Abstract
Background and Aims.
Increased colonic serotonin (5-HT) level and decreased serotonin reuptake transporter (SERT) expression in irritable bowel syndrome (IBS) may contribute to diarrhea and visceral hypersensitivity. We investigated whether mucosal SERT is modulated by gut microbiota via a mast cell–prostaglandin E2 (PGE2) pathway.
Methods.
C57Bl/6 mice received intracolonic infusion of fecal supernatant (FS) from healthy controls (HC) or diarrhea-predominant IBS (IBS-D) patients. The role of mast cells was studied in mast cell–deficient (W/Wv) mice. Colonic organoids and/or mast cells were used for in vitro experiments. SERT expression was measured by quantitative PCR and Western blot. Visceromotor responses (VMR) to colorectal distension (CRD) and colonic transit were assessed.
Results.
Intracolonic infusion of IBS-D FS in mice caused an increase in mucosal 5-HT compared to HC FS, accompanied by ~50% reduction in SERT expression. Mast cell stabilizers, COX-2 inhibitors, and PGE2 receptor antagonist prevented SERT downregulation. Intracolonic infusion of IBS-D FS failed to reduce SERT expression in mast cell deficient (W/Wv) mice. This response was restored by mast cell reconstitution. The downregulation of SERT expression evoked by IBS FS was prevented by lipopolysaccharide antagonist LPS-RS and a bacterial trypsin inhibitor. In vitro LPS treatment caused increased COX-2 expression and PGE2 release from cultured mouse mast cells. Intracolonic infusion of IBS-D FS in mice reduced colonic transit, increased fecal water content, and increased VMR to CRD. Ondansetron prevented these changes.
Conclusions.
Fecal LPS acting in concert with trypsin in IBS-D patients, stimulates mucosal mast cells to release PGE2, which downregulates mucosal SERT, resulting in increased mucosal 5-HT. This may contribute to diarrhea and abdominal pain common in IBS.
Keywords: SERT, IBS, colonic mucosa, mast cells
Introduction
Serotonin (5-HT) is a key paracrine and neurocrine signaling molecule in the gut that plays an essential role in regulating gastrointestinal motility, secretion, vasodilation, nociception and inflammation.1,2 The increased postprandial plasma 5-HT levels in patients with diarrhea-predominant irritable bowel syndrome (IBS-D),3–5 and significant symptom relief from treatment with 5-HT receptor antagonists6,7 suggests that dysregulated mucosal 5-HT activity may contribute to IBS pathogenesis. Most enteric 5-HT is synthesized by enterochromaffin cells8 through the rate-limiting enzyme tryptophan hydroxylase 1 (TPH1) and released in response to mechanical9 and chemical10 stimuli in the gut lumen. The termination of 5-HT signaling relies almost exclusively on intracellular transport by the serotonin selective reuptake transporter (SERT), which is expressed in all intestinal epithelial cells.11–12 The main enzyme responsible for the degradation of 5-HT in colonic tissues is monoamine oxidase A (MAOA), which is encoded by the MAOA gene. Decreased SERT expression in the gut of IBS patients, as reported by several studies,13–15 may contribute to the increased 5-HT level in the colon of IBS patients.4,5 Further, SERT null mice exhibit watery diarrhea with enhanced colonic motility, and visceral hypersensitivity.16 All these observations support the idea that impaired uptake of 5-HT by gut epithelial cells resulting in increased 5-HT in the colon may play an important role in IBS pathogenesis. However, to date, little is known about the mechanism responsible for the downregulation of SERT in IBS patients.
Decreased SERT expression is evident in mice infected by enteropathogenic Escherichia coli,17 whereas treatment with Lactobacillus rhamnosus GG supernatant reversed decreased SERT expression in a post-infectious IBS model.18 Elevated colonic SERT expression in germ-free mice was reversed by a fecal material inoculation,19 suggesting gut microbiota and dysbiosis may play a role in regulating SERT expression in the gut.
Mucosal mast cells sense external stimuli and release effectors that may cause IBS symptoms.20–22 Drugs with mast cell stabilizing properties seem to relieve pain and diarrhea in IBS.23,24 We have shown that intracolonic administration of mucosal supernatants from IBS-D patients induces visceral hypersensitivity in rats by increasing the synthesis and release of prostaglandin E2 (PGE2) from mast cells, which, in turn, activates dorsal ganglion neurons.25 In a preliminary study, a substantial elevation of mucosal 5-HT in response to IBS-D supernatant treatment was significantly reversed by the mast cell stabilizer cromolyn sodium – an intriguing suggestion of cross talk between mucosal mast cells and 5-HT signaling in the gut. This prompted investigation of the mechanisms by which fecal microbiota modulates colonic SERT expression.
Methods
Please refer to the Supplementary Material for comprehensive details.
Patients
14 IBS-D patients meeting Rome III criteria (7 females, 7 males), aged 43 to 59 years, were recruited from the University of Michigan, Division of Gastroenterology and Hepatology outpatient and primary care clinics. 14 age-matched healthy individuals served as controls. Consents were obtained from all participants, and the study was approved by the University of Michigan Human Research Protection Program. Each participant provided fecal samples for fecal supernatant (FS) preparation, as previously described (see Supplementary Material).26 6 IBS-D patients were recruited to study the effect of FS on SERT expression before and after low-FODMAP treatment. Patients’ demographics and symptoms, and the study design were previously reported in detail.26
Animals
All animal procedures were performed in accordance with National Institutes of Health guidelines and approved by the University of Michigan Institutional Animal Care & Use Committee. C57B16 and mast cell–deficient WBB6F1/J-KitW/KitW-v/J (W/Wv) and TLR4-deficient C3H/HeJ male mice (aged 6 wk) were obtained from Jackson Laboratories (Bar Harbor, ME). Animals were housed 3 per cage in a controlled environment (12-h daylight cycle, lights off at 18:00), with free access to food and water. The animals were euthanized with CO2 in accordance with University of Michigan guidelines and protocols.
Intracolonic infusions
Intracolonic infusions were performed with a flexible plastic tube (18-gauge, 3 in, Instech Laboratories, Plymouth Meeting, PA) inserted into the distal colon 3 cm from the anus. Fecal supernatant (FS, 0.3 mL) or PBS was infused slowly for 1 min. In separate studies, intracolonic injection of LPS-RS, or intraperitoneal injection of different agents, as detailed in the Results, were administered 30 min before FS infusion. Mice were subjected to further experiments or killed for specimen collection 18 h after intracolonic FS infusion.
Collection of mucosal and blood specimens and assays of supernatants
Colonic specimens from experimental animals were collected as previously described,26 or as specified in the Results. Blood samples collected by cardiac puncture were placed into heparin-coated tubes. Plasma was collected after centrifugation (4,000 g, 5 min). ELISA assays of 5-HT (ADI900175, Enzo Life Sciences, Ann Arbor, MI) and PGE2 (#500141, Cayman Chemical, Ann Arbor, MI) were performed.
Murine colonic organoid culture
Murine colonic crypt isolation and culture followed the manufacturer’s method (https://www.stemcell.com/intestinal-epithelial-organoid-culture-with-intesticult-organoid-growth-medium-mouse-lp.html). See Supplementary Material.
Mast cell culture, co-culture, and reconstitution
Selective reconstitution of mast cells in mast cell–deficient W/Wv mice was conducted according to previously described methods (see Supplementary Material).25,27
Mast cell specific TLR4 deficient mice were prepared by reconstituting W/Wv mice with bone marrow-derived MC from TLR4 deficient mice. For colonic organoid–mast cell co-culture, murine colonic organoids were cultured as described.28 Mast cells were added to 24-well plates (10,000 cells/well) and incubated with organoids for 24 h. After treatment, mast cells were removed from the co-culture by low-speed centrifugation (200 g/min). Organoids were collected for further analysis. For mast cell reconstitution, mast cell–deficient W/Wv mice received a tail vein injection of 5 × 106 cultured mast cells and were studied 4 wk later (see Supplementary Material).
Colorectal distension and electromyography recording
Visceromotor responses (VMR) were recorded by quantifying reflex contractions of the abdominal musculature induced by colorectal distension (CRD) (see Supplementary Material).
Colonic transit
Colonic transit time was measured using the bead expulsion assay, as previously described.29 Colon patency was carefully monitored with a flexible plastic tube (18-gauge, 3 in, Instech Laboratories) inserted 3 cm into the colon before a 3-mm glass ball was inserted transanally with blunt surgical forceps and carefully advanced 3 cm into the colon with the plastic tube. The animal was observed in a box. Colonic transit time was the time from glass ball insertion to its expulsion.
Free water content
Fresh animal droppings were collected in Petri dishes to determine the wet weight, and oven-dried to constant weight at 105°C before being weighed again to determine the dry weight. Fecal water percentage is the difference between wet and dry weights.
Agonists and antagonists
Described in the Supplementary Material.
Western blot analysis
Western blots against SERT (catalog #702076) and TPH1 (#PA1–777) (Thermo Fisher Scientific, Waltham, MA) were conducted as described in the Supplementary Material.
Reverse transcriptase–PCR studies
Gene expressions of SERT, TPH1, COX-2, MAOA, and Chga were measured by RT–PCR (see Supplementary Material). Primer sets are provided in Supplementary Table 1.
Immunofluorescence staining
Sections of colon tissue were used for immunohistochemistry studies as described previously (see the Supplemental Methods)
Statistical analysis
Differences of quantified data were compared using Student t test for comparison between 2 groups, or one-way ANOVA for more than 2 groups. Results are expressed as mean ± SEM. P < 0.05 was considered statistically significant.
Results
Intracolonic administration of IBS-D patient fecal supernatant causes increased 5-HT and decreased SERT expression in mouse colon
Our previous studies demonstrated that intracolonic administration of fecal supernatant from IBS-D patients leads to increased visceral hypersensitivity.26 We used this model to determine if fecal material from IBS-D patients affects colonic 5-HT levels.
Fecal supernatant (FS) (0.3 mL) from IBS-D patients and healthy controls (HC) was collected as previously described.26 These samples were intracolonically (IC) administered to naive mice, and plasma and distal colonic samples were collected 18 h after treatment. In response to intracolonic infusion of FS from IBS-D patients, the 5-HT level in mouse mucosa increased to 10.94 ± 1.71 ng·mL−1·mg−1 compared to 5.52 ± 1.91 ng·mL−1·mg−1 after infusion of FS from HC (P < 0.01, n =8–10) (Figure 1A). A similar increase was measured in platelet-poor serum 5-HT levels in mice treated with IBS-D FS compared to HC FS (P < 0.01, n = 14–16) (Supplementary Figure 1A).
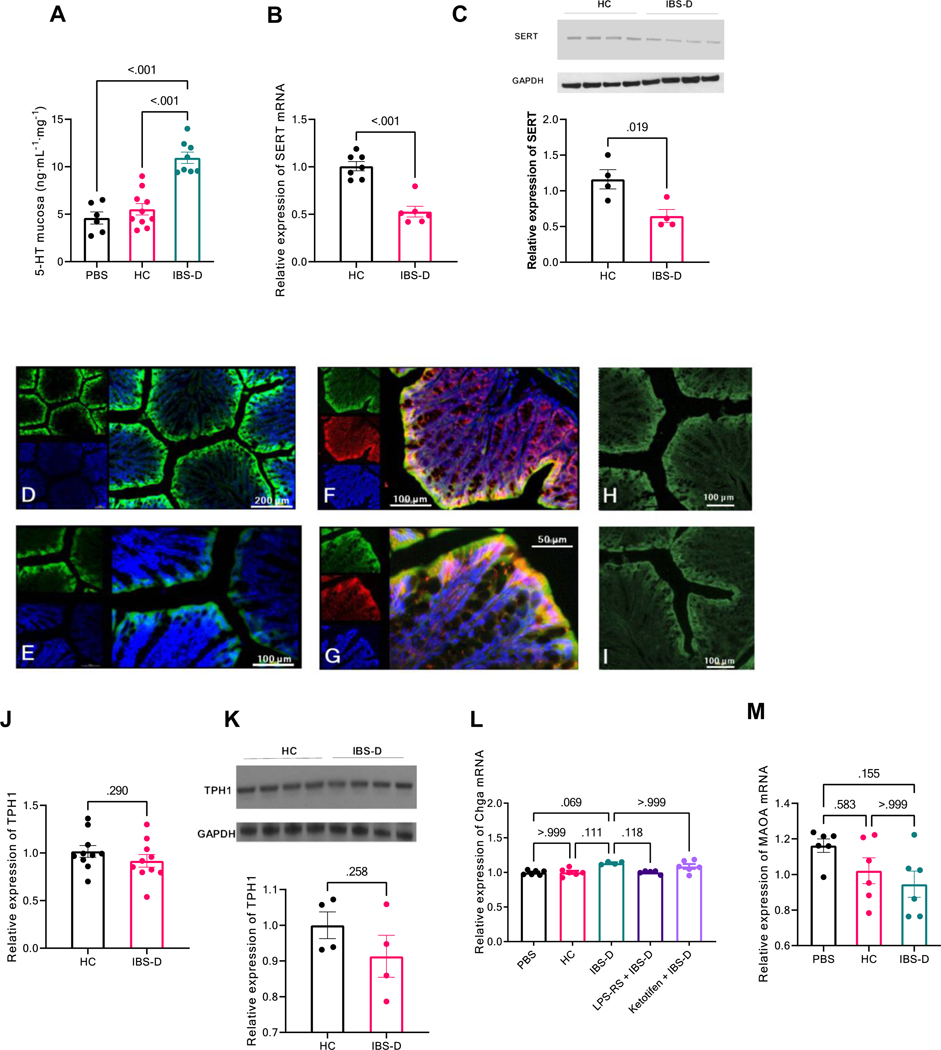
Figure 1. Increased 5-HT level and decreased SERT expression in mouse colon induced by IBS-D patient fecal supernatant.
(A) 5-HT release from distal colonic mucosa in mice 18 h after intracolonic infusion of fecal supernatant (FS) from healthy controls (HC) or IBS-D patients. Results are expressed as mean ± SEM, *P < 0.01 from HC, Student t test, n = 6–9 in each group with duplicated measurements. (B) RT–PCR of SERT in distal colon from mice treated with intracolonic infusion of HC or IBS-D FS. Results are expressed as mean ± SEM, *P < 0.01 from HC, Student t test, n = 6–7 in each group. (C) Western blot of SERT in distal colon from mice treated with HC or IBS-D FS, quantified by densitometry analysis. Results are expressed as mean ± SEM, *P = 0.02 from HC, Student t test, n = 4 in each group. (D,E) Immunofluorescent staining for SERT was performed on sections of normal mouse colon. SERT staining is in green contrasting with blue counterstained nuclei. SERT is principally expressed on the surface area of colon mucosa, but not in the lamina propria. (F,G) Immunofluorescent staining for SERT and E-cadherin was performed on sections of normal mouse colon. SERT staining is in green and E-cadherin in red, contrasting with blue counterstained nuclei. SERT is principally expressed in the mature differentiated epithelial cells where E-cadherin is primarily localized. Immunofluorescent staining for SERT in normal (H) and IBS-D FS injected mouse colon (I). The average fluorescence intensity was lower in mouse colon after IBS-D FS treatment. (J) RT–PCR of TPH1 in distal colon from mice treated with HC or IBS-D FS. Results are expressed as mean ± SEM, *P = 0.29 from HC, Student t test, n = 10 in each group. (K) Western blot of TPH1 in distal colon from mice treated with HC or IBS-D FS, quantified by densitometry analysis. Results are expressed as mean ± SEM, *P = 0.26 from HC, Student t test, n = 4 in each group. (L and M) RT-PCR of Chag and MAOA in distal colon from mice treated with HC or IBS-D FS. Results are expressed as mean ± SEM. *P = 0.11 from HC for Chag, *P > 0.99 from HC for MAOA, Student t test, n = 6 in each group.
Increased 5-HT in the colonic mucosa may be due to enhanced synthesis of 5-HT by enterochromaffin cells regulated by TPH1,8 and/or reduced reuptake of 5-HT from the lamina propria by epithelial cells mediated by serotonin reuptake transporter (SERT). Our RT–PCR data showed intracolonic infusion of IBS-D FS inhibited SERT mRNA level by 47.9%, compared to HC FS (P < 0.01, n = 6–7, Figure 1B). Similar results were observed with SERT expression at the protein level (=0.02, n = 4, Figure 1C). Notably, intracolonic infusion of HC FS had no effect on SERT mRNA level, compared to PBS (P = 0.93 n = 4–7, Supplementary Figure 1B). Consequently, HC FS was used as control in later experiments. Immunofluorescent staining for SERT was performed on sections of normal mouse colon. SERT immunoreactivities are localized mainly in the colonic epithelial cells where E-cadherin is located. Intracolonic administration of IBS-D FS caused a reduction in SERT fluorescence intensity compared to controls (14.8 ± 3.2 vs. 21.5 ± 4.0, P = 0.026, Mann-Whitney two-sided test, n=6) (Figure 1D-I). These findings confirmed our PCR and Western Blot observations. In contrast to SERT, intracolonic infusion of IBS-D FS did not affect TPH1 mRNA (P = 0.29, n = 10, Figure 1D) or protein level (Figure 1E). To determine if the formation and release of 5-HT–containing secretory granules are affected in our IBS mouse model, we used RT–PCR to quantify Chga mRNA, which encodes Chga, a neuroendocrine secretory protein that is released with 5-HT. Intracolonic infusion of IBS-D FS did not affect Chga gene expression, compared to HC FS (Figure 1F). The main enzyme responsible for the catabolic degradation of 5-HT in colonic tissue is monoamine oxidase A (MAOA). RT–PCR showed that intracolonic infusion of IBS-D FS had no effect on MAOA mRNA level in mice colon, compared to HC FS (Figure 1G). Together, these observations suggest that decreased 5-HT reuptake, rather than increased synthesis, elevates mucosal 5-HT in our IBS mouse model.
IBS-D fecal supernatant downregulates colonic SERT expression in a mast cell–dependent manner
To examine the signaling pathway regulating SERT expression in IBS, we established an ex vivo system with murine colon organoids, as previously described.30 The organoids were treated with IBS-D FS (1:1000 dilution) for 18 h; no change was observed in SERT expression, compared to HC FS (P > 0.99, n = 6, Figure 2A). This difference from our in vivo results suggests that cell types other than epithelial cells – perhaps mucosal immune cells – may be required to mediate downregulation of SERT expression in response to IBS-D FS. We hypothesize that mucosal SERT expression is modulated by gut microbiota via a mast cell–mediated process in IBS.
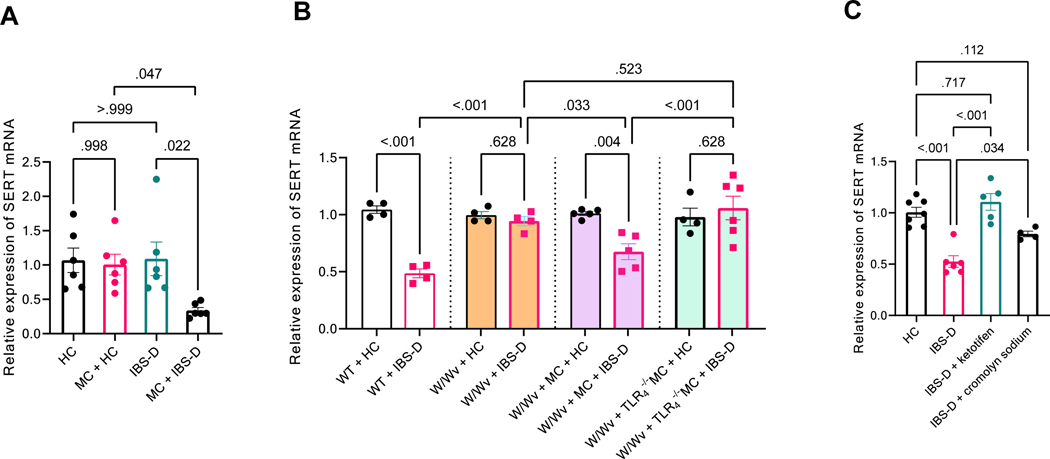
Figure 2. Decreased colonic SERT expression induced by IBS-D fecal supernatant is mast cell–dependent.
(A) RT–PCR of SERT in murine colonic organoids alone or co-cultured with mast cells (MC), treated with fecal supernatant (FS) from HC or IBS-D patients. Results are expressed as mean ± SEM, two-way ANOVA, n = 6 in each group. (B) colonic SERT expression in response to intracolonic infusion of IBS-D FS in wild-type (WT), W/Wv, W/Wv mice reconstituted with bone marrow–derived MC from wild-type mice, and W/Wv mice reconstituted with bone marrow–derived MC from TLR deficient mice. Results are expressed as mean ± SEM, one-way ANOVA, n = 4–6 in each group. (C) Decreased colonic SERT expression evoked by intracolonic IBS-D FS infusion was abolished by ketotifen or cromolyn sodium pretreatment (30 min before infusion, IP). Results are expressed as mean ± SEM, one-way ANOVA, n = 4–7 in each group.
To test this hypothesis, bone marrow–derived mast cells were harvested and differentiated in vitro, as previously described.27 These mature mast cells were added to murine colonic organoids before FS infusion, and then removed by low-speed centrifugation before the organoids were collected for further analysis. As shown in Figure 2A, mast cell co-culture alone without IBS-D FS did not affect SERT expression in murine colonic organoids (P> 0.99, n = 6). IBS-D FS infusion in the mast cell co-culture caused a 75.1% reduction in SERT expression in the colonic organoids, compared to HC FS (P = 0.02, n = 6).
To demonstrate conclusively the critical role of mast cells in regulating SERT expression in our IBS rodent model, we measured the colonic SERT level in mast cell–deficient (W/Wv) mice. As shown in Figure 2B, in contrast to the reduced SERT expression in wild-type littermates, intracolonic infusion of IBS-D FS did not affect SERT expression in W/Wv mice (P = 0.75, n = 4). Reconstitution of bone marrow–derived mast cells in W/Wv mice, as described previously,25 restored the SERT response induced by intracolonic infusion of IBS-D FS (P = 0.01, n = 4–5). On the other hand, reconstitution of bone marrow-derived mast cells obtained from the TLR4 deficient mice in W/Wv mice did not affect SERT response to IBS-D FS (Figure 2B). In addition, pretreating wild-type mice with mast cell stabilizers ketotifen (1 mg/kg, ip) or cromolyn sodium (30 mg/kg, ip) 30 min before intracolonic IBS-D FS infusion largely abolished the inhibitory effect of IBS-D FS on colonic SERT expression (P < 0.01, n = 5–6 and P = 0.04, n = 4–6, respectively, Figure 2C). Cromolyn sodium treatment also significantly prevented the elevation of 5-HT in the colonic mucosa and platelet-poor serum of wild-type mice treated with intracolonic IBS-D FS infusion (Supplementary Figures 1A and 2).
Mast cells regulate colonic SERT expression in response to IBS-D fecal supernatant via COX–PGE2 pathway
Previous studies have shown that mast cell–released mediators, including histamine, trypsin, and prostaglandin E2, are increased in the colonic mucosa of IBS-D patients and in rat colon in response to intracolonic administration of IBS-D supernatant.25,31 Here, we studied the involvement of these mediators in IBS-D FS–induced SERT downregulation. As shown in Figure 3A, pretreatment with tryptase inhibitor nafamostat mesylate (10 mg/kg, ip) or histamine H1 receptor antagonist ebastine (10 mg/kg, ip) failed to prevent downregulation of SERT induced by IBS-D FS (P = 0.99, n = 3–6 and P = 0.20, n = 4–6, respectively). In contrast, blocking PGE2 receptor EP2 by the EP2-specific antagonist PF-04418948 (10 mg/kg, ip) significantly diminished the inhibitory effect of IBS-D FS on colonic SERT expression (P = 0.01, n = 3–6). On the other hand, intraperitoneal administration of antagonists of EP1 (ONO-8130, 10 mg/kg), EP3 (L-798106, 10 mg/kg) or EP4 (ONO-AE3–208, 10 mg/kg) were without effects on SERT expression (Figure 3G). Similarly, PF-04418948 or cromolyn sodium treatment reversed IBS-D FS–induced elevation of colonic mucosal 5-HT (Supplementary Figure 2). These data suggest that PGE2, but not histamine or tryptase, released from mast cells mediates the downregulation of SERT in response to IBS-D FS.
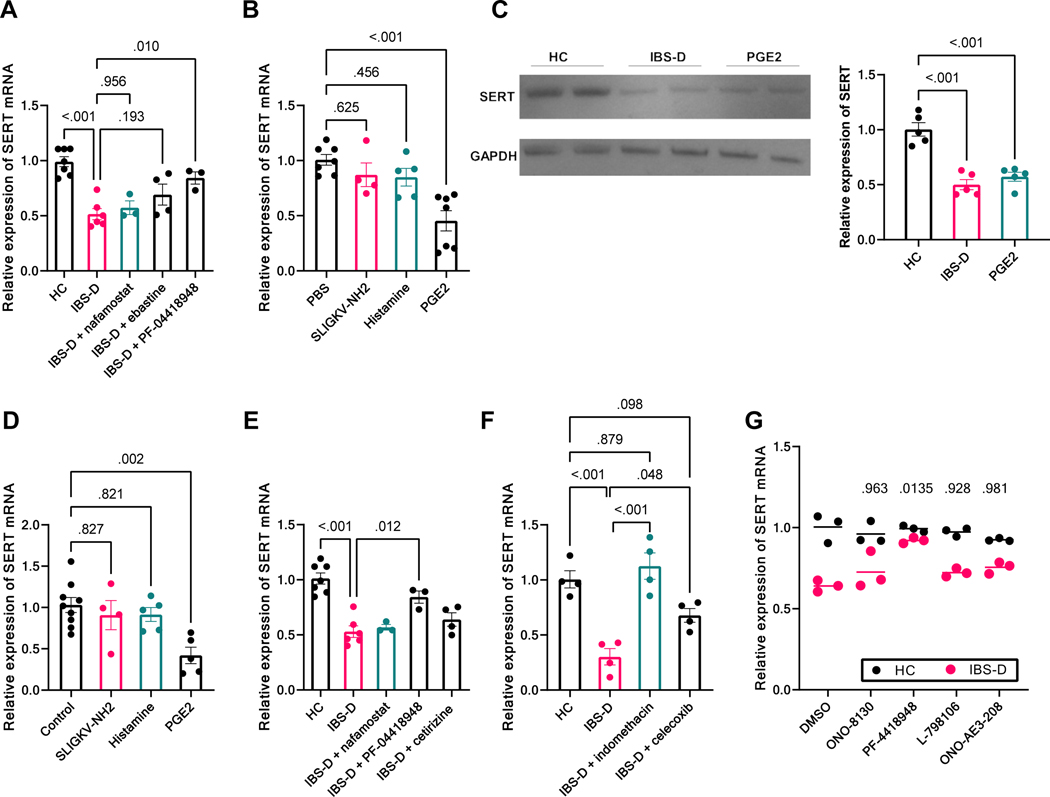
Figure 3. PGE2 is the mast cell mediator regulating colonic SERT expression in response to IBS-D supernatant.
(A) SERT expression in colonic mucosa of mice treated with HC or IBS-D FS with or without pretreatment (30 min before infusion, ip) with nafamostat mesylate, ebastine, or PF-04418948. Results are expressed as mean ± SEM, one-way ANOVA, n = 3–7 in each group. (B) SERT expression in colonic mucosa of mice treated with intracolonic infusion of PBS, SLIGKV-NH2, histamine, or PGE2 for 18 h. Results are expressed as mean ± SEM, one-way ANOVA, n = 4–7 in each group. (C) Western blot of SERT in distal colon from mice treated with intracolonic infusion of HC FS, IBS-D FS, or PGE2. Results are expressed as mean ± SEM, one-way ANOVA, n = 5 in each group. (D) SERT expression in murine colonic organoids after treatment with PBS, SLIGKV-NH2, histamine, or PGE2 for 18 h. Results are expressed as mean ± SEM, one-way ANOVA, n = 4–9 in each group. (E) IBS-D FS–induced SERT downregulation in the ex vivo murine colonoid–mast cell co-culture system was reversed by PF-04418948 (administered simultaneously with IBS-D FS to culture media). Results are expressed as mean ± SEM, one-way ANOVA, n = 3–7 in each group. (F) IBS-D FS–induced SERT downregulation in the ex vivo murine colonoid–mast cell co-culture system was reversed by indomethacin or celecoxib (administered simultaneously with IBS-D FS to culture media). Results are expressed as mean ± SEM, one-way ANOVA, n = 4 in each group. (G) Pretreatment with specific antagonists of EP1 (ONO-8130, ip., 10 mg/kg), EP3 (L-798106, ip., 10 mg/kg), and EP4 (ONO-AE3–208, ip., 10 mg/kg) failed to prevent downregulation of SERT induced by IBS-D FS (2way ANOVA with Dunnett’s Post Hoc test with vehicle group, n=3, P values as marked). In contrast, blocking PGE2 receptor EP2 by the EP2-specific antagonist PF-04418948 (10 mg/kg, ip) significantly diminished the inhibitory effect of IBS-D FS on colonic SERT expression (2way ANOVA with Dunnett’s Post Hoc test with vehicle group, P = 0.01, n = 3).
To demonstrate PGE2 is capable of regulating colonic SERT expression, mice were treated with intracolonic administration of PGE2 (100 μg/kg), the PAR2 receptor agonist SLIGKV-NH2 (100 μg/mouse), or histamine (300 μg/mouse) for 18 h before colonic samples were analyzed. As shown in Figure 3B, PAR2 agonist or histamine did not affect SERT expression in comparison to control (P = 0.62, n = 4–7 and P = 0.46, n = 5–7, respectively). In contrast, intracolonic administration of PGE2 decreased SERT mRNA by 55.3% (P < 0.01, n = 7) and protein by 47.5% (Figure 3C), mimicking the in vivo effect of IBS-D FS on SERT expression. Similar to IBS-D FS, PGE2 treatment also increased the 5-HT level in platelet-poor serum (Supplementary Figure 3).
To determine if the effect of PGE2 on SERT expression is mast cell–dependent, murine colonic organoids were treated with PGE2 (10 nM/well), SLIGKV-NH2 (100 μM/well), or histamine (10 μM/well). As shown in Figure 3D, similar to its effect in vivo, PGE2 significantly downregulated SERT expression in murine colonic organoids by 61.0% (P = < 0.01, n = 5–9) in the absence of mast cells, whereas the PAR2 agonist or histamine had no effect (P = 0.83, n = 4–9 and P = 0.82, n = 5–9, respectively). EP2 antagonist PF-04418948 (10 μM/well) significantly reversed the effect of IBS-D FS on SERT expression in our mast cell–colonoid co-culture model (P = 0.01, n = 3–6, Figure 3E). Collectively, these data suggest that PGE2 is the downstream effector of mast cells, directly regulating colonic epithelial cell SERT expression.
To provide further evidence that PGE2 synthesized and released from mast cells is critical to mediate SERT expression, we pretreated the mast cell–colonoid co-culture with the nonselective COX inhibitor indomethacin (50 μM/well) or the selective COX-2 inhibitor celecoxib (50 μM/well) before IBS-D FS stimulation. As shown in Figure 3F, blocking PGE2 synthesis in mast cells with indomethacin completely reversed the inhibitory effect of IBS-D FS on SERT expression (P < 0.01, n = 4). Similarly, selectively inhibiting COX-2 with celecoxib reversed the downregulation of SERT evoked by IBS-D FS (P = 0.048, n = 4). Taken together, these data suggest that in response to IBS-D FS, mast cells synthesize and release PGE2, which, in turn, downregulates SERT expression in colonic epithelial cells through activating the EP2 receptor.
LPS in IBS-D fecal supernatant mediates mast cell activation and SERT downregulation
Our previous study showed that an increased LPS level in fecal samples from patients with IBS-D is responsible for IBS-D FS–induced visceral hypersensitivity (VH) in rats.26 Both fecal LPS level and FS-induced VH were normalized when the rats were treated with FS from IBS patients after a 4-wk low-FODMAP diet.26 Interestingly, in the current study, intracolonic infusion of FS from IBD-S patients before and after a 4-wk low-FODMAP diet revealed normalized colonic SERT levels in mice treated with FS from IBS-D patients who responded to the low-FODMAP diet (P = 0.04, n = 8 in each group with duplicated measurements, Figure 4A). We therefore hypothesized that LPS is a key component in the IBS-D FS to activate mast cells to regulate SERT expression.
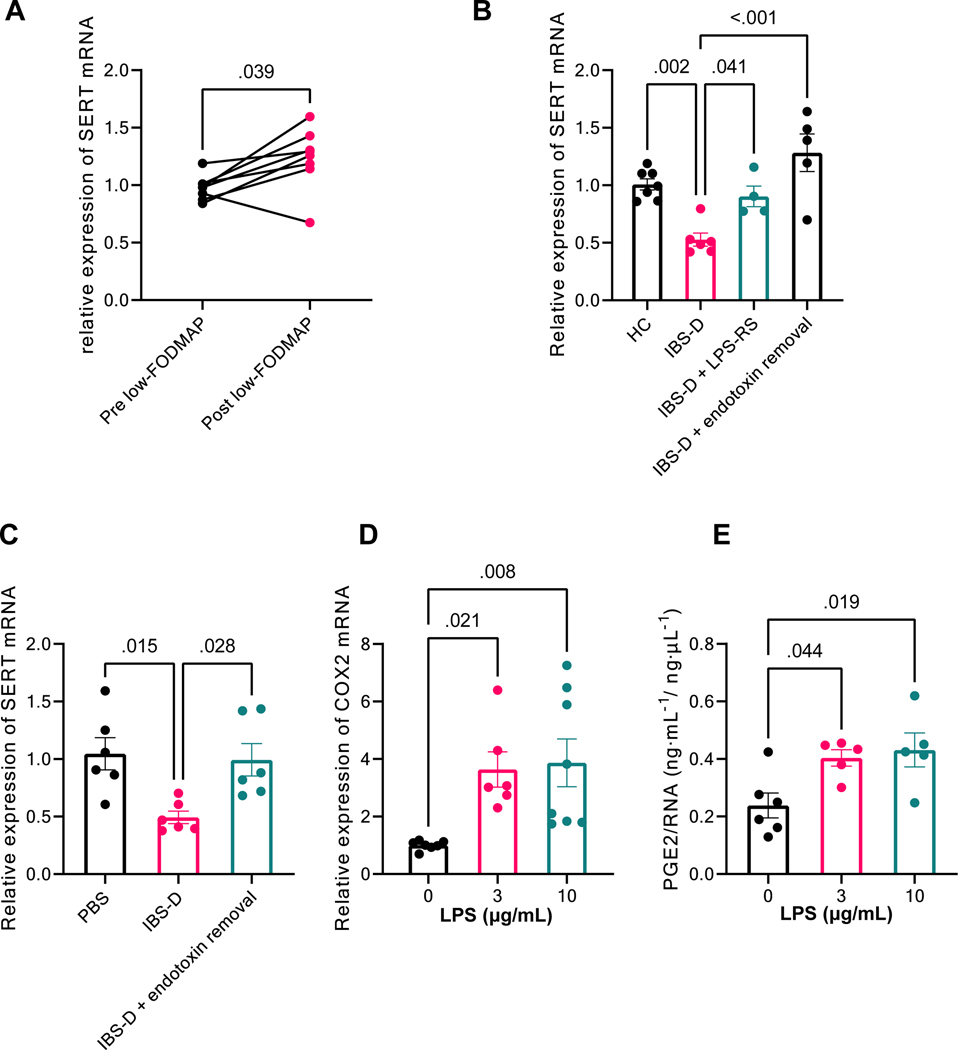
Figure 4. LPS is a required component in the IBS-D fecal supernatant for mast cell activation and SERT downregulation.
(A) Intracolonic infusion of FS from IBS-D patients who responded to 4-wk low-FODMAP treatment resulted in increased SERT expression in mouse colon compared to infusion of FS from the same patients before low-FODMAP. Results are expressed as individual values, Wilcoxon signed-rank test, n = 8 with duplicated measurements. (B) SERT expression in colonic mucosa of mice treated with HC FS, IBS-D FS, pretreatment with LPS-RS (30-min intracolonic infusion) followed by IBS-D FS or IBS-D FS pretreated with endotoxin removal. Results are expressed as mean ± SEM, one-way ANOVA, n = 4–7 in each group. (C) SERT expression in murine colonic organoids co-cultured with mast cells, treated with PBS, IBS-D FS, or IBS-D FS pretreated with endotoxin removal. Results are expressed as mean ± SEM, one-way ANOVA, n = 6 in each group. (D) RT–PCR of COX-2 in cultured bone marrow–derived mast cells treated with LPS for 4 h (0, 3, 10 μg/mL). Results are expressed as mean ± SEM. one-way ANOVA, n = 6–8 in each group. (E) Release of PGE2 from mast cells treated with LPS for 4 h (0, 3, 10 μg/mL). Results are expressed as mean ± SEM. one-way ANOVA, n = 5–6 in each group.
To test this hypothesis, mice were treated with intracolonic administration of the potent TLR4 antagonist lipopolysaccharide from Rhodobacter sphaeroides (LPS-RS) (1.0 mg/kg) 30 min before IBS-D FS treatment. As shown in Figure 4B, IBS-D FS–induced mucosal SERT downregulation was prevented by LPS-RS (P = 0.04, n = 4–6). Similarly, pretreating the IBS-D FS with endotoxin removal completely abolished its inhibitory effect on SERT expression (P < 0.01, n = 5–6) (Figure 4B). Moreover, after endotoxin removal, IBS-D FS failed to exert any ex vivo effect on SERT expression in mice colonic organoids co-cultured with mast cells (P = 0.03, n = 6, Figure 4C). These data suggest that LPS is the key component in the IBS-D FS to modulate SERT expression via mast cell activation. Furthermore, in vitro studies demonstrated a dose-dependent increase in COX-2 expression (Figure 4D) and PGE2 release (Figure 4E) from cultured mouse mast cells in response to a 4-h LPS treatment (3 and 10 μg/mL).
To further delineate the role of LPS in mediating SERT expression, we treated naïve mice with intracolonic administration of LPS (50 μg/mL) for 18 h; significant changes in colonic SERT expression were not detected (P > 0.99, n = 4–6, Supplementary Figure 4A). This finding suggests that LPS is essential, but alone, insufficient, to activate mast cells to regulate SERT expression in vivo. Another important component in the FS of IBS-D patients is trypsin, which has been reported to be elevated in IBS patients.25 We showed that heating the IBS-D FS for 5 min at 95°C, which deactivates peptides such as proteinases but not LPS,32 completely abolished the inhibitory effects of IBS-D FS when administered to the colon of naïve mice (P < 0.01, n = 4, Supplementary Figure 4B). Similarly, pretreating IBS-D FS with a soybean trypsin inhibitor (2.5 mg/mL) or bacterial protease inhibitor cocktail (Sigma-Aldrich, 0.1 mL/mL) abolished the inhibitory effects on colonic SERT expression (P < 0.01, n = 3–4, Supplementary Figure 4B). In separate studies, we showed that intracolonic administration of trypsin-3 (10 units/mouse) failed to affect colonic SERT expression (P > 0.99, n = 4); whereas intracolonic infusion of LPS (50 μg/mouse) combined with trypsin-3 (10 units/mouse) caused a 25% reduction of SERT expression compared to control (P = 0.01, n = 5–6) (Supplementary Figure 4A) These findings suggest that LPS acting in concert with trypsin mediates the downregulation of colonic SERT expression.
Dysregulated mucosal SERT–serotonin pathway contributes to IBS-D FS–induced diarrhea and visceral hypersensitivity
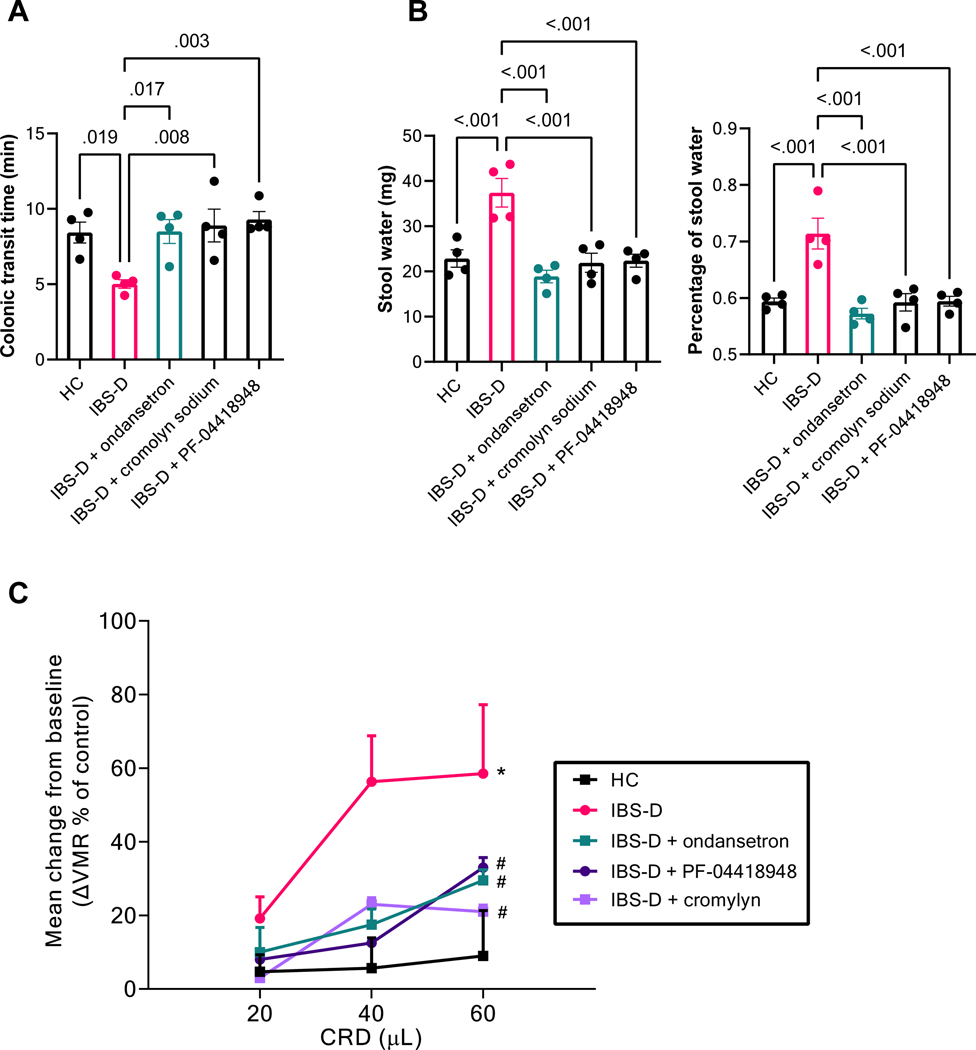
To demonstrate the pathophysiological significance of decreased SERT expression and increased mucosal 5-HT level induced by the FS from IBS-D patients, we showed that intracolonic infusion of IBS-D FS in mice reduced colonic transit 30% (P = 0.02, n = 4, Figure 5A), increased fecal water content 25% (P < 0.01, n = 4, Figure 5B), and increased VMR to CRD 2–3 fold (P < 0.001, n = 6, Figure 5C). Pretreatment with 5-HT3 antagonist ondansetron (5 mg/kg, ip) prevented these changes, indicating the increased 5-HT level in the mucosa is responsible for the enhanced colonic motility, intestinal secretion, and visceral hypersensitivity in our IBS-D model. Importantly, pretreatment with mast cell stabilizer cromolyn sodium (30 mg/kg, ip), or EP2-specific antagonist PF-04418948 (10 mg/kg, ip), which decreased mucosal 5-HT level by reversing the downregulated SERT expression by IBD-FS, also blocked IBS-D FS–induced increase in motility, secretion, and visceral hypersensitivity (Figure 5).
Figure 5. IBS-D fecal supernatant causes diarrhea and visceral hypersensitivity in a mast cell–, PGE2-, and 5-HT–dependent manner.
(A) Colonic transit time in mice treated with intracolonic infusion of HC or IBS-D FS, with or without pretreatment (30 min before infusion, IP) with ondansetron, cromolyn sodium, or PF-0441894. Results are expressed as mean ± SEM, one-way repeated measures ANOVA, n = 4. (B) Stool water weight (left) and percentage (right) of mice treated with intracolonic infusion of HC or IBS-D FS, with or without pretreatment (30 min before infusion, IP) with ondansetron, cromolyn sodium, or PF-0441894. Results are expressed as mean ± SEM, one-way repeated measures ANOVA, n = 4. (C) VMR to CRD in mice treated with intracolonic administration of HC or IBS-D FS, with or without pretreatment (30 min before infusion, IP) with ondansetron, cromolyn sodium, or PF-0441894. Results are expressed as mean ± SEM, *P < 0.05 from HC, #P < 0.05 from IBS-D, two-way repeated measures ANOVA, n = 6.
Discussion
In the intestinal epithelium, 5-HT acts as a neurotransmitter and a paracrine signaling molecule to initiate and modulate the major physiological processes in the gut, including secretion, motility, vasodilation, inflammation, and pain sensation. The intensity and duration of 5-HT action ultimately depends on the efficiency of 5-HT inactivation. Due to a relative lack of intercellular degradative enzymes, the termination of 5-HT signaling relies almost exclusively on the SERT-dependent translocation of 5-HT from the lamina propria. Generally, SERT activity and extracellular 5-HT concentration are inversely correlated. Acute inhibition of SERT by the selective serotonin reuptake inhibitor (SSRI) increases colonic motility and contraction, which are associated with abdominal cramping.33 Over long periods, however, excessive accumulation of 5-HT can lead to 5-HT receptor desensitization and diminished 5-HT action.
A significant decrease in SERT expression was reported in biopsy specimens from IBS patients, compared to healthy individuals.13,14,34 This occurred without overt mucosal inflammation in our IBS rodent model. Others have reported decreased SERT levels in post-infectious or water avoidance stress-induced IBS animal models.18,35 A decrease in SERT expression in IBS may have important pathophysiological consequences as shown by the demonstration that mice lacking the high-affinity 5-HT transporter exhibited increased intestinal motility and abnormal expression of cation transporters. 16 Our current studies showed for the first time in an IBS rodent model a causal relationship between a decrease in SERT expression and enhanced colonic motility and intestinal secretion accompanied by the development of visceral hypersensitivity.
The mechanisms regulating SERT expression in the gastrointestinal tract are not clear. Previous studies with intestinal cell lines suggest that proinflammatory signaling such as TNFα and INFγ,36 as well as native immunity triggers including TLR237 and NOD238 are able to downregulate SERT in vitro. It has also been proposed that SERT expression is affected by epigenetic factors39 and microRNAs.40 However, the exact mechanism by which SERT is regulated in the setting of IBS remains largely unknown. To the best of our knowledge, our studies showed for the first time that in response to gut dysbiosis, luminal endotoxins in the setting of IBS stimulate intestinal mast cells to synthesize and release PGE2, which, in turn, acts on the gut epithelium to downregulate SERT, resulting in increased mucosal 5-HT. This new observation is supported by the following findings: 1) intracolonic administration of fecal supernatant from IBS-D patients to naïve mice caused a marked decrease in SERT expression and an increase of 5-HT level in the colonic mucosa; 2) these changes were prevented by mast cell stabilizers ketotifen and cromolyn sodium, or the selective PGE2 receptor antagonist PF-0441894; Furthermore, the downregulation of SERT expression evoked by IBS-D FS was not observed in mast cell deficient (W/Wv ) mice but the response was restored by mast cells reconstitution in these W/Wv mice; 3) intracolonic administration of PGE2 but not histamine or PAR2 agonist downregulated mucosal SERT expression; 4) the inhibitory effect of IBS-D fecal supernatant or LPS on SERT expression in murine colonic organoids required the presence of bone marrow–derived mast cells and the enzymatic activities of COX-2 to synthesize PGE2; 5) the downregulation of SERT expression evoked by IBS-D FS was prevented by pretreating the FS with LPS antagonist and trypsin inhibitor.
The lamina propria is rich in mast cells, which sense stimuli from both the lumen interface and within the mucosa and transmit signals to adjacent cells through releasing a plethora of mediators. Histamine and proteases – two bioactive substances presynthesized and stored in cytoplasmic granules of mast cells – have been implicated in visceral hypersensitivity,25,41,42 intestinal hypersecretion,43 and barrier dysfunction.44 In our study, we showed that neither histamine nor tryptase exhibit a direct effect on intestinal SERT expression, in vivo or in vitro (Figure 3).
In addition to degranulation of presynthesized mediators, activation of mast cells also leads to de novo synthesis and release of neoformed mediators, such as prostanoids, by the action of COX.45 Prostaglandins, especially PGE2, play an important role in the development of visceral hypersensitivity in IBS by activating the EP2 receptor on dorsal root ganglion neurons.46 Among the four PGE2 receptor subtypes, EP2 is differentially expressed in the epithelia of human colonic mucosa.47 The direct function of PGE2 on colonic epithelium is unclear. In this study, we identified a novel function of PGE2 in regulating SERT expression in colonic epithelial cells.
To identify which component(s) in the IBS-D fecal supernatant triggered mast cell activation in our rodent model, we found that downregulation of SERT induced by IBS-D FS was preventable by LPS antagonist LPS-RS or removal of LPS from the fecal FS. This suggests mediation by fecal LPS. Our in vitro studies further supported this possibility, as LPS was able to activate mast cells to release PGE2 through the induction of the COX-2 enzyme, and to downregulate SERT expression in murine organoids when co-cultured with mast cells. However, intracolonic infusion of LPS failed to downregulate SERT expression, suggesting LPS is required, but not sufficient to cause SERT downregulation in vivo. IBS-D patients also have elevated fecal trypsin, 48 which can alter epithelial function.46 In this study, we showed that heating the IBS-D FS, which deactivates peptides such as proteinases but not LPS, completely abolished the inhibitory effect of IBS-D FS on colonic SERT expression. Furthermore, pretreatment of IBS-D FS with a soybean trypsin inhibitor or bacterial protease inhibitor cocktail also prevented downregulation of SERT expression. Taken together, these observations suggest that fecal trypsin acts in concert with LPS to modulate colonic SERT expression.
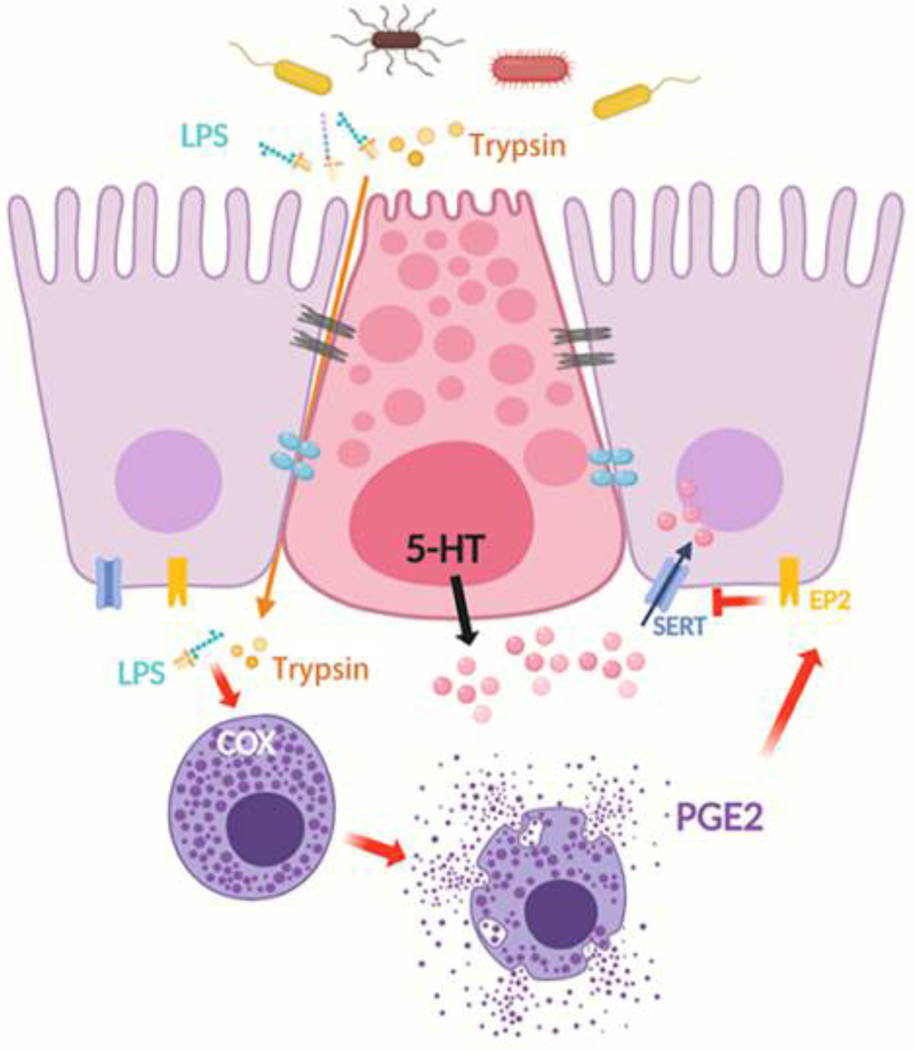
Based on our findings, we would like to propose the following working model to explain the mechanism responsible for the decreased mucosal SERT expression in IBS. Fecal material in the colon of IBS patients contains increased amounts of LPS and other bioactive substances, such as trypsin. On entry to the lamina propria, LPS acting in concert with trypsin increases COX-dependent PEG2 synthesis and release from activated mucosal mast cells. PGE2, in turn, acts on EP2 receptors in the epithelial cells to downregulate SERT expression, eventually leading to increased mucosal 5-HT availability and the symptom-triggering actions of 5-HT, resulting in increased motility, intestinal secretion, and visceral hypersensitivity in IBS patients. This may contribute to diarrhea and abdominal pain often observed in IBS (Figure 6).
Figure 6. Mucosal SERT is modulated by gut microbiota via mast cell–COX–PGE2 pathway in IBS.
Proinflammatory bacterial products such as LPS and trypsin, among others, are increased in the gut lumen of IBS patients. On entry to the lamina propria, these mediators activate the mucosal mast cells to increase COX-dependent PEG2 synthesis and release, which acts on EP2 receptors on the epithelial cells to downregulate SERT expression, leading to increased mucosal 5-HT availability, which stimulates motility, epithelial secretion, and visceral sensitivity via intrinsic and extrinsic sensory neuronal networks.
Supplementary Material
What You Need to Know.
Background and Context
IBS patients have increased colonic serotonin level and decreased serotonin reuptake transporter expressions. We investigated whether mucosal serotonin reuptake transporter is modulated by gut microbiota via a mast cell-prostaglandin E2 pathway.
New Findings
Fecal lipopolysaccharide acting in concert with trypsin in IBS-D patients stimulates mucosal mast cells to release PGE2, which downregulates mucosal serotonin reuptake transporter, resulting in increased mucosal 5HT.
Limitations
We limited our studies to IBS-D patients. Future studies should compare and contrast findings in IBS-C patients as the pathophysiology between these two groups of IBS patients may be quite different.
Impact
Gut dysbiosis may contribute to dysregulation of serotonin metabolic pathway resulting in increased serotonin in the colonic mucosa. This may contribute to diarrhea and abdominal pain in IBS patients.
Lay summary
Dysregulation of microbial composition in the colon may result in abnormal serotonin metabolism in the colon contributing to diarrhea and abdominal pain often observed in IBS patients.
Acknowledgments
Funding
The studies were supported by the National Institutes of Health grants R01DK110436 and P30DK34933.
Abbreviations used in this paper:
- COX
cyclooxygenases
- CRD
colorectal distension
- EP2
prostaglandin E2 receptor 2
- HC
healthy controls
- IBS
irritable bowel syndrome
- IBS-D
diarrhea-predominant IBS
- LPS
lipopolysaccharide
- LPS-RS
lipopolysaccharide from Rhodobacter sphaeroides
- MOAA
monoamine oxidase A
- PAR2
protease-activated receptor 2
- PGE2
prostaglandin E2
- SERT
serotonin reuptake transporter
- RT-PCR
reverse transcriptase–polymerase chain reaction
- TPH1
tryptophan hydroxylase 1
- VH
visceral hypersensitivity
- VMR
visceromotor response
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 2013;10:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spohn SN, Mawe GM. Non-conventional features of peripheral serotonin signalling - the gut and beyond. Nat Rev Gastroenterol Hepatol 2017;14:412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houghton LA, Atkinson W, Whitaker RP, et al. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut 2003;52:663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunlop SP, Coleman NS, Blackshaw E, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol 2005;3:349–357. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson W, Lockhart S, Whorwell PJ, et al. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology 2006;130:34–43. [DOI] [PubMed] [Google Scholar]
- 6.Andresen V, Montori VM, Keller J, et al. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol 2008;6:545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of alosetron for the treatment of irritable bowel syndrome in women and men: A meta-analysis of eight randomized, placebo-controlled, 12-week trials. Clin Ther 2008;30:884–901. [DOI] [PubMed] [Google Scholar]
- 8.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 2007;132:397–414. [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Knutson K, Alcaino C, et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol 2017;595:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellono NW, Bayrer JR, Leitch DB, et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 2017;170:185–198.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wade PR, Chen J, Jaffe B, et al. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci 1996;16:2352–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen JX, Pan H, Rothman TP, et al. Guinea pig 5-HT transporter: Cloning, expression, distribution, and function in intestinal sensory reception. Am J Physiol 1998;275:G433–448. [DOI] [PubMed] [Google Scholar]
- 13.Foley S, Garsed K, Singh G, et al. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology 2011;140:1434–1443 e1. [DOI] [PubMed] [Google Scholar]
- 14.Kerckhoffs AP, ter Linde JJ, Akkermans LM, et al. SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am J Physiol Gastrointest Liver Physiol 2012;302:G1053–1060. [DOI] [PubMed] [Google Scholar]
- 15.Faure C, Patey N, Gauthier C, et al. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology 2010;139:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JJ, Li Z, Pan H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci 2001;21:6348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esmaili A, Nazir SF, Borthakur A, et al. Enteropathogenic Escherichia coli infection inhibits intestinal serotonin transporter function and expression. Gastroenterology 2009;137:2074–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao YN, Feng LJ, Liu YY, et al. Effect of Lactobacillus rhamnosus GG supernatant on serotonin transporter expression in rats with post-infectious irritable bowel syndrome. World J Gastroenterol 2018;24:338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut 2016;65:155–168. [DOI] [PubMed] [Google Scholar]
- 21.Albert-Bayo M, Paracuellos I, Gonzalez-Castro AM, et al. Intestinal mucosal mast cells: Key modulators of barrier function and homeostasis. Cells 2019;8:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Song J, Hou X. Mast cells and irritable bowel syndrome: From the bench to the bedside. J Neurogastroenterol Motil 2016;22:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klooker TK, Braak B, Koopman KE, et al. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut 2010;59:1213–1221. [DOI] [PubMed] [Google Scholar]
- 24.Stefanini GF, Saggioro A, Alvisi V, et al. Oral cromolyn sodium in comparison with elimination diet in the irritable bowel syndrome, diarrheic type. Multicenter study of 428 patients. Scand J Gastroenterol 1995;30:535–541. [DOI] [PubMed] [Google Scholar]
- 25.Grabauskas G, Wu X, Gao J, et al. Prostaglandin E2, produced by mast cells in colon tissues from patients with irritable bowel syndrome, contributes to visceral hypersensitivity in mice. Gastroenterology 2020;158:2195–2207 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou SY, Gillilland M 3rd, Wu X, et al. FODMAP diet modulates visceral nociception by 24popolysaccharide-mediated intestinal inflammation and barrier dysfunction. J Clin Invest 2018;128:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rijnierse A, Koster AS, Nijkamp FP, et al. Critical role for mast cells in the pathogenesis of 2,4-dinitrobenzene-induced murine colonic hypersensitivity reaction. J Immunol 2006;176:4375–4384. [DOI] [PubMed] [Google Scholar]
- 28.Mizutani T, Clevers H. Primary intestinal epithelial organoid culture. Methods Mol Biol 2020;2171:185–200. [DOI] [PubMed] [Google Scholar]
- 29.Koslo RJ, Burks TF, Porreca F. Centrally administered bombesin affects gastrointestinal transit and colonic bead expulsion through supraspinal mechanisms. J Pharmacol Exp Ther 1986;238:62–67. [PubMed] [Google Scholar]
- 30.Mahe MM, Aihara E, Schumacher MA, et al. Establishment of Gastrointestinal Epithelial Organoids. Curr Protoc Mouse Biol 2013;3:217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology 2007;132:26–37. [DOI] [PubMed] [Google Scholar]
- 32.Coelho AM, Vergnolle N, Guiard B, et al. Proteinases and proteinase-activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology 2002;122:1035–1047. [DOI] [PubMed] [Google Scholar]
- 33.Tack J, Broekaert D, Corsetti M, et al. Influence of acute serotonin reuptake inhibition on colonic sensorimotor function in man. Aliment Pharmacol Ther 2006;23:265–274. [DOI] [PubMed] [Google Scholar]
- 34.Spiller R, Lam C. An update on post-infectious irritable bowel syndrome: Role of genetics, immune activation, serotonin and altered microbiome. J Neurogastroenterol Motil 2012;18:258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi HL, Liu CH, Ding LL, et al. Alterations in serotonin, transient receptor potential channels and protease-activated receptors in rats with irritable bowel syndrome attenuated by Shugan decoction. World J Gastroenterol 2015;21:4852–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foley KF, Pantano C, Ciolino A, et al. IFN-gamma and TNF-alpha decrease serotonin transporter function and expression in Caco2 cells. Am J Physiol Gastrointest Liver Physiol 2007;292:G779–G784. [DOI] [PubMed] [Google Scholar]
- 37.Latorre E, Layunta E, Grasa L, et al. Intestinal serotonin transporter inhibition by Toll-like receptor 2 activation. A feedback modulation. PLoS One 2016;11:e0169303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Layunta E, Latorre E, Forcen R, et al. NOD2 Modulates Serotonin Transporter and Interacts with TLR2 and TLR4 in Intestinal Epithelial Cells. Cell Physiol Biochem 2018;47:1217–1229. [DOI] [PubMed] [Google Scholar]
- 39.Gill RK, Kumar A, Malhotra P, et al. Regulation of intestinal serotonin transporter expression via epigenetic mechanisms: role of HDAC2. Am J Physiol Cell Physiol 2013;304:C334–C341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baudry A, Mouillet-Richard S, Schneider B, et al. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science 2010;329:1537–1541. [DOI] [PubMed] [Google Scholar]
- 41.Wouters MM, Balemans D, Van Wanrooy S, et al. Histamine receptor H1-mediated sensitization of TRPV1 mediates visceral hypersensitivity and symptoms in patients with irritable bowel syndrome. Gastroenterology 2016;150:875–887 e9. [DOI] [PubMed] [Google Scholar]
- 42.Buhner S, Schemann M. Mast cell-nerve axis with a focus on the human gut. Biochim Biophys Acta 2012;1822:85–92. [DOI] [PubMed] [Google Scholar]
- 43.Crowe SE, Luthra GK, Perdue MH. Mast cell mediated ion transport in intestine from patients with and without inflammatory bowel disease. Gut 1997;41:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bueno L, Fioramonti J. Protease-activated receptor 2 and gut permeability: A review. Neurogastroenterol Motil 2008;20:580–587. [DOI] [PubMed] [Google Scholar]
- 45.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 2000;69:145–182. [DOI] [PubMed] [Google Scholar]
- 46.Grabauskas G, Wu X, Turgeon D, et al. Mo2033 Marked elevation in mucosal proinflammatory PGE2 is responsible for pain in diarrhea predominant IBS (IBS-D) patients. Gastroenterology 2015;148:S–775. [Google Scholar]
- 47.Olsen Hult LT, Kleiveland CR, Fosnes K, et al. EP receptor expression in human intestinal epithelium and localization relative to the stem cell zone of the crypts. PLoS One 2011;6:e26816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tooth D, Garsed K, Singh G, et al. Characterization of faecal protease activity in irritable bowel syndrome. Gut 2014; 63:753–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.