Abstract
Acute ischemic stroke (AIS) is the most common type of stroke. Fingolimod is a sphingosine analog that acts on sphingosine‐1‐phosphate receptors (S1PR). Recently, the safety and efficacy of fingolimod in both patients with intracerebral hemorrhage and patients with AIS have been investigated in proof‐of‐concept trials. In this review, we performed a meta‐analysis to evaluate the efficacy and safety of fingolimod for AIS. This study was conducted according to the PRISMA (Preferred Reporting Items for Systemic review and Meta‐Analysis) statement. We searched for publications on the PubMed, Embase, Cochrane Central Register of Controlled Trials, Clinical trials, CNKI, Wanfang Data, VIP, CBM up to August 2021. We compiled five studies; a main meta‐analysis forest plots were conducted for the values of the proportion of patients whose modified Rankin scale (MRS) score was 0–1 at day 90. There were heterogeneities in each study; the method of sensitivity analysis was performed. A sensitivity analysis was performed with a mean difference (MD) of the efficacy of fingolimod plus standardized treatment versus standardized treatment alone. Random effect model is used for meta‐analysis regardless of the I2 index. The analysis was carried out for categorical variables using the risk ratio (RR), LogRR, and its 95% CI. The methodological quality of each randomized controlled trial (RCTs) was assessed according to the Cochrane Collaboration tool to assess the risk of bias (ROB). A meta‐analysis of five studies with 228 participants was conducted. The risk ratio of patients whose MRS score was 0–1 at day 90 between fingolimod plus standardized treatment and standardized treatment alone was 2.59 (95%CI, 1.48–4.56). The Fingolimod plus standard treatment group decreased infarct growth and improved clinical function than the standard treatment.
Keywords: acute ischemic stroke, fingolimod, meta‐analysis, modified rankin scale
The efficacy and safety of fingolimod plus standardized treatment versus standardized treatment alone for acute ischemic stroke: a systematic review and meta‐analysis.

Abbreviations
- AEs
adverse events
- AIS
Acute ischemic stroke
- BBB
blood‐brain barrier
- BL
Bin Liu
- CI
confidence interval
- CNS
Central nervous system
- CZ
Chenhui Zhao
- FJ
Feng Jiang
- JY
Jun Yuan
- JZ
Jin Zhen
- MD
mean difference
- MeSH
Medical Subject Heading
- ML
Min Li
- MRS
Modified Rankin Scale
- MS
multiple sclerosis
- MW
Meiling Wang
- NIHSS
National Institutes of Health Stroke Scale
- NL
Na Li
- PB
Peng Bai
- PEDro
The Physiotherapy Evidence Database
- PRISMA
Preferred Reporting Items for Systemic Reviews and Meta‐Analysis
- PW
Ping Wang
- RCTs
Randomized controlled trials
- RR
risk ratio
- RZ
Runxiu Zhu
- S1PR
sphingosine‐1‐phosphate receptors
- SAEs
serious adverse events
- tPA
tissue plasminogen activator
- YY
Yuan Yao
- ZL
Zihong Liang
1. INTRODUCTION
Acute ischemic stroke (AIS) is the most common type of stroke. It has the characteristics of high morbidity, high mortality, and high disability, which seriously endangers the health and life of patients. 1 Effective treatment after AIS will directly affect the prognosis of patients. 1
Disabling stroke outcomes make it the second leading cause of death worldwide after cardiac ischemia. Therapy for AIS centers first on rapid revascularization of arterial territories, with additional focus on managing blood pressure and cerebral edema. 2 Revascularization is currently achieved by the intravenous administration of tissue plasminogen activator (tPA) and intravascular therapy. However, the benefit of tPA is highly time dependent, considering that pooled analysis has documented loss of benefit beyond 4.5 h from onset of symptoms. 2 , 3 , 4 Although numerous neuroprotective clinical trials have been conducted, no significant breakthrough has been made to improve the outcome of stroke patients. 2 , 5 , 6
Cerebral ischemia‐induced cell death swiftly activates the immune system and initiates inflammation within the brain. 7 , 8 , 9 , 10 , 11 In an early phase, these immune responses appear to exacerbate neurovascular dysfunction by promoting thrombus formation and accumulation of blood components in the cerebral microvasculature. 11 , 12 , 13 These changes subsequently exacerbate the ischemic cascade catalyzing neural cell death in the penumbra, resulting in the extension of infarction, which potentially limits the efficacy of pharmacologic or mechanical reperfusion. 11 , 14 , 15 , 16
Fingolimod is a sphingosine analog that acts on sphingosine‐1‐phosphate receptors (S1PR). It was approved by the US. Food and Drug Administration in 2010 as the first oral disease‐modifying therapy for the relapsing–remitting form of multiple sclerosis (MS). 17 , 18 Fingolimod inhibits the egress of lymphocytes from lymph nodes and limits their recirculation. 18 , 19 Additional effects on the integrity of the blood–brain barrier (BBB) and direct action on neurons and glia that bear sphingosine‐1‐phosphate receptor may also contribute to its beneficial attributes in MS. 18 , 20 , 21 , 22 Recently, the safety and efficacy of fingolimod in both patients with intracerebral hemorrhage and patients with AIS have been investigated in proof‐of‐concept trials. 2 , 18 Fingolimod limited the expansion of infarct volume and ameliorated hemorrhagic transformation in patients with acute ischemic stroke who received intravenous alteplase within 4.5 h after stroke onset, 11 , 18 Meanwhile, in patients with acute anterior circulation occlusion who are >4.5 h after disease onset, fingolimod significantly improved the clinical outcome, reduced secondary lesion growth, and decreased microvascular permeability. 18 In this systematic review, we performed a meta‐analysis to evaluate the efficacy and safety of fingolimod for acute ischemic stroke.
2. MATERIALS AND METHODS
2.1. Protocol and registration
Our protocol was registered prospectively with the Prospero website (CRD42021272343), the prospective international register of systematic reviews available at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021272343.
2.2. Literature search
This search was restricted only to articles published in the English and Chinese language. We searched for publications on the PubMed, Embase, Cochrane Central Register of Controlled Trials, Clinical trials, CNKI, Wanfang Data, VIP, CBM up to August 2021. We did keyword and Medical Subject Heading (MeSH) searches for our theme, and MeSH terms, keywords, and their synonyms related to "Fingolimod hydrochloride" and "Cerebrovascular Disorders." A flowchart of the search strategy is shown in Figure 1. One of us used a standardized form of data extraction to extract data; another person checked it, revisited the data that did not match, and resolved the differences through discussion and consensus.
FIGURE 1.
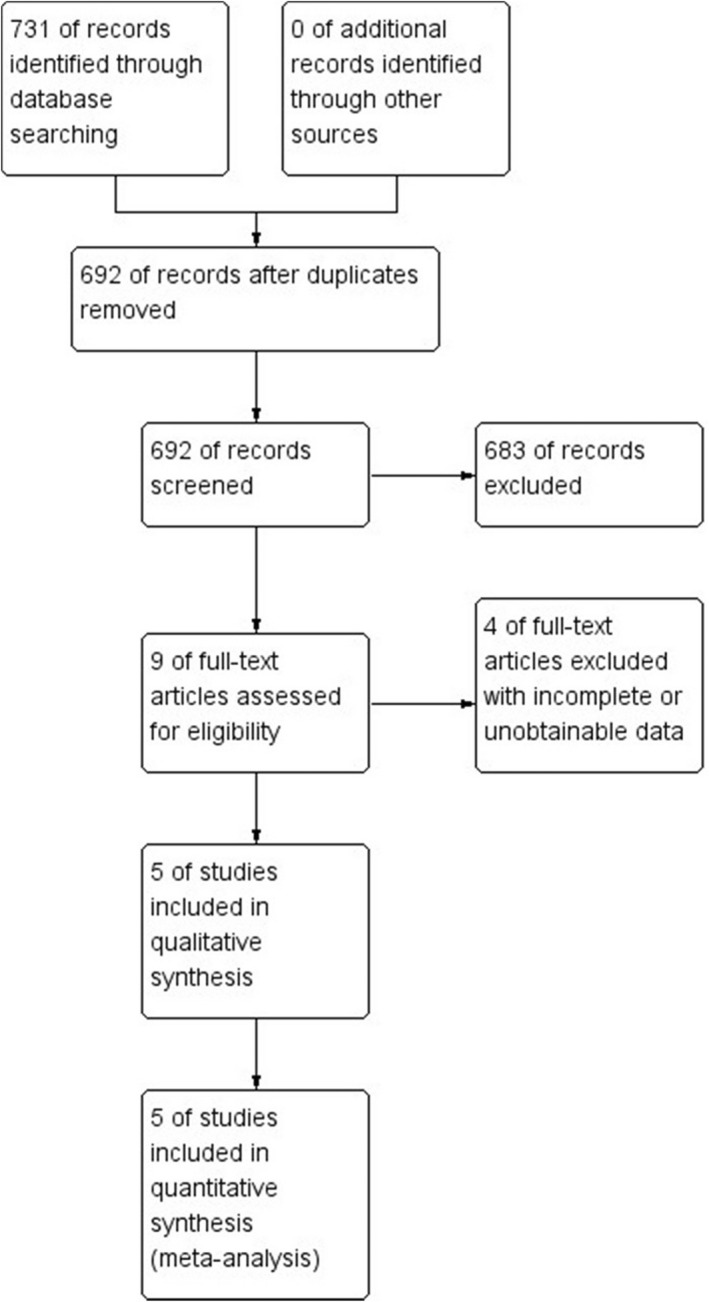
Flow chart presenting the process of the study selection for fingolimod meta‐analysis.
2.3. Inclusion and exclusion criteria for the literature
Studies were included if they fulfilled the following criteria: (1) Published English and Chinese randomized controlled trial in various journals regardless of whether the blind method was used or not; (2) >18 years of age; (3) acute onset of focal neurological deficit consistent with acute ischemic stroke; (4) fingolimod was given 0.5 mg of the drug orally once daily, for three consecutive days plus standardized treatment in the test group, standardized treatment was given in control group (standard treatment adhered to current American Heart Association guidelines including the intravenous administration of tPA, intravascular therapy, antiplatelet drugs, and so on). Exclusion criteria: (1) Case reports and studies that included fewer than two patients, review, meta‐analysis; (2) studies from which no data are provided or data are otherwise not extractable; (3) preexisting neurologic disability (a score greater than 2 on the MRS); (4) for studies published in more than one report, the most comprehensive and up‐to‐date version will be used.
2.4. Main variables
Among the five articles selected, we extracted the values of the proportion of patients whose MRS score was 0–1 at day 90, the mean difference in the change in MRS score at day 90, the change in the National Institutes of Health Stroke Scale (NIHSS) score at 24 h, the change in NIHSS score at day 7, the change in NIHSS score at day 90, relative infarct lesion growth at 24 h, relative infarct lesion growth at day 7, the incidence of complications/adverse events.
2.5. Data abstraction
The titles and abstracts of studies retrieved during the searches were screened for duplicates by two independent reviewers (PB and PW). Potentially relevant full texts were then screened according to our inclusion and exclusion criteria. The final included studies were then collated, and the two reviewers used standardized data extraction formats to extract the data. After extraction, both reviewers matched their data with each other and revisited papers where disagreements arose. Any discrepancies were resolved through discussion with other team members. The extracted data included the following: first author, study design, site of study, year of publication, language, number of patients receiving fingolimod, the values of variables. If required data were missing, not reported in the paper, or reported in an unusual form, the corresponding authors of the respective papers were contacted for clarification. Supplementary material associated with the main paper was also explored in such cases.
2.6. Risk of bias assessment and quality of evidence
Two authors (PB and PW) individually assessed the methodological quality of RCTs using the Cochrane Collaboration tool for assessing the risk of bias. 2324 The criteria were selected a priori and included: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting (including reporting of all outcomes and specifying a primary outcome), and (7) other bias. The evaluated domains were judged as low risk, high risk, or unclear bias per established criteria. In the case of evaluation discrepancies, the authors discussed and came to an agreement. We also assessed risk of bias in the included studies in duplicate (PB and PW), using the PEDro scale for quality. This instrument has been shown to have acceptably high reliability and validity. 25
2.7. Statistical analysis
Data analysis of efficacy was performed using statistical software provided by Revman5.3. Data analysis of safety was performed using statistical software provided by Stata16.0. For continuous variables, mean difference (MD) is adopted as the effective index, and the point estimated value and 95% confidence interval (CI) of each effect quantity are given. For the data of median, maximum, and minimum values mentioned in the included study, combined analysis is carried out after transformation according to the formula. 26 The analysis was carried out for categorical variables using the risk ratio (RR), LogRR, and its 95% CI. The heterogeneity included in the study was analyzed by the X2 test (the test level was axiom 0.1) and evaluated with the I2 index. The random effect model is used for meta‐analysis regardless of the I2 index.
The sensitivity analysis was to remove the individual studies in turn, then to reconduct the meta‐analysis and evaluate the difference between the results after the exclusion and the original combined results. A p‐value of <0.05 was considered statistically significant. 24
2.8. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in https://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY, 27 and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20. 28
3. RESULTS
3.1. Study identification and selection
The database search identified 731 records by searching PubMed, Embase, Cochrane Central Register of Controlled Trials, Clinical trials, CNKI, Wanfang Data, VIP, CBM database dated until August 2021. After removing duplicates, 692 titles were initially screened, and nine theme‐related abstracts were selected for further screening. Four studies were excluded because data were not available. Finally, five studies were included in this systematic review (Figure 1). 1 , 2 , 11 , 18 , 29 four used the values of the proportion of patients whose MRS score was 0–1 at day 90 in total, one used the mean difference in the change in MRS score at day 90, three used the change in NIHSS score at 24 h, two used the change in NIHSS score at day 7, two used the change in NIHSS score at day 90, two used the relative infarct lesion growth at 24 h, two used the relative infarct lesion growth at day 7.
3.2. Study characteristics and quality assessment
Table 1 lists detailed information from the five included studies. The included studies were published between 2014 and 2019. The number of participants per study ranged from 22 to 90, with a total number of 228. Patients who have received fingolimod were recorded in 114 of 228 (50%). All studies were randomized controlled trials.
TABLE 1.
Clinical and demographic characteristics of 228 patients from five studies included in the systematic review
| Reference (study) | Research type | Patient No | Country | Language | Interventions | Outcome measures |
|---|---|---|---|---|---|---|
| Zhang Liantao (2019) | RCTs | 90 | China | English | FTY720 ST | ④⑦ |
| De‐Cai Tian (2018) | RCTs | 46 | China | English | FTY720 ST | ①② |
| Zilong Zhu (2015) | RCTs | 47 | China | English | FTY720 ST | ①②⑤ |
| Ying Fu (2014) | RCTs | 22 | China | English | FTY720 ST | ①③④⑥ |
| De‐Cai Tian (2017) | RCTs | 23 | China | Chinese | FTY720 ST | ①②③⑤⑥ |
RCTs: randomized clinical trials, FTY720:fingolimod, ST: standardized treatment, ① the proportion of patients whose MRS score was 0,1 at day 90, ② the change of NIHSS scores over 24 h, ③ the change of NIHSS scores at day 7, ④ the change of NIHSS scores at day 90, ⑤ relative infarct lesion growth over 24 h, ⑥ relative infarct lesion growth at day 7, ⑦ the mean difference in the change in MRS score at day 90.
Table 2 lists the characteristics of the patients in the included trials from the five included studies. The total number of patients is 228. There were 134 males and 94 females, with an average age of (63.125 ± 10.995) years.
TABLE 2.
The characteristics of the patients in the included trials
| Age of participants | Male percentage | ||||
|---|---|---|---|---|---|
| Reference (study) | Patient no | ||||
| Test group | Control group | Test group | Control group | ||
| Zhang Liantao (2019) 1 | 90 | 63.31 ± 11.65 | 65.46 ± 11.70 | 31/45 | 26/45 |
| De‐Cai Tian (2018) 11 | 46 | 67 ± 6.8 | 65 ± 13 | 9/23 | 7/23 |
| Zilong Zhu (2015) 18 | 47 | 60 ± 12.5 | 59 ± 7.51 | 13/22 | 17/25 |
| Ying Fu (2014) 44 | 22 | 62.3 ± 8.0 | 54.7 ± 11.0 | 8/11 | 9/11 |
| De‐Cai Tian (2017) 29 | 23 | 66.2 ± 8.2 | 63.1 ± 11.1 | 7/13 | 7/10 |
| Total | 228 | 63.55 ± 10.5 | 62.7 ± 11.5 | 68/114 | 66/114 |
Figure 2 shows the risk of bias assessment of the five randomized trials; two trials described adequate methods of random sequence generation; one trial described allocation concealment. In four trials, the participants were blinded. The rate of dropout was low in all trials. None of these studies had incomplete outcome data or selective outcome reporting. All five studies had no other bias.
FIGURE 2.
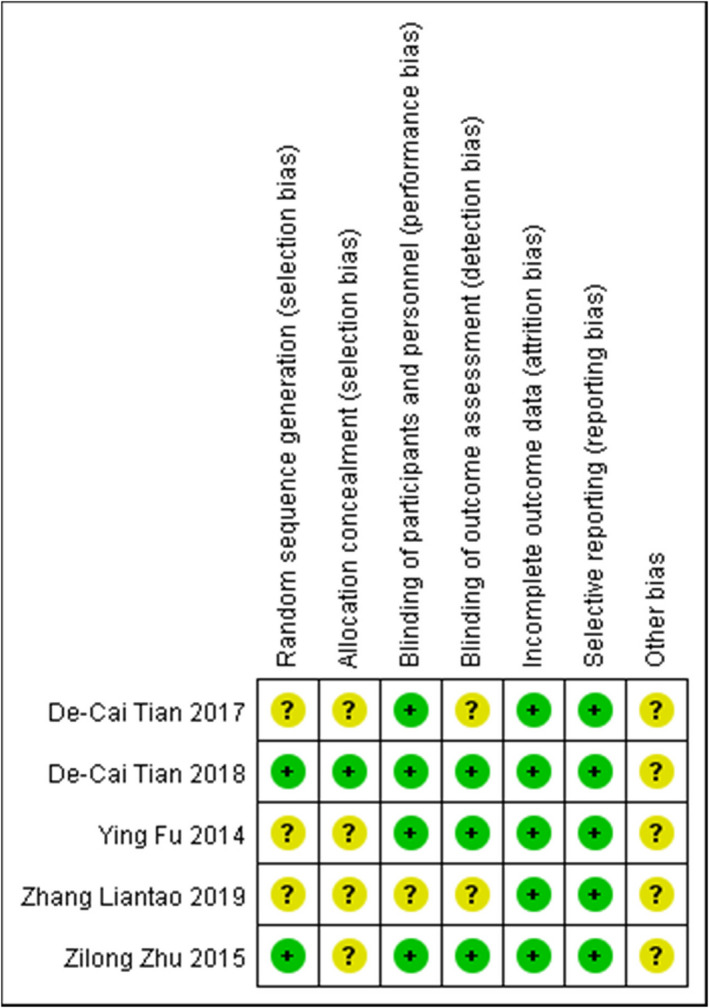
Risk of bias summary for included studies. A “+” stands for low risk, “–” for high risk, and “?” for unclear risk.
Table 3 lists the risk of bias in the included studies in duplicate (PB and PW), using the PEDro(The Physiotherapy Evidence Database)scale for quality.
TABLE 3.
PEDro score of the trials included
| PEDro score | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference (study) | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | Total |
| ZhangLiantao (2019) | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 5 |
| De‐Cai Tian (2018) | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 9 |
| Zilong Zhu (2015) | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Ying Fu (2014) | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| De‐Cai Tian (2017) | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 6 |
PEDro: The Physiotherapy Evidence Database. The PEDro scale criteria are as follows: (1) random allocation, (2) concealed allocation, (3) baseline comparability, (4) blinding of patients, (5) blinding of therapist, (6) blinding of assessor, (7) adequate follow‐up, (8) intention‐to‐treat analysis, (9) between‐group comparison, (10) point estimate and variability. 0, absent; 1, present.
Figure 3, including five articles, shows a forest plot of the risk ratio of patients whose MRS score was 0–1 at day 90, the mean difference in the change in MRS score at day 90 between fingolimod plus standardized treatment, and standardized treatment alone. Figure 3A, including four articles, shows a forest plot of the risk ratio of the proportion of patients whose MRS score was 0–1 at day 90 between fingolimod plus standardized treatment and standardized treatment alone. This finding suggested that the risk ratio of the proportion of patients whose MRS score was 0–1 at day 90 between fingolimod plus standardized treatment and standardized treatment alone was 2.59 (95%CI, 1.48–4.56). A random‐effect model was used. Sensitivity analyses were performed by removing each study in turn and reanalyzed it. No studies were found to significantly affect heterogeneity. Figure 3B, including one article, shows a forest plot of the mean difference in the change in MRS score at day 90. The mean difference in MRS scores change at day 90 of fingolimod plus standardized treatment versus standardized treatment alone was −0.50 (95%CI, −0.93 to −0.07). A random‐effect model was used.
FIGURE 3.
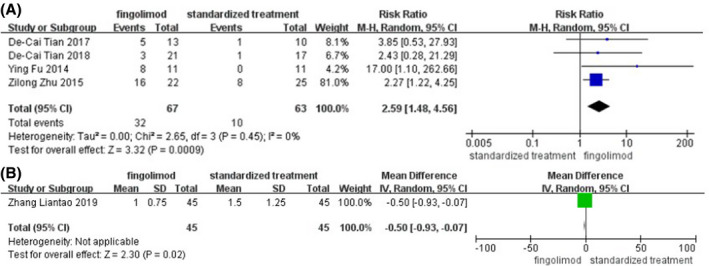
(A) Forest plot of the risk ratio of the proportion of patients whose MRS score was 0–1 at day 90, (B) the mean difference in the change in MRS score at day 90 between fingolimod plus standardized treatment and standardized treatment alone.
Figure 4 shows a forest plot of the mean difference in the change in NIHSS score at 24 h, NIHSS score at day 7, NIHSS score at day 90, relative infarct lesion growth at 24 h, relative infarct lesion growth at day 7 between fingolimod plus standardized treatment and standardized treatment alone. Figure 4A, including three articles, shows a forest plot of the mean difference in the change in NIHSS score at 24 h between fingolimod plus standardized treatment and standardized treatment alone. This finding suggested that the mean difference in NIHSS score change at 24 h of fingolimod plus standardized treatment versus standardized treatment alone was 2.78 (95%CI, 1.46–4.10). A random‐effect model was used. Sensitivity analyses were performed by removing each study in turn and reanalyzed it. The application of sensitivity analysis showed that the study by De‐Cai Tian et al. (2017) significantly affected heterogeneity. Figure 4B, including two articles, shows a forest plot of the mean difference in the change in NIHSS score at day 7 between fingolimod plus standardized treatment and standardized treatment alone. This finding suggested that the mean difference in NIHSS score change at day 7 of fingolimod plus standardized treatment versus standardized treatment alone was 2.59 (95%CI, −0.27 to 7.26). A random‐effect model was used. Figure 4C, including two articles, shows a forest plot of the mean difference in the change in NIHSS score at day 90 between fingolimod plus standardized treatment and standardized treatment alone. This finding suggested that the mean difference in NIHSS score change at day 90 of fingolimod plus standardized treatment versus standardized treatment alone was 3.98(95%CI, 1.15–6.80). A random‐effect model was used. Figure 4D, including two articles, shows a forest plot of the mean difference in the change in relative infarct lesion growth at 24 h between fingolimod plus standardized treatment and standardized treatment alone. This finding suggested that the mean difference in relative infarct lesion growth change at 24 h of fingolimod plus standardized treatment versus standardized treatment alone was −26.46 (95%CI, −43.64 to −9.28). A random‐effect model was used. Figure 4E, including two articles, shows a forest plot of the mean difference in the change in relative infarct lesion growth at day 7 between fingolimod plus standardized treatment and standardized treatment alone. This finding suggested that the mean difference in relative infarct lesion growth change at day 7 of fingolimod plus standardized treatment versus standardized treatment alone was −17.42 (95%CI, −32.67–−2.18). A random‐effect model was used.
FIGURE 4.
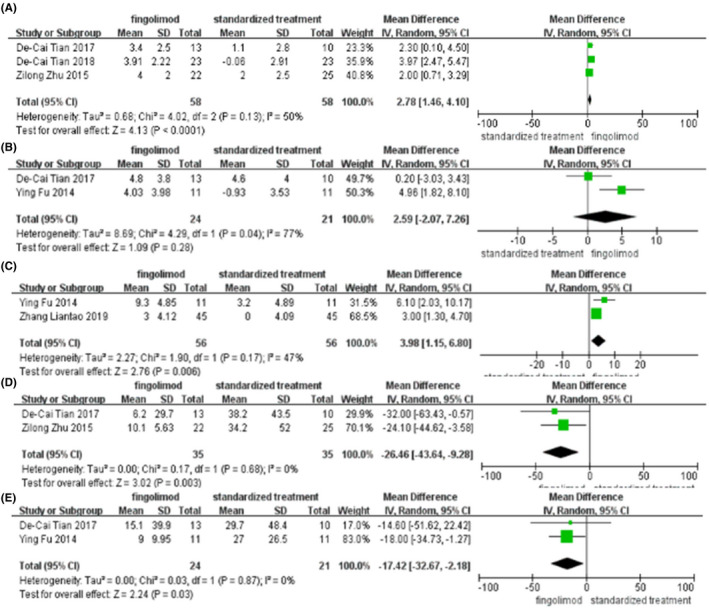
(A) Forest plot of the mean difference in the change in NIHSS score at 24 h, (B) NIHSS score at day 7, (C) CNIHSS score at day 90, (D) relative infarct lesion growth at 24 h, (E) relative infarct lesion growth at day 7 between fingolimod plus standardized treatment and standardized treatment alone.
3.3. Safety outcomes
We combined the data retrieved from the five trials for serious adverse events (SAEs) and adverse events (AEs) such as deaths, myocardial infarctions, recurrent strokes, hernia, hemorrhage of the digestive tract, fever (>38°C), hemorrhagic transformation at 24 h, lung infection, urinary tract infection, herpes virus infection, abnormal laboratory liver function test, gastrointestinal disorders, arrhythmia, and macular edema. The collected data of common AEs are displayed in Table 4. Data analysis was performed using statistical software provided by Stata16.0. We did not find any significant difference between the fingolimod and standardized treatment groups in terms of SAEs and AEs.
TABLE 4.
Safety outcomes in the meta‐analysis
| No. of studies | LogRR | 95%CI | p value | |
|---|---|---|---|---|
| Complications | ||||
| Deaths | 5 | –1.08 | –2.59–0.43 | 0.16 |
| Myocardial infarctions | 5 | 0.28 | –1.37–1.92 | 0.74 |
| Recurrent strokes | 5 | 0.26 | –1.39–1.91 | 0.75 |
| Hernia | 5 | –0.97 | –2.02–0.07 | 0.07 |
| Hemorrhage of the digestive tract | 5 | –0.72 | –2.00–0.56 | 0.27 |
| Hemorrhagic transformation at 24 h | 2 | 0.94 | –0.20–2.08 | 0.11 |
| Fever (>38°C) | 4 | –0.09 | –0.89–0.71 | 0.82 |
| Event | ||||
| All events | ||||
| At least one adverse event | 3 | –0.12 | –0.85–0.61 | 0.75 |
| Any serious adverse event | 4 | –0.06 | –1.99–1.87 | 0.95 |
| Frequent or special interest adverse events | ||||
| Lung infection | 5 | 0.06 | –0.58–0.69 | 0.86 |
| Urinary tract infection | 5 | 0.02 | –0.95–0.99 | 0.97 |
| Abnormal laboratory liver function test | 3 | –0.04 | –2.26–2.18 | 0.97 |
| Gastrointestinal disorders | 3 | –0.04 | –2.26–2.18 | 0.97 |
| Herpes virus infection | 4 | –0.03 | –1.96–1.90 | 0.98 |
| Arrhythmia | 3 | 0.67 | –1.33–2.66 | 0.51 |
| Macular edema | 3 | –0.04 | –2.26–2.18 | 0.97 |
Abbreviations: RR, relative risk; CI, confidence interval.
4. DISCUSSION
This meta‐analysis included five trials to assess the efficacy and safety of fingolimod in patients with AIS. Recently, the effectiveness and safety of fingolimod in patients with AIS have been investigated in some RCTs. 1 , 2 , 11 , 18 , 29 This systematic review and meta‐analysis provide data to support the efficacy and safety of fingolimod for AIS.
4.1. The efficacy of Fingolimod
Our meta‐analysis presented that fingolimod resulted in the decrease of infarct growth and improved clinical function. Our primary endpoint here is based on a proportion of patients with MRS 0–1 at 90 days, decrease in NIHSS score at 24 h, decrease in NIHSS score at day 7, decrease in NIHSS score at 90 days, relative infarct lesion growth at 24 h, and relative infarct lesion growth at 7 days. The sensitivity analysis with analyses of the decrease in NIHSS score at 24 h and at day 7 showed that the study by De‐Cai Tian et al. (2017) significantly affected heterogeneity. It showed no statistically significant differences between fingolimod and standardized treatment in NIHSS score at day 7.
Studies have shown critical linkages between various immunomodulatory mechanisms in ischemic stroke. 18 , 30 Ischemic stroke involves neuronal dysfunction and complex interactions between other cells, including vascular endothelial cells, BBB, extracellular matrix, and immune system. 31 , 32 , 33 Early clinical observations suggest a link between inflammation and ischemic stroke. Inflammation predisposes people to ischemic stroke and directly leads to many pathological changes. 32 , 34 , 35 , 36 , 37 Further understanding of the relationship between immunity and brain tissue in ischemic stroke is helpful to develop new immunomodulatory therapy.
Fingolimod significantly reduced infarct expansion at 24 h. Fingolimod not only inhibits lymphocyte infiltration into the brain parenchyma and protects brain tissue from secondary injury but also, at an earlier stage, by reducing the number of cells accumulating in the brain microvasculature. Inhibit the formation of capillary‐inflammatory thrombosis and protect the function of the CNS. 38 , 39 , 40 , 41 In addition, fingolimod also targets intrinsic cells of the CNS, including vascular endothelial cells. It produces nonimmune effects, thereby protecting brain tissue to some extent. The effect of fingolimod on vascular endothelial cells can inhibit the proinflammatory and thrombotic states of endothelial cells and improve the integrity of BBB. 38 , 42
4.2. The safety of Fingolimod
Our meta‐analysis showed no significant difference in the incidence of complications and adverse events between fingolimod and the standard treatment. Because of the brief fingolimod treatment, this drug does not necessarily produce an immune‐deficient state.
4.3. Strengths and limitations
Our meta‐analysis aimed to present efficacy and safety data on humans. To date, there was a previous meta‐analysis of 17 experimental articles on fingolimod (580 animals), and the main goal was to update the evidence how fingolimod affects experimental stroke. 43 No meta‐analysis has been published about the effects and safety of fingolimod for AIS on humans. Limitations of this study: (1) Although the search strategy is relatively complete, it does not rule out that eligible articles are not included. (2) A large sample of studies lacked in the included studies. (3) The fact that it only includes randomized controlled trials. (4) It is not distinguished patients who receive different standard treatments such as the intravenous administration of tPA, intravascular therapy, antiplatelet drugs, and so on. (5) Four of the included trials came from the same group of investigators. (6) Four of the excluded studies’ data are not extractable. The records with unobtainable data may cause bias in the results. (7) None of the included trials were double‐blinded (most had a PROBE design). (8) High heterogeneity across studies should not be neglected, though a random‐effects model was used for adjustment. Nonetheless, results were broadly similar even if sensitivity analysis which decreased the heterogeneity were performed. Inherent limitations in the majority of meta‐analyses, such as lack of access to raw data and the variety in definitions of outcomes in the included studies, are unavoidable. None of the included studies was adequately sized to evaluate the proposed primary endpoint. (9) The entire data were derived from patients in China. More studies are needed that include other ethnic groups. (10) Different inclusion/exclusion criteria and follow‐up periods in the included studies led to high heterogeneity. (11) The treatment of five included studies did not cover intravascular therapy. (12) Five studies included only phase 1 or early phase 2 trials with very small sample sizes. (13) The quality of studies is poor. (14) The sample sizes for each of the analysis are small. (15) Four five studies are from the same group and all from China. RCTs with greater patient numbers will be needed for future studies. (16) The method for infarct growth measured in the trials was not uniformity.
5. CONCLUSION
The Fingolimod plus standard treatment group decreased infarct growth and improved clinical function than the standard treatment. There was no significant difference in the incidence of complications and adverse events between the standard treatment group and fingolimod plus standard treatment group. Our study shows that these early results are promising; larger studies in different patient populations are needed to validate the studies.
DISCLOSURE
The authors declare no conflict of interest.
6. ETHICS APPROVAL STATEMENT
As this was a systematic review, no ethics approval was sought.
AUTHOR CONTRIBUTIONS
PB, PW, FJ, BL, ML designed the study, searched and screened the literature, extracted and analyzed the data, drafted the manuscript. PB, JZ, YY, NL collated the data, guided manuscript writing. JY, CZ, ZL, MW revised the manuscript.
7. DATA AVAIL ABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Supporting information
Appendix S1
Appendix S2
ACKNOWLEDGMENTS
This work was supported in part by the Doctoral Research start‐up fund of Inner Mongolia Autonomous Region People’s Hospital (Grant No. 2021BS04), the Natural Science Foundation of Inner Mongolia Autonomous Region of China (Grant No. 2020MS03063), the Natural Science Foundation of Inner Mongolia Autonomous Region of China (Grant No.2018MS8009), and the Natural Science Foundation of Inner Mongolia Autonomous Region of China (Grant No.2019MS08077). The authors thank Chenhui Zhao of the First Affiliated Hospital of Wannan Medical College for their excellent technical support. We are thankful to Zihong Liang and Meiling Wang from Inner Mongolia People's Hospital for their assistance in figure composition.
Bai P, Zhu R, Wang P, et al. The efficacy and safety of fingolimod plus standardized treatment versus standardized treatment alone for acute ischemic stroke: A systematic review and meta‐analysis. Pharmacol Res Perspect. 2022;10:e00972. doi: 10.1002/prp2.972
Funding information
This work was also funded by the Doctoral Research startup fund of Inner Mongolia Autonomous Region People's Hospital (Grant No. 2021BS04), the Natural Science Foundation of Inner Mongolia Autonomous Region of China (Grant No. 2020MS03063), the Natural Science Foundation of Inner Mongolia Autonomous Region of China (Grant No.2018MS8009), and the Natural Science Foundation of Inner Mongolia Autonomous Region of China (Grant No.2019MS08077).
REFERENCES
- 1. Liantao Z, Jing Z, Lingling L, Hua L. Efficacy of fingolimod combined with alteplase in acute ischemic stroke and rehabilitation nursing. Pa J Pharm Sci. 2019;32(1(Special)):413–419. [PubMed] [Google Scholar]
- 2. Fu Y, Hao J, Zhang N, et al. Fingolimod for the treatment of intracerebral hemorrhage: a 2‐arm proof‐of‐concept study. JAMA Neurology. 2014;71(9):1092‐1101. [DOI] [PubMed] [Google Scholar]
- 3. Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317‐1329. [DOI] [PubMed] [Google Scholar]
- 4. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695‐1703. [DOI] [PubMed] [Google Scholar]
- 5. Chamorro Á, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nat Rev Neurol. 2012;8(7):401‐410. [DOI] [PubMed] [Google Scholar]
- 6. Ahmad M, Graham SH. Inflammation after stroke: mechanisms and therapeutic approaches. Transl Stroke Res. 2010;1(2):74‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gan Y, Liu Q, Wu W, et al. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc Natl Acad Sci USA. 2014;111(7):2704‐2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Q, Jin W‐N, Liu Y, et al. Brain ischemia suppresses immunity in the periphery and brain via different neurogenic innervations. Immunity. 2017;46(3):474‐487. [DOI] [PubMed] [Google Scholar]
- 9. Shi K, Wood K, Shi FD, Wang X, Liu Q. Stroke‐induced immunosuppression and poststroke infection. Stroke Vasc Neurol. 2018;3(1):34‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fu Y, Liu Q, Anrather J, Shi FD. Immune interventions in stroke. Nat Rev Neurol. 2015;11(9):524‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tian D‐C, Shi K, Zhu Z, et al. Fingolimod enhances the efficacy of delayed alteplase administration in acute ischemic stroke by promoting anterograde reperfusion and retrograde collateral flow. Ann Neurol. 2018;84(5):717‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Langhauser F, Göb E, Kraft P, et al. Kininogen deficiency protects from ischemic neurodegeneration in mice by reducing thrombosis, blood‐brain barrier damage, and inflammation. Blood. 2012;120(19):4082‐4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kleinschnitz C, Kraft P, Dreykluft A, et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121(4):679‐691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kraft P, Göb E, Schuhmann MK, et al. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo‐inflammation but not by direct neuroprotection. Stroke. 2013;44(11):3202‐3210. [DOI] [PubMed] [Google Scholar]
- 15. Zhang L, Zhang ZG, Chopp M. The neurovascular unit and combination treatment strategies for stroke. Trends Pharmacol Sci. 2012;33(8):415‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fisher M, Saver JL. Future directions of acute ischaemic stroke therapy. Lancet Neurol. 2015;14(7):758‐767. [DOI] [PubMed] [Google Scholar]
- 17. Cohen JA, Chun J. Mechanisms of fingolimod's efficacy and adverse effects in multiple sclerosis. Ann Neurol. 2011;69(5):759‐777. [DOI] [PubMed] [Google Scholar]
- 18. Zhu Z, Fu Y, Tian D, et al. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation. 2015;132(12):1104‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Massberg S, von Andrian UH. Fingolimod and sphingosine‐1‐phosphate–modifiers of lymphocyte migration. N Engl J Med. 2006;355(11):1088‐1091. [DOI] [PubMed] [Google Scholar]
- 20. Sanchez T, Estrada‐Hernandez T, Paik J‐H, et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor‐induced vascular permeability. J Biol Chem. 2003;278(47):47281‐47290. [DOI] [PubMed] [Google Scholar]
- 21. Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. Targeting blood‐brain barrier sphingolipid signaling reduces basal P‐glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci USA. 2012;109(39):15930‐15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hait NC, Wise LE, Allegood JC, et al. Active, phosphorylated fingolimod inhibits histone deacetylases and facilitates fear extinction memory. Nat Neurosci. 2014;17(7):971‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tugwell P, Tovey D. Prisma 2020. J Clini Epidemiol. 2021;134:A5‐A6. 10.1016/j.jclinepi.2021.04.008 [DOI] [PubMed] [Google Scholar]
- 25. Gibson W, Wand BM, O'Connell NE. Transcutaneous electrical nerve stimulation (TENS) for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;9(9):Cd011976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019; 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018;46(D1):D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alexander SPH, Kelly E, Mathie A, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: introduction and other protein targets. Br J Pharmacol. 2019;176(Suppl 1):S1–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. 田德财 . 免疫调节剂芬戈莫德联合 tPA治疗发病 4.5‐6h 的急性缺血性脑卒中的探索研究 2017.
- 30. Yang Y, Rosenberg GA. Blood‐brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42(11):3323‐3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ajmo CT, Collier LA, Leonardo CC, et al. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol. 2009;218(1):47‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J Clin Investig. 2010;120(5):1368‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10(12):826‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bråtane BT, Cui H, Cook DJ, Bouley J, Tymianski M, Fisher M. Neuroprotection by freezing ischemic penumbra evolution without cerebral blood flow augmentation with a postsynaptic density‐95 protein inhibitor. Stroke. 2011;42(11):3265‐3270. [DOI] [PubMed] [Google Scholar]
- 35. Russo MV, McGavern DB. Immune surveillance of the CNS following infection and injury. Trends Immunol. 2015;36(10):637‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abbott NJ, Rönnbäck L, Hansson E. Astrocyte‐endothelial interactions at the blood‐brain barrier. Nat Rev Neurosci. 2006;7(1):41‐53. [DOI] [PubMed] [Google Scholar]
- 37. Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. NeuroMol Med. 2010;12(2):193‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW. Pattern recognition receptors and central nervous system repair. Exp Neurol. 2014;258:5‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wolf SA, Boddeke HW, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol. 2017;79:619‐643. [DOI] [PubMed] [Google Scholar]
- 40. Butovsky O, Jedrychowski MP, Moore CS, et al. Identification of a unique TGF‐β‐dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17(1):131‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Block ML, Zecca L, Hong JS. Microglia‐mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57‐69. [DOI] [PubMed] [Google Scholar]
- 42. Thornton P, McColl BW, Greenhalgh A, Denes A, Allan SM, Rothwell NJ. Platelet interleukin‐1alpha drives cerebrovascular inflammation. Blood. 2010;115(17):3632‐3639. [DOI] [PubMed] [Google Scholar]
- 43. Dang C, Lu Y, Li Q, Wang C, Ma X. Efficacy of the sphingosine‐1‐phosphate receptor agonist fingolimod in animal models of stroke: an updated meta‐analysis. Intern J Neurosci. 2021;131(1):85‐94. [DOI] [PubMed] [Google Scholar]
- 44. Fu Y, Zhang N, Ren LI, et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci USA. 2014;111(51):18315‐18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2


