflects no disease burden (28, 29). The study was approved by the University of Minnesota Institutional Review Board and conducted in accordance with the Declaration of Helsinki.
Experimental Protocol
Participants completed one experimental session, during which a description of study design was provided, and written informed consent was obtained. During the experimental session, participants completed a volitional peak exercise protocol on an upright stationary bicycle. The exercise protocol included three-minutes of a baseline rest period, followed by three-minute graded stages at a speed of 65–70 RPM, and of incrementally increasing intensity. (FSHD wattage: 20-watts/stage; control wattage: 40 to 60-watts/stage). Study participants performed a cycling ergometer test to volitional fatigue using a 20-watt protocol for adults with FSHD and a 40 to 60-watt protocol based on participants’ fitness level and experience cycling (i.e., experience with cycling and greater physical activity levels would start at 80 watts). Control participants and adults with FSHD were then compared 1) at an absolute workload of 60 watts (n=17, FSHD: 9, 2 females; Control: 8, 2 females), 2) at a relative workload corresponding with the stage at which they achieved 40% of their VO2peak (n=22, FSHD: 11, 4 females; Control: 11, 4 females), and 3) at VO2peak. The absolute level of 60 watts was chosen for two reasons: 1) absolute work levels provide insight to how adults with FSHD perform during functional daily activities compared to control participants and 2) it was the wattage that most participants completed. A moderate intensity workload (40% of peak) was chosen to investigate how measures between two groups compared at a relative submaximal workload. The test was terminated when the participants exhibited either a plateau in oxygen consumption (VO2) or could no longer maintain cadence speed.
Physiologic Monitoring and Data Collection
Breath-by-breath gas exchange and measures of ventilation (VE; VE = respiratory rate (RR) * tidal volume (VT)), respiratory exchange ratio (RER) and partial pressure of end tidal carbon dioxide (PETCO2), were collected throughout rest and during exercise with a Medgraphics metabolic cart (Ultima System CardiO2, Medical Graphic, St. Paul, MN, USA). The gas analyzer was calibrated according to manufacturing guidelines before each test, using calibration gases of 5% CO2, 12% oxygen (O2) and balanced nitrogen (N2). Gas volumes were measured through a Prevent® flow sensor using a 3-Liter calibration syringe and corrected for ambient conditions prior to each test. The VE to VO2 production (VE/VCO2 slope), a pathologic indicator of cardiopulmonary disease and known marker of increased mortality risk (30, 31), was calculated as follows:
Heart rate was measured via 12-lead ECG. Blood pressure was measured manually through sphygmomanometry at rest and following each exercise stage; likewise, oxygen pulse (O2 pulse ;VO2/HR), a validated surrogate marker of stroke volume at rest and with physical activity (32), was calculated for each of these time points. Dyspnea and rating of perceived exertion (RPE; scale 6–20) were measured at the end of each stage during the exercise protocol. To account for the influence of LM on exercise intolerance, a total-body dual-energy x-ray absorptiometry (DXA) scan (Lunar iDXA, GE Healthcare, Chicago, IL, USA) was conducted; assessment was performed using a three-compartment model (bone mineral content, fat mass (FM), LM. Female participants completed a urine human chorionic gonadotropin (hCG) test (Clinical Guard, Atlanta, GA, USA) to determine they were not pregnant.
Statistical Analysis
Statistical analyses were performed using SPSS v26.0 (SPSS, Inc., Chicago, IL, USA); significance defined as an α-level of p<0.05 for all comparisons. Data is reported as group averages (mean ± standard deviation); distribution normality was assessed and parametric vs. non-parametric methods were used as appropriate. A mixed-model repeated-measures ANOVA was used to determine if people with FSHD responded differently to controls (group effect) at rest, and during relative intensities of exercise (40% of VO2peak, VO2peak) (time effect). When an interaction (time × group) was observed, a Bonferroni correction was calculated to determine significance between time points and groups. Because the 60W absolute workload did not include all participants, a separate test to determine differences in means was conducted; an independent samples t-test was used when data was normally distributed, and a Mann-Whitney U test was performed when data was not normally distributed. Associations between continuous variables were identified with the Pearson product-moment correlation; a stepwise linear regression model was used to determine whether measures of body composition or cardiopulmonary function could predict exercise intolerance differentially between the groups (dependent variable: VO2peak (mL/min), independent variables: leg LM (LLM (kg)), % leg LM (LLM), %WBFM, VO2/HR ((mL/beat)). An ANCOVA was performed to assess whether VO2peak was confounded by differing levels of physical activity between study groups. Due to large differences in standard deviation between groups, effect size was calculated using Glass’s delta (Δ), whereby:
RESULTS
Subject Characteristics
FSHD and control participants were similar in age (47±13 vs. 46±13 years, p=0.86), height (1.78±0.1 vs. 1.72±0.1 m, p=0.19), weight (84.8±11.4 vs. 80.6±17.1 kg, p=0.50), and BMI (27.0±4.0 vs. 26.8±4.1 kg/m2, p=0.94). In the FSHD group, one participant self-reported as Hispanic, the remaining 10 self-identified as non-Hispanic white (6 men, 4 women); racial self-identification by control study participants was as follows: Hispanic: 2, non-Hispanic white: 8 (4 men, 4 women), black: 1. A lower amount of daily physical activity was reported among people with FSHD, as compared with controls (activity metabolic index score; 41.8±65.1 vs. 252.6±146.1 kcal/day, p<0.01). Additional self-reported measures include the FSHD Health Index (HI) score, values of which are shown in Table 1.
Table 1:
Self-reported measures of functionality in FSHD.
| FSHD Health Index (HI) Category | Mean Score (n=11) |
|---|---|
| Shoulder and arm function | 36.7±24.2 |
| Fatigue | 31.2±21.2 |
| Mobility and ambulation | 29.0±21.2 |
| Social performance | 27.2±23.7 |
| Core strength and function | 23.5±17.3 |
| Body image | 22.4±22.7 |
| Activity limitation | 21.7±13.5 |
| Social satisfaction | 21.2±20.2 |
| Emotional health | 19.7±15.3 |
| Pain | 17.0±10.8 |
| Hand and finger function | 13.5±21.2 |
| Communication | 11.3±15.2 |
| Gastrointestinal function | 6.6±10.1 |
| Cognitive function | 4.8±10.6 |
| Total FSHD-HI Score | 23.9±13.8 |
Body Composition
Lean mass.
Measures of LM in FSHD are in Table 2. Absolute measures of LM, including whole-body LM (WBLM), trunk LM (TLM), LLM, arm LM (ARMLM), and the combined appendicular region (ALM; sum of fat- and bone-free tissue in the arms and legs (33)) were all similar between FSHD and control groups (p>0.05 for all). However, the % whole-body LM (%WBLM) among people with FSHD was found to be 13% lower than in controls (p=0.03), a finding which was furthermore seen among male (p=0.045) but not female (p=0.41) study participants. Similarly, the FSHD group exhibited a %LLM that was 16% lower than that observed in controls (p=0.02); this finding was replicated among male FSHD-control pairs (p=0.03), though not in females (p=0.34). Multiple measures of LM were found to be correlated with self-reported physical activity in the FSHD group (WBLM: r=0.60, p=0.049; LLM: r=0.62, p=0.04; %WBLM: r=0.66, p=0.03), though these relationships were not present among controls or when groups were combined.
Table 2:
Measures of body composition in FSHD
| FSHD | FSHD (Range) | 95% CI | CTL | CTL (Range) | 95% CI | |
|---|---|---|---|---|---|---|
| Lean mass (kg) | ||||||
| Whole-body lean mass | 47.9±7.9 | 40.5–67.6 | 42.6–53.2 | 58.2±12.6 | 33.9–78.7 | 45.1–64.0 |
| % whole-body lean mass | 58.8±10.2 | 46.3–82.1 | 51.9–65.6 | 67.6±7.0* | 52.7–75.8 | 62.9–72.3 |
| % whole-body lean mass: Men | 59.3±11.5 | 49.7–82.1 | 48.6–69.9 | 70.0±5.2* | 61.1–75.8 | 65.2–74.7 |
| % whole-body lean mass: Women | 57.9±9.0 | 46.3–67.5 | 43.6–72.2 | 63.5±8.7 | 52.7–71.0 | 49.7–77.3 |
| Trunk lean mass | 23.7±3.5 | 19.8–32.2 | 21.3–26.1 | 27.1±5.5 | 15.2–34.8 | 21.2–29.7 |
| Leg lean mass | 15.5±3.8 | 11.6–24.1 | 12.9–18.0 | 19.9±4.2 | 12.0–26.5 | 15.4–21.8 |
| % leg lean mass | 60.8±11.1 | 48.5–84.8 | 53.4–68.3 | 72.2±8.9* | 50.7–82.3 | 66.2–78.2 |
| % leg lean mass: Men | 62.5±13.0 | 48.5–84.8 | 50.5–74.6 | 77.0±4.4* | 68.6–82.3 | 72.9–81.1 |
| % leg lean mass: Women | 57.8±7.4 | 49.7–67.6 | 46.0–69.7 | 63.9±9.1 | 50.7–71.1 | 49.5–78.4 |
| Arm lean mass | 5.3±1.0 | 4.4–7.8 | 4.6–6.0 | 7.8±2.5 | 3.6–12.5 | 5.3–8.9 |
| Appendicular lean mass | 20.7±4.6 | 16.4–31.9 | 17.6–23.9 | 27.7±6.7 | 15.6–39.0 | 20.8–30.7 |
| Fat mass (kg) | ||||||
| Whole-body fat mass | 32.1±12.0 | 11.1–49.6 | 24.1–40.2 | 22.8±6.7* | 13.4–35.8 | 18.2–27.2 |
| Trunk fat mass | 17.4±9.1 | 4.7–31.5 | 11.3–23.5 | 12.8±4.9 | 5.1–21.8 | 9.1–15.4 |
| Leg fat mass | 10.1±2.9 | 4.3–14.9 | 8.2–12.1 | 6.6±1.6* | 4.9–11.7 | 5.5–8.4 |
| % leg fat mass | 39.2±11.2 | 15.2–51.5 | 31.7–46.7 | 28.7±10.0* | 17.7–49.3 | 22.1–35.5 |
| % leg fat mass: Men | 37.4±13.1 | 15.2–51.5 | 25.4–49.5 | 24.7±8.4* | 17.7–43.0 | 16.9–32.5 |
| % leg fat mass: Women | 42.2±7.4 | 32.4–50.3 | 30.3–54.0 | 36.0±9.1 | 28.9–49.3 | 21.6–50.5 |
| Arm fat mass | 3.2±1.1 | 1.2–4.9 | 2.5–3.9 | 2.6±0.8 | 1.5–4.2 | 2.0–3.1 |
unless otherwise indicated, data is for combined analyses of men and women;
indicates p<0.05.
Fat mass.
Measures of FM in FSHD are in Table 2. People with FSHD were found to have an absolute volume of whole-body FM (WBFM) that was 42% higher than controls (p=0.03). Furthermore, in people with FSHD, the lower body did appear to be more affected, with the absolute volume of leg FM (LFM) reaching a level that was 46% higher than in controls (p<0.01); the relative proportion of leg FM (%LFM) was likewise different between groups, with FSHD participants exhibiting a value that was 37% higher than in controls (p=0.03). Furthermore, sex-driven differences in %LFM were observed, as values were higher for male FSHD-control pairs (p=0.05), but not female counterparts (p=0.34). Absolute values of trunk FM (TFM) and arm FM (ARMFM) were similar between FSHD and control groups (p>0.05 for both).
Cardiopulmonary Function during Relative Workloads
When working at maximal intensities, VO2 was 32% lower (p<0.01, Figure 1A) in FSHD, an observation that remained when controlling for physical activity level (p=0.02). Similarly, wattage was 55% lower in the FSHD group both at VO2peak (p<0.01, Figure 1B), and when working at a relative intensity of 40% of VO2peak (41.8±30.3 vs. 92.7±32.6 watts, p=0.01). Ratings of perceived exertion and dyspnea were similar between FSHD and control groups when working at both submaximal, relative intensities and at VO2peak (p>0.05 for all, Figures 2A–B).
Figure 1:
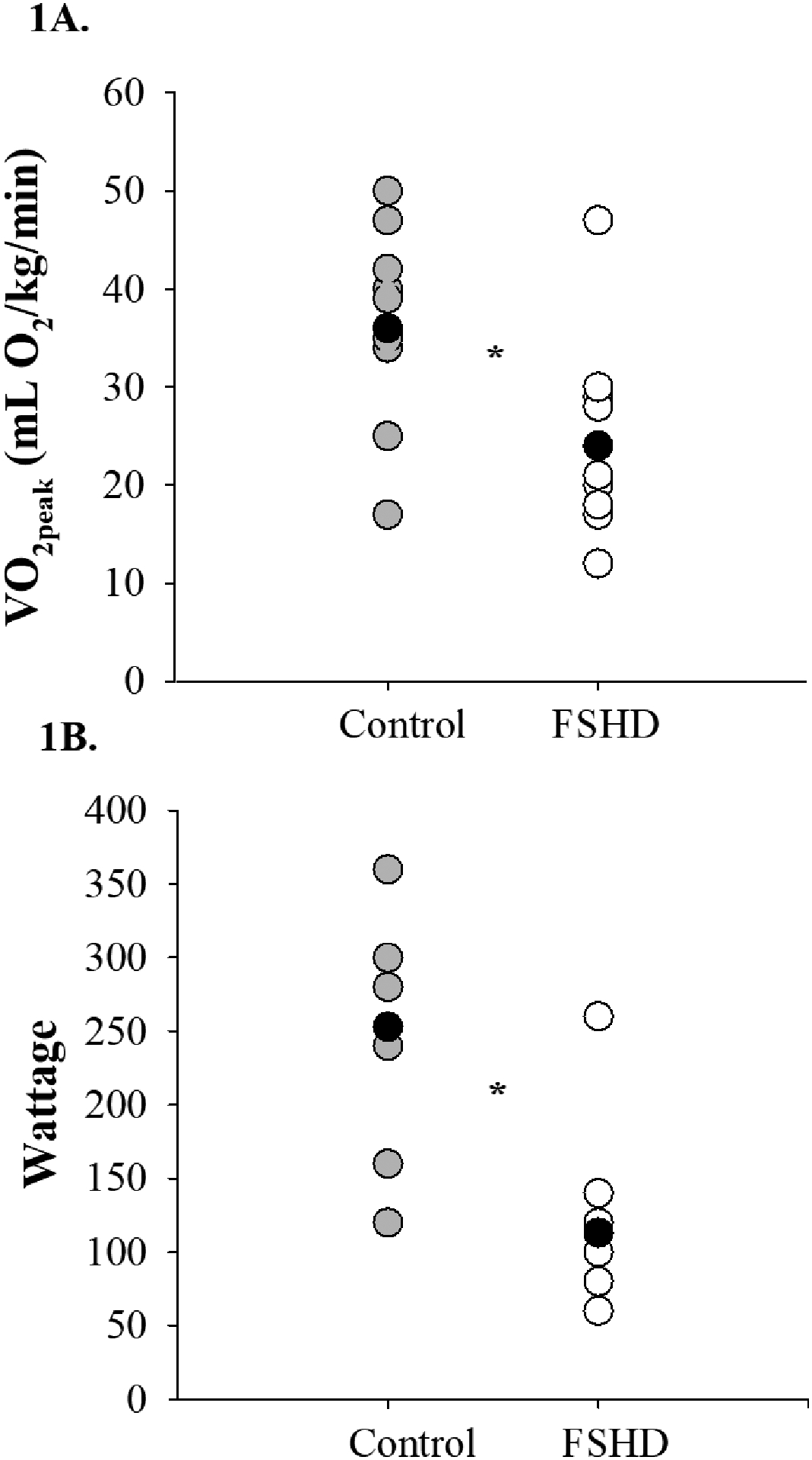
Peak exercise capacity in FSHD. 1A) VO2peak was lower than that observed in controls; 1B) Peak wattage was lower among people with FSHD (VO2, volume of oxygen consumption; *p<0.01 for both).
Figure 2:
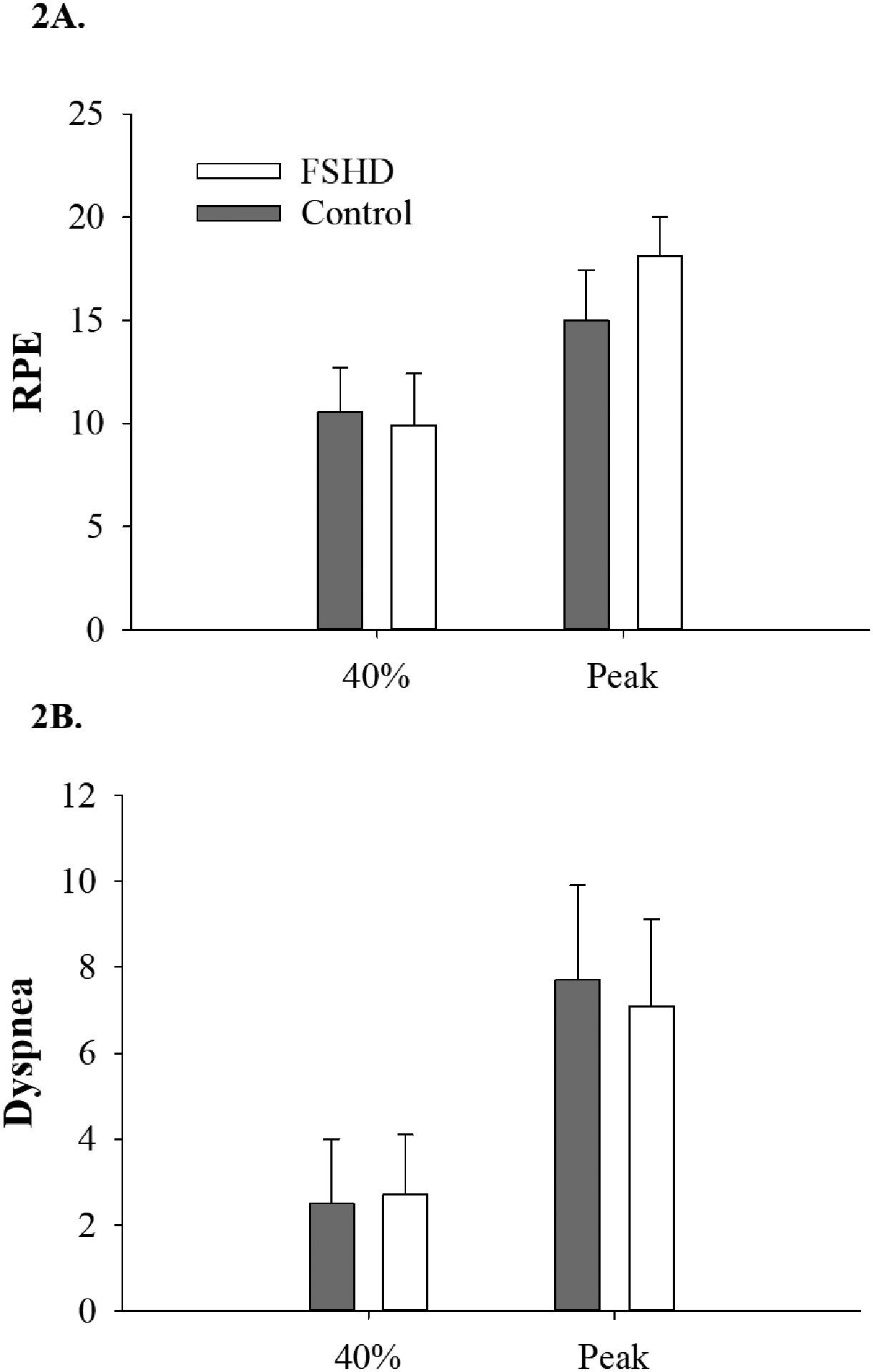
Self-reported exertion levels in FSHD. 2A) During relative work, ratings of perceived exertion did not differ between people with FSHD and controls; 2B) The FSHD group had self-reported levels of breathlessness that were similar to controls, when working at a relative intensity (RPE, rating of perceived exertion; 40%, submaximal exercise at 40% of VO2peak; Peak, VO2peak; p>0.05 for both).
VE increased (time effect, p<0.01) more in the controls compared to adults with FSHD (time × group interaction, p<0.01) with a trend for the controls to have a higher VE at 40% of VO2peak (p=0.053), and greater VE at VO2peak (p<0.01, Figure 3A). RR was greater for adults with FSHD compared with controls (time × group interaction, p=0.03) at rest (p=0.01) but increased similarly throughout submaximal and peak exercise (p>0.05 for both, Figure 3B). VT increased (time effect, p<0.01) similarly (time × group interaction, p=0.32) for both groups, but was overall attenuated in adults with FSHD compared with controls (group effect, p=0.02, Figure 3C). Differences in PETCO2 were noted at various stages of the exercise protocol (time effect, p<0.01); similarly, between groups (time × group interaction, p=0.41), with no main effect of group (p=0.10, Table 3).
Figure 3:
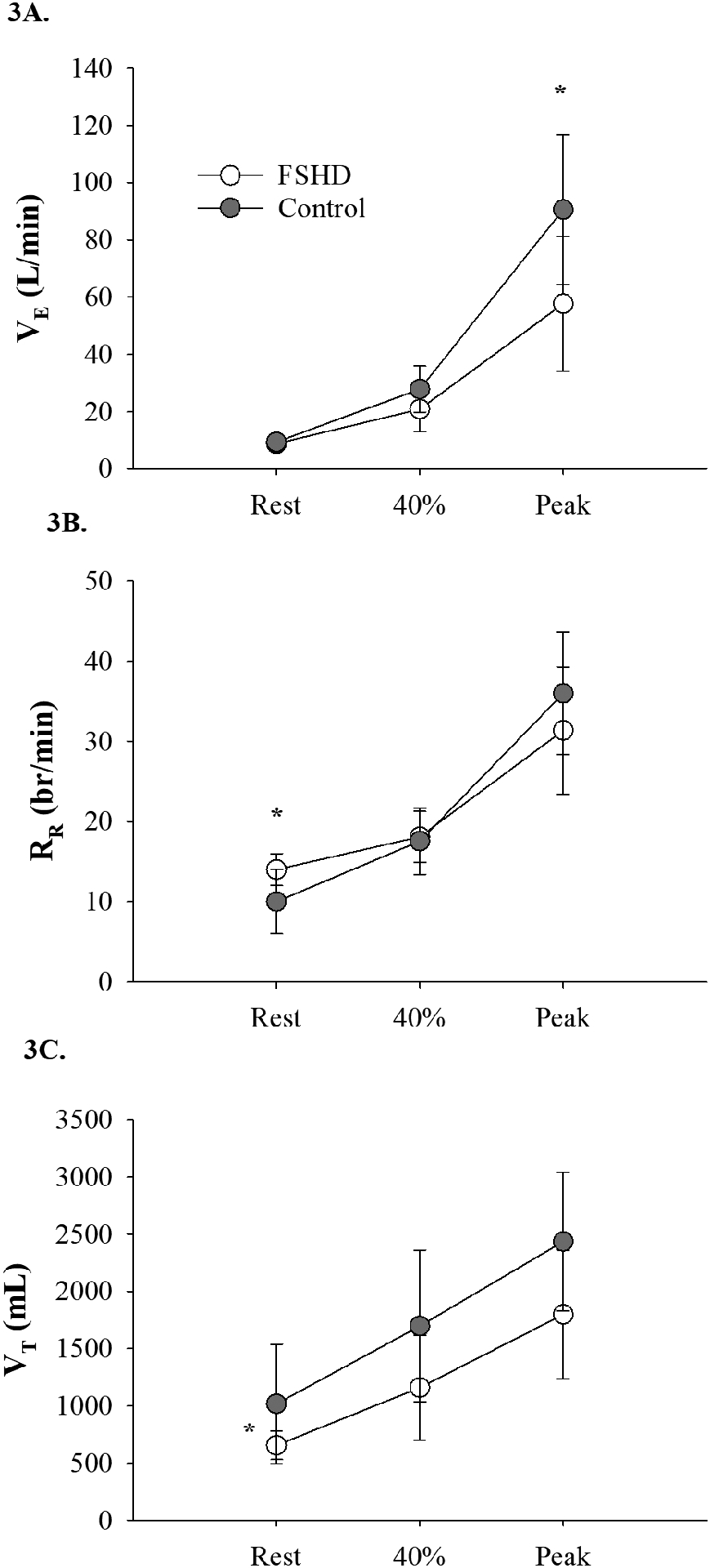
Measures of ventilation during exercise in FSHD. 3A) VE response during maximal exercise was significantly lower in FSHD; 3B) People with FSHD demonstrated a RR that was significantly higher than controls while at rest, but increased similarly throughout exercise; 3C) Overall, VT is lower among people with FSHD, as compared with controls) (VE, minute ventilation; RR, respiratory rate; VT, tidal volume; 40%, submaximal exercise at 40% of VO2peak; Peak, VO2peak; *p<0.05).
Table 3:
Measures of cardiopulmonary (CP) function in FSHD
| FSHD | Range (FSHD) | 95% CI | CTL | Range (CTL) | 95% CI | |
|---|---|---|---|---|---|---|
| Rest | ||||||
| HR (bpm) | 79±13 | 57–101 | 71–88 | 74±14 | 56–100 | 65–84 |
| SBP (mmHg) | 130±9 | 114–142 | 123–136 | 122±11 | 102–136 | 114–129 |
| DBP (mmHg) | 83±6 | 70–92 | 79–87 | 82±9 | 64–92 | 76–88 |
| MAP (mmHg) | 99±5 | 91–106 | 96–102 | 94±10 | 79–107 | 88–10 |
| VO2/HR (mL/beat) | 3.1±0.6 | 2.4–4.5 | 2.7–3.6 | 3.7±1.3 | 1.4–6.1 | 2.8–4.6 |
| RER | 0.95±0.1 | 0.85–1.18 | 0.87–1.03 | 0.98±0.2 | 0.82–1.30 | 0.88–1.08 |
| PETCO2 (mmHg) | 38.0±3.1 | 30.6–41.2 | 35.9–40.1 | 40.1±4.9 | 34.2–49.7 | 36.8–43.4 |
| Submax exercise (40% of VO 2 peak) | ||||||
| HR (bpm) | 103±18 | 77–134 | 91–115 | 106±17 | 86–128 | 95–118 |
| SBP (mmHg) | 148±16 | 114–142 | 138–158 | 148±23 | 102–136 | 132–163 |
| DBP (mmHg) | 89±8 | 70–92 | 84–95 | 85±9 | 64–92 | 79–90 |
| MAP (mmHg) | 109±10 | 91–106 | 102–115 | 106±11 | 79–107 | 99–114 |
| VO2/HR (mL/beat) | 7.5±2.3 | 4.7–11.9 | 5.9–9.1 | 11.0±4.5* | 4.1–18.0 | 21.7–26.9 |
| RER | 0.95±0.1 | 0.75–1.10 | 0.88–1.02 | 0.90±0.1 | 0.80–1.0 | 0.85–0.95 |
| PETCO2 (mmHg) | 42.4±4.4 | 32.6–48.8 | 39.4–45.4 | 46.1±5.3 | 38.0–54.3 | 42.5–49.6 |
| VE/VCO2 slope | 25.2±3.4 | 21.2–33.4 | 22.9–27.5 | 24.3±3.8 | 20.0–30.6 | 21.7–26.9 |
| Submax exercise (60-watts) | ||||||
| HR (bpm) | 114±23 | 90–165 | 97–132 | 96±16 | 76–125 | 83–109 |
| SBP (mmHg) | 166±18 | 144–196 | 152–180 | 138±18* | 102–166 | 123–153 |
| DBP (mmHg) | 92±11 | 70–104 | 84–100 | 86±6 | 76–94 | 80–91 |
| MAP (mmHg) | 116±12 | 99–135 | 107–125 | 105±7* | 96–116 | 99–110 |
| VO2/HR (mL/beat) | 8.9±1.5 | 6.7–10.6 | 7.8–10.0 | 8.9±2.9 | 4.1–12.4 | 6.9–11.3 |
| RER | 1.01±0.1 | 0.87–1.31 | 0.90–1.12 | 0.90±0.1 | 0.76–1.0 | 0.82–0.98 |
| PETCO2 (mmHg) | 41.0±6.3 | 25.8–46.0 | 36.2–45.8 | 41.6±4.0 | 37.5–50.0 | 38.3–45.0 |
| VE/VCO2 slope | 27.6±8.1 | 20.8–48.2 | 21.3–33.8 | 25.8±3.7 | 21.7–30.6 | 22.7–29.0 |
| VE (L/min) | 33.6±14.7 | 23.0–68.0 | 22.3–44.9 | 25.9±3.7* | 18.0–32.0 | 18.5–26.3 |
| RR (br/min) | 24±11 | 15–50 | 16–32 | 17±5* | 12–25 | 13–21 |
| VT (mL) | 1405±217 | 1180–1796 | 1238–1572 | 1430±329 | 856–1985 | 1154–1705 |
| RPE | 13.4±2.9 | 10–18 | 11.2–15.7 | 8.2±2.1* | 7–13 | 6.5–10.0 |
| Dyspnea | 4.2±2.0 | 1–8 | 2.7–5.7 | 1.5±1.5* | 0–4 | 0.2–2.8 |
| Maximal exercise | ||||||
| HR (bpm) | 143±28 | 109–185 | 124–162 | 152±28 | 92–190 | 134–171 |
| SBP (mmHg) | 187±22 | 158–232 | 171–202 | 194±30 | 140–238 | 173–214 |
| DBP (mmHg) | 95±11 | 70–106 | 88–102 | 94±112 | 79–110 | 87–103 |
| MAP (mmHg) | 125±13 | 99–141 | 116–134 | 123±123 | 69–148 | 108–138 |
| VO2/HR (mL/beat) | 11.1±3.3 | 7.3–16.5 | 8.9–13.3 | 17.5±6.6* | 6.6–30.3 | 13.1–21.9 |
| RER | 1.12±0.1 | 0.84–1.29 | 1.02–1.22 | 1.18±0.1 | 0.93–1.40 | 1.08–1.27 |
| PETCO2 (mmHg) | 38.6±5.0 | 25.8–44.1 | 35.2–42.0 | 42.6±5.4 | 35.0–52.0 | 38.9–46.2 |
| VE/VCO2 slope | 31.4±6.0 | 26.5–48.2 | 27.4–35.4 | 30.6±8.5 | 21.9–54.2 | 24.9–36.3 |
L, liters; min, minute; br, breaths; mL, milliliters; bpm, beats per minute; mmHg, millimeters of mercury;
indicates p<0.05.
Systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), heart rate (HR), and respiratory exchange ratio (RER) all increased throughout the exercise protocol (time effect, p<0.01 for all), in a similar fashion across stages, and between groups (time × group interaction, group effect, p>0.05 for all, Table 3). O2 pulse increased (time effect, p<0.01) to a greater extent in controls (time × group interaction, p<0.01), as demonstrated by a higher O2 pulse at 40% of VO2peak (p=0.03) and at VO2peak (p<0.01); overall, O2 pulse was shown to be elevated in controls, as compared with FSHD participants (group effect, p=0.02, Table 3).
Cardiopulmonary Function during an Absolute Submaximal Workload
A sub-analysis was conducted with individuals who completed 60 watts of lower extremity cycling exercise. Adults with FSHD demonstrated a VO2 that was 21% higher than in controls (FSHD: 1017.2±141.2 vs. Control: 840.5±187.1 mL/min, p=0.04). Likewise, the FSHD group exhibited a VE and RR that was 55% and 41% higher, respectively, than in controls (p<0.05 for both, Table 3), though differences in VT between groups at this exercise intensity were absent (p=0.86, Table 3). HR, DBP, O2 pulse, PETCO2, and VE/VCO2 slope were similar between individuals with FSHD and controls (p>0.05 for all, Table 3); though trending towards significance, RER likewise did not differ between FSHD and control participants (p=0.09). Conversely, SBP and MAP were 20% and 10% higher, respectively, among people with FSHD, as compared with the control group (p<0.05 for both, Table 3). RPE was 63% higher and dyspnea was 180% higher in the FSHD group compared with controls (p<0.01 for both, Table 3).
Associations and predictors of exercise intolerance
A regression analysis examining a primary predictor of exercise intolerance failed to find interactions between the FSHD and control group using resting O2 pulse, or various measures of body composition (%WBFM, LLM, %LLM) in the model, thus suggesting that none of these variables could independently predict VO2peak between the groups (p>0.05 for all). However, as correlations between VO2peak and multiple cardiopulmonary and compositional measures were noted in combined analysis (%LLM: p<0.01, r=0.90; LLM: p<0.01, r=0.84; O2 pulse at VO2peak: p<0.01, r=0.79; %WBFM: p<0.01, r=−0.78; resting O2 pulse: p<0.01, r=0.76; HR at VO2peak: p=0.03, r=0.46; VE at VO2peak: r=0.80, p<0.001; and VT at VO2peak: r=0.77, p<0.01), it is likely that these factors did limit exercise performance to some degree for both groups or alternatively were a function of their exercise performance. Moreover, when groups were assessed separately, differences in the strength of the correlations on VO2peak were observed, signifying potential variations in the mechanisms of this limitation between FSHD and control participants. In the control group, VO2peak was most strongly related to resting O2 pulse (p<0.01, r=0.90), followed by %LLM: p<0.01, r=0.89; LLM: p<0.01, r=0.79; O2 pulse at VO2peak: p=0.01, r=0.72; %WBFM: p=0.01, r=−0.70; VE at VO2peak: r=0.74, p=0.01 and VT at VO2peak: r=0.61, p=0.04). Among people with FSHD, %LLM was most strongly related to VO2peak (p<0.01, r=0.89), though a relationship between VO2peak and other factors was likewise found (LLM: p<0.01, r=0.87; O2 pulse at VO2peak: p<0.01, r=0.77; %WBFM: p<0.01, r=−0.74; VE at VO2peak: r=0.68, p=0.02; and VT at VO2peak: r=0.75, p<0.01). Interestingly, self-reported measures of functionality, as indicated via the FSHD-HI survey, were found to be negatively correlated with VO2peak (activity limitation: p<0.01, r=−0.78; total FSHD-HI score: p=0.03, r=−0.65), thus suggesting a strong association between perceived and objective impairments in people with FSHD.
DISCUSSION
This study is the first to identify a greater exercise intolerance among people with FSHD, as reflected by a VO2peak that was 32% lower than controls. Additionally, the presence of exercise intolerance in the FSHD group is further supported by self-reported RPE and dyspnea which were 63% and 180% higher, respectively, at an absolute, submaximal workload, than among control participants. Importantly, our study revealed that the strongest limiting factor for peak exercise capacity in adults with FSHD was lean mass, indicating that those with lower lean mass have lower exercise capacity. In contrast, the strongest correlation for VO2peak in controls was resting O2 pulse, indicating that stroke volume, was likely the limiting factor to peak exercise levels in controls. Finally, we observed an attenuated cardiopulmonary response at both the relative submaximal and peak exercise workload, while during the absolute intensity exercise, VO2 and measures of cardiopulmonary function were elevated in the FSHD group. These findings suggest that during activities of daily living, such as stair-climbing or yard work, people with FSHD will likely require greater work (VO2) and will feel as if the exercise is harder, while leaving them more breathless than individuals without FSHD. Further, a blunted hemodynamic and pulmonary response during relative exercise (submax 40% of peak and at VO2peak) suggest physical limitations to exercise in FSHD when compared with age- and sex-matched controls. Indeed, the FSHD-HI activity limitation score accounted for 60% of the variability in the VO2peak.
Exercise Intolerance in FSHD
Although previous work on exercise intolerance among people with FSHD is sparse, the available literature yield results that support our findings. In research by Morse et al., people with FSHD were found to have a 28% lower distance traveled during the assisted 6-minute cycle test than that seen in controls (4); this variable was used as a surrogate measure for VO2peak, thereby matching our own mean difference in exercise capacity of 32%. However, ratings of dyspnea and fatigue were not measured by Morse et al., making it difficult to ascertain whether exercise intolerance was truly present in this study.
Mechanisms of Exercise Intolerance
Exercise intolerance has been described as a syndrome which “coalesces as dysfunction across multiple physiologic systems (34),” including those at both the peripheral and central levels. Identifying and addressing unique mechanisms of exercise intolerance, such as those related to body composition and hemodynamic function among people with FSHD, is an important first step in the development of therapeutic interventions designed to treat the condition.
Peripheral mechanisms.
A combination of peripheral factors, including alterations in muscle volume, fiber type, and metabolism, are believed to play a primary role in the development of exercise intolerance (35–37). These contributors to exercise intolerance may especially impact clinical populations, where a low volume of muscle mass, a reduced rate of maximal force production, and associations between VO2peak and measures of LM (calf muscle volume: r=0.48; mid-arm muscle volume: r=0.36) have been widely reported (8–10). Our research coincides with these observations, as we report that among people with FSHD, %LLM and LLM were strongly associated with VO2peak, suggesting that the presence of exercise intolerance among these participants was related primarily to losses in LM in the lower body. While a transition from a fast-glycolytic to slow-oxidative phenotype has been reported among people with FSHD, fiber typing was not performed in this study, and therefore, it is difficult to determine the extent to which this phenomenon may have contributed to exercise intolerance among our participants (38). RER is a measure of whole-body substrate metabolism and because skeletal muscle is the largest energy source for maintaining physical activity, skeletal muscle is a major determinant of RER (39, 40). RER, however, did not differ between groups at rest or during relative exercise and was not correlated with VO2peak among either FSHD or control participants. There was however a trend for RER to be greater in FSHD compared with controls at absolute exercise levels, indicating a shift to more anaerobic energy sources during a similar workload in this population. These observations suggest that muscle fiber type, metabolic pathways, and preferred energy may not be the dominant factor in the development of exercise intolerance in dystrophic populations. Although not the strongest contributor, it is important to acknowledge that %LLM was correlated with VO2peak in the controls as well, indicating the importance of muscle mass to exercise capacity.
Hemodynamic mechanisms.
Prominent mechanisms of exercise intolerance are Q, a value which is driven by the combined influences of heart rate and stroke volume (41). While measures of Q have not previously been studied among people with FSHD, other dystrophic groups, including those with Duchenne muscular dystrophy (DMD), have exhibited a submaximal Q that is 50% lower than that seen in controls, when assessed proportionately to resting values (42). Importantly, this observation seems to be driven primarily by a stroke volume that is 20% lower than in controls, as differences in heart rate during physical activity between DMD and control groups do not appear to present (42). These observations align with ours, in which people with FSHD had a HR that did not differ from control counterparts either at rest, or at any stage in the exercise protocol. While we did not directly measure stroke volume in our study, a surrogate measure—O2 pulse—was similar between groups during absolute, submaximal exercise workloads (60 watts) and at rest; notably, resting O2 pulse was strongly associated with VO2peak among controls, though not in people with FSHD. Moreover, people with FSHD were found to have an exercise O2 pulse that was 32% lower than in controls when working at 40% of VO2peak and at peak exercise, suggesting that when exercising at the same relative intensity, stroke volume may be reduced, but likely a result of the lower exercise capacity and wattage performed by the FSHD group. Finally, similarities in O2 pulse at other exercise workloads, and a lack of correlations between this marker and exercise intolerance in the FSHD group, suggests that this factor is not responsible for the presence of the phenomenon, and that other mechanisms (i.e., LM atrophy) indeed play a larger role.
Additional pulmonary mechanisms that could contribute to exercise intolerance include ventilatory dysfunction, particularly in people with chronic disease. In work by Morse et al., the low exercise capacity demonstrated by people with FSHD was accompanied by a corresponding low VE during the later stages of a relative, maximal intensity exercise test (4). In addition, significant inspiratory and expiratory muscle weakness has been demonstrated in adult with FSHD (43). While ventilatory efficiency (VE/VCO2 slope) was similar between FSHD and control groups at all exercise intensities, our study found variations in ventilatory function between groups, which manifested differently, at various intensities and workloads. In fact, during relative submaximal exercise, people with FSHD were found to have a VE response that trended lower than in controls; similarly, VE was 37% lower in the FSHD group at VO2peak. This observed reduction in VE at relative workloads may be a result of shallow breathing, as VT was lower in the FSHD group during relative exercise, though RR and PETCO2 were not affected. Conversely, VE was 55% higher among people with FSHD when working at an absolute intensity of 60-watts, due to a faster RR, as VT and PETCO2 did not differ between groups. Further, both peak VE and VT correlated with VO2peak in the groups when combined and when analyzed separately. Collectively, the observed reductions in VT at rest and during exercise combined with the previously demonstrated respiratory weakness (43) may in part contribute to exercise intolerance in adults with FSHD.
The cardiovascular response to exercise, may also contribute to exercise intolerance. In our study, all measures of arterial pressure, including SBP, DBP, and MAP, were similar between FSHD and control groups at rest, and during both submaximal and maximal relative workloads and likely did not contribute to exercise intolerance in the FSHD group. When working at an absolute intensity, however, people with FSHD were found to have a SBP and MAP that were 20% and 10% higher, respectively, than in the control group. This suggests that the elevated cardiovascular response in FSHD may be at least partially explained by a lower volume of LLM, thus making the same absolute volume of work proportionately harder. This theory is supported by the elevated VO2 and ventilatory response during absolute work in the FSHD group, though physical inactivity and other hemodynamic irregularities also likely play a contributory role.
Limitations
Limitations of our research include a small number of study participants, particularly of females. Additionally, nine of 11 FSHD-control pairs in our study were matched by race, though two non-Hispanic white males with FSHD were partnered with controls who self-identified as belonging to a dissimilar racial group (black: 1, Hispanic: 1). While differences in VO2max between races have been documented, these observations have primarily been made among Hispanic and non-Hispanic black females (44), whereas no significant differences in VO2max among males of differing races have been observed (44). Therefore, we believe that the divergent ethnic and racial backgrounds among two male FSHD-control pairs had minimal influence on primary markers of exercise intolerance.
Validated research supporting the use of O2 pulse as a surrogate measure of stroke volume among people with FSHD or other forms of muscular dystrophy have not been completed; as such, it is difficult to interpret the extent to which cardiovascular function may contribute to exercise intolerance in adults with FSHD. Finally, we did not control for medication use in our study, and in one instance, blood pressure-lowering medications (calcium-channel blockers) were used by a female control participant, but not her FSHD partner (Table 4). When examining individual values for this participant, we found that MAP at rest and VO2peak was 11 and 12 mmHg higher, respectively, as compared to in the control group overall. Thus, her MAP was elevated vs reduced when compared with the control group and likely did not influence our findings.
Table 4:
Medication use among study participants.
| Medication class | FSHD | Control |
|---|---|---|
| Angiotensin-converting enzyme (ACE) inhibitors | 1 | 1 |
| Antacids | 1 | 0 |
| Anticholinergics/antimuscarinics | 1 | 0 |
| Antifungals | 1 | 0 |
| Atypical antipsychotics | 0 | 1 |
| Calcium-channel blockers | 0 | 1 |
| Corticosteroids | 1 | 0 |
| HMG-CoA reductase inhibitors | 1 | 1 |
| Lincomycin antibiotics | 0 | 1 |
| Norepinephrine-dopamine reuptake inhibitors | 1 | 0 |
| NSAIDs | 2 | 0 |
| Proton pump inhibitors | 1 | 1 |
| Selective serotonin and norepinephrine reuptake inhibitors (SNRIs) | 1 | 0 |
| Selective serotonin receptor agonists (SSRAs) | 1 | 0 |
| Selective serotonin reuptake inhibitors (SSRIs) | 1 | 1 |
| Supplements | 8 | 2 |
| Tricyclic antidepressants | 1 | 0 |
| Vasodilators | 1 | 0 |
| Total medications | 23 | 9 |
CONCLUSIONS
The results of this study are important, as they show that people with FSHD are more likely to suffer from exercise intolerance than age- and sex-matched controls, especially when performing work at absolute and peak exercise levels; furthermore, we have shown that the mechanisms contributing to activity limitation between groups may be different. While resting O2 pulse is a primary influence on VO2peak among control groups, the lack of a significant correlation between these factors in people with FSHD suggests that in this population, it has little effect on exercise capacity; likewise, other resting and peak aspects of hemodynamic function (HR, SBP, DBP, MAP) are not shown to be drivers of VO2peak in this clinical group. Instead, markers of LM were found to be the strongest driver of VO2peak in FSHD, primarily as a result of muscle atrophy in the lower body. Collectively, adults with FSHD, exhibit muscle atrophy, a result of overexpression of the DUX4 gene, leading to functional limitations, demonstrated by the FSHD-HI, with both contributing to lower exercise capacity in adults with FSHD. These findings suggest that targeted therapeutic interventions, such as those which specifically address unique changes in muscle mass, may be a tangible way to address the presence of exercise intolerance at least partially among people with FSHD, thereby significantly improving both physical functionality and quality of life. As such, we believe that future research in this area should focus on investigating further mechanisms of exercise intolerance, such as muscle metabolism and fiber type distribution, among people with FSHD, as well as identifying the potential ways in which exercise training may be used as a functional tool in the management of the condition.
ACKNOWLEDGEMENTS
We declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by ACSM.
Special thanks to Dr. Julian Wolfson, for assistance with the statistical approach.
We would like to thank all the individuals who participated in this study, particularly those who traveled a long distance to contribute to this research.
Funding
This study was supported in part by a FLEXfund grant from Friends of FSH Research, the National Institutes of Arthritis and Musculoskeletal and Skin Diseases (R01 AR055685), and the University of Minnesota’s NIH Clinical and Translational Science Award (UL1TR002494).
Conflict of interest and funding statement:
The authors of this research have no disclosures or competing interests. This study was supported in part by a FLEXfund grant from Friends of FSH Research, the National Institutes of Arthritis and Musculoskeletal and Skin Diseases (R01 AR055685), and the University of Minnesota’s NIH Clinical and Translational Science Award (UL1TR002494). We declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by ACSM.
Footnotes
Conflicts of interest
The authors of this research have no disclosures or competing interests.
Data availability
The data that support the findings of this study are available from the corresponding author, upon request.
REFERENCES
- 1.Lim KRQ, Nguyen Q, Yokota T. DUX4 Signalling in the Pathogenesis of Facioscapulohumeral Muscular Dystrophy. Int J Mol Sci. 2020;21(3):729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang LH, Tawil R. Facioscapulohumeral Dystrophy. Curr Neurol Neurosci Rep. 2016;16(7):66. [DOI] [PubMed] [Google Scholar]
- 3.Statland JM, Tawil R. Facioscapulohumeral Muscular Dystrophy. Continuum (Minneap Minn). 2016;22(6, Muscle and Neuromuscular Junction Disorders):1916–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morse CI, Bostock EL, Twiss HM, Kapp LH, Orme P, Jacques MF. The cardiorespiratory response and physiological determinants of the assisted 6-minute handbike cycle test in adult males with muscular dystrophy. Muscle Nerve. 2018;58(3):427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens S, Gutin B. Exercise intolerance. Pediatr Rev. 2000;21(1):6–9. [DOI] [PubMed] [Google Scholar]
- 6.Smith JR, Joyner MJ, Curry TB, Borlaug BA, Keller-Ross ML, Van Iterson EH, et al. Locomotor muscle group III/IV afferents constrain stroke volume and contribute to exercise intolerance in human heart failure. J Physiol. 2020;598(23):5379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocha A, Arbex FF, Sperandio PA, Mancuso F, Marillier M, Bernard AC, et al. Exercise intolerance in comorbid COPD and heart failure: the role of impaired aerobic function. Eur Respir J. 2019;53(4):1802386. [DOI] [PubMed] [Google Scholar]
- 8.Anker SD, Swan JW, Volterrani M, Chua TP, Clark AL, Poole-Wilson PA, et al. The influence of muscle mass, strength, fatigability and blood flow on exercise capacity in cachectic and non-cachectic patients with chronic heart failure. Eur Heart J. 1997;18(2):259–69. [DOI] [PubMed] [Google Scholar]
- 9.Cicoira M, Zanolla L, Franceschini L, Rossi A, Golia G, Zamboni M, et al. Skeletal muscle mass independently predicts peak oxygen consumption and ventilatory response during exercise in noncachectic patients with chronic heart failure. J Am Coll Cardiol. 2001;37(8):2080–5. [DOI] [PubMed] [Google Scholar]
- 10.Minotti JR, Christoph I, Oka R, Weiner MW, Wells L, Massie BM. Impaired skeletal muscle function in patients with congestive heart failure. Relationship to systemic exercise performance. J Clin Invest. 1991;88(6):2077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haykowsky MJ, Brubaker PH, Morgan TM, Kritchevsky S, Eggebeen J, Kitzman DW. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J Gerontol A Biol Sci Med Sci. 2013;68(8):968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306(9):H1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina AJ, Bharadwaj MS, Van Horn C, Nicklas BJ, Lyles MF, Eggebeen J, et al. Skeletal Muscle Mitochondrial Content, Oxidative Capacity, and Mfn2 Expression Are Reduced in Older Patients With Heart Failure and Preserved Ejection Fraction and Are Related to Exercise Intolerance. JACC Heart Fail. 2016;4(8):636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. [DOI] [PubMed] [Google Scholar]
- 15.Kasugai A Using exercise tolerance to predict mortality in hemodialysis patients. Journal of the Medical Society of Toho University. 2002;50:73–80. [Google Scholar]
- 16.Ness KK, Plana JC, Joshi VM, Luepker RV, Durand JB, Green DM, et al. Exercise Intolerance, Mortality, and Organ System Impairment in Adult Survivors of Childhood Cancer. J Clin Oncol. 2020;38(1):29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12(12):1995–2004. [DOI] [PubMed] [Google Scholar]
- 18.Baek SJ, Nam GE, Han KD, Choi SW, Jung SW, Bok AR, et al. Sarcopenia and sarcopenic obesity and their association with dyslipidemia in Korean elderly men: the 2008–2010 Korea National Health and Nutrition Examination Survey. J Endocrinol Invest. 2014;37(3):247–60. [DOI] [PubMed] [Google Scholar]
- 19.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–48. [DOI] [PubMed] [Google Scholar]
- 20.Barnabei MS, Martindale JM, Townsend D, Metzger JM. Exercise and muscular dystrophy: implications and analysis of effects on musculoskeletal and cardiovascular systems. Compr Physiol. 2011;1(3):1353–63. [DOI] [PubMed] [Google Scholar]
- 21.Kakulas BA. Dystrophinopathies in Exercise Intolerance: Springer, Paris; 1999. [Google Scholar]
- 22.Milone M, Liewluck T, Winder TL, Pianosi PT. Amyloidosis and exercise intolerance in ANO5 muscular dystrophy. Neuromuscul Disord. 2012;22(1):13–5. [DOI] [PubMed] [Google Scholar]
- 23.Vera KA, McConville M, Kyba M, Keller-Ross ML. Sarcopenic Obesity in Facioscapulohumeral Muscular Dystrophy. Front Physiol. 2020;11:1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haller RG, Lewis SF. Pathophysiology of exercise performance in muscle disease. Med Sci Sports Exerc. 1984;16(5):456–9. [DOI] [PubMed] [Google Scholar]
- 25.Lof M, Olausson H, Bostrom K, Janerot-Sjöberg B, Sohlstrom A, Forsum E. Changes in basal metabolic rate during pregnancy in relation to changes in body weight and composition, cardiac output, insulin-like growth factor I, and thyroid hormones and in relation to fetal growth. Am J Clin Nutr. 2005;81(3):678–85. [DOI] [PubMed] [Google Scholar]
- 26.Dewey KG. Energy and protein requirements during lactation. Annu Rev Nutr. 1997;17:19–36. [DOI] [PubMed] [Google Scholar]
- 27.Richardson MT, Leon AS, Jacobs DR, Ainsworth BE, Serfass R. Comprehensive evaluation of the Minnesota Leisure Time Physical Activity Questionnaire. J Clin Epidemiol. 1994;47(3):271–81. [DOI] [PubMed] [Google Scholar]
- 28.Hamel J, Johnson N, Tawil R, Martens WB, Dilek N, McDermott MP, et al. Patient-Reported Symptoms in Facioscapulohumeral Muscular Dystrophy (PRISM-FSHD). Neurology. 2019;93(12):e1180–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson NE, Quinn C, Eastwood E, Tawil R, Heatwole CR. Patient-identified disease burden in facioscapulohumeral muscular dystrophy. Muscle Nerve. 2012;46(6):951–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson RJT, Yates DRA, Walkington JP, Davies SJ. Ventilatory inefficiency adversely affects outcomes and longer-term survival after planned colorectal cancer surgery. Br J Anaesth. 2019;123(2):238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neder JA, Alharbi A, Berton DC, Alencar MC, Arbex FF, Hirai DM, et al. Exercise Ventilatory Inefficiency Adds to Lung Function in Predicting Mortality in COPD. COPD. 2016;13(4):416–24. [DOI] [PubMed] [Google Scholar]
- 32.Crisafulli A, Piras F, Chiappori P, Vitelli S, Caria MA, Lobina A, et al. Estimating stroke volume from oxygen pulse during exercise. Physiol Meas. 2007;28(10):1201–12. [DOI] [PubMed] [Google Scholar]
- 33.Merlini L, Vagheggini A, Cocchi D. Sarcopenia and sarcopenic obesity in patients with muscular dystrophy. Front Aging Neurosci. 2014;6:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Craig JC, Colburn TD, Caldwell JT, Hirai DM, Tabuchi A, Baumfalk DR, et al. Central and peripheral factors mechanistically linked to exercise intolerance in heart failure with reduced ejection fraction. Am J Physiol Heart Circ Physiol. 2019;317(2):H434–H44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keller-Ross ML, Johnson BD, Carter RE, Joyner MJ, Eisenach JH, Curry TB, et al. Improved Ventilatory Efficiency with Locomotor Muscle Afferent Inhibition is Strongly Associated with Leg Composition in Heart Failure. Int J Cardiol. 2016;202:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipkin DP, Jones DA, Round JM, Poole-Wilson PA. Abnormalities of skeletal muscle in patients with chronic heart failure. Int J Cardiol. 1988;18(2):187–95. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81(2):518–27. [DOI] [PubMed] [Google Scholar]
- 38.Celegato B, Capitanio D, Pescatori M, Romualdi C, Pacchioni B, Cagnin S, et al. Parallel protein and transcript profiles of FSHD patient muscles correlate to the D4Z4 arrangement and reveal a common impairment of slow to fast fibre differention and a general deregulation of MyoD-dependent genes. Proteomics. 2006;6(19):5303–21. [DOI] [PubMed] [Google Scholar]
- 39.Ramos-Jiménez A, Hernández-Torres RP, Torres-Durán PV, Romero-Gonzalez J, Mascher D, Posadas-Romero C, et al. The Respiratory Exchange Ratio is Associated with Fitness Indicators Both in Trained and Untrained Men: A Possible Application for People with Reduced Exercise Tolerance. Clin Med Circ Respirat Pulm Med. 2008;2:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wasserman K, Beaver WL, Whipp BJ. Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation. 1990;81(1 Suppl):II14–30. [PubMed] [Google Scholar]
- 41.Ehrman J, Gordon P, Visich P, Keteyian S. Clinical Exercise Physiology. Champaign, IL: Human Kinetics; 2013. [Google Scholar]
- 42.Power LC, Gusso S, Hornung TS, Jefferies C, Derraik JGB, Hofman PL, et al. Exercise Cardiac Magnetic Resonance Imaging in Boys With Duchenne Muscular Dystrophy Without Cardiac Disease. Pediatr Neurol. 2021;117:35–43. [DOI] [PubMed] [Google Scholar]
- 43.Henke C, Spiesshoefer J, Kabitz HJ, Herkenrath S, Randerath W, Brix T, et al. Respiratory muscle weakness in facioscapulohumeral muscular dystrophy. Muscle Nerve. 2019;60(6):679–86. [DOI] [PubMed] [Google Scholar]
- 44.Wang CY, Haskell WL, Farrell SW, Lamonte MJ, Blair SN, Curtin LR, et al. Cardiorespiratory fitness levels among US adults 20–49 years of age: findings from the 1999–2004 National Health and Nutrition Examination Survey. Am J Epidemiol. 2010;171(4):426–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon request.


