Abstract
Maturation of [FeFe]-hydrogenase (HydA) involves synthesis of a CO, CN−, and dithiomethylamine (DTMA)-coordinated 2Fe subcluster that is inserted into HydA to make the active hydrogenase. This process requires three maturation enzymes: the radical S-adenosyl-l-methionine (SAM) enzymes HydE and HydG, and the GTPase HydF. In vitro maturation with purified maturation enzymes has been possible only when clarified cell lysate was added, with the lysate presumably providing essential components for DTMA synthesis and delivery. Here we report maturation of [FeFe]-hydrogenase using a fully defined system that includes components of the glycine cleavage system (GCS), but no cell lysate. Our results reveal for the first time an essential role for the aminomethyl-lipoyl-H-protein of the GCS in hydrogenase maturation and the synthesis of the DTMA ligand of the H-cluster. In addition, we show that ammonia is the source of the bridgehead nitrogen of DTMA.
Keywords: hydrogenase maturation, lipoyl-H-protein, biosynthesis, glycine cleavage system, dithiomethylamine
Graphical Abstract
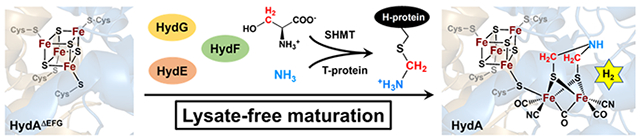
The aminomethyl-lipoyl-H-protein (Hmet) of the glycine cleavage system (GCS) is shown to play a key role in maturation of the [FeFe]-hydrogenase. Including Hmet, or Hmet plus other components of the GCS, allows maturation by purified HydE, HydF, and HydG in the absence of cell lysate for the first time.
[FeFe]-hydrogenase (HydA) catalyzes the reversible reduction of protons to H2 at a complex metal cluster, the H-cluster, composed of a [4Fe-4S] cluster bridged via a cysteine thiolate to a [2Fe] subcluster coordinated by CO, CN− and dithiomethylamine (DTMA) ligands (Figure 1).[1] [FeFe]-hydrogenases can catalyze rates of H2 production that are approximately 100x higher than other hydrogenases, making HydA an appealing target for biohydrogen production technologies.[2] Early efforts to heterologously express [FeFe]-hydrogenase, however, led to inactive enzyme, a major obstacle to practical applications of this enzyme.[3] Subsequent studies showed that three maturation enzymes, HydE, HydF, and HydG, were required to produce an active [FeFe]-hydrogenase.[4]
Figure 1.
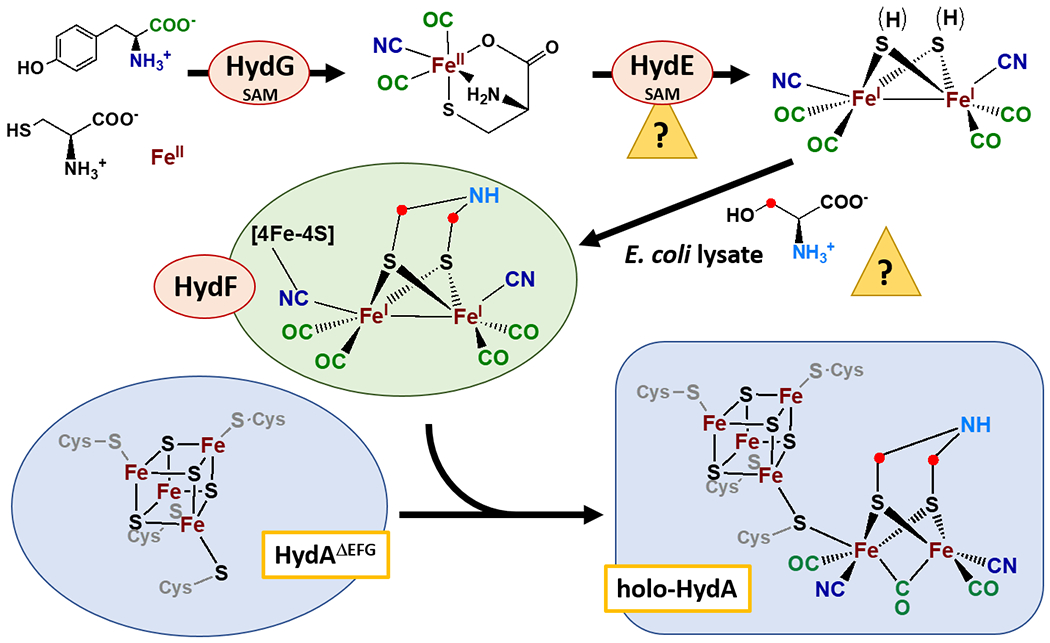
Current model for biological maturation of the H-cluster of the [FeFe]-hydrogenase. Question marks indicate steps that are not fully understood.
HydF is a GTPase that binds redox-active iron-sulfur clusters and serves as a scaffold for assembly of a 2Fe precursor ([2Fe]F) delivered to HydA to yield active hydrogenase.[5] HydF can be loaded with [2Fe]F enzymatically by co-expression with HydE and HydG, yielding a [2Fe]F subcluster resembling the [2Fe] subcluster of the H-cluster ([2Fe]H).[6] In an alternative semi-synthetic approach, pre-formed synthetic [2Fe] clusters coordinated by CO, CN−, and DTMA can be used to load HydF, which then can activate HydA.[7] HydG synthesizes the CO and CN− ligands from tyrosine,[8] and also provides iron in the form of a [Fe(Cys)(CO)2(CN)] synthon.[9] HydE binds a synthetic analog of this synthon, and uses it as a substrate to catalyze an adenosylation reaction,[10] and may convert two of these adenosylated Fe(I) synthon units to a [2Fe] subcluster precursor.[11] The origin of the DTMA ligand is not well understood. It was thought to arise from HydE catalysis[12] but now appears to be formed independent of HydE.[11] Maturation of HydAΔEFG (HydA expressed in the absence of the maturase enzymes) can be achieved in vitro by combining extracts of E. coli cells overexpressing the individual maturases, or by using purified maturase enzymes together with E. coli cell lysate.[8d, 13] In work to date, the requirement for the E. coli lysate (20% to 100% v/v) has been absolute.[4c, 4d, 8d, 9a, 11, 13–14]
We report here development of a fully defined enzymatic system for maturation of HydA using HydE, HydF, and HydG, their substrates and reductants, and components of the glycine cleavage system (GCS, Scheme S1),[15] but without E. coli lysate. The results reveal a key role for the aminomethyl-lipoyl-H-protein (Hmet) as a precursor of the DTMA ligand of the H-cluster.
To better understand the chemistry of H-cluster maturation, we pursued fractionation of E. coli cell lysate to identify component(s) responsible for its requirement during maturation. These efforts revealed one or more components of a low-molecular weight (LMW) fraction (10 – 15 kDa, Figure S1) of lysate could also support maturation. Essential component(s) from E. coli lysate were inactivated by heat/acid treatment, suggesting they may be proteins (Figure S2). Shotgun proteomics revealed the presence of carrier proteins including the acyl-carrier protein (ACP) in the LMW fraction, suggesting the involvement of a small carrier protein in hydrogenase maturation, perhaps in the still poorly understood synthesis of the DTMA ligand, as suggested by Juan Fontecilla-Camps (personal communication). However, no detectable HydA activity was observed following in vitro HydA maturation with holo- or lipoyl-ACP in place of E. coli cell lysate, and with purified HydE, HydF, HydG, SAM, and other small molecule components (see SI).
We next considered the H-protein of the GCS (Scheme S1), as a potential key component of HydA maturation. Lipoylated H-protein acts as a carrier between protein components of the GCS (Scheme S1), and can be in the disulfide form (Hox), the reduced form (Hred), or the aminomethyl form (Hmet). Maturation reactions carried out with Hred in the absence of cell lysate provided no detectable hydrogenase activity (Figure 2). Hmet was synthesized enzymatically from Hox using the P-protein, PLP, and glycine (Scheme S1), purified, and verified via mass spectrometry (Figure S3). HydA maturation with Hmet added to our defined system resulted in active HydA, as evidenced by H2 gas production (Figure 2). Increasing the amount of Hmet provided a significant increase in H2 (Figure 2), demonstrating that HydA activation is dependent on Hmet. To our knowledge, this is the first report of biological HydAΔEFG conversion to active hydrogenase in the absence of cell lysate, and points to Hmet as a previously unrecognized essential component.
Figure 2.
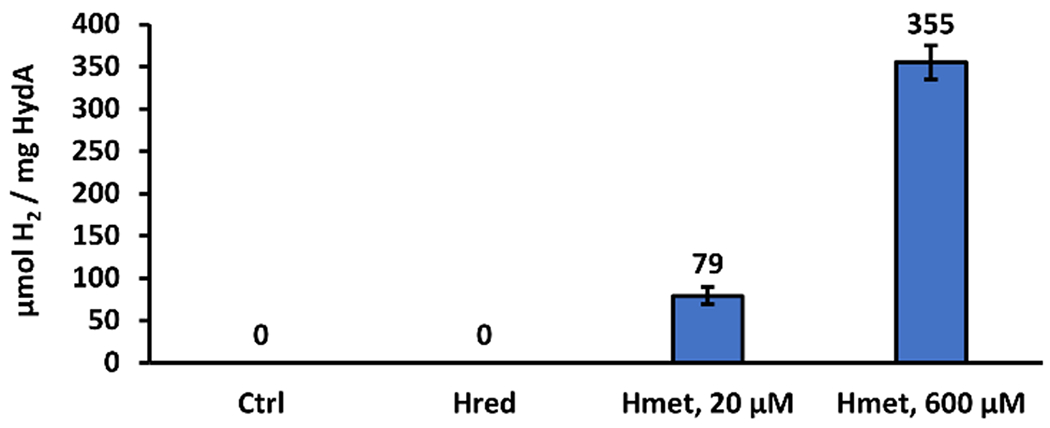
Maturation of HydA without lysate, using the GCS H-protein. Without H-protein (Ctrl) or with lipoyl-H-protein (Hred) no active HydA is formed. Addition of aminomethyl-lipoyl-H-protein (Hmet) provides maturation of HydA in a concentration-dependent manner. Assays for H2 were run for 24 h to allow H2 to accumulate. Conditions as reported in the SI.
In these experiments, the level of activation of HydAΔEFG in the presence of Hmet is nonetheless low, requiring 24 h incubation to detect small amounts of H2. The low hydrogenase activity may be due to instability of Hmet, which is known to readily hydrolyze, especially in the presence of partner proteins.[16] We reasoned that if this is so, regeneration of Hmet in situ would improve the hydrogenase maturation. Regeneration of Hmet from Hred in situ under the reducing conditions required for maturation was accomplished by including aminomethyltransferase (T-protein), serine hydroxymethyltransferase (SHMT), serine, and NH4+ (Scheme 1) in the maturation mixture. HydA maturation under these conditions yielded dramatically improved hydrogenase activity, with further refinement as described below providing specific activities as high as 390 μmol/min/mg HydA (Figure 3); in comparison, holo-HydA is reported to have a specific activity of ~700 μmol/min/mg.[17]
Scheme 1.
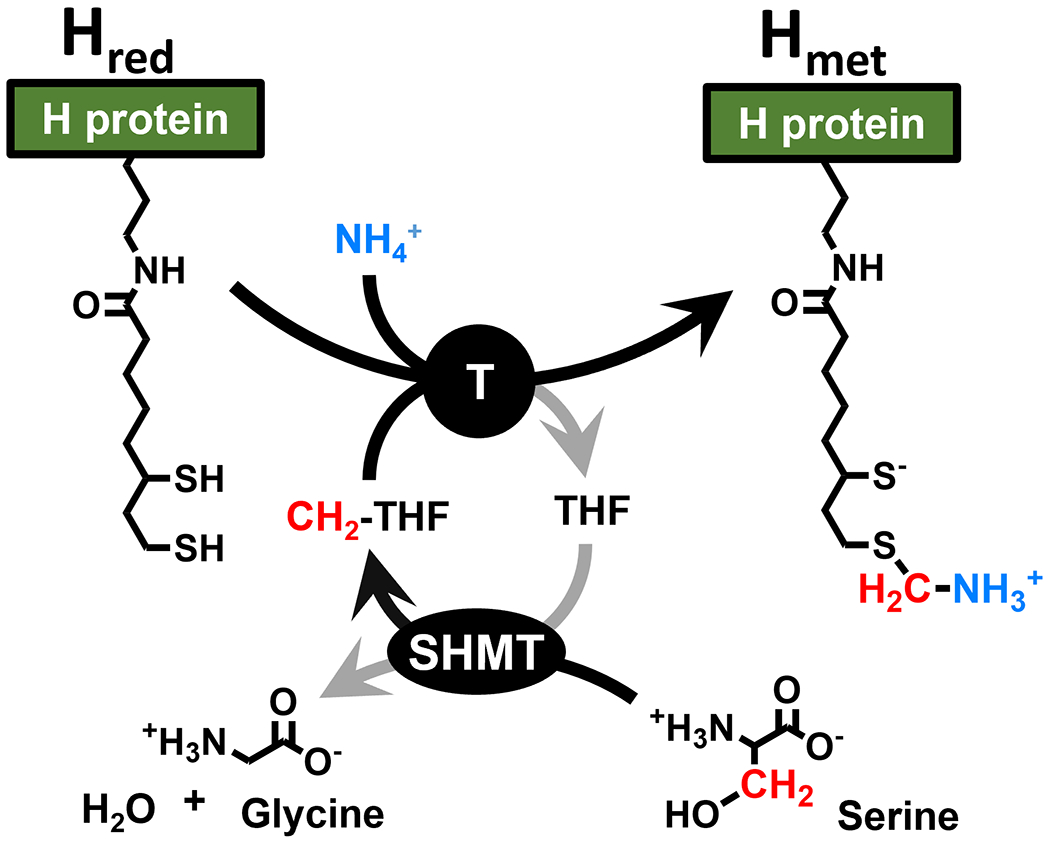
In situ generation of Hmet from Hred via SHMT and the T-protein of the GCS (see Scheme S1 for the full GCS). All reactions are reversible.
Figure 3.
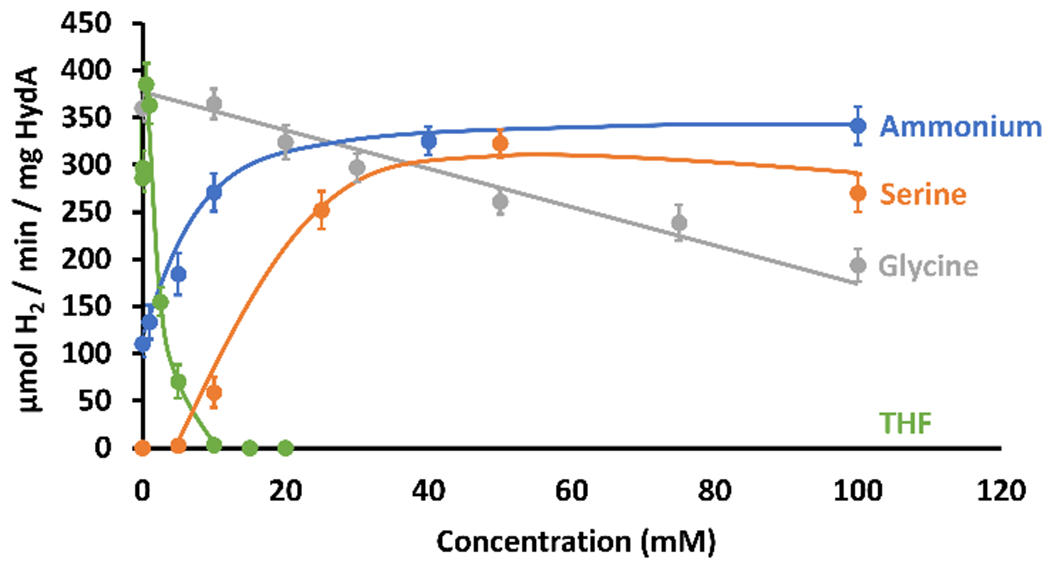
Maturation of HydA in the presence of the T-protein and SHMT. Concentrations of key components were varied to test the hypothesis of the involvement of the GCS and Hmet during hydrogenase maturation. Conditions as reported in the SI. Reactions contained no lysate.
To further probe the involvement of Hmet of the GCS during HydA maturation, we examined the dependence of maturation on concentrations of the Hmet regeneration components (Figure 3). Maturation is dependent on both serine and NH4+, with high concentrations required for optimal activity, consistent with the equilibria outlined in Scheme 1. Glycine has an inverse effect on maturation (Figure 3), consistent with the role of SHMT in producing methylene-tetrahydrofolate (CH2-THF, Scheme 1). The maturation observed without added NH4+ is attributed to co-purification of NH4+ with T-protein, consistent with the well-defined NH4+ binding site in this protein.[18] THF also impacts maturation, increasing activity at low concentrations followed by a rapid decrease with increasing THF concentration (Figures 3, S5), consistent with THF involvement in both SHMT and T-protein equilibria; the dominant effect of THF is on the T-protein equilibrium, driving it towards Hred and away from Hmet (Scheme 1). Maturation works without added THF because THF co-purifies with the T-protein. The dependence of HydA maturation on these components required for generation of Hmet in situ further support a key role for Hmet in HydA maturation; these components were provided by lysate in prior lysate-dependent maturations.[4c, 4d, 8d, 9a, 13b, 13c, 14]
Our results suggest that the GCS, and specifically Hmet, provides a precursor for DTMA biosynthesis. We propose that the aminomethyl group of Hmet is the source of the C and N of the C-N-C framework of DTMA. Given that the aminomethyl of Hmet is derived from the β-C of serine and NH4+ (Scheme 1), we set out to probe incorporation of isotopic labels from these sources into the DTMA of matured HydA. Maturation reactions were carried out in the absence of lysate (see SI), with [13C3,15N]-serine or 15NH4Cl included in place of the unlabeled component. HydA was purified from the maturation mixture and oxidized with thionin to give an electron paramagnetic resonance (EPR) spectrum typical of the Hox state of holo-HydA (Figure S6). Electron nuclear double resonance (ENDOR) spectroscopy was used to look for incorporation of isotopic labels into the DTMA of the H-cluster. For the [13C3,15N]-serine samples, the spectrum collected at ~ g1 shows two distinct 13C hyperfine signals (Figure 4, red) associated with the two carbons of the DTMA ligand, as reported previously for HydA matured in the presence of lysate.[14b] The presence of signals with different hyperfine splittings for the two DTMA carbons is consistent with the prior work demonstrating their origins from serine and asymmetry in the DTMA ligand environment in holo-HydA.[14b, 19] However, this sample prepared without lysate shows no 15N ENDOR response, indicating that 15N from [13C3,15N]-serine is not present in the DTMA of HydA matured lysate-free (Figure 4, black).
Figure 4.
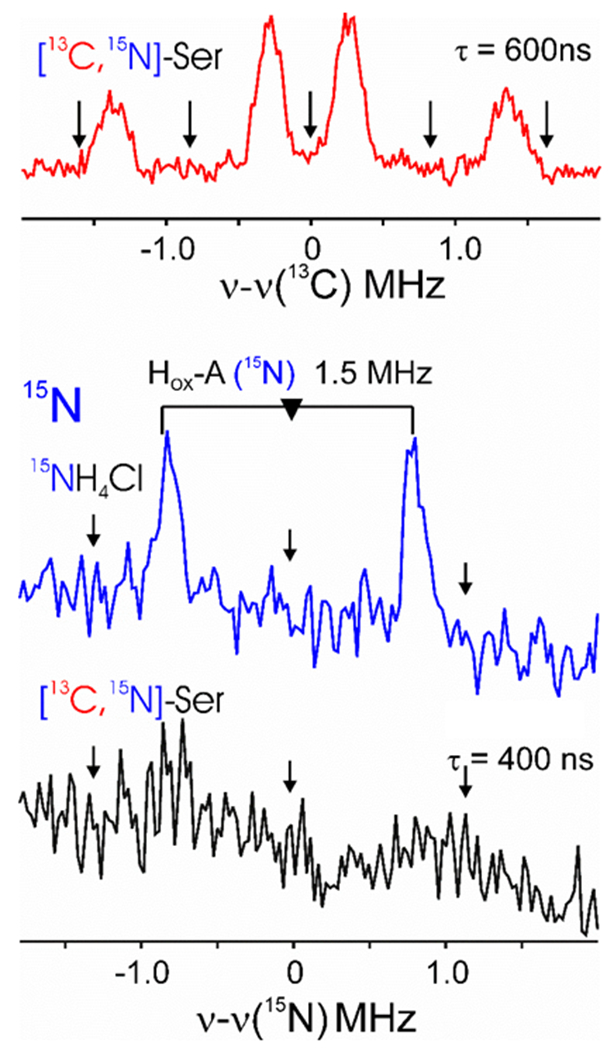
35 GHz pulsed 13C and 15N ENDOR of HydA matured in the absence of lysate. Top, 13C Mims ENDOR spectrum at g=2.093 of HydA matured with [13C3,15N]-serine. Bottom, 15N Mims ENDOR spectra at g=2.004 of HydA matured with 15NH4Cl (blue) and with [13C3,15N]-serine (black). Mims holes are indicated with arrows. Microwave frequency, 34.605 GHz; rep time, 50 ms; T = 2 K. For a complete characterization of these signals, see ref 15b.
In our defined maturation, the T-protein uses NH4+ to provide the N for the aminomethyl group of Hmet (Scheme 1), and thus NH4+ should be the source of the N of DTMA. 15N ENDOR spectra of HydA matured in the presence of 15NH4Cl reveals this to be true, as the corresponding 15N coupling of 1.5 MHz is observed (Figure 4, blue). Prior work showed that both N and C of DTMA could be derived from serine, but this was in the presence of E. coli cell lysate (72% v/v).[14b] Given that cell lysate contains enzymes such as serine dehydratase that can liberate NH4+ from serine, it is likely that the observed incorporation of 15N from serine in the prior work was a result of 15NH4+ liberation due to the presence of lysate.
We demonstrate the first maturation of HydA using a defined enzymatic system, thereby revealing an essential role for Hmet and the GCS in building the DTMA ligand of the H-cluster. Hmet alone, when added to lysate-free maturation components, is sufficient to provide active HydA (Figure 2), revealing the key role for Hmet during H-cluster biosynthesis. Inclusion of an Hmet regeneration system (T-protein, SHMT, serine, and NH4+) affords high levels of HydA activation, with the roles of T-protein and SHMT highlighted by the dependence of activation on the concentrations of key species (Figure 3). 13C/15N-ENDOR spectroscopy of HydA matured using this defined system confirms that the carbon atoms of DTMA originate from serine, while the nitrogen comes from NH4+ (Figure 4), consistent with our previous prediction[20] and with the known chemistry of the GCS during Hmet formation from Hred (Scheme 1).
The recent report that HydA can be matured in the presence of HydF, a synthetic [Fe2(SH)2(CO)4(CN)2]2− complex, and cell lysate,[11] together with the results presented here, lead us to propose that Hmet interacts with the HydF-[Fe2(SH)2(CO)4(CN)2]2− complex to synthesize the dimethylamine backbone of DTMA. In this model, the aminomethyl group of Hmet is functionally equivalent to the formaldehyde and ammonia used to synthesize the C-N-C DTMA backbone in the [2Fe]H model reported by Li and Rauchfuss.[21] We propose that during biological maturation, each sulfur of the [2Fe]F precursor cluster [Fe2(SH)2(CO)4(CN)2]2− nucleophilically attacks the C of an aminomethyl group of Hmet, resulting in transfer of two aminomethyl groups from two Hmet to the bridging sulfides of the HydF-bound [2Fe]F precursor cluster. Condensation of the aminomethyl moieties with loss of NH4+ would then form DTMA (Scheme 2). In this model, the sulfurs of DTMA are derived from the precursor diiron cluster, not from the lipoyl group of Hmet; use of a seleno-lipoyl-H-protein during maturation could test this hypothesis.
Scheme 2.
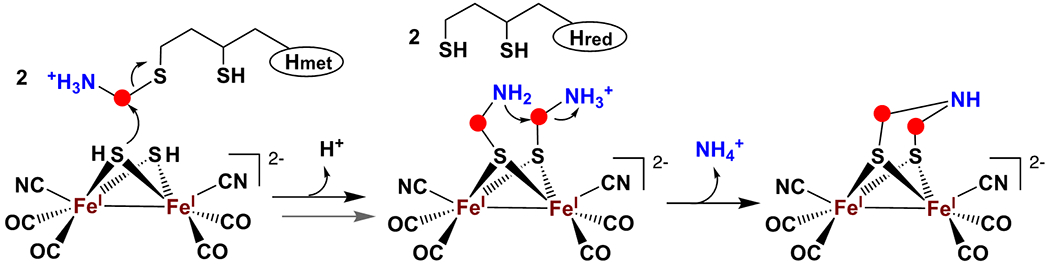
Proposed pathway for formation of the DTMA ligand of the [2Fe]F cluster on HydF.
Supplementary Material
Acknowledgements
This work was funded by the U.S. DOE (DE-SC0005404 to J.B.B. and E.M.S.). S.J.B acknowledges funding from the NSF (MCB-1716686), and B.M.H from the NIH GM 111097. The Mass Spectrometry Facility was in part funded by the MJ Murdock Charitable Trust and the NIH (P20GM103474 and S10OD28650). The authors thank Prof. Inger Andersson (Uppsala U.) for the gift of pBAD-HisA-slr0293, and Juan Fontecilla-Camps, Roman Rohac, and Yvain Nicolet for helpful discussions.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].a) Lubitz W, Ogata H, Rudiger O, Reijerse E, Chem. Rev 2014, 114, 4081–4148; [DOI] [PubMed] [Google Scholar]; b) Schilter D, Camara JM, Huynh MT, Hammes-Schiffer S, Rauchfuss TB, Chem. Rev 2016, 116, 8693–8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].a) Mészáros LS, Németh B, Esmieu C, Ceccaldi P, Berggren G, Angew. Chem 2018, 130, 2626–2629; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Rodriquez-Maciá P, Galle LM, Bjornsson R, Lorent C, Zebger I, Yoda Y, Cramer SP, DeBeer S, Span I, Birrell JA, Angew. Chem. Int. Ed 2020, 59, 16786–16794; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Schuchmann K, Chowdhury NP, Müller V, Front. Microbiol 2018, 9, 2911; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Wegelius A, Khanna N, Esmieu C, Barone GD, Pinto F, Tamagnini P, Berggren G, LIndblad P, Energy Environ. Sci 2018, 11, 3163–3167; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Land H, Ceccaldi P, Mészáros LS, Lorenzi M, Redman HJ, Senger M, Stripp ST, Berggren G, Chem. Sci 2019, 10, 9941–9948; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Li T, Jiang Q, Huang J, Aitchison CM, Huang F, Yang M, Dykes GF, He H-L, Wang Q, Sprick RS, Cooper AI, Liu L-N, Nat. Commun 2020, 11, 5448; [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Birrell JA, Rudiger O, Reijerse EJ, Lubitz W, Joule 2017, 1, 61–76; [Google Scholar]; h) Esmieu C, Raleiras P, Berggren G, Sustain. Energ. Fuels 2018, 2, 724–750; [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Reeve HA, Ash PA, Park H, Huang AL, Posidias M, Tomlinson C, Lenz O, Vincent KA, Biochem. J 2017, 474, 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a) Voordouw G, Hagen WR, Kruse-Wolters KM, van Berkel-Arts A, Veeger C, European journal of biochemistry / FEBS 1987, 162, 31–36; [DOI] [PubMed] [Google Scholar]; b) Atta M, Meyer J, Biochim. Biophys. Acta - Prot. Struct 2000, 1476, 368–371. [DOI] [PubMed] [Google Scholar]
- [4].a) Posewitz MC, King PW, Smolinski SL, Zhang L, Seibert M, Ghirardi ML, J. Biol. Chem 2004, 279, 25711–25720; [DOI] [PubMed] [Google Scholar]; b) Posewitz MC, King PW, Smolinski SL, Smith RD, Ginley AR, Ghirardi ML, Seibert M, Biochem. Soc. Trans 2005, 33, 102–104; [DOI] [PubMed] [Google Scholar]; c) King PW, Posewitz MC, Ghirardi ML, Seibert M, J. Bacteriol 2006, 188, 2163–2172; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) McGlynn SE, Ruebush SS, Naumov A, Nagy LE, Dubini A, King PW, Broderick JB, Posewitz MC, Peters JW, J. Biol. Inorg. Chem 2007, 12, 443–447. [DOI] [PubMed] [Google Scholar]
- [5].a) Brazzolotto X, Rubach JK, Gaillard J, Gambarelli S, Atta M, Fontecave M, J. Biol. Chem 2006, 281, 769–774; [DOI] [PubMed] [Google Scholar]; b) McGlynn SE, Shepard EM, Winslow MA, Naumov AV, Duschene KS, Posewitz MC, Broderick WE, Broderick JB, Peters JW, FEBS Lett. 2008, 582, 2183–2187; [DOI] [PubMed] [Google Scholar]; c) Shepard EM, McGlynn SE, Bueling AL, Grady-Smith C, George SJ, Winslow MA, Cramer SP, Peters JW, Broderick JB, Proc. Natl. Acad. Sci. U.S.A 2010, 107, 10448–10453; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Czech I, Silakov A, Lubitz W, Happe T, FEBS Lett. 2010, 584, 638–642; [DOI] [PubMed] [Google Scholar]; e) Németh B, Land H, Magnuson A, Hofer A, Berggren G, J. Biol. Chem 2020, 295, 11891–11901; [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Caserta G, Pecqueur L, Adamska-Venkatesh A, Papini C, Roy S, Artero V, Atta M, Reijerse E, Lubitz W, Fontecave M, Nat. Chem. Biol 2017, 13, 779–784. [DOI] [PubMed] [Google Scholar]
- [6].a) Czech I, Stripp S, Sanganas O, Leidel N, Happe T, Haumann M, FEBS Lett. 2011, 585, 225–230; [DOI] [PubMed] [Google Scholar]; b) Scott AG, Szilagyi RK, Mulder DW, Ratzloff MW, Byer AS, King PW, Broderick WE, Shepard EM, Broderick JB, Dalton Trans. 2018, 47, 9521–9535. [DOI] [PubMed] [Google Scholar]
- [7].a) Berggren G, Adamska A, Lambertz C, Simmons TR, Esselborn J, Atta M, Gambarelli S, Mouesca JM, Reijerse E, Lubitz W, Happe T, Artero V, Fontecave M, Nature 2013, 499, 66–69; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Németh B, Esmieu C, Redman HJ, Berggren G, Dalton Trans. 2019, 48, 5978–5986; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Artero V, Berggren G, Atta M, Caserta G, Roy S, Pecqueur L, Fontecave M, Acc. Chem. Res 2015, 48, 2380–2387; [DOI] [PubMed] [Google Scholar]; d) Németh B, Senger M, Redman HJ, Ceccaldi P, Broderick J, Magnuson A, Stripp ST, Haumann M, Berggren G, J. Biol. Inorg. Chem 2020, 25, 777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a) Driesener RC, Challand MR, McGlynn SE, Shepard EM, Boyd ES, Broderick JB, Peters JW, Roach PL, Angew. Chem. Int. Ed. Engl 2010, 49, 1687–1690; [DOI] [PubMed] [Google Scholar]; b) Shepard EM, Duffus BR, McGlynn SE, Challand MR, Swanson KD, Roach PL, Peters JW, Broderick JB, J. Am. Chem. Soc 2010, 132, 9247–9249; [DOI] [PubMed] [Google Scholar]; c) Shepard EM, Impano S, Duffus BR, Pagnier A, Duschene KS, Betz JN, Byer AS, Galambas A, McDaniel EC, Watts H, McGlynn SE, Peters JW, Broderick WE, Broderick JB, Dalton Trans. 2021, 50, 10405–10422; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Kuchenreuther JM, George SJ, Grady-Smith CS, Cramer SP, Swartz JR, PLoS ONE 2011, 6, e20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Kuchenreuther JM, Myers WK, Suess DLM, Stich TA, Pelmenschikov V, Shiigi SA, Cramer SP, Swartz JR, Britt RD, George SJ, Science 2014, 343, 424–427; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Suess DLM, Bürstel I, De La Paz L, Kuchenreuther JM, Pham CC, Cramer SP, Swartz JR, Britt RD, Proc. Natl. Acad. Sci. U. S. A 2015, 112, 11455–11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].a) Tao L, Pattenaude SA, Joshi S, Begley TP, Rauchfuss TB, Britt RD, J. Am. Chem. Soc 2020, 142, 10841–10848; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Rohac R, Martin L, Liu L, Basu D, Tao L, Britt RD, Rauchfuss TB, Nicolet Y, J. Am. Chem. Soc 2021, 143, 8499–8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang Y, Tao L, Woods TJ, Britt RD, Rauchfuss TB, J. Am .Chem. Soc 2022, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shepard EM, Mus F, Betz J, Byer A, Duffus BR, Peters JW, Broderick JB, Biochemistry 2014, 53, 4090–4104. [DOI] [PubMed] [Google Scholar]
- [13].a) Boyer ME, Stapleton JA, Kuchenreuther JM, Wang C.-w., Swartz JR, Biotechnol. Bioengin 2007, 99, 59–67; [DOI] [PubMed] [Google Scholar]; b) Kuchenreuther JM, Stapleton JA, Swartz JR, PLoS ONE 2009, 4, e7565; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kuchenreuther JM, Britt RD, Swartz JR, PLoS ONE 2012, 7, e45850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].a) Rao G, Pattenaude SA, Alwan K, Blackburn NJ, Britt RD, Proc. Natl. Acad. Sci. U. S. A 2019, 116, 20850–20855; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Rao G, Tao L, Britt RD, Chemical Science 2020, 11, 1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Douce R, Bourguignon J, Neuburger M, Rébeillé F, Trends Plant Sci. 2001, 167–176. [DOI] [PubMed] [Google Scholar]
- [16].Guilhaudis L, Simorre J-P, Blackledge M, Marion D, Gans P, Neuburger M, Douce R, Biochemistry 2000, 39, 4259–4266. [DOI] [PubMed] [Google Scholar]
- [17].a) Kamp C, Silakov A, Winkler M, Reijerse EJ, Lubitz W, Happe T, Biochim. Biophys. Acta - Bioenergetics 2008, 1777, 410–416; [DOI] [PubMed] [Google Scholar]; b) Girbal L, von Abendroth G, Winkler M, Benton PMC, Meynial-Salles I, Croux C, Peters JW, Happe T, Soucaille P, Appl. Environ. Microbiol 2005, 71, 2777–2781; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Kuchenreuther JM, Grady-Smith CS, Bingham A, George SJ, Cramer SP, Swartz JR, PLoS ONE 2010, 5, e15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Okamura-Ikeda K, Hosaka H, Maita N, Fujiwara K, Yoshizawa AC, Nakagawa A, Taniguchi H, J. Biol. Chem 2010, 285, 18684–18692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Reijerse EJ, Pelmenschikov V, Birrell JA, Richers CP, Kaupp M, Rauchfuss TB, Cramer SP, Lubitz W, J. Phys. Chem. Lett 2019, 10, 6794–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Betz JN, Boswell NW, Fugate CJ, Holliday GL, Akiva E, Scott AG, Babbitt PC, Peters JW, Shepard EM, Broderick JB, Biochemistry 2015, 54, 1807–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li H, Rauchfuss TB, J. Am. Chem. Soc 2002, 124, 726–727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


