Abstract
Background:
Social vulnerability indicators are associated with health care inequities and may similarly impede ongoing participation in research studies. We evaluated the association of social vulnerability indicators and research participant attrition in a trial focused on reducing health disparities.
Methods:
Self-identified White (W) or African-American/Black (AAB) adults enrolled in the HYpertension and VALUEs (HYVALUE) trial, a randomized trial testing a values affirmation intervention on medication adherence, from February 2017 - September 2019 were included. The self-reported measures of social vulnerability indicators included: (i) AAB race; (ii) female gender; (iii) no health insurance; (iv) unemployment; (v) a high school diploma or less; and (vi) financial-resource strain. Full attrition was defined as not completing at least one 3- or 6-month follow-up study visit. Log binomial regression models adjusted for age, sex, race, medical co-morbidities, and the other social vulnerability indicators to estimate the relative risk (RR) of each social vulnerability indicator with study attrition.
Results:
Among 825 participants, the mean age was 63.3 years (±11.7 years), 60% were women, 54% were AAB, and 97% reported at least one social vulnerability. Overall, 21% participants had full attrition after study enrollment. After adjustment for all other social vulnerabilities, only financial-resource strain remained consistently associated with full attrition (RR 1.71, 95% CI 1.28–2.29). In a secondary analysis of partial attrition (completed only one follow-up visit), financial-resource strain (RR 1.40, 95% CI: 1.09–1.81) and being uninsured (RR 1.54, 95% CI; 1.01–2.34) were associated with partial attrition.
Conclusions:
In a trial aimed at reducing disparities in medication adherence, participants who reported financial-resource strain had a higher risk of participant attrition independent of race or sex. Our findings suggest that efforts to retain diverse populations in clinical trials should extend beyond race and sex to consider other social vulnerability indicators.
Registration:
Keywords: Study attrition, social vulnerability, healthcare disparities, African Americans, hypertension
Introduction
Historically, research participants from socially vulnerable populations have not been adequately represented in biomedical research studies.1–6 Underrepresented groups in the U.S., such as members of racial and ethnic minority groups and women, often face health and health care inequities and representation of socially vulnerable populations is important to the generalizability of research findings.7 The 1993 National Institutes of Health (NIH) Revitalization Act mandated that women and racial and ethnic minority groups be included in federally funded research studies – yet their representation in biomedical research studies remains low.8–10
Recruitment practices to improve participation of women and ethnic and racial minorities in research studies has been the primary focus in biomedical research to improve diversity in representation of research participants. However, little is known about a potentially related problem, differential attrition of socially vulnerable participants after study enrollment. Attrition of research participants can introduce bias in research findings and decrease generalizability of biomedical research findings to the public. If differential attrition mainly occurs among socially vulnerable populations in biomedical research studies, the opportunity to understand how biomedical research findings may impact socially vulnerable populations is impeded.11–13 Understanding factors associated with differential attrition not only among racial and ethnic minorities and women, but among those with other social vulnerabilities, is key to addressing the issue of diverse representation in clinical studies.
Social vulnerability can be operationalized as the accumulation or convergence of interrelated social factors, as described by Andersen’s framework, “Behavioral Model of Health Services Use”.14 The use of health services is influenced by predisposing factors (e.g., socio-cultural characteristics), enabling factors (e.g., logical aspects of obtaining care) and need (e.g., perceptions of health).15–17 Social vulnerability indicators, such as minority race and ethnicity, female gender, financial-resource strain, lack of health insurance, unemployment and low educational attainment, are associated with a higher risk of health and health care inequities.15,17–24 Although Andersen’s framework of health services use may not consistently apply to all social vulnerability indicators, (e.g., female gender as a barrier to healthcare is not consistently shown in the literature) it is a useful framework to understand potential causal pathways to health and health care inequities.23,24 These same social vulnerability indicators may influence recruitment and retention of study participants in biomedical research.8,9 A better understanding of different aspects of social vulnerability indicators and their associations with the risk of attrition in research studies can help biomedical researchers plan their retention strategies.
To explore whether social vulnerability indicators are associated with participant attrition in a randomized trial, we examined the relationship between participant self-identified race, gender, financial-resource strain, insurance status, employment status, and educational attainment with enrollee attrition over 6 months in the HYVALUE trial. The HYVALUE trial targeted enrollment of self-identified White (W) and African-American/Black (AAB) participants with uncontrolled hypertension from three large health systems in the United States. We hypothesized that social vulnerability indicators would be associated with increased attrition for individuals participating in the trial.
Methods
Data Source and Study Population
The study population included enrolled research participants in the HYVALUE trial.25 The HYVALUE trial was a patient-level, blinded randomized controlled trial that examined whether a values-affirmation intervention improved medication adherence (primary outcome) by targeting racial stereotype threat.25 Values-affirmation interventions ask participants to write about their core values, such as their relationship with friends or family, religious values, or artistic ability. Values-affirmation interventions have demonstrated effectiveness at decreasing academic achievement gaps among minority students, and the HYVALUE trial tested the efficacy of a values-affirmation exercise to improve medication adherence among AAB participants. Between February 1, 2017 to December 31, 2019, the trial enrolled participants who self-identified as non-Hispanic AAB or W race and ethnicity, with uncontrolled hypertension in primary care practices from 3 different health care systems; two in Colorado and one in the mid-Atlantic region.25 Institutional review boards at each health care system approved study procedures, and participants gave written informed consent prior to enrolling in the study. The authors declare that all supporting data are available within the article and its online supplementary files.
AAB and W participants were eligible for the HYVALUE trial if they were over 21 years of age, had a diagnosis of hypertension within 24 months of study recruitment, had an elevated blood pressure (systolic blood pressure >140mmHg or diastolic blood pressure >90mmHg) in the prior 12 months, were taking medications for hypertension, and had an upcoming primary care appointment at the time of recruitment into the HYVALUE trial.25 At the enrollment visit and prior to the intervention, participants were given a short questionnaire administered by trained study staff that evaluated socio-demographic factors that included measures of social vulnerability. For the intervention trial, all participants completed a brief writing exercise focused on values prior to their primary care visit. The only difference was that intervention arm participants self-affirmed their values (i.e., wrote about why their values are important to them) whereas the control arm participants did not (i.e., wrote about why values that are not important to them might be important to someone else).25
Study participants were asked to complete follow-up visits at 3- and 6-months to collect study outcomes including questionnaires, pill counts and blood pressure measurements. Study coordinators used several strategies to encourage retention of participants including: scheduling future follow-up visits with the study team at time of initial enrollment, collecting updated contact information including an alternate contact, inquiring about preferred method for study communication, allowing for flexibility in scheduling of visits, calling study participants with reminders, sending post card and email reminders, coordinating study visits with scheduled clinic appointments and offering follow-up visits over the phone and/or links to online surveys.26–29 Ongoing attempts were made to schedule follow-up visits unless a participant asked to no longer be contacted, or died. Participants were given a gift card incentive of $20 for completing each study visit.
A total of 960 participants were enrolled in the HYVALUE trial. We only included participants enrolled by September 15, 2019 (due for 6-month follow-up visit by March 15, 2020) for our analyses to avoid attrition that may have been driven by the COVID-19 pandemic. Fifty participants (5%) with missing social vulnerability measures were also excluded from our analysis (Figure S1 in the Data Supplement).
Social Vulnerability Indicators
Our social vulnerability indicator measures were collected by in-person surveys at trial enrollment prior to implementing randomization to the values affirmation intervention. Measures selected were based on the Behavioral Model of Health Services Use and empirically evaluated characteristics associated with health and health care inequities.14–16 The social vulnerability indicators included: (i) being of AAB race; (ii) female or other gender; (iii) no health insurance; (iv) unemployment and age less than 65 years; and (v) limited education, defined as no more than a high school diploma or GED; and (vi) financial-resource strain. Financial-resource strain was determined by participants’ self-reported level of difficulty paying for very basics like food, housing, medical care, and heating within the 3 months prior to enrolling into the trial.30–32 Participants rated their difficulty paying for basic items along an ordinal scale: very hard, somewhat hard, and not hard at all. Because follow-up rates were similar between participants who reported paying for basics was “somewhat hard” or “very hard” (Table S1 in the Data Supplement), we categorized difficulty paying for basics into two categories for our analysis: financial-resource strain (very hard and somewhat hard paying for basics) and financial stability (not hard at all paying for basics).
Ascertainment of Attrition Outcome
Our main outcome was participant attrition defined as failure to complete a study follow-up visit. The attrition outcome was categorized into three mutually exclusive categories: no attrition participants completed both three-month and six-month follow-up study visits; full attrition participants did not complete both three-month and six-month follow-up study visits; and partial attrition participants only completed a three-month or a six-month follow-up study visit but not both. Study participants were only provided the option to conduct their follow-up study visit over the phone if conducting the follow-up visit in person was not feasible. The only difference between phone and in-person follow-up visits was the collection of blood pressure and pill count measurements for in-person study visits.
Statistical Analysis
Bivariate analyses were used to examine the effect of each social vulnerability indicator on attrition. The mean and standard deviation were estimated for continuous variables and counts and percentages were used for dichotomous variables. In the primary analysis, relative risk (RR) of full attrition versus no attrition or partial attrition was estimated using unadjusted and adjusted log-binomial models. To understand how the attrition groups compare to no attrition, in secondary analysis, we compared partial attrition to no attrition and full attrition to no attrition, removing participants in the respective third group. When comparing RR within each vulnerability domain, the factor that we hypothesized would be the non-vulnerable indicator (e.g., more than high school education) was the referent. Finally, to account for possible effects of our intervention on attrition, in additional analysis, we stratified our adjusted primary models comparing full attrition to those with no or partial attrition by treatment arm (intervention versus control arm).
The adjusted log-binomial models accounted for all social vulnerability indicators and participant medical co-morbidities. Patient-level co-morbidities evaluated were history of circulatory system disease, congestive heart failure, cardiac arrhythmia, renal failure, major depression disorder, and current smoker. Data for our clinical variables were exported from electronic medical records using International Classification of Diseases (ICD-9 or ICD-10) Clinical Modification (CM) diagnoses codes collected at the time of participant study enrollment (Table S2 in the Data Supplement). Circulatory system disease consisted of the following: ischemic heart disease, peripheral vascular disease, and cerebrovascular disease. Cardiac arrhythmia was defined as having a diagnosis code for any type of arrhythmia (e.g., atrial fibrillation or supraventricular tachycardia). In the adjusted log-binomial models, the number of additional medical co-morbidities beyond hypertension were included. Categories were: 0 additional co-morbidities, only 1 additional co-morbidity, 2 additional co-morbidities, and at least 3 or more co-morbidities. Only baseline measures at the time of participant study enrollment were used for analyses. All analyses were performed using SAS version 9.4.
Results
Of the 960 enrolled HYVALUE study participants, 85 were excluded for enrolling after September 15, 2019, and 50 were excluded due to incomplete data on the social vulnerability indicators, leaving a final study population of 825 participants (Figure S1 in the Data Supplement). The most common missing social vulnerability indicator was the financial-resource strain measure (n=32).
The mean age of the included 825 study participants was 63.3 years, women comprised 60% of the study population, and most participants were AAB race (54%). For the other social vulnerability indicator categories: 28% participants had limited education, 42% experienced some level of financial-resource strain, 21% were unemployed, and 5% had no health insurance (Table 1). A total of 800 (97%) of the participants had at least one of the six social vulnerability indicators studied, 650 (79%) had two or more. Beyond hypertension, 71% of participants had at least 1 additional medical comorbidity (Table 1).
Table 1.
Baseline Characteristics of HYVALUE Study Participants Overall and According to Attrition Group
| All (N=825) |
No Attrition (N=465) |
Partial Attrition* (N=187) |
Full Attrition† (N=173) |
|
|---|---|---|---|---|
| Age, mean (SD) | 63.3 (11.9) | 64.9 (10.9) | 61.8 (13.1) | 60.4 (12.6) |
| Age | ||||
| Under 65, No. (%) | 430 (52) | 219 (47) | 101 (54) | 110 (64) |
| 65 and over, No. (%) | 395 (48) | 246 (53) | 86 (46) | 63 (36) |
| Race | ||||
| White, No. (%) | 383 (46) | 236 (51) | 82 (44) | 65 (38) |
| African-American/Black, No. (%) | 442 (54) | 229 (49) | 105 (56) | 108 (62) |
| Sex | ||||
| Male, No. (%) | 329 (40) | 181 (39) | 74 (40) | 74 (43) |
| Female, No. (%) | 496 (60) | 284 (61) | 113 (60) | 99 (57) |
| Education | ||||
| Some college or higher, No. (%) | 594 (72) | 345 (74) | 141 (75) | 108 (62) |
| High school or less, No. (%) | 231 (28) | 120 (26) | 46 (25) | 65 (38) |
| Financial-resource strain | ||||
| No difficulty paying for basics, No. (%) | 482 (58) | 306 (66) | 102 (54) | 74 (43) |
| Difficulty paying for basics, No. (%) | 343 (42) | 159 (34) | 85 (45) | 99 (57) |
| Employment | ||||
| Employed or retired, No. (%) | 648 (79) | 372 (80) | 150 (80) | 126 (73) |
| Unemployed, No. (%) | 177 (21) | 93 (20) | 37 (20) | 47 (27) |
| Health insurance | ||||
| Insured, No. (%) | 783 (95) | 446 (96) | 173 (93) | 164 (95) |
| Uninsured, No. (%) | 42 (5) | 19 (4) | 14 (7) | 9 (5) |
| Circulatory system disease‡ | ||||
| No (%) | 556 (67) | 310 (66) | 126 (67) | 120 (69) |
| Yes (%) | 269 (33) | 155 (33) | 61 (33) | 53 (31) |
| Cardiac arrhythmia§ | ||||
| No (%) | 668 (81) | 375 (81) | 154 (82) | 139 (80) |
| Yes (%) | 157 (19) | 90 (19) | 33 (18) | 34 (20) |
| Congestive heart failure | ||||
| No (%) | 730 (89) | 422 (91) | 166 (89) | 142 (82) |
| Yes (%) | 95 (12) | 43 (9) | 21 (11) | 31 (18) |
| Valvular Heart Disease | ||||
| No (%) | 757 (92) | 418 (90) | 179 (96) | 160 (93) |
| Yes (%) | 68 (8) | 47 (10) | 8 (4) | 13 (8) |
| Diabetes | ||||
| No (%) | 579 (70) | 341 (73) | 123 (66) | 115 (67) |
| Yes (%) | 246 (30) | 124 (27) | 64 (34) | 58 (34) |
| Depression | ||||
| No (%) | 619 (75) | 358 (77) | 137 (73) | 124 (72) |
| Yes (%) | 206 (25) | 107 (23) | 50 (26) | 49 (28) |
| Current smoker | ||||
| No (%) | 702 (86) | 414 (90) | 152 (82) | 136 (81) |
| Yes (%) | 113 (14) | 46 (10) | 34 (18) | 33 (20) |
| Mean systolic BP at baseline | 139.5 (18) | 138.9 (18) | 141.0 (19) | 139.5 (19) |
| Mean diastolic BP at baseline | 82.4 (12) | 81.3 (11) | 83.5 (14) | 83.9 (12) |
| Number of additional comorbidities | ||||
| 0 (%) | 239 (29) | 138 (30) | 58 (31) | 43 (25) |
| 1 (%) | 284 (34) | 164 (35) | 56 (30) | 64 (37) |
| 2 (%) | 142 (17) | 87 (19) | 28 (15) | 27 (16) |
| 3+ (%) | 160 (19) | 76 (16) | 45 (24) | 39 (23) |
Partial attrition indicates study participants only completed one of two follow-up study visits.
Full attrition indicates study participants completed none of the follow-up study visits.
Circulatory system disease consisted of the following: ischemic heart disease, peripheral vascular disease, and cerebrovascular disease.
Cardiac arrhythmia defined as having an International Classification of Diseases 9th or 10th edition Clinical Modification diagnosis code for any type of arrhythmia (e.g., atrial fibrillation, supraventricular).
Demographic characteristics in the three categories of attrition are also shown in Table 1. Of the included study participants, 465 (56%) participants had no attrition, 187 (23%) had partial attrition and 173 (21%) had full attrition. Table 1 shows that there was a higher percentage of AAB race, low educational attainment, unemployment, and financial-resource strain for participants with full attrition than participants with partial or no attrition. No differences in baseline social vulnerability indicators were found by treatment arm (data not shown).
In unadjusted primary analysis for risk of full attrition versus partial or no attrition, of the six social vulnerability indicators we hypothesized would predict attrition, four were significant in the direction we expected: unemployment, low educational attainment, financial-resource strain, and AAB race (Figure 1). Two (being uninsured and female gender) were not significant predictors of full attrition. After adjustment for all other vulnerable social vulnerability indicators and medical comorbidities, financial-resource strain remained as a unique predictor of full attrition (RR = 1.71, 95% CI 1.28–2.29). In additional analysis stratifying the primary models by treatment arm, financial-resource strain was associated with full attrition in both arms, yet AAB race was associated with full attrition in the control arm only (Table S3 in the Data Supplement).
Figure 1.
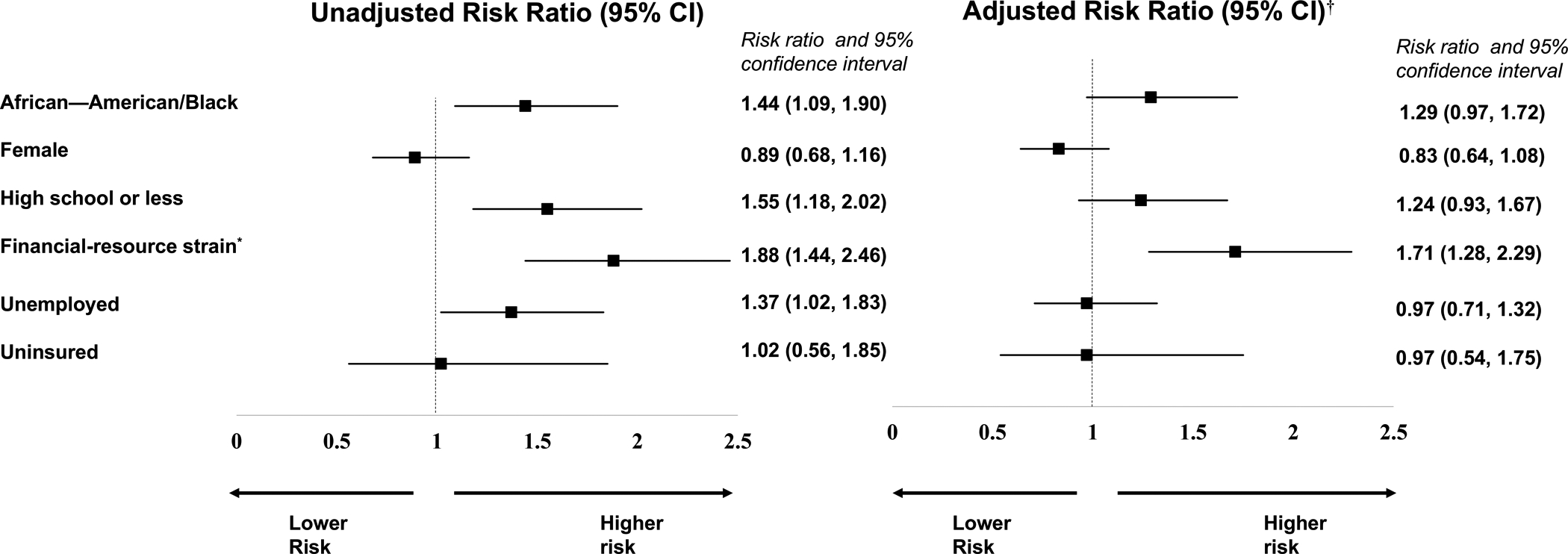
Unadjusted and adjusted risk ratios of social vulnerabilities with full attrition.
Log binomial regression models compared participants with full attrition to those with partial attrition or no attrition.
*Financial-resource strain was determined by participants’ self-reported level of difficulty paying for very basics like food, housing, medical care, and heating within the 3 months prior to enrolling into the trial. Participants who reported paying for basics was ‘somewhat hard’ or ‘very hard’ were categorized as having financial-resource strain.
†The adjusted log-binomial models controlled for other social vulnerable indicators and the number of participant medical co-morbidities.
In unadjusted secondary analysis for risk of partial attrition versus no attrition (full attrition excluded), financial-resource strain and being uninsured were significant predictors in the direction we expected (Figure 2). Four domains (AAB race, unemployment, education, and female gender) were not significant predictors of partial attrition compared to no attrition. In the fully adjusted model, financial-resource strain (RR=1.40, 95% CI 1.09–1.81) and being uninsured (RR=1.54, 95% CI 1.01–2.34) remained unique predictors of increased risk of partial attrition versus not attrition. In adjusted secondary analysis for risk of full attrition verses no attrition (partial attrition excluded), financial-resource strain (RR=1.83, 95% CI 1.39–2.40 and AAB race (RR=1.33, 95% CI 1.02–1.74) were significantly associated with full attrition (Figure 3).
Figure 2.
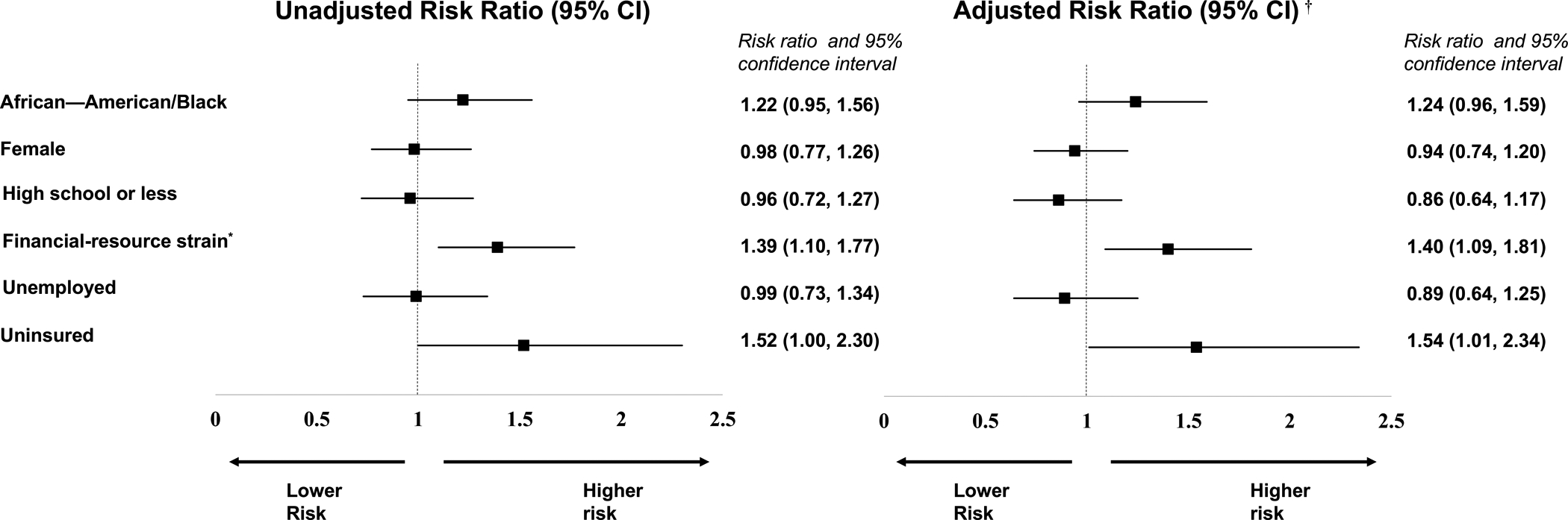
Unadjusted and adjusted risk ratios of social vulnerabilities with partial attrition.
Log binomial regression models compared participants with partial attrition (attended at least one study visit) to participants with no attrition.
*Financial-resource strain was determined by participants’ self-reported level of difficulty paying for very basics like food, housing, medical care, and heating within the 3 months prior to enrolling into the trial. Participants who reported paying for basics was ‘somewhat hard’ or ‘very hard’ were categorized as having financial-resource strain.
†The adjusted log-binomial models controlled for other social vulnerability indicators and the number of participant medical co-morbidities.
Figure 3.
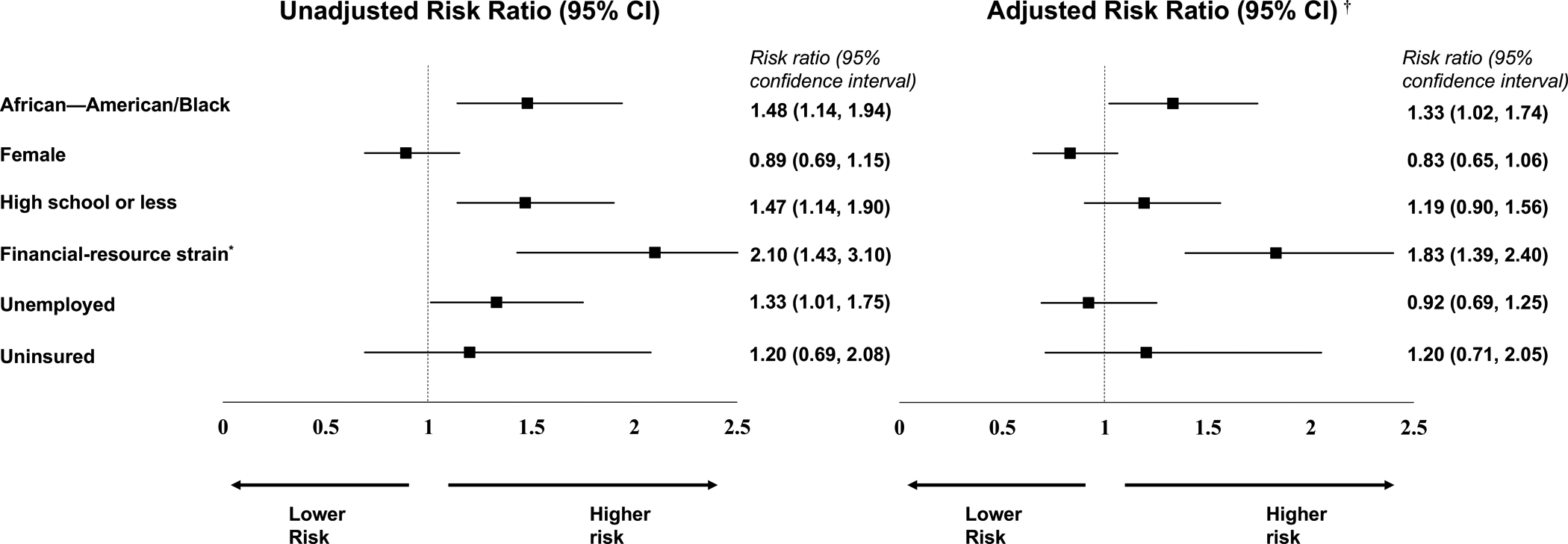
Unadjusted and adjusted risk ratios of social vulnerabilities for full attrition versus no attrition.
Log binomial regression models compared participants with full attrition to participants with no attrition.
*Financial-resource strain was determined by participants’ self-reported level of difficulty paying for very basics like food, housing, medical care, and heating within the 3 months prior to enrolling into the trial. Participants who reported paying for basics was ‘somewhat hard’ or ‘very hard’ were categorized as having financial-resource strain.
†The adjusted log-binomial models controlled for vulnerable social indicators and the number of participant medical co-morbidities.
Discussion
In this retrospective study of 825 research participants in the HYVALUE trial, we explored the association between social vulnerability indicators and participant attrition from a randomized trial, as defined by completion of study visits. Four out of five participants reported at least two of the six social vulnerability indicators measured in this study. We found that financial-resource strain – difficulty paying for the basics in the last 3 months – was consistently associated with a higher risk of partial or full attrition after adjusting for all other social vulnerability indicators. Importantly, we found no significant independent associations between participant gender, education, employment, and insurance status with full study attrition, although being uninsured had a unique association with partial study attrition. AAB race was significantly associated with full attrition (versus no attrition) in our secondary analysis. In a population with a high burden of social vulnerabilities, our study suggests that when controlling for other social vulnerability indicators, financial-resource strain remained consistently associated with complete loss to follow-up.
Our finding of an association between financial-resource strain and risk of attrition in medical and translational research builds on prior work looking at measures of financial resources. For example, measures such as low family income and unemployment status have both been shown to be associated with participant attrition in health behavior interventions implemented in primary care settings.33–37 We uniquely evaluated this association using a simple self-reported question endorsed by the Institute of Medicine as a measure or financial-resource strain instead of the common approach of asking about household income.30 Income questions on surveys are limited by participant non-response, accuracy in those who are retired and the use of income thresholds that may not accurately reflect a participant’s ability to actually pay for their basic needs.38–41 Financial-resource strain accounts for both household income and household expenses and may occur across a range of income levels.40 We asked participants about difficulty in paying for their basic needs such as housing in the last 3 months and found a strong association with this measure and the risk of both partial and full study attrition. Importantly, this measure of financial-resource strain was a better predictor of study attrition than other indicators such as employment status.
Our findings also build on work that demonstrated a similar relationship between financial-resource strain and non-adherence to medications – factors influencing the relationship between financial-resource strain and non-adherence to medications likely influence attrition in research.40,42 The association between a participant’s financial-resource strain and attrition in biomedical research studies may be due to the need to balance the financial demands (e.g. time away from work) and resources (e.g. transportation to visits, childcare) required to participate in biomedical research.43–45 In other words, financial-resource strain may force individuals to choose between unpaid time off work to attend a study versus working to pay a utility bill or skipping a meal to pay for transportation to a study visit.40 Therefore, our findings suggest asking about the difficulty of paying for basic needs may provide useful insights into how financial-resource strain may influence the ability of participants to complete future study visits.
Another important contribution of this study is the focus on study attrition among those who have already completed the enrollment visit in a research study. Prior studies have primarily focused on the link between social vulnerability indicators and willingness to initially participate in biomedical research.1,3,4,6,46–48 The direction of these results is mixed and differs based on the social vulnerability indicator – e.g., AAB populations’ mistrust of biomedical research.4 Although these studies show some social vulnerability indicators as barriers to willingness to participate in biomedical research, many studies show that these barriers may not change the likelihood of actual research participation. For example, despite several studies suggesting AAB individuals report less willingness to consider participating in research, studies have shown that when approached, AAB individuals are actually just as likely to agree to participate in biomedical research as non-minoritized populations.3,49–52 Our study adds to this prior work by focusing on how social vulnerability indicators may affect study attrition, a less studied area of research that occurs after barriers to initial participation have been overcome.
Attrition of biomedical research participants can lead to selection bias and limit generalizability of research findings. Although common, attrition of research participants in biomedical research interventions has been historically underreported or largely ignored.13,53 13 Differential study participant attrition according to social vulnerability indicators is particularly concerning in studies focused on reducing health disparities in populations who may also have a disproportionate burden of social vulnerability indicators. The HYVALUE trial population included a racially diverse (54% AAB and 46% W) population with a high prevalence of social vulnerability indicators; 97% of the study population reported at least one social vulnerability measure. Several strategies have been shown effective at increasing participant retention such as providing financial resources via transportation vouchers or reimbursement and providing flexibility in the modes of study data collection including via mail, online or in person.26,29,54 The HYVALUE trial deployed many of these strategies to limit study attrition, yet, complete study attrition was still ~20%. Future studies targeting populations with a high prevalence of social vulnerability indicators should consider these and other strategies to effectively lower attrition rates.
A major strength of our study is the socio-demographic diversity and high burden of social vulnerability indicators among study participants. The HYVALUE trial was designed to be pragmatic and was implemented in conjunction with an existing service (i.e., primary care clinic visit).13 Therefore, our results provide important insights into study attrition in pragmatic clinical trials and how social vulnerability indicators may lead to attrition bias in biomedical research.
Our study also has limitations. Measures of vulnerability were self-reported and only collected at baseline; therefore, we cannot assess for potential changes in social vulnerability indicators over our observation period. Also, measures used to evaluate social vulnerability indicators were limited to the study questions available for analysis and not exhaustive of all potential social vulnerability indicators. In addition, we had low rates of uninsured participants (5%) and we were underpowered to examine the association with insurance status and attrition. Also, our study findings reflect an intervention designed to address disparities in medication adherence among AAB participants, and measures implemented within the study may have led to AAB participants having less attrition or differential attrition by study arm. In our additional exploratory analysis, the relationship between participant race and full attrition was only seen in the control arm suggesting this relationship may have been influenced by our intervention (Table III in the Data Supplement). Yet, our study was not powered to measure the effects of the social vulnerability indicator measures on risk of attrition by study arm and these findings warrant future examination. Furthermore, our adjusted log binomial models simultaneously controlled for other measured social vulnerability indicators that might be considered potential mediators – i.e., some social vulnerability indicators may mediate others. Our modeling approach may have led to an underestimation of effect size for any one social vulnerability indicator. Last, our results may not be generalizable to other research designs such as randomized controlled trial pharmaceutical interventions or other racial-ethnic populations beyond AAB and W Americans.
The results of our study have important implications for current national priorities in the United States related to the recruitment of underrepresented populations into biomedical research. The NIH Revitalization Act passed in 1993 placed a national priority on recruitment of women and members of racial and ethnic minority groups. Racial and ethnic minority and women representation in federally funded research has improved since 1993, but challenges still exist, especially in industry-funded research.3 Our findings suggest that these challenges continue after study enrollment. Although race and ethnicity and sex and gender were the primary foci of the NIH Revitalization Act, we find that race and sex were not consistently associated with retention and attrition of research participants. AAB race was significantly associated with risk of full attrition compared to group with no attrition but not with full attrition compared to partial or no attrition after adjusting for other social vulnerability indicators. The only social vulnerability indicator consistently associated with study attrition was financial-resource strain. To improve our understanding of factors that contribute to retention and attrition, our findings suggest social vulnerability indicators beyond race or gender should be considered to improve participation of diverse populations.
Conclusions
In an ongoing prospective trial among a population of patients with a high burden of social vulnerability indicators, we found that financial-resource strain was uniquely associated with a higher risk of study attrition. Our study suggests that trials with diverse populations should consider retention plans that are attentive to challenges of socially disadvantaged participants.
Supplementary Material
Figure S1. Flow chart of final study population.
Table S1. Comparison of categorical financial-resource strain by type of follow up achieved.
Table S2. ICD-9 and ICD-10 CM Codes Used to Define Clinical Variables to Adjust for in the Log-Binomial Models.
Table S3. Comparison between adjusted risk ratios for full attrition to no attrition or partial attrition by treatment arm (intervention vs control arm).
Acknowledgments
The authors would like to acknowledge the contribution of the following individuals to the study conduct and data collection for this project: Cozette Boakye, Cassandra Bryant, Suzanne Dircksen, Hilde Heyn, Jennifer McCance, Courtney Anderson, Amanda Skenadore, Christine Truong, and Leslie Wright. We would also like to thank participants of the HYVALUE trial.
Sources of Funding
The HYVALUE trial was an investigator-initiated study funded by the National Heart, Lung, and Blood Institute of the NIH under Award Number R01 HL133343. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Abbreviations:
- AAB
African American/Black
- CM
Clinical Modification
- ICD-9
International Classification of Diseases Ninth Revision
- NIH
National Institute of Health
- RR
Risk ratio
- U.S.
United States
- W
White
Footnotes
Disclosures
None
References
- 1.Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore). 2008;87(1):1–9. [DOI] [PubMed] [Google Scholar]
- 2.Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Annals of epidemiology. 2002;12(4):248–256. [DOI] [PubMed] [Google Scholar]
- 3.Fisher JA, Kalbaugh CA. Challenging assumptions about minority participation in US clinical research. Am J Public Health. 2011;101(12):2217–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbie-Smith G, Thomas SB, St George DM. Distrust, race, and research. Archives of internal medicine. 2002;162(21):2458–2463. [DOI] [PubMed] [Google Scholar]
- 5.Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. The New England journal of medicine. 2000;343(7):475–480. [DOI] [PubMed] [Google Scholar]
- 6.Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S. Attitudes and beliefs of African Americans toward participation in medical research. J Gen Intern Med. 1999;14(9):537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.In: Baciu A, Negussie Y, Geller A, Weinstein JN, eds. Communities in Action: Pathways to Health Equity. Washington (DC)2017. [PubMed] [Google Scholar]
- 8.Freedman LS, Simon R, Foulkes MA, et al. Inclusion of women and minorities in clinical trials and the NIH Revitalization Act of 1993--the perspective of NIH clinical trialists. Control Clin Trials. 1995;16(5):277–285; discussion 286–279, 293–309. [DOI] [PubMed] [Google Scholar]
- 9.Oh SS, Galanter J, Thakur N, et al. Diversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled. PLoS Med. 2015;12(12):e1001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104(2):e16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewin A, Brondeel R, Benmarhnia T, Thomas F, Chaix B. Attrition Bias Related to Missing Outcome Data: A Longitudinal Simulation Study. Epidemiology. 2018;29(1):87–95. [DOI] [PubMed] [Google Scholar]
- 12.Barry AE. How attrition impacts the internal and external validity of longitudinal research. J Sch Health. 2005;75(7):267–270. [DOI] [PubMed] [Google Scholar]
- 13.Amico KR. Percent total attrition: a poor metric for study rigor in hosted intervention designs. Am J Public Health. 2009;99(9):1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grabovschi C, Loignon C, Fortin M. Mapping the concept of vulnerability related to health care disparities: a scoping review. BMC Health Serv Res. 2013;13:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aday LA, Andersen R. A framework for the study of access to medical care. Health Serv Res. 1974;9(3):208–220. [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36(1):1–10. [PubMed] [Google Scholar]
- 17.Aday LA, Andersen RM. Equity of access to medical care: a conceptual and empirical overview. Med Care. 1981;19(12):4–27. [PubMed] [Google Scholar]
- 18.Andersen R Health status indices and access to medical care. Am J Public Health. 1978;68(5):458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor DG, Aday LA, Andersen R. A social indicator of access to medical care. J Health Soc Behav. 1975;16(1):39–49. [PubMed] [Google Scholar]
- 20.Flores G, Tomany-Korman SC. Racial and ethnic disparities in medical and dental health, access to care, and use of services in US children. Pediatrics. 2008;121(2):e286–298. [DOI] [PubMed] [Google Scholar]
- 21.Manuel JI. Racial/Ethnic and Gender Disparities in Health Care Use and Access. Health Serv Res. 2018;53(3):1407–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClendon J, Essien UR, Youk A, et al. Cumulative Disadvantage and Disparities in Depression and Pain Among Veterans With Osteoarthritis: The Role of Perceived Discrimination. Arthritis Care Res (Hoboken). 2021;73(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson-KFF Health System Tracker. Peterson Center on Healthcare and Kaiser Family Foundation. https://www.healthsystemtracker.org/brief/covid-19-is-the-number-one-cause-of-death-in-the-u-s-in-early-2021/. Published 2021. Accessed March 1, 2021.
- 24.Services CfMM. Gender Disparities in Health Care in Medicare Advantage. In:2017.
- 25.Daugherty SL, Vupputuri S, Hanratty R, et al. Using Values Affirmation to Reduce the Effects of Stereotype Threat on Hypertension Disparities: Protocol for the Multicenter Randomized Hypertension and Values (HYVALUE) Trial. JMIR Res Protoc. 2019;8(3):e12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zweben A, Fucito LM, O’Malley SS. Effective Strategies for Maintaining Research Participation in Clinical Trials. Drug Inf J. 2009;43(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grape A, Rhee H, Wicks M, Tumiel-Berhalter L, Sloand E. Recruitment and retention strategies for an urban adolescent study: Lessons learned from a multi-center study of community-based asthma self-management intervention for adolescents. J Adolesc. 2018;65:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zook PM, Jordan C, Adams B, et al. Retention strategies and predictors of attrition in an urban pediatric asthma study. Clin Trials. 2010;7(4):400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicholson LM, Schwirian PM, Groner JA. Recruitment and retention strategies in clinical studies with low-income and minority populations: Progress from 2004–2014. Contemp Clin Trials. 2015;45(Pt A):34–40. [DOI] [PubMed] [Google Scholar]
- 30.Adler NE, Stead WW. Patients in context--EHR capture of social and behavioral determinants of health. The New England journal of medicine. 2015;372(8):698–701. [DOI] [PubMed] [Google Scholar]
- 31.Puterman E, Haritatos J, Adler NE, Sidney S, Schwartz JE, Epel ES. Indirect effect of financial strain on daily cortisol output through daily negative to positive affect index in the Coronary Artery Risk Development in Young Adults Study. Psychoneuroendocrinology. 2013;38(12):2883–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall MH, Matthews KA, Kravitz HM, et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- 33.Gustavson K, von Soest T, Karevold E, Roysamb E. Attrition and generalizability in longitudinal studies: findings from a 15-year population-based study and a Monte Carlo simulation study. BMC Public Health. 2012;12:918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ejiogu N, Norbeck JH, Mason MA, Cromwell BC, Zonderman AB, Evans MK. Recruitment and retention strategies for minority or poor clinical research participants: lessons from the Healthy Aging in Neighborhoods of Diversity across the Life Span study. Gerontologist. 2011;51 Suppl 1:S33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blumenthal DS, Sung J, Coates R, Williams J, Liff J. Recruitment and retention of subjects for a longitudinal cancer prevention study in an inner-city black community. Health Serv Res. 1995;30(1 Pt 2):197–205. [PMC free article] [PubMed] [Google Scholar]
- 36.Lamers F, Hoogendoorn AW, Smit JH, et al. Sociodemographic and psychiatric determinants of attrition in the Netherlands Study of Depression and Anxiety (NESDA). Compr Psychiatry. 2012;53(1):63–70. [DOI] [PubMed] [Google Scholar]
- 37.Molewyk Doornbos M, Zandee GL, Timmermans B, et al. Factors impacting attrition of vulnerable women from a longitudinal mental health intervention study. Public Health Nurs. 2020;37(1):73–80. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Egerter S, Cubbin C, Takahashi ER, Braveman P. Potential implications of missing income data in population-based surveys: an example from a postpartum survey in California. Public Health Rep. 2007;122(6):753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turrell G Income non-reporting: implications for health inequalities research. J Epidemiol Community Health. 2000;54(3):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyles CR, Seligman HK, Parker MM, et al. Financial Strain and Medication Adherence among Diabetes Patients in an Integrated Health Care Delivery System: The Diabetes Study of Northern California (DISTANCE). Health Serv Res. 2016;51(2):610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Essig L, Winter JK Item Non-Response to Financial Questions in Household Surveys: An Experimental Study of Interviewer and Mode Effects. The Journal of Applied Public Economics. 2009;30:24. [Google Scholar]
- 42.Strickland JC, Stoops WW, Kincer MA, Rush CR. The impact of financial strain on medication non-adherence: Influence of psychiatric medication use. Psychiatry Res. 2019;271:389–395. [DOI] [PubMed] [Google Scholar]
- 43.Keller CS, Gonzales A, Fleuriet KJ. Retention of minority participants in clinical research studies. West J Nurs Res. 2005;27(3):292–306. [DOI] [PubMed] [Google Scholar]
- 44.Brown DR, Fouad MN, Basen-Engquist K, Tortolero-Luna G. Recruitment and retention of minority women in cancer screening, prevention, and treatment trials. Annals of epidemiology. 2000;10(8 Suppl):S13–21. [DOI] [PubMed] [Google Scholar]
- 45.Janson SL, Alioto ME, Boushey HA, Asthma Clinical Trials N. Attrition and retention of ethnically diverse subjects in a multicenter randomized controlled research trial. Control Clin Trials. 2001;22(6 Suppl):236S–243S. [DOI] [PubMed] [Google Scholar]
- 46.Svensson K, Ramirez OF, Peres F, Barnett M, Claudio L. Socioeconomic determinants associated with willingness to participate in medical research among a diverse population. Contemp Clin Trials. 2012;33(6):1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoberman A, Shaikh N, Bhatnagar S, et al. Factors that influence parental decisions to participate in clinical research: consenters vs nonconsenters. JAMA Pediatr. 2013;167(6):561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trauth JM, Musa D, Siminoff L, Jewell IK, Ricci E. Public attitudes regarding willingness to participate in medical research studies. J Health Soc Policy. 2000;12(2):23–43. [DOI] [PubMed] [Google Scholar]
- 49.Wendler D, Kington R, Madans J, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3(2):e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyer S, Woldu HG, Sheets LR. Sociodemographic diversity in cancer clinical trials: New findings on the effect of race and ethnicity. Contemp Clin Trials Commun. 2021;21:100718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katz RV, Green BL, Kressin NR, Claudio C, Wang MQ, Russell SL. Willingness of minorities to participate in biomedical studies: confirmatory findings from a follow-up study using the Tuskegee Legacy Project Questionnaire. J Natl Med Assoc. 2007;99(9):1052–1060. [PMC free article] [PubMed] [Google Scholar]
- 52.Katz RV, Green BL, Kressin NR, et al. Exploring the “legacy” of the Tuskegee Syphilis Study: a follow-up study from the Tuskegee Legacy Project. J Natl Med Assoc. 2009;101(2):179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dumville JC, Torgerson DJ, Hewitt CE. Reporting attrition in randomised controlled trials. BMJ. 2006;332(7547):969–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annu Rev Public Health. 2006;27:1–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart of final study population.
Table S1. Comparison of categorical financial-resource strain by type of follow up achieved.
Table S2. ICD-9 and ICD-10 CM Codes Used to Define Clinical Variables to Adjust for in the Log-Binomial Models.
Table S3. Comparison between adjusted risk ratios for full attrition to no attrition or partial attrition by treatment arm (intervention vs control arm).


