Abstract
Background:
Biomarkers have the potential to provide clinical guidance, but there is limited data for biomarkers in metastatic hormone sensitive prostate cancer (mHSPC).
Methods:
We performed a retrospective multicenter review from Winship Cancer Institute at Emory University and Georgia Cancer Center for Excellence at Grady Memorial Hospital (2014 – 2020) in the United States of America (USA). We collected demographics, disease characteristics, and laboratory data, including complete blood counts (CBC) at the start of upfront therapy. We evaluated overall survival (OS) and progression-free survival (PFS) associated with baseline lab values.
Results:
165 patients were included with a median follow-up time of 33.5 months (mo). 105 (63.6%) had Gleason scores of 8–10 and 108 (65.9%) were classified as high-volume disease. 92 patients received upfront docetaxel (55.8%) and 73 received upfront abiraterone (44.2%). Univariate analyses (UVA) and multivariable analyses (MVA) identified worse clinical outcomes (CO) associated with elevated basophils and basophil-to-lymphocyte ratio (BLR). Based on MVA, elevated basophils (defined as ≥0.1, optimal cut) were associated with a hazard ratio (HR) of 3.51 (95% CI 1.65–7.43, p 0.001) for OS and HR of 1.88 (95% CI 1.05–3.38, p 0.034) for PFS. Our MVA also found that BLR ≥ 0.0142 was associated with HR 2.11 (95% CI 1.09–4.10, p 0.028) for OS; however, PFS was not statistically significant.
Conclusion:
We conclude that elevated baseline basophils and BLR are associated with worse clinical outcomes in mHSPC. Although results require further validation, BLR is a potential prognostic biomarker.
Keywords: castration-sensitive prostate cancer, hormone-sensitive prostate cancer, biomarkers, basophils, basophil to lymphocyte ratio
1. Introduction
Prostate cancer (PCa) remains the most commonly diagnosed malignancy among men in the United States with an estimated 42% increase in new metastatic PCa cases from 2015 – 2025 [1–4]. Metastatic hormone sensitive prostate cancer (mHSPC) is a complex disease to manage due to a rapidly evolving treatment landscape in addition to the increasing incidence of disease. Current therapies include androgen deprivation therapy (ADT) with either upfront abiraterone (ABI), docetaxel (DOC), enzalutamide (ENZA), and apalutamide (APA), whereas ADT alone was the mainstay of treatment until 2015 [5–7].
Readily available data in routine labs, including complete blood counts (CBCs), can reflect inflammatory changes due to acute phase reactants that are associated with disease progression, serving as surrogates of the immune system-tumor interaction [8–12]. Studies in other subsets of PCa, such as metastatic castration resistant PCa (mCRPC) and in different malignancies have reported worse clinical outcomes (CO) with increases in leukocytes, such as neutrophils, and the neutrophil-to-lymphocyte ratio (NLR) [13–18]. Other leukocyte subsets, such as basophils, have not been studied as extensively. There is some data in other malignancies, specifically bladder and pancreatic cancer, showing that higher basophils are associated with tumor recurrence and decreased overall survival (OS), respectively [19–20].
To identify potential prognostic biomarkers in mHSPC, we compiled a demographically diverse patient database that included CBC data at initiation of upfront treatment in mHSPC, then evaluated for associations with CO, specifically OS and progression-free survival (PFS).
2. Material and Methods
2.1. Patients and Data
PCa patients’ records were compiled from pharmacy databases at Winship Cancer Institute of Emory University and Grady Cancer Center for Excellence (2014 – 2020) in the United States of America (USA). Patients treated with either docetaxel (DOC) or abiraterone (ABI) in the upfront setting were identified and included in the study if they did not receive any other prior systemic therapy. Institutional Review Board approval was obtained. Data collected included demographics, treatments, outcomes, and labs. For labs, we focused on baseline CBC, defined as the time prior to or just after starting upfront therapy. Based on the CBC, we calculated NLR and basophil-to-lymphocyte ratio (BLR). Each of those measurements were dichotomized as high vs. low at the location that maximized the log-rank test for OS using a bias-adjusted log-rank test searching algorithm [21]. The patient list was last reviewed in September 2021. At that time, the data was updated regarding disease progression and the last date of follow-up.
2.2. Definitions
Patients were classified as high-volume disease based on the CHAARTED criteria of visceral metastases or ≥ 4 bone lesions with ≥1 beyond axial skeleton [22].
Normal labs were set at the following values: hemoglobin (hgb) 12.9 – 16.1 (gm/dl), platelets (plt) 150–400 (103/μL), neutrophils 0.67–6.41 (103/μL), lymphocytes 0.72–3.29 (103/μL), basophils 0–0.07 (103/μL).
Clinical outcomes included: OS (time from drug initiation to death) and PFS (time from drug initiation to biochemical progression, radiographic progression, or death; whichever occurred first). Patient deaths were confirmed by reviewing both the state of Georgia obituary database and electronic health record (EHR). Cases were censored at the last follow-up if there were no events. Biochemical progression was based on an increase in PSA on two consecutive measurements with the first measurement noted as time of progression, or if PSA nadir was <4 then the PSA >4 was used as time of progression.
2.3. Statistical Analysis
Statistical analysis was conducted using SAS Version 9.4, and SAS macros [23]. The significance level was set at P < 0.05. Descriptive statistics for each variable were reported. The univariate associations (UVA) and multivariable analyses (MVA) for OS or PFS was tested by Cox proportional hazard model with hazard ratio (HR) and its 95% confidence interval (CI) being reported. Variables controlled in the MVA were drug, race, age, Gleason score, disease volume, and ECOG status. Due to the limited number of events and total sample size, we focus on controlling with a few selected important confounders based on existing knowledge. The optimal cut off value for a continuous biomarker was derived relative to PFS using bias-adjusted log-rank test after examining all possible cuts in the data space. [24] The association of interest was also examined in subgroups by race and treatment using interaction terms in MVA model.
3. Results
3.1. Overview
165 patients were included with a median follow-up time of 33.5 months (mo) (95% CI 29.3 – 37.2 mo). 92 patients received upfront DOC (55.8%) and 73 received upfront ABI (44.2%). 105 (63.6%) had Gleason scores of 8–10 and 108 (65.9%) were classified as high-volume disease (per CHAARTED trial criteria) [Table 1]. The most significant results were an association of elevated basophils and BLR with worse clinical outcomes, notably OS [Tables 2 – 4, Figures 1 and 2]. Additionally, our results found decreased OS for low hgb (<12.9 associated with HR of 2.33, 95% CI 1.18–4.62, p 0.015). There was no significant change in clinical outcomes based on platelets or NLR. The full UVAs can be found in Tables 2 and 3.
Table 1.
Baseline Characteristics by Baseline BLR and Baseline Basophils at Optimal Cut.
| Baseline Basophils at optimal cutΨ | Baseline BLR at optimal cut Ω | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Level | Below N=68 | Above N=97 | p-value* | Below N=141 | Above N=24 | p-value* |
| Drug under investigation | Docetaxel | 76 (53.90) | 16 (66.67) | 0.244 | 38 (55.88) | 54 (55.67) | 0.978 |
| Abiraterone | 65 (46.10) | 8 (33.33) | 30 (44.12) | 43 (44.33) | |||
| Race | Black | 68 (48.23) | 21 (87.50) | <0.001 | 51 (75.00) | 38 (39.18) | <0.001 |
| Non-Black | 73 (51.77) | 3 (12.50) | 17 (25.00) | 59 (60.82) | |||
| Age at diagnosis | <65 | 73 (51.77) | 16 (66.67) | 0.176 | 40 (58.82) | 49 (50.52) | 0.292 |
| >=65 | 68 (48.23) | 8 (33.33) | 28 (41.18) | 48 (49.48) | |||
| PSA at diagnosis at | Above | 73 (52.9) | 18 (75) | 0.044 | 44 (64.71) | 47 (50) | 0.063 |
| optimal cut 37.2 (ng/mL) | Below | 65 (47.1) | 6 (25) | 24 (35.29) | 47 (50) | ||
| Total Gleason score | 7 | 22 (15.60) | 1 (4.17) | 0.282 | 10 (14.71) | 13 (13.4) | 0.743 |
| 8–10 | 89 (63.12) | 16 (66.67) | 41 (60.29) | 64 (65.98) | |||
| Unknown | 30 (21.28) | 7 (29.17) | 17 (25.00) | 20 (20.62) | |||
| ECOG at time of starting treatment a | 0 | 71 (50.35) | 4 (16.67) | 0.002 | 29 (42.65) | 46 (47.42) | 0.829 |
| 1 | 53 (37.59) | 12 (50.00) | 28 (41.18) | 37 (38.14) | |||
| 2 | 17 (12.06) | 8 (33.33) | 11 (16.18) | 14 (14.43) | |||
| Number of distant metastases b | 0–1 | 41 (29.08) | 3 (12.50) | 0.090 | 20 (29.41) | 24 (24.74) | 0.504 |
| 2–4+ | 100 (70.92) | 21 (87.50) | 48 (70.59) | 73 (75.26) | |||
| Disease volume c | High | 90 (63.83) | 18 (78.26) | 0.176 | 50 (73.53) | 58 (60.42) | 0.081 |
| Low | 51 (36.17) | 5 (21.74) | 18 (26.47) | 38 (39.58) | |||
Eastern Cooperative Oncology Group (ECOG) performance status, ranging from 0 to 5, with lower scores indicating better functionality.
Number of anatomical locations (lymph nodes = 1, bone = 1, liver = 1, lung = 1, brain = 1)
Disease volume is classified as high-volume disease based on CHAARTED criteria of visceral metastases or ≥ 4 bone lesions with ≥1 beyond axial skeleton
The p-value is calculated by Chi-square test or Fisher’s exact test, wherever appropriate.
“Below” defined as <0.1. “Above” defined as ≥0.1, with 0.1 being optimal cut.
“Below” defined as <0.0142, “Above” defined as ≥ 0.0142, with 0.0142 being optimal cut
Table 2.
UVA for mOS in mHSPC Based on Baseline Lab Values.
| Variable | N | Hazard Ratio (95% CI)‡ | P-value* | |
|---|---|---|---|---|
| Hgb Status (Normal: 12.9 – 16.1) | Abnormal | 97 | 2.33 (1.18–4.62) | 0.015 |
| Normal | 68 | - | - | |
| Hgb at Optimal Cut (11.8) | Above | 106 | 0.28 (0.15–0.51) | <0.001 |
| Below | 59 | - | - | |
| Platelets Status (Normal: 150 – 400) | Abnormal | 25 | 0.64 (0.25–1.63) | 0.348 |
| Normal | 140 | - | - | |
| Platelets at Optimal Cut (259) | Above | 56 | 2.11 (1.17–3.82) | 0.013 |
| Below | 109 | - | - | |
| Neutrophils Status (Normal: 0.67 – 6.41) | Abnormal | 18 | 1.33 (0.56–3.17) | 0.513 |
| Normal | 147 | - | - | |
| Neutrophils at Optimal Cut (3.08) | Above | 102 | 1.04 (0.56–1.93) | 0.897 |
| Below | 63 | - | - | |
| Lymphocytes Status (Normal: 0.72 – 3.29) | Abnormal | 26 | 1.05 (0.49–2.25) | 0.910 |
| Normal | 139 | - | - | |
| Lymphocytes at Optimal Cut (2.7) | Above | 13 | 0.84 (0.29–2.44) | 0.745 |
| Below | 152 | - | - | |
| Monocytes Status (Normal: 0.14 – 0.71) | Abnormal | 24 | 0.65 (0.26–1.66) | 0.369 |
| Normal | 141 | - | - | |
| Monocytes at Optimal Cut (0.53) | Above | 57 | 1.25 (0.68–2.32) | 0.472 |
| Below | 108 | - | - | |
| Eosinophils Status (Normal: 0.05 – 0.29) | Abnormal | 67 | 1.30 (0.72–2.36) | 0.379 |
| Normal | 98 | - | - | |
| Eosinophils at Optimal Cut (0.11) | Above | 75 | 0.54 (0.29–1.02) | 0.057 |
| Below | 89 | - | - | |
| Basophils Status (Normal: 0 – 0.07) | Abnormal | 29 | 3.20 (1.69–6.07) | <0.001 |
| Normal | 136 | - | - | |
| Basophils at Optimal Cut (0.1) | Above | 24 | 3.69 (1.91–7.12) | <0.001 |
| Below | 141 | - | - | |
| NLR at Optimal Cut (1.67) | Above | 121 | 0.94 (0.49–1.82) | 0.864 |
| Below | 44 | - | - | |
| NER at Optimal Cut (23) | Above | 76 | 1.08 (0.55–2.10) | 0.822 |
| Below | 63 | - | - | |
| PLR at Optimal Cut (135.5) | Above | 104 | 1.82 (0.93–3.55) | 0.079 |
| Below | 61 | - | - | |
| MLR at Optimal Cut (0.396) | Above | 52 | 2.40 (1.31–4.39) | 0.005 |
| Below | 113 | - | - | |
| BLR at Optimal Cut (0.0142) | Above | 97 | 1.35 (0.73–2.50) | 0.338 |
| Below | 68 | - | - |
The p-value is calculated by Cox proportional hazard model; CI - 95% confidence interval.
Hgb = hemoglobin, NLR = neutrophil-to-lymphocyte ratio, NER = neutrophil-to-eosinophil ratio, PLR = platelet-to-lymphocyte ratio, MLR = monocyte-to-lymphocyte ratio, BLR = basophil-to-lymphocyte ratio
Table 4.
UVA and MVA of CO Associated with Baseline Basophils and BLR in mHSPC.
| UVA | MVA† | |||||||
|---|---|---|---|---|---|---|---|---|
| OS | PFS | OS | PFS | |||||
| HR (CI‡) | p-value | HR (CI‡) | p-value | HR (CI‡) | p-value | HR (CI‡) | p-value | |
| Basophils ≥ 0.1 (Above) vs. <0.1 (Below optimal cut) | 3.69 (1.91–7.12) | <0.001 | 2.39 (1.42–4.01) | 0.001 | 3.51 (1.65–7.43) | 0.001 | 1.88 (1.05–3.38) | 0.034 |
| BLR ≥ 0.0142 (Above) vs. < 0.0142 (Below optimal cut) | 1.35 (0.73–2.50) | 0.338 | 0.76 (0.51–1.14) | 0.187 | 2.11 (1.09–4.10) | 0.028 | 0.87 (0.55–1.35) | 0.526 |
The MVA was built in Cox proportional hazard model by controlling for drug, race, age at diagnosis, disease volume, ECOG, and Gleason.
95% confidence interval
BLR = basophil-to-lymphocyte ratio
Figure 1. Kaplan-Meier (KM) Plots for overall survival (OS) and progression free survival (PFS) for Baseline Basophils at Optimal Cut.
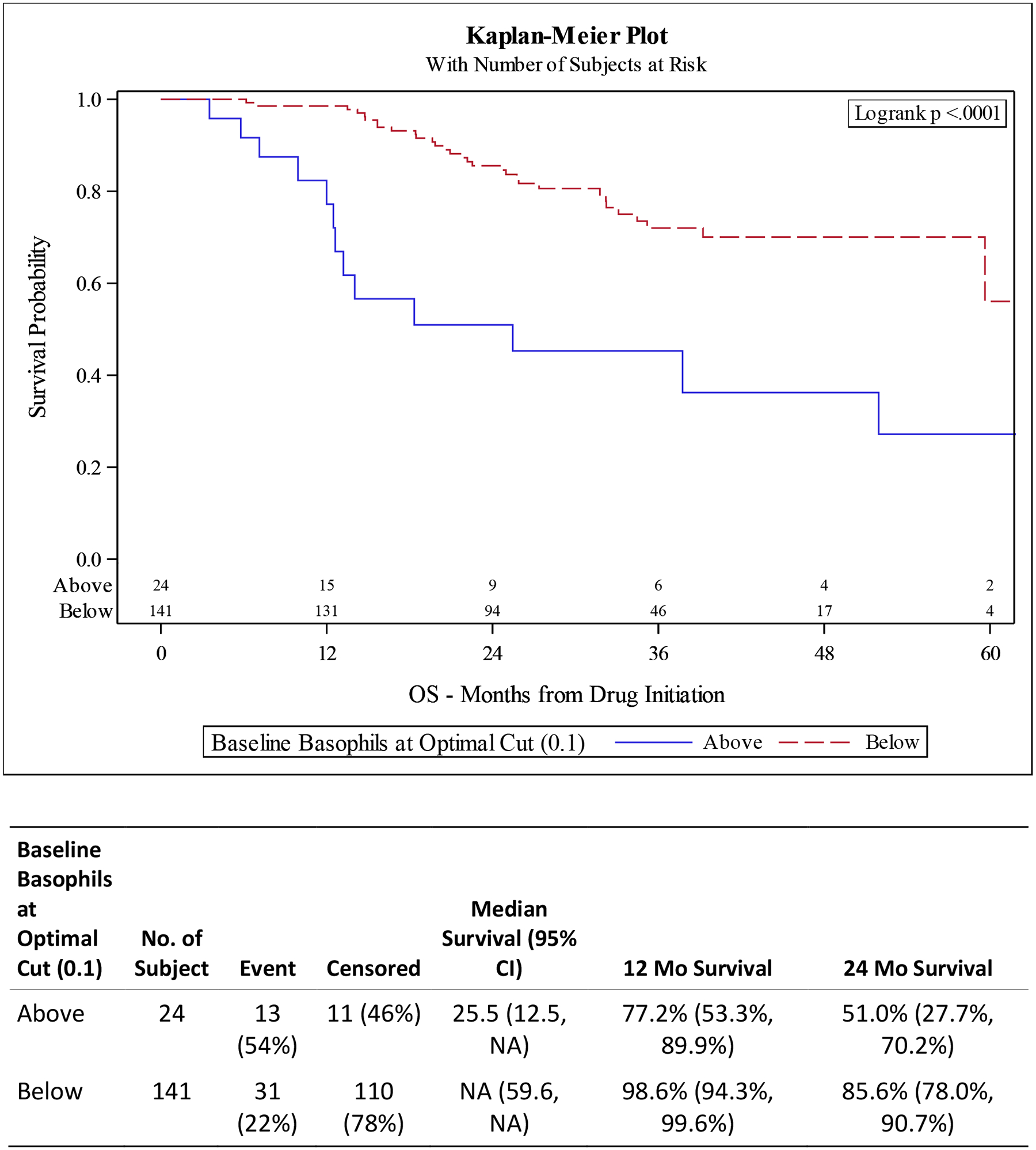
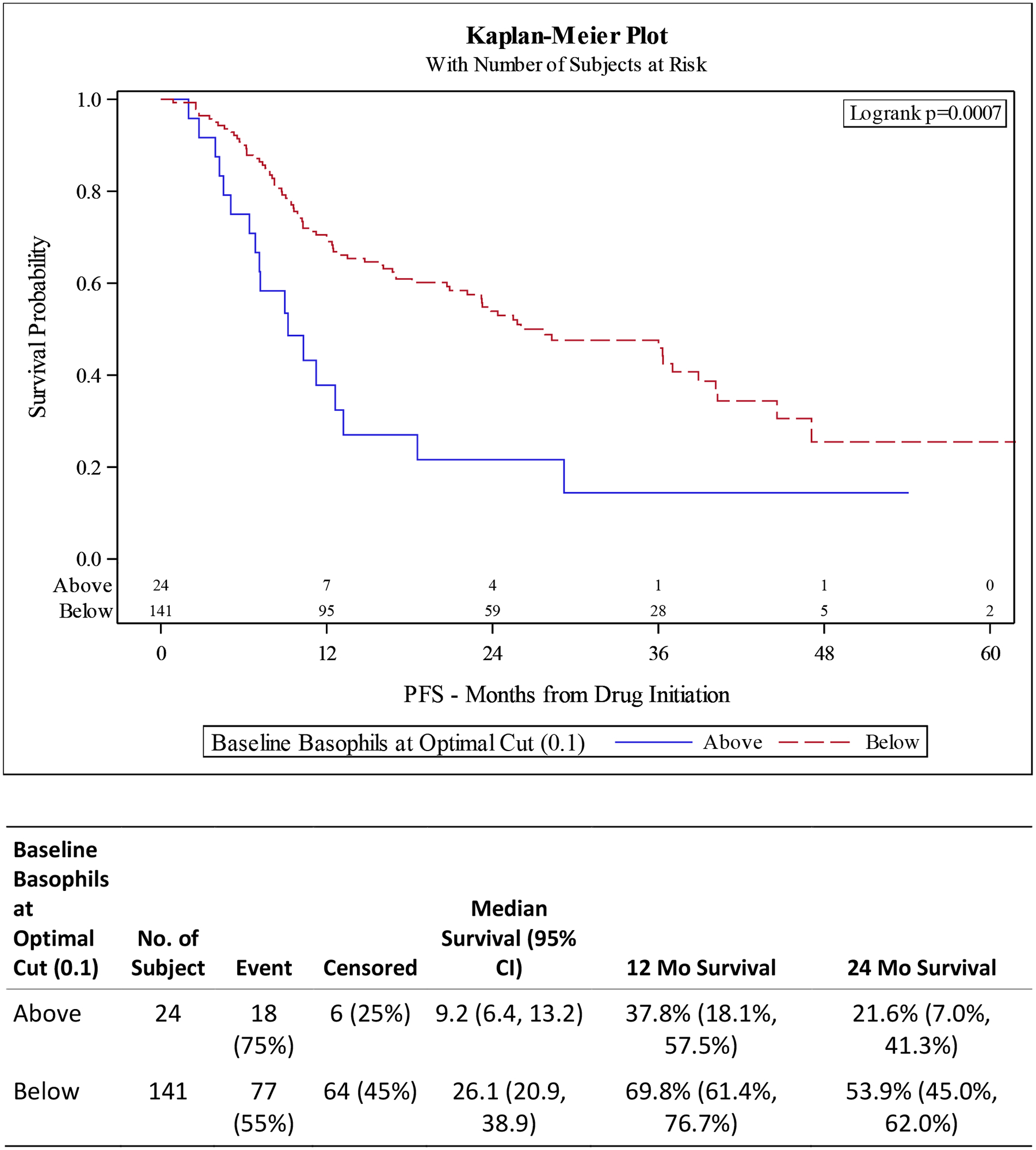
A) OS improved for patients with lower baseline basophils (p <0.0001). B) PFS improved for patients with lower baseline basophils (p 0.0007).
Figure 2. Kaplan-Meier (KM) Plots for overall survival (OS) and progression free survival (PFS) for Basophil-to-Lymphocyte Ratio (BLR) at Optimal Cut.
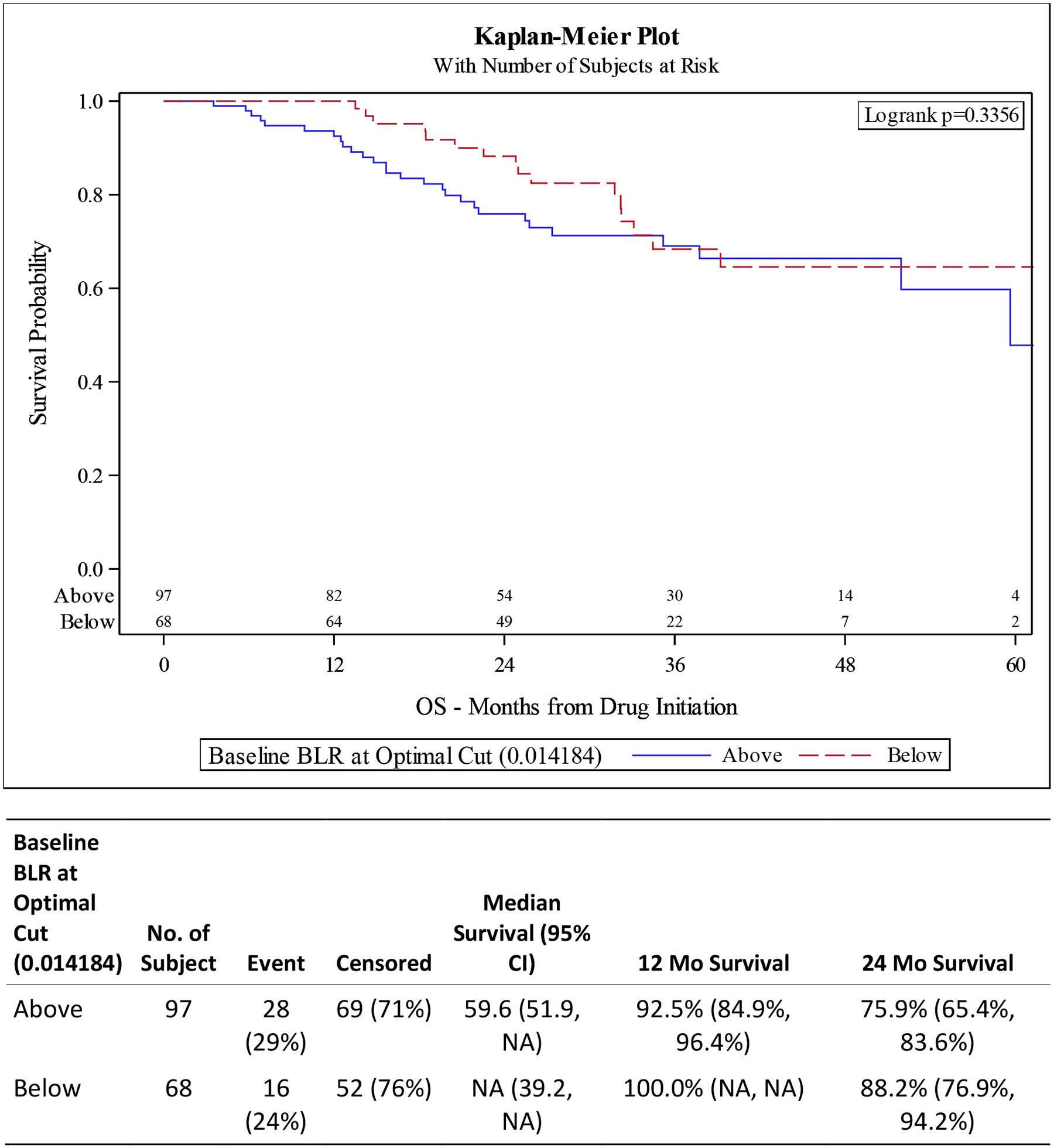
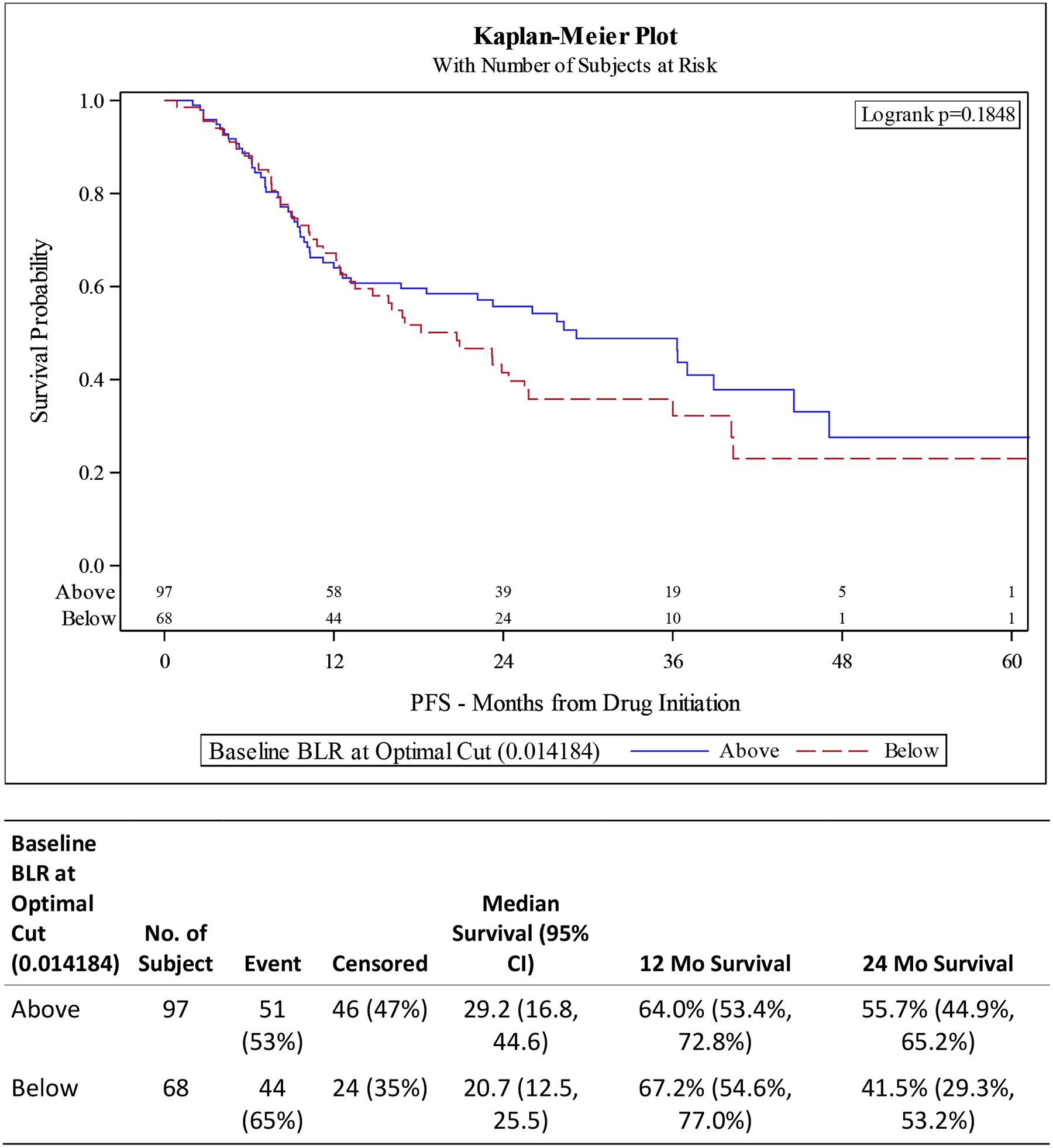
A) OS is not statistically different between BLR high vs low groups (p 0.3356). B) PFS is not statistically different based on BLR (p 0.1848).
Table 3.
UVA for Progression Free Survival (PFS) in mHSPC Based on Baseline Lab Values.
| Variable | N | Hazard Ratio (95% CI)‡ | P-value* | |
|---|---|---|---|---|
| Hgb Status (Normal: 12.9 – 16.1) | Abnormal | 97 | 1.77 (1.15–2.71) | 0.010 |
| Normal | 68 | - | - | |
| Hgb at Optimal Cut (11.8) | Above | 106 | 0.34 (0.23–0.51) | <0.001 |
| Below | 59 | - | - | |
| Platelets Status (Normal: 150 – 400) | Abnormal | 25 | 0.78 (0.43–1.40) | 0.408 |
| Normal | 140 | - | - | |
| Platelets at Optimal Cut (259) | Above | 56 | 2.05 (1.36–3.08) | <0.001 |
| Below | 109 | - | - | |
| Neutrophils Status (Normal: 0.67 – 6.41) | Abnormal | 18 | 1.29 (0.70–2.36) | 0.417 |
| Normal | 147 | - | - | |
| Neutrophils at Optimal Cut (3.08) | Above | 102 | 0.81 (0.54–1.22) | 0.312 |
| Below | 63 | - | - | |
| Lymphocytes Status (Normal: 0.72 – 3.29) | Abnormal | 26 | 1.04 (0.62–1.76) | 0.878 |
| Normal | 139 | - | - | |
| Lymphocytes at Optimal Cut (2.7) | Above | 13 | 2.07 (1.10–3.90) | 0.023 |
| Below | 152 | - | - | |
| Monocytes Status (Normal: 0.14 – 0.71) | Abnormal | 24 | 0.87 (0.50–1.51) | 0.617 |
| Normal | 141 | - | - | |
| Monocytes at Optimal Cut (0.53) | Above | 57 | 0.89 (0.57–1.37) | 0.587 |
| Below | 108 | - | - | |
| Eosinophils Status (Normal: 0.05 – 0.29) | Abnormal | 67 | 1.39 (0.93–2.08) | 0.109 |
| Normal | 98 | - | - | |
| Eosinophils at Optimal Cut (0.11) | Above | 75 | 0.58 (0.38–0.88) | 0.011 |
| Below | 89 | - | - | |
| Basophils Status (Normal: 0 – 0.07) | Abnormal | 29 | 1.97 (1.20–3.25) | 0.007 |
| Normal | 136 | - | - | |
| Basophils at Optimal Cut (0.1) | Above | 24 | 2.39 (1.42–4.01) | 0.001 |
| Below | 141 | - | - | |
| NLR at Optimal Cut (1.67) | Above | 121 | 0.72 (0.47–1.12) | 0.149 |
| Below | 44 | - | - | |
| NER at Optimal Cut (23) | Above | 76 | 1.35 (0.85–2.13) | 0.199 |
| Below | 63 | - | - | |
| PLR at Optimal Cut (135.5) | Above | 104 | 1.55 (1.00–2.40) | 0.049 |
| Below | 61 | - | - | |
| MLR at Optimal Cut (0.396) | Above | 52 | 1.52 (1.00–2.33) | 0.052 |
| Below | 113 | - | - | |
| BLR at Optimal Cut (0.0142) | Above | 97 | 0.76 (0.51–1.14) | 0.187 |
| Below | 68 | - | - | |
The p-value is calculated by Cox proportional hazard model; CI - 95% confidence interval.
Hgb = hemoglobin, NLR = neutrophil-to-lymphocyte ratio, NER = neutrophil-to-eosinophil ratio, PLR = platelet-tolymphocyte ratio, MLR = monocyte-to-lymphocyte ratio, BLR = basophil-to-lymphocyte ratio
3.2. Overall Survival for Basophils and Basophil-To-Lymphocyte Ratio
UVA for OS shows decreased survival for patients with elevated basophils (defined as ≥ optimal cut of 0.1) (HR 3.69 95% CI 1.91–7.12, p <0.001) and high BLR (defined as ≥ optimal cut of 0.0142) (HR 1.35, 95% CI 0.73–2.50, p 0.338). These findings are confirmed in our MVA with elevated basophils having a HR of 3.51 (95% CI 1.65–7.43) p 0.001) and high BLR having HR of 2.11 (95% CI 1.09–4.10 p 0.028) (Tables 2 and 4).
3.3. Progression-Free Survival for Basophils and Basophil-To-Lymphocyte Ratio
UVA for PFS associated with elevated basophils had a HR 2.39 (95% CI 1.42–4.01 p 0.001) with MVA showing HR 1.88 (95% CI 1.05–3.38, p 0.034). High BLR was not statistically significant in either the UVA (HR 0.76, 95% CI 0.51–1.14, p 0.187) or MVA (HR 0.87, 95% CI 0.55–1.35, p 0.526) after controlling for drug, race, age at diagnosis, disease volume, ECOG status, and Gleason score (Tables 3 and 4, Figures 1 and 2).
3.4. Overall Survival and Progression Free Survival for Baseline Basophils and Basophil-To-Lymphocyte Ratio: Subgroup Analyses
3.4.1. Stratified by Race
Given the diversity of our patient population, we decided to stratify our results based on race. MVA for OS showed worse survival in Black patients with high BLR compared to low BLR (HR 2.23, 95% CI 1.02–4.89, p 0.045). OS was also worse in Black patients with elevated basophils (HR 3.70 (1.62–8.45), p 0.002). There was no significant difference in PFS in Black patients with high vs low BLR or normal vs elevated basophils. There was also no significant difference in OS or PFS in non-Black patients with elevated basophils or high BLR (Supplemental Materials).
3.4.2. Stratified by Upfront Therapy
MVA for OS showed survival was worse in patients who had high BLR and were treated with DOC (HR 2.64 (95% CI 1.08–6.41, p 0.032) as well as patients with elevated basophils treated with DOC (HR 3.95 (95% CI 1.49–10.51, p 0.006). There was no association with abiraterone on basophil or BLR status and survival outcomes (Supplemental Materials).
4. Discussion
Our study evaluated potential biomarkers in a diverse population of mHSPC patients within readily available clinical labs. We found that elevated basophils and BLR are associated with worse clinical outcomes (Figure 1, Table 4). MVA data revealed that patients with elevated baseline basophils were more than three times as likely to have shorter survival compared to those with normal baseline values (Table 4). Utilizing prognostic markers, such as baseline basophils and BLR, can aid in navigating the evolving treatment landscape of mHSPC by helping predict more aggressive, high-volume disease.
We further stratified results based on race and choice of upfront therapy. Elevated basophils and BLR is associated with worse OS more often for Black patients compared to Non-Black patients (supplemental table B1 and B2). Additionally, elevated basophils and BLR is associated with worse OS more often for patients treated with upfront DOC compared to ABI (supplemental table D1 and D2). These results may be due to a higher incidence of high-volume disease in Black patients and high-volume disease being treated more often with DOC.
Current literature regarding basophils and BLR in prostate cancer is limited with one study reporting no association of basophils with Gleason score [26]. At the time of publication, there was no literature discussing basophils or BLR specifically in mHSPC. However, basophils have been evaluated as a biomarker in other malignancies. In bladder cancer, higher basophils were associated with recurrence after tumor resection and bacillus Calmette-Guerin (BCG) administration [19]. In pancreatic cancer, a higher percentage of basophils in tumor-draining lymph nodes were associated with worse OS (HR 8.51, 95% CI 1.04–69.33, p 0.04) and PFS (HR 11.07, 95% CI 1.38–88.60, p 0.02) [20]. Additionally, the same study from De Monte el al. found that basophil-deficient mice do not fully develop pancreatic tumors after orthotopic transplantation of pancreatic tumor cells. Although these findings are consistent with our results in mHSPC showing that patients have worse CO with higher basophils and BLR, basophils are not always associated with worse outcomes in all malignancies. For example, melanoma patients with higher basophils who were receiving immune checkpoint inhibitors (ICIs) had improved OS (HR 2.33 for basophils <0.06, 95% CI 1.30 – 4.19, p 0.005) [27]. In non-metastatic colorectal cancer, low baseline basophils are associated with more aggressive disease and worse survival outcomes [28, 29]. These differences in outcomes associated with basophils compared to our data may be related to tumor type, extent of disease, and types of prior treatments such as ICIs.
Other components of the CBC, such as NLR, have more data for use as biomarkers. High NLR has been associated with worse clinical outcomes in most solid tumors, including PCa [10, 16–18, 30, 31]. Within mCRPC, NLR has been used in prognostic scoring models and for predicting response to ABI and ENZA [16–18, 31]. In a study of all metastatic PCa without specification for subtype, a higher pretreatment NLR was associated with disease progression and decreased survival [32]. For our study population of mHSPC, NLR was not associated with any significant changes to OS or PFS.
The biological rationale for the association of improved clinical outcomes with changes in leukocytes and lymphocytes is likely related to tumor-driven systemic inflammation. Acute phase reactants are upregulated leading to elevated leukocytes and platelets while decreasing hemoglobin and lymphocytes [15]. Neutrophils activate the innate and adaptive immune system to promote macrophage recruitment and differentiation, angiogenesis, and tumorigenesis [14]. Monocytes are also recruited to tumors and differentiate to tumor-associated macrophages, which then contribute to tumor growth, local immune suppression, and ultimately metastasis [33]. Lymphocytes are involved in immunosurveillance, so decreased lymphocytes, which contribute to higher NLR or monocyte to lymphocyte ratio (MLR), could indicate an ineffective immune response to the tumor leading to evasion of immunosurveillance resulting in unchecked progression [11–13, 16, 30, 34]. We suspect that similar mechanisms involving changes in acute phase reactants also lead to elevated basophils and BLR, but the exact role of basophils in disease progression is not well defined.
Our study is the first to identify baseline basophils and BLR as promising prognostic biomarkers in mHSPC, a disease that has minimal biomarker data, and is unique in that our patient population included 54% Black patients and 46% non-Black patients. However, there are some limitations to our study. Our patient population lacked additional diversity from other racial or ethnic groups such as Hispanic and Asian groups. We also did not include data on patients treated with ENZA or APA due to limited sample size given these therapies have recently been approved in mHSPC. Additionally, preliminary findings of the PEACE trial suggest better outcomes in high volume mHSPC treated with docetaxel, abiraterone, and ADT [25]. Other limitations are inherent to a retrospective study, such as, working within the confines with the electronic medical records (EMR), evaluating real world data, and being unable to control all confounding factors in our analyses. Further study is needed to validate our findings in a larger population, with additional ethnicities, inclusive of all approved upfront therapies, and with a longer follow up time.
5. Conclusions
Elevated baseline basophils and BLR were associated with worse OS and PFS in a demographically diverse population of patients with mHSPC treated with upfront DOC or ABI. Our study is the first to identify baseline basophils and BLR as prognostic biomarkers. This is one of few studies evaluating biomarkers in mHSPC. Although our findings require further validation, basophils and BLR may have the potential to help guide prognostication in mHSPC.
Supplementary Material
Highlights.
We found that metastatic hormone sensitive prostate cancer patients with high baseline basophils and basophil-to-lymphocyte ratios (BLR) had worse outcomes.
Basophils ≥ 0.1 has a hazard ratio (HR) of 3.51 for OS and HR of 1.88 for PFS
BLR ≥ 0.0142 has a HR 2.11 for OS. There was no significant difference for PFS.
We conclude that basophils may help predict patient outcomes and could serve as a prognostic biomarker.
Funding:
This work was supported by National Institutes of Health/ National Cancer Institute and the Biostatistics and Bioinformatics Shared Resource of the Winship Cancer Institute of Emory University under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest:
M.A. Bilen has acted as a paid consultant for and/or as a member of the advisory boards of Exelixis, Bayer, BMS, Eisai, Pfizer, AstraZeneca, Janssen, Calithera Biosciences, Genomic Health, Nektar, and Sanofi and has received grants to his institution from Xencor, Bayer, Bristol-Myers Squibb, Genentech/Roche, Seattle Genetics, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Genome & Company, AAA, Peloton Therapeutics, and Pfizer for work performed as outside of the current study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cancer Stat Facts: Common Cancer Sites. National Cancer Institute. https://seer.cancer.gov/statfacts/html/common.html. Accessed April 14, 2021.
- 2.Dalela D, Sun M, Diaz M, Karabon P, Seisen T, Trinh Q-D, et al. Contemporary Trends in the Incidence of Metastatic Prostate Cancer Among US Men: Results from Nationwide Analyses. Eur Urol Focus. 2019; 5(1): 77–90. doi: 10.1016/j.euf.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Kelly SP, Anderson WF, Rosenberg PS, Cook MB. Past, Current, and Future Incidence Rates and Burden of Metastatic Prostate Cancer in the United States. Eur Urol Focus. 2018; 4(1): 121–127. doi: 10.1016/j.euf.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiner AB, Matulewicz RS, Eggener SE, Schaeffer EM. Increasing Incidence of Metastatic Prostate Cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis. 2016; 19(4): 395–397. doi: 10.1038/pcan.2016.30. [DOI] [PubMed] [Google Scholar]
- 5.Hahn AW, Higano CS, Taplin M-E, et al. Metastatic Castration Sensitive Prostate Cancer: Optimizing Patient Selection and Treatment. Am Soc Clin Oncol Educ Book. 2018; 38: 363–371. doi: 10.1200/EDBK_200967. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong AJ, Szmulewitz RZ, Petrylak DP, Ryan CJ, Agarwal N. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019;37(32):2974–2986. doi: 10.1200/JCO.19.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide Treatment and Metastasis-free Survival in Prostate Cancer. N Engl J Med. 2018; 378:1408–1418. doi: 10.1056/NEJMoa1715546. [DOI] [PubMed] [Google Scholar]
- 8.Petrylak DP, Crawford ED. Biomarkers for the Management of Castration-Resistant Prostate Cancer: We Are Not There Yet. Target Oncol. 2017. Aug;12(4):401–412. doi: 10.1007/s11523-017-0500-y. [DOI] [PubMed] [Google Scholar]
- 9.Tian S, Lei Z, Gong Z, Sun Z, Xu D, Piao M. Clinical implication of prognostic and predictive biomarkers for castration-resistant prostate cancer: a systematic review. Cancer Cell Int. 2020;20:409. Published 2020 Aug 26. doi: 10.1186/s12935-020-01508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terada N, Akamatsu S, Kobayashi T, Inoue T, Ogawa O, Antonarakis ES. Prognostic and predictive biomarkers in prostate cancer: latest evidence and clinical implications. Ther Adv Med Oncol. 2017;9(8):565–573. doi: 10.1177/1758834017719215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilen MA, Martini DJ, Liu Y, Lewis C, Collins HH, Shabto JM, et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer. 2019;125(1):127–134.doi: 10.1002/cncr.31778. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-Related Inflammation and Treatment Effectiveness. Lancet Oncol. 2014;15:e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 14.Selders GS, Fetz AE, Radic MZ, Bowlin GL. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen Biomater. 2017;4(1):55–68. doi: 10.1093/rb/rbw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulhar R, Ashraf MA, Jialal I. Physiology, Acute Phase Reactants. [Updated 2020 May 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK519570/ [PubMed] [Google Scholar]
- 16.Templeton AJ, Pezaro C, Omlin A, McNamara MG, Leibowitz-Amit R, Vera-Badillo FE, et al. Simple prognostic score for metastatic castration-resistant prostate cancer with incorporation of neutrophil-to-lymphocyte ratio. Cancer. 2014; 120, 21: 3346–3352. doi: 10.1002/cncr.28890. [DOI] [PubMed] [Google Scholar]
- 17.Boegemann M, Schlack K, Thomes S, Steinestel J, Rahbar K, Semjonow A, et al. The Role of the Neutrophil to Lymphocyte Ratio for Survival Outcomes in Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Abiraterone. Int J Mol Sci. 2017;18(2):380. doi: 10.3390/ijms18020380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawahara T, Kato M, Tabata K, Kojima I, Yamada H, Kamihira O, et al. A high neutrophil-to-lymphocyte ratio is a poor prognostic factor for castration-resistant prostate cancer patients who undergo abiraterone acetate or enzalutamide treatment. BMC Cancer. 2020. Sep 25;20(1):919. doi: 10.1186/s12885-020-07410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferro M, Lorenzo GD, Vartolomei MD, Bruzzese D, Cantiello F, Lucarelli G, et al. Absolute Basophil Count is Associated with Time to Recurrence in Patient with High-Grade T1 Bladder Cancer Receiving Bacillus Calmette-Guerin After Transurethral Resection of the Bladder Tumor. World J Urol. 2020; 38(1): 143–150. doi: 10.1007/s00345-019-02754-2. [DOI] [PubMed] [Google Scholar]
- 20.De Monte L, Wormann S, Brunetto E, Heltai S, Magliacane G, Reni M, et al. Basophil Recruitment into Tumor-Draining Lymph Nodes Correlates with Th2 Inflammation and Reduced Survival in Pancreatic Cancer Patients. Cancer Res. 2016; 76(7): 1792–803. doi: 10.1158/0008-5472.CAN-15-1801-T. [DOI] [PubMed] [Google Scholar]
- 21.Mandrekar JN M SJ, Cha SS Cutpoint Determination Methods in Survival Analysis using SAS. SAS Users Group International; 282003. [Google Scholar]
- 22.Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015. Aug 20;373(8):737–46. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Nickleach D, Zhang C, Switchenko JM, Kowalski J. Carrying Out Streamlined Routine Data Analyses With Reports for Observational Studies: Introduction to a Series of Generic SAS® Macros. F1000Res. 2018; 7: 1955. doi: 10.12688/f1000research.16866.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandrekar JN, Mandrekar SJ, Cha SS. Cutpoint determination methods in survival analysis using SAS [abstract 261–28.]. Proceedings of the 28th SAS Users Group International Conference (SUGI 2003); March 30 to April 2, 2003; Seattle, Washington. Available at: https://www2.sas.com/proceedings/sugi28/261-28.pdf. [Google Scholar]
- 25.Fizazi K, Carles Galceran J, Foulon S, Roubaud G, McDermott R, Fléchon A, Tombal B, Supiot S, Berthold DR, Ronchin P, Kacso G, Gravis Mescam G, Calabro’ F, Berdah JF, Hasbini A, Silva M, Thiery-Vuillemin A, Latorzeff I, Rieger I, & Bossi A (2021). LBA5 a phase III trial with a 2×2 factorial design in men with de novo metastatic castration-sensitive prostate cancer: Overall survival with abiraterone acetate plus prednisone in peace-1. Annals of Oncology, 32. 10.1016/j.annonc.2021.08.2099 [DOI] [Google Scholar]
- 26.Hayashi T, Fujita K, Tanigawa G, Kawashima A, Nagahara A, Ujike T, et al. Oncotarget. 2017; 8(21): 35255–35261. doi: 10.18632/oncotarget.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosner S, Kwong E, Shoushtari AN, Friedman CF, Betof AS, Brady MS, et al. Peripheral Blood Clinical Laboratory Variable Associated with Outcomes Following Combination Nivolumab and ipilimumab Immunotherapy in Melanoma. Cancer Med. 2018; 7(3): 690–7. doi: 10.1002/cam4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Q, Luo D, Sanjun C, Li Q, Li X. Circulating Basophil Count as a Prognostic Marker of Tumor Aggressiveness and Survival Outcomes in Colorectal Cancer. Clin Transl Med. 2020; 9: 6. doi: 10.1186/s40169-019-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei Y, Zhang X, Wang G, Zhou Y, Luo M, Wang S, et al. The Impacts of Pretreatment Circulating Eosinophils and Basophils on Prognosis of Stage I-III Colorectal Cancer. Asia Pac J Clin Oncol. 2018; 14(5): e243–251. doi: 10.1111/ajco.12871. [DOI] [PubMed] [Google Scholar]
- 30.Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, et al. Prognostic Role of Neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014; 106(6): dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 31.Sun Z, Ju Y, Han F, Sun X, Wang F. Clinical Implication of Pretreatment Inflammatory Biomarkers as Independent Prognostic Indicators in Prostate Cancer. J Clin Lab Anal. 2018; 32(3): e22277. doi: 10.1002/jcla.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawahara T, Yokomizo Y, Ito Y, Ito H, Ishiguro H, Teranishi J-I, et al. Pretreatment neutrophil-to-lymphocyte ratio predicts the prognosis in patients with metastatic prostate cancer. BMC Cancer. 2016;16:111. doi: 10.1186/s12885-016-2134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richard DM, Hettinger J, Feuerer M. Monocytes and Macrophages in Cancer: Development and Functions. Cancer Microenviron. 2013. Aug; 6(2): 179–191. doi: 10.1007/s12307-012-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan Y, Xiong H, Feng Y, Liao G, Tong T, Pang J. Revealing the prognostic landscape of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in metastatic castration-resistant prostate cancer patients treated with abiraterone or enzalutamide: a meta-analysis. Prostate Cancer Prostatic Dis. 2020; 23(2):220–231. doi: 10.1038/s41391-020-0209-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


