Abstract
Objectives:
Recent results from “ORAL Surveillance” trial have raised concerns regarding the cardiovascular safety of tofacitinib in patients with rheumatoid arthritis (RA). We further examined this safety concern in the real-world setting.
Methods:
We created two cohorts of RA patients initiating treatment with tofacitinib or tumor necrosis factor inhibitors (TNFI) using de-identified data from Optum Clinformatics (2012–2020), IBM MarketScan (2012–2018), and Medicare (parts A, B, and D, 2012–2017) claims databases: 1) A “real-world evidence (RWE) cohort” consisting of routine care patients; and 2) A “RCT-duplicate cohort” mimicking inclusion and exclusion criteria of the ORAL surveillance trial to calibrate results against the trial findings. Cox proportional hazards models with propensity score fine stratification weighting were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for composite outcome of myocardial infarction and stroke and accounting for 76 potential confounders. Database-specific effect estimates were pooled using fixed effects models with inverse-variance weighting.
Results:
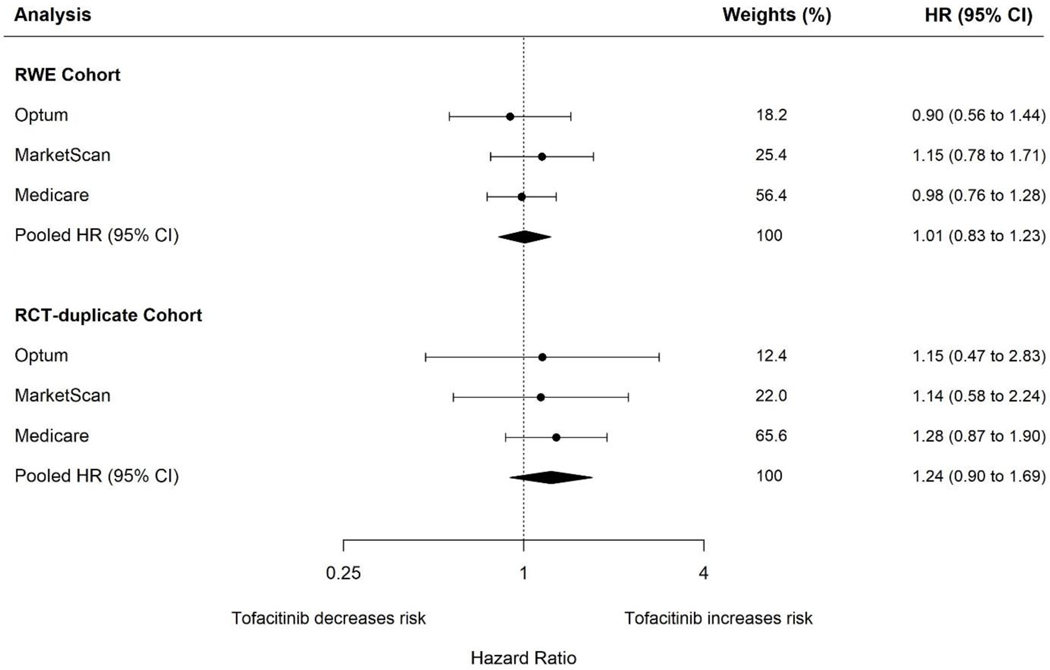
In the RWE cohort, 102,263 patients were identified of whom 12,852 (12.6%) initiated tofacitinib. The pooled weighted HR (95% CI) comparing tofacitinib with TNFI was 1.01 (0.83 to 1.23) in RWE cohort and 1.24 (0.90 to 1.69) in RCT-duplicate cohort which aligned closely with ORAL-surveillance results (HR: 1.33, 95% CI: 0.91 to 1.94).
Conclusions:
We did not find evidence for an increased risk of cardiovascular outcomes with tofacitinib in RA patients treated in the real-world setting; however, tofacitinib was associated with an increased risk of cardiovascular outcomes, albeit statistically non-significant, in RA patients with cardiovascular risk factors.
Keywords: Tofacitinib, rheumatoid arthritis, cardiovascular outcomes
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease that affects approximately 0.2% of adults worldwide.1 RA is characterized by systemic inflammation that leads to joint damage and extra-articular manifestations including cardiovascular (CV) disease that adversely impact morbidity and mortality. Janus kinase (JAK) inhibitors (consisting of tofacitinib, baricitinib, upadacitinib) are a class of targeted synthetic disease modifying anti-rheumatic drugs (DMARDs) that are increasingly used for management of patients diagnosed with moderate to severely active rheumatoid arthritis.2 3 Tofacitinib, first approved in United States in 2012, is the most commonly prescribed JAK inhibitor.2 4
Tofacitinib has been associated with improved disease control in RA patients with similar efficacy when compared with other biological DMARDs such as adalimumab.5–7 However, recent reports have from the “ORAL Surveillance” post-marketing safety trial have indicated a potential for increased risk of major adverse CV events (MACE) with tofacitinib, in comparison with tumor necrosis factor inhibitors (TNFI), among RA patients at least 50 years of age and with at least one risk factor for cardiovascular disease (HR: 1.33, 95% CI: 0.91 to 1.94).8–10 Thus, the aim of this study was to conduct a large population-based observational study to further examine the risk of CV outcomes with tofacitinib in RA patients treated in routine clinical care settings.
Methods
Data Sources and Study Design
We conducted a new user, active comparator cohort study (Supplemental Figure 1) using claims data from the Optum Clinformatics (November 2012-June 2020), IBM MarketScan (November 2012- December 2018), and Medicare (parts A, B, and D, November 2012- December 2017) databases.11 The Optum and MarketScan claims databases capture de-identified record of over 200 million and 78 million commercially insured patients respectively in the United States. Medicare is a federal health insurance program and provides healthcare coverage for residents of the U.S. aged at least 65 years and patient aged less than 65 with a disability status as ascertained by U.S. Social Security Administration. All three data sources provide longitudinal information including patient demographics, inpatient and outpatient medical diagnoses and procedures, and prescription dispensing records. The protocol for this study was registered on clinicaltrials.gov (NCT04772248) and reporting followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.12 13 The study protocol received approval (IRB #2011P002580-207) from the Institutional Review Board of Brigham and Women’s Hospital (Boston, Massachusetts). Requirement for patient informed consent was waived because all personal identifiers were removed from each of the datasets to ensure patient confidentiality. Signed data license agreements were obtained for all data sources.
Study population
The base population consisted of patients initiating treatment on tofacitinib or a TNFI (infliximab, adalimumab, certolizumab pegol, etanercept, and golimumab). Cohort entry date corresponded to first TNFI or tofacitinib dispensation (“index drug”) with a minimum of 365 days of continuous enrollment in health plan prior to and including the cohort entry date. Patients required at least two diagnosis codes for RA in any setting during the 365 days baseline period (between 7 and 365 days apart).14 A previous validation study demonstrated a positive predictive value of 86% for this claims-based algorithm which combines two diagnosis codes for RA with one DMARD dispensing record.14 To ensure the inclusion of new users, we excluded TNFI users with a prescription of index TNFI and tofacitinib users with prescription of tofacitinib in the 365 days prior to cohort entry date. We also excluded patients with a prescription of tofacitinib and TNFI on cohort entry date, patients missing data on age or gender, and those with admission to nursing facility or hospice on or prior to cohort entry date. TNFI users with history of use of any JAK inhibitor or with prescriptions for multiple agents from the TNFI class on cohort entry date were also excluded. Finally, tofacitinib users with prescriptions of other approved JAK inhibitors (i.e. baricitinib or upadacitinib) on or at any point prior to cohort entry date were excluded.
From this source population of RA patients initiating treatment with tofacitinib or TNFI, we created two study cohorts. The first cohort, “real-world evidence (RWE)”, included all RA patients from routine care. The RWE cohort included patients at least 18 years of age in MarketScan and Optum (≥65 in Medicare) at cohort entry date. The second cohort, “RCT-duplicate cohort”, mimicked the inclusion and exclusion criteria of the ORAL surveillance trial.9 This study population was used to calibrate our findings and ensure comparability with the ORAL Surveillance trial results.8 10 The RCT-duplicate cohort was restricted to patients at least 50 years of age (65 in Medicare) with at least one methotrexate dispensation in six months prior to cohort entry date. This cohort was also restricted to patients with at least one CV risk factor including history of smoking, hypertension, dyslipidemia, diabetes mellitus, ischemic heart disease, or family history of ischemic heart disease. Patients hospitalized with infections in the 30-days prior to cohort entry date and pregnant patients were excluded from the RCT-duplicate cohort.
Exposure and Outcome Definition
We used an as-treated exposure definition whereby patients were followed from treatment initiation for study outcomes until treatment discontinuation or switch, insurance disenrollment, death, or end of the study period, whichever occurred first. The primary endpoint was defined as a composite CV outcome consisting of hospitalizations for myocardial infarction (MI) or stroke. Patients were followed for CV outcomes on the day after the cohort entry date (Supplemental Figure 1). Individual CV outcomes were also examined independently as secondary outcomes including MI, stroke, heart failure hospitalization, and coronary revascularization. We also examined the risk of all-cause mortality as an additional secondary outcome. Finally, we examined the risk of herpes zoster as a positive control outcome as previous studies have established an increased risk of herpes zoster with tofacitinib.15 16
Covariate Assessment
We assessed 76 potential confounders (75 in MarketScan) during the baseline covariate assessment period defined as the 365 days prior to treatment initiation (full list of covariates are included in Supplemental Methods).
Statistical Analysis
Descriptive statistics were used to summarize baseline characteristics for each study cohort. Crude incidence rates and corresponding 95% confidence intervals (CI) were reported for each study outcome. Propensity score (PS) fine stratification weighting was used to account for measured confounders in this study (details outlined in Supplemental Methods).17 Standardized differences (%) were used to assess the balance in individual covariates between two treatment groups before and after PS fine-stratification weighting.18 19 Cox proportional hazards model were used to estimate hazard ratios and corresponding 95% confidence intervals (CI) accounting for potential confounders using PS fine stratification weights and using an as-treated exposure definition in the primary analysis. Robust variance estimation was used to calculate 95% CI to account for weighting. We also assessed crude and weighted difference in rates and corresponding 95% CI when comparing tofacitinib with TNFI using Poisson regression. Effect estimates were pooled across three databases using fixed effects model with inverse variance weighting. We examined cumulative incidence of composite CV outcomes and the corresponding 95% CI separately for each treatment group. We used the Aetion Evidence Platform for cohort construction.20 Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R (R Foundation for Statistical Computing, Vienna, Austria).
Secondary and Sensitivity analyses
Pre-specified subgroup analyses were conducted based on age (≤65 and > 65), sex, and baseline cardiovascular disease (RWE cohorts only). In addition, we examined the risk of CV outcomes by stratifying by unique number of previous agents of bDMARDs (i.e. 0 vs ≥1). Secondary analysis was also conducted by using an intention-to-treat exposure definition whereby patients were censored 365 days after initiation of treatment with tofacitinib or TNFI. We also conducted 1:1 PS matching where each patient initiating tofacitinib was matched with a patient initiating TNFI using nearest neighbor greedy matching without replacement using a caliper of 0.025 on the natural scale of the PS.21 22 Finally, sensitivity analyses were conducted by restricting the TNFI comparator group in RWE and RCT-duplicate cohorts to only adalimumab and etanercept users, the specific TNFI which were the comparator in the ORAL Surveillance trial.9 10
Results
RWE Cohort
Study Population
The Consort diagrams for construction of the RWE and RCT-duplicate cohorts are outlined in Supplemental Tables 1 - 6. In RWE cohort, 28,568, 34,083, and 39,612 patients who met the inclusion and exclusion criteria were identified from Optum, MarketScan, and Medicare respectively of whom 13.2%, 15.6%, and 9.5% initiated treatment on tofacitinib (Supplemental Table 7). The mean age, in years, comparing tofacitinib and TNFI users was 56.8 vs 54.6 in Optum, 54.7 vs 52.7 in MarketScan, and 72.1 vs 72.2 in Medicare. The majority of patients in RWE cohort were female across the three databases (77% to 79%). The prevalence of CVD risk factors and previous use of co-medications was slightly higher in tofacitinib users compared with TNFI users (Supplemental Table 7).
In RWE cohort, 13% of patients in Optum, 10% in MarketScan, and 31% in Medicare had a history of cardiovascular disease. There were no discernable differences across most markers of healthcare utilization when comparing tofacitinib and TNFI users (Supplemental Table 7). Overall, PS fine stratification achieved excellent covariate balance with standardized differences close to zero across all covariates (Table 1, Supplemental Table 8).
Table 1.
Select baseline characteristics of RWE rheumatoid arthritis patients initiating tofacitinib or tumor necrosis factor inhibitors after propensity score fine stratification weighting
| Optum | MarketScan* | Medicare | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Tofacitinib (N=3,761) | TNFI (N=24,688) | SD (%) | Tofacitinib (N=5,298) | TNFI (N=28,727) | SD (%) | Tofacitinib (N=3,782) | TNFI (N=35,816) | SD (%) |
| Demographics | |||||||||
| Age; mean (std) | 56.8 (12.5) | 57.1 (13.2) | −2.6 | 54.7 (11.5) | 55.0 (12.0) | −1.9 | 72.1 (5.6) | 72.2 (5.6) | −1.3 |
| Female Gender; n (%) | 3,043 (80.9) | 20,046 (81.2) | −0.7 | 4,333 (81.8) | 23,503 (81.8) | −0.1 | 3,134 (82.9) | 29,819 (83.3) | −1.0 |
| White race; n (%) | 2,395 (63.7) | 15,691 (63.6) | 0.3 | - | - | - | 3,026 (80.0) | 28,449 (79.4) | 1.4 |
| Black race; n (%) | 412 (11.0) | 2,737 (11.1) | −0.4 | - | - | - | 410 (10.8) | 4,051 (11.3) | −1.5 |
| Asian race; n (%) | 103 (2.7) | 643 (2.6) | 0.8 | - | - | - | 85 (2.2) | 810 (2.3) | −0.1 |
| Hispanic race; n (%) | 471 (12.5) | 3,127 (12.7) | −0.4 | - | - | - | 126 (3.3) | 1224 (3.4) | −0.5 |
| RA related variables | |||||||||
| Number of unique bDMARDs; mean (std) | 1.6 (0.7) | 1.6 (0.7) | 2.4 | 1.8 (0.8) | 1.8 (0.8) | 1.6 | 1.6 (0.7) | 1.6 (0.7) | 2.2 |
| Non-biologic DMARDs | |||||||||
| Number of distinct csDMARDs | 1.0 (0.8) | 1.0 (0.8) | 0.3 | 1.0 (0.8) | 1.0 (0.8) | 1.8 | 1.1 (0.8) | 1.1 (0.8) | 0.8 |
| Any csDMARD use; n (%) | 2,723 (72.4) | 17,851 (72.3) | 0.2 | 3,989 (75.3) | 21,451 (74.7) | 1.4 | 2,889 (76.4) | 27,253 (76.1) | 0.7 |
| Methotrexate; n (%) | 1,731 (46.0) | 11,244 (45.5) | 1.0 | 2,722 (51.4) | 14,582 (50.8) | 1.2 | 1,954 (51.7) | 18,239 (50.9) | 1.5 |
| Hydroxychloroquine; n (%) | 933 (24.8) | 6,143 (24.9) | −0.2 | 1,254 (23.7) | 6,639 (23.1) | 1.3 | 950 (25.1) | 8,915 (24.9) | 0.5 |
| Leflunomide; n (%) | 799 (21.2) | 5,273 (21.4) | −0.3 | 1,065 (20.1) | 5,756 (20.0) | 0.2 | 828 (21.9) | 7,935 (22.2) | −0.6 |
| Sulfasalazine; n (%) | 388 (10.3) | 2,558 (10.4) | −0.1 | 491 (9.3) | 2,604 (9.1) | 0.7 | 418 (11.1) | 3,988 (11.1) | −0.3 |
| Glucocorticoid use | |||||||||
| Prior use of oral glucocorticoids (365 days); n (%) | 2,814 (74.8) | 18,489 (74.9) | −0.2 | 3,896 (73.5) | 21,185 (73.7) | −0.5 | 2,846 (75.3) | 26,944 (75.2) | 0.1 |
| Recent use of Oral Glucocorticoids (60 days); n (%) (60 days) |
1,898 (50.5) | 12,458 (50.5) | 0.0 | 2,625 (49.5) | 14,274 (49.7) | −0.3 | 2,115 (55.9) | 20,072 (56.0) | −0.2 |
| Cumulative dose of oral steroids in mg; mean (std) | 934.5 (1,485.5) | 935.6 (5,973.1) | 0.0 | 1,952.8 (25,666.3) | 2,094 (26,335.2) | −0.5 | 1,024.5 (1,195.2) |
1,019.6 (1,279.5) |
0.4 |
| CVD Risk Factors | |||||||||
| Obesity; n (%) | 882 (23.5) | 5,876 (23.8) | −0.8 | 810 (15.3) | 4392 (15.3) | 0.0 | 581 (15.4) | 5,446 (15.2) | 0.4 |
| Smoking; n (%) | 749 (19.9) | 4,925 (20.0) | −0.1 | 465 (8.8) | 2,566 (8.9) | −0.6 | 972 (25.7) | 9,180 (25.6) | 0.2 |
| Atrial Fibrillation; n (%) | 154 (4.1) | 1,038 (4.2) | −0.6 | 140 (2.6) | 752 (2.6) | 0.1 | 397 (10.5) | 3,723 (10.4) | 0.3 |
| Coronary artery disease; n (%) | 381 (10.1) | 2,564 (10.4) | −0.8 | 425 (8.0) | 2,394 (8.3) | −1.1 | 904 (23.9) | 8,477 (23.7) | 0.5 |
| Type 2 diabetes mellitus; n (%) | 805 (21.4) | 5,353 (21.7) | −0.7 | 835 (15.8) | 4,563 (15.9) | −0.3 | 1,162 (30.7) | 10,918 (30.5) | 0.5 |
| Heart failure; n (%) | 192 (5.1) | 1,324 (5.4) | −1.2 | 175 (3.3) | 960 (3.3) | −0.2 | 450 (11.9) | 4,267 (11.9) | 0.0 |
| Hypertension; n (%) | 1,966 (52.3) | 13,075 (53.0) | −1.4 | 2,355 (44.5) | 12,922 (45.0) | −1.1 | 3,110 (82.2) | 29,417 (82.1) | 0.3 |
| Hyperlipidemia; n (%) | 1,619 (43.0) | 10,706 (43.4) | −0.6 | 2,002 (37.8) | 10,937 (38.1) | −0.6 | 2,569 (67.9) | 24,187 (67.5) | 0.8 |
| Stroke or transient ischemic attack; n (%) | 92 (2.4) | 605 (2.5) | 0.0 | 113 (2.1) | 620 (2.2) | −0.2 | 134 (3.5) | 1,255 (3.5) | 0.2 |
| Peripheral vascular disease; n (%) | 163 (4.3) | 1,103 (4.5) | −0.6 | 141 (2.7) | 776 (2.7) | −0.3 | 442 (11.7) | 4,166 (11.6) | 0.2 |
| Venous thromboembolism; n (%) | 102 (2.7) | 699 (2.8) | −0.7 | 141 (2.7) | 765 (2.7) | 0.0 | 103 (2.7) | 996 (2.8) | −0.4 |
| Other comorbidities | |||||||||
| Chronic liver disease; n (%) | 273 (7.3) | 1,792 (7.3) | 0.0 | 315 (5.9) | 1,696 (5.9) | 0.2 | 317 (8.4) | 2,985 (8.3) | 0.2 |
| Chronic kidney disease (Stage 3+); n (%) | 212 (5.6) | 1,428 (5.8) | −0.6 | 168 (3.2) | 926 (3.2) | −0.3 | 442 (11.7) | 4,207 (11.7) | −0.2 |
| COPD; n (%) | 599 (15.9) | 3,992 (16.2) | −0.7 | 629 (11.9) | 3,454 (12.0) | −0.5 | 1,041 (27.5) | 9,955 (27.8) | −0.6 |
| Inflammatory bowel disease; n (%) | 63 (1.7) | 415 (1.7) | 0.0 | 68 (1.3) | 353 (1.2) | 0.5 | 50 (1.3) | 461 (1.3) | 0.3 |
| Psoriasis; n (%) | 170 (4.5) | 1,100 (4.5) | 0.3 | 169 (3.2) | 885 (3.1) | 0.6 | 119 (3.1) | 1,028 (2.9) | 1.6 |
| Cancer (excluding NMSC); n (%) | 484 (12.9) | 3,267 (13.2) | −1.1 | 692 (13.1) | 3,783 (13.2) | −0.3 | 789 (20.9) | 7,438 (20.8) | 0.2 |
| Combined Comorbidity Index; mean (std) | 1.2 (2.0) | 1.2 (2.0) | −0.8 | 0.7 (1.5) | 0.7 (1.5) | −0.7 | 1.8 (2.4) | 1.9 (2.4) | −0.4 |
| Frailty score; mean (std) | 0.2 (0.0) | 0.2 (0.0) | −0.8 | 0.1 (0.0) | 0.1 (0.0) | −1.5 | 0.2 (0.0) | 0.2 (0.0) | 0.0 |
-Full Tables describing all patient characteristics before and after PS-weighting are provided in the Supplementary Material.
-Abbreviation: bDMARD-biological disease modifying anti-rheumatic drugs, COPD- Chronic obstructive pulmonary disease, CRP-C-reactive protein, csDMARDs-conventional synthetic disease modifying anti-rheumatic drugs, NMSC-Non-melanoma skin cancer, RA-rheumatoid arthritis, SD- standardized difference, std-standard deviation, TNFI- tumor necrosis factor inhibitors
Data for race is not available in MarketScan
Primary Outcome
The crude incidence rates of the primary CV endpoint per 100 person-years (95% CI) for tofacitinib and TNFI users were 0.73 (0.47 to 1.09) and 0.61 (0.51 to 0.72) in Optum, 0.75 (0.52 to 1.05) and 0.52 (0.44 to 0.61) in MarketScan, and 2.14 (1.66 to 2.70) and 1.86 (1.71 to 2.02) in Medicare (Table 2).
Table 2.
Incidence rate, crude hazard ratio, and corresponding 95% confidence intervals for the primary composite cardiovascular outcome in RWE and RCT-duplicate cohort of rheumatoid arthritis patients initiating treatment with tofacitinib or tumor necrosis factor inhibitors
| Data source | Exposure group | Sample Size | Events | Total person years of follow-up | Crude Incidence Rate (95% CI)* | Crude Incidence Rate Difference (95% CI)* | Crude HR (95% CI) |
|---|---|---|---|---|---|---|---|
| RWE Cohort | |||||||
| Optum | TNFI | 24,805 | 143 | 23,458 | 0.61 (0.51 to 0.72) | Ref | Ref |
| Tofacitinib | 3,763 | 24 | 3,273 | 0.73 (0.47 to 1.09) | 0.12 (−0.19 to 0.43) | 1.21 (0.78 to 1.86) | |
| MarketScan | TNFI | 28,776 | 141 | 27,257 | 0.52 (0.44 to 0.61) | Ref | Ref |
| Tofacitinib | 5,307 | 35 | 4,655 | 0.75 (0.52 to 1.05) | 0.23 (−0.03 to 0.50) | 1.44 (0.99 to 2.09) | |
| Medicare | TNFI | 35,830 | 562 | 30,277 | 1.86 (1.71 to 2.02) | Ref | Ref |
| Tofacitinib | 3,782 | 69 | 3,229 | 2.14 (1.66 to 2.70) | 0.28 (−0.25 to 0.81) | 1.15 (0.89 to 1.48) | |
| Pooled | TNFI | 89,411 | 846 | 80,992 | 1.24 (1.16 to 1.33) | Ref | Ref |
| Tofacitinib | 12,852 | 128 | 11,157 | 1.31 (1.10 to 1.56) | 0.20 (0.01 to 0.39) | 1.23 (1.02 to 1.48) | |
| RCT-Duplicate Cohort | |||||||
| Optum | TNFI | 6,077 | 56 | 5,932 | 0.94 (0.71 to 1.23) | Ref | Ref |
| Tofacitinib | 801 | 10 | 752 | 1.33 (0.64 to 2.45) | 0.39 (−0.47 to 1.25) | 1.43 (0.73 to 2.81) | |
| MarketScan | TNFI | 6,920 | 55 | 6,857 | 0.80 (0.60 to 1.04) | Ref | Ref |
| Tofacitinib | 1,151 | 13 | 1,069 | 1.22 (0.65 to 2.08) | 0.41 (−0.28 to 1.11) | 1.50 (0.82 to 2.74) | |
| Medicare | TNFI | 18,576 | 289 | 16,241 | 1.78 (1.58 to 2.00) | Ref | Ref |
| Tofacitinib | 1,545 | 32 | 1,338 | 2.39 (1.64 to 3.38) | 0.61 (−0.24 to 1.47) | 1.35 (0.93 to 1.94) | |
| Pooled | TNFI | 31,573 | 400 | 29,030 | 1.46 (1.32 to 1.61) | Ref | Ref |
| Tofacitinib | 3,497 | 55 | 3,159 | 1.83 (1.41 to 2.39) | 0.46 (0.01 to 0.92) | 1.39 (1.05 to 1.85) | |
-Abbreviations: CI-Confidence interval, HR-Hazard ratio, RCT-randomized controlled trial, Ref-Reference, RWE-Real world evidence, TNFI-tumor necrosis factor inhibitors
-All estimates were pooled using fixed effects models with inverse variance weighting
Per 100 person-years
In the primary analysis, the pooled weighted HR (95% CI) for CV outcomes when comparing tofacitinib with TNFI was 1.01 (0.83 to 1.23) with weighted rate difference (95% CI) corresponding to 0.02 (−0.19 to 0.23) CV events per 100 person-years (Figure 1 and Supplemental Table 9). Correspondingly, there was no differences in cumulative incidence of composite CV outcomes when comparing tofacitinib with TNFI in any of the three databases (Supplemental Figure 2). Among tofacitinib users, the median (interquartile range) months to CV events was 6.9 (2.9 to 16.3) in Optum, 5.1 (2.0 to 12.3) in MarketScan, and 6.0 (2.0 to 13.4) in Medicare. Among TNFI users, the median (interquartile range) months to CV events was 7.5 (2.7 to 17.5) in Optum, 6.1 (2.5 to 11.7) in MarketScan, and 6.3 (2.4 to 15.1) in Medicare.
Figure 1.
Forest plot of propensity score fine stratification weighted hazard ratios and corresponding 95% confidence intervals for composite cardiovascular outcomes when comparing tofacitinib with tumor necrosis factor inhibitors in patients with rheumatoid arthritis in RWE cohort (top panel) and RCT-duplicate cohort (bottom panel)
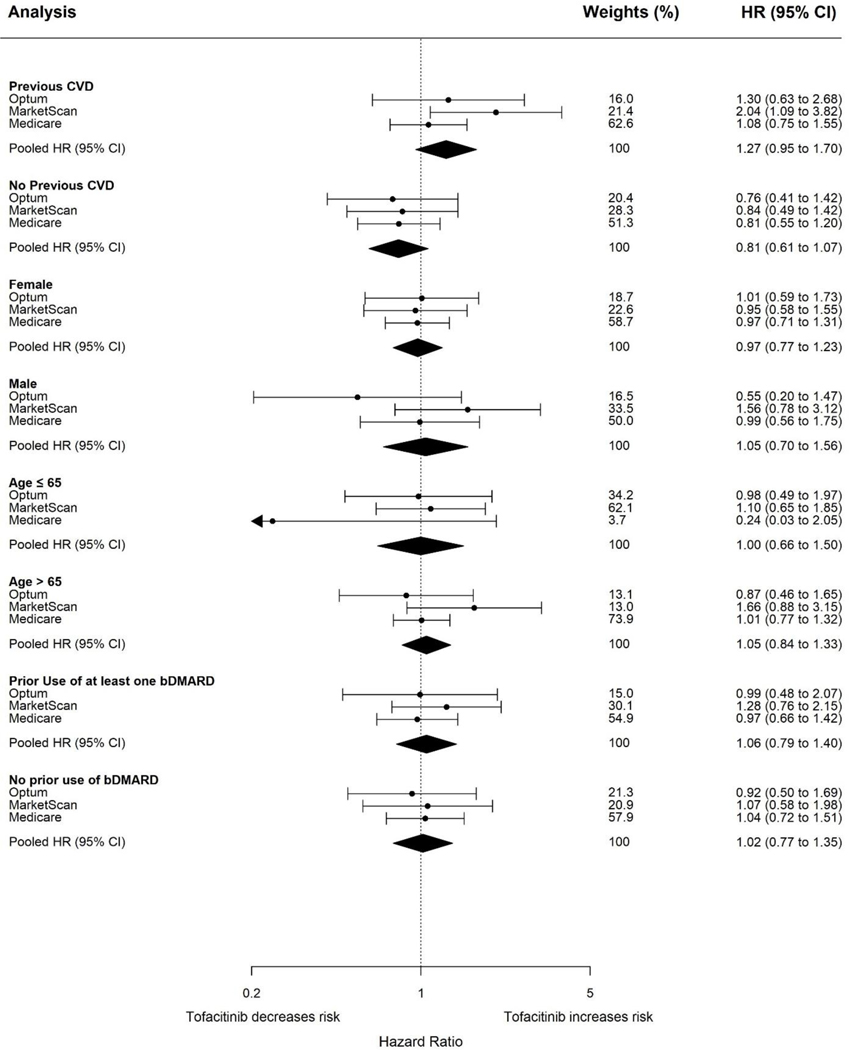
In subgroup analyses, the pooled weighted HR (95% CI) was 1.27 (0.95 to 1.70) and 0.81 (0.61 to 1.07) among patients with and without history of cardiovascular disease respectively (Figure 2 and Supplemental Table 9). The pooled weighted HR (95% CI) among patients ≤ 65 years of age was 1.00 (0.66 to 1.50) and 1.05 (0.84 to 1.33) for patient aged more than 65 years. No association was observed across other subgroups including among males (pooled weighted HR: 1.05, 95% CI: 0.70 to 1.56), females (pooled weighted HR: 0.97, 95% CI: 0.77 to 1.23), patients with previous use of biological DMARDs (pooled weighted HR: 1.06, 95% CI: 0.79 to 1.40), and patients without previous use of biological DMARDs (pooled weighted HR: 1.02, 95% CI: 0.77 to 1.35)(Supplemental Table 9). Consistent results were observed across other sensitivity and secondary analyses including PS matching, intention-to-treat exposure definition, and restriction of the TNFI comparator to adalimumab and etanercept users (Supplemental Table 9).
Figure 2.
Forest plot of propensity score fine stratification weighted hazard ratios and corresponding 95% confidence intervals for composite cardiovascular outcomes for subgroup analyses in RWE study cohort
Secondary outcomes
For individual CV outcomes, the pooled weighted HR (95% CI) was 1.04 (0.82 to 1.33) for MI, 0.93 (0.66 to 1.31) for stroke, 1.07 (0.79 to 1.46) for heart failure hospitalization, and 1.04 (0.78 to 1.40) for coronary revascularization (Supplemental Table 10) when comparing tofacitinib users with TNFI users. The pooled weighted HR (95% CI) was 1.20 (0.98 to 1.46) for all-cause mortality. For the positive control outcome, we successfully replicated the known association between tofacitinib and risk of herpes zoster (pooled weighted HR 1.98, 95% CI: 1.78 to 2.19).
RCT-duplicate Cohort
Study Population
In the RCT-duplicate cohort, 6,878, 8,071, and 20,121 patients were identified from Optum, MarketScan, and Medicare, respectively, of whom 11.6%, 14.3%, and 7.7% initiated treatment with tofacitinib (Supplemental Table 11). Overall, PS fine stratification weighting achieved excellent covariate balance in this study population with standardized differences close to zero for all covariates (Supplemental Table 12).
Primary outcome
The crude incidence rates of the primary CV endpoint per 100 person-years (95% CI) for tofacitinib and TNFI users were 1.33 (0.64 to 2.45) and 0.94 (0.71 to 1.23) in Optum, 1.22 (0.65 to 2.08) and 0.80 (0.60 to 1.04) in MarketScan, and 2.39 (1.64 to 3.38) and 1.78 (1.58 to 2.00) in Medicare (Table 2). In the primary analysis, the pooled weighted HR (95% CI) for primary CV outcome was 1.24 (0.90 to 1.69) corresponding to a pooled weighted rate difference (95% CI) of 0.28 (−0.24 to 0.80) CV events per 100 person-years when comparing tofacitinib users with TNFI users (Figure 1 and Supplemental Table 13). The cumulative incidence of CV outcomes was similar when comparing with tofacitinib with TNFI users in Optum and MarketScan but was slightly higher among tofacitinib users in Medicare, albeit with wide confidence intervals for these analyses (Supplemental Figure 3). The median months (interquartile range) to CV events among tofacitinib users was 6.9 (2.6 to 14.2) in Optum, 6.0 (2.8 to 11.8) in MarketScan, and 5.2 (1.7 to 12.4) in Medicare. Among TNFI users, the median (interquartile range) months to CV events was 6.9 (3.0–11.0) in Optum, 7.1 (2.8–12.5) in MarketScan, and 6.8 (2.2 to 15.8) in Medicare. In sensitivity analysis restricting comparator to adalimumab and etanercept (Supplemental Table 13), the pooled weighted HR (95%) CI for primary CV outcome was 1.32 (0.94 to 1.86).
Discussion
Overall, in this large population-based study, tofacitinib in comparison with TNFI was not associated with risk of composite CV outcome in RA patients treated in real world settings (pooled weighted HR: 1.01, 95% CI: 0.83 to 1.23). Results from the RCT-duplicate cohort were consistent with those reported from the ORAL surveillance trial (pooled weighted HR: 1.24, 95% CI: 0.90 to 1.69 vs trial: 1.33, 95% CI: 0.91 to 1.94).8 10 An increased risk of CV outcomes was also observed among RWE patients with history of cardiovascular disease (pooled weighted HR: 1.27, 95% CI: 0.95 to 1.70) but not those without history of CVD (pooled weighted HR: 0.81, 95% CI: 0.61 to 1.07).
The findings from previous studies examining the association between tofacitinib and CV outcomes have been discordant. Recent reports from the ORAL Surveillance trial have indicated that both 5mg and 10mg twice daily dose of tofacitinib, in comparison with TNFI, were associated with increased risk of major adverse CV events (HR: 1.24, 95% CI: 0.81 to 1.91 and HR: 1.43, 95% CI: 0.94 to 2.18 respectively).8 10 This trial consisted 4,362 patients who were at least 50 years of age, with one at least one risk factor for cardiovascular disease, and with a background of treatment with methotrexate.9 10 Results from a recent meta-analysis of RCTs, excluding ORAL surveillance, were inconclusive for the association between tofacitinib and CV risk in patients with RA (Odds Ratio: 1.29, 95% CI: 0.40 to 4.13) or chronic plaque psoriasis (Odds Ratio: 3.61, 95% CI: 0.71 to 18.43) due to low event rates.23 In contrast, among patients enrolled in the CORRONA RA registry in the United States, tofacitinib, in comparison with biological DMARDs (including TNFI and non-TNFI biologics), was not associated with an increased risk of MACE which was defined as MI, stroke, transient ischemic attack, or CV death (HR: 0.61: 0.34 to 1.06).24 Our results suggest that the association between tofacitinib and CV outcomes may be modified by baseline CV risk. In patients from our RWE cohort who had no underlying CV risk factors or history (87% of patients in Optum, 90% in MarketScan, and 69% in Medicare), we noted no detectable impact of tofacitinib treatment on risk of adverse CV outcomes. However, among patients with CV risk factors or history, estimates consistently suggested a potentially elevated risk. We recommend continuing research to better understand risk-benefit tradeoffs of this important treatment option in a wide range of RA patients.
Overall, there is no known direct mechanism that would explain a detrimental effect of tofacitinib on risk of CV outcomes. In phase II and III trials, both doses of tofacitinib (5mg or 10mg) either as monotherapy or in combination with non-biologic DMARDs were associated with 15 to 20% increase in low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C levels) when comparing four weeks after treatment initiation with baseline. The changes in LDL-C and HDL-C levels persisted 12 months after treatment initiation.25 However, the levels of the ratio of total-cholesterol: HDL-C and LDL-C: HDL-C levels, which are more reliable predictors of CV events, did not change.25 In another study with 46 RA patients, tofacitinib was not associated with changes in carotid intima-media thickness when comparing 54 weeks after treatment initiation with baseline (1.09 ± 0.69 and 1.08 ± 0.78 mm respectively).26 Additional mechanistic studies will be required in light of potential increased risk of CV outcomes associated with tofacitinib in ORAL Surveillance trial.
This study has strengths and limitations. First, we conducted a large multi-database population-based studies with more than 102,000 patients, a sample size larger than the ORAL Surveillance trial and CORRONA RA registry study in the United States.8 10 24 Second, we comprehensively assessed the risk of individual CV outcomes including MI, stroke, heart failure, and coronary revascularization. Third, we employed a new user design active comparator design to control for confounding by disease severity and circumvent prevalent-user bias.11 Fourth, we calibrated our results using the RCT-duplicate cohort to assess the validity of the study and ensure that are results were comparable with those of the ORAL surveillance post-marketing trial.8 10 Fifth, we found an increased risk of herpes zoster infection which was included as a positive control outcome, consistent with previous studies.15 16 Finally, the study protocol was pre-registered prior to conducting our study.12 Our study has some limitations. First, residual confounding by factors not captured in administrative claims including RA activity is possible. However, we used an active comparator group (i.e., TNF inhibitors) and adjusted for 76 confounders (75 in MarketScan) including multiple variables that may serve as proxies for RA disease severity. Reassuringly, a recent study using the CORRONA RA registry, a prospective disease-based registry inclusive of 50,605 RA patients across 177 private and academic practices in the United States, demonstrated that RA patients who are treated with tofacitinib are comparable to patients treated with biological DMARDs in regards to RA-related variables including clinical disease activity index.24 In addition, we observed approximately equal distribution in time to CV events throughout follow-up after treatment initiation among tofacitinib and TNFI users in RWE and RCT-duplicate cohorts. Second, exposure misclassification is possible due to incomplete adherence to study drugs. To minimize exposure misclassification, we implemented an as-treated exposure definition where the occurrence of study outcomes was assessed while patients were on treatment. Finally, we could not assess the risk of CV outcomes with newer JAK inhibitors including baricitinib and upadacitinib and thus additional studies will be required to examine the risk of CV outcomes with these newer agents.
Overall, in this multi-database population-based study, we did not find evidence for an increased risk of CV outcomes with tofacitinib, in comparison with TNFI, among RA patients treated in the real-world setting. However, concordant with results from ORAL Surveillance safety trial, tofacitinib, in comparison with TNFI, was associated with an elevated risk of CV outcomes, though statistically non-significant, in RA patients with CV risk factors or a history of cardiovascular disease. Thus, an elevated risk of CV outcomes cannot be ruled out in patients with CV risk factors or history of cardiovascular disease.
Supplementary Material
KEY MESSAGE.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Recently released topline findings from “ORAL Surveillance” post-marketing trial have raised concerns that tofacitinib, in comparison with tumor necrosis factor inhibitors, may increase the risk of cardiovascular disease in patients with rheumatoid arthritis.
WHAT THIS STUDY ADDS
In this multi-database, population-based study including 102,263 rheumatoid arthritis patients, tofacitinib was not associated with an increased risk of cardiovascular outcomes when compared with tumor necrosis factor inhibitors.
A numerically increased risk of cardiovascular outcomes was observed in older patients with cardiovascular risk factors or history of cardiovascular disease.
HOW MIGHT THIS IMPACT ON CLINICAL PRACTICE OR FUTURE DEVELOPMENTS?
In this study in real-world setting, tofacitinib, in comparison with tumor necrosis factor inhibitors, was not associated with increased risk of cardiovascular outcomes, although an increased risk of cardiovascular outcomes with tofacitinib cannot be ruled out in patients with cardiovascular risk factors or history of cardiovascular disease.
Acknowledgments and affiliations:
RJD has received research grants to the Brigham and Women’s Hospital from Bayer, Novartis, and Vertex for unrelated projects. SCK has received research grants to the Brigham and Women’s Hospital from Roche/Genentech, Pfizer, Bristol-Myers Squibb, Roche, and AbbVie for unrelated studies. All other authors have no conflict of interests to disclose. This study was funded by internal sources of the Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital & Harvard Medical School, Boston, MA, USA. SCK is supported by the National Institutes of Health (NIH) grant - K24AR078959. FK is supported by a postdoctoral fellowship from Fonds de recherche du Québec-Santé (FRQS). This work was previously presented at American College of Rheumatology Convergence 2021 Meeting (Abstract 1939): Khosrow-Khavar F, Kim S, Lee H, Lee S, and Desai R. Risk of Cardiovascular Outcomes in Patients Treated with Tofacitinib: First Results from the Safety of TofAcitinib in Routine Care Patients with Rheumatoid Arthritis (STAR-RA) Study. 2021.
Footnotes
Patient and Public Involvement statement:
There was no involvement of patients or public entities in conception and execution of this study.
Data Sharing:
No additional data available.
References
- 1.Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis 2019;78(11):1463–71. doi: 10.1136/annrheumdis-2019-215920 [published Online First: 2019/09/13] [DOI] [PubMed] [Google Scholar]
- 2.Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol 2021;73(7):1108–23. doi: 10.1002/art.41752 [published Online First: 2021/06/09] [DOI] [PubMed] [Google Scholar]
- 3.Singh JA, Saag KG, Bridges SL Jr., et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2016;68(1):1–25. doi: 10.1002/acr.22783 [published Online First: 2015/11/08] [DOI] [PubMed] [Google Scholar]
- 4.Desai RJ, Solomon DH, Jin Y, et al. Temporal Trends in Use of Biologic DMARDs for Rheumatoid Arthritis in the United States: A Cohort Study of Publicly and Privately Insured Patients. Journal of managed care & specialty pharmacy 2017;23(8):809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang F, Sun L, Wang S, et al. Efficacy and Safety of Tofacitinib, Baricitinib, and Upadacitinib for Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Mayo Clin Proc 2020;95(7):1404–19. doi: 10.1016/j.mayocp.2020.01.039 [published Online First: 2020/06/06] [DOI] [PubMed] [Google Scholar]
- 6.van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367(6):508–19. doi: 10.1056/NEJMoa1112072 [published Online First: 2012/08/10] [DOI] [PubMed] [Google Scholar]
- 7.Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017;390(10093):457–68. doi: 10.1016/S0140-6736(17)31618-5 [published Online First: 2017/06/21] [DOI] [PubMed] [Google Scholar]
- 8.Pfizer Inc. Pfizer Shares Co-Primary Endpoint Results from Post-Marketing Required Safety Study of XELJANZ® (tofacitinib) in Subjects with Rheumatoid Arthritis (RA) 2021. [Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-shares-co-primary-endpoint-results-post-marketing accessed February 1, 2021.
- 9.Pfizer Inc. Safety Study Of Tofacitinib Versus Tumor Necrosis Factor (TNF) Inhibitor In Subjects With Rheumatoid Arthritis 2014. [Available from: https://clinicaltrials.gov/ct2/show/NCT02092467.
- 10.Ytterberg SR, Bhatt DL, Mikuls T, et al. Safety and Efficacy of Tofacitinib vs TNF Inhibitors in RA Patients Aged 50 Years or Older with One or More Cardiovascular Risks: Results from a Phase 3b/4 Randomized Safety Trial. ACR Convergence 2021. [Google Scholar]
- 11.Lund JL, Richardson DB, Sturmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep 2015;2(4):221–28. doi: 10.1007/s40471-015-0053-5 [published Online First: 2016/03/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai RJ. Safety of TofAcitinib in Routine Care Patients With Rheumatoid Arthritis (STAR-RA)- Cardiovascular Endpoints 2021. [Available from: https://clinicaltrials.gov/ct2/show/NCT04772248.
- 13.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X [published Online First: 2007/12/08] [DOI] [PubMed] [Google Scholar]
- 14.Kim SY, Servi A, Polinski JM, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther 2011;13(1):R32. doi: 10.1186/ar3260 [published Online First: 2011/02/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee EB, Fleischmann R, Hall S, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 2014;370(25):2377–86. doi: 10.1056/NEJMoa1310476 [published Online First: 2014/06/19] [DOI] [PubMed] [Google Scholar]
- 16.Pawar A, Desai RJ, Gautam N, et al. Risk of admission to hospital for serious infection after initiating tofacitinib versus biologic DMARDs in patients with rheumatoid arthritis: a multidatabase cohort study. Lancet Rheumat 2020;2(2):e84–e98. doi: 10.1016/S2665-9913(19)30137-7 [DOI] [PubMed] [Google Scholar]
- 17.Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ 2019;367:l5657. doi: 10.1136/bmj.l5657 [published Online First: 2019/10/28] [DOI] [PubMed] [Google Scholar]
- 18.Franklin JM, Rassen JA, Ackermann D, et al. Metrics for covariate balance in cohort studies of causal effects. Stat Med 2014;33(10):1685–99. doi: 10.1002/sim.6058 [published Online First: 2013/12/11] [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Communications in Statistics - Simulation and Computation 2008;38(6):1228–34. [Google Scholar]
- 20.Aetion Inc. Aetion Evidence Platform® (2020). Software for real-world data analysis. [Available from: http://www.aetion.com
- 21.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med 2014;33(6):1057–69. doi: 10.1002/sim.6004 [published Online First: 2013/10/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786 [published Online First: 2011/08/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie W, Xiao S, Huang Y, et al. Effect of tofacitinib on cardiovascular events and all-cause mortality in patients with immune-mediated inflammatory diseases: a systematic review and meta-analysis of randomized controlled trials. Ther Adv Musculoskelet Dis 2019;11:1759720X19895492. doi: 10.1177/1759720X19895492 [published Online First: 2020/01/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kremer JM, Bingham CO, 3rd, Cappelli LC, et al. Postapproval Comparative Safety Study of Tofacitinib and Biological Disease-Modifying Antirheumatic Drugs: 5-Year Results from a United States-Based Rheumatoid Arthritis Registry. ACR Open Rheumatol 2021;3(3):173–84. doi: 10.1002/acr2.11232 [published Online First: 2021/02/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charles-Schoeman C, Gonzalez-Gay MA, Kaplan I, et al. Effects of tofacitinib and other DMARDs on lipid profiles in rheumatoid arthritis: implications for the rheumatologist. Semin Arthritis Rheum 2016;46(1):71–80. doi: 10.1016/j.semarthrit.2016.03.004 [published Online First: 2016/04/16] [DOI] [PubMed] [Google Scholar]
- 26.Kume K, Amano K, Yamada S, et al. Tofacitinib improves atherosclerosis despite up-regulating serum cholesterol in patients with active rheumatoid arthritis: a cohort study. Rheumatol Int 2017;37(12):2079–85. doi: 10.1007/s00296-017-3844-9 [published Online First: 2017/10/17] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.