Abstract
The prefrontal cortex (PFC) is intimately associated with behavioral characteristics of alcohol use disorders, including high motivation to drink and difficulty with moderation. Thus, continued mechanistic research investigating PFC cells and targets altered by ethanol experiences should inform translational efforts to craft new, efficacious treatments. Inhibitory interneurons expressing parvalbumin (PV-INs) comprise only a minor fraction of cells within PFC, yet these cells are indispensable for coordinating PFC ensemble function, oscillatory activity, and subcortical output. Based on this, PV-INs represent an exciting target for the rational design of breakthrough treatments for alcohol use disorders. Here, we assessed experience-dependent physiological adaptations via ethanol place conditioning. By manipulating the timing of administration relative to conditioning sessions, equivalent ethanol exposure can form either rewarding or aversive memories in different individuals. Here, we found that female mice and male mice on a C57BL/6J background display conditioned place preference (CPP) or aversion (CPA) to an intoxicating dose of ethanol (2 g/kg, intraperitoneal) without overt differences between sexes. Ethanol reward learning was associated with decreased PV-IN excitability in deep layer prelimbic PFC, whereas PV-INs from CPA mice were not different from controls. Furthermore, PV-INs from mice in the CPP group, but not the CPA group, displayed potentiated excitatory synaptic strength that emerged during one-week of abstinence. Taken together, these findings illustrate that synaptic and intrinsic adaptations associated with ethanol can depend on an individual’s experience. These studies provide further context and support for PFC PV-INs as intriguing targets for modulating alcohol associations.
Keywords: electrophysiology, addiction, drug abuse, preclinical model, fast-spiking interneuron, plasticity
Introduction
Breakthrough treatments are needed to help alleviate suffering caused by maladaptive drinking and alcohol use disorders (AUDs) (Grant et al., 2017; Whiteford et al., 2013). In turn, the rational development of new pharmacological or brain stimulation-based treatment options will be driven by a better understanding of the molecular and neurocircuit mechanisms through which ethanol exposure alters brain function. Difficulty in controlling drinking and high cue-associated motivation for alcohol are each associated with prefrontal cortex (PFC) function (Blaine et al., 2020; Courtney et al., 2016; Grusser et al., 2004; Koob, 2014; Yang et al., 2021). Conversely, population-level analyses have detected differences in PFC structure and function related to heavy drinking or diagnosis of an AUD (Medina et al., 2008; Sorg et al., 2012; Wang et al., 2016). Together, these basic findings motivate continued translational studies to identify cells and targets within PFC that undergo neuroadaptations following chronic alcohol exposure.
Preclinical studies indicate that PFC pyramidal cells regulate drinking and other motivated behaviors through long-range projections to subcortical structures (Diehl et al., 2020; Klenowski, 2018; Marcus et al., 2020; Seif et al., 2013; Siciliano et al., 2019). Nonetheless, inhibitory interneurons expressing parvalbumin (PV-INs) are indispensable for coordinating pyramidal cell activity and PFC output (Ferguson and Gao, 2018). PV-INs have been described as “basket cells” based on their distinguishing synapses that encase the cell bodies of nearby pyramidal cells (Bartos and Elgueta, 2012). These synapses exert rapid, high-fidelity feedforward inhibition to orchestrate neuronal ensembles and synchronize network activity (Atallah et al., 2012; Sohal et al., 2009). Based on these properties, PV-INs represent a potential anatomical substrate involved in reduced alcohol cravings induced by PFC-directed transcranial magnetic stimulation (Herremans et al., 2015; Philip et al., 2020; Salling and Martinez, 2016). In addition, their unique features confer PV-INs with tremendous potential for developing microcircuit-specific pharmacological interventions. Thus, their neurobiological properties indicate that PV-INs represent an exciting target for the development of breakthrough psychiatric treatments.
Convergent studies show that manipulating PV-IN function affects drinking in multiple models (Melon et al., 2018; Patton et al., 2021; Radke et al., 2017); but conversely, how does ethanol experience alter PV-IN function? In high-exposure models of dependence, ethanol experience is associated with decreased PFC PV-IN excitability one-day into withdrawal (Hughes et al., 2020) and impaired dopamine-evoked modulation at one-week withdrawal (Trantham-Davidson et al., 2014). By contrast, our laboratory has detected increased PFC PV-IN excitability during one-day abstinence from ethanol in volitional models (Joffe et al., 2020b) where only moderate blood ethanol concentrations (BECs) are attained (Hwa et al., 2011; Salling et al., 2018). We also detected sex-dependent adaptations to synaptic physiology, whereby PV-INs from female mice displayed decreased excitatory synaptic strength following intermittent access to ethanol, whereas comparable cells from male mice exhibited increased synaptic strength. On the other hand, alterations to PV-IN synaptic physiology have not been reported in models of higher ethanol exposure. Building off this literature, we sought to evaluate whether physiological adaptations to PFC PV-INs occur following specific ethanol experiences.
Place conditioning offers one way to assess experience-dependent physiological adaptations. Individuals have unique experiences, relationships, and memories with alcohol, in part due to its combination of rewarding and aversive properties. Place conditioning offers a versatile means to assess the subjective effects of ethanol in mice. Extensive research from the Cunningham lab (Cunningham et al., 2002; Cunningham et al., 2006; Cunningham et al., 2003), as well as from others more recently (Pati et al., 2019), has demonstrated that ethanol administration can facilitate conditioned place preference (CPP) or conditioned place aversion (CPA) depending on the timing of administration. That is, ethanol delivery immediately prior to exposure to the conditioned stimulus (CS+) can engender CPP, whereas administration following CS+ exposure leads to conditioned place aversion (CPA). By manipulating the timing of ethanol delivery, rewarding and aversive memories may be formed following a controlled and equivalent level of ethanol exposure.
Here, we conditioned separate groups of mice to display either ethanol CPP or CPA by repeatedly administering an intoxicating dose of ethanol (2 g/kg intraperitoneal) either immediately before or after conditioning sessions, respectively. Female mice and male mice on a C57BL/6J background readily displayed CPP or CPA without overt differences between sexes. Despite equivalent exposure to ethanol, CPP mice, but not CPA mice, displayed decreased PV-IN excitability in layer 5 prelimbic cortex. Moreover, ethanol reward learning was specifically associated with increased excitatory synaptic strength on PV-INs and the phenotype emerged during the first week of abstinence from ethanol. These findings illustrate that ethanol-induced neuronal adaptations can vary based on behavioral experience and suggest that PFC PV-INs represent an intriguing target for modulating persistent ethanol reward memories.
Material and Methods
Mice
Mice were housed 2-5 per cage on a standard light cycle (on at 6:00 am). C57BL/6J mice obtained from The Jackson Laboratory were used to establish ethanol place conditioning in initial studies. For electrophysiology experiments, mice were bred and maintained in-house as described in previous studies (Joffe et al., 2020b). In brief, transgenic mice (on a C57BL/6J background) expressing tdTomato fluorescent protein in PV-INs were bred by crossing female homozygous PV-Cre mice (Hippenmeyer et al., 2005) (Jackson Laboratories, Stock No: 017320) with male homozygous Rosa26-loxP-STOP-loxP-CAG-tdTomato “Ai9” mice (Jackson Laboratories, Stock No: 007909). Mice were classified as female or male by their external genitalia and these studies are limited by this definition. Mice were 8-10 weeks at the onset of studies. Mice underwent 2-3 days of handling without injections before experiments. All experiments were approved by the Institutional Animal Care and Use Committee, Vanderbilt University.
Place conditioning
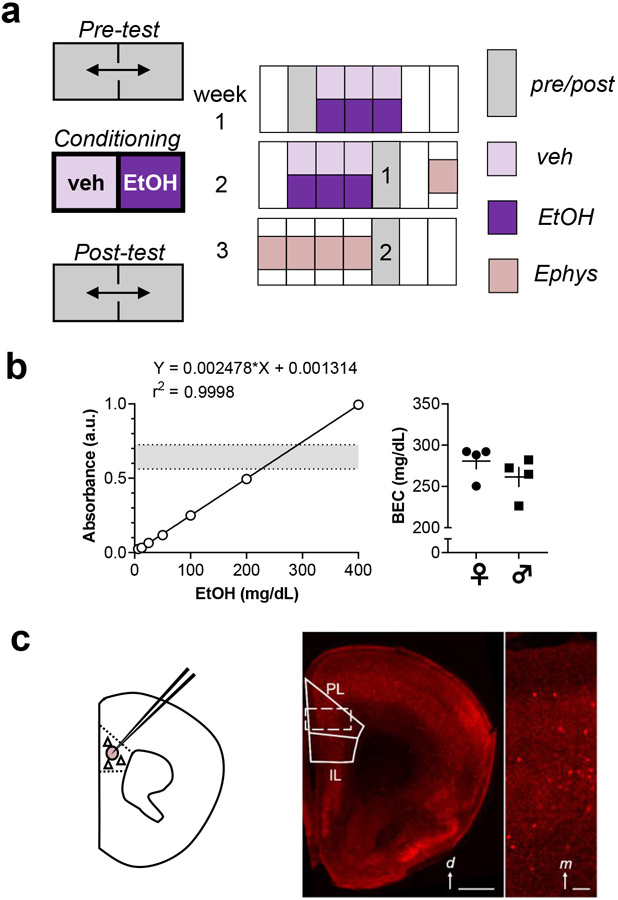
Place conditioning methods were adapted from previous studies and protocols (Cunningham et al., 2006; Pati et al., 2019). Experiments were performed with mixed-sex cohorts and contained balanced numbers of mice across conditioning groups (control, CPP, and CPA). Experiments consisted of a pre-test, 12 saline/ethanol pairings over 6 days (conditioning), and a post-test (Figure 1a). Animals were then sacrificed for electrophysiology 2-7 days later (i.e. 3-8 days following the last ethanol injection). To evaluate the durability of ethanol place conditioning, mice in the validation studies underwent a second post-test session one week after the first. Mice were conditioned in a commercially available, 27.3 x 27.3 x 20.3 cm two-chambered apparatus (Med Associates Inc.). An insert with 2 rooms containing distinct metal grid floors (thick bars; thin crosswise grid) was inserted into an open field chamber (Med Associates) and mouse position was tracked using infrared detection. During pre-test trials, mice were allowed to freely explore the 2 distinct sides of the chamber for 20 minutes to obtain baseline data. As we have found previously (Joffe et al., 2017), C57BL/6J mice generally display an innate preference for the thin crosswise grid floor at baseline. Based on this, we employed a biased place conditioning design. The ethanol-paired conditioned stimulus (CS+) was assigned to each CPP subject as its initially non-preferred side, while the CS+ for each CPA mouse was set to its initially preferred side. Thus, most CPP mice were assigned ethanol injections to be paired with the side with thick bars while most CPA mice received ethanol pairings associated with the side with the crosswise grid. CS+ side assignments were randomized and counterbalanced for control mice. Each conditioning session lasted 5 minutes. On each conditioning day, all mice received a vehicle injection (0.9% saline, intraperitoneal) immediately prior to being placed on the non-paired (CS-) side of the apparatus during a morning session. CS+ conditioning sessions were then performed each day >4 hours later in the afternoon to mitigate residual effects of ethanol from influencing CS- conditioning trials. CPP mice received ethanol (2 g/kg, 26.5% v/v, 10 mL/kg) immediately before being placed on the CS+ side; CPA mice received ethanol immediately after being removed from the CS+ side, prior to return to homecage; and control mice received a vehicle injection prior to being placed on the CS+ side. During 20-minute post-test trials, mice were allowed access to both sides of the conditioning chamber to re-assess their place preferences. The difference in time spent on the CS+ side between the post-test and the pre-test, normalized to the length of the test sessions (1200 seconds), was evaluated as a primary measurement of ethanol reward/aversion learning. The time spent at rest during the test sessions was also evaluated as a measurement related to aversion learning.
Figure 1. Methodology.
(a) Place conditioning experiments consisted of a 20-minute pre-test; 12, 5-minute saline/ethanol pairings over 6 days; and 2, 20-minute post-tests separated by one week. Animals sacrificed for electrophysiology only underwent the first post-test session. Control mice received a saline vehicle injection before all conditioning sessions. Conditioned place preference (CPP) mice received 2 g/kg ethanol i.p. immediately prior to each 5-minute ethanol conditioning session in the afternoons. Conditioned place aversion (CPA) mice received ethanol immediately after each afternoon conditioning session. (b) Blood ethanol concentrations (BECs) were obtained using an enzymatic colorimetric assay. Left, standard curve displaying high correlation between ethanol concentration and 340-nm absorbance. Dotted lines and shaded area represent the range of values obtained from experimental samples. Right, summarized data displaying intoxication-level BECs obtained from mice 5 minutes after systemic ethanol injection. Female mice (circles) and male mice (squares) displayed comparable BECs (281±10 vs 261±12 mg/dL; t-test: t6 = 1.2, n.s.). N = 4 mice per group. (c) In some studies, acute brain slices were prepared for whole-cell patch-clamp electrophysiology from mice expressing tdTomato fluorescent protein in interneurons expressing parvalbumin (PV-INs). Left, schematic indicating breeding strategy and recording location in prelimbic (PL) prefrontal cortex (PFC). Right, representative image displaying interspersed tdTomato fluorescence throughout neocortex. d, dorsal; IL, infralimbic Scale bar 1 mm. Inset, digital zoom displaying fluorescent PV-INs within PL PFC. m, medial. Scale bar 100 μm.
Blood ethanol concentration (BEC) assessment
BECs were determined from a subset of mice to validate systemic levels of ethanol consistent with intoxication (Figure 1b). Mice received identical ethanol injections as administered in place conditioning studies (2 g/kg, 0.9% saline, intraperitoneal), 5 minutes prior to sacrifice. Trunk blood was collected following decapitation under deep isoflurane anesthesia. Whole blood samples were collected in BD Vacutainer® heparin tubes. From heparin tubes, 0.2 mL of blood samples were added to 1.8 mL trichloroacetic acid solution (6.25% w/v), mixed thoroughly, and centrifuged for 5 minutes at 2000g to extract plasma. Ethanol concentrations were assessed following the instructions on a commercially available kit (Pointe Scientific Alcohol Reagent Kit, A750439). For each sample, 10 μL of supernatant was diluted 1:10 in 0.9% sterile saline. Then, 10 μl of each dilution was added to a single well within a 96-well plate. Reagent containing ethanol dehydrogenase was formulated and warmed according to packaged instructions in water and 200 μL was added to each well. After 5 minutes incubation, the plate was analyzed using FlexStation 3 Multi-Mode Microplate Reader. A standard curve was plotted from serial dilutions of 400 mg/dL ethanol standard (Sigma E-036) at 200, 100, 50, 25, 12.5, and 6.25 mg/dL in saline. Blank control wells (saline + reagent) were used to obtain a baseline control absorbance subtracted from standard curve and experimental subject absorbances. All samples were evaluated in duplicate.
Electrophysiology
Acute prelimbic PFC slices were prepared as described (Di Menna et al., 2018). Mice were deeply anesthetized with isoflurane and decapitated. Brains were removed without perfusion and slices were prepared using N-methyl-D-glucamine (NMDG) solution. Immediately after preparation, 300 μM coronal slices recovered for 10 minutes in 30-32°C NMDG solution, (in mM): 93 NMDG, 20 HEPES, 2.5 KCl, 0.5 CaCl2, 10 MgCl2, 1.2 NaH2PO4, 25 glucose, 5 Na-ascorbate, and 3 Na-pyruvate. Slices were then maintained for >1 hour in 22-24°C artificial cerebrospinal fluid, (in mM): 119 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 1 NaH2PO4, 11 glucose, and 26 NaHCO3. Membrane properties were assessed in current-clamp using a potassium-based internal solution (in mM): 125 K-gluconate, 4 NaCl, 10 HEPES, 4 MgATP, 0.3 NaGTP, 10 Tris-phosphocreatine. Pipette tips filled with internal solution measured 3-6 MΩ in artificial cerebrospinal fluid. PV-INs in layer 5 were targeted by tdTomato fluorescence, and all PV-INs displayed the characteristic membrane properties of fast-spiking interneurons. After obtaining whole-cell access, cells were dialyzed for 5 minutes while in voltage clamp at −80 mV. PV-INs were then switched to current clamp and a series of 21, 1-sec current injections were applied. Protocols and analyses have been thoroughly described in previous publications (Joffe et al., 2020a; Joffe et al., 2020b). Injections began at −150 pA, were incremented at 25 pA, and ended at +350 pA. Membrane resistance was calculated as the slope of the membrane potential hyperpolarization in response to three sequential current injections (−25, −50, −75 pA). Spikes per current injection were detected using a threshold search. Rheobase was defined as the minimum current injection needed to evoke one action potential during this current step procedure. Cells were then switched to voltage clamp configuration, and spontaneous excitatory synaptic transmission was collected over two minutes. Interneurons were clamped at −80 mV, near the chloride reversal potential, to electrically isolate spontaneous excitatory postsynaptic currents (sEPSCs) (Joffe et al., 2020b). sEPSCs were detected with a pre-defined template search optimized for the fast kinetics of EPSCs on PV-INs. Slice treatment and data collection protocols were identical across sexes and treatment conditions, which were always controlled with littermates. For each cohort, recordings were performed over one week and we counterbalanced sexes/conditions by recording from two different groups most days. The experimenter was blinded to the group (control/CPP/CPA) but blinding by sex was not possible.
Imaging
Representative brain slice images were obtained using a Zeiss LSM 710 microscope META Inverted provided by Vanderbilt Cell Imaging Shared Resource.
Statistics
The number of cells and/or mice is indicated by “n” and/or “N” respectively, in each figure legend. No more than four cells were recorded from one mouse. Data are presented as mean ± standard error or as box plots displaying median, interquartile range, and range. Linear least squares best-fit regression, two-way ANOVA, and three-way ANOVA were used as appropriate and performed using GraphPad Prism. Where significant (α: 0.05) main effects or interactions were observed, we used Sidak post-hoc comparisons to assess specific differences. All statistical findings are displayed in the figures or legends.
Results
Ethanol administration can induce conditioned place preference and place aversion
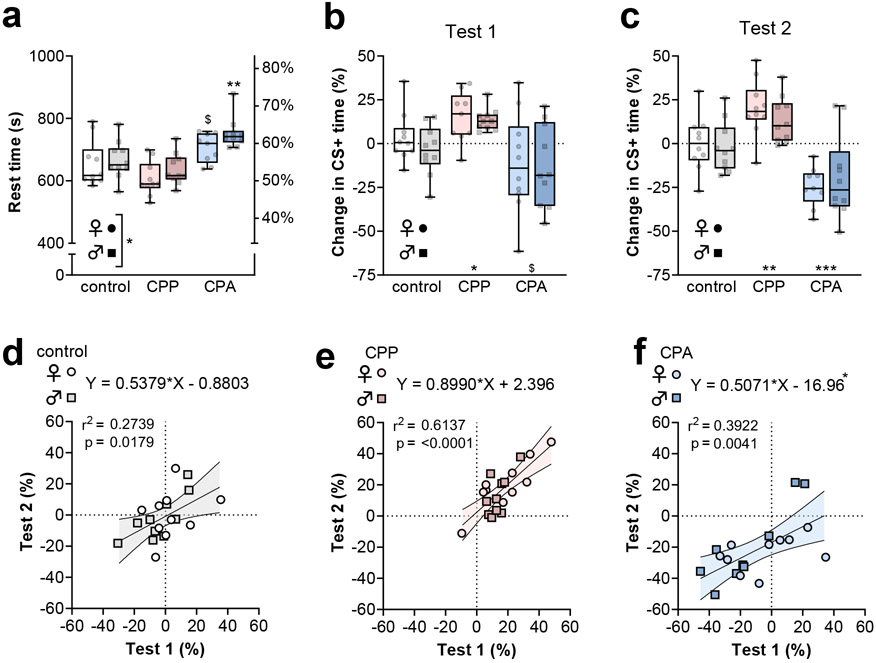
While previous studies have generally been limited to male mice of the DBA2J substrain, we aimed to leverage congenic transgenic strains and therefore first sought to characterize whether CPP and CPA could be readily elicited in C57BL/6J mice of all sexes. Ethanol conditioning was conducted during 6 sessions over 2 weeks. Following an initial place conditioning pre-test, we administered an intoxicating dose of ethanol (2 g/kg, i.p.) to C57BL/6J mice immediately before or after being confined to the CS+ side of the apparatus for a 5-minute conditioning sessions (Figure 1a). The change in time spent on the CS+ side during the post-test, relative to the baseline day, was taken as a measurement related to ethanol reward or aversion memory. Consistent with previous findings from place aversion studies, mice from the CPA group spent more time at rest during the post-tests (Figure 2a). In addition, CPA mice displayed a strong trend towards CS+ avoidance (Figure 2b), suggesting the formation of aversive ethanol memories. By contrast, mice from the CPP group spent more time on the CS+ side of the apparatus than saline-saline controls, consistent with the formation of ethanol reward memories.
Figure 2. Conditioned place preference (CPP) and conditioned place aversion (CPA) are elicited by ethanol administration.
(a) Time at rest during second post-test. Female mice displayed less time at rest than males (Two-way ANOVA: F1,53 = 4.1, *:p<0.05 main effect of sex). CPA was associated with increased time at rest (Two-way ANOVA: F2,53 = 17.5, p<0.001 main effect of group; $:p<0.1, **:p<0.01, post-test vs same-sex control). (b) Mouse sex did not affect place conditioning during the first post-test (Two-way ANOVA: F1,50 = 0.5, n.s. main effect of sex). CPP mice spent more time on the ethanol-paired (CS+) side, while CPA mice avoided the CS+ side of the test apparatus (Two-way ANOVA: F2,50 = 10.4, p<0.001 main effect of group; *:p<0.05, $:p<0.1 post-test vs mixed-sex control). (c) Mouse sex was not associated with place conditioning during the second post-test (Two-way ANOVA: F1,53 = 0.1, n.s. main effect of sex). CPP mice spent more time on the CS+ side and CPA mice avoided that side of the test apparatus (Two-way ANOVA: F2,53 = 27.5, p<0.0001 main effect of group, **:p<0.01, ***:p<0.001 post test vs mixed-sex control). In control subjects (d), the CPP group (e), and the CPA group (f), the times spent on the CS+ side during the first and second post-tests were correlated. Comparable slopes of the best-fit lines were observed (ANOVA: F2,53 = 1.2, n.s.), but the y-intercepts of the best-fit lines differed across groups (ANOVA: F2,55 = 13.0, p<0.0001). The 95% confidence interval for the y-intercept for the CPA best-fit line did not contain the origin [−24.9 to −9.0]. N = 9-10 mice per group.
Mice were returned to their home cages for one week and we then conducted a second post-test to examine the stability of ethanol associations. During this test, CPP mice retained place preference and CPA mice displayed significant avoidance behavior (Figure 2c), suggesting retained or potentially enhanced ethanol memories following one-week. No sex differences were detected relating to CS+ time during either session. As we planned to evaluate PFC physiology across a range of recording days, we felt it was important to assess the stability of ethanol place conditioning expression across the 2 sessions. Significant correlations were observed between CS+ time in the two test sessions in all groups of mice (Figure 2d-1f), suggesting high stability of ethanol memories within individuals. While the 95% confidence interval of the linear regressions for control and CPP groups each contained the origin, the best-fit line for the CPA group was shifted down by 17 points (Figure 2f). These data suggest that, on average, CPA mice avoided the CS+ side more during the second test than the first, consistent with an incubation of ethanol aversion memory. Together, these data confirm that ethanol intoxication can induce both CPP and CPA in C57BL/6J mice of all sexes. We next aimed to investigate how these experiences would alter PFC physiology.
Ethanol reward learning decreases PV-IN excitability
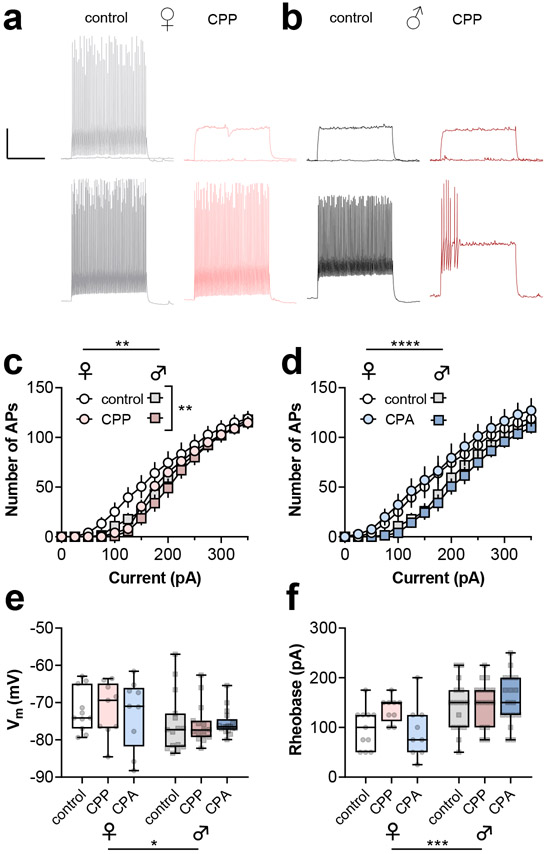
To examine whether adaptations to PFC PV-INs are associated with ethanol reward or aversion learning, we prepared acute brain slices from PV-tdTomato mice within one week following ethanol CPP or CPA (Figure S1). All tdTomato-expressing cells displayed characteristic properties of fast-spiking interneurons in deep layer prelimbic PFC, including rapid maximal firing frequencies and minimal spike frequency adaptation (Figure 3a and 3b). Relative to controls, PV-INs from CPP mice displayed a right-shifted spike-firing curve in response to current injections (3a-3c). By contrast, PV-INs from CPA mice displayed comparable spiking-current input-output curves related to controls (Figure 3d). In addition, and consistent with previous studies (Joffe et al., 2020b), PV-INs from female mice fired action potentials in response to lower current injections than PV-INs from male mice in both CPP and CPA analyses (Figures 3a-3d). Secondary analyses revealed a depolarized resting membrane potential (Figure 3e) and lower rheobase (Figure 3f) in PV-INs from female mice, without any effect of or interaction with ethanol experience. PV-INs from female mice also displayed a trend towards greater membrane resistance than PV-INs from male mice, but no effect of ethanol or interaction was detected (172 ± 9 vs 154 ± 5 MΩ, Two-way ANOVA: F1,66 = 3.5, p<0.07 main effect of sex, data not shown). These findings collectively suggest that hypoactivity of PV-INs may contribute to the expression of ethanol reward learning. Nonetheless, excitatory drive onto PV-INs is crucial in regulating their activity in vivo (Bartos and Elgueta, 2012; Gonzalez-Burgos et al., 2015), and we proceeded to examine excitatory drive onto PV-INs following ethanol CPP and CPA.
Figure 3. Ethanol CPP decreases PV-IN current-evoked spiking.
(a) Top, Representative PV-IN current-clamp recordings from control (left, grey/black) and CPP mice (right, red). Traces depict responses to current injections. The responses to 0 pA and 100 pA injections are shown on top and the responses to 200 pA are shown on the bottom. Scale bars 20 mV, 0.5 s. (b) CPP decreased spike-firing in response to current injections (RM Three-way ANOVA: F14,616 = 2.2, **:p<0.01 CPP x current interaction). PV-INs from female mice were more excitable than those from male mice. (RM Three-way ANOVA: F14,616 = 2.3, **:p<0.01 sex x current interaction). (c) PV-INs from female mice were more excitable than those from male mice (RM Three-way ANOVA: F14,616 = 5.7, ****:p<0.0001 sex x current interaction), but no effect of CPA was detected (RM Three-way ANOVA: F14,616 = 0.5, n.s. CPA x current interaction). The same control data are presented in panels B and C. (d) PV-INs from female mice were more depolarized than those from male mice (Two-way ANOVA: F1,66 = 5.2, *:p<0.05 main effect of sex), but no effect of ethanol conditioning was detected. (e) PV-INs from female mice displayed lower threshold currents to fire (Two-way ANOVA: F1,66 = 14.5, ***:p<0.001 main effect of sex), but no effect of ethanol conditioning was detected. n/N = 9-15 cells from 3-5 mice per group.
Ethanol reward learning enhances postsynaptic strength onto PV-INs
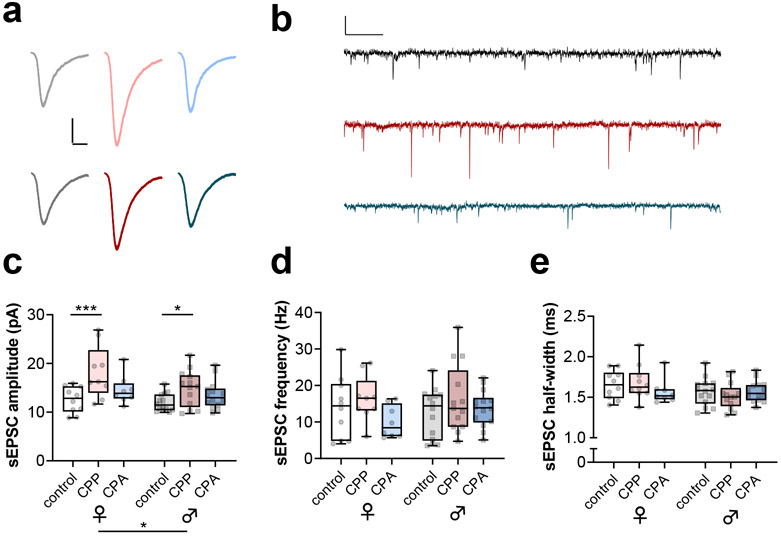
We electrically isolated spontaneous excitatory postsynaptic currents (sEPSCs) by recording in voltage-clamp configuration near the reversal potential for inhibitory currents. sEPSCs from PV-INs displayed fast rise and decay kinetics consistent with synaptic currents recorded from fast-spiking interneurons (Figure 4a and 4b) (Bartos and Elgueta, 2012; Gonzalez-Burgos et al., 2015; Miyamae et al., 2017; Povysheva et al., 2013). Interestingly, we observed greater sEPSC amplitude in PV-INs from CPP mice relative to controls (Figure 4c), suggesting that ethanol reward learning is associated with increased function of postsynaptic AMPA receptors on PV-INs. While we also observed greater PV-IN sEPSC amplitude in female mice relative to male mice, no interactions with place conditioning group were detected. By contrast, no differences in sEPSC frequency (Figure 4d) or decay kinetics (Figure 4e) were associated with ethanol experience. Together, these findings suggest that sEPSC amplitude, a measurement associated with postsynaptic strength, may be specifically related to ethanol reward learning. We next aimed to evaluate whether this adaptation, and reduced excitability of PV-INs (Figure 3), may be correlated with behavioral indices related to ethanol reward learning.
Figure 4. Ethanol CPP increases postsynaptic strength on PV-INs.
(a) Averaged representative traces of spontaneous excitatory postsynaptic currents (sEPSCs) from PV-INs from female mice (top) and male (mice) from control (black), CPP (red), or CPA (blue) groups. Scale bars 5 pA, 2 ms. (b) Representative timecourses of sEPSCs. Scale bars 20 pA, 200 ms. (c) Ethanol CPP increased the amplitude of sEPSCs from PV-INs relative to controls (Two-way ANOVA: F2,62 = 9.7, p<0.001 main effect of group, *:p<0.05, ***:p<0.001 post-tests vs same-sex control). PV-INs from male mice had smaller sEPSC amplitude than those from female mice (Two-way ANOVA: F1,62 = 4.1, *:p<0.05 main effect of sex). (d) No effect of ethanol conditioning or mouse sex on the frequency of sEPSCs from PV-INs. (e) Differences in sEPSC decay time were not detected. n/N = 8-14 cells from 3-5 mice per group.
PFC PV-IN synaptic strength increases during abstinence from ethanol
Despite all mice receiving the same dose of ethanol, individuals display differences in place conditioning related to their experience. We reasoned that the rich interindividual variability would afford opportunities to assess relationships between discrete physiological parameters and ethanol memories; however, we detected no significant correlations with respect to the change in CS+ time. Data for the key synaptic parameters and rheobase are displayed in Figure S2 and S3, respectively.
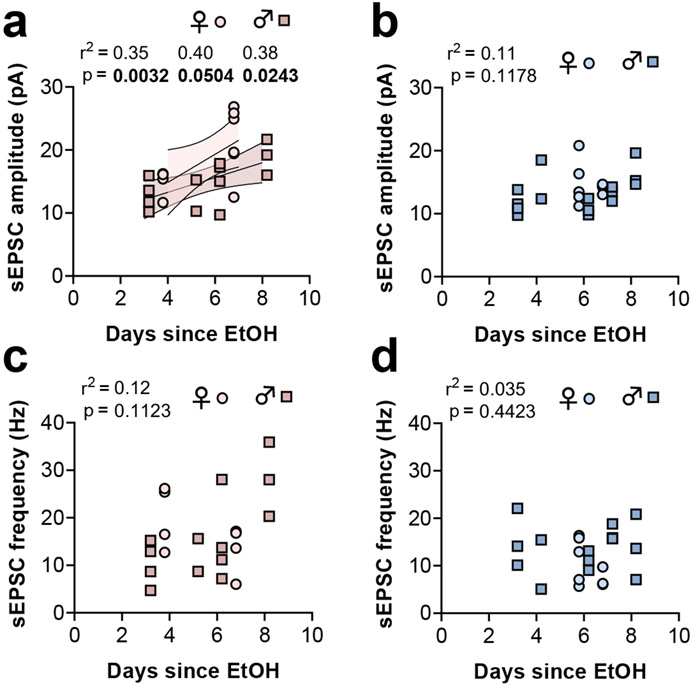
Our experimental design also provided considerable variability with respect to the duration of abstinence from ethanol, as recordings were made between 3 and 8 days following the final ethanol injection. Abstinence from ethanol and other drugs is often associated with dynamic time-dependent adaptations, so we assessed potential relationships between PV-IN synaptic strength and number of days since the last ethanol administration. Within the CPP group, PV-IN sEPSC amplitude was positively associated with the amount of elapsed time since the last exposure to ethanol (Figure 5a). Because sEPSC amplitude was greater in PV-INs from female mice than from male mice, we isolated these data by sex in a follow-up analysis. The positive association between sEPSC amplitude and elapsed time remained when the analysis was restricted to either female mice or male mice, suggesting that abstinence from ethanol may be an important component of the enduring synaptic potentiation on PV-INs. Importantly, no relationship between sEPSC amplitude and the time since ethanol exposure was detected within the CPA group, again indicating that ethanol-induced synaptic potentiation on PV-INs depends on the formation of reward memories. Finally, no relationship between sEPSC frequency and duration of ethanol abstinence was detected in mice from the CPP or CPA groups (Figure 5c and 5d).
Figure 5. Potentiated excitatory drive onto PFC PV-INs emerges during abstinence.
(a) A positive correlation was observed between the length of time elapsed since the last ethanol reward pairing and PV-IN sEPSC amplitude. The relationship was detected in all mice and in female mice (light red fill) and male mice (dark red fill) when separated. The error lines and fill denote the 95% confidence interval for each best-fit line. (b) No relationship between sEPSC amplitude and the length of time elapsed since the last ethanol aversion pairing. (c) No relationship between sEPSC frequency and the length of time elapsed since the last ethanol reward pairing. (d) No relationship between sEPSC frequency and the length of time elapsed since the last ethanol aversion pairing. n/N = 8-14 cells from 3-5 mice per group.
Discussion
Alcohol’s effects are pleiotropic and heterogenous, providing great motivation for preclinical studies designed to associate specific physiological endpoints with discrete behavioral adaptations. With this in mind, we leveraged place conditioning to assess how rewarding and aversive ethanol experiences modify PFC PV-IN function in mice. PV-INs from female mice and male mice in the CPP, and not the CPA group, displayed decreased excitability and increased excitatory input, illustrating that the valence of formed associations represents an essential component of neuroadaptations induced by ethanol.
The present findings help clarify complex adaptations to PV-IN excitability following ethanol exposure (Hughes et al., 2020; Joffe et al., 2020b; Trantham-Davidson et al., 2014). When provided intermittent access to ethanol, rodents typically drink to attain low-to-moderate BECs approaching the common legal limit for intoxication within the United States, 80 mg/dL (Hwa et al., 2011; Salling et al., 2018). In this model, about one-third of PFC interneurons in male rats displayed increased Fos expression at one-day abstinence (George et al., 2012). At the same timepoint, previous studies from our group demonstrated enhanced PV-IN excitably in all mice (Joffe et al., 2020b), providing a potential physiological correlate for increased intermediate early gene expression in rats. By contrast, the present findings, along with previous studies following chronic ethanol gavage in rats (Hughes et al., 2020), demonstrate that decreased excitability of PV-INs can occur during the first week of abstinence following repeated ethanol intoxication where BECs above 250 mg/dL are repeatedly reached. Thus, ethanol may exert biphasic effects on PV-IN excitability depending on the level of exposure. Similarly, a recent report demonstrated that interneurons expressing somatostatin are activated by low doses of ethanol but strongly inhibited by high doses (Li et al., 2021). While analogous dose-response studies examining PV-INs have not yet been reported, moderate-to-high concentrations of ethanol (25-50 mM; corresponding to 115-230 mg/dL) disrupts PV-IN up-state activity in slice culture (Woodward and Pava, 2009). While these findings from reduced systems might suggest generalizable effects of ethanol across PFC interneuron subtypes, that hypothesis is not supported by previous work identifying interneuron subtype-specific adaptations following ethanol experiences (Hughes et al., 2020; Joffe et al., 2020b) . Nonetheless, it is highly likely that other types of PFC interneurons undergo adaptations following ethanol place conditioning and this hypothesis should be explored in future research.
We found that PV-INs from CPP mice displayed selectively enhanced sEPSC amplitude consistent with a postsynaptic enhancement of AMPA receptor function. PV-IN excitatory synapses display minimal postsynaptic NMDA receptor currents (Bogart and O'Donnell, 2018; Matta et al., 2013; McGarry and Carter, 2016). Thus, AMPA receptor function is critical for modulating phasic excitation of PV-INs and integrating these cells within local microcircuit networks (Bartos et al., 2007; Cobb et al., 1995; Fuchs et al., 2007; Gonzalez-Burgos et al., 2015; Knoblich et al., 2019; Royer et al., 2012). While these basic neurobiological studies suggest that adaptations to synaptic strength onto PV-INs are likely to confer major effects at the neurocircuit and behavioral level, future studies will need to be conducted to test this hypothesis. The present studies are also limited by a relatively cursory assessment of AMPA receptor function, and future work should address quantal release parameters, subunit stoichiometry, and short- and long-term plasticity.
We initially designed the present studies to map discrete physiological adaptations across individual differences in ethanol place conditioning. Despite this aim, we detected no correlations between any PV-IN parameter and change in CS+ time. On the other hand, PV-IN sEPSC amplitude correlated positively with the duration of abstinence from ethanol. Comparable place preferences, however, were observed at both ends of the time range examined for electrophysiology studies. Together, these findings seem to imply that PV-IN AMPA receptor function is not particularly relevant for reward memory. Instead, could synaptic strength onto PV-INs encode another aspect of ethanol experience? One potential hypothesis for future studies is that PV-IN AMPA receptor potentiation is a neural correlate for affective disturbances in abstinence, phenomena that often take several days or longer to manifest in rodents (Holleran and Winder, 2017). Alternatively, potentiated PV-IN synaptic strength might encode motivation to drink or cue reactivity. In patients with an AUD, changes in PFC responses to stress images and alcohol cues have been linked with the duration of abstinence and are associated with subsequent heavy drinking later in treatment (Blaine et al., 2020). Based on this, the delayed potentiation of PV-IN synaptic strength may relate to a progressive enhancement in ethanol-seeking motivation, often referred to as an incubation of craving (Alonso-Caraballo et al., 2021; Pickens et al., 2011). Longitudinal studies examining PV-IN function during a wide variety of behaviors across ethanol exposure and abstinence would be ideal to better understand these exciting hypotheses.
In conclusion, these studies describe adaptations to PFC PV-IN excitability and synaptic strength that occurred following ethanol CPP. These changes did not emerge following ethanol CPA, indicating specific associations with reward learning. Continued research into the neurobiological mechanisms contributing to these adaptations, as well as their behavioral relevance, may help to develop novel treatments for AUDs.
Supplementary Material
Highlights.
C57BL/6J mice develop ethanol conditioned place preference (CPP) or aversion (CPA)
CPP decreases excitability of prelimbic parvalbumin-expressing interneurons (PV-INs)
CPP increases excitatory synaptic currents onto PV-INs in a time-dependent manner
Despite ethanol experience, PV-INs from CPA mice did not differ from controls
Effects of ethanol were comparable in PV-INs from female and male mice
Acknowledgements
The authors thank Dipanwita Pati and Melanie Pina for helpful discussions related to the place conditioning methodology. We also thank members of the Vanderbilt WCNDD, Vanderbilt VCAR, and University of Pittsburgh Medical Center TNP for stimulating discussions.
Funding and Declaration of Interest
This work was supported by National Institutes of Health grants R01MH062646 and R37NS031373 to PJC, R37AA019455 and R01DA042475 to DGW, R00AA025403 to KAJ, and K99/R00AA027806 to MEJ. Some experiments were performed using the Vanderbilt University Medical Center Murine Neurobehavior Core and Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, DK59637 and Ey08126). PJC receives research support from Acadia Pharmaceuticals and Boehringer Ingelheim. All other authors declare no potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
PJC receives research support from Acadia Pharmaceuticals and Boehringer Ingelheim and is an inventor on multiple patents for allosteric modulators of metabotropic glutamate receptors. All other authors declare no potential conflicts of interest.
References
- Alonso-Caraballo Y, Guha SK, and Chartoff EH (2021). The neurobiology of abstinence-induced reward-seeking in males and females. Pharmacol Biochem Behav 200, 173088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah BV, Bruns W, Carandini M, and Scanziani M (2012). Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron 73, 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, and Elgueta C (2012). Functional characteristics of parvalbumin- and cholecystokinin-expressing basket cells. J Physiol 590, 669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, and Jonas P (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8, 45–56. [DOI] [PubMed] [Google Scholar]
- Blaine SK, Wemm S, Fogelman N, Lacadie C, Seo D, Scheinost D, and Sinha R (2020). Association of Prefrontal-Striatal Functional Pathology With Alcohol Abstinence Days at Treatment Initiation and Heavy Drinking After Treatment Initiation. Am J Psychiatry 177, 1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogart LJ, and O'Donnell P (2018). Multiple long-range inputs evoke NMDA currents in prefrontal cortex fast-spiking interneurons. Neuropsychopharmacology 43, 2101–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, and Somogyi P (1995). Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 378, 75–78. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Schacht JP, Hutchison K, Roche DJ, and Ray LA (2016). Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict Biol 21, 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Clemans JM, and Fidler TL (2002). Injection timing determines whether intragastric ethanol produces conditioned place preference or aversion in mice. Pharmacol Biochem Behav 72, 659–668. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, and Groblewski PA (2006). Drug-induced conditioned place preference and aversion in mice. Nat Protoc 1, 1662–1670. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Smith R, and McMullin C (2003). Competition between ethanol-induced reward and aversion in place conditioning. Learn Behav 31, 273–280. [DOI] [PubMed] [Google Scholar]
- Di Menna L, Joffe ME, Iacovelli L, Orlando R, Lindsley CW, Mairesse J, Gressens P, Cannella M, Caraci F, Copani A, et al. (2018). Functional partnership between mGlu3 and mGlu5 metabotropic glutamate receptors in the central nervous system. Neuropharmacology 128, 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl MM, Iravedra-Garcia JM, Moran-Sierra J, Rojas-Bowe G, Gonzalez-Diaz FN, Valentin-Valentin VP, and Quirk GJ (2020). Divergent projections of the prelimbic cortex bidirectionally regulate active avoidance. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BR, and Gao WJ (2018). PV Interneurons: Critical Regulators of E/I Balance for Prefrontal Cortex-Dependent Behavior and Psychiatric Disorders. Front Neural Circuits 12, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, et al. (2007). Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron 53, 591–604. [DOI] [PubMed] [Google Scholar]
- George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, and Koob GF (2012). Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci U S A 109, 18156–18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Cho RY, and Lewis DA (2015). Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry 77, 1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, et al. (2017). Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001-2002 to 2012-2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 74, 911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, et al. (2004). Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 175, 296–302. [DOI] [PubMed] [Google Scholar]
- Herremans SC, Van Schuerbeek P, De Raedt R, Matthys F, Buyl R, De Mey J, and Baeken C (2015). The Impact of Accelerated Right Prefrontal High-Frequency Repetitive Transcranial Magnetic Stimulation (rTMS) on Cue-Reactivity: An fMRI Study on Craving in Recently Detoxified Alcohol-Dependent Patients. PLoS One 10, e0136182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, and Arber S (2005). A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol 3, e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holleran KM, and Winder DG (2017). Preclinical voluntary drinking models for alcohol abstinence-induced affective disturbances in mice. Genes Brain Behav 16, 8–14. [DOI] [PubMed] [Google Scholar]
- Hughes BA, Crofton EJ, O'Buckley TK, Herman MA, and Morrow AL (2020). Chronic ethanol exposure alters prelimbic prefrontal cortical Fast-Spiking and Martinotti interneuron function with differential sex specificity in rat brain. Neuropharmacology 162, 107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, and Miczek KA (2011). Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res 35, 1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Santiago CI, Oliver KH, Maksymetz J, Harris NA, Engers JL, Lindsley CW, Winder DG, and Conn PJ (2020a). mGlu2 and mGlu3 Negative Allosteric Modulators Divergently Enhance Thalamocortical Transmission and Exert Rapid Antidepressant-like Effects. Neuron 105, 46–59 e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Vitter SR, and Grueter BA (2017). GluN1 deletions in D1- and A2A-expressing cell types reveal distinct modes of behavioral regulation. Neuropharmacology 112, 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Winder DG, and Conn PJ (2020b). Contrasting sex-dependent adaptations to synaptic physiology and membrane properties of prefrontal cortex interneuron subtypes in a mouse model of binge drinking. Neuropharmacology, 108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenowski PM (2018). Emerging role for the medial prefrontal cortex in alcohol-seeking behaviors. Addict Behav 77, 102–106. [DOI] [PubMed] [Google Scholar]
- Knoblich U, Huang L, Zeng H, and Li L (2019). Neuronal cell-subtype specificity of neural synchronization in mouse primary visual cortex. Nat Commun 10, 2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2014). Neurocircuitry of alcohol addiction: synthesis from animal models. Handb Clin Neurol 125, 33–54. [DOI] [PubMed] [Google Scholar]
- Li M, Cabrera-Garcia D, Salling MC, Au E, Yang G, and Harrison NL (2021). Alcohol reduces the activity of somatostatin interneurons in the mouse prefrontal cortex: A neural basis for its disinhibitory effect? Neuropharmacology 188, 108501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DJ, Bedse G, Gaulden AD, Ryan JD, Kondev V, Winters ND, Rosas-Vidal LE, Altemus M, Mackie K, Lee FS, et al. (2020). Endocannabinoid Signaling Collapse Mediates Stress-Induced Amygdalo-Cortical Strengthening. Neuron 105, 1062–1076 e1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta JA, Pelkey KA, Craig MT, Chittajallu R, Jeffries BW, and McBain CJ (2013). Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neurosci 16, 1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry LM, and Carter AG (2016). Inhibitory Gating of Basolateral Amygdala Inputs to the Prefrontal Cortex. J Neurosci 36, 9391–9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, and Tapert SF (2008). Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res 32, 386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melon LC, Nasman JT, John AS, Mbonu K, and Maguire JL (2018). Interneuronal delta-GABAA receptors regulate binge drinking and are necessary for the behavioral effects of early withdrawal. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamae T, Chen K, Lewis DA, and Gonzalez-Burgos G (2017). Distinct Physiological Maturation of Parvalbumin-Positive Neuron Subtypes in Mouse Prefrontal Cortex. J Neurosci 37, 4883–4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pati D, Pina MM, and Kash TL (2019). Ethanol-induced conditioned place preference and aversion differentially alter plasticity in the bed nucleus of stria terminalis. Neuropsychopharmacology 44, 1843–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton MS, Heckman M, Kim C, Mu C, and Mathur BN (2021). Compulsive alcohol consumption is regulated by dorsal striatum fast-spiking interneurons. Neuropsychopharmacology 46, 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Sorensen DO, McCalley DM, and Hanlon CA (2020). Non-invasive Brain Stimulation for Alcohol Use Disorders: State of the Art and Future Directions. Neurotherapeutics 17, 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, and Shaham Y (2011). Neurobiology of the incubation of drug craving. Trends Neurosci 34, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povysheva NV, Zaitsev AV, Gonzalez-Burgos G, and Lewis DA (2013). Electrophysiological heterogeneity of fast-spiking interneurons: chandelier versus basket cells. PLoS One 8, e70553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Jury NJ, Delpire E, Nakazawa K, and Holmes A (2017). Reduced ethanol drinking following selective cortical interneuron deletion of the GluN2B NMDA receptors subunit. Alcohol 58, 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, and Buzsaki G (2012). Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci 15, 769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, Jane Skelly M, Avegno E, Regan S, Zeric T, Nichols E, and Harrison NL (2018). Alcohol consumption during adolescence in a mouse model of binge drinking alters the intrinsic excitability and function of the prefrontal cortex through a reduction in the hyperpolarization-activated cation current. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salling MC, and Martinez D (2016). Brain Stimulation in Addiction. Neuropsychopharmacology 41, 2798–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, et al. (2013). Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci 16, 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Noamany H, Chang CJ, Brown AR, Chen X, Leible D, Lee JJ, Wang J, Vernon AN, Vander Weele CM, et al. (2019). A cortical-brainstem circuit predicts and governs compulsive alcohol drinking. Science 366, 1008–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, and Deisseroth K (2009). Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg SF, Taylor MJ, Alhassoon OM, Gongvatana A, Theilmann RJ, Frank LR, and Grant I (2012). Frontal white matter integrity predictors of adult alcohol treatment outcome. Biol Psychiatry 71, 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, Floresco SB, and Chandler LJ (2014). Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. J Neurosci 34, 3706–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fan Y, Dong Y, Ma M, Ma Y, Dong Y, Niu Y, Jiang Y, Wang H, Wang Z, et al. (2016). Alterations in Brain Structure and Functional Connectivity in Alcohol Dependent Patients and Possible Association with Impulsivity. PLoS One 11, e0161956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, et al. (2013). Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382, 1575–1586. [DOI] [PubMed] [Google Scholar]
- Woodward JJ, and Pava MJ (2009). Effects of ethanol on persistent activity and up-States in excitatory and inhibitory neurons in prefrontal cortex. Alcohol Clin Exp Res 33, 2134–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Meng YJ, Tao YJ, Deng RH, Wang HY, Li XJ, Wei W, Hua Y, Wang Q, Deng W, et al. (2021). Functional Connectivity of Nucleus Accumbens and Medial Prefrontal Cortex With Other Brain Regions During Early-Abstinence Is Associated With Alcohol Dependence and Relapse: A Resting-Functional Magnetic Resonance Imaging Study. Front Psychiatry 12, 609458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.