Abstract
Introduction
Paraganglioma and pheochromocytoma are uncommon conditions that affect around 1.5–9 patients per million. The most frequent symptoms are headache, hypertension and diaphoresis; however, palpitations or tachycardia could be present. Malignancy is not frequent, and when is suspected, positron emission tomography (PET) should be performed. Surgery it's the gold standard treatment, with acceptable rates of morbidity and mortality.
Presentation of the case
A 33-year-old woman presented to private practice with long-standing symptoms consisting of asthenia, adynamia, and sensation of palpable masses in the neck. Due to her medical history and imaging findings, urine metanephrines were obtained, showing high values of adrenaline 6.69 (μg/24 h), noradrenaline 130.09 (μg/24 h), dopamine 262.59 (μg/24 h). PET was performed to identify hyperfunctioning masses in other locations, finding bilateral carotid hypermetabolic masses and a nodular lesion anterior to the aortoiliac bifurcation, probably malignant. Laparoscopic retroperitoneal tumor resection was performed by a laparoscopic and metabolic surgeon, with intraoperative findings of a vascularized mass (30 × 25 mm) closely related to the left aortoiliac bifurcation and peritoneal fluid.
Discussion
Paragangliomas are rare tumors that frequently produce catecholamines with varied symptoms. Diagnosis requires patient history, laboratory studies including 24-hour urine-metanephrines and plasma metanephrine levels. Imaging such as CT, MRI and PET scan are necessary. Perioperative management needs to be performed and surgery is the basis of the treatment in patients with localized disease. Metastatic disease has a 50% mortality at 5 years and requires a different approach.
Conclusion
Paraganglioma is a rare and complex entity that requires a multidisciplinary approach.
Keywords: Paraganglioma, Laparoscopy, Diagnosis, Case report, Surgery
Highlights
-
•
Paragangliomas are rare tumors that produce catecholamines with varied symptoms.
-
•
Diagnosis requires a detailed history, laboratory and imaging studies.
-
•
Perioperative management needs to be performed by a multidisciplinary board.
-
•
Surgery is the cornerstone treatment of this pathology.
1. Introduction
Tumors arising from the chromaffin cells are extremely rare, approximately affecting between 1.5 and 9 per million people in some series of cases. Around 85–90% of these tumors are derived from cells of the adrenal medulla and are known as pheochromocytomas (PHEO); on the other hand, 15–10% of the cases arise from de chromaffin cells of sympathetic or parasympathetic tissue and are termed extra-adrenal pheochromocytomas or paragangliomas (PGL) [1], [2], [3], [4]. In most cases, parasympathetic PGLs involve the glossopharyngeal nerves in the carotid bodies, followed by the localization around the ear in the “glomus tympanicum” [5]. In cases of tumors involving sympathetic ganglia, the most common location is the abdomen, in prevertebral and paravertebral tissue, the wall of the urinary bladder, and 53% of the cases involving the organ of Zuckerkandl (situated in the distal abdominal aorta) [5], [6].
These tumors commonly present in the emergency room with hypertension, occasional headaches, and diaphoresis; other symptoms include palpitations, tachycardia and dyspnea explained by an unusual discharge of catecholamines activity in the serum (nor-epinephrine, dopamine, and epinephrine) [4], [5], [6].
In the majority of the cases, these tumors are not malignant; but this feature cannot be defined in a pre-operative way unless there are extensions of the disease. Other clinical features involve the myocardium chronic illness due to a persistent exposition to catecholamines such as myocardiopathy or derived left ventricle dilatation, but these features are uncommon [4].
In most of the cases (90%), PGL is sporadic, and is not associated with hereditary conditions. However, a small proportion could be related to familial syndromes [4], [7]. Recently, the association of succinate dehydrogenase gene mutation has shown to be associated with Leigh's syndrome [4], [7]. Other hereditary conditions such as Von Hippel Lindau, Type 2 multiple endocrine neoplasia or neurofibromatosis type 1 are related to specific mutations and are most commonly associated with PGL [4], [7].
Gold standard management of this entity, its surgical resection (unless metastases are present at diagnosis), and overall survival it's estimated to be similar to the unexposed population [4], [8]. Perioperative management and a multidisciplinary approach need to be done in order to achieve good postoperative results [4], [8]. To the present, the literature reports a few cases of surgical resection of PGLs located in the organ of Zuckerkandl due to technical difficulty [8], [9], [10].
2. Presentation of the case
After ethical and institutional approval, previous informed consent was filled, following SCARE guidelines [11]. A 33-year-old woman presented to the private practice with long-standing symptoms consisting of asthenia, adynamia, and sensation of palpable masses in the neck. The patient had no gastrointestinal or respiratory symptoms, no dysphagia or odynophagia. Relevant medical history included open cholecystectomy and an extensive family history of paragangliomas in different locations of the body that affected the father, two paternal uncles, and cousins. Physical examination findings include high blood pressure, bilateral soft, mobile, non-painful masses of approximately 30 × 30 mm in the neck without changes in skin color. The abdomen had a supraumbilical laparotomy scar, there were no palpable masses, pain, or signs of peritoneal irritation.
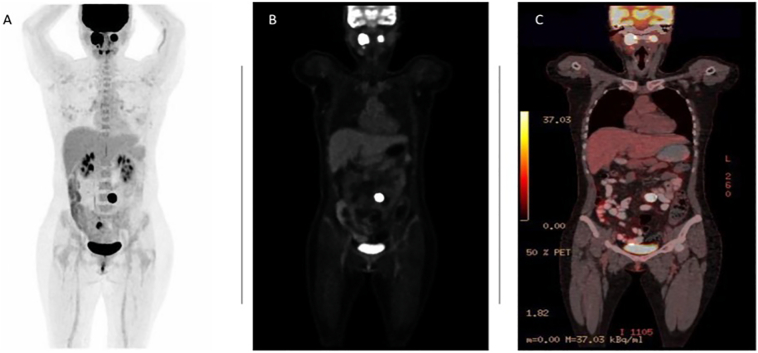
While the complete blood count, liver profile, kidney function, thyroid hormones and urinalysis were normal, the angiography of the neck vessels showed findings compatible with bilateral vagal paragangliomas with small involvement of the carotid body, the largest on the right side, with approximate dimensions of 36.3 × 17.3 × 34.3 mm in diameter (Fig. 1). Due to her medical history and imaging findings, urine metanephrines were obtained, showing high values of adrenaline 6.69 (μg/24 h), noradrenaline 130.09 (μg/24 h), dopamine 262.59 (μg/24 h), indoleacetic hydroxy acid 4.2 (μg/24 h), and serotonin 391 ng/ml. Positron emission tomography (PET) was performed in order to identify hyperfunctioning masses in other locations, finding bilateral carotid hypermetabolic masses and a nodular lesion anterior to the aortoiliac bifurcation, probably malignant (Fig. 2). Considering an extra-adrenal paraganglioma as probable, the patient was referred to an endocrinologist, in order to initiate antihypertensive therapy with alpha-adrenergic blockers and ingestion of large amounts of fluids a week prior the procedure.
Fig. 1.
Axial Computed Tomography Angiography of the neck vessels showing findings of bilateral vagal paragangliomas with small involvement of the carotid body, the largest on the right side (see red asterisks in A and B).
Fig. 2.
PET/CT with maximum grayscale projection, demonstrating bilateral vagal paragangliomas and a nodular lesion anterior to the aortoiliac bifurcation (see A). 18F-FDG-PET/CT revealing strongly FDG-avid masses in bilateral neck and in the vicinity of the left aortoiliac bifurcation (see B). PET/CT fusion image showing abnormal increase of tumor metabolism (see C).
Laparoscopic retroperitoneal tumor resection was performed by a laparoscopic and metabolic surgeon, with intraoperative findings of a vascularized mass (30 × 25 mm) in the vicinity of the left aortoiliac bifurcation and peritoneal fluid (Fig. 3), both samples were sent to pathology. During the intraoperative manipulation of the mass, the patient did not present variations in the blood pressure, nor was antihypertensive medication necessary and no unexpected complications occurred. Immediate postoperative follow-up was done in the Intensive Care Unit (ICU) to closely monitor the blood pressure. The patient had no complications and was transferred to an inpatient postoperative floor. After two days of inpatient care, the patient was discharged with analgesic management.
Fig. 3.
Vascularized mass anterior to the left aortoiliac bifurcation (see A). Intraoperative photos depicting the dissection of the mass (see B and C). Surgical specimen obtained (see D).
Pathology revealed a tumor of 30 × 25 × 20 mm, well defined, with a fibrous capsule, the mitotic activity was 1–2 mitoses per 10 fields, absent necrosis, multiple cells with vesicular oval nuclei, clear cytoplasm, poorly defined borders, pleomorphism, enlarged nuclei, and no lymphovascular invasion. Immunohistochemical studies showed intense and diffuse reactivity cells of the lesion with chromogranin and synaptophysin, with a cell proliferation index (Ki67) of 2%, reactive S100 protein, and non-reactive CAM 5.2 marker. Peritoneal reaction fluid was reported without malignancy. Overall findings correspond to a paraganglioma.
Follow-up at 30–60 days does not show any complication, the patient returned to the private practice with surgical wounds in good condition, no pain, tolerating diet, and adequate intestinal transit. The patient was discharged from our service and continued her follow-up with the head and neck surgery department.
3. Discussion
Paragangliomas are rare tumors, with an incidence of 2 to 8 per million people in the world. They frequently produce catecholamines and their symptoms can be very varied [12]. Patients may present with the classic triad of sweating, headache, palpitations, and other symptoms that may include anxiety, syncope, high blood pressure, and even hypertensive crisis [12], [13], [14]. This pathology accounts for 0.2% of arterial hypertension cases [12], [13]. Approximately, a quarter of paragangliomas are malignant, defined by the World Health Organization as paragangliomas with the presence of distant metastases [12].
Diagnosis requires a detailed patient history as well as laboratory and imaging studies [1]. Laboratory studies must include 24-hour urine metanephrines measurement and plasma metanephrine levels, which together have over 90% of sensitivity for patients with pheochromocytoma/paraganglioma. In our case, the patient showed increased serum adrenaline, noradrenaline, and dopamine values, reaching the diagnosis of increased catecholamine production [15].
Imaging studies for the location and characterization of the tumor should be obtained [4], [15]. Computed Tomography (CT) and nuclear Magnetic Resonance Imaging (MRI) are preferred since 75% of tumors are located in the adrenal glands [4], [15]. Additional useful studies, such as I-metaiodobenzylguanidine, have many false positives because 50% of patients with adequate adrenal glands have symmetrical or asymmetric enlargement of their size [4], [15]. Finally, PET scan with 18F-fluorodeoxyglucose constitutes the best tool for the localization of the pathology, with a sensitivity that ranges from 74 to 100% [4], [15], for which its use is recommended by the endocrine society. Nevertheless, it may not distinguish paragangliomas from other malignancies. In our case, initial diagnoses were performed by angiography of the neck, however, due to clinical suspicion, PET scan shows hypermetabolic tumors in the aortoiliac bifurcation, and benefits the diagnosis of probable malignancy [4], [15], [16].
Perioperative management, in this case, needs to be performed with a multidisciplinary board, including an endocrinologist, surgery department, and anesthesiologist; due to the increased risk of hypertensive crisis or episodes of unexpected hypotension [4], for that reason, medical interventions are focused on maintaining adequate intravascular volume and achieving alpha-beta blocking with anti-hypertensive treatments [4]. In our case, perioperative management was performed by an endocrinologist with anti-hypertensive medication and a high-volume input; this approach leads to a decreased risk of hypertensive crisis and postoperative shock [4].
Surgery is the cornerstone of treatment in patients with localized disease and it can be performed through an open or a minimally invasive approach [13]. Due to the excess of catecholamines, perioperative management used to be treacherous, with initial mortality rates of 30 to 45%. Mostly due to poor blood pressure control, still, with improvement in medical care and surgical techniques, the perioperative mortality rate has decreased to 0–2.9% [17].
Metastatic disease has a 50% mortality at 5 years and therefore has a different treatment approach [18]. In the absence of resectable disease, chemotherapy, I-MIBG scintigraphy and radiation are options to control the patient symptoms [18]. Useful chemotherapeutic agents include cyclophosphamide, vincristine, and dacarbazine (CVD), which is the most commonly used [13]. Results from different studies using CVD, show a complete or partial response in 4 to 37% of patients [19]. In our case, PET scan ruled out metastatic disease, and surgical resection was done without any complication at 30–60 days of follow-up.
4. Conclusion
Paragangliomas are solid tumors with high morbidity but low mortality. A multidisciplinary approach in the perioperative, intraoperative and postoperative period needs to be done in order to achieve good results.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
Ethical approval of the institutional committee was made previous publication.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Guarantor
Lina M Rodríguez, MD, MPH.
Research registration number
None.
CRediT authorship contribution statement
Ricardo Nassar, MD: Participated in drafting the article and revising it critically for important intellectual content.
Lina M. Rodríguez, MD, MPH: Participated in drafting the article and revising it critically for important intellectual content.
Felipe Girón, MD, MSc: Participated in drafting the article and revising it critically for important intellectual content.
Carlos Rey, MD: Participated in drafting the article and revising it critically for important intellectual content.
David Venegas, MD: Participated in drafting the article and revising it critically for important intellectual content.
Ricardo E. Núñez-Rocha: Made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data.
Declaration of competing interest
Authors do not declare any conflict of interest.
Acknowledgments
To our patient.
References
- 1.Beard C.M., Sheps S.G., Kurland L.T., et al. Occurrence of pheochromocytoma in Rochester, Minnesota, 1950 through 1979. Mayo Clin. Proc. 1983;58:802–804. [PubMed] [Google Scholar]
- 2.Stenstrom G., Svardsudd K. Pheochromocytoma in Sweden 1958 –1981. An analysis of the National Cancer Registry Data. Acta Med. Scand. 1986;220:225–232. [PubMed] [Google Scholar]
- 3.Hartley L., Perry-Keene D. Phaeochromocytoma in Queensland–1970 – 83. Aust. N. Z. J. Surg. 1985;55:471–475. [PubMed] [Google Scholar]
- 4.Joynt K.E., Moslehi J.J., Baughman K.L. Paragangliomas. Cardiol. Rev. 2009;17(4):159–164. doi: 10.1097/crd.0b013e3181a6de. [DOI] [PubMed] [Google Scholar]
- 5.Asa S.L., Ezzat S., Mete O. The diagnosis and clinical significance of paragangliomas in unusual locations. J. Clin. Med. 2018 Sep 13;7(9):280. doi: 10.3390/jcm7090280. PMID: 30217041; PMCID: PMC6162705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renard J., Clerici T., Licker M., Triponez F. Pheochromocytoma and abdominal paraganglioma. J. Visc. Surg. 2011 Dec;148(6):e409–e416. doi: 10.1016/j.jviscsurg.2011.07.003. Epub 2011 Sep 8 PMID: 21862435. [DOI] [PubMed] [Google Scholar]
- 7.Amar L., Bertherat J., Baudin E., et al. Genetic testing in pheochromocytoma or functional paraganglioma. J. Clin. Oncol. 2005;23:8812–8818. doi: 10.1200/JCO.2005.03.1484. [DOI] [PubMed] [Google Scholar]
- 8.Navarrete A., Almenara R., Momblán D., Lacy A. Resección laparoscópica de paraganglioma en el órgano de zuckerkandl guiado por sonda gamma con 123 I-metayodobencilguanidina. Cir. Esp. 2017;95(4):239–241. doi: 10.1016/j.ciresp.2016.10.0. [DOI] [PubMed] [Google Scholar]
- 9.Xu W., Li H., Ji Z., Yan W., Zhang Y., Zhang X. Retroperitoneal laparoscopic management of paraganglioma: A single institute experience. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149433. 5. Nakamoto R., Nakamoto Y, Ishimori T, Togashi K. Clinical. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salgaonkar H., Behera R.R., Sharma P.C., Chadha M., Katara A.N., Bhandarkar D.S. Laparoscopic resection of a large paraganglioma arising in the organ of zuckerkandl: report of a case and review of the literature. J. Minim. Access Surg. 2016;12:378–381. doi: 10.4103/0972-9941.169990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., SCARE Group The SCARE 2020 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020 Dec;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. Epub 2020 Nov 9. PMID: 33181358. [DOI] [PubMed] [Google Scholar]
- 12.Gunawardane P.T.K., Grossman A. Phaeochromocytoma and paraganglioma. Adv. Exp. Med. Biol. 2017;956:239–259. doi: 10.1007/5584_2016_76. PMID: 27888488. [DOI] [PubMed] [Google Scholar]
- 13.Fishbein L. Pheochromocytoma and paraganglioma: genetics, diagnosis, and treatment. Hematol. Oncol. Clin. North Am. 2016 Feb;30(1):135–150. doi: 10.1016/j.hoc.2015.09.006. Epub 2015 Oct 23 PMID: 26614373. [DOI] [PubMed] [Google Scholar]
- 14.Fishbein L., Merrill S., Fraker D.L., et al. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann. Surg. Oncol. 2013;20:1444–1450. doi: 10.1245/s10434-013-2942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenders J.W., Duh Q.Y., Eisenhofer G., et al. Pheochromocytoma and paragan- glioma: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014;99:1915–1942. doi: 10.1210/jc.2014-1498. [DOI] [PubMed] [Google Scholar]
- 16.Mozley P.D., Kim C.K., Mohsin J., et al. The efficacy of iodine-123-MIBG as a screening test for pheochromocytoma. J. Nucl. Med. 1994;35:1138–1144. [PubMed] [Google Scholar]
- 17.Bruynzeel H., Feelders R.A., Groenland T.H., et al. Risk factors for hemodynamic instability during surgery for pheochromocytoma. J. Clin. Endocrinol. Metab. 2010;95:678–685. doi: 10.1210/jc.2009-1051. [DOI] [PubMed] [Google Scholar]
- 18.Ayala-Ramirez M., Feng L., Johnson M.M., et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic Para- gangliomas: primary tumor size and primary tumor location as prognostic indica- tors. J. Clin. Endocrinol. Metab. 2011;96:717–725. doi: 10.1210/jc.2010-1946. [DOI] [PubMed] [Google Scholar]
- 19.Niemeijer N.D., Alblas G., van Hulsteijn L.T., et al. Chemotherapy with cyclophospha- mide, vincristine and dacarbazine for malignant paraganglioma and pheochro- mocytoma: systematic review and meta-analysis. Clin. Endocrinol. 2014;81:642–651. doi: 10.1111/cen.12542. [DOI] [PubMed] [Google Scholar]