Abstract
16S rRNA-targeted in situ hybridization combined with confocal laser scanning microscopy was used to elucidate the spatial distribution of microbes within two types of methanogenic granular sludge, mesophilic (35°C) and thermophilic (55°C), in upflow anaerobic sludge blanket reactors fed with sucrose-, acetate-, and propionate-based artificial wastewater. The spatial organization of the microbes was visualized in thin sections of the granules by using fluorescent oligonucleotide probes specific to several phylogenetic groups of microbes. In situ hybridization with archaeal- and bacterial-domain probes within granule sections clearly showed that both mesophilic and thermophilic granules had layered structures and that the outer layer harbored mainly bacterial cells while the inner layer consisted mainly of archaeal cells. Methanosaeta-, Methanobacterium-, Methanospirillum-, and Methanosarcina-like cells were detected with oligonucleotide probes specific for the different groups of methanogens, and they were found to be localized inside the granules, in both types of which dominant methanogens were members of the genus Methanosaeta. For specific detection of bacteria which were previously detected by whole-microbial-community 16S ribosomal DNA (rDNA)-cloning analysis (Y. Sekiguchi, Y. Kamagata, K. Syutsubo, A. Ohashi, H. Harada, and K. Nakamura, Microbiology 144:2655–2665, 1998) we designed probes specific for clonal 16S rDNAs related to unidentified green nonsulfur bacteria and clones related to Syntrophobacter species. The probe designed for the cluster closely related to Syntrophobacter species hybridized with coccoid cells in the inner layer of the mesophilic granule sections. The probe for the unidentified bacteria which were clustered with the green nonsulfur bacteria detected filamentous cells in the outermost layer of the thermophilic sludge granule sections. These results revealed the spatial organizations of methanogens and uncultivated bacteria and their in situ morphologies and metabolic functions in both mesophilic and thermophilic granular sludges.
Granular sludge in upflow anaerobic sludge blanket (UASB) reactors harbors several metabolic groups of microbes for complete mineralization of organic matter (11). The microorganisms are packed as a spherical biofilm, forming an interesting microbial ecosystem with a characteristic internal architecture. This unique biofilm has been intensively studied, and a number of unique phenomena have been reported.
One feature of the granules is the spatial organization of the microorganisms. Usually, the inner layer consists mostly of aceticlastic methanogens and the outer layer is comprised of fermentative bacteria (7, 13). Immunohistochemical techniques have demonstrated the layered structure and the juxtaposition of syntrophs and methanogens in the consortia (6, 12, 24). Furthermore, in situ hybridization analysis of several methanogenic and sulfidogenic granules demonstrated the proximity of these organisms in mesophilic granules (8, 9). However, the mosaic of microbes within the granules, especially thermophilic sludge granules, has not been completely investigated. Specifically, location at the bacterial genus and species levels in the consortia is hardly understood.
Recently, we described whole-community 16S ribosomal DNAs (rDNAs) in the mesophilic and thermophilic methanogenic granular sludges adapted to sucrose-, acetate-, and propionate-containing wastewater by using a PCR-based cloning approach (18). In this analysis, a number of unidentifiable clones were found in both granules. This indicates that a large portion of the microbial community members in the granules have not been characterized and their spatial organization is unknown.
In this study, we used the in situ hybridization technique combined with confocal laser scanning microscopy (CLSM) to visualize the locations of several microorganisms of particular interest in both mesophilic and thermophilic granular sludges which were detected in our previous 16S rDNA cloning analysis. Initially, oligonucleotide probes specific to Bacteria and several phylogenetic groups of methanogens were used to characterize and compare the overall microbial topographies in both types of granules. Second, some unidentifiable bacteria, which were considered to be major bacterial components in the granules in the previous 16S rDNA-cloning analysis, were visualized by specifically designed and fluorescently labeled probes to reveal their in situ morphologies and locations in the granules.
MATERIALS AND METHODS
Operation of UASB reactors.
Granules were collected from two laboratory-scale UASB reactors (13-liter capacity) operated at mesophilic (35°C) and thermophilic (55°C) temperatures as described previously (18) (Fig. 1). Both reactors were fed with the synthetic substrate containing sucrose, acetate, propionate, and peptone or yeast extract (chemical oxygen demand [COD] ratio, 4.5:2.25:2.25:1) over 2 years of operation. The substrate concentration was 2,000 mg of COD/liter for the mesophilic reactor and 4,000 mg of COD/liter for the thermophilic reactor.
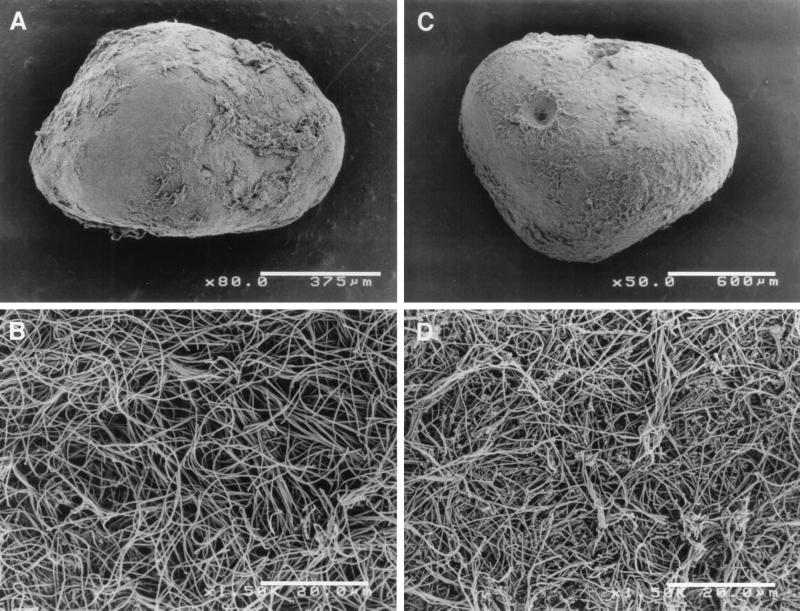
FIG. 1.
Scanning electron micrographs of mesophilic (A and B) and thermophilic (C and D) sludge granules at low magnification (A and C) and their surfaces at higher magnification (B and D).
Fixation and sectioning of the granules.
The granule samples were gently washed with phosphate-buffered saline (PBS [0.13 M NaCl, 10 mM Na2HPO4, pH 7.2]), and allowed to settle naturally. Whole granules were then fixed with 4% paraformaldehyde in PBS and left for 6 h at 4°C. The granules were then exposed to 50% ethanol in PBS for 12 h at 4°C. To allow probes to penetrate the cells in the thermophilic granule samples, five freeze-and-thaw cycles (−80 to 60°C) were done after fixation. The fixed granules were dehydrated by successive passages through 50, 80, and 100% ethanol (three times), 50:50 (vol/vol) ethanol-xylene, and 100% xylene (three times) and embedded in melted paraffin wax. Serial sections 10 to 15 μm thick were cut with a rotary microtome and mounted on gelatin-coated glass slides. The sections were dewaxed through 100% xylene (two times) and 100% ethanol (two times) and dried at room temperature.
In situ hybridization.
The 16S rRNA-targeted oligonucleotide probes used in this study are listed in Table 1. They comprised domain-specific probes for Bacteria and Archaea (3, 19); order-, family-, and genus-specific probes for several phylogenetic groups of methanogens (14); and a genus-specific probe for Desulfobulbus (5). For more specific detection of unidentified bacteria in both types of granules, we designed the following two probes: (i) SYB701, specific for clone MUG28 (closely related to Syntrophobacter species [10]) as described in our previously study (18) (5′-AAATGCAGTTTCCAATGCAC-3′; Escherichia coli positions, 701 to 720), and (ii) GNSB633, specific for clones MUG9 and TUG8, -9, and -10 (possibly classified in the green nonsulfur bacteria), as described previously (18) (5′-TAGCCCGCCAGTCTTGAACG-3′; E. coli positions, 633 to 652). For in situ hybridization, the probes were labeled with either Cy-5 or rhodamine (see below).
TABLE 1.
Fluorescently labeled oligonucleotide probes used in this study
| Probe name | OPD namea | Target group | Probe sequence (5′ to 3′) | Reference |
|---|---|---|---|---|
| EUB338 | S-D-Bact-0338-a-A-18 | Bacteria | GCTGCCTCCCGTAGGAGT | 3 |
| ARC915 | S-D-Arch-0915-a-A-20 | Archaea | GTGCTCCCCCGCCAATTCCT | 19 |
| MG1200 | S-O-Mmic-1200-a-A-21 | Methanomicrobiales | CGGATAATTCGGGGCATGCTG | 14 |
| MB1174 | S-F-Mbac-1174-a-A-22 | Methanobacteriaceae | TACCGTCGTCCACTCCTTCCTC | 14 |
| MS1414 | S-F-Msar-1414-a-A-21 | Methanosarcinaceae | CTCACCCATACCTCACTCGGG | 14 |
| MX825 | S-F-Msae-0825-a-A-23 | Methanosaetaceae | TCGCACCGTGGCCGACACCTAGC | 14 |
| D660 | S-G-Dsbb-0660-a-A-20 | Desulfobulbus | GAATTCCACTTTCCCCTCTG | 5 |
| SYB701 | S-*-MUG28-0701-a-A-20 | rDNA clone MUG28 | AAATGCAGTTTCCAATGCAC | This study |
| GNSB633 | S-*-GNSB-0633-a-A-20 | rDNA clones in the green nonsulfur bacteria | TAGCCCGCCAGTCTTGAACG | This study |
OPD, oligonucleotide probe database (1).
Hybridizations were performed at 46°C for 10 h with hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl [pH 7.2], 0.01% sodium dodecyl sulfate) containing 5 ng of each labeled probe/μl (2). The hybridization stringency was adjusted by adding formamide to the hybridization buffer (5% for EUB338 and MG1200; 10% for SYB701; 20% for MX825, D660, and GNSB633; and 35% for ARC915, MB1174, and MS1414). The washing step was done at 48°C for 30 min with washing buffer containing the same components as the hybridization buffer except for the probes. For double staining of the sections, Cy-5- and rhodamine-labeled probes were used simultaneously, with the probe requiring a higher concentration of formamide for high stringency hybridized and washed first, followed by the other probe for the second hybridization. The sections hybridized with the probes were observed with a CLSM (Olympus FLUOVIEW BX50).
Dot blot hybridization.
Dot blot hybridization was performed to estimate the specificity of the designed probes. Digoxigenin-labeled SYB701 and GNSB633 probes were used for the hybridization and were detected with the DIG nucleic acid detection kit (Boehringer Mannheim) essentially according to the manufacturer’s instructions. For determination of probe specificity, rDNAs from the following reference organisms were used: Desulfovibrio vulgaris Marburg (DSM2119); Syntrophobacter wolinii (DSM2245) in coculture with Desulfovibrio sp. strain G11; Desulfobulbus propionicus MUD (DSM6523); and rDNA clones MUG28 (the target of the SYB701 probe), MUG9, TUG8, TUG9, and TUG10 (the targets of GNSB633), and MUG6, MUG7, MUG8, TUG6, and TUG7 (nontarget green nonsulfur bacteria) (18). Dot blot hybridization was performed with the same hybridization and washing buffers used in whole-cell in situ hybridization. The optimal formamide concentrations for the newly designed probes were determined by changing the formamide concentrations in the buffers. In addition, rhodamine-labeled SYB701 probe was used for in situ hybridization with the above-mentioned organisms for the probe check.
SEM.
Scanning electron microscope (SEM) observation was performed with a HITACHI-S4500 SEM operating as described previously (22).
RESULTS
In situ hybridization with Methanobacterium cells in thermophilic granules.
In our previous study, epifluorescence microscopy revealed that a number of F420-autofluorescent curved rods morphologically resembling Methanobacterium were present in thermophilic sludge granules (18). However, they could not be detected by the usual in situ hybridization protocol, probably due to the lack of penetration of probes into the cells (9, 15). Attempts to detect hydrogen-consuming methanogens, except Methanosarcina-like cells, were unsuccessful in the sections from thermophilic sludge granules, whereas hydrogen-consuming methanogens could be easily detected in the mesophilic sludge granules. To determine whether Methanobacterium cells hybridized with a Methanobacteriaceae-specific probe (MB1174), we isolated some thermophilic Methanobacterium strains from the thermophilic granules by using hydrogen and carbon dioxide as the energy and carbon sources, respectively. Neither Methanobacteriaceae-specific probe (MB1174) nor Archaea universal probe (ARC915) hybridized with these strains. Several attempts to detect the strains by modifying the protocol, i.e., increasing the concentration of sodium dodecyl sulfate in the hybridization buffer (from 0.01 to 0.1 or 1%) or adding proteinase K pretreatment before hybridization, were made, but no improvement was observed. We eventually used freeze-thaw cycles (−80 and 60°C) for the strains after fixation with 4% paraformaldehyde. Three to five freeze-thaw cycles significantly enhanced hybridization with both ARC915 and MB1174 probes, which resulted in detection of almost 100% of the cells (data not shown). Hybridization with other methanogens, such as Methanosaeta, was not influenced by this treatment (data not shown).
Overall structure of the mesophilic and thermophilic granules.
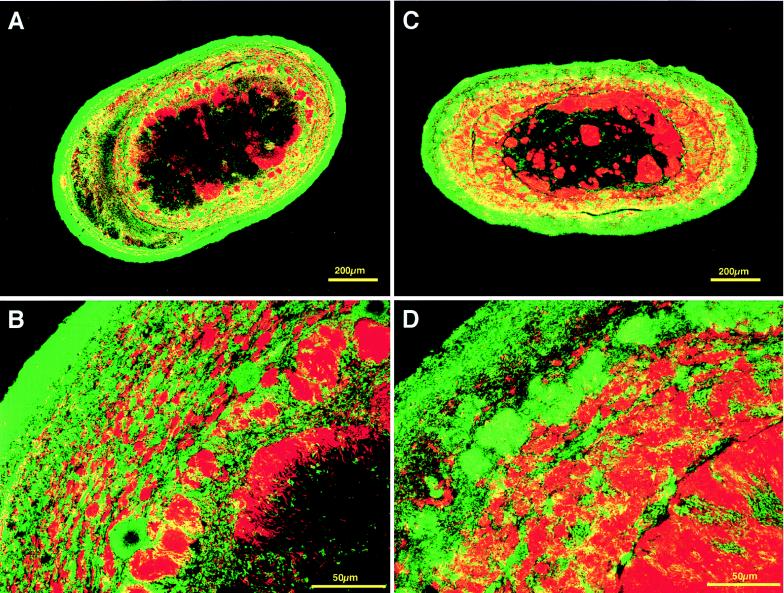
To visualize all Bacteria and Archaea, Cy-5-labeled EUB338 probe (3) and rhodamine-labeled ARC915 probe (19) were used simultaneously in sections of both types of granular sludge. As shown in Fig. 2, both types of granule showed a characteristic structure; the outer layer was dominated by bacterial cells ca. 50 μm thick, whereas the inner layer was occupied mainly by archaeal cells. Both types of granule had large dark nonstaining centers, in which neither archaeal nor bacterial signals could be found. The nonstaining center was always observed in large granules (exceeding about 0.5 mm in diameter), but smaller granules rarely had it.
FIG. 2.
In situ hybridization of sections from mesophilic and thermophilic granules viewed by CLSM. The sections were simultaneously hybridized with Cy-5-labeled bacterial-domain probe (EUB338) (green) and rhodamine-labeled archaeal-domain probe (ARC915) (red). Mesophilic (A and B) and thermophilic (C and D) sludge granules at low magnification (A and C) and at higher magnification (B and D) are shown.
Archaea in granules.
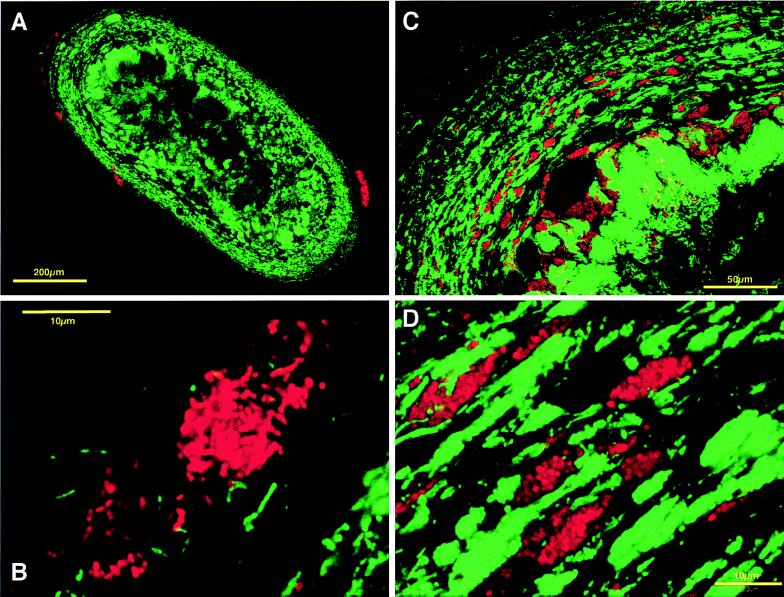
In situ hybridization with MX825 probe in both mesophilic and thermophilic sludge granules revealed that Methanosaeta cells predominated among Archaea cells (Fig. 3A and D). They formed a large number of microcolonies inside the granules. The Methanosaeta cells and bacterial cells formed multiple layers that seemed like annular growth rings. The remaining portion of archaeal cells in the mesophilic granules consisted mainly of Methanobacterium- (or Methanobrevibacter)-like cells and Methanospirillum-like cells, which hybridized with MB1174 and MG1200 probes (14), respectively (Fig. 3B and C). Methanobacterium-like cells detected by MB1174 probe were much less numerous than Methanosaeta cells, but closer observation revealed that Methanobacterium-like cells and bacterial cells formed aggregates in which they were closely juxtaposed (Fig. 3B). Methanospirillum-like cells detected with MG1200 probe were distributed evenly inside the granules, although they were not as numerous as Methanosaeta cells (Fig. 3C). The MS1414 probe detected very few cells, suggesting that Methanosarcina-type methanogens were not frequent in the mesophilic granules.
FIG. 3.
In situ hybridization of sections from mesophilic and thermophilic granules viewed by CLSM. The sections were simultaneously hybridized with Cy-5-labeled bacterial probe (EUB338) (green) and rhodamine-labeled probe for several phylogenetic groups of methanogens (red). (A to C) Mesophilic sludge granule sections; (D to F) thermophilic sludge granule sections. (A and D) Sections hybridized with MX825 probe for the genus Methanosaeta (red) and bacterial probe (green). (B and E) Sections hybridized with MB1174 probe for the family Methanobacteriaceae (red) and bacterial domain probe (green). The inset in panel B is a magnification of a microcolony of coccoid bacterial cells and Methanobacteriaceae. (C) Mesophilic section hybridized with MG1200 probe (red) for the order Methanomicrobiales and bacterial probe (green). (F) Thermophilic section hybridized with MS1414 probe (red) for the family Methanosarcinaceae and bacterial probe (green), indicating a microcolony of Methanosarcina-like organisms (inset).
In contrast with the mesophilic granules, the thermophilic granules contained denser populations of Methanobacterium-like cells detected with the MB1174 probe (Fig. 3E). They were distributed widely over the granules and sometimes formed microcolonies. In addition, some signals from methanogens morphologically similar to Methanosarcina species were observed in the sections with MS1414 probe (Fig. 3F).
Bacteria in the granules.
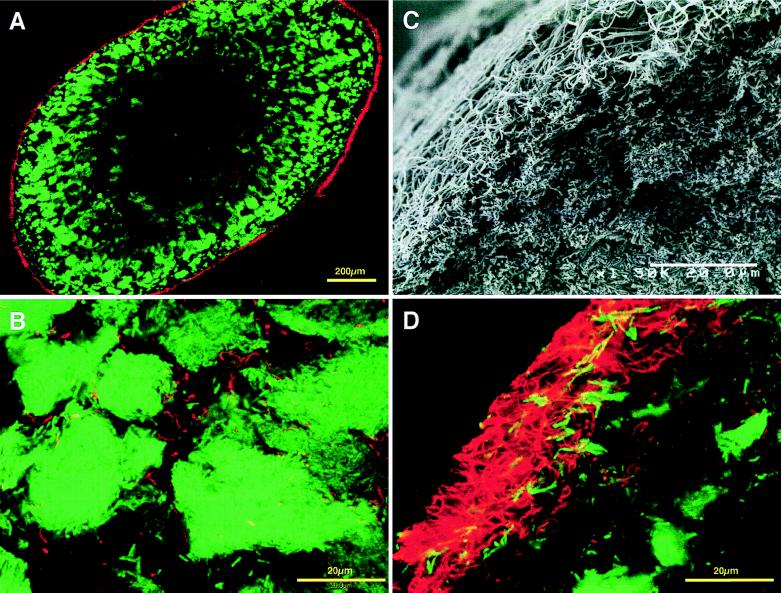
From our previous 16S rDNA-cloning analysis, several known bacteria were expected to be present in both mesophilic and thermophilic sludge granules (18). First, 16S rDNA clones belonging to the genus Desulfobulbus were targeted for in situ hybridization. We used D660 probe, which is specific for the genus Desulfobulbus (5). This probe matches the clones MUG35 and MUG36, which are closely related to previously known Desulfobulbus species. MUG35 and MUG36 clones accounted for approximately 8% of the total clone library for the mesophilic sludge granules (18). In situ hybridization with the D660 probe detected irregular-coccus-shaped bacteria in only the outer layer of sections from the mesophilic sludge granules (see Fig. 5A and B).
FIG. 5.
In situ hybridization of sections from a mesophilic granule viewed by CLSM. (A) View of a mesophilic sludge granule section which was simultaneously hybridized with Cy-5-labeled archaeal-domain probe (ARC915) (green) and rhodamine-labeled D660 probe for the genus Desulfobulbus (red). (B) Higher magnification of mesophilic granule section shown in panel A showing the outer layer of the section. (C) Mesophilic granule section hybridized with Cy-5-labeled archaeal probe (ARC915) (green) and rhodamine-labeled SYB701 probe for the clone MUG28 (red). (D) Higher magnification of mesophilic granule section shown in panel C showing the inner layer.
Next, a probe (SYB701) was designed for clone MUG28, which was determined in the cloning analysis to be closely related to the Syntrophobacter species and strain MPOB (10) (Fig. 4A). This clone accounted for 5% of the total mesophilic clone library (18). The probe contains at least three mismatches for other known 16S rRNAs, including all Syntrophobacter species, and its specificity was verified, since it did not react with one of its closest relatives, S. wolinii (4) by either dot blot hybridization or whole-cell in situ hybridization (data not shown). The application of this probe to the mesophilic sludge granule sections showed that a number of coccoid cells, showing morphology similar to that of previously described Syntrophobacter species (Fig. 5C and D), were detected inside the granules. The probe detected no signal in the thermophilic sludge granules under the same hybridization conditions.
FIG. 4.
Phylogenetic (neighbor-joining [16]) tree of rDNA clones previously detected by community 16S rDNA-cloning analysis of the mesophilic and thermophilic granular sludges (18). The targets of the probes (SYB701 and GNSB633) designed in this study are indicated by brackets. MUG, clones detected from the mesophilic granules; TUG, clones detected from the thermophilic granules. The numbers in parentheses indicate the number of identical clones obtained per number of clones analyzed. The numbers at nodes represent bootstrap values resampled 100 times. (A) 16S rDNA clones related to Syntrophobacter species in the delta subclass of the class Proteobacteria and the targets of the probe SYB701. (B) 16S rDNA clones among the green nonsulfur bacteria and the target organisms for the GNSB633 probe.
In both mesophilic and thermophilic sludge granules, some unknown microbes related to the green nonsulfur bacteria were expected to be present (18). We also designed a probe specific for the clones TUG8, TUG9, TUG10, and MUG9, which are related to the green nonsulfur bacteria. These clones accounted for 2% of the mesophilic and 16% of the thermophilic granule clone libraries (18) (Fig. 4B). The probe had at least four mismatches with other known 16S rRNAs, and the specificity of the probe was checked by dot blot hybridization with all rDNA plasmid clones previously obtained from the granules as relatives of green nonsulfur bacteria (data not shown). Hybridization using this probe in both types of granule sections showed that a number of filamentous cells were distinctively detected in the outermost layer of the thermophilic sludge granule section (Fig. 6). A few cells which hybridized with the probe were also observed inside the granules (Fig. 6B). In the mesophilic granule section, a few cells morphologically similar to the hybridized cells in the thermophilic sludge granule section hybridized with the probe only inside the granule (data not shown). Although the mesophilic granules were also covered with filamentous organisms resembling the thermophilic ones (Fig. 1B), the probe reacted with few of the filaments.
FIG. 6.
Scanning electron micrograph and in situ hybridization of section from a thermophilic sludge granule viewed by CLSM. (A) thermophilic sludge granule section simultaneously hybridized with Cy-5-labeled archaeal-domain probe (ARC915) (green) and rhodamine-labeled GNSB633 probe for the clones in the green nonsulfur bacteria cluster (red). (B) Higher magnification of the granule section shown in panel A showing the inside of the section. (C) Scanning electron micrograph of a thermophilic sludge granule section showing the external layer. (D) Higher magnification of the thermophilic sludge granule section shown in panel A showing the outermost layer, similar to panel C.
DISCUSSION
The spatial distributions of Bacteria and Archaea within sucrose- and short-chain fatty acid (acetate and propionate)-treating mesophilic and thermophilic sludge granules was elucidated by applying the in situ hybridization technique. Both the mesophilic and thermophilic granular sludges received almost-identical synthetic substrates; hence, both granules perform similar metabolic functions, but they contain phylogenetically different organisms (18).
To prepare the sections, we used paraffin wax for embedding the granules; cutting sections ≥10 μm thick, which is too thick for usual epifluorescence microscopy, is preferable in order to maintain the structure of the granules in situ. However, application of CLSM allowed easy viewing of granules with 10- to 15-μm-thick sections. In the detection of some thermophilic methanogens by in situ hybridization, we faced a problem regarding the penetration of oligonucleotide probes into the cells, particularly thermophilic Methanobacterium cells. To solve this problem, we employed freeze-thaw cycles to the fixed cells before hybridization, resulting in the improvement of probe penetration. This pretreatment was expected to destroy the cells which had weak walls, such as gram-negative cells, but a large part of the cells of the methanogen Methanosaeta concilii, for instance, were not influenced by this treatment (data not shown).
Overall microbial distributions in both types of sludge granules were clearly visualized by these pretreatments combined with CLSM. A multiple-microbial-layer structure in the granules was demonstrated by applying double staining to the sections with bacterial-domain probe (EUB338) and archaeal-domain probe (ARC915). This observation showed that the outer layer was dominated by bacterial cells and the inner layer consisted mainly of archaeal cells in both the mesophilic and thermophilic sludges. Similar layered structures were reported on carbohydrate-containing wastewater-treating granules by electron microscopy (13), immunolabeling (12, 24), and in situ hybridization techniques (9). Our observations of the overall microbial distributions give an additional and more evident demonstration of the ordered microbial structure of mesophilic granules. Furthermore, it is clearly demonstrated that the thermophilic granules have similar distinctive layered structures. An in situ hybridization study of granule sections by Harmsen et al. indicated that sucrose-fed mesophilic granules contained three layers, of which the innermost consisted of large cavities and inorganic materials (9). A large nonstaining part located in the centers of the granule sections was also observed in both types of our granules, particularly in large granules. SEM of the centers in sections of both types of granules revealed that the nonstaining part contained not only cellular materials but probably inorganic materials as well (data not shown). The nonstaining center might be formed as a result of the accumulation of metabolically inactive cells, decaying cells, and inorganic materials, which can no longer obtain substrates due to the limitation of diffusion into the center.
The dominant member of the Archaea in both granules was the genus Methanosaeta, which hybridized with the MX825 probe (14). This was in good agreement with other reports (9, 12, 13, 17, 22–24) and with our previous findings that a large number of clones related to the genus Methanosaeta were detected in both types of sludge granules, based on the 16S rDNA-cloning analysis (18). Some microcolonies were detected by the MS1414 probe in both mesophilic and thermophilic granules, which are morphologically and phylogenetically similar to Methanosarcina. The microcolonies were more frequent in the thermophilic than the mesophilic granules.
From our 16S rDNA-cloning analysis of the same granules, several organisms were expected to be phylogenetically related to certain cultivated strains of Bacteria (18). For the detection and localization of these bacteria, we focused on three clades in the bacterial domain for detection by in situ hybridization. First, we targeted the clade which contained the Desulfobulbus species. Nine of 115 total clones in our mesophilic sludge granule library were assigned to this clade. The D660 probe (5) was used for hybridization with mesophilic granule sections. The detected cells were morphologically similar to the known Desulfobulbus species and were located in only the outer layer of the section. The influent wastewater for the mesophilic granules contained approximately 300 mg of propionate/liter and 80 mg of sulfate/liter. Approximately 3% of overall COD removed could be oxidized by sulfate-reduction; hence, the detected cells might contribute to propionate oxidation coupled with sulfate reduction.
Secondly, we focused on the clones which were closely related with Syntrophobacter species. Harmsen et al. reported that strain MPOB, a syntrophic propionate-oxidizing strain, was located inside the sucrose-fed mesophilic UASB granules, in which the organisms were juxtaposed with Methanobrevibacter-like methanogens (9). As we expected, the designed probe (SYB701) detected a number of coccoid cells, which had a morphology similar to that of strain MPOB and Syntrophobacter species (4, 20, 25), in the inner layer of the mesophilic sludge granule sections. When the hybridized cells and Methanobacterium (or Methanobrevibacter)-like cells were double stained, it was found that they formed an aggregate, suggesting that the cells detected by SYB701 probe might be propionate-oxidizing syntrophs related to the known Syntrophobacter species. Such proximity between a hydrogen-producing fatty-acid oxidizer and a hydrogen-utilizing methanogen is necessary to make the less energetically favorable propionate oxidation reaction possible. Actually, when an enrichment culture with propionate as the sole carbon source was made for the mesophilic sludge granules, this type of cell could be detected frequently by the probe in the culture (data not shown).
Finally, we focused on the clones in the green nonsulfur bacteria clade. The clade, targeted by the GNSB633 probe, contained 18 of 110 total clones in the thermophilic sludge granule clone library and two of 115 total clones in the mesophilic sludge granule clone library (Fig. 4B). When the probe was applied to the sections of both types of granule, only filamentous cells could be detected. Thermophilic sludge granule sections contained a large amount of the cells detected, especially in the outermost layer of the sections, whereas mesophilic sludge granule sections contained only a few of these cells. This observation is consistent with the frequency of appearance of clones in the previous 16S rDNA-cloning analysis (18). In the thermophilic granules, it was observed that long, thin filamentous bacteria, which were apparently different from Methanosaeta, were predominant on the surfaces of the granules (Fig. 1D). This type of organism might be responsible for the granulation of sludge and the preservation of its structure (22). Our data revealed the phylogenetic position of the organism, but metabolic and functional information about this organism is still not clearly known. However, since (i) the filamentous organisms occupy the outermost layer of the granules and (ii) this type of organism was frequently observed in thermophilic granules treating carbohydrate-based wastewater (21, 22), it is suggested that these cells probably use the primary substrate, i.e., sucrose, in the anaerobic degradation of organic matter in the wastewater. We tried to enrich the filaments from the thermophilic sludge granules by using conventional batch culture with sucrose as the sole energy source, but all attempts were unsuccessful. The other interesting finding was that although the mesophilic granules were also covered with filamentous organisms morphologically similar to the thermophilic filaments (Fig. 1B), almost none of these cells reacted with the GNSB633 probe. This observation suggests that the filamentous cells in the mesophilic sludge granules are phylogenetically different from the thermophilic ones.
In this study, we describe the localization of microbes in two types of granules which were adapted to sucrose-, acetate-, and propionate-based artificial wastewater at different temperatures. The in situ hybridization technique in combination with CLSM revealed a unique microbial architecture of known bacteria and methanogens in the granules. Furthermore, in situ hybridization with the probes designed by using our previous 16S rDNA-cloning data not only uncovered the in situ morphologies of unidentified (or currently unculturable) bacteria but also imply possible in situ metabolic functions of these unidentified bacteria from their spatial location and architecture in the granules without cultivation. This approach will provide further insight into the structures and functions of microbial consortia and give us valuable information for isolation and cultivation of currently unculturable microbes.
ACKNOWLEDGMENTS
We thank Roderick Mackie at the University of Illinois at Urbana-Champaign for his critical reading of the manuscript. We also thank Kazuaki Syutsubo for his stimulating interest in this work, Tadashi Tagawa for his help with UASB reactors and histological techniques, and Hirokazu Oseki and Tetsuki Hidano for their help with in situ hybridization and CLSM work.
This study was financially supported by research grant 97Ea11-011 of the Proposal-Based R & D Program of the New Energy and Industrial Technology Development Organization (NEDO), Japan.
REFERENCES
- 1.Alm E W, Oerther D B, Larsen N, Stahl D A, Raskin L. The oligonucleotide probe database. Appl Environ Microbiol. 1996;62:3557–3559. doi: 10.1128/aem.62.10.3557-3559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann R I. In situ identification of micro-organisms by whole cell hybridization with rRNA-targeted nucleic acid probes, section 3.3.6. In: Akkermans A D L, van Elsas J D, editors. Molecular microbial ecology manual. London, England: Kluwer Academic Publishers; 1995. pp. 3.3.6/1–3.3.6/15. [Google Scholar]
- 3.Amann R I, Binder B J, Olson R J, Chisholm S W, Devereux R, Stahl D A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boone D R, Bryant M P. Propionate-degrading bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from methanogenic ecosystems. Appl Environ Microbiol. 1980;40:626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devereux R, Kane M D, Winfrey J, Stahl D A. Genus- and group-specific hybridization probes for determinative and environmental studies of sulfate-reducing bacteria. Syst Appl Microbiol. 1992;15:601–609. [Google Scholar]
- 6.Grotenhuis J T C, Smit M, Plugge C M, Yuansheng X, van Lammeren A A M, Stams A J M, Zehnder A J B. Bacteriological composition and structure of granular sludge adapted to different substrates. Appl Environ Microbiol. 1991;57:1942–1949. doi: 10.1128/aem.57.7.1942-1949.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guiot S R, Pauss A, Costerton J W. A structured model of the anaerobic granule consortium. Water Sci Tech. 1992;25:1–10. [Google Scholar]
- 8.Harmsen H J M, Akkermans A D L, Stams A J M, de Vos W M. Population dynamics of propionate-oxidizing bacteria under methanogenic and sulfidogenic conditions in anaerobic granular sludge. Appl Environ Microbiol. 1996;62:2163–2168. doi: 10.1128/aem.62.6.2163-2168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harmsen H J M, Kengen H M P, Akkermans A D L, Stams A J M, de Vos W M. Detection and localization of syntrophic propionate-oxidizing bacteria in granular sludge by in situ hybridization using 16S rRNA-based oligonucleotide probes. Appl Environ Microbiol. 1996;62:1656–1663. doi: 10.1128/aem.62.5.1656-1663.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harmsen H J M, Kengen H M P, Akkermans A D L, Stams A J M. Phylogenetic analysis of two syntrophic propionate-oxidizing bacteria in enrichment cultures. Syst Appl Microbiol. 1995;18:67–73. [Google Scholar]
- 11.Lettinga G. Anaerobic digestion and wastewater treatment systems. Antonie Leeuwenhoek. 1995;67:3–28. doi: 10.1007/BF00872193. [DOI] [PubMed] [Google Scholar]
- 12.Macario A J L, Visser F A, van Lier J B, Conway de Macario E. Topography of methanogenic subpopulations in a microbial consortium adapting to thermophilic conditions. J Gen Microbiol. 1991;137:2179–2189. [Google Scholar]
- 13.MacLeod F A, Guiot S R, Costerton J W. Layered structure of bacterial aggregates produced in an upflow anaerobic sludge bed and filter reactor. Appl Environ Microbiol. 1990;56:1598–1607. doi: 10.1128/aem.56.6.1598-1607.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raskin L, Stromley J M, Rittmann B E, Stahl D A. Group-specific 16S rRNA hybridization probes to describe natural communities of methanogens. Appl Environ Microbiol. 1994;60:1232–1240. doi: 10.1128/aem.60.4.1232-1240.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roller C, Wagner M, Amann R, Ludwig W, Schleifer K H. In situ probing of Gram-positive bacteria with high G+C content using 23S rRNA-targeted oligonucleotides. Microbiology. 1994;140:2849–2858. doi: 10.1099/00221287-140-10-2849. [DOI] [PubMed] [Google Scholar]
- 16.Saito N, Nei M. The neighbor-joining method: a new method for constructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt J E, Ahring B K. Granular sludge formation in upflow anaerobic sludge blanket (UASB) reactors. Biotechnol Bioeng. 1996;49:229–246. doi: 10.1002/(SICI)1097-0290(19960205)49:3<229::AID-BIT1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 18.Sekiguchi Y, Kamagata Y, Syutsubo K, Ohashi A, Harada H, Nakamura K. Phylogenetic diversity of mesophilic and thermophilic granular sludges determined by 16S rRNA gene analysis. Microbiology. 1998;144:2655–2665. doi: 10.1099/00221287-144-9-2655. [DOI] [PubMed] [Google Scholar]
- 19.Stahl D A, Amann R. Development and application of nucleic acid probes. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. New York, N.Y: John Wiley & Sons, Inc.; 1991. pp. 205–248. [Google Scholar]
- 20.Stams A J M, van Dijk J B, Dijkema C, Plugge C M. Growth of syntrophic propionate-oxidizing bacteria with fumarate in the absence of methanogenic bacteria. Appl Environ Microbiol. 1993;59:1114–1119. doi: 10.1128/aem.59.4.1114-1119.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uemura S, Harada H. Inorganic composition and microbial characteristics of methanogenic granular sludge grown in a thermophilic upflow anaerobic sludge blanket reactor. Appl Microbiol Biotechnol. 1995;43:358–364. [Google Scholar]
- 22.Uemura S, Harada H. Microbial characteristics of methanogenic sludge consortia developed in thermophilic UASB reactors. Appl Microbiol Biotechnol. 1993;39:654–660. [Google Scholar]
- 23.van Lier J B, Grolle K C F, Stams A J M, Conway de Macario E, Lettinga G. Start-up of a thermophilic upflow anaerobic sludge bed (UASB) reactor with mesophilic granular sludge. Appl Microbiol Biotechnol. 1992;37:130–135. doi: 10.1007/BF00174217. [DOI] [PubMed] [Google Scholar]
- 24.Visser F A, van Lier J B, Macario A J L, Conway de Macario E. Diversity and population dynamics of methanogenic bacteria in a granular consortium. Appl Environ Microbiol. 1991;57:1728–1734. doi: 10.1128/aem.57.6.1728-1734.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallrabenstein C, Hauschild E, Schink B. Syntrophobacter pfennigii sp. nov., new syntrophically propionate-oxidizing anaerobe growing in pure culture with propionate and sulfate. Arch Microbiol. 1995;164:346–352. [Google Scholar]