Abstract
The blood-brain barrier (BBB) continues to represent one of the most significant challenges for successful drug-based treatments of neurological disease. Mechanical modulation of the BBB using focused ultrasound and microbubbles has shown considerable promise in enhancing therapeutic delivery to the brain, but questions remain regarding possible long-term effects of such forced disruption. This review examines the available evidence for inflammation associated with ultrasound-induced BBB disruption, and potential strategies for managing such inflammatory effects to improve both the efficacy and safety of therapeutic ultrasound in neurological applications.
Keywords: Blood-brain barrier, neurovasculature, focused ultrasound, microbubbles, cavitation, sterile inflammation, immunomodulation, immunoprivilege, neurological disease
Challenges for treatment of central nervous system (CNS) diseases
A significant proportion of the global population is diagnosed annually with some form of neurological disorder or disease – 16.5% of global deaths can be attributed to CNS diseases [1]. There have consequently been many efforts to develop effective CNS-acting compounds and biomolecules. Unfortunately, despite being one of the more heavily funded areas of research in the pharmaceutical industry, CNS drug discovery and development is associated with a low rate of return. Although there is an abundance of promising in vivo animal data from pharmacokinetic and pharmacodynamic studies, very few drug candidates show comparable efficacy in human trials [2,3]. Amongst the reasons for this are key differences in anatomy and physiology between humans and animal models including of the blood-brain barrier (BBB) [4].
Conventional methods of structural modification for small compounds have produced only modest improvements in terms of BBB penetration [5]. Consequently, in recent years, there has been increased interest in drug delivery methods to the brain based on local permeabilization of the BBB using focused ultrasound (FUS), especially in combination with microbubbles (MB). These methods have shown considerable promise, with several first-in-human clinical trials reporting successful outcomesi, ii, iii [6–8]. There are, however, important safety concerns relating to mechanical disruption of the BBB, specifically in relation to the metabolic and physiological pathways required for brain homeostasis. If the permeability of the BBB is modulated to increase drug extravasation, it is imperative to understand the potential consequences of that disruption, especially in neurological conditions in which BBB may already be compromised.
This paper aims to provide an overview of the evidence for ultrasound induced neuroinflammation, its implications, and strategies by which adverse effects could potentially be mitigated to maximize the benefit-risk ratio in clinical applications.
A brief overview of the blood-brain barrier
The BBB provides both a physical and a physiological barrier between the brain parenchyma and the bloodstream (Figure 1). It is composed primarily of microvascular endothelial cells supported by pericytes and astrocytic foot processes [9]. The BBB prevent entrance of exogenous toxins and agents from the bloodstream into the brain parenchyma and maintains separation between the CNS and the peripheral nervous system (PNS). Given the BBB’s ability to selectively determine the passage of biomolecules and chemicals, its role in homeostasis, in multiple diseases, and in accurate evaluation of drug efficacy, are topics of great interest for clinicians and researchers.
Figure 1. An overview of the cellular composition of the brain vasculature.
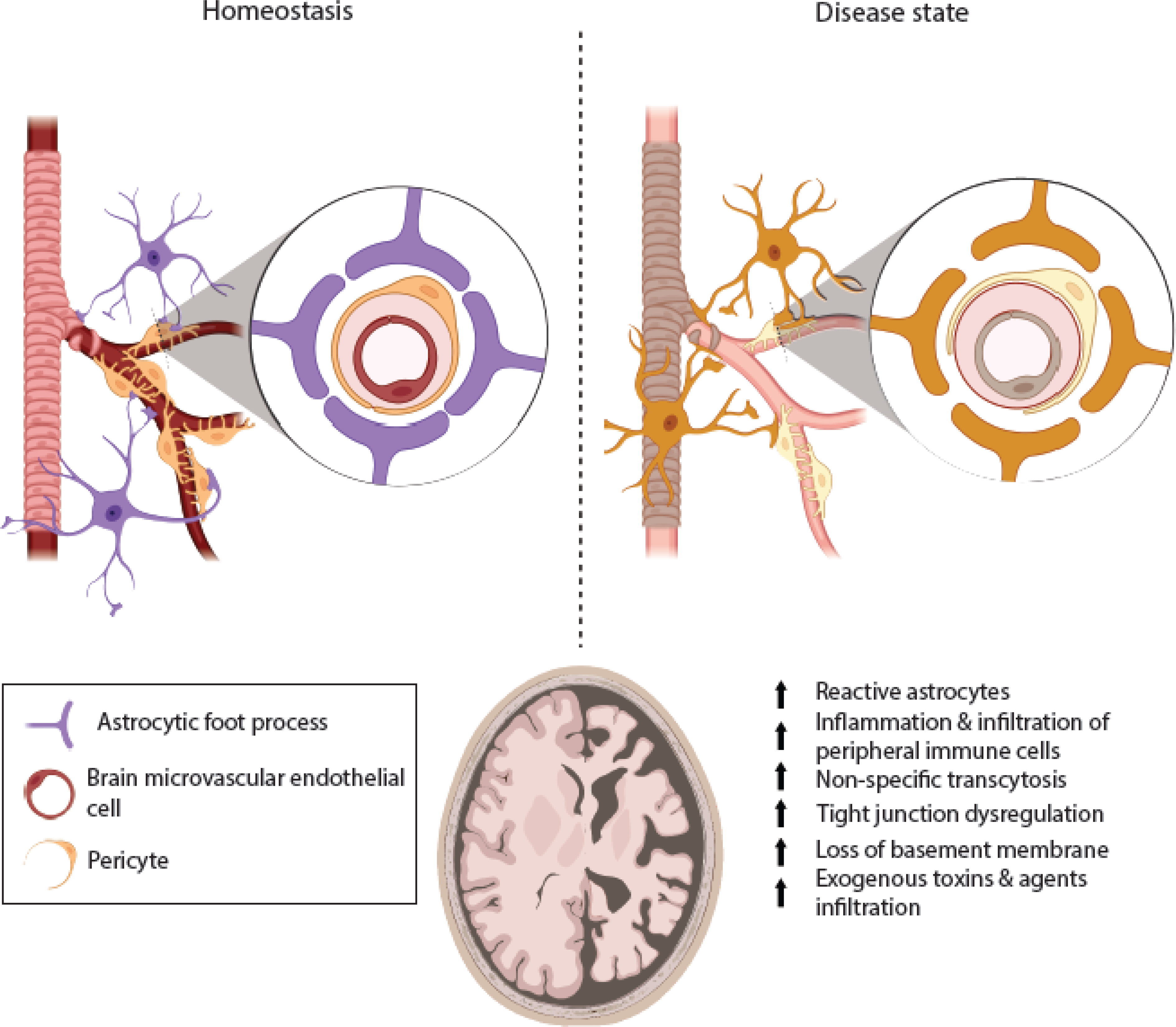
The blood brain barrier (BBB) is composed of brain endothelial cells supported by pericytes and endfoot processes of astrocytes. The microvascular endothelial cells form continuous tight junctions with one another, and the astrocytes and pericytes support the vascular network along with the basement membrane lining along the basolateral aspect of the endothelia. In homeostasis, the BBB prevents harmful toxins and agents from entering the central nervous system. This is essential because neurons are especially sensitive to microenvironmental changes. In many neurological diseases, the ability of endothelial cells to form tight junctions is compromised, pericytes’ ability to effectively support the vascular network is impaired, and reactive astrocytes signal and interact with microglia, the resident brain “macrophages.” There is an increase in local inflammation that leads to further leakage and dysregulation of tight junction complexes, that can allow for chemotaxis of peripheral immune cells. In extreme circumstances, the increased permeability can be so severe that it allows exogenous agents to enter the brain parenchyma, which can be devastating for neurons and the relevant network near the disrupted BBB. Image created through Biorender.
Potential side effects of BBB disruption
There is a growing body of data demonstrating an important role for the BBB in mediating CNS diseases [10–13]. In developing drug delivery methodologies that disrupt the BBB, it is therefore important to consider the downstream effects of modulating the BBB (Figure 1). For example, BBB breakdown is known to coincide with peripheral immune cell infiltration and the inflammation of the brain parenchyma in diseases such as multiple sclerosis (MS) [14,15]. There is also some evidence of BBB involvement in the progression of other neurological diseases, such as lysosomal storage disorders [16–19]. For intensively studied neurological diseases such as Parkinson’s (PD) and Alzheimer’s disease (AD), there is now experimental evidence showing that the BBB may play an active role early on in their etiology [20–22]. In PD, accumulation of α-synuclein has been shown to be the dominant pathophysiology that leads to clinical manifestations observed in patients. While there have been previous reports of neurovascular impairment in PD patients, recent data have shown that with α-synuclein overexpression in mice, BBB integrity is also compromised [23]. In AD research, the accumulation of amyloid-β plaques and neurofibrillary tangles has been a central theme over the past few decades. In recent years, however, there has been increased interest in the effects of neurovascular factors as well [24]. For example, it has been proposed that a compromised BBB could allow passage of exogenous toxins and agents into the brain, leading to inflammatory responses that could cause plaque and tangle formation as a byproduct [25]. There is also evidence of a correlation between BBB degradation in AD tissue and bacterial and viral infiltration leading to an innate immune response cascade [26][27,28]. At the time of writing, these represent areas of considerable uncertainty and debate. For example, it has yet to be established whether BBB dysfunction is a causative agent in the disease processes, or a symptom of disease progression. These questions, nevertheless, have important implications for drug discovery, design, and development, as well as for preclinical in vivo drug evaluation. Leukocytic infiltration through the BBB is known to drive the pathophysiology in neuroimmune diseases [29], while non-specific transcytosis and tight junction dysregulation are upregulated in response to changes in the microenvironment surrounding neurons, e.g., during stroke [30]. The emerging use of alternative drug delivery techniques that modify the BBB integrity thus has to be balanced against the fact that many of the patients being treated may already have neurovascular complications and/or clinical symptoms that are driven by BBB dysfunction, as much as by neuronal dysregulation [11]. It is therefore critical to investigate the mechanism(s) behind BBB opening via ultrasound-mediated cavitation and the consequences of this manipulation, especially for the treatment of non-terminal diseases, for which patients may receive multiple treatments over several years.
Ultrasound and microbubble-mediated BBB opening
Initial studies.
FUS was first used therapeutically for tissue ablation. In this type of procedure, a large, single element, spherically focused transducer, operating at a center frequency between 0.5 and 10 MHz generates a region of sufficient intensity to cause tissue denaturation. Typically, the focal region of a FUS transducer is ~16 mm3 which enables good spatial control of energy deposition. FUS can rapidly destroy tissue via a range of both mechanical and thermal effects. A common side effect of high intensity FUS is cavitation, i.e. the formation and subsequent oscillation of bubbles as a result of the changes in tissue temperature and pressure. The presence of these bubbles can be beneficial, for example accelerating the rate of heating and promoting mechanical erosion [31,32]. Cavitation is, however, a stochastic process and it was found that similar benefits could be achieved at much lower ultrasound intensities by injecting a suspension of pre-existing MB into the target tissue. This was particularly important in early, preclinical studies of BBB opening using FUS to mitigate the risk of collateral damage [33]. MB-mediated BBB opening was reported as a possible alternative drug delivery technique as well as a theranostic some two decades ago, when FUS was used with contrast agents and magnetic resonance imaging (MRI) to open and detect BBB opening in rabbits [34]. Subsequent studies in mice, rats, and rabbits, focused on the observed bioeffects, which included: vascular wall damage, ischemia and tissue necrosis [35]. The findings suggested that limiting parameters such as the acoustic pressure amplitude and pulse duration, would be critical in producing therapeutic effects with minimal adverse reactions [36,37]. There have also been multiple follow-up studies investigating the mechanism behind BBB opening [38]. Technical details regarding the physics of ultrasound and microbubbles can be found in Box 1.
Box 1.
Focused Ultrasound (FUS) and microbubbles (MB).
Ultrasound is widely used in diagnostic imaging as it is non-ionizing and facilitates real-time imaging of anatomical structures within the body. A linear or curvilinear array of transducers is used to transmit and receive short pulses at frequencies between 2–18 MHz. The received signals provide information about the nature and location of internal structures. While some features within the body can be easily distinguished by ultrasound, this is not the case for blood vessels and consequently gas microbubbles (MBs) have been used for over 2 decades as a contrast agent to improve imaging of the vasculature.
Being filled with gas, MB are highly compressible and hence respond strongly to the mechanical perturbations imposed by a sound field. The fluctuating pressure causes the MB to volumetrically oscillate and re-radiate the incident energy at multiple frequencies. This nonlinear response can be detected by an ultrasound transducer and is fundamental to both microbubble imaging and real-time control of BBB opening. In therapeutic applications, the oscillations of the MB are thought to mechanically stimulate BBB opening and thus locally enhance drug uptake.
The attenuation of ultrasound in most tissues increases with frequency via a power law relationship and leads to increased heat deposition due to viscous absorption. Thus, for FUS+MB, lower frequencies (~1 MHz) than those used in imaging are used to prevent off target heating of surrounding tissue especially of bony structures such as the skull. Therapeutic applications also typically employ longer pulses than those used in imaging to increase the probability of generating the desired biological effect.
Therapeutic applications.
Over the last two decades, the therapeutic potential of FUS+MB has been explored for a range of neurological conditions (excluding cancer) in pre-clinical models including the delivery of quercetin-modified sulfur nanoparticles to minimize endoplasmic reticulum (ER) stress in AD [39], BDNF retrovirus also for treatment of AD [40], curcumin and neurotrophic factors for treatment of PD [41,42] and to increase laronidase uptake as part of enzyme replacement therapy (ERT) in an animal model of mucopolysaccharidosis type I disease [43]. In clinical trials, FUS+MB with MRI guidance have been shown to enable localized BBB opening in amyotrophic lateral sclerosis (ALS) [6]i, AD ii, and PD iii patients. Several studies, however, have highlighted potential risks associated with FUS+MB. These include neuroinflammation, which is discussed in more detail in the next section.
Ultrasound-induced neuroinflammation
Identification of sterile inflammation as a possible bioeffect of FUS+MB for BBB opening.
At the low frequencies (<1 MHz) required for efficient transmission of ultrasound through the skull, the probability of inertially driven bubble collapse is higher due to the prolonged rarefactional period. In vitro and modeling studies suggest that this can lead to significant and permanent biological damage in the local tissue [44,45]. In addition, studies in rats from recent years have indicated that FUS+MB can induce sterile inflammation [46], a possibility that requires more extensive and rigorous investigation. In some applications, stimulation of an immune response may be beneficial, e.g. it has been suggested that FUS+MB may contribute to killer T cell activation and infiltration in tumors [43,47]. Similar approaches have been suggested for brain-specific tumors such as glioblastoma multiforme (GBM), an aggressive brain cancer with very poor prognosis [48].
Since the identification of FUS as a promising alternative delivery technique for CNS therapeutics, there has been extensive assessment of its safety. Table S1 provides a summary of selected studies using FUS+MB for BBB opening. Prior to 2017, a primary focus of the research on FUS for BBB opening was identifying acoustic parameters that minimize visible red blood cell (RBC) extravasation, as assessed by histological analyses in mice, rats and rabbits [36,49–54]. In recent years, studies, primarily in rats, have begun to address FUS-induced CNS inflammation in more detail, over time periods between 24h and 6 weeks post-ultrasound treatment and there have been several reviews and discussions of sterile inflammation as a response to BBB opening [55]. An area that requires further investigation, however, is the relationship between inflammation and the acoustic exposure parameters. There has been considerable investigation of how the selection of acoustic parameters affects the degree of BBB permeabilisation and how this relates to extravasation of differently sized molecules [51,56], but it remains to be examined whether there is a corresponding modulation of sterile inflammatory effects.
Mechanisms.
FUS+MB exposure has been shown to permeabilize the blood brain barrier through the disruption of tight junction protein complexes between endothelial cells – thought to be facilitated by oscillating MB along the endothelial surfaces [57,58]. Localized disruption allows blood-borne components such as circulating therapeutics or albumin to diffuse into the brain parenchyma. In addition to the formation of paracellular holes, neurovascular units may also be stimulated by the oscillating MBs. In rodent studies, this has been shown to stimulate a neuroinflammatory cascade, which upregulates the expression of chemokines, cytokines, and other relevant trophic factors [59–61] (Figure 2).
Figure 2. Biological effects of FUS+MB via disruption of the BBB.
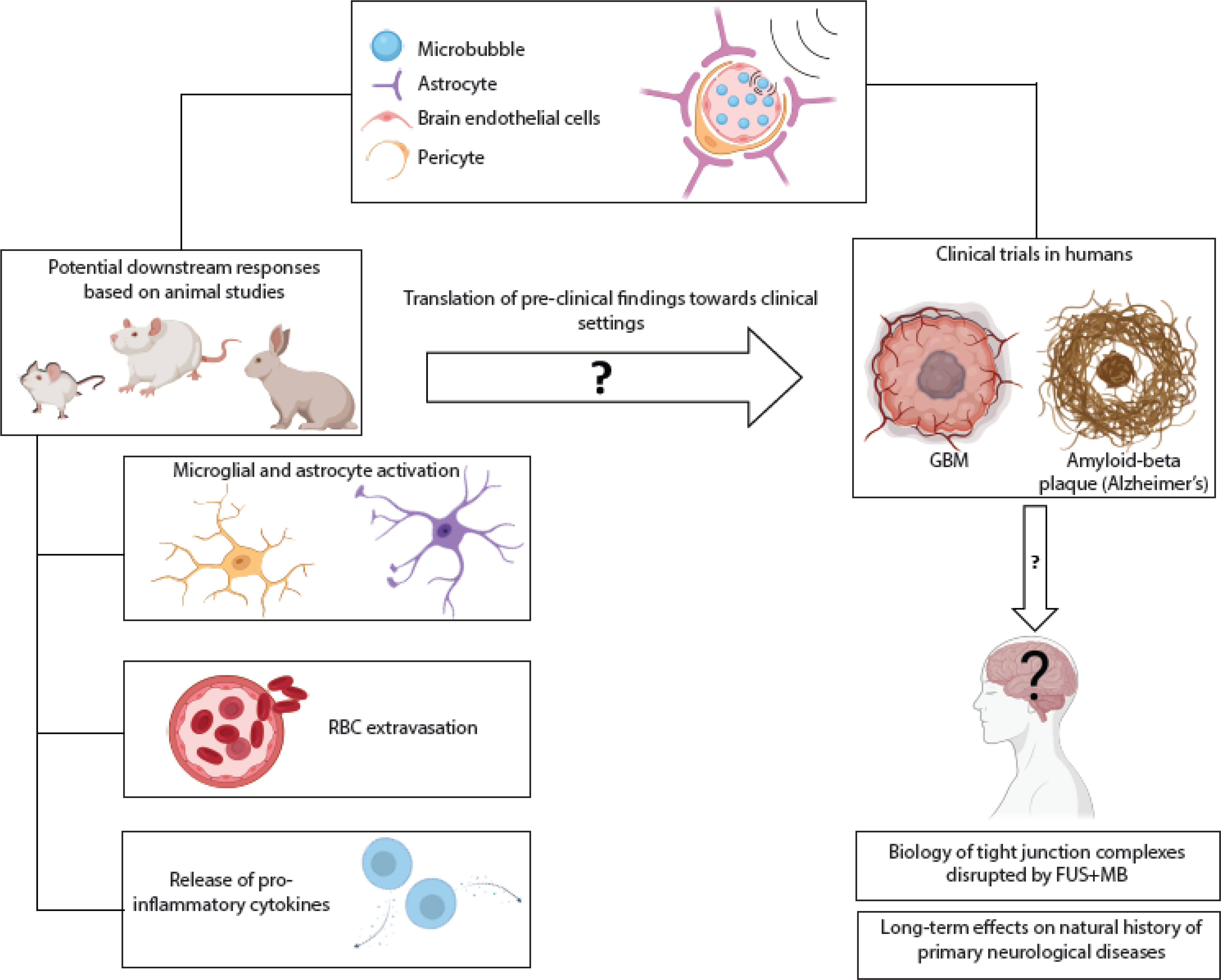
The combination of FUS+MB has shown considerable promise as a drug delivery strategy but further understanding of the downstream effects is required. Depending on the ultrasound exposure conditions, a number of biological effects have been observed in preclinical rodent models, including: activation of microglia and astrocytes increasing with increasing acoustic pressure [60]; extravasation of RBCs, which can be minimized through appropriate adjustment of acoustic parameters and/or MB size [51, 56]; and release of cytokines in brain regions contralateral to the hemisphere treated with FUS+MB [46]. It has yet to be seen if these effects occur in humans. To date, clinical studies of FUS+MB applications have focused on treatment of GBM iv, vi, dissolution of protein aggregates in Alzheimer’s disease ii [7], and alleviation of symptoms in Parkinson’s disease iii, xi [8]. Further work is needed to examine potential longer-term biological effects, particularly as the range of clinical application is broadened, and clinical trials involve repeated treatments or younger populations ix, xiii, xiv,xv. Abbreviations: FUS: focused ultrasound; MB: microbubbles; RBC: red blood cell; GBM: glioblastoma. Image created through Biorender.
Several studies have suggested that permanent tissue damage is avoidable when the appropriate ultrasound settings and MB dose are used (Table S1). In view of these findings, subject-specific, pre-operative planning should be considered as a possible path to reduce tissue damage. In addition, active monitoring of MB response allows potential real-time feedback and control of the treatment by modifying the peak negative pressure and/or pulsing regime of the FUS, as exemplified in a recent preclinical study in non-human primates [62]. However, even when using the minimum acoustic settings to cause BBB permeabilization, it is conceivable that a sterile inflammatory response can still occur, although this requires further investigation. Studies in rats have shown that sterile inflammation following FUS-mediated BBB permeabilization is mediated through the NFkB pathway, with evidence of endothelial activation (high ICAM-1 expression) and a cytokine cascade including production of tumor necrosis factor, a potent inflammatory cytokine, elevated even at 24 hours post sonication [63]. There have been studies looking at providing prophylactic treatment (i.e., anti-integrin α4β1/VLA-4) to mitigate possible immune infiltration or responses [64–66], but this has not been investigated specifically in the context of FUS+MB treatment for CNS diseases.
In recent years, significant efforts have been made to understand the mechanisms underpinning observable bioeffects following BBB opening at the cellular and molecular levels [67–69]. In particular, there have been several studies investigating specific immunomodulatory pathways [46,60,61,63,70] in microglia and astrocytes. Interestingly, there has been less investigation of the role played by endothelial cells and pericytes in potentially inducing the inflammatory cascade post-FUS+MB treatment. This is despite evidence suggesting that these cells and their interactions are critical to the process [71–76]. Better understanding of the initial physiological responses produced by endothelial cells and pericytes (with and without astrocyte and microglia activity) will be critical in assessing the cell-type specific effects of FUS+MB as well as the cell-cell crosstalk that ultimately generates tissue-level neuroinflammation.
It has also been shown that in rats, an innate immune response can be activated for up to 6 days after FUS+MB exposure, as evidenced by infiltration of CD68+ monocytes/macrophages [46]. Infiltration of the CNS by peripheral monocytes/macrophages is a hallmark of tissue damage that cannot be managed through microglial activation alone, and can be indicative of impending fibrosis [77], and potentially long-term implications. Even in cases where BBB integrity is restored within 24 hours post-sonication, the neuroinflammatory response does not always subside [70,78]. Additionally, FUS+MB exposure has been shown to reduce P-glycoprotein (Pgp) (encoded by the ABCB1 gene) expression; this may allow for increased retention of therapeutics in the parenchyma, which could have immediate therapeutic benefits, but the downstream physiological effects should be further investigated, as Pgp expression and regulation are closely associated with pro-inflammatory and anti-inflammatory cytokine expression and release. [79,80]. Additionally, while there have been preliminary studies showing that FUS does not necessarily lead to tight junction complex damage [81], it has yet to be seen how non-homeostatic changes to the microenvironment may induce or facilitate biological changes in the integrity of the tight junctions or cellular membranes.
Clinical studies of the neuroinflammatory effects from FUS+MB treatment.
Table 1 presents an overview of recent clinical trials using FUS+MB in a range of CNS conditions, together with details of any inflammatory (or anti-inflammatory) pathways, where these are explicitly mentioned. In non-neurological conditions, FUS+MB exposure has been shown to stimulate immune responses that may be beneficial e.g. in metastatic cancer; or in other cases, to directly induce anti-inflammatory effects at the target site [82–85]. Recent studies have also shown that immunomodulation can be successfully used to treat GBM; and that FUS+MB can be effective in inducing targeted immune effects and to deliver immunotherapeutics with promising results [86,87]. It has yet to be determined, however, whether an immunomodulatory approach is appropriate for treatment of non-oncological CNS diseases [88–94]. While most current clinical trials report no significant inflammation post-FUS+MB treatment (Table 1), the evaluation of potential neuroinflammation in many of these studies is limited, lacking for instance molecular biomarker data in regard to cytokine levels in the cerebrospinal fluid (CSF) or tissue biopsies.
Table 1.
Overview of clinical trials using FUS+MB for the treatment or diagnosis of CNS disease.
| Clinical trial (NIH reference) | Clinical trial phase | Condition of interest | Study description & role of inflammation as modulator or side effect |
|---|---|---|---|
| NCT02343991 vi | Phase not applicable | Brain tumors | Evaluate whether FUS can increase passage of tumor-specific biomarkers into the vasculature, improving the quality of liquid biopsy [106] |
| NCT02253212 iv | Phase III | Glioblastoma (recurrent) | Evaluate BBB opening tolerated by patients before delivery of chemotherapeutics; discusses anticancer immune response in the context of other organ-specific cancers (e.g., breast cancer) and studies in other species (e.g., mouse models) in [107] |
| NCT02986932 ii | Phase I | Alzheimer’s disease | Reduction of pathological protein aggregate in AD; no mention of inflammation as modulator or in post-treatment evaluation [7] |
| NCT03321487 i | Phase not applicable | Amyotrophic lateral sclerosis | Evaluation of BBB opening in primary motor cortex; MRI imaging show transient disruption via gadolinium perfusion [6]; the authors reported no significant inflammation 30 days post-procedure. |
| NCT03551249 vii | Phase not applicable | Glioma | Establishing safety profile for patients using FUS+MB as first line of therapy (standard chemotherapy); no mention of inflammation as immunomodulator for glioma treatment. |
| NCT03608553 iii | Phase I | Parkinson’s disease dementia | Performed BBB opening in parieto-occipito-temporal regions of the patients’ brains; no adverse effects reported [8]; no mention of inflammation |
| NCT03616860 viii | Phase I | Glioma | Evaluating FUS+MB to increase quality of liquid biopsy via increasing tumor biomarker perfusion into vasculature through transient BBB opening [108]; no mention of inflammation as possible modulator |
| NCT03671889 v | Phase II | Alzheimer’s disease | Evaluation of focal, transient BBB opening in the hippocampus; found indications of perivenous blood-meningeal permeability post-barrier disruption which may be indicative of tissue healing process (in the context of inflammation) [100] |
| NCT03782194 ix | Phase not applicable | Anxiety, obsessive compulsive disorder, posttraumatic stress disorder | Investigate whether usage of FUS pulsation can influence amygdala function to improve emotion regulation |
| NCT04118764 x | Phase not applicable | Alzheimer’s disease | Prospective study done with non-human primates in which eosinophil count increased; low acoustic pressure leads to minimal inflammatory cell density [109] |
| NCT04370665 xi | Phase not applicable | Parkinson’s disease | Delivering imiglucerase using Exablate MRgFUS system and Definity to open the BBB; no mention of inflammation |
| NCT04526262 xii | Phase not applicable | Alzheimer’s disease | Evaluated plaque removal and cognitive functions post-FUS+MB treatment (repeated opening) [110]; no mention of inflammation specific to the study |
| NCT04620460 xiii | Phase not applicable | Schizophrenia | Investigate whether FUS pulsation can modulate cortical function; no mention of immunomodulation as mechanistic target |
| NCT04804709 xiv | Phase I | Progressive diffuse midline glioma (DMG) | Evaluate whether FUS+MB delivery of Panobinostat through transient BBB opening is safe (phase I); no discussion on immunomodulation as possible mechanism |
| NCT05089786 xv | Phase II | Treatment-resistant neurologic and psychiatric indications | To evaluate whether FUS can improve clinical measurements in neurological and psychiatric disorders; no discussion of inflammation |
Strategies for mitigating sterile inflammation in ultrasound-mediated therapy
As mentioned earlier, the use of FUS+MB has shown promising results for treatment of glioblastoma [95–97]. For as long as uncertainties remain over its long-term safety, however, the case for using FUS+MB in non-terminal CNS conditions is less clear [98]. There have been several studies looking at the immediate and short-term consequences of FUS treatment in humans, but these studies have focused primarily on functional measures designed to observe whether there were rises in biomarkers of concern [99,100]. To the best of the authors’ knowledge, long-term follow up studies of the treated patients are still lacking. Such studies are inevitably difficult to perform, due to the complexity of neurological diseases and desegregation of confounding factors that may influence interpretation of clinical data. Examining long-term, post-procedure effects in animal models could provide one step towards addressing these complex issues.
There are already data showing that ultrasound-induced BBB disruption can induce inflammatory responses even at low acoustic intensities [46,61,101,102] and ultimately, the clinical applicability of FUS+MB is dependent upon understanding the underpinning mechanisms and the immediate, as well as long-term effects, of both single and multiple FUS+MB treatments. For example, it is critical to determine whether repeated treatment can produce adverse effects unrelated to the natural history of the neurological disease being treated. This is particularly important when identifying treatments for genetic and hereditary disorders in which many in the diagnosed population are pediatric patients.
Non-mechanical modulation of the BBB has also been shown to induce neuroinflammatory effects, indicating these are not specific to FUS+MB. For example, the use of d-mannitol, an osmotic agent that has been widely used for modulating intracranial pressure, has been reported to increase pro-inflammatory cytokines [103]. The opening of adjacent endothelial cells can induce a response from both astrocytes and microglial cells [60], such as a cascade of chemokines that encourage homing and chemotaxis of peripheral immune cells that are circulating in the neurovasculature, especially near the meninges [98]. A potential advantage of FUS+MB over d-mannitol is that the effects of FUS+MB can be much more easily localized to specific regions of the brain and their corresponding vasculature.
A key consideration for the use of FUS+MB in neurological disorders is the degree to which adverse reactions post-procedure can present and whether there are pre- or post-operative measures to minimize such effects. Some studies have shown that corrective measures can be taken post-treatment to inhibit an immune response using drugs such as dexamethasone [104]. In a similar manner, another group recently reported that the type of anesthetic used prior to FUS+MB disruption of the BBB can influence gene expression in the brain [105]. Such differences may not have immediate implications post-procedure but are likely to be critical in understanding how cells respond long after the initial acute disruption of the BBB.
Concluding remarks and future perspectives
This review has sought to examine the current literature on the role of the BBB in mediating sterile inflammation following exposure to FUS+MB. The number of studies that have evaluated sterile inflammation associated with FUS+MB, either in vitro or in vivo, is relatively small; and while the technology shows great promise, there is a need to accelerate our understanding of the downstream physiological responses (Figure 2). This need is becoming increasingly pressing as the range of applications for FUS+MB mediated BBB permeabilization increases and is extended into non-terminal conditions. Further work is needed to elucidate the pathways associated with such reactive inflammatory responses when the BBB is disrupted (see Outstanding Questions). Addressing this knowledge gap will hopefully encourage further discourse on potential improvements to FUS+MB-mediated treatments for neurological conditions, to maximize their benefit-risk ratio.
Outstanding Questions.
Can some of the hallmarks of neurological disease be attributed to neurovascular dysfunction as much as to neuronal dysregulation?
What are the biological effects of mechanical modulation of the BBB produced by focused ultrasound (FUS) and microbubbles (MB)?
Clinically, FUS+MB have so far been applied primarily as a treatment for terminal conditions such as glioblastoma. If, however, they are applied in the future to non-terminal CNS diseases, what are the potential long-term adverse effects that should be considered by clinicians and researchers?
How would a course of several FUS+MB treatments affect the long-term integrity of the BBB?
Should there be a strategic algorithm or pipeline in place for determining appropriate use of FUS+MB?
How can the potential adverse effects of neuroinflammation arising from FUS+MB disruption of the BBB be minimized? Can pre- or post-operative strategies be developed to contain or mitigate such effects?
Supplementary Material
Highlights.
The blood-brain barrier plays both a physical and a physiological “gate keeping” role in maintaining brain homeostasis.
In recent years, there has been increasing interest in understanding the role of the blood-brain barrier in neurological disorders that were traditionally considered to be neuron-centric, for instance Parkinson’s and Alzheimer’s disease.
Alternative drug delivery techniques, such as focused ultrasound (FUS), are emerging as powerful tools to bypass the blood-brain barrier and facilitate treatment of neurological conditions.
To enable widespread clinical use of these techniques, there is an urgent need to investigate and address the associated safety concerns, for example, the consequences of sterile inflammation that may be induced by barrier disruption.
Acknowledgement
The authors would like to acknowledge BioRender for providing the templates to create the figures provided in the main text. OJ and MF are supported by the National Center for Advancing Translational Sciences. SRB and JAF are supported by the Clinical Center; JAF is supported by the National Institute of Biomedical Imaging and Bioengineering. MLD is supported by the Kennedy Trust for Rheumatology Research.
Resources
Declaration of interests
Authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Feigin VL, Abajobir AA, Abate KH, Abd-Allah F, Abdulle AM, Abera SF, Abyu GY, Ahmed MB, Aichour AN, Aichour I, Aichour MTE, Akinyemi RO, Alabed S, Al-Raddadi R, Alvis-Guzman N, Amare AT, Ansari H, Anwari P, Ärnlöv J, Asayesh H, Asgedom SW, Atey TM, Avila-Burgos L, Frinel E, Avokpaho GA, Azarpazhooh MR, Barac A, Barboza M, Barker-Collo SL, Bärnighausen T, Bedi N, Beghi E, Bennett DA, Bensenor IM, Berhane A, Betsu BD, Bhaumik S, Birlik SM, Biryukov S, Boneya DJ, Bulto LNB, Carabin H, Casey D, Castañeda-Orjuela CA, Catalá-López F, Chen H, Chitheer AA, Chowdhury R, Christensen H, Dandona L, Dandona R, Veber G A de, Dharmaratne SD, Do HP, Dokova K, Dorsey ER, Ellenbogen RG, Eskandarieh S, Farvid MS, Fereshtehnejad S-M, Fischer F, Foreman KJ, Geleijnse JM, Gillum RF, Giussani G, Goldberg EM, Gona PN, Goulart AC, Gugnani HC, Gupta R, Hachinski V, Gupta R, Hamadeh RR, Hambisa M, Hankey GJ, Hareri HA, Havmoeller R, Hay SI, Heydarpour P, Hotez PJ, Jakovljevic M, (Michael) B, Javanbakht M, Jeemon P, Jonas JB, Kalkonde Y, Kandel A, Karch A, Kasaeian A, Kastor A, Keiyoro PN, Khader YS, Khalil IA, Khan EA, Khang Y-H, Tawfih A, Khoja A, Khubchandani J, Kulkarni C, et al. 2017. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015 Lancet Neurol. 16 877–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pound P and Ram R 2020. Are researchers moving away from animal models as a result of poor clinical translation in the field of stroke? An analysis of opinion papers BMJ Open Sci. 4 e100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cummings J 2018. Lessons Learned from Alzheimer Disease: Clinical Trials with Negative Outcomes Clin. Transl. Sci. 11 147–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].O’Brown NM, Pfau SJ and Gu C 2018. Bridging barriers: a comparative look at the blood–brain barrier across organisms Genes Dev. 32 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Danon JJ, Reekie TA and Kassiou M 2019. Challenges and Opportunities in Central Nervous System Drug Discovery Trends Chem. 1 612–24 [Google Scholar]
- [6].Abrahao A, Meng Y, Llinas M, Huang Y, Hamani C, Mainprize T, Aubert I, Heyn C, Black SE, Hynynen K, Lipsman N and Zinman L 2019. First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound Nat. Commun. 10 4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, Herrmann N, Heyn C, Aubert I, Boutet A, Smith GS, Hynynen K and Black SE 2018. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound Nat. Commun.9 2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gasca-Salas C, Fernández-Rodríguez B, Pineda-Pardo JA, Rodríguez-Rojas R, Obeso I, Hernández-Fernández F, del Álamo M, Mata D, Guida P, Ordás-Bandera C, Montero-Roblas JI, Martínez-Fernández R, Foffani G, Rachmilevitch I and Obeso JA 2021. Blood-brain barrier opening with focused ultrasound in Parkinson’s disease dementia Nat. Commun. 12 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Abbott NJ, Rönnbäck L and Hansson E 2006. Astrocyte-endothelial interactions at the blood-brain barrier Nat. Rev. Neurosci. 7 41–53 [DOI] [PubMed] [Google Scholar]
- [10].Song K, Li Y, Zhang H, An N, Wei Y, Wang L, Tian C, Yuan M, Sun Y, Xing Y, Gao Y and Santibañez JF 2020 Oxidative Stress-Mediated Blood-Brain Barrier (BBB) Disruption in Neurological Diseases Oxid. Med. Cell. Longev. 2020 [Google Scholar]
- [11].Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M and Zlokovic BV. 2015. Blood-brain barrier breakdown in the aging human hippocampus Neuron 85 296–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, Harrington MG, Pa J, Law M, Wang DJJ, Jacobs RE, Doubal FN, Ramirez J, Black SE, Nedergaard M, Benveniste H, Dichgans M, Iadecola C, Love S, Bath PM, Markus HS, Salman RA, Allan SM, Quinn TJ, Kalaria RN, Werring DJ, Carare RO, Touyz RM, Williams SCR, Moskowitz MA, Katusic ZS, Lutz SE, Lazarov O, Minshall RD, Rehman J, Davis TP, Wellington CL, González HM, Yuan C, Lockhart SN, Hughes TM, Chen CLH, Sachdev P, O’Brien JT, Skoog I, Pantoni L, Gustafson DR, Biessels GJ, Wallin A, Smith EE, Mok V, Wong A, Passmore P, Barkof F, Muller M, Breteler MMB, Román GC, Hamel E, Seshadri S, Gottesman RF, van Buchem M A, Arvanitakis Z, Schneider JA, Drewes LR, Hachinski V, Finch CE, Toga AW, Wardlaw JM and Zlokovic BV. 2019. Vascular dysfunction-The disregarded partner of Alzheimer’s disease Alzheimers. Dement. 15 158–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nation DA, Sweeney MD, Montagne A, Sagare AP, D’Orazio LM, Pachicano M, Sepehrband F, Nelson AR, Buennagel DP, Harrington MG, Benzinger TLS, Fagan AM, Ringman JM, Schneider LS, Morris JC, Chui HC, Law M, Toga AW and Zlokovic BV. 2019 Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction Nat. Med. 2019. 252 25 270–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shimizu F, Nishihara H and Kanda T 2018. Blood–brain barrier dysfunction in immuno-mediated neurological diseases Immunol. Med. 41 120–8 [DOI] [PubMed] [Google Scholar]
- [15].Spencer JI, Bell JS and DeLuca GC 2018. Vascular pathology in multiple sclerosis: reframing pathogenesis around the blood-brain barrier J. Neurol. Neurosurg. Psychiatry 89 42–52 [DOI] [PubMed] [Google Scholar]
- [16].Jeyakumar M, Thomas R, Elliot-Smith E, Smith DA, Van der Spoel AC, D’Azzo A, Perry VH, Butters TD, Dwek RA and Platt FM 2003. Central nervous system inflammation is a hallmark of pathogenesis in mouse models of GM1 and GM2 gangliosidosis Brain 126 974–87 [DOI] [PubMed] [Google Scholar]
- [17].Begley D, Pontikis C and Scarpa M 2008. Lysosomal storage diseases and the blood-brain barrier Curr. Pharm. Des. 14 1566–80 [DOI] [PubMed] [Google Scholar]
- [18].Bellettato CM and Scarpa M 2018. Possible strategies to cross the blood-brain barrier Ital. J. Pediatr 44 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Edelmann MJ and Maegawa GHB 2020. CNS-Targeting Therapies for Lysosomal Storage Diseases: Current Advances and Challenges Front. Mol. Biosci. 7 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Profaci CP, Munji RN, Pulido RS and Daneman R 2020. The blood–brain barrier in health and disease: Important unanswered questions J. Exp. Med. 217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ivanidze J, Skafida M, Pandya S, Patel D, Osborne JR, Raj A, Gupta A, Henchcliffe C and Dyke JP 2020. Molecular Imaging of Striatal Dopaminergic Neuronal Loss and the Neurovascular Unit in Parkinson Disease Front. Neurosci. 14 528809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lochhead JJ, Yang J, Ronaldson PT and Davis TP 2020. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders Front. Physiol. 11 914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Elabi O, Gaceb A, Carlsson R, Padel T, Soylu-Kucharz R, Cortijo I, Li W, Li J-Y and Paul G 2021 Human α-synuclein overexpression in a mouse model of Parkinson’s disease leads to vascular pathology, blood brain barrier leakage and pericyte activation Sci. Reports 2021. 111 11 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lin Z, Sur S, Liu P, Li Y, Jiang D, Hou X, Darrow J, Pillai JJ, Yasar S, Rosenberg P, Albert M, Moghekar A and Lu H 2021. Blood–Brain Barrier Breakdown in Relationship to Alzheimer and Vascular Disease Ann. Neurol. 90 227–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Singhrao SK and Harding A 2020. Is Alzheimer’s disease a polymicrobial host microbiome dysbiosis? Expert Rev. Anti. Infect. Ther. 18 275–7 [DOI] [PubMed] [Google Scholar]
- [26].Vigasova D, Nemergut M, Liskova B and Damborsky J 2021. Multi-pathogen infections and Alzheimer’s disease Microb. Cell Factories 2021 201 20 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Poole S, Singhrao SK, Kesavalu L, Curtis MA and Crean S 2013. Determining the Presence of Periodontopathic Virulence Factors in Short-Term Postmortem Alzheimer’s Disease Brain Tissue J. Alzheimer’s Dis. 36 665–77 [DOI] [PubMed] [Google Scholar]
- [28].Carrasco L, Alonso R, Pisa D and Rabano A 2017. Alzheimer’s Disease and Fungal Infection Handbook of Infection and Alzheimer’s Disease vol 5, ed Miklossy J (Amsterdam: IOS Press; ) pp 281–94 [Google Scholar]
- [29].Kipnis J and Filiano AJ 2017 The central nervous system: privileged by immune connections Nat. Rev. Immunol. 2017. 182 18 83–4 [DOI] [PubMed] [Google Scholar]
- [30].Storelli F, Billington S, Kumar AR and Unadkat JD 2021. Abundance of P-Glycoprotein and Other Drug Transporters at the Human Blood-Brain Barrier in Alzheimer’s Disease: A Quantitative Targeted Proteomic Study Clin. Pharmacol. Ther. 109 667–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Anon 2000. Section 6—Mechanical Bioeffects in the Presence of Gas-Carrier Ultrasound Contrast Agents J. Ultrasound Med. 19 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Coussios CC, Farny CH, Ter Haar G and Roy RA 2007. Role of acoustic cavitation in the delivery and monitoring of cancer treatment by high-intensity focused ultrasound (HIFU) Int. J. Hyperthermia 23 105–20 [DOI] [PubMed] [Google Scholar]
- [33].Hynynen K and Jolesz FA 1998. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull Ultrasound Med. Biol. 24 275–83 [DOI] [PubMed] [Google Scholar]
- [34].Hynynen K, McDannold N, Vykhodtseva N and Jolesz FA 2001. Noninvasive MR Imaging–guided Focal Opening of the Blood-Brain Barrier in Rabbits Radiology 220 640–6 [DOI] [PubMed] [Google Scholar]
- [35].Hynynen K, McDannold N, Martin H, Jolesz FA and Vykhodtseva N 2003. The threshold for brain damage in rabbits induced by bursts of ultrasound in the presence of an ultrasound contrast agent (Optison) Ultrasound Med. Biol. 29 473–81 [DOI] [PubMed] [Google Scholar]
- [36].McDannold N, Vykhodtseva N, Raymond S, Jolesz FA and Hynynen K 2005. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits Ultrasound Med. Biol. 31 1527–37 [DOI] [PubMed] [Google Scholar]
- [37].Hynynen K, McDannold N, Sheikov NA, Jolesz FA and Vykhodtseva N 2005. Local and reversible blood–brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications Neuroimage 24 12–20 [DOI] [PubMed] [Google Scholar]
- [38].Sheikov N, McDannold N, Vykhodtseva N, Jolesz F and Hynynen K 2004. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles Ultrasound Med. Biol. 30 979–89 [DOI] [PubMed] [Google Scholar]
- [39].Liu Y, Gong Y, Xie W, Huang A, Yuan X, Zhou H, Zhu X, Chen X, Liu J, Liu J and Qin X 2020. Microbubbles in combination with focused ultrasound for the delivery of quercetin-modified sulfur nanoparticles through the blood brain barrier into the brain parenchyma and relief of endoplasmic reticulum stress to treat Alzheimer’s disease Nanoscale 12 6498–511 [DOI] [PubMed] [Google Scholar]
- [40].Wang F, Wei X-X, Chang L-S, Dong L, Wang Y-L and Li N-N 2021. Ultrasound Combined With Microbubbles Loading BDNF Retrovirus to Open Blood–Brain Barrier for Treatment of Alzheimer’s Disease Front. Pharmacol. 12 615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang N, Yan F, Liang X, Wu M, Shen Y, Chen M, Xu Y, Zou G, Jiang P, Tang C, Zheng H and Dai Z 2018. Localized delivery of curcumin into brain with polysorbate 80-modified cerasomes by ultrasound-targeted microbubble destruction for improved Parkinson’s disease therapy Theranostics 8 2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lin CY, Lin YC, Huang CY, Wu SR, Chen CM and Liu HL 2020. Ultrasound-responsive neurotrophic factor-loaded microbubble- liposome complex: Preclinical investigation for Parkinson’s disease treatment J. Control. Release 321 519–28 [DOI] [PubMed] [Google Scholar]
- [43].Hsu Y-H, Liu R-S, Lin W-L, Yuh Y-S, Lin S-P and Wong T-T 2017 Transcranial pulsed ultrasound facilitates brain uptake of laronidase in enzyme replacement therapy for Mucopolysaccharidosis type I disease Orphanet J. Rare Dis. 2017. 121 12 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mancia L, Vlaisavljevich E, Yousefi N, Rodriguez M, Ziemlewicz TJ, Lee FT, Henann D, Franck C, Xu Z and Johnsen E 2019. Modeling tissue-selective cavitation damage Phys. Med. Biol. 64 225001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Miller MW, Miller DL and Brayman AA 1996. A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective Ultrasound Med. Biol. 22 1131–54 [DOI] [PubMed] [Google Scholar]
- [46].Kovacs ZI, Kim S, Jikaria N, Qureshi F, Milo B, Lewis BK, Bresler M, Burks SR and Frank JA 2017. Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation Proc. Natl. Acad. Sci. 114 E75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Joiner JB, Pylayeva-Gupta Y and Dayton PA 2020. Focused Ultrasound for Immunomodulation of the Tumor Microenvironment J. Immunol. 205 2327–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cohen-Inbar O, Xu Z and Sheehan JP 2016. Focused ultrasound-aided immunomodulation in glioblastoma multiforme: A therapeutic concept J. Ther. Ultrasound 4 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hynynen K, McDannold N, Vykhodtseva N, Raymond S, Weissleder R, Jolesz FA and Sheikov N 2006. Focal disruption of the blood-brain barrier due to 260-kHz ultrasound bursts: a method for molecular imaging and targeted drug delivery J. Neurosurg. 105 445–54 [DOI] [PubMed] [Google Scholar]
- [50].Choi JJ, Wang S, Brown TR, Small SA, Duff KEK and Konofagou EE 2008. Noninvasive and transient blood-brain barrier opening in the hippocampus of Alzheimer’s double transgenic mice using focused ultrasound Ultrason. Imaging 30 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Choi JJ, Feshitan JA, Baseri B, Wang S, Tung YS, Borden MA and Konofagou EE 2010. Microbubble-size dependence of focused ultrasound-induced bloodBrain barrier opening in mice in vivo IEEE Trans. Biomed. Eng. 57 145–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jordão JF, Ayala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin JA, Hynynen K and Aubert I 2010. Antibodies Targeted to the Brain with Image-Guided Focused Ultrasound Reduces Amyloid-β Plaque Load in the TgCRND8 Mouse Model of Alzheimer’s Disease PLoS One 5 e10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Burgess A, Ayala-Grosso CA, Ganguly M, Jordão JF, Aubert I and Hynynen K 2011. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier PLoS One 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tung Y-S, Vlachos F, Feshitan JA, Borden MA and Konofagou EE 2011. The mechanism of interaction between focused ultrasound and microbubbles in blood-brain barrier opening in mice J. Acoust. Soc. Am. 130 3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Todd N, Angolano C, Ferran C, Devor A, Borsook D and McDannold N 2020. Secondary effects on brain physiology caused by focused ultrasound-mediated disruption of the blood–brain barrier J. Control. Release 324 450–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Chen H and Konofagou EE 2014. The size of blood-brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure J. Cereb. Blood Flow Metab. 34 1197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sirsi S and Borden M 2009. Microbubble Compositions, Properties and Biomedical Applications Bubble Sci. Eng. Technol. 1 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Beekers I, Vegter M, Lattwein KR, Mastik F, Beurskens R, van der Steen AFW, de Jong N, Verweij MD and Kooiman K 2020. Opening of endothelial cell–cell contacts due to sonoporation J. Control. Release 322 426–38 [DOI] [PubMed] [Google Scholar]
- [59].McMahon D, Lassus A, Gaud E, Jeannot V and Hynynen K 2020. Microbubble formulation influences inflammatory response to focused ultrasound exposure in the brain Sci. Reports 101 10 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Pascal A, Li N, Lechtenberg KJ, Rosenberg J, Airan RD, James ML, Bouley DM and Pauly KB 2020. Histologic evaluation of activation of acute inflammatory response in a mouse model following ultrasound-mediated blood-brain barrier using different acoustic pressures and microbubble doses Nanotheranostics 4 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kovacs ZI, Burks SR and Frank JA 2018. Focused ultrasound with microbubbles induces sterile inflammatory response proportional to the blood brain barrier opening: Attention to experimental conditions Theranostics 8 8 2245–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kamimura HA, Flament J, Valette J, Cafarelli A, Badin RA, Hantraye P and Larrat B 2018. Feedback control of microbubble cavitation for ultrasound-mediated blood–brain barrier disruption in non-human primates under magnetic resonance guidance: J. Cereb. Blood Flow Metab. 39 1191–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].McMahon D and Hynynen K 2017. Acute inflammatory response following increased blood-brain barrier permeability induced by focused ultrasound is dependent on microbubble dose Theranostics 7 3989–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Breuer J, Korpos E, Hannocks M-J, Schneider-Hohendorf T, Song J, Zondler L, Herich S, Flanagan K, Korn T, Zarbock A, Kuhlmann T, Sorokin L, Wiendl H and Schwab N 2018. Blockade of MCAM/CD146 impedes CNS infiltration of T cells over the choroid plexus J. Neuroinflammation 151 15 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jurberg AD, Chaves B, Pinho LG, Silva J H M da, Savino W and Cotta-de-Almeida V 2021. VLA-4 as a Central Target for Modulating Neuroinflammatory Disorders Neuroimmunomodulation 28 213–21 [DOI] [PubMed] [Google Scholar]
- [66].Savino W, Chaves B, Bonomo AC and Cotta-de-Almeida V 2021. Integrin-directed antibody-based immunotherapy: focus on VLA-4 Immunother. Adv. 1 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sheikov N, McDannold N, Sharma S and Hynynen K 2008. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium Ultrasound Med. Biol. 34 1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Chang JW, Wu MT, Song WS and Yang FY 2020. Ultrasound Stimulation Suppresses LPS-Induced Proinflammatory Responses by Regulating NF-κB and CREB Activation in Microglial Cells Cereb. Cortex 30 4597–606 [DOI] [PubMed] [Google Scholar]
- [69].Chen S, Nazeri A, Baek H, Ye D, Yang Y, Yuan J, Rubin JB and Chen H 2022. A review of bioeffects induced by focused ultrasound combined with microbubbles on the neurovascular unit J. Cereb. Blood Flow Metab. 42 3–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sinharay S, Tu T-W, Kovacs ZI, Schreiber-Stainthorp W, SUndby M, Zhang X, Papadakis GZ, Reid WC, Frank JA and Hammoud DA 2019. In vivo imaging of sterile microglial activation in rat brain after disrupting the blood-brain barrier with pulsed focused ultrasound: [18F]DPA-714 PET study J. Neuroinflammation 16 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Smyth LCD, Rustenhoven J, Park TIH, Schweder P, Jansson D, Heppner PA, O’Carroll SJ, Mee EW, Faull RLM, Curtis M and Dragunow M 2018. Unique and shared inflammatory profiles of human brain endothelia and pericytes J. Neuroinflammation 15 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yamamoto S, Niida S, Azuma E, Yanagibashi T, Muramatsu M, Huang TT, Sagara H, Higaki S, Ikutani M, Nagai Y, Takatsu K, Miyazaki K, Hamashima T, Mori H, Matsuda N, Ishii Y and Sasahara M 2015. Inflammation-induced endothelial cell-derived extracellular vesicles modulate the cellular status of pericytes Sci. Reports 2015 51 5 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hurtado-Alvarado G, Cabañas-Morales AM and Gómez-Gónzalez B 2014. Pericytes: Brain-immune interface modulators Front. Integr. Neurosci. 7 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jeong AK, Tran ND, Li Z, Yang F, Zhou W and Fisher MJ 2006. Brain endothelial hemostasis regulation by pericytes J. Cereb. Blood Flow Metab. 26 209–17 [DOI] [PubMed] [Google Scholar]
- [75].Rustenhoven J, Jansson D, Smyth LC and Dragunow M 2017. Brain Pericytes As Mediators of Neuroinflammation Trends Pharmacol. Sci. 38 291–304 [DOI] [PubMed] [Google Scholar]
- [76].Rudziak P, Ellis CG and Kowalewska PM 2019 Role and molecular mechanisms of pericytes in regulation of leukocyte diapedesis in inflamed tissues Mediators Inflamm. 2019. 4123605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Dorrier CE, Aran D, Haenelt EA, Sheehy RN, Hoi KK, Pintarić L, Chen Y, Lizama CO, Cautivo KM, Weiner GA, Popko B, Fancy SPJ, Arnold TD and Daneman R 2021. CNS fibroblasts form a fibrotic scar in response to immune cell infiltration Nat. Neurosci. 2021 242 24 234–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Zhao R, Jiang J, Li H, Chen M, Liu R, Sun S, Ma D, Liang X and Wang S 2018. Phosphatidylserine-microbubble targeting-activated microglia/macrophage in inflammation combined with ultrasound for breaking through the blood-brain barrier J. Neuroinflammation 1 15 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Aryal M, Fischer K, Gentile C, Gitto S, Zhang Y-Z and Mcdannold N 2017. Effects on P-Glycoprotein Expression after Blood-Brain Barrier Disruption Using Focused Ultrasound and Microbubbles PLoS One 1 12 e01166061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Torres-Vergara P and Penny J 2018. Pro-inflammatory and anti-inflammatory compounds exert similar effects on P-glycoprotein in blood–brain barrier endothelial cells J. Pharm. Pharmacol. 70 713–22 [DOI] [PubMed] [Google Scholar]
- [81].Kugelman TL, Karakatsani ME, Choi CS, Nimi Y, Agalliu D and Konofagou EE 2020. Safe Focused Ultrasound-Mediated Blood-Brain Barrier Opening and Repair is Not Mediated by Tight Junction Degradation. SSRN: 10.2139/ssrn.3599080 [DOI] [Google Scholar]
- [82].Sheybani ND and Price RJ 2019. Perspectives on Recent Progress in Focused Ultrasound Immunotherapy Theranostics 9 7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Sheybani ND, Witter AR, Thim EA, Yagita H, Bullock TNJ and Price RJ 2020. Combination of thermally ablative focused ultrasound with gemcitabine controls breast cancer via adaptive immunity J. Immunother. Cancer 8 e001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yuan J, Ye D, Chen S and Chen H 2021. Therapeutic Ultrasound-Enhanced Immune Checkpoint Inhibitor Therapy Front. Phys. 9 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Alkins R, Burgess A, Kerbel R, Wels WS and Hynynen K 2016. Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival Neuro. Oncol. 18 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Beccaria K, Sabbagh A, de Groot J, Canney M, Carpentier A and Heimberger AB 2021. Blood–brain barrier opening with low intensity pulsed ultrasound for immune modulation and immune therapeutic delivery to CNS tumors J. Neurooncol. 151 65–73 [DOI] [PubMed] [Google Scholar]
- [87].Malo CS, Khadka RH, Ayasoufi K, Jin F, AbouChehade JE, Hansen MJ, Iezzi R, Pavelko KD and Johnson AJ 2018. Immunomodulation mediated by anti-angiogenic therapy improves CD8 T cell immunity against experimental glioma Front. Oncol. 8 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Dantzer R 2018. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa Physiol. Rev. 98 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bennett FC and Molofsky A V 2019. The immune system and psychiatric disease: a basic science perspective Clin. Exp. Immunol. 197 294–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Tansey MG and Romero-Ramos M 2019. Immune system responses in Parkinson’s disease: Early and dynamic Eur. J. Neurosci. 49 364–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].O’Reilly ML and Tom VJ 2020. Neuroimmune System as a Driving Force for Plasticity Following CNS Injury Front. Cell. Neurosci. 14 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Tchessalova D, Posillico CK and Tronson NC 2018. Neuroimmune activation drives multiple brain states Front. Syst. Neurosci. 12 39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH and Kipnis J 2015. Structural and functional features of central nervous system lymphatic vessels Nat. 2015 5237560 523 337–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J and Reich DS 2017. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI Elife 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Meng Y, Reilly RM, Pezo RC, Trudeau M, Sahgal A, Singnurkar A, Perry J, Myrehaug S, Pople CB, Davidson B, Llinas M, Hyen C, Huang Y, Hamani C, Suppiah S, Hynynen K and Lipsman N 2021. MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases Sci. Transl. Med. 13 4011. [DOI] [PubMed] [Google Scholar]
- [96].Chen K-T, Lin Y-J, Chai W-Y, Lin C-J, Chen P-Y, Huang C-Y, Kuo JS, Liu H-L and Wei K-C 2020. Neuronavigation-guided focused ultrasound (NaviFUS) for transcranial blood-brain barrier opening in recurrent glioblastoma patients: clinical trial protocol Ann. Transl. Med. 8 673–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wei HJ, Upadhyayula PS, Pouliopoulos AN, Englander ZK, Zhang X, Jan CI, Guo J, Mela A, Zhang Z, Wang TJC, Bruce JN, Canoll PD, Feldstein NA, Zacharoulis S, Konofagou EE and Wu CC 2021. Focused Ultrasound-Mediated Blood-Brain Barrier Opening Increases Delivery and Efficacy of Etoposide for Glioblastoma Treatment Int. J. Radiat. Oncol. 110 539–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Poon C, Pellow C and Hynynen K 2021. Neutrophil recruitment and leukocyte response following focused ultrasound and microbubble mediated blood-brain barrier treatments Theranostics 11 1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].D’Haese P-F, Ranjan M, Song A, Haut MW, Carpenter J, Dieb G, Najib U, Wang P, Mehta RI, Chazen JL, Hodder S, Claassen D, Kaplitt M and Rezai AR 2020. β-Amyloid Plaque Reduction in the Hippocampus After Focused Ultrasound-Induced Blood–Brain Barrier Opening in Alzheimer’s Disease Front. Hum. Neurosci. 14 593672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Mehta RI, Carpenter JS, Mehta RI, Haut MW, Ranjan M, Najib U, Lockman P, Wang P, D’haese P-F and Rezai AR 2021. Blood-Brain Barrier Opening with MRI-guided Focused Ultrasound Elicits Meningeal Venous Permeability in Humans with Early Alzheimer Disease Radiology 298 654–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kovacs ZI, Tu T-W, Sundby M, Qureshi F, Lewis BK, Jikaria N, Burks SR and Frank JA 2018. MRI and histological evaluation of pulsed focused ultrasound and microbubbles treatment effects in the brain Theranostics 8 4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ji R, Karakatsani ME, Burgess M, Smith M, Murillo MF and Konofagou EE 2021. Cavitation-modulated inflammatory response following focused ultrasound blood-brain barrier opening J. Control. Release 337 458–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Burks SR, Kersch CN, Witko JA, Pagel MA, Sundby M, Muldoon LL, Neuwelt EA and Frank JA 2021. Blood–brain barrier opening by intracarotid artery hyperosmolar mannitol induces sterile inflammatory and innate immune responses Proc. Natl. Acad. Sci. 118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].McMahon D, Oakden W and Hynynen K 2020. Investigating the effects of dexamethasone on blood-brain barrier permeability and inflammatory response following focused ultrasound and microbubble exposure Theranostics 10 1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Mathew AS, Gorick CM, Thim EA, Garrison WJ, Klibanov AL, Miller GW, Sheybani ND and Price RJ 2021. Transcriptomic response of brain tissue to focused ultrasound-mediated blood–brain barrier disruption depends strongly on anesthesia Bioeng. Transl. Med. 6 e10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, Heyn C, Alkins R, Trudeau M, Sahgal A, Perry J and Hynynen K 2019. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study Sci. Rep. 321 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, Karachi C, Leclercq D, Lafon C, Chapelon JY, Capelle L, Cornu P, Sanson M, Hoang-Xuan K, Delattre JY and Idbaih A 2016. Clinical trial of blood-brain barrier disruption by pulsed ultrasound Sci Transl Med; 343 8: 343re2. [DOI] [PubMed] [Google Scholar]
- [108].Meng Y, Pople CB, Suppiah S, Llinas M, Huang Y, Sahgal A, Perry J, Keith J, Davidson B, Hamani C, Amemiya Y, Seth A, Leong H, Heyn CC, Aubert I, Hynynen K and Lipsman N 2021. MR-guided focused ultrasound liquid biopsy enriches circulating biomarkers in patients with brain tumors Neuro. Oncol. 23 1789–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Pouliopoulos AN, Kwon N, Jensen G, Meaney A, Niimi Y, Burgess MT, Ji R, McLuckie AJ, Munoz FA, Kamimura HAS, Teich AF, Ferrera VP and Konofagou EE 2021. Safety evaluation of a clinical focused ultrasound system for neuronavigation guided blood-brain barrier opening in non-human primates Sci. Reports 2021 111 11 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Park SH, Baik K, Jeon S, Chang WS, Ye BS and Chang JW 2021. Extensive frontal focused ultrasound mediated blood–brain barrier opening for the treatment of Alzheimer’s disease: a proof-of-concept study Transl. Neurodegener. 44 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


