Abstract
Introduction:
Observational research suggests that periodontitis affects psoriasis. However, observational studies are prone to reverse causation and confounding, which hampers drawing causal conclusions and the effect direction. We applied the Mendelian randomization (MR) method to comprehensively assess the potential bi-directional association between periodontitis and psoriasis.
Methods:
We used genetic instruments from the largest available genome-wide association study (GWAS) of European descent for periodontitis (17,353 cases, 28,210 controls) to investigate the relationship with psoriasis (13,229 cases, 21,543 controls), and vice versa. The Causal Analysis Using Summary Effect estimates (CAUSE) and inverse variance weighted (IVW) MR analyses were used for the primary analysis. Robust MR approaches were used as sensitivity analyses.
Results:
Both univariable methods CAUSE and IVW MR analyses revealed neither an impact of periodontitis on psoriasis (CAUSE odds ratio (OR)=1.00, P=1.00; IVW OR=1.02. P=0.6247) nor vice versa (CAUSE OR=1.01, P=0.5135; IVW OR=1.00, P=0.7070). The null association was corroborated by pleiotropy-robust methods with odds ratios close to one and P-values > 0.59. Overall, MR analyses did not suggest an effect of periodontitis on psoriasis. Similarily, there was no evidence to support an effect of psoriasis on periodontitis.
Conclusion:
Within the limits of this MR study, the outcomes neither supported periodontitis affecting psoriasis nor psoriasis affecting periodontitis.
Keywords: periodontitis, psoriasis, Mendelian randomization, epidemiology
INTRODUCTION
Periodontitis is a microbially-associated inflammatory disease of the tooth-supporting tissue that affects approximately 50% of the adult population, with 10% suffering from severe periodontitis (Bernabe et al., 2020). Periodontitis is associated with the presence of a dysbiotic microbial community in a susceptible host (Hajishengallis, Chavakis, & Lambris, 2020). Although bacteria are required in the pathogenesis, it is the host inflammatory response to the microbial challenge that drives immune cell-mediated self-degradation of periodontal tissue resulting in tooth loss (van Dyke, Bartold, & Reynolds, 2020). Psoriasis is a chronic papulosquamous inflammatory skin disease often accompanied with concomitant comorbidities approximately prevalent in 2–4% of the general population (Christophers, 2001). A distinct configuration of the skin microbiota have been shown for psoriasis patients characterized by co-occuring bacterial communities (Fyhrquist et al., 2019). The exact role of bacteria, streptococci in particular, in the pathogenesis of psoriasis remains unresolved, an abnormal immune response to dysbiotic skin microbiota has been proposed as mechanistic link (Langan et al., 2018; Langan et al., 2019; Liang et al., 2021).
Whereas an imbalance in the periodontal microbiota and the inflammatory response of the host explains local tissue destruction, it is unclear whether this imbalanced relationship can link periodontal disease with extra-oral comorbidities such as psoriasis. Several observational studies proposed an association between periodontitis and psoriasis, which has been substantiated by meta-analyses (Ungprasert, Wijarnpreecha, & Wetter, 2017) (Zhang, Gu, Xie, & Su, 2020) estimating pooled odds ratios (OR) ranging from of 1.55 (95% confidence interval (CI), 1.35–177) to 2.87 (95% CI, 1.75–4.69). In both periodontitis and psoriasis the predominant immune response is neutrophilic (Christophers, 2017) and further common etiopathogenesis pathways have been hypothesized (Falcao & Bullón, 2019) Proinflammatory cytokines play key roles in both periodontitis and plaque psoriasis. The available evidence from immunological and genetic studies highlighted the immunological circuits that converge on adaptive immune pathways involving IL-17, IL-23, TH17, TNF-alpha and TNF-gamma in psoriasis and periodontitis (Griffiths, Armstrong, Gudjonsson, & Barker, 2021; Hawkes, Chan, & Krueger, 2017; Marchesan et al., 2020). IL-17, TNF-alpha and IL-36 contribute to amplification of self-sustaining inflammatory circuits and influx of neutrophils in psoriasis.
The emerging findings that inflammatory processes in different peripheral tissues may be interlinked may improve our mechanistic understanding of comorbidities. A recent meta-analysis including eight studies reported a significantly worse periodontal health among psoriasis patients (Qiao et al., 2019)
However, both diseases share several risk factors such as smoking, alcohol consumption, stress and immune depression (Sharma, Raman, & Pradeep, 2015) which might act as confounders leading to spurious correlation and are difficult to control in observational studies. In particular, smoking and alcohol consumption have been recently reported to be causally associated with periodontits (Baumeister et al., 2021)
Mendelian randozimation (MR) is an alternative approach to account for observational bias (Burgess, Foley, & Zuber, 2018; Burgess et al., 2019). MR relies on the natural, random assortment of genetic variants during meiosis yielding a random distribution of genetic variants in a population. Individuals are naturally assigned at birth to inherit a genetic variant that affects a risk factor or disease susceptibility, or not inherit such a variant. The natural randomization that occurs in the generation of each individual’s genetic makeup is analogous to the design of a randomized trial. MR uses instrumental variable (IV) analysis where the genetic variants are the instruments, analogous to the random assignment to treatment and control groups in a randomized trial. Because the genetic variants are typically unassociated with confounders, differences in the outcome between those who carry the variant and those who do not can be attributed to differences in risk factor or disease susceptibility. Thus, MR provides a reliable understanding of the influence of modifiable exposures on the trait of interest compared to traditional observational studies which are susceptible to confounding or reverse causation (Richmond & Davey Smith, 2021). We carried out a bidirectional MR study on the association of periodontitis and psoriasis and carried out a series of sensitivity analyses to account for pleiotropic single nucleotide polymorphisms (SNPs) associated with potential confounding factors.
MATERIALS AND METHODS
In MR analyses, genetic variants strongly associated with an exposure will be exploited as IVs to investigate the potential causal effect of an exposure with an outcome of interest (Burgess et al., 2018). Due to the random assignment of genetic variants at conception, MR estimates are not biased by confounding, reverse causation, and measurement error. Most commonly, inference is based on SNPs as IVs identified by genome-wide association studies (GWAS) (Burgess et al., 2018). For the validity of each IV, three main assumptions have to be fulfilled: (1) robust association between the instrument and the exposure (‘relevance’), (2) instruments affects the outcome through the exposure (‘exclusion restriction’) and the genetic variant is not associated with confounders of the exposure-outcome association (‘exchangability’) (Labrecque & Swanson, 2018). To satisfy the first MR assumption, we selected SNPs associated with the exposure at a significance threshold of P<5×10−6 for periodontitis and P<5×10−8 for psoriasis. From these set of SNPs, we select only independent instruments with the lowest p-value by calculating pairwise linkage disequilibrium (LD) and omitting SNPs with r2≥0.001 (Burgess et al., 2019). Further verification of the first assumption was made by computing the F-statistic and the proportion of the explained phenotypic variance by all SNPs (Burgess & Thompson, 2011).
We performed two-sample MR analysis based on summary statistics from the largest available GWAS on periodontitis and psoriasis including 17,353 periodontitis cases and 28,210 controls (Shungin et al., 2019) based on the GLIDE consortium (Shungin et al., 2015) as well as 13,229 psoriasis cases and 21,543 controls (Tsoi et al., 2017). Both GWAS were conducted in people of European Caucasian descent. Periodontitis cases were classified by either the Centers for Disease and Control and Prevention/American Academy of Periodontology case definition (CDC/AAP) (Page & Eke, 2007) or the Community Periodontal Index (CPI) (World Health Organization, 2013), which is based on probing depths and number of deep periodontal pockets (Shungin et al., 2015). Psoriasis was diagnosed by experienced physicians. All included GWAS obtained ethical review board approval and informed consent as described in the respective original manuscript (Shungin et al., 2019; Tsoi et al., 2017).
For analyses, genetic variants from different studies were harmonized with regard to their effects, and palindromic SNPs (i.e. SNPs whose alleles consist of a base and its complementary base) were excluded. In order to maintain consistency in SNPs used as IVs across different analysis, we only used variants available for all examined traits and did not replace missing variants by proxies.
Statistical analyses
Statistical Power was calculated according to Brion et al. (Brion, Shakhbazov, & Visscher, 2013), which exploits asymptotic theory and derives the power via the non-centrality parameter from the respective asymptotic χ2-distribution. As primary analysis we applied the Causal Analysis Using Summary Effect estimates (CAUSE) approach (Morrison, Knoblauch, Marcus, Stephens, & He, 2020), which has been demonstrated to outperform other established methods to detect causal relationships in the presence of pleiotropy (Wang, Gao, Fan, Xue, & Zhou, 2021). In addition, we carried out a series of sensitivity analyses including pleiotropy-robust methods.
First, we harmonized summary statistics results to ensure effect size alignment and to prohibit strand mismatch. Then we carried out CAUSE, which incorporates all genetic variants after clumping and thereby increases statistical power, with periodontitis as exposure and psoriasis as outcome and vice versa. CAUSE used genome-wide summary statistics to disentangle causality (i.e., SNPs affect psoriasis through their effect on periodontitis) from correlated pleiotropy (i.e., violation of the MR exchangeability assumption whereby SNPs are associated with periodontitis and psoriasis through a shared heritable factor), while taking into account uncorrelated horizontal pleiotropy (an exclusion restriction violation where the SNPs associate with periodontitis through separate mechanisms). It uses Bayesian modeling to assess whether the sharing model (the model that fixes the causal effect at zero) fits the data at least as well as the causal model (the model that allows a causal effect different from zero) (Morrison et al., 2020).
To complete the picture, we carried out the standard inverse-variance weighted (IVW) MR with the multiplicative random effects model (Burgess et al., 2019) for which valid estimates of the exposure on the outcome are based on all three fulfilled assumptions. (Labrecque & Swanson, 2018)Weak instrument-exposure association might reduce the plausibility of MR relevance assumption. Therefore, we examined F statistics of the instruments and calculated the explained phenotypic variance. Violations of assumptions (2) and (3) can occur through horizontal pleiotropy, i.e. instruments exert an effect on the outcome independent of the exposure. To investigate pleiotropy, we searched for previously reported associations of instruments with the shared risk factors, in particular smoking and alcohol consumption, in PhenoScanner (www.phenoscanner.medschl.cam.ac.uk/) and the GWAS Catalog (www.ebi.ac.uk/gwas/). In addition, we performed a suite of pleiotropy-robust methods (weighted median, robust adjusted profile score (RAPS), radial regression, MR pleiotropy residual sum and outlier (MR-PRESSO)) to address the issue of pleiotropy (Slob & Burgess, 2020). Finally, we examined the heterogeneity of the ratio estimators using Cohran’s Q, I2 statistic, and the MR Egger intercept, and performed leave-one-SNP-out analysis.
All analyses were performed using the packages cause (1.2.0), MendelianRandomization (0.5.1), TwoSampleMR (0.5.6) and MR-PRESSO (1.0) in R, version 4.0.5. Reporting follows the STROBE-MR statement (Skrivankova et al., 2021). The study protocol was not pre-registered.
RESULTS
Descriptive statistics on phenotypes from studies included in the GWAS on exposure and outcome, including the number of individuals suffering from periodontitis classified by CDC/AAP and CPI, are presented in Table S1.
The CAUSE analysis showed no effect of periodontitis on psoriasis (OR=1.0, 95% credible interval (CredIn)=0.16–6.17, P=1.00). Similarily, CAUSE did not indicate an effect of psoriasis on periodontitis (OR=1.01, 95% CredIn=0.97–1.04, P=0.5135). Similar effect estimates were produced when the IVW method was used (Table 1, Figure 1).
Table 1.
Mendelian randomization estimates for the relationship between genetically instrumented periodontitis and psoriasis and vice versa
| Outcome | Exposure | Method | OR | (95% CI) | P value |
|---|---|---|---|---|---|
| psoriasis | periodontitis | Inverse variance weighted | 1.017 | (0.95–1.088) | 0.6247 |
| Weighted median | 1.020 | (0.926–1.123) | 0.6868 | ||
| Robust adjusted profile score | 1.019 | (0.947–1.098) | 0.6098 | ||
| IVW radial | 1.017 | (0.956–1.082) | 0.5918 | ||
| MR-PRESSO | 1.017 | (0.956–1.082) | 0.5935 | ||
| periodontitis | psoriasis | Inverse variance weighted | 0.995 | (0.967–1.023) | 0.7070 |
| Weighted median | 1.000 | (0.959–1.044) | 0.9837 | ||
| Robust adjusted profile score | 0.995 | (0.965–1.026) | 0.7471 | ||
| IVW radial | 0.995 | (0.967–1.023) | 0.7014 | ||
| MR-PRESSO | 0.995 | (0.967–1.023) | 0.7033 |
IVW, inverse variance weighted; OR, odds ratio; CI, confidence interval; MR-PRESSO, MR Pleiotropy RESidual Sum and Outlier.
Figure 1.
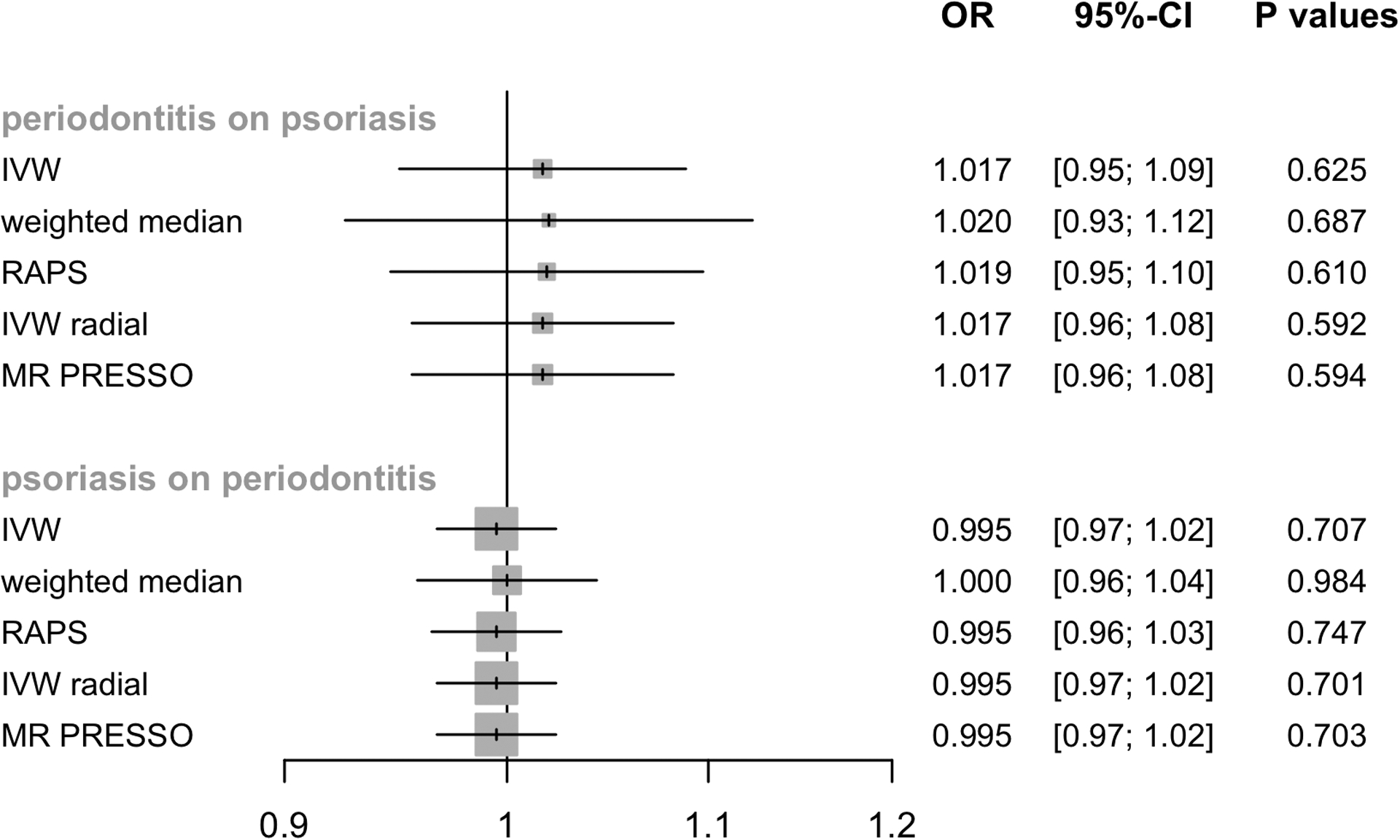
Mendelian randomization estimates for the relationship between genetically instrumented periodontitis and psoriasis and vice versa. IVW, inverse variance weighted; RAPS, Robust adjusted profile score; MR-PRESSO, MR Pleiotropy RESidual Sum and Outlier; OR, odds ratio; CI, confidence interval.
Pleiotropy-robust methods corroborated the findings of no effect of periodontitis on psoriasis (0.5918≤P≤0.6868) or vice versa (0.7014≤P≤0.9837, Table 1). The 76 and 46 SNPs selected als IVs for periodontitis and psoriasis explained 3.1% and 14.6% of the variance, respectively (Table S2). The F-statistics ranged between 16.5–25.1 and 29.9–918.3 for periodontitis and psoriasis (Table S2). Both, the explained phenotypic variance and the high F-statistics of the variants verifies MR assumption (1). No substantial heterogeneity between Wald ratios in the IVW estimate was observed (Table 2) and leave-one-out analysis did not reveal any leverage points with high influence (Table S3). Using the PhenoScanner and GWAS Catalog, we also did not find any genetic instrument of periodontits and psoriasis to be associated with any potential confounder (Table S4). Pleiotropy robust MR methods showed similar results to IVW (Table 1, Figure 1). Low or no heterogeneity, no indication of potential confounding SNPs and similar results by pleiotropy robust analyses implicitly reassures that MR assumptions (2) and (3) hold.
Table 2.
Heterogeneity of Wald ratios and MR-Egger test for directional pleiotropy
| Heterogeneity | |||||
|---|---|---|---|---|---|
| Exposure | Outcome | Q | df | I2 | p value |
| psoriasis | periodontitis | 42.3 | 44 | 0.04 | 0.5443 |
| MR-Egger test for directional pleiotropy | |||||
| Exposure | Outcome | Intercept | se | p value | |
| psoriasis | periodontitis | −0.0009 | 0.0054 | 0.8680 | |
Q, heterogeneity statistic Q; df, degree of freedom; se, standard error
On a 5% significance level, our analyses had a power of >87% to detect a causal effect of OR=1.2 of periodontitis on psoriasis and a power of >96% for a causal effect of OR=1.1 of psoriasis on periodontitis (Table S5).
DISCUSSION
The results of the MR study do not support evidence for an effect of periodontitis on psoriasis or for an effect of psoriasis on periodontitis. The MR analysis, the CAUSE in particular, was powered to detect small effects, and produced consistent estimates using different MR techniques. Our MR estimates contradict the available observational studies, which suggested a bidirectional association of periodontitis and psoriasis (Qiao et al., 2019; Ungprasert et al., 2017; Zhang et al., 2020). Although several pathogenic links between both diseases have been suggested due to shared common immunologic response (Dalmády, Kemény, Antal, & Gyulai, 2020; Griffiths et al., 2021; Hawkes et al., 2017; Marchesan et al., 2020), our MR study does not support a causal link in either direction, as the estimated relative risks were close to zero. One explanation could be that previously observed associations between periodontitis and psoriasis is coincidental or thwarted by an unknown confounder. Moreover, a causal link between periodontitis and psoriasis or conversely cannot be established in observational studies because most patients with periodontitis exhibit systemic health problems. Periodontitis is often accompanied by a range of systemic diseases (Hajishengallis & Chavakis, 2021; Holmstrup et al., 2017; Teles, Wang, Hajishengallis, Hasturk, & Marchesan, 2021) and most psoriatic patients suffer from numerous comorbidities (Pearce et al., 2005) including rheumatological, cardiovascular and psychiatric complications (Greb et al., 2016). Therefore, it is possible that these comorbidities, and in particular those sharing inflammatory pathways, contribute to the association between periodontitis and psoriasis.
Nevertheless, the applied complementary MR approaches yielded homogeneous results with point estimates slightly above and below 1 for both hypotheses - psoriasis influencing periodontitis and vice versa - with highly overlapping confidence intervals. Consequently, it is unlikely that periodontitis and psoriasis are causally related. If this is the case, the strength of the effects are unlikely to be of clinical significance. The study had limitations. MR based on genetic summary statistics limits the range of analyses. However, based on the observed and consistent negative results from several complementary approaches with effect estimates close to one, it is unlikely that the finding is distorted by any form of bias. MR analyses are less susceptible to bias involving violation of MR assumptions and provide robust evidence when effects are very small or absent (VanderWeele et al., 2014). The CAUSE analysis exploits the full range of genetic variants with no evidence for a causal relationship between periodontitis and psoriasis. The wide credible intervals for these estimates may suggest low power to rule out very small effects. However, the null association was corroborated by univariable and pleiotropy-robust MR approaches. The genetic instruments explained 3.4% and 14.6% of the phenotypic variance of periodontitis and psoriasis with minimum F statistics of 16.48 and 29.94, consistent with the absence of weak instrument bias. One should keep in mind that MR studies are located at the interface between observational and interventional studies and therefore in the evidence-based pyramid below randomized clinical trials (RCTs) and systematic reviews of RCTs (Davies, Holmes, & Davey Smith, 2018). Another limitation is that the partial CPI commonly underestimates severe periodontitis and overestimates less-severe periodontitis (Baelum, Fejerskov, Manji, & Wanzala, 1993), which miss-identified the numerator of the MR Wald ratio estimator.
CONCLUSION
In conclusion, our study does not indicate that periodontitis causally affects psoriasis or conversely. However, caution is warranted before generalization of the findings and further studies are needed to finally clarify the subject. We stress on the importance of combining evidence from multiple lines of observational and experimental research that align to strengthen causal inference (Munafò, Higgins, & Smith, 2021). In-vivo studies of animal models would thus further strengthen the evidence on putative bidirectional causation between periodontitis and psoriasis.
Supplementary Material
CLINICAL RELEVANCE.
Scientific rational for the study:
Previous observational research suggested a relationship between periodontitis and psoriasis. Observational estimates on the association of periodontitis and psoriasis might be subject to confounding or reverse causation.
Principal findings:
Application of complementary Mendelian randomization approaches to investigate causality did not indicate an effect between periodontitis and psoriasis, and vice versa.
Practical implications:
The study results do not suggest that treatment for periodontal disease or psoriasis would improve comorbid disease activity.
Acknowledgments:
This work received no specific financial support. JTE was supported by awards R01AR042742, R01AR050511, R01AR054966, R01AR063611, and R01AR065183 from the National Institutes of Health, and by the Ann Arbor VA Hospital. LCT was supported by an award (K01AR072129) from the National Institutes of Health, and by awards from the Dermatology Foundation, the National Psoriasis Foundation, and the Arthritis National Research Foundation.
Conflicts of Interest Statement:
All authors disclose no conflict of interest and no financial support.
Data Availability Statement:
The summary statistics for the periodontitis GWAS are available at https://data.bris.ac.uk/data/dataset/2j2rqgzedxlq02oqbb4vmycnc2. The GWAS summary statistics for psoriasis without the 23andMe component can be made available on request. Code Availability: the analysis code in R is available on request.
REFERENCES
- Baelum V, Fejerskov O, Manji F, & Wanzala P (1993). Influence of CPITN partial recordings on estimates of prevalence and severity of various periodontal conditions in adults. Community Dentistry and Oral Epidemiology, 21(6), 354–359. 10.1111/j.1600-0528.1993.tb01098.x [DOI] [PubMed] [Google Scholar]
- Baumeister S-E, Freuer D, Nolde M, Kocher T, Baurecht H, Khazaei Y, … Holtfreter B (2021). Testing the association between tobacco smoking, alcohol consumption, and risk of periodontitis: A Mendelian randomization study. Journal of Clinical Periodontology. [DOI] [PubMed] [Google Scholar]
- Bernabe E, Marcenes W, Hernandez CR, Bailey J, Abreu LG, Alipour V, … Kassebaum NJ (2020). Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. Journal of Dental Research, 99(4), 362–373. 10.1177/0022034520908533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion M-JA, Shakhbazov K, & Visscher PM (2013). Calculating statistical power in Mendelian randomization studies. International Journal of Epidemiology, 42(5), 1497–1501. 10.1093/ije/dyt179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, … Theodoratou E (2019). Guidelines for performing Mendelian randomization investigations. Wellcome Open Research, 4, 186. 10.12688/wellcomeopenres.15555.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Foley CN, & Zuber V (2018). Inferring Causal Relationships Between Risk Factors and Outcomes from Genome-Wide Association Study Data. Annual Review of Genomics and Human Genetics, 19, 303–327. 10.1146/annurev-genom-083117-021731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, & Thompson SG (2011). Avoiding bias from weak instruments in Mendelian randomization studies. International Journal of Epidemiology, 40(3), 755–764. 10.1093/ije/dyr036 [DOI] [PubMed] [Google Scholar]
- Christophers E (2001). Psoriasis--epidemiology and clinical spectrum. Clinical and Experimental Dermatology, 26(4), 314–320. 10.1046/j.1365-2230.2001.00832.x [DOI] [PubMed] [Google Scholar]
- Christophers E (2017). Periodontitis and risk of psoriasis: Another comorbidity. Journal of the European Academy of Dermatology and Venereology: JEADV, 31(5), 757–758. 10.1111/jdv.14249 [DOI] [PubMed] [Google Scholar]
- Dalmády S, Kemény L, Antal M, & Gyulai R (2020). Periodontitis: A newly identified comorbidity in psoriasis and psoriatic arthritis. Expert Review of Clinical Immunology, 16(1), 101–108. 10.1080/1744666X.2019.1700113 [DOI] [PubMed] [Google Scholar]
- Davies NM, Holmes MV, & Davey Smith G (2018). Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ (Clinical Research Ed.), 362, k601. 10.1136/bmj.k601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcao A, & Bullón P (2019). A review of the influence of periodontal treatment in systemic diseases. Periodontology 2000, 79(1), 117–128. 10.1111/prd.12249 [DOI] [PubMed] [Google Scholar]
- Fyhrquist N, Muirhead G, Prast-Nielsen S, Jeanmougin M, Olah P, Skoog T, … Alenius H (2019). Microbe-host interplay in atopic dermatitis and psoriasis. Nature Communications, 10(1), 4703. 10.1038/s41467-019-12253-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, … Gottlieb AB (2016). Psoriasis. Nature Reviews. Disease Primers, 2, 16082. 10.1038/nrdp.2016.82 [DOI] [PubMed] [Google Scholar]
- Griffiths CEM, Armstrong AW, Gudjonsson JE, & Barker JNWN (2021). Psoriasis. Lancet (London, England), 397(10281), 1301–1315. 10.1016/S0140-6736(20)32549-6 [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, & Chavakis T (2021). Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nature Reviews. Immunology, 21(7), 426–440. 10.1038/s41577-020-00488-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Chavakis T, & Lambris JD (2020). Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontology 2000, 84(1), 14–34. 10.1111/prd.12331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes JE, Chan TC, & Krueger JG (2017). Psoriasis pathogenesis and the development of novel targeted immune therapies. The Journal of Allergy and Clinical Immunology, 140(3), 645–653. 10.1016/j.jaci.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrecque J, & Swanson SA (2018). Understanding the Assumptions Underlying Instrumental Variable Analyses: A Brief Review of Falsification Strategies and Related Tools. Current Epidemiology Reports, 5(3), 214–220. 10.1007/s40471-018-0152-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan EA, Griffiths CEM, Solbach W, Knobloch JK, Zillikens D, & Thaçi D (2018). The role of the microbiome in psoriasis: Moving from disease description to treatment selection? The British Journal of Dermatology, 178(5), 1020–1027. 10.1111/bjd.16081 [DOI] [PubMed] [Google Scholar]
- Langan EA, Künstner A, Miodovnik M, Zillikens D, Thaçi D, Baines JF, … Knobloch JK (2019). Combined culture and metagenomic analyses reveal significant shifts in the composition of the cutaneous microbiome in psoriasis. The British Journal of Dermatology, 181(6), 1254–1264. 10.1111/bjd.17989 [DOI] [PubMed] [Google Scholar]
- Liang X, Ou C, Zhuang J, Li J, Zhang F, Zhong Y, & Chen Y (2021). Interplay Between Skin Microbiota Dysbiosis and the Host Immune System in Psoriasis: Potential Pathogenesis. Frontiers in Immunology, 12, 764384. 10.3389/fimmu.2021.764384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesan JT, Girnary MS, Moss K, Monaghan ET, Egnatz GJ, Jiao Y, … Swanson KV (2020). Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontology 2000, 82(1), 93–114. 10.1111/prd.12269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J, Knoblauch N, Marcus JH, Stephens M, & He X (2020). Mendelian randomization accounting for correlated and uncorrelated pleiotropic effects using genome-wide summary statistics. Nature Genetics, 52(7), 740–747. 10.1038/s41588-020-0631-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Higgins JPT, & Smith GD (2021). Triangulating Evidence through the Inclusion of Genetically Informed Designs. Cold Spring Harbor Perspectives in Medicine, 11(8). 10.1101/cshperspect.a040659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RC, & Eke PI (2007). Case definitions for use in population-based surveillance of periodontitis. Journal of Periodontology, 78(7 Suppl), 1387–1399. 10.1902/jop.2007.060264 [DOI] [PubMed] [Google Scholar]
- Pearce DJ, Morrison AE, Higgins KB, Crane MM, Balkrishnan R, Fleischer AB, & Feldman SR (2005). The comorbid state of psoriasis patients in a university dermatology practice. The Journal of Dermatological Treatment, 16(5–6), 319–323. 10.1080/09546630500335977 [DOI] [PubMed] [Google Scholar]
- Qiao P, Shi Q, Zhang R, E L, Wang P, Wang J, & Liu H (2019). Psoriasis Patients Suffer From Worse Periodontal Status-A Meta-Analysis. Frontiers in Medicine, 6, 212. 10.3389/fmed.2019.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond RC, & Davey Smith G (2021). Mendelian Randomization: Concepts and Scope. Cold Spring Harbor Perspectives in Medicine. Advance online publication. 10.1101/cshperspect.a040501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Raman A, & Pradeep AR (2015). Association of chronic periodontitis and psoriasis: Periodontal status with severity of psoriasis. Oral Diseases, 21(3), 314–319. 10.1111/odi.12271 [DOI] [PubMed] [Google Scholar]
- Shungin D, Cornelis MC, Divaris K, Holtfreter B, Shaffer JR, Yu Y-H, … Franks PW (2015). Using genetics to test the causal relationship of total adiposity and periodontitis: Mendelian randomization analyses in the Gene-Lifestyle Interactions and Dental Endpoints (GLIDE) Consortium. International Journal of Epidemiology, 44(2), 638–650. 10.1093/ije/dyv075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungin D, Haworth S, Divaris K, Agler CS, Kamatani Y, Keun Lee M, … Johansson I (2019). Genome-wide analysis of dental caries and periodontitis combining clinical and self-reported data. Nature Communications, 10(1), 2773. 10.1038/s41467-019-10630-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slob EAW, & Burgess S (2020). A comparison of robust Mendelian randomization methods using summary data. Genetic Epidemiology, 44(4), 313–329. 10.1002/gepi.22295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrivankova Veronika W.; Richmond Rebecca C.; Woolf Benjamin A. R.; Davies Neil M.; Swanson Sonja A.; VanderWeele Tyler J. et al. (2021). Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ, 375, n2233. 10.1136/bmj.n2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles F, Wang Y, Hajishengallis G, Hasturk H, & Marchesan JT (2021). Impact of systemic factors in shaping the periodontal microbiome. Periodontology 2000, 85(1), 126–160. 10.1111/prd.12356 [DOI] [PubMed] [Google Scholar]
- Tsoi LC, Stuart PE, Tian C, Gudjonsson JE, Das S, Zawistowski M, … Elder JT (2017). Large scale meta-analysis characterizes genetic architecture for common psoriasis associated variants. Nature Communications, 8, 15382. 10.1038/ncomms15382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungprasert P, Wijarnpreecha K, & Wetter DA (2017). Periodontitis and risk of psoriasis: A systematic review and meta-analysis. Journal of the European Academy of Dermatology and Venereology : JEADV, 31(5), 857–862. 10.1111/jdv.14051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ, Tchetgen Tchetgen EJ, Cornelis M, & Kraft P (2014). Methodological challenges in mendelian randomization. Epidemiology, 25, 427–435. 10.1097/EDE.0000000000000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke TE, Bartold PM, & Reynolds EC (2020). The Nexus Between Periodontal Inflammation and Dysbiosis. Frontiers in Immunology, 11, 511. 10.3389/fimmu.2020.00511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gao B, Fan Y, Xue F, & Zhou X (2021). Mendelian randomization under the omnigenic architecture. Briefings in Bioinformatics. Advance online publication. 10.1093/bib/bbab322 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2013). Oral Health Surveys: Basic Methods (5th ed.). Nonserial Publications. Geneva: World Health Organization. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=847173 [Google Scholar]
- Zhang X, Gu H, Xie S, & Su Y (2020). Periodontitis in patients with psoriasis: A systematic review and meta-analysis. Oral Diseases. Advance online publication. 10.1111/odi.13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The summary statistics for the periodontitis GWAS are available at https://data.bris.ac.uk/data/dataset/2j2rqgzedxlq02oqbb4vmycnc2. The GWAS summary statistics for psoriasis without the 23andMe component can be made available on request. Code Availability: the analysis code in R is available on request.


