Abstract
Circadian rhythm disruption is associated with immune system disturbance and has been observed in many health problems where chronic-inflammation acts as a major contributor. We aim to examine whether rest-activity circadian rhythm is associated with chronic inflammation using white blood-cell-based inflammatory indices including white blood cell (WBC) count, neutrophil count, neutrophil-to-lymphocyte ratio (NLR) and systemic immune-inflammation index (SII). We analyzed the data from 8089 adults (age≥20) with at least 4 days of validated accelerometer recordings and a valid WBC count from the National Health and Nutrition Examination Survey (NHANES) 2011–2014. Non parametric rest-activity circadian rhythm parameters were derived from the accelerometer recordings. In the models adjusting multiple covariates, a one-quantile increase in relative amplitude (i.e. more robust circadian rhythm) was associated with 1×108 cells/L decrease in WBC number (95% CI: 5×107 to 1.5×108, P<0.001), 7×107 cells/L decrease in neutrophils (95% CI: 3×107 to 1.1×108, P=0.003) and 15.2×109 /L decrease in SII (95% CI: 6×109 /L to 20×109/L, P=0.019). Consistent results were also observed for the association of M10 value and L5 value with these inflammatory indices. Our results indicated that blunted rest-activity circadian rhythm is associated with increased white blood-cell-based inflammatory indices in adults, suggesting interventions aiming at enhancing circadian rhythm by lifestyle programs may be a novel approach to improve the general health.
Keywords: Rest-activity rhythm, circadian rhythm, WBC count, inflammation
Introduction
In mammals, the internal circadian timing system is entrained to the 24-h light-dark cycle through light signals reaching a master clock located within the hypothalamus. With the relatively recent widespread availability of electrical lighting, humans have the ability to extend wakefulness activities far into the night. This capability to alter the timing of wakefulness can result in a misalignment between behavior and endogenous circadian physiology. Circadian misalignment or disruption (1) is especially pronounced in night-shift workers and is thought to be responsible for their well-documented health problems including hypertension, sleep disturbance, diabetes and cardiovascular disease (2). Circadian disruption can also occur in a “real world” setting, mainly caused by individuals’ choices or constraints on daily schedules of sleep-wake cycle and activities (such as physical activity, food intake, etc.) (3).
The mammalian molecular circadian clock machinery is present in virtually all cell types and can directly regulate the trafficking of immune cells (4). Previous studies (5–7) have found that dramatic circadian misalignment plays critical roles in inflammatory process under laboratory settings, indicating the interface between circadian rhythm and immune homeostasis may have a wide range of implications for public health and individual risk of diseases. However, whether long-term mild disruption of circadian rhythm in the daily real-world setting (i.e. a combination of the interaction between circadian timing and behavior factors) affects our inflammatory status is less studied.
Rest-activity rhythm is one of the most prominent outputs of the circadian system and is now commonly used to assess circadian function in both animal and humans (8). Several measures calculated from rest-activity cycles are thought to reflect the strength and timing of the circadian systems in real life and these measurements have been associated with morbidity and mortality and changes in response to interventions (9–11). Few studies have tested whether participants with impaired rest-activity rhythm have increased chronic inflammation. Only two recent studies with one in elder men (≥65 years) (12) and one in children (13) reported that rest-activity rhythm disruption was associated with high levels of C-reactive protein (CRP) and other inflammatory markers. In addition to CRP which has been used as a biomarker of chronic inflammation, white blood-cell-based markers such as elevated white blood cell (WBC) count (14–17) and certain WBC subtypes especially neutrophil count (18,19) have also been extensively studied as chronic inflammatory indices and linked with incident CVD, cancer and mortality. More recently, several new white blood-cell-based composite indices such as neutrophil-to-lymphocyte ratio (NLR) (20,21) and systemic immune-inflammation index (SII, calculated based on the neutrophil, lymphocyte and platelet counts) (22,23) have also been identified as markers predicting CVD incidence and mortality in the general population. In this study, we aim to test whether impaired rest-activity rhythm is associated with these white blood-cell based inflammatory markers that are established predictors of mortality in the general population in a nationally representative sample of US adults (≥20 years).
Methods
Study Population
The National Health and Nutrition Examination Survey (NHANES) is a research program designed to assess the health and nutritional status of individuals in the United States. Wrist accelerometers data were available in the 2011–2014 NHANES study cycle. A multistage probability sampling design was used in NHANES study to produce a weighted, representative sample of the US population (24). The National Center for Health Statistics Research Ethics Review Board approved all NHANES protocols, and all participants gave informed consent. Our sample included adults aged ≥20 years old that had at least 4 days of validated accelerometer recordings from NHANES 2011–2014 cycles [dataset (25)]. Participants who were pregnant at screening were excluded. Of the 8355 validated participants, we excluded 262 with missing WBC data and 4 with extreme WBC values (>30.0 × 109). Figure 1 illustrates the flow of participants selected for inclusion in this analysis (n = 8089).
Figure 1.
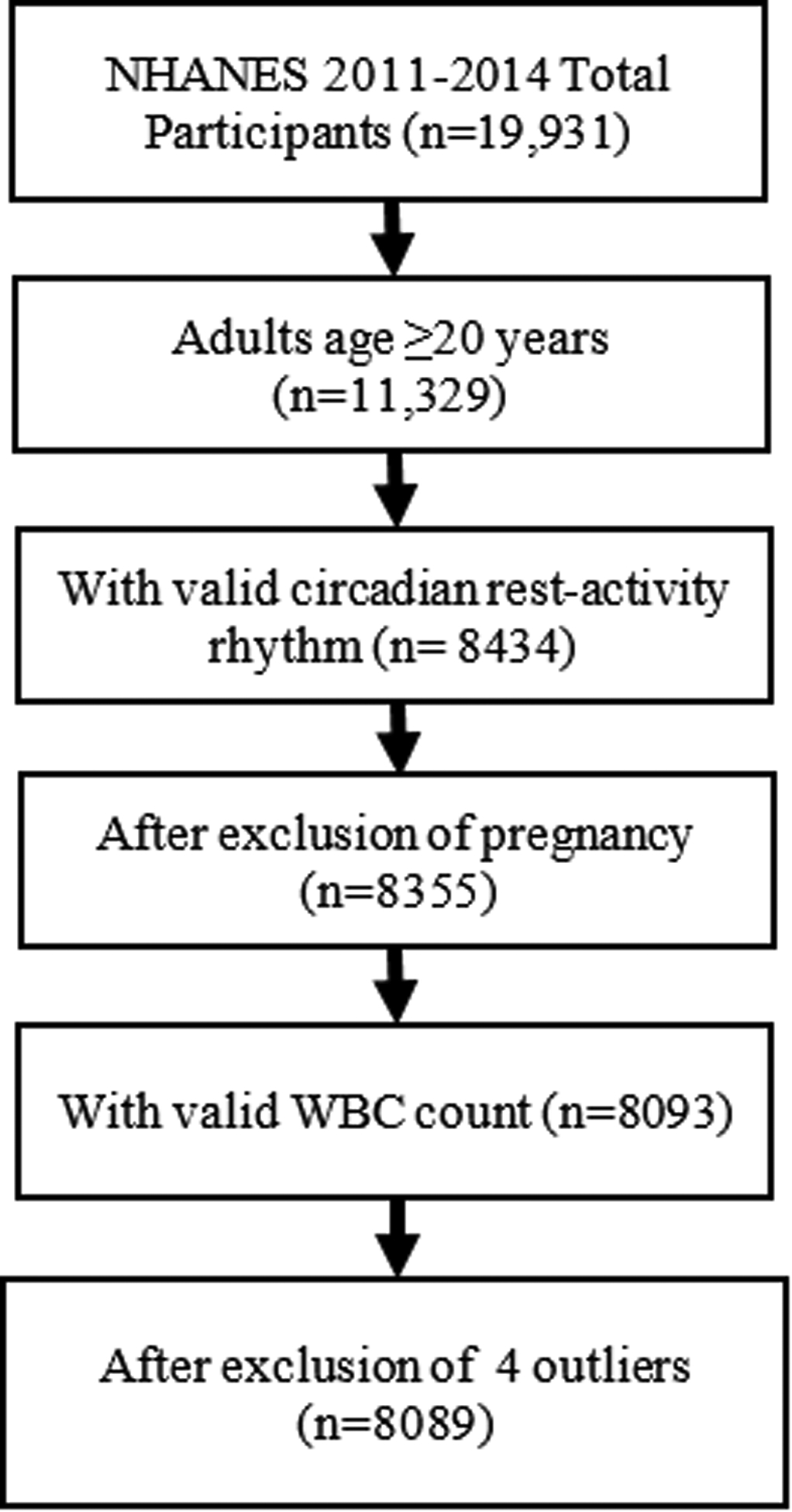
Flowchart for inclusion of study participants
Measurement of WBC Count and Its Subtypes
According to the protocol of NHANES 2011–2014 cycle, the Beckman Coulter method of counting and sizing, in combination with an automatic diluting and mixing device for sample processing, and a single beam photometer for hemoglobinometry was used for count of blood cells from peripheral blood sample obtained during the day of the participant’s exam in the NHANES Mobile Examination Center (MEC). In this study, our primary white blood-cell-based inflammatory markers are WBC count, neutrophil count, NLR and SII. The SII level was calculated from the numbers of platelet, neutrophil and lymphocyte using the following formula: SII=platelet × neutrophil/lymphocyte count and expressed as ×109/L.
Accelerometer Recording and Data Preprocessing
All participants aged 6 years and older during the 2011–2012 cycle and all participants aged 3 years and older during the 2013–2014 cycle were asked to wear an accelerometer (ActiGraph Model GT3X+, ActiGraph of Pensacola, FL) on the day of their exam in the NHANES MEC and to keep wearing it all day and night for 7 consecutive days. We focused on participants aged ≥20 years with valid accelerometer recordings defined below. The device was worn on the non-dominant wrist, if possible. Raw signals obtained on the x-, y-, or z-axes every 1/80 of a second (80 Hz) were processed, flagged and summarized at the minute level and released by NHANES in November 2020. These summary measures in the minute summary file (PAXMIN) are specified in Monitor-Independent Movement Summary (MIMS) units, which is a non-proprietary, open-source, device-independent universal summary metric developed by researchers at Northeastern University. MIMS triaxial value (variable name: PAXMTSM) at the every minute level was used to calculate rest-activity rhythms. MIMS triaxial values were changed to missing (i.e. a value of 0) if they met any of the following conditions: (1) PAXMTSM is coded as “−0.01”; (2) estimated wake/sleep/wear status during the minute (variable name PAXPREDM) is coded as “Non wear”; or (3) minute data quality flag count (variable name PAXQFM) is larger than “0”. R package “accelmissing” was used to impute the missing count values in the accelerometer data with the following pre-processing steps: (1) the minimum minutes of missing interval were defined as 60 minutes; (2) the valid days were defined as more than 16 hours of wearing; and (3) the minimum number of valid days that the subject should have was defined as 4 days.
Rest-Activity Rhythm Parameters
R package “nparACT” was used to compute the following nonparametric variables of rest-activity rhythms, which have been extensively described before(26,27): (1) Interdaily stability (IS), which estimates how closely the 24-hour rest–activity pattern follows the 24-hour light–dark cycle (IS ≃ 0 for Gaussian noise, IS ≃ 1 for perfect stability); (2) Intradaily variability (IV), which quantifies the fragmentation of the 24-hour rhythm (IV ≃ 0 for a perfect sine wave, IV ≃ 2 for Gaussian noise); (3) The relative amplitude (RA), which is the relative difference between M10 (the average activity of the ten consecutive hours with the highest activity) and L5 (the average activity of the five consecutive hours with the lowest activity) in an average 24h from midnight to midnight (RA=(M10−L5)/(M10+L5)). It is a nonparametric measure of the amplitude of rest-activity rhythm with higher RAs indicating more robust 24-hour rest–activity oscillations, reflecting both higher activity when awake and relatively lower activity during the night; (4) Onset time of the M10 (M10 start time), which indicates the starting time of the peak activity; and (5) Onset time of the L5 (L5 start time), which provides an indication of the starting time of nadir activity.
Covariates
Self-reported information about demographic factors regarding age, sex, race (i.e., Non-Hispanic (NH) White, NH Black, Mexican American and other race— including other Hispanic, Asian and other race), smoking status, alcohol drinking, and family income-to-poverty ratio were collected. Current smokers were defined when individuals reported a consumption of ≥100 cigarettes during their lifetime and were still currently smoking. Participants were categorized into ideal, intermediate, or poor leisure-time physical activity levels based on whether they met the AHA recommendations (28) for weekly activity: ideal, 75 minutes or more of vigorous activity or 150 minutes or more of moderate activity or 150 minutes or more of combined moderate and vigorous physical activity; intermediate, more than 0 minutes of physical activity but less than recommendations; and poor, 0 minutes of physical activity. Self-reported presence of chronic disorders including history of CVD (i.e. congestive heart failure, coronary heart disease, angina pectoris and heart attack), hypertension, stroke, diabetes and cancer were also included as study covariates.
Statistical Analysis
STATA (v16) was used to perform survey data analysis to account for complex survey design and produce representative estimates of the US population. Four-year survey weights were calculated and used in all analyses to adjust for unequal selection probability and non-response bias in accordance with NHANES analytical guidelines. Descriptive statistics were presented as population means, and standard deviations for continuous variables and weighted proportions for categorical variables. Survey weighted multiple linear regressions were used to assess the association of rest-activity rhythm parameters with WBC count, neutrophil count, NLR, and SII. Age, sex and race were included as covariates unless otherwise indicated. Because a one-unit change in RA, IS or IV would reflect the difference between the extreme lower and upper ends of the range, they were divided into quartiles for the regression models. Log transformed outcomes were used in the regression models to make them normally distributed when reporting the P values. We tested 3 models with increased number of covariates. Base model (model 1) included age, sex, and race as covariates. Model 2 further adjusted ratio of family income to poverty level, smoking status and physical activity. Model 3 further included BMI and history of CVD, cancer, stroke, diabetes and hypertension as covariates. Statistical significance was set at P < 0.05. To ensure that a possible effect of collinearity was not present between the independent variables in the models, the variance inflation factor was calculated in the model. A value greater than 10 indicates such a problem is present. In all the models that we tested, the variance inflation factors were less than 1.5. The interaction between sex and rest-activity rhythm parameters was also tested to examine whether the associations of white blood-cell-based inflammatory markers with rest-activity circadian rhythm parameters were modified by sex. Sensitivity analyses were conducted on the sub-group consisting of those without a recent illness in the past 30 days: a head or chest cold, stomach or intestinal illness with vomiting, diarrhea, flu, pneumonia or ear infection.
Results
Our analytical sample included 8089 participants aged ≥ 20 years (mean ±SD: 50.3 ± 17.4), representing 171 million noninstitutionalized residents of the United States. Demographic and general health factors as well as rest-activity rhythm parameters are presented in Table 1.
Table 1.
Descriptive characteristics of the study sample (n =8089)
| Variables | Statistics |
|---|---|
| Age, years, mean (SD) | 49.0 (16.8) |
| Female, N (%) | 4159 (52.0) |
| Race, N (%) | |
| NH White | 3347 (67.6) |
| NH Black | 1863 (10.9) |
| Mexican American | 964 (8.4) |
| Other | 1915 (13.1) |
| Current smoker, N (%) | 1572 (19.1) |
| Recent illness, N (%) | 1615 (19.5) |
| CVD, N (%) | 699 (7.5) |
| Cancer, N (%) | 767 (10.9) |
| Stroke, N (%) | 329 (3.2) |
| Diabetes, N (%) | 1107 (10.5) |
| Hypertension, N (%) | 3130 (35.1) |
| Physical activity, N (%) | |
| Poor | 4120 (46.6) |
| Intermediate | 1329 (17.4) |
| Ideal | 2640 (36.0) |
| BMI (kg/m2), mean (SD) | 29.2 (6.9) |
| Reference, N (%) | 2422 (29.3) |
| Overweight, N (%) | 2572 (33.1) |
| Obese, N (%) | 3019 (37.6) |
| Ratio of family income to poverty, mean (SD) | 2.9 (1.7) |
| WBC count (109 cells/L), mean (SD) | 7.1 (2.1) |
| Neutrophils (109 cells/L), mean (SD) | 4.3 (1.7) |
| Lymphocytes (109 cells/L), mean (SD) | 2.1 (0.7) |
| Platelets (109 /L), mean (SD) | 235.3 (58.7) |
| NLR, mean (SD) | 2.3 (1.2) |
| SII (109 /L), mean (SD) | 533.1 (325.4) |
| Rest-activity parameters, mean (SD) | |
| RA | 0.85 (0.12) |
| RA-Q1 | 0.66 (0.13) |
| RA-Q2 | 0.84 (0.02) |
| RA-Q3 | 0.90 (0.01) |
| RA-Q4 | 0.94 (0.01) |
| IS | 0.59 (0.14) |
| IV | 0.71 (0.23) |
| M10 | 14.4 (4.2) |
| L5 | 1.2 (1.1) |
% and means (SD) were weight adjusted. NH: Non-Hispanic; BMI, body mass index; RA, relative amplitude; IS, interdaily stability; IV, intradaily variability; M10, the average activity level of the most active continuous 10-h period; L5, the average activity level of the least active continuous 5-h period; NLR, neutrophil-lymphocyte ratio; SII, systemic immune-inflammation index
The associations of rest activity circadian rhythm parameters with WBC count, neutrophil count, NLR and SII were presented in Table 2. Across three models, lower relative amplitude was significantly associated with higher levels of WBC and neutrophil count as well as higher levels of SII. In the fully adjusted regression model (i.e. model 3), a one-quantile increase in relative amplitude was associated with 1×108 cells/L decrease in WBC number (95% CI: 5×107 to 1.5×108, P<0.001), 7×107 cells/L decrease in neutrophils (95% CI: 3×107 to 1.10, P=0.003) and 15.2×109/L (95% CI: 6×109 /L to 20×109/L, P=0.019) decrease in SII. A negative relationship between NLR and relative amplitude was observed in model 1 and 2, however, the significance disappeared in model 3. Increased intradaily variability (i.e. increased rhythm fragmentation) was associated with increased numbers of WBC, neutrophils, NLR and SII in model 2. Decreased stability of the rhythm (IS) was associated with increased numbers of WBC and neutrophils in model 1. In consistent with the findings for relative amplitude, lower levels of M10 in all 3 models and higher levels of L5 in model 1 and model 2 (with the exception of NLR) were also significantly associated with higher levels of inflammatory indices (Table 2). We did not observe any significant associations of the M10 start time or L5 start time with these selected white blood-cell-based inflammatory markers (Table S1). The associations with other WBC subtypes (i.e. lymphocyte count and monocyte count) and other white blood-cell-based composite indices (i.e. ratio of neutrophil to WBC and ratio of monocyte to WBC) were listed in supplementary Table 2. There was no significant interactions between sex and rest activity circadian rhythm parameters on WBC count, neutrophil count, NLR and SII, indicating the findings were consistent across sex. These results were not impacted by exclusion of participants with recent illnesses (data not shown).
Table 2.
Associations of rest-activity parameters with white blood-cell-based inflammatory markers
| Dependent variables | Rest-activity rhythm parameters | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| Beta (95% CI) | P | Beta (95% CI) | P | Beta (95% CI) | P | ||
| WBC* | RA | −0.26 (−0.31 −0.21) | <0.001 | −0.16 (−0.22, −0.11) | <0.001 | −0.10 (−0.15, −0.05) | <0.001 |
| IV | −0.01 (−0.06, 0.04) | 0.920 | 0.05 (−0.0004, 0.10) | 0.038 | 0.03 (−0.03, 0.08) | 0.260 | |
| IS | −0.07 (−0.12, −0.02) | 0.012 | −0.04 (−0.09, 0.01) | 0.114 | −0.01 (−0.06, 0.03) | 0.570 | |
| M10 | −0.06 (−0.07, −0.04) | <0.001 | −0.05 (−0.06, −0.04) | <0.001 | −0.03 (−0.04, −0.02) | <0.001 | |
| L5 | 0.19 (0.13, 0.25) | <0.001 | 0.1 (0.04, 0.15) | 0.001 | 0.07 (0.02, 0.12) | 0.008 | |
| Neutrophils † | RA | −0.19 (−0.23, −0.15) | <0.001 | −0.12 (−0.16, −0.07) | <0.001 | −0.07 (−0.11, −0.03) | 0.003 |
| IV | 0.01 (−0.03, 0.04) | 0.366 | 0.04 (0.004, 0.08) | 0.013 | 0.02 (−0.02, 0.06) | 0.115 | |
| IS | −0.05 (−0.08, −0.01) | 0.007 | −0.02 (−0.06, 0.01) | 0.108 | −0.003 (−0.04, 0.03) | 0.642 | |
| M10 | −0.05 (−0.06, −0.03) | <0.001 | −0.04 (−0.05, −0.03) | <0.001 | −0.02 (−0.04, −0.01) | <0.001 | |
| L5 | 0.13 (0.08, 0.18) | <0.001 | 0.07 (0.02, 0.11) | 0.006 | 0.05 (−0.002, 0.09) | 0.054 | |
| NLR‡ | RA | −0.08 (−0.11, −0.05) | <0.001 | −0.07 (−0.1, −0.04) | 0.002 | −0.05 (−0.08, −0.01) | 0.064 |
| IV | 0.04 (−0.002, 0.07) | 0.057 | 0.04 (0.009, 0.08) | 0.031 | 0.03 (−0.007, 0.06) | 0.252 | |
| IS | −0.02 (−0.05, 0.01) | 0.250 | −0.01 (−0.04, 0.02) | 0.551 | 0.0004 (−0.03, 0.03) | 0.716 | |
| M10 | −0.02 (−0.03, −0.01) | <0.001 | −0.02 (−0.03, −0.01) | <0.001 | −0.01 (−0.02, −0.004) | 0.009 | |
| L5 | 0.05 (0.02, 0.07) | 0.014 | 0.03 (0.005, 0.06) | 0.090 | 0.03 (−0.002, 0.06) | 0.243 | |
| SII‡ | RA | −25.0 (−33.4, −16.5) | <0.001 | −20.2 (−29.0, −11.5) | 0.001 | −15.2 (−24.4, −6.0) | 0.019 |
| IV | 6.7 (−1.6, 14.9) | 0.104 | 9.3 (1.1, 17.2) | 0.035 | 6.0 (−2.4, 14.0) | 0.199 | |
| IS | −4.5 (−12.7, 3.8) | 0.383 | −2.8 (−12.3, 6.6) | 0.650 | 0.22 (−9.4, 9.5) | 0.756 | |
| M10 | −6.0 (−7.9, −4.0) | <0.001 | −4.9 (−6.9, −3.0) | <0.001 | −3.2 (−5.1, −1.4) | 0.023 | |
| L5 | 13.8 (6.4, 21.2) | 0.005 | 9.2 (1.9, 16.4) | 0.038 | 7.4 (0.05, 14.8) | 0.124 | |
P values were calculated using log-transformed WBC count as outcome; N=8089 for model 1, N=7486 for model 2, N=7413 for model 3
P values were calculated using log-transformed neutrophil count as outcome; N=8074 for model 1, N=7471 for model 2, N=7400 for model 3
P values were calculated using log-transformed values as outcome; N=8074 for model 1, N=7471 for model 2, N=7394 for model 3
Model 1: Adjusted for age, sex, race
Model 2: Adjusted for age, sex, race, ratio of family income to poverty level, physical activity, and smoking
Model 3: Adjusted for age, sex, race, ratio of family income to poverty level, physical activity, smoking, BMI, CVD, cancer, stroke, diabetes and hypertension
Discussion
In the present study of a nationally representative adult sample, we observed that lower relative amplitude of the rest-activity rhythm (i.e. decreased robustness of circadian rhythm) was associated with increased numbers of white blood cells, increased numbers of neutrophils, as well as increased levels of SII, indicating that mild disruption of circadian rhythm in the real-world setting is associated with increased inflammatory states. The findings for M10 value and L5 value, the two determinants of relative amplitude, were in consistent with the findings of RA with lower M10 value and higher L5 value associated with worse inflammatory indices. These associations were independent of sociodemographic factors and the significance persisted after the exclusion of individuals with acute inflammatory disorders in the past month. Given the fact that chronic, low-grade activation of pro-inflammatory process occurs within many chronic diseases and that blunted rest-activity rhythm was associated with an increased risk of CVD events (11), cancer (29) and diabetes (30), in an effort to avoid the spurious associations caused by adjustment for multiple confounders, we further excluded the participants with inflammatory chronic diseases including CVD, cancer, and diabetes. The finding stays the same, this is, impaired robustness of rest-activity circadian rhythm was associated with increased inflammatory states with increased numbers of WBC as well as SII. We did not observe significant results in the associations of the timing of rest-activity cycles (i.e. M10 or L5 starting time) with the number of WBC, neutrophil or SII across different models, indicating that our body may have higher levels of adaptive capacity or resilience in the phase shift of the rhythm caused by individuals’ choices or constraints on daily schedules.
It has been well established that the circadian rhythm regulates the immune system and previous studies have linked the disruption of circadian rhythm with increased inflammation (31). Hitoshi Inokawa et al. (32) showed that constant circadian disruption induced by chronic jet-lag paradigms in wild mice caused significant early mortality, and was associated with immune senescence and consequent chronic inflammation, suggesting an important regulatory role of the circadian system. Research from Khoa et al. found that myeloid cell-specific deletion of clock gene Bmal1 in mice induces exaggerated inflammation responses to infection or high-fat diet, directly prove the role of circadian rhythm in immune responses (33). Recently, similar results has been found in human studies. Xiao et al (12) observed significant relationship between weaker rest-activity rhythms (indexed by reduced relative amplitude) and higher levels of inflammatory markers such as CRP, IL-6, and TNF- α in fasting blood in Caucasian men aged ≥65 years. Similarly, in another small study in children (N=121), Qian et al. (13) found that CRP levels were inversely associated with relative amplitudes in which 1 SD increase of relative amplitude was linked to 32.6% decrease in CRP levels in saliva samples. The present study not only confirms the previous findings in a nationally representative samples using white blood-cell-based markers as biomarkers of systemic inflammation, but also extends the findings to both males and females, multiple race groups and a broad age range. We also observed that a lower M10 value, an index of lower daily activity, and a higher L5 value, an index of higher activity during night (or sleep period) were associated with worse inflammatory markers. This is in consistent with previous epidemiology studies which have observed that physical inactivity and disturbed sleep are associated with increased inflammatory markers (34–36).
Our study has some limitations that should be considered during interpretation. The current study cannot explain the temporal relationships or causal inferences between factors, although our goal in this study was to explore the associations of mild disruption of circadian rhythm in the real-world setting with inflammation rather than to infer causality. However, longitudinal studies with accelerometer data and inflammation markers obtained on multiple visits will provide more information in explaining the nature and direction of causality. Second, shift work status/recent travel over time zones was not obtained from participants of NHANSE 2011–2014 cycle, therefore, a sensitive test by excluding these participants cannot be performed. Furthermore, it has been long recognized that blood count of leukocytes vary in a circadian manner with the amplitude for leukocyte count of 0.78×109/L, and for neutrophils of 0.04 × 109/L (37). While detection of the leukocytes at controlled time points of the day in the future study are warranted and will provide more solid evidence on the association between circadian rhythm and chronic inflammation, sample collections in the NHANES MEC were conducted in a random order, therefore, the oscillation nature of the leucocytes is likely to increase the measure error and weaken the associations we found here, not exaggerate them. In addition, other inflammation markers, such as CRP level, were not available in NHANES 2011–2014 cycles to further confirm the association of activity rhythm disruption with chronic inflammation. Although we do not anticipate the results would be different from those of white blood-cell-based inflammatory markers, assessing the relationship between rest-activity rhythm and different components of the immune system in a comprehensive way is warranted. Also, we cannot examine whether the association of rest-activity rhythm with inflammation status is different in different race groups because of lack of sufficient data.
In conclusion, our study found that mild disruption of circadian rhythm indexed by the weakened rest-activity rhythm was associated with indicators of low-grade inflammation in adults from a national representative sample. Future studies could focus on understanding of the nature of the circadian biology of inflammatory conditions, testing interventions targeting improving rest-activity rhythm through the modifications of lifestyle and behavioral factors to improve the general health and incorporating characteristics of rest-activity rhythm into algorithms to better predict inflammation-related chronic disease risks.
Supplementary Material
Acknowledgments
X. W. receives funding from NIH DK117365 and AHA SFRN863620 and S. S. receives funds from NIH HL143440 and MD013307.
References
- 1.Vetter C Circadian disruption: What do we actually mean? Eur J Neurosci 2020;51:531–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith MR, Eastman CI. Shift work: health, performance and safety problems, traditional countermeasures, and innovative management strategies to reduce circadian misalignment. Nat Sci Sleep 2012;4:111–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry 2014;26:139–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Druzd D, de Juan A, Scheiermann C. Circadian rhythms in leukocyte trafficking. Semin Immunopathol 2014;36:149–162 [DOI] [PubMed] [Google Scholar]
- 5.Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci 2010;1193:48–59 [DOI] [PubMed] [Google Scholar]
- 6.Boudjeltia KZ, Faraut B, Stenuit P, Esposito MJ, Dyzma M, Brohee D, Ducobu J, Vanhaeverbeek M, Kerkhofs M. Sleep restriction increases white blood cells, mainly neutrophil count, in young healthy men: a pilot study. Vasc Health Risk Manag 2008;4:1467–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinges DF, Douglas SD, Zaugg L, Campbell DE, McMann JM, Whitehouse WG, Orne EC, Kapoor SC, Icaza E, Orne MT. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. The Journal of clinical investigation 1994;93:1930–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003;26:342–392 [DOI] [PubMed] [Google Scholar]
- 9.Tranah GJ, Blackwell T, Ancoli-Israel S, Paudel ML, Ensrud KE, Cauley JA, Redline S, Hillier TA, Cummings SR, Stone KL, Study of Osteoporotic Fractures Research G. Circadian activity rhythms and mortality: the study of osteoporotic fractures. J Am Geriatr Soc 2010;58:282–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paudel ML, Taylor BC, Ancoli-Israel S, Blackwell T, Stone KL, Tranah G, Redline S, Cummings SR, Ensrud KE, Osteoporotic Fractures in Men S. Rest/activity rhythms and mortality rates in older men: MrOS Sleep Study. Chronobiol Int 2010;27:363–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paudel ML, Taylor BC, Ancoli-Israel S, Stone KL, Tranah G, Redline S, Barrett-Connor E, Stefanick ML, Ensrud KE. Rest/activity rhythms and cardiovascular disease in older men. Chronobiol Int 2011;28:258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao Q, Qian J, Evans DS, Redline S, Lane NE, Ancoli-Israel S, Scheer F, Stone K, Osteoporotic Fractures in Men Study G. Cross-Sectional and Prospective Associations of Rest-Activity Rhythms with Circulating Inflammatory Markers in Older Men. J Gerontol A Biol Sci Med Sci 2022; 77(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian J, Martinez-Lozano N, Tvarijonaviciute A, Rios R, Scheer F, Garaulet M. Blunted rest-activity rhythms link to higher body mass index and inflammatory markers in children. Sleep 2021;44(5):zsaa256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allin KH, Bojesen SE, Nordestgaard BG. Inflammatory biomarkers and risk of cancer in 84,000 individuals from the general population. Int J Cancer 2016;139:1493–1500 [DOI] [PubMed] [Google Scholar]
- 15.Lassale C, Curtis A, Abete I, van der Schouw YT, Verschuren WMM, Lu Y, Bueno-de-Mesquita HBA. Elements of the complete blood count associated with cardiovascular disease incidence: Findings from the EPIC-NL cohort study. Sci Rep 2018;8:3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabat GC, Kim MY, Manson JE, Lessin L, Lin J, Wassertheil-Smoller S, Rohan TE. White Blood Cell Count and Total and Cause-Specific Mortality in the Women’s Health Initiative. Am J Epidemiol 2017;186:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruggiero C, Metter EJ, Cherubini A, Maggio M, Sen R, Najjar SS, Windham GB, Ble A, Senin U, Ferrucci L. White blood cell count and mortality in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol 2007;49:1841–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welsh C, Welsh P, Mark PB, Celis-Morales CA, Lewsey J, Gray SR, Lyall DM, Iliodromiti S, Gill JMR, Pell J, Jhund PS, Sattar N. Association of Total and Differential Leukocyte Counts With Cardiovascular Disease and Mortality in the UK Biobank. Arterioscler Thromb Vasc Biol 2018;38:1415–1423 [DOI] [PubMed] [Google Scholar]
- 19.Shah AD, Denaxas S, Nicholas O, Hingorani AD, Hemingway H. Neutrophil Counts and Initial Presentation of 12 Cardiovascular Diseases: A CALIBER Cohort Study. J Am Coll Cardiol 2017;69:1160–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Eliot M, Koestler DC, Wu WC, Kelsey KT. Association of Neutrophil-to-Lymphocyte Ratio With Mortality and Cardiovascular Disease in the Jackson Heart Study and Modification by the Duffy Antigen Variant. JAMA Cardiol 2018;3:455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang J, Chang Y, Ahn J, Oh S, Koo DH, Lee YG, Shin H, Ryu S. Neutrophil-to-lymphocyte ratio and risk of lung cancer mortality in a low-risk population: A cohort study. Int J Cancer 2019;145:3267–3275 [DOI] [PubMed] [Google Scholar]
- 22.Li H, Wu X, Bai Y, Wei W, Li G, Fu M, Jie J, Wang C, Guan X, Feng Y, Meng H, Li M, He M, Zhang X, Guo H. Physical activity attenuates the associations of systemic immune-inflammation index with total and cause-specific mortality among middle-aged and older populations. Sci Rep 2021;11:12532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin Z, Wu Q, Chen S, Gao J, Li X, Zhang X, Zhou Y, He D, Cheng Z, Zhu Y, Wu S. The Associations of Two Novel Inflammation Indexes, SII and SIRI with the Risks for Cardiovascular Diseases and All-Cause Mortality: A Ten-Year Follow-Up Study in 85,154 Individuals. J Inflamm Res 2021;14:131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat 1 2013:1–37 [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) 2021. [article online], 2020. Available from https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
- 26.Cespedes Feliciano EM, Quante M, Weng J, Mitchell JA, James P, Marinac CR, Mariani S, Redline S, Kerr J, Godbole S, Manteiga A, Wang D, Hipp JA. Actigraphy-Derived Daily Rest-Activity Patterns and Body Mass Index in Community-Dwelling Adults. Sleep 2017;40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goncalves BSB, Adamowicz T, Louzada FM, Moreno CR, Araujo JF. A fresh look at the use of nonparametric analysis in actimetry. Sleep Med Rev 2015;20:84–91 [DOI] [PubMed] [Google Scholar]
- 28.Han L, You D, Ma W, Astell-Burt T, Feng X, Duan S, Qi L. National Trends in American Heart Association Revised Life’s Simple 7 Metrics Associated With Risk of Mortality Among US Adults. JAMA Netw Open 2019;2:e1913131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milanti A, Chan DNS, Li C, So WKW. Actigraphy-measured rest-activity circadian rhythm disruption in patients with advanced cancer: a scoping review. Support Care Cancer 2021;29:7145–7169 [DOI] [PubMed] [Google Scholar]
- 30.Xiao Q, Qian J, Evans DS, Redline S, Lane NE, Ancoli-Israel S, Scheer F, Stone K, Osteoporotic Fractures in Men Study G. Cross-sectional and Prospective Associations of Rest-Activity Rhythms With Metabolic Markers and Type 2 Diabetes in Older Men. Diabetes Care 2020;43:2702–2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carter SJ, Durrington HJ, Gibbs JE, Blaikley J, Loudon AS, Ray DW, Sabroe I. A matter of time: study of circadian clocks and their role in inflammation. J Leukocyte Biol 2016;99:549–560 [DOI] [PubMed] [Google Scholar]
- 32.Inokawa H, Umemura Y, Shimba A, Kawakami E, Koike N, Tsuchiya Y, Ohashi M, Minami Y, Cui G, Asahi T, Ono R, Sasawaki Y, Konishi E, Yoo SH, Chen Z, Teramukai S, Ikuta K, Yagita K. Chronic circadian misalignment accelerates immune senescence and abbreviates lifespan in mice. Sci Rep 2020;10:2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 2013;341:1483–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgos I, Richter L, Klein T, Fiebich B, Feige B, Lieb K, Voderholzer U, Riemann D. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun 2006;20:246–253 [DOI] [PubMed] [Google Scholar]
- 35.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab 2010;24:775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsons TJ, Sartini C, Welsh P, Sattar N, Ash S, Lennon LT, Wannamethee SG, Lee IM, Whincup PH, Jefferis BJ. Physical Activity, Sedentary Behavior, and Inflammatory and Hemostatic Markers in Men. Med Sci Sports Exerc 2017;49:459–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sennels HP, Jorgensen HL, Hansen AL, Goetze JP, Fahrenkrug J. Diurnal variation of hematology parameters in healthy young males: the Bispebjerg study of diurnal variations. Scand J Clin Lab Invest 2011;71:532–541 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


