Abstract
Feed efficiency is one of the economically important traits for the cattle industry that affects profit (feed costs) and the environment (production of manure and methane). Due to that feed efficiency is driven by multi-factors, mechanisms contributing to the animal to animal variation in this trait have not been well defined, limiting the development of precision feeding strategies to improve the herd production efficiency. Rumen microbial fermentation and volatile fatty acids (VFA) production have been recently reported to be associated with cattle feed efficiency, however the roles of rumen epithelial function in feed efficiency are less studied although the rumen epithelium has an important function in VFA absorption and metabolism which can affect host feed efficiency. Rumen epithelium is colonized with a diverse microbial population, termed epimural microbiota, which has proposed functions in tissue development, barrier and inflammation, urea transport, and oxygen scavenging, suggesting that they can affect rumen epithelial functions and subsequently cattle feed efficiency. Especially, prospective functions of epimural microbiota, enhanced rumen immunity and increased rumen epithelial thickness, might contribute to less nutritional requirement for tissue recuperation. Thus, the understanding of the functions of rumen epithelium, epimural microbiota, and rumen epithelial host-microbe interactions is essential to identify their roles in contributing to feed efficiency. In this review, we will focus on to date research findings on the structure of rumen epithelium, epimural microbiota, and epithelial host-microbe interactions together with their functions and how these are associated with feed efficiency, aiming to provide insights on future directions to study rumen epithelial host-microbe interactions and improve the rumen functions in cattle.
Keywords: Ruminant, Feed efficiency, Rumen epithelium, Epimural microbiota, Microbiome
1. Introduction
There are approximately one billion cattle, and sixty million tons of beef and veal produced annually worldwide (USDA, 2021). In cattle operations, feed accounts for a large proportion (40% to 65%) of total production costs (Kahn and Cottle, 2014; Taylor et al., 2016). Therefore, reducing the feed intake of beef cattle without the decrease in growth performance or enhancing the production rate with a similar amount of feed intake could increase the profitability of farmers. Furthermore, the amount of consumed feed affects the emission of enteric greenhouse gas and the production of manure which causes environmental problems (Boaitey et al., 2017; Brunes et al., 2021). As such, improving cattle feed efficiency has been identified as one of the prioritized tasks for both research and industrial communities.
Researchers have observed varied growth performance and feed intake of animals within a population who are reared on the same farm and/or with similar genetic background, indicating that such differences can be directly associated with their varied feed efficiency, which is usually defined as the efficiency of the conversion from feed to animal growth or production (Nkrumah et al., 2006; Bonilha et al., 2017; Elolimy et al., 2018). There are several measures of feed efficiency including feed conversion ratio (FCR), residual average daily gain (RG), and residual feed intake (RFI) (Brunes et al., 2021). Recently, RFI has attracted more attention than other feed efficiency measurements because of its moderate heritability (h2 = 0.33 ± 0.01) (Berry and Crowley, 2013), and independence of the animal body weight/size (Kenny et al., 2018). Variation in RFI can be affected by various biological processes including intake of feed, digestion and absorption of feed, host metabolism, activity, and thermoregulation (Richardson and Herd (2004)). Among them, digestion of feed by rumen microbiota and absorption of volatile fatty acids (VFA) required for animal growth by rumen epithelial tissue accounts for about 15% variation of RFI in beef cattle (Richardson and Herd (2004); Elolimy et al., 2018). It is evident that rumen epithelial tissue plays a vital role in nutrient absorption and the recent study revealed that expression of genes involved in the nutrient absorption differed between cattle with feed efficiency (Elolimy et al., 2018) suggesting that epithelial function can affect cattle production efficiency. However, the regulatory mechanisms of its roles in cattle feed efficiency are not well defined due to the challenges to studying its physiological and metabolic functions in vivo. This review will focus on the rumen epithelial functions (tissue and its associated microbiota) and to date findings on their roles in cattle feed efficiency, with the aim to provide knowledge for future strategies to improve cattle feed efficiency through enhancing the rumen functions.
2. Structure of rumen epithelium
Ruminants have developed their own morphological characteristics with multi-chambered stomach including the rumen, reticulum, omasum, and abomasum, being one of the novel characteristics compared to monogastric mammals (Chen et al., 2019). Among them, the rumen is the biggest and the main place where the feed is digested and fermented by its symbiotic microbes to produce VFA as the main end fermentation products. Nutrients like VFA, minerals, and so on are absorbed through the rumen epithelial wall and then utilized by the animals. Because over 75% of ruminal VFA are absorbed through the rumen epithelium and utilized as an energy resource (Church, 1974), the rumen epithelial absorption plays a key role in ruminant's growth.
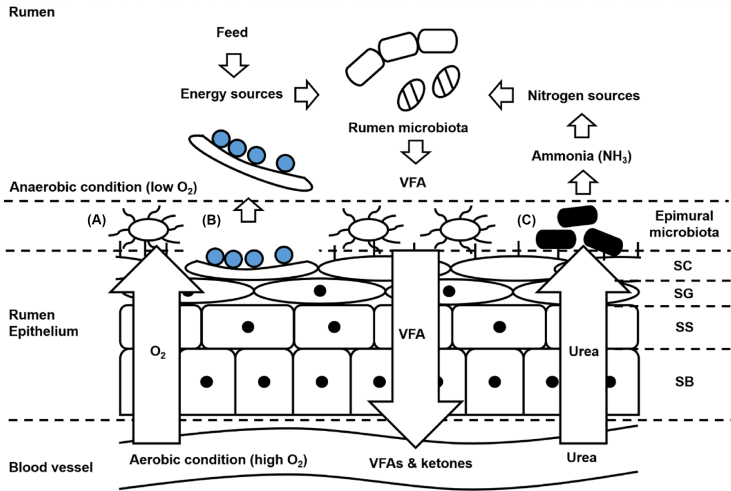
The rumen epithelium is a stratified squamous epithelium composed of four cell layers: 1) stratum basale, 2) stratum spinosum, 3) stratum granulosum, and 4) stratum corneum (Baldwin and Connor, 2017) (Fig. 1). The stratified squamous epithelium structure is usually found in organs that are closely in contact with the external environment like esophagus, mouth, and skin (Chruścik et al., 2021). Chen et al. (2019) identified that rumen epithelium has a similar gene expression pattern with esophageal epithelium rather than intestinal epithelium, supporting the theory that rumen has evolved as an extension of the esophagus. Rumen epithelium has a stratum corneum layer as a protective barrier like esophagus due to the exposure to extensive microbes in the rumen (Fig. 1) and a number of papillae that extend the surface area to absorb nutrients efficiently like intestine (Meyer et al., 2014). Therefore, the bidirectional evolution of the structure suggests that rumen epithelium has evolved as the multi-functioned structure for digestion and absorption of feed and protection from the invasion of microbes. Moreover, it has been revealed that different ruminant species (e.g., addax, bison, red deer, and moose) have differences in rumen epithelial structure (e.g. papillation and foregut size), physiology (e.g. rumen content stratification) and fiber digestibility (Pe'rez-Barberı´a, 2004; Clauss et al., 2009). Based on the studies, we could suggest that differences in rumen structure between differentially evolved ruminant species might affect rumen physiology and feed digestibility. Hence, considering rumen structure including the degree of papillation and foregut size as a main factor might be worthwhile for feed digestibility and efficiency studies in the future.
Fig. 1.
The structure of rumen epithelium and function of epimural microbiota: (A) oxygen scavenging, (B) tissue recycling, (C) urea digestion. SC = stratum corneum; SG = stratum granulosum; SS = stratum spinosum; SB = stratum basale; VFA = volatile fatty acids.
As one of the four layers, the stratum corneum is a keratinized cell layer directly faced to the lumen. The stratum corneum prevents microbes and exogenous toxic compounds including endotoxins and biogenic amines from penetrating the rumen epithelium as a physical barrier similar to the mucus layer in the intestine (Baaske et al., 2020). The stratum corneum layer consists of keratinized cells through the keratinization of horny cells transformed from the granular cells in stratum granulosum which can't proliferate due to their release of the cell organelles including a nucleus, ribosome, mitochondria, Golgi apparatus, and mucous granules during the keratinization process (Lavker and Matoltsy, 1970). Therefore, the preventions of direct colonization of rumen microbes on the cells of stratum granulosum and contact with toxic compounds are critical for the keratinization to form the functional stratum corneum. Subsequently, the tight junction formed in the stratum granulosum allows the selective absorption of nutrients (Aschenbach et al., 2019) as well as acts as the second physical barrier. Cells in stratum granulosum connect each other tightly by tight junction (TJ) proteins including claudin-1 and zonula occludens-1 (ZO-1) and gap junction molecules like connexin 43 (Graham and Simmons, 2005). The TJ and gap junction proteins are also expressed in stratum spinosum and stratum basale layers, but their intensities are reduced towards the basal membrane of rumen epithelium (Graham and Simmons, 2005). The expression of these TJ at both gene and protein levels has been observed in rumen epithelial tissues from multiple ruminant species (Graham and Simmons, 2005; Stumpff et al., 2011; Sun et al., 2018; Trabi et al., 2020), however, none of these studies investigated the expression at each stratum layer and whether there is a difference in TJ profiles among different layers. In the meantime, the cells in the stratum spinosum layer have tortuous shape and become oval in shape near the stratum granulosum and the stratum basale connects to the stratum spinosum with desmosomes that consists of columnar cells, and the cells have many mitochondria (Steven and Marshall, 1970). The density of cell mitochondria which produces ATPs as an energy source for biochemical reactions occurred in the cell increases towards the basal lamina (Graham and Simmons, 2005), providing the energy needed for transportation of nutrients (VFA, ketones, lactate, urea, minerals, and so on) to the blood stream, VFA metabolism (ketogenesis), tissue morphogenesis, pH regulation and other systemic metabolisms (e.g., hormonal or immune-related metabolisms). These suggest that the energy metabolisms are actively conducted in the stratum basale which are important to maintaining rumen epithelial homeostasis. The unique structure of rumen epithelial layers reveals the complexity of the regulatory mechanisms in its developmental, physiological, and metabolic processes at each layer. However, these have not been well studied due to the difficulty in separating each rumen epithelial layer.
The development of rumen epithelium starts from fetal stage. Arias et al. (1978) reported that the rumen tissue of a 90-day bovine fetal was already distinguished into mucosa, muscularis, and serosa with a stratified-cubodial epithelium including 2 different epithelial layers (basal and superficial; 10 cell layers) before birth. Postnatally, the rumen epithelium of neonatal ruminants (30 d after birth) had lower cell layers (6 cell layers) and more differentiated structure, a stratified squamous epithelium (Arias et al., 1978; Nwaogu and Ezeasor, 2008). Increased intake of solid feed particles during early life stimulates the increased rumen mass, papillary growth, and keratinization. Physical stimuli of solid feed increase rumen weight and chemical stimuli of fermentation products such as VFA promotes papillary growth and keratinization (Baldwin and Connor, 2017). Coinciding with the morphological development of rumen epithelium, metabolic function of rumen epithelial cells changes. While whole body tissues including the rumen epithelium use glucose as a main energy source during the milk-fed stage, rumen epithelial cells utilize VFA like butyrate as a primary energy source and ketogenic precursors after weaning (Baldwin and Jesse (1992); Baldwin and Connor, 2017). To date, there have been a little evidence that difference in the rumen development of ruminants can influence feed efficiency. Lam et al. (2018) showed that efficient cattle had thicker rumen epithelial tissues, especially epithelium on the ventral blind sac, compared with inefficient cattle. In regard to the rumen thickness, feed diet composition and fermentation products like butyrate could affect rumen epithelium thickening (Suárez et al., 2007; Diao et al., 2019). Although the relationship between rumen thickness and feed efficiency has not been understood yet, rumen developmental parameters such as papillae length, papillae width, and rumen epithelium thickness would provide fundamental information as indicators and connectors between feed digestibility, rumen development, and feed efficiency.
3. Functions of rumen epithelium
In addition to function acting as a protective barrier, rumen epithelium plays a key role in nutrient absorption. Specifically, VFA, the major microbial fermentation products, are absorbed through the following several mechanisms: 1) bicarbonate-dependent transport, 2) passive diffusion, 3) nitrate sensitive VFA absorption, 4) proton-coupled dissociated VFA transport, and 5) electrogenic VFA transport (Penner, 2014). Among them, bicarbonate-dependent transport and passive diffusion account for the highest proportion of VFA absorption (42% to 57% and 29% to 59%, respectively) (Penner et al., 2009; Schurmann, 2013; Penner, 2014). As the ruminal VFA production is concomitant with the high level of protons released, the epithelial VFA absorption is related to the transportation of protons or anions to regulate intraruminal pH (Gäbel et al., 2002), which can directly affect the microbial activities. Intraruminal dissociated VFA (VFA−) are exchanged with bicarbonate (HCO3-) through the bicarbonate-dependent transporter on the apical and basolateral membranes and transfer of HCO3- into the lumen to increase/maintain the intraruminal pH (Dengler et al., 2014). Undissociated VFA are diffused passively through the epithelial cell membrane and protons released from undissociated VFA are exported to extracellular space to regulate intracellular pH by Na/H exchanger (Penner, 2014). The absorbed acetate and propionate are utilized as energy sources or precursors for fatty acid synthesis and gluconeogenesis, and most absorbed butyrate (95%) is metabolized into ketone bodies like acetoacetate and D-3-hydroxybutyrate in rumen epithelium, which are energy sources for ruminants (Gäbel et al., 2002).
Rumen epithelium also plays roles in transporting nutrients such as ammonia and urea. In the rumen, microbes also metabolize dietary nitrogen sources and produce microbial proteins and ammonia as end-products. Ammonia is then absorbed as lipophilic form (NH3) by rumen epithelium through passive diffusion or ionized form (NH4+) via putative potassium channels depending on the intraruminal pH (Abdoun et al., 2006). Additionally, ammonia can be produced by microbial degradation of recycled urea from saliva or rumen epithelium. Urea can be produced in the liver as an end product of nitrogen metabolism and then transferred into the rumen through saliva, passive diffusion across the rumen epithelium, and urea transporters on the apical and basal membranes of the rumen epithelium (Getahun et al., 2019). The identified expression of urea transporter genes (urea transporter-B1 [UT-B1] and urea transporter-B2 [UT-B2]), and proteins in the rumen epithelium of sheep (Ritzhaupt et al., 1997, 1998; Lu et al., 2014) and the existence of UT-B1 and UT-B2 in the rumen epithelium of cattle (Marini and Van Amburgh, 2003; Stewart et al., 2005) suggest that UT-driven urea recycling can be one of the major mechanisms affecting rumen nitrogen metabolism. In addition to UT-B, Røjen et al. (2011) identified the expressions of aquaporins (AQP) 3, 7, and 10 genes, and their protein abundance in Holstein cows. Walpole et al. (2015) identified the role of UT-B and AQP in urea transport through the rumen epithelium in vitro using chamber and rumen tissues from Holstein calves. Lu et al. (2015) identified that ruminal VFA and acidic pH stimulate the expression of UT-B transporter and VFA receptors (G protein-coupled receptor 4 [GPR4] and G protein-coupled receptor 41 [GPR41]) of goat rumen epithelium. Furthermore, a high level of VFA caused by non-fiber carbohydrate diet induced increase in rumen microbial protein synthesis and decrease in urinal urea-N excretion (Lu et al., 2019), suggesting that increase of UT-B transporter expression induced by VFA may improve host nitrogen utilization efficiency. However, further research is needed to reveal the exact regulatory mechanisms of urea transporters and their relationship with VFA metabolism. We suggest that the application of immunocytochemistry with Western blot analysis would be helpful to understand how four rumen epithelial cell layers contribute to the urea transport across the rumen epithelium.
In addition, the function of lipid absorption through the rumen epithelium has been recently suggested by Zhao and colleagues (Zhao et al., 2017). In the study, genes related to lipid metabolism including lipid accumulation (fatty acid binding protein 4 [FABP4]), transportation (ATP binding cassette subfamily A member 1 [ABCA1]), cholesterol synthesis (3-hydroxy-3-methylglutaryl CoA synthase 1 [HMGCS1], 3-hydroxy-3-methylglutaryl CoA reductase [HMGCR], and farnesyl diphosphate synthase [FDPS]), and transcription factor (sterol regulatory element binding transcription factor 2 [ SREBF2]) were differentially expressed in the rumen epithelium of beef cattle during the dietary transition from high forage to high grain and the variation of the expression of these genes was related to the individual difference in intraruminal pH shift in response to the dietary change. Also, Sun et al. (2021) identified that a lamb group fed high concentrate diet (milk powder with concentrate starter) up-regulated the expression genes including fatty acid 2-hydroxylase (FA2H), tribbles pseudokinase 3 (TRIB3), 3-oxoacyl-ACP synthase, mitochondrial (OXSM), MLX interacting protein like (MLXIPL), acyl-CoA thioesterase 7 (ACOT7), podoplanin (PDPN), hydroxyacyl-CoA dehydrogenase (HADH), and 2,4-dienoyl-CoA reductase 2 (DECR2) and down-regulated the expression of genes including phospholipase A2 group III (PLA2G3), arachidonate lipoxygenase 3 (ALOXE3), 3-hydroxyacyl-CoA dehydratase 1 (HACD1), and acyl-CoA synthetase medium chain family member 1 (ACSM1) that involved in lipid transport and metabolism compared to the lamb fed control diet (milk powder only). These suggests that ruminal lipids derived from plants and microbes could be absorbed across the rumen epithelium and utilized by the epithelial cells for cell growth and proliferation. Although the absorptive capacity of ruminal lipids through the rumen epithelium might be important for maintaining rumen epithelial homeostasis, there have been few studies about lipid metabolism in the rumen epithelium highlighting the need to research area for comprehensive knowledge on the rumen epithelial functions.
4. Rumen epithelium and cattle feed efficiency
Researchers have identified that there is significant animal-to-animal variation in the growth rate and feed efficiency among animals raised under similar conditions such as with the same breed and age, and under the same diet and farm environment (Nkrumah et al., 2006: 306 Continental × British hybrid steers; Bonilha et al., 2017: 61 Nellore bulls; Elolimy et al., 2018: 149 Red Angus cattle). A recent comprehensive review on the biological determinants of feed efficiency summarizes that many factors including animals’ eating behavior, physical activity and digestibility, rumen microbiome and methane emission, energy metabolism at both cellular and animal levels, protein turnover, body composition, and endocrine system can contribute to the animal-to-animal variation of feed efficiency (Cantalapiedra-Hijar et al., 2018). Among these factors, rumen-related factors such as feed digestion and absorption of nutrients can contribute significantly to feed efficiency, which has attracted much attention recently. As the VFA producers, the rumen microbiome has been firstly reported to be associated with cattle feed efficiency since 2008 (Guan et al., 2008) and other studies have commonly reported that several rumen microbial taxa belonged to the family Prevotellaceae, Lachnospiraceae, Ruminococcaceae were related with feed efficiency traits: average daily gain (ADG), gain-to-feed ratio (G:F), or RFI (Myer et al., 2015; Li and Guan (2017); Paz et al., 2018; Freetly et al., 2020; Lopes et al., 2021). Furthermore, Li et al. (2019b) showed that there were 59 heritable rumen microbial taxa (56 bacterial and 3 archaeal taxa) such as Ruminococcus (h2 = 0.16 ± 0.08), unclassified Clostridiales (h2 = 0.25 ± 0.09), and Methanobacterium (h2 = 0.23 ± 0.08) and some of heritable microbial features like Firmicutes-to-Bacteroidetes ratio, unclassified Clostridiales, unclassified Christensenellaceae and unclassified (Mogibacteriaceae) were strongly correlated with feed efficiency traits (FCR, ADG, and dry matter intake (DMI); not RFI or backfat-adjusted RFI [RFIf]). However, many of these studies have focused on rumen digesta associated microbiota and their functions in feed efficiency, and less attention has paid to the rumen epithelium. Therefore, in this review, we will focus on the to-date findings of rumen epithelium and their functions in cattle feed efficiency.
4.1. Rumen VFA absorption in cattle with different feed efficiency
As VFA are absorbed by the rumen epithelia, their absorption capacity and efficiency can directly affect the ruminal pH, microbial VFA production, and host energy metabolism. Guan et al. (2008) reported that low RFI (high feed efficiency) steers had higher total VFA (P = 0.059; 96.74 vs. 55.35 mmol/L) and butyrate (P < 0.001; 14.54 vs. 3.35 mmol/L) concentrations. However, such observations in differences of VFA concentration between low and high RFI groups were inconsistent among studies (Hernandez-Sanabria et al., 2012, Hernandez-Sanabria et al., 2010; Fitzsimons et al., 2014; McDonnell et al., 2016; Lage et al., 2020) which could be due to the lack of knowledge in ruminal VFA absorptions as well as the difference in diet, age and breed of animals as well as sample collection methods among studies. It is important to be aware that ruminal VFA concentration is the result of VFA production, absorption, washout, and interconversion rate of VFA (Bedford et al., 2020). A recent study by Lam et al. (2018) reported that cattle with high feed efficiency (low RFI cattle) had no difference in intraruminal VFA concentration and rumen weight while they had thicker rumen epithelium wall (136 vs. 126 μm) than cattle with low feed efficiency (high RFI cattle). The thinner ruminal epithelial wall may allow better passive nutrient absorption, providing more energy for host animals. Future study on the rumen wall physical structure and its relationship with nutrient absorption and cattle feed efficiency is warranted.
Recent studies have reported that the high VFA absorption rate may compensate for the less dry matter intake of low RFI (feed efficient) animals by facilitating rumen fermentation through the fast removal of fermentation end-products (Gäbel et al., 2002; Elolimy et al., 2018). Indeed, Kong et al. (2016) found that the rumen epithelium of low RFI steers (RFI = −1.4 to −2.33 kg/d) had a higher expression of genes involved in the remodeling of the epithelial adherent junctions (dynamin 2 [DNM2], hepatocyte growth factor-regulated tyrosine kinase substrate [HGS], actin beta [ACTB], tubulin beta class I [TUBB], and tubulin alpha 4a [TUBA4A]), protein synthesis (ubiquitin A-52 residue ribosomal protein fusion product 1 [UBA52], ribosomal protein L36 [RPL36], mitogen-activated protein kinase 1 [MAPK1], ribosomal protein L18a [RPL18A], ribosomal protein S15 [RPS15], ribosomal protein L10 [RPL10]), cytoskeletal dynamics, cell growth, proliferation, apoptosis, and cell migration (Ras homolog family member G [RHOG], cofilin 1 [CFL1], ACTB, myosin light chain 12B [MYL12B], G protein subunit beta 1 [GNB1], G protein subunit beta 2 [GNB2], MAPK1) than those of high RFI steers (RFI = 1.32 to 3.23 kg/d) (Table 1). All these functions may increase paracellular permeability allowing faster passive absorption of VFA. While there were no significant differences in expression of genes involved in VFA absorption and metabolism in Kong's study, Elolimy et al. (2018) reported that the rumen epithelia of low RFI animals (RFI = −2.69 ± 0.58 kg/d) had greater expression of genes involved in VFA absorption (solute carrier family 16 member 3 [SLC16A3], solute carrier family 9 member A1 [SLC9A1], and hypoxia-inducible factor 1 subunit alpha [HIF1A]), ketogenesis (3-hydroxy-3-methylglutaryl CoA lyase [HMGCL], 3-hydroxy-3-methylglutaryl CoA synthase 2 [HMGCS2], and 3-hydroxybutyrate dehydrogenase 1 [BDH1]), and other metabolisms (lactate dehydrogenase A [LDHA] and peroxisome proliferator activated receptor delta [PPARD]) compared to those of high RFI group (RFI = 3.08 ± 0.55 kg/d). The expression of monocarboxylate transporter 4 (SLC16A3), chloride anion exchanger (solute carrier family 26 member 3 [SLC26A3]), and HIF1A has been reported to be stimulated by n-butyrate and hypoxia condition (Dengler et al., 2015). These suggest that butyrate-producing and facultative epimural associated microbes (defined as epimural microbiota and will be discussed in detail in the later sections) could play a role in epithelial VFA absorption via affecting the expression of these transporters. The increased expression of genes involved in VFA metabolism including ketogenesis (HMGCL, HMGCS2, and BDH1), and lactate production through pyruvate derived from propionate (Remond et al., 1995) (LDHA) in low RFI animals (Elolimy et al., 2018), suggested that the rumen epithelium of cattle with high feed efficiency are under the hypoxia condition. However, there are very limited studies to understand rumen epithelial metabolism and how hypoxia could affect its VFA absorptions.
Table 1.
Up-regulated genes of rumen epithelium in low RFI group compared to high RFI group.
| Gene | Metabolism | Fold change1 | Animal | Reference |
|---|---|---|---|---|
| Dynamin 2 (DNM2) | Remodeling of the epithelial adherens junctions | 1.17 | Hereford × Angus steers | Kong et al. (2016) |
| Hepatocyte growth factor-regulated tyrosine kinase substrate (HGS) | Remodeling of the epithelial adherens junctions | 1.26 | Hereford × Angus steers | |
| Actin beta (ACTB) | Remodeling of the epithelial adherens junctions/Cell migration | 1.24 | Hereford × Angus steers | |
| Tubulin, beta class 1 (TUBB5) | Remodeling of the epithelial adherens junctions | 1.28 | Hereford × Angus steers | |
| Tubulin alpha 4a (TUBA4A) | Remodeling of the epithelial adherens junctions | 1.43 | Hereford × Angus steers | |
| Ubiquitin A-52 residue ribosomal protein fusion product 1 (UBA52) | Protein synthesis | 1.24 | Hereford × Angus steers | |
| Ribosomal protein L36 (RPL36) | Protein synthesis | 1.28 | Hereford × Angus steers | |
| Mitogen-activated protein kinase 1 (MAPK1) | Protein synthesis/Cell migration | 1.30 | Hereford × Angus steers | |
| Similar to rRibosomal protein L 18a, ribosomal protein L18a (RPL18A) | Protein synthesis | 1.24 | Hereford × Angus steers | |
| Ribosomal protein S15 (RPS15) | Protein synthesis | 1.29 | Hereford × Angus steers | |
| Ribosomal protein L10 (RPL10) | Protein synthesis | 1.40 | Hereford × Angus steers | |
| Ras homolog family member G (RHOG) | Cell migration | 1.39 | Hereford × Angus steers | |
| Cofilin 1 (non-muscle) (CFL1) | Cell migration | 1.26 | Hereford × Angus steers | |
| Myosin light chain 12B (MYL12B) | Cell migration | – | Hereford × Angus steers | |
| G protein subunit beta 1 (GNB1) | Cell migration | 1.18 | Hereford × Angus steers | |
| G protein subunit beta 2 (GNB2) | Cell migration | 1.24 | Hereford × Angus steers | |
| Monocarboxylate transporter 4 (SLC16A3) | VFA absorption | 1.13 | Red Angus steers and heifers | Elolimy et al. (2018) |
| Solute carrier family 9 member a1 (SLC9A1) | VFA absorption | 1.01 | Red Angus steers and heifers | |
| Hypoxia inducible factor 1 subunit alpha (HIF1A) | VFA absorption | 1.01 | Red Angus steers and heifers | |
| Hydroxymethylglutaryl-CoA lyase, mitochondrial (HMGCL) | Ketogenesis | 1.09 | Red Angus steers and heifers | |
| Hydroxymethylglutaryl-CoA synthase, mitochondrial (HMGCS2) | Ketogenesis | 1.17 | Red Angus steers and heifers | |
| D-beta-hydroxybutyrate dehydrogenase, mitochondrial (BDH1) | Ketogenesis | 1.06 | Red Angus steers and heifers | |
| Lactate dehydrogenase A (LDHA) | Pyruvate metabolism | 1.06 | Red Angus steers and heifers | |
| Peroxisome proliferator activated receptor delta (PPARD) | Other metabolic pathways | 1.08 | Red Angus steers and heifers |
RFI = residual feed intake; VFA = volatile fatty acids.
Fold change is gene expression in low RFI relative to high RFI.
Overall, although these 2 pilot studies on rumen epithelial transcriptome (Kong et al., 2016; Elolimy et al., 2018) used animals with different breeds and sex (Hereford x Angus, steers in Kong et al., 2016 vs Red Angus, steers and heifers in Elolimy et al., 2018), it is evident that genes related to VFA absorption through passive transfer or transporters in the rumen epithelial tissues expressed differently between high and low RFI animals, suggesting the cattle with higher feed efficiency might have enhanced nutrient absorptive capacity. Therefore, the difference in rumen wall thickness, the passage rate together with the rumen microbiome all directly affect the VFA concentrations detected in the rumen. Further research on VFA dynamics is needed to verify the contribution of the VFA absorptive capacity of rumen epithelium in cattle feed efficiency. Additionally, future rumen epithelial transcriptome study for each of the four cell layers to understand rumen epithelial function in relation to cell types is required. Each cell layer might have different function and gene expression pattern under different conditions (growth stage, diets, management, and so on). Based on previous study (Graham and Simmons, 2005), we could speculate that the stratum basale layer mainly expresses genes involved in nutrient transport and ketogenesis, on the other hand, the stratum granulosum layer expresses genes related with keratinization, cell–cell junction, and microbe interaction.
4.2. Rumen epithelial nitrogen metabolism in cattle with different feed efficiency
Although there is no study yet reporting the rumen epithelial functions in nitrogen metabolism and its relationship with feed efficiency, a couple of recent studies showed a potential difference in nitrogen metabolism in cattle is associated with the variation in feed efficiency. For example, Asher et al. (2018) reported that the longissimus muscles of low RFI calves had a higher protein/fat ratio than that in high RFI calves, suggesting that there were differences in nitrogen or energy metabolism related to protein and fat accretion in the muscle of cattle with different feed efficiency. In addition, a researcher has reported that nitrogen excretion differed between high and low RFI Murrah buffalo heifers and low RFI heifers excreted less nitrogen in both urine and feces than high RFI heifers (Sharma et al., 2018). Although no differences were found in fecal and urine nitrogen excretion between Brahman steers with high and low feed efficiency by Carmona et al. (2020), the authors reported that efficient animals classified by residual gain excreted a lower amount of nitrogen in both urine and feces (g/100 g N intake) than inefficient animals. Based on these studies, it suggests that overall nitrogen metabolism in cattle can affect feed efficiency. We speculate that N recycling in the rumen system (both contents and tissue) can directly affect the ruminal nitrogen metabolism like microbial crude protein (MCP) production as well as epithelial function. Although there have been a limited number of studies related with nitrogen metabolism compared to carbohydrate metabolism in the rumen, the presence of urea recycling and the stimulation of urea transporter expression by ruminal VFA (Lu et al., 2015) could suggest the importance of rumen nitrogen metabolism and its balance with carbohydrate metabolism for feed efficiency in ruminants. Therefore, future research on ruminal nitrogen metabolism and feed efficiency is essential to understand the role of carbohydrate and nitrogen metabolism balance (nutrient synchronization) in cattle feed efficiency.
4.3. Systemic role of rumen epithelium in feed efficiency
In addition to VFA absorption and nitrogen metabolism, above mentioned studies also revealed that the rumen epithelia of cattle with high feed efficiency have more active tissue morphogenesis and VFA metabolism (Kong et al., 2016; Elolimy et al., 2018; Lam et al., 2018). The greater metabolic and functional activity of rumen epithelium may produce more end-products like β-hydroxybutyrate and acetoacetate and affect the expression of endocrine molecules like insulin (Manns and Boda, 1967), insulin-like growth factor-1 (IGF-1), and leptin (Kiani, 2013) in other tissues and/or organs that improve the animal production systemically (Cantalapiedra-Hijar et al., 2018). Sun et al. (2018) identified that the rumen epithelial tissue of lambs fed milk and starter feed differentially expressed IGF-1 and IGF-1 receptor genes compared to lambs fed milk. Stick et al. (1998) reported that serum IGF-1 concentration was associated with feed efficiency (G:F; P = 0.04) and Nascimento et al. (2015) showed that efficient Nellore cattle had higher concentration of plasma insulin and IGF-1 rather than inefficient cattle. In addition to the rumen epithelium (Lam et al., 2018), small intestine (Montanholi et al., 2013), and liver (Montanholi et al., 2017) of efficient animals had greater morphogenetic traits (nuclei number or area), suggesting that tissues of efficient animals have greater metabolic and functional activity. Although greater metabolic activities in whole body tissues are consistent with cellular energy-consuming processes (mitochondrial proton leakage, ion pumping, and protein turnover) and the processes contribute a higher proportion (about 67%) to animal feed efficiency variation (Richardson and Herd (2004); Cantalapiedra-Hijar et al., 2018), the active morphogenesis and metabolisms might be the reflection of more active anabolic processes for animal growth. Future studies to understand the systemic interaction between rumen epithelium and other organs are vital to determine its systemic role in affecting feed efficiency.
5. Rumen epithelial microbiota (epimural microbiota) and feed efficiency
Rumen is an important organ for ruminants where feed fermentation occurs by rumen microbiota. The rumen microbes ferment feed particles that animals can't degrade by their own enzymes and provide fermentation products to the host ruminants. Rumen microbiota are distinguished into three fractions according to their location in the rumen: 1) liquid-associated microbiota, 2) solid-associated microbiota, and 3) rumen epithelium-associated or rumen epithelial microbiota. Rumen epithelial microbiota, defined as epimural microbiota, are the microorganisms that colonize on the rumen epithelium by attaching to the rumen epithelial cells strongly or loosely. Although it has less than 1% of the total rumen microbial population and low contribution to VFA production (Sadet et al., 2007), they are the ones may directly interact with host and their roles in contributing to rumen epithelial functions can directly influence ruminant productivity. To date, the role of epimural microbial community in ruminant physiology has been underestimated and less studied compared to the rumen liquid- and solid-associated microbiota.
With the development of high throughput sequencing, there have been many studies to reveal the associations between rumen microbiome and diet and feed fermentation (Henderson et al., 2015; Furman et al., 2020), host genetics (Li et al., 2019b), rumen health (Petri et al., 2013b), methane emission (Difford et al., 2018), and feed efficiency (Li and Guan (2017)). It has been reported that cattle with high feed efficiency have lower microbial diversity and richness (Guan et al., 2008), a lower abundance of active bacterial families of Lachnospiraceae, Lactobacillaceae, and Veillonellaceae (Li and Guan (2017)), and a lower abundance of archaeal species of Methanobrevibacter smithii (Carberry et al., 2014) in the rumen contents compared to inefficient animals. However, there have been a small number of rumen epimural microbiome studies (Jiao et al., 2015; Mann et al., 2018) and most of them mainly related to subacute ruminal acidosis (SARA) (Petri et al., 2013a, 2019; Liu et al., 2015; McCann et al., 2016; Li et al., 2019a; Abbas et al., 2020). To date, the role of epimural microbiome in cattle feed efficiency has not been reported.
5.1. Composition of epimural microbiota
The rumen epithelium allows the colonization of rumen epithelial attached microbes (epimural microbiota) whose composition and function have been studied since the 1960s (Rahman and Decker, 1966; Wallace et al., 1979). Epimural microbes attach to the rumen epithelial cells by their fimbriae, flagella, glycocalyx, or other surface structures (McCowan et al., 1978; Semjén and Gálfi, 1990; Galfi et al., 1998; Klemm et al., 2010). Although the bacteria, as the main members of epimural microbiota, have been mostly studied, there have been several studies to identify epimural archaeal, fungal, and protozoan communities (Ishaq et al., 2017; Holman and Gzyl, 2019). The epimural bacterial community has a greater variation in richness (α-diversity Shannon–Wiener and Simpson indices) among individual animals compared to rumen solid-associated and liquid-associated bacteria (De Mulder et al. (2017); Anderson et al., 2021). Through a meta-analysis, Anderson et al. (2021) identified the core epimural bacterial taxa from 17 different studies with phyla Firmicutes (41.8%), Proteobacteria (20.0%), and Bacteroidetes (19.6%), and genera Campylobacter (8.4%), Prevotella (6.0%), Christensenellaceae R-7 group (5.8%), Butyrivibrio (5.4%), and a previously uncultured genus of the family Neisseriaceae (3.7%) being dominant. From the same study, 2 archaeal taxa (Methanobrevibacter and Methanomethylophilaceae) were also identified as the core members of the epimural microbiota in addition to bacteria. Although Ishaq et al. (2017) reported the diversity and relative abundance of epimural fungal and protozoal communities, further studies are essential to determine whether they are the true members of epimural community as widely reported for the epimural bacteria and archaea. Future studies are needed to study the eukaryotic microbes attached to the rumen epithelium and to identify their functions and roles in rumen function.
5.2. Function of epimural microbiota
The functions of epimural microbiota, especially for the epimural bacteria, have been identified as follows: 1) tissue recycling, 2) urea hydrolysis, and 3) oxygen scavenging (Cheng et al., 1979). McCowan et al. (1978) observed some of the epimural bacteria could digest keratinized epithelial cells, suggesting they could stimulate sequential removal of the keratinized epithelial cells and stimulation of the formation of new keratinized cell layers in the stratum corneum, leading to improved growth and absorptive capacity of the rumen epithelium. Moreover, the dead cells can be used as microbial nutrients to enhance microbial growth and subsequently feed digestion and fermentation (Cheng et al., 1979). Therefore, tissue recycling by epimural microbiota might affect the feed efficiency of animals by recycling the host-derived nutrients and improving the cell turnover of the rumen epithelium. In addition, epimural bacteria are known to be involved in urea cycling. The degradation of urea is mainly induced by ureolytic bacteria attached to the rumen epithelium or ureolytic bacteria detached from the rumen epithelium and found in rumen fluid (Wallace, 1979; Wallace et al., 1979). Wallace et al. (1979) showed that epimural ureolytic bacteria can provide a normal range of ureolytic activity and the urease activity of epimural bacteria regulates urea transport across the rumen epithelium affecting nitrogen recycling. It has been known that several rumen bacterial taxa such as members in the genera of Actinomyces, Bacillus, Butyrivibrio, Bifidobacterium, Haemophilus, Neisseria, Peptostreptococcus, Prevotella, Pseudomonas, Ruminococcus, Streptococcus, Succinivibrio, Treponema, and unclassified Succinivibrionaceae had ureolytic activity (Wozny et al., 1977; Barr et al., 1980; Jin et al., 2016). However, future research is needed to identify if these ureolytic bacterial species are derived from epimural bacterial community and whether the ureolytic function of epimural microbes can affect feed efficiency.
In addition to tissue recycling and urea digestion, the function of oxygen scavenging by facultative epimural microbes might influence the feed efficiency of animals because maintaining the strict anaerobic condition is essential for the digestion and fermentation of feed by obligated anaerobes. Through the rumen epithelium, oxygen might be diffused from host tissue to the luminal surface of the rumen and makes rumen epithelial surface a good environment for facultative epimural anaerobes (Cheng et al., 1979; Mann et al., 2018). Epimural bacterial community had a higher abundance of Proteobacteria phylum in which many facultative bacterial taxa like Campylobacter than that in a ruminal content-associated bacterial community (Liu et al., 2016). Furthermore, Tan et al. (2021) identified that low RFI steers had a higher abundance of active Campylobacteraceae and Neisseriaceae which members belonged to these 2 families are known to have oxygen scavenging function. We speculate that oxygen scavenging microbes may affect not only rumen fermentation but also rumen epithelial metabolisms by hypoxia condition. It has been revealed that high grain feeding can affect epimural bacteria and the resulting lower pH can cause the lesion of epithelium. Some studies revealed that when animals were under acidosis condition or fed concentrate diet the expression of genes involved in glutathione metabolism in rumen epithelial tissue were increased (Gholizadeh et al., 2020; Sun et al., 2021). Glutathione metabolism pathway is an antioxidant pathway that detoxifies the oxygen-derived free radicals using glutathione peroxidase and transferase enzymes (Gholizadeh et al., 2020). Although it is unclear if oxygen scavenging function of epimural microbes is related to glutathione metabolism of rumen epithelial cells, we speculate that high concentrations of VFA derived from rumen fermentation and rumen epithelial lesion can cause oxidative stress and then glutathione metabolism may be activated against the oxidative stress. Although the results of transcriptome study with low and high RFI groups (Elolimy et al., 2018) did not show DEGs involved in glutathione metabolism, the expression of HIF1A was up-regulated in low RFI group (Kong et al., 2016). The up-regulated expression of HIF1A can then induce expressions of genes that promote cell survival during hypoxia. Furthermore, HIF1A has a role in wound healing (Hong et al., 2014). Based on the references, we can assume that oxygen scavenging or invasion of epimural microbes into rumen epithelium may cause hypoxia condition by removing oxygen or inducing injury in rumen epithelium, respectively. In addition to facultative epimural microbes, the active rumen epithelial metabolisms, as well as the thickness of the rumen wall, might contribute to the hypoxia condition. Even though the reasons why facultative anaerobes are abundant and activated and their roles in feed efficiency have not been understood, it's worth studying the role of oxygen scavenging microbes in hypoxia and how it can affect the cattle feed efficiency in the future.
5.3. Interactions between epimural microbiota and host
The colonization of commensal microbes on the gut epithelium activates gut innate and adaptive immune systems by affecting cellular turnover and barrier function of the epithelium and prevents the pathogen from penetrating the epithelium through the protective barrier (Yoo et al., 2020). Compared to the studies in human gut microbiota and intestinal immune system, there has been limited research about the relationship between epimural microbiota and rumen epithelial barrier and immune functions.
Recent study also suggested that epimural microbiota could play a role in the rumen epithelial wall development and immune functions (inflammation and barrier functions) through direct interactions with the host (Malmuthuge et al., 2019; Lin et al., 2019). VFA, especially butyrate and propionate, derived from microbial fermentation may stimulate the growth of rumen epithelium throughout indirect response of hormones like insulin, epidermal growth factor, and IGF-1 (Diao et al., 2019). We speculate that some epimural microbes can produce butyrate or propionate to affect the growth of rumen papillae although the absolute concentrations of VFA from epimural microbiota are lower than those produced from rumen contents-associated microbiota. The increased papillae length and/or surface area might increase the VFA absorption rate and contribute to feed efficiency. In addition, Petri et al. (2019) reported that the abundances of genera Alkalibaculum, Anaerorhabdus, Coprococcus, and Dethiobacter were positively correlated to the expression of genes involved in pH regulation [solute carrier family 9 member A2 (SLC9A2; previous symbol: NHE2) and solute carrier family 9 member A3 (SLC9A3; previous symbol: NHE3)] and VFA transport [solute carrier family 16 member 1 (SLC16A1; alternative symbol: MCT1)], suggesting that epimural microbial community might interact with the rumen epithelial cells and affect the rumen epithelial function through altering the gene expression. However, how the epimural bacteria influence gene expression needs further study.
Chen et al., 2012 reported that acidosis resistant cattle had different epimural bacterial diversity and higher expressions of toll like receptor 2 (TLR2) and toll like receptor 4 (TLR4) in the rumen epithelia than those of acidosis susceptible cattle, suggesting that susceptibility to SARA may be associated with the activated immunity of the epithelial tissue which is induced by epimural microbiota. Activated immune systems of rumen epithelium might reduce energy for the recovery from severe inflammation, which eventually improve feed efficiency of host animals. Furthermore, microbial products like lipopolysaccharide (LPS), and microorganism-associated molecular patterns (MAMP) might stimulate the expression of genes related to immune activity (Garcia et al., 2017), although there have been few studies about LPS and MAMP of epimural microbiota. Most Gram-negative produce LPS as their cell membrane structure and also MAMP which are virulence factors including outer membrane parts like peptidoglycans, lipopeptides, porins, flagellin, and bacterial DNA. These microbial factors can affect gut epithelial gene expression involved in immunity and other metabolisms like cell proliferation (Garcia et al., 2017). Rumen epithelial microbiota could also produce LPS and MAMP and interact with the epithelial tissues. Therefore, future research is needed to determine which kinds of LPS and MAMPs epimural microbes have and whether they can be detected or interact with rumen epithelial tissue receptors.
In summary, balanced composition of epimural microbial community (commensal, pathogenic, VFA producing, and ureolytic microbes) may influence feed efficiency by regulating rumen epithelial functions. Unfortunately, interactions between epimural microbiota and rumen epithelial cells have not been fully understood yet due to the difficulty of the cultivation of epimural microbes and collection of epithelial samples to study their functional changes temporally and spatially in vivo. Future studies on rumen epithelium, epimural microbiota, and their interaction are essential to identify the roles of rumen epithelium in cattle feed efficiency.
5.4. Research approaches/methods to study epimural microbiome
Methods to study epimural microbiota have evolved significantly since the 1960s. The early studies identified the urease activity of epimural bacteria (Rahman and Decker, 1966; Houpt and Houpt, 1968). After 1970s, rumen wall-associated bacteria were isolated to study their functions in ureolysis (Wallace et al., 1979), oxygen scavenging (Mead and Jones, 1981), and digestion of dead epithelial tissue (Dinsdale et al., 1980). In the 1990s, the adherence assays using cultured rumen epithelial cells were developed to validate the attachment and/or colonization of isolated bacterial strains from rumen epithelium (Semjén and Gálfi, 1990; Galfi et al., 1998; Štyriak et al., 1992, 1994). Since the 2000s, 16S rDNA gene amplicon sequencing method has been used for the identification of epimural microbial profiles at the DNA level (to detect the presence of the taxa) and at the cDNA/RNA level (to detect metabolically active taxa). The amplicon sequencing of 16S rDNA revealed that the rumen epimural bacterial community significantly differs from digesta associated microbiota and the diversity of epimural bacterial community was higher than that of digesta associated microbiota in young and adult cattle (Li et al., 2012; Malmuthuge et al., 2014). Additionally, amplicon-seq based next-generation sequencing techniques have been used to assess the changes in epimural community in response to rumen development (Jiao et al., 2015) high-grain diet (Liu et al., 2015; Wetzels et al., 2015; Zhang et al., 2017), and locations within the rumen (Sbardellati et al., 2020). Although the taxonomic composition of epimural community has been widely studied using amplicon-seq (also termed as metataxonomics), this approach could only obtain information about the composition and diversity of epimural microbial community, which lacks the knowledge on their activity or functions as well as how they are related to host function. Although researchers have used PICRUSt (phylogenetic investigation of communities by reconstruction of unobserved states) (Langille et al., 2013) to predict gene abundance of rumen epimural microbiota (Zhang et al., 2017; Seddik et al., 2019), PICRUSt could only predict the function based on 16S rRNA gene from known culturable species. With only a small number of the rumen epithelial bacterial being isolated and sequenced, such prediction can be largely biased.
Nowadays, researchers started to study the rumen epimural microbiome using the combined omics (metataxonomics, metagenomics, metatranscriptomics, and host transcriptomics) approaches. Recent attempts have been made to study the host-epimural microbiota interaction metataxonomic analysis to assess epimural bacterial community and transcriptomic analysis of rumen epithelium, with the main focus on SARA pathogenesis (Li et al., 2019a; Petri et al., 2018, 2019, 2020). During SARA, the relative abundances of Olsenella, Desulfovibrio, and Fusobacterium were significantly increased which were positively correlated with the expression of genes involved in tissue remodeling (Li et al., 2019a). Similarly, Petri et al. (2019) identified that the abundances of Alkalibaculum, Anaerorhabdus, Coprococcus, and Dethiobacter were positively correlated with expressions of genes involved in pH regulation (NHE2 and NHE3) and VFA absorption (MCT1). In addition, Mann et al. (2018) firstly identified the metatranscriptome profile of epimural microbiome of Holstein cows in response to SARA challenge using total RNA sequencing (5.9 M reads/sample). This study was able to identify the highly expressed microbial genes involved in energy, nitrogen, and carbohydrate metabolisms. Another recent study with a deeper total RNA-seq (80M reads/sample) has allowed the identification of active epimural bacteria and methanogen together with host transcriptome and revealed the difference between high and low RFI steers (Tan et al., 2021). However, these studies only reported the correlations, and the causal relationships between epimural bacteria and host gene expression have not been identified. Further studies at the protein expression level would be essential to know how interaction between rumen epithelium and epimural microbiota can affect host feed efficiency. Also, rumen morphological and physiological studies should be done to identify the effect of rumen and papillae size on VFA absorption and feed efficiency.
There have been several challenges on epimural microbiome research as most of the studies to date: 1) had small population sizes; 2) with different experimental factors (species, diet, age, geographic regions) which does not allow the metanalysis; 3) with different sampling time, and rumen locations; 4) applied sequencing approaches and analytical methods; 5) result variation between individual animals; and 6) identified a large number of unidentified microbes. To achieve a comprehensive understanding of epimural community, future studies with uniformed methods and factors should be considered together with the efforts that should be made on culturomics to identify previously uncultured epimural microbes.
6. Future directions to study epithelial host-microbe interactions for improving feed efficiency
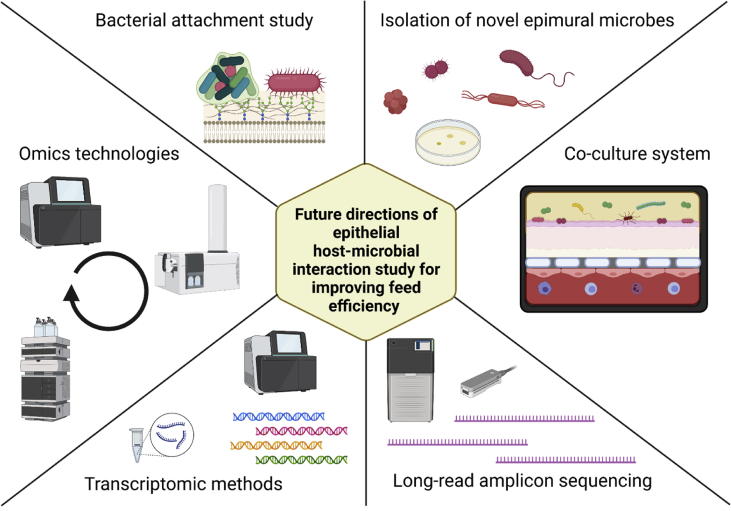
Despite the development of technologies, it has been difficult to study the epimural microbiota and epimural host-microbe interactions because of several limitations including its low microbial density, difficulty in sampling tissue, high animal expense (difficulty in using gnotobiotic animals), and difficulty in separating epimural microbes from the host epithelium in addition to the above-mentioned challenges. Future research from various aspects is needed to elucidate the regulatory mechanisms of host-microbe interactions in the rumen epithelia for the development of novel strategies to improve rumen function as a whole (Fig. 2).
Fig. 2.
Future directions of epithelial host-microbe interaction study for improving feed efficiency (Created with BioRender.com).
Firstly, the adherence of epimural microbiota to rumen epithelial cells makes it hard to separate from the host. To detach epimural microbes from rumen epithelium, several proteases or chemicals which destroy the various types of bacterial adherence are needed. Using the new technologies, researchers can do culture-dependent experiments with the isolated epimural microbial species which might be involved in host-microbe interaction and discuss host-microbe interaction in more depth with their potential mechanisms.
Secondly, lots of epimural microbial species have not been isolated and characterized yet so it is hard to interpret the results of epimural microbial profile based on the widely used marker amplicon sequencing. Many researchers in rumen microbiology have tried to identify novel rumen microbes and accumulate their reference genomes through the projects like Hungate 1,000 project to fulfill the lack of rumen microbial collection (Creevey et al., 2014; Stewart et al., 2019). Also, culture-dependent studies are needed to understand rumen epithelial host-microbe interactions. To date, only a few recent studies have identified novel rumen bacteria from the rumen epithelium (Hong et al., 2020; Na et al., 2021), and continuous efforts to isolate novel epimural microbes are essential for epimural microbiome study.
Thirdly, although the recent molecular methods such as genome and transcriptome sequencing have allowed the characterization of unculturable microbes and host gene expression, there are several difficulties to study rumen epithelial host-microbe interactions. Standard short-read amplicon sequencing like the Illumina platform can provide limited information about microbial taxonomy beyond genus level and different members within the same genus can have very different functions. Recently developed long-read amplicon sequencing technologies might provide microbial taxonomic information at species level or even strain level (Callahan et al., 2021; Karst et al., 2021) that can provide insights into the real functions of each taxon in the epimural microbiota.
Fourthly, methods are lacking to study rumen epithelial function spatially and temporally in vivo. The rumen is large in volume with an average size of 70 to 100 L (Bickhart and Weimer, 2018) and spatially distinguished as several sacs like ventral sac, dorsal sac, and cranial sac, suggesting that each location of rumen may have different physiological conditions influenced by host physiology and rumen contents (McCowan et al., 1980; Sbardellati et al., 2020). Although Sbardellati et al. (2020) identified that there were differences in epimural microbial profile among four ruminal locations, it is unclear whether this contributes to the spatial function of rumen epithelium and epimural microbiota. Recently, the isolation of rumen epithelial cells has become a useful tool to identify rumen epithelial metabolisms like an immune response (Zhan et al., 2019; Reisinger et al., 2021). Co-culture of epimural bacteria with rumen epithelial cells might be a valuable model to study rumen epithelial host-microbe interactions. However, the co-culture system should maintain anaerobic conditions on the apical side and aerobic conditions on the basal membrane of rumen epithelial cells (Sasaki et al., 2020). Furthermore, organoids, multicellular in vitro culture systems, have been developed for monogastric animals (Duque-Correa et al., 2020; Lee et al., 2021) which might be used to replace isolated rumen epithelial cell culture systems in the future.
Fifthly, although transcriptome profiling of both epimural microbiota and rumen epithelium might help to elucidate the host-microbe interaction mechanisms related with feed efficiency. There have been recent efforts to apply transcriptomic methods to epimural microbial study (Mann et al., 2018; Tan et al., 2021) but the methodology has not been fully developed yet because the separation of epimural microbial transcriptome from host transcriptome. Recently, single cell RNA-seq has been applied to study functions of different cell types under the various conditions, especially diseased status (Vijay et al., 2020). However, no study yet has applied such method to study host and epimural microbial cells and this could be difficult due to their tight attachment and a large number of rumen epithelial cells. Furthermore, the separation of each rumen epithelial layer might be helpful to understand rumen epithelial function deeply because the four layers of stratified squamous structure have their own characteristics after differentiation. To overcome such limitations, developing methods for degrading cell–cell junction between rumen epithelial cells and host-microbial attachment should be essential. Additionally, transcriptome studies with the newly developed coculture systems might solve the problem by controlling the number of epimural microbes or rumen epithelial cells. In addition, using microbiome DNA enrichment kits could increase the sequencing depth of epimural samples, and developing epimural microbiome-specific bioinformatics pipeline by clustering dominant and specific taxonomy in epimural microbial community could improve the speed and accuracy of analysis for epimural microbiome study.
Lastly, another limitation of epimural host-microbe interaction study is a lack of methods to determine a causal relationship. Omics technologies enable us to understand comprehensive aspects of the epimural community, but the absence of mechanisms hides the causal relationship between host-epimural microbe interactions. To identify mechanisms of host-microbe interaction or microbial metabolisms, culture-dependent methods with single microbe and application of genetic technologies like gene knock-out are essential for epimural microbiota study.
7. Conclusion
In summary, recent studies have identified that gut epithelium is not just an absorptive organ but also a functional mediator between gut microbiota and host animals, which can regulate gut homeostasis through the crosstalk with gut microbiota and immune systems (protective barrier, innate and adaptive epithelial immunity). Research on rumen epithelial host-microbe interactions in coincide with ruminant intestinal studies highlights their important roles in rumen homeostasis and functions. Future research is needed to understand rumen epithelial host-microbe interactions and identify the role of epimural microbiota in ruminant feed efficiency. Moreover, rumen epithelial host-microbe interaction research might provide new insights to the host-microbe interaction in not only gut but also other tissues like skin due to its unique structure and function.
Author contributions
Sang Weon Na: writing - original draft; Le Luo Guan: conceptualization, writing - review & editing, funding acquisition, supervision
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
The authors acknowledge the funding support by Alberta Beef Producer/Canadian Cattleman’s Association (BCRC) (FRG.16.19) as well as NSERC discovery grant for the financial support.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Abbas W., Keel B.N., Kachman S.D., Fernando S.C., Wells J.E., Hales K.E., et al. Rumen epithelial transcriptome and microbiome profiles of rumen epithelium and contents of beef cattle with and without liver abscesses. J Anim Sci. 2020;98 doi: 10.1093/jas/skaa359. skaa359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdoun K., Stumpff F., Martens H. Ammonia and urea transport across the rumen epithelium: a review. Anim Health Res Rev. 2006;7:43–59. doi: 10.1017/S1466252307001156. [DOI] [PubMed] [Google Scholar]
- Anderson C.J., Koester L.R., Schmitz-Esser S. Rumen epithelial communities share a core bacterial microbiota: a meta-analysis of 16s rrna gene illumina miseq sequencing datasets. Front Microbiol. 2021;12:539. doi: 10.3389/fmicb.2021.625400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias J., Cabrera R., Valencia A. Observations on the histological development of the bovine rumen papillae morphological changes due to age. Anat Histol Embryol. 1978;7:140–151. doi: 10.1111/j.1439-0264.1978.tb00664.x. [DOI] [PubMed] [Google Scholar]
- Aschenbach J.R., Zebeli Q., Patra A.K., Greco G., Amasheh S., Penner G.B. Symposium review: the importance of the ruminal epithelial barrier for a healthy and productive cow. J Dairy Sci. 2019;102:1866–1882. doi: 10.3168/jds.2018-15243. [DOI] [PubMed] [Google Scholar]
- Asher A., Shabtay A., Cohen-Zinder M., Aharoni Y., Miron J., Agmon R., et al. Consistency of feed efficiency ranking and mechanisms associated with inter-animal variation among growing calves. J Anim Sci. 2018;96(3):990–1009. doi: 10.1093/jas/skx045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baaske L., Gäbel G., Dengler F. Ruminal epithelium: a checkpoint for cattle health. J Dairy Res. 2020;87:322–329. doi: 10.1017/S0022029920000369. [DOI] [PubMed] [Google Scholar]
- Baldwin R., Jesse B. Developmental changes in glucose and butyrate metabolism by isolated sheep ruminal cells. J Nutr. 1992;122:1149–1153. doi: 10.1093/jn/122.5.1149. [DOI] [PubMed] [Google Scholar]
- Baldwin R.L., Connor E.E. Rumen function and development. Vet Clin North Am Food Anim Pract. 2017;33:427–439. doi: 10.1016/j.cvfa.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Barr M.E., Mann S.O., Richardson A.J., Stewart C.S., Wallace R.J. Establishment of ureolytic staphylococci in the rumen of gnotobiotic lambs. J Appl Microbiol. 1980;49(2):325–330. doi: 10.1111/j.1365-2672.1980.tb05131.x. [DOI] [PubMed] [Google Scholar]
- Bedford A., Beckett L., Harthan L., Wang C., Jiang N., Schramm H., et al. Ruminal volatile fatty acid absorption is affected by elevated ambient temperature. Sci Rep. 2020;10:1–11. doi: 10.1038/s41598-020-69915-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry D., Crowley J. Cell biology symposium: genetics of feed efficiency in dairy and beef cattle. J Anim Sci. 2013;91:1594–1613. doi: 10.2527/jas.2012-5862. [DOI] [PubMed] [Google Scholar]
- Bickhart D., Weimer P. Symposium review: host–rumen microbe interactions may be leveraged to improve the productivity of dairy cows. J Dairy Sci. 2018;101:7680–7689. doi: 10.3168/jds.2017-13328. [DOI] [PubMed] [Google Scholar]
- Boaitey A., Goddard E., Mohapatra S., Crowley J. Feed efficiency estimates in cattle: the economic and environmental impacts of reranking. Sustain Agric Res. 2017;6(2):35–47. [Google Scholar]
- Bonilha S.F.M., Branco R.H., Mercadante M.E.Z., dos Santos Gonçalves Cyrillo J.N., Monteiro F.M., Ribeiro E.G. Digestion and metabolism of low and high residual feed intake nellore bulls. Trop Anim Health Prod. 2017;49:529–535. doi: 10.1007/s11250-017-1224-9. [DOI] [PubMed] [Google Scholar]
- Brunes L.C., Baldi F., Lopes F., Lôbo R.B., Espigolan R., Costa M., et al. Selection criteria for feed efficiency-related traits and their association with growth, reproductive and carcass traits in nelore cattle. Anim Prod Sci. 2021;61:1633–1642. [Google Scholar]
- Callahan B.J., Grinevich D., Thakur S., Balamotis M.A., Yehezkel T.B. Ultra-accurate microbial amplicon sequencing with synthetic long reads. Microbiome. 2021;9:1–13. doi: 10.1186/s40168-021-01072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalapiedra-Hijar G., Abo-Ismail M., Carstens G., Guan L., Hegarty R., Kenny D.A., et al. Biological determinants of between-animal variation in feed efficiency of growing beef cattle. Animal. 2018;12:s321–s335. doi: 10.1017/S1751731118001489. [DOI] [PubMed] [Google Scholar]
- Carberry C.A., Waters S.M., Kenny D.A., Creevey C.J. Rumen methanogenic genotypes differ in abundance according to host residual feed intake phenotype and diet type. Appl Environ Microbiol. 2014;80:586–594. doi: 10.1128/AEM.03131-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona P., Costa D., Silva L. Feed efficiency and nitrogen use rankings of bos indicus steers differ on low and high protein diets. Anim Feed Sci Technol. 2020;263:114493. [Google Scholar]
- Chen Y., Oba M., Guan L.L. Variation of bacterial communities and expression of toll-like receptor genes in the rumen of steers differing in susceptibility to subacute ruminal acidosis. Vet Microbiol. 2012;159:451–459. doi: 10.1016/j.vetmic.2012.04.032. [DOI] [PubMed] [Google Scholar]
- Chen L., Qiu Q., Jiang Y., Wang K., Lin Z., Li Z., et al. Large-scale ruminant genome sequencing provides insights into their evolution and distinct traits. Science. 2019;364 doi: 10.1126/science.aav6202. [DOI] [PubMed] [Google Scholar]
- Cheng K., McCowan R., Costerton J. Adherent epithelial bacteria in ruminants and their roles in digestive tract function. Am J Clin Nutr. 1979;32:139–148. doi: 10.1093/ajcn/32.1.139. [DOI] [PubMed] [Google Scholar]
- Chruścik A., Kauter K., Windus L., Whiteside E. Fundamentals of anatomy and physiology. University of Southern Queensland; 2021. 3.2 epithelial tissue.https://usq.pressbooks.pub/anatomy/chapter/epithelial-tissue/ [Google Scholar]
- Church D. vol. 1. 1974. (Digestive physiology and nutrition of ruminants). [Google Scholar]
- Clauss M., Fritz J., Bayer D., Nygren K., Hammer S., Hatt J.-M., et al. Physical characteristics of rumen contents in four large ruminants of different feeding type, the addax (addax nasomaculatus), bison (bison bison), red deer (cervus elaphus) and moose (alces alces) Comp Biochem Physiol Mol Integr Physiol. 2009;152:398–406. doi: 10.1016/j.cbpa.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Creevey C.J., Kelly W.J., Henderson G., Leahy S.C. Determining the culturability of the rumen bacterial microbiome. Microb Biotechnol. 2014;7:467–479. doi: 10.1111/1751-7915.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mulder T., Goossens K., Peiren N., Vandaele L., Haegeman A., De Tender C., et al. Exploring the methanogen and bacterial communities of rumen environments: solid adherent, fluid and epimural. FEMS Microbiol Ecol. 2017;93:fiw251. doi: 10.1093/femsec/fiw251. [DOI] [PubMed] [Google Scholar]
- Dengler F., Rackwitz R., Benesch F., Pfannkuche H., Gäbel G. Bicarbonate-dependent transport of acetate and butyrate across the basolateral membrane of sheep rumen epithelium. Acta Physiol. 2014;210:403–414. doi: 10.1111/apha.12155. [DOI] [PubMed] [Google Scholar]
- Dengler F., Rackwitz R., Benesch F., Pfannkuche H., Gäbel G. Both butyrate incubation and hypoxia upregulate genes involved in the ruminal transport of scfa and their metabolites. J Anim Physiol Anim Nutr. 2015;99:379–390. doi: 10.1111/jpn.12201. [DOI] [PubMed] [Google Scholar]
- Diao Q., Zhang R., Fu T. Review of strategies to promote rumen development in calves. Animals. 2019;9:490. doi: 10.3390/ani9080490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difford G.F., Plichta D.R., Løvendahl P., Lassen J., Noel S.J., Højberg O., et al. Host genetics and the rumen microbiome jointly associate with methane emissions in dairy cows. PLoS Genet. 2018;14:e1007580. doi: 10.1371/journal.pgen.1007580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinsdale D., Cheng K., Wallace R., Goodlad R. Digestion of epithelial tissue of the rumen wall by adherent bacteria in infused and conventionally fed sheep. Appl Environ Microbiol. 1980;39:1059–1066. doi: 10.1128/aem.39.5.1059-1066.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Correa M.A., Maizels R.M., Grencis R.K., Berriman M. Organoids–new models for host–helminth interactions. Trends Parasitol. 2020;36:170–181. doi: 10.1016/j.pt.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elolimy A.A., Abdelmegeid M.K., McCann J.C., Shike D.W., Loor J.J. Residual feed intake in beef cattle and its association with carcass traits, ruminal solid-fraction bacteria, and epithelium gene expression. J Anim Sci Biotechnol. 2018;9:1–13. doi: 10.1186/s40104-018-0283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons C., Kenny D., Fahey A., McGee M. Feeding behavior, ruminal fermentation, and performance of pregnant beef cows differing in phenotypic residual feed intake offered grass silage. J Anim Sci. 2014;92:2170–2181. doi: 10.2527/jas.2013-7438. [DOI] [PubMed] [Google Scholar]
- Freetly H.C., Dickey A., Lindholm-Perry A.K., Thallman R.M., Keele J.W., Foote A.P., et al. Digestive tract microbiota of beef cattle that differed in feed efficiency. J Anim Sci. 2020;98 doi: 10.1093/jas/skaa008. skaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman O., Shenhav L., Sasson G., Kokou F., Honig H., Jacoby S., et al. Stochasticity constrained by deterministic effects of diet and age drive rumen microbiome assembly dynamics. Nat Commun. 2020;11:1–13. doi: 10.1038/s41467-020-15652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gäbel G., Aschenbach J., Müller F. Transfer of energy substrates across the ruminal epithelium: implications and limitations. Anim Health Res Rev. 2002;3:15–30. doi: 10.1079/ahrr200237. [DOI] [PubMed] [Google Scholar]
- Galfi P., Neogrady S., Semjen G., Bardocz S., Pusztai A. Attachment of different escherichia coli strains to cultured rumen epithelial cells. Vet Microbiol. 1998;61:191–197. doi: 10.1016/s0378-1135(98)00175-8. [DOI] [PubMed] [Google Scholar]
- Garcia M., Bradford B., Nagaraja T. Invited review: ruminal microbes, microbial products, and systemic inflammation. Prof Anim Sci. 2017;33:635–650. [Google Scholar]
- Getahun D., Getabalew M., Zewdie D., Alemneh T., Akeberegn D. Urea metabolism and recycling in ruminants. Biomed J Sci Tech Res. 2019;20:14790–14796. [Google Scholar]
- Gholizadeh M., Fayazi J., Asgari Y., Zali H., Kaderali L. Reconstruction and analysis of cattle metabolic networks in normal and acidosis rumen tissue. Animals. 2020;10(3):469. doi: 10.3390/ani10030469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham C., Simmons N.L. Functional organization of the bovine rumen epithelium. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R173–R181. doi: 10.1152/ajpregu.00425.2004. [DOI] [PubMed] [Google Scholar]
- Guan L.L., Nkrumah J.D., Basarab J.A., Moore S.S. Linkage of microbial ecology to phenotype: correlation of rumen microbial ecology to cattle's feed efficiency. FEMS Microbiol Lett. 2008;288:85–91. doi: 10.1111/j.1574-6968.2008.01343.x. [DOI] [PubMed] [Google Scholar]
- Henderson G., Cox F., Ganesh S., Jonker A., Young W., Janssen P.H. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci Rep. 2015;5:1–15. doi: 10.1038/srep14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Sanabria E., Goonewardene L.A., Wang Z., Durunna O.N., Moore S.S., Guan L.L. Impact of feed efficiency and diet on adaptive variations in the bacterial community in the rumen fluid of cattle. Appl Environ Microbiol. 2012;78(4):1203–1214. doi: 10.1128/AEM.05114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Sanabria E., Guan L.L., Goonewardene L.A., Li M., Mujibi D.F., Stothard P., et al. Correlation of particular bacterial PCR-denaturing gradient gel electrophoresis patterns with bovine ruminal fermentation parameters and feed efficiency traits. Appl Environ Microbiol. 2010;76(19):6338–6350. doi: 10.1128/AEM.01052-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman D.B., Gzyl K.E. A meta-analysis of the bovine gastrointestinal tract microbiota. FEMS Microbiol Ecol. 2019;95:fiz072. doi: 10.1093/femsec/fiz072. [DOI] [PubMed] [Google Scholar]
- Hong S.J., Do Hyun Kim D.J.S., Jung M.N.H., Baik M. Psii-7 complete genome analysis of a novel mucolytic bacterium, prevotella mucinisolvens sp. Nov., isolated from bovine rumen epithelium. J Anim Sci. 2020;98:406. [Abstract)] [Google Scholar]
- Hong W.X., Hu M.S., Esquivel M., Liang G.Y., Rennert R.C., McArdle A., et al. The role of hypoxia-inducible factor in wound healing. Adv Wound Care. 2014;3:390–399. doi: 10.1089/wound.2013.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houpt T.R., Houpt K.A. Transfer of urea nitrogen across the rumen wall. Am J Physiol. 1968;214:1296–1303. doi: 10.1152/ajplegacy.1968.214.6.1296. [DOI] [PubMed] [Google Scholar]
- Ishaq S.L., AlZahal O., Walker N., McBride B. An investigation into rumen fungal and protozoal diversity in three rumen fractions, during high-fiber or grain-induced sub-acute ruminal acidosis conditions, with or without active dry yeast supplementation. Front Microbiol. 2017;8:1943. doi: 10.3389/fmicb.2017.01943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J., Huang J., Zhou C., Tan Z. Taxonomic identification of ruminal epithelial bacterial diversity during rumen development in goats. Appl Environ Microbiol. 2015;81:3502–3509. doi: 10.1128/AEM.00203-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D., Zhao S., Wang P., Zheng N., Bu D., Beckers Y., Wang J. Insights into abundant rumen ureolytic bacterial community using rumen simulation system. Front Microbiol. 2016;7:1006. doi: 10.3389/fmicb.2016.01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn L., Cottle D. 1st ed. Csiro Publishing; Clayton: 2014. Beef cattle production and trade. [Google Scholar]
- Karst S.M., Ziels R.M., Kirkegaard R.H., Sørensen E.A., McDonald D., Zhu Q., et al. High-accuracy long-read amplicon sequences using unique molecular identifiers with nanopore or pacbio sequencing. Nat Methods. 2021;18:165–169. doi: 10.1038/s41592-020-01041-y. [DOI] [PubMed] [Google Scholar]
- Kenny D., Fitzsimons C., Waters S., McGee M. Invited review: improving feed efficiency of beef cattle–the current state of the art and future challenges. Animal. 2018;12:1815–1826. doi: 10.1017/S1751731118000976. [DOI] [PubMed] [Google Scholar]
- Kiani A. Temporal changes in plasma concentration of leptin, igf-1, insulin and metabolites under extended fasting and re-feeding conditions in growing lambs. Int J Endocrinol Metabol. 2013;11:34. doi: 10.5812/ijem.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P., Vejborg R.M., Hancock V. Prevention of bacterial adhesion. Appl Microbiol Biotechnol. 2010;88:451–459. doi: 10.1007/s00253-010-2805-y. [DOI] [PubMed] [Google Scholar]
- Kong R.S., Liang G., Chen Y., Stothard P., Guan L.L. Transcriptome profiling of the rumen epithelium of beef cattle differing in residual feed intake. BMC Genom. 2016;17:1–16. doi: 10.1186/s12864-016-2935-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lage C., Coelho S., Diniz Neto H., Malacco V., Rodrigues J., Sacramento J., et al. Relationship between feed efficiency indexes and thermography, blood, and ruminal parameters in pre-weaning dairy heifers. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S., Munro J., Zhou M., Guan L., Schenkel F., Steele M., et al. Associations of rumen parameters with feed efficiency and sampling routine in beef cattle. Animal. 2018;12:1442–1450. doi: 10.1017/S1751731117002750. [DOI] [PubMed] [Google Scholar]
- Langille M.G., Zaneveld J., Caporaso J.G., McDonald D., Knights D., Reyes J.A., et al. Predictive functional profiling of microbial communities using 16s rrna marker gene sequences. Nat Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavker R.M., Matoltsy A.G. Formation of horny cells: the fate of cell organelles and differentiation products in ruminal epithelium. J Cell Biol. 1970;44(3):501–512. doi: 10.1083/jcb.44.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.-R., Yang H., Lee S.-I., Haq I., Ock S.-A., Wi H., et al. Robust three-dimensional (3d) expansion of bovine intestinal organoids: an in vitro model as a potential alternative to an in vivo system. Animals. 2021;11:2115. doi: 10.3390/ani11072115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Zhou M., Adamowicz E., Basarab J.A. Characterization of bovine ruminal epithelial bacterial communities using 16s rrna sequencing, pcr-dgge, and qrt-pcr analysis. Vet Microbiol. 2012;155:72–80. doi: 10.1016/j.vetmic.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Li F., Guan L.L. Metatranscriptomic profiling reveals linkages between the active rumen microbiome and feed efficiency in beef cattle. Appl Environ Microbiol. 2017;83 doi: 10.1128/AEM.00061-17. e00061-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Gelsinger S., Edwards A., Riehle C., Koch D. Transcriptome analysis of rumen epithelium and meta-transcriptome analysis of rumen epimural microbial community in young calves with feed induced acidosis. Sci Rep. 2019;9:1–13. doi: 10.1038/s41598-019-40375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Li C., Chen Y., Liu J., Zhang C., Irving B., et al. Host genetics influence the rumen microbiota and heritable rumen microbial features associate with feed efficiency in cattle. Microbiome. 2019;7:1–17. doi: 10.1186/s40168-019-0699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Xie F., Sun D., Liu J., Zhu W., Mao S. Ruminal microbiome-host crosstalk stimulates the development of the ruminal epithelium in a lamb model. Microbiome. 2019;7:1–16. doi: 10.1186/s40168-019-0701-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J-h, Bian G-r, Zhu W-y, Mao S-y. High-grain feeding causes strong shifts in ruminal epithelial bacterial community and expression of toll-like receptor genes in goats. Front Microbiol. 2015;6:167. doi: 10.3389/fmicb.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Jh, Zhang Ml, Zhang Ry, Zhu Wy, Mao Sy. Comparative studies of the composition of bacterial microbiota associated with the ruminal content, ruminal epithelium and in the faeces of lactating dairy cows. Microb Biotechnol. 2016;9:257–268. doi: 10.1111/1751-7915.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes D.R.G., de Souza Duarte M., La Reau A.J., Chaves I.Z., de Oliveira Mendes T.A., Detmann E., et al. Assessing the relationship between the rumen microbiota and feed efficiency in nellore steers. J Anim Sci Biotechnol. 2021;12:1–17. doi: 10.1186/s40104-021-00599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Stumpff F., Deiner C., Rosendahl J., Braun H., Abdoun K., et al. Modulation of sheep ruminal urea transport by ammonia and ph. Am J Physiol Regul Integr Comp Physiol. 2014;307:R558–R570. doi: 10.1152/ajpregu.00107.2014. [DOI] [PubMed] [Google Scholar]
- Lu Z., Gui H., Yao L., Yan L., Martens H., Aschenbach J.R., et al. Short-chain fatty acids and acidic ph upregulate ut-b, gpr41, and gpr4 in rumen epithelial cells of goats. Am J Physiol Regul Integr Comp Physiol. 2015;308:R283–R293. doi: 10.1152/ajpregu.00323.2014. [DOI] [PubMed] [Google Scholar]
- Lu Z., Shen H., Shen Z. Effects of dietary-scfa on microbial protein synthesis and urinal urea-n excretion are related to microbiota diversity in rumen. Front Physiol. 2019;10:1079. doi: 10.3389/fphys.2019.01079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmuthuge N., Griebel P.J., Guan L.L. Taxonomic identification of commensal bacteria associated with the mucosa and digesta throughout the gastrointestinal tracts of preweaned calves. Appl Environ Microbiol. 2014;80:2021–2028. doi: 10.1128/AEM.03864-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmuthuge N., Liang G., Guan L.L. Regulation of rumen development in neonatal ruminants through microbial metagenomes and host transcriptomes. Genome Biol. 2019;20:1–16. doi: 10.1186/s13059-019-1786-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann E., Wetzels S.U., Wagner M., Zebeli Q., Schmitz-Esser S. Metatranscriptome sequencing reveals insights into the gene expression and functional potential of rumen wall bacteria. Front Microbiol. 2018;9:43. doi: 10.3389/fmicb.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns J., Boda J. Insulin release by acetate, propionate, butyrate, and glucose in lambs and adult sheep. Am J Physiol. 1967;212:747–755. doi: 10.1152/ajplegacy.1967.212.4.747. [DOI] [PubMed] [Google Scholar]
- Marini J., Van Amburgh M. Nitrogen metabolism and recycling in holstein heifers. J Anim Sci. 2003;81:545–552. doi: 10.2527/2003.812545x. [DOI] [PubMed] [Google Scholar]
- McCann J.C., Luan S., Cardoso F.C., Derakhshani H., Khafipour E., Loor J.J. Induction of subacute ruminal acidosis affects the ruminal microbiome and epithelium. Front Microbiol. 2016;7:701. doi: 10.3389/fmicb.2016.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowan R., Cheng K., Bailey C., Costerton J. Adhesion of bacteria to epithelial cell surfaces within the reticulo-rumen of cattle. Appl Environ Microbiol. 1978;35:149–155. doi: 10.1128/aem.35.1.149-155.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowan R., Cheng K., Costerton J. Adherent bacterial populations on the bovine rumen wall: distribution patterns of adherent bacteria. Appl Environ Microbiol. 1980;39:233–241. doi: 10.1128/aem.39.1.233-241.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell R., Hart K., Boland T., Kelly A., McGee M., Kenny D. Effect of divergence in phenotypic residual feed intake on methane emissions, ruminal fermentation, and apparent whole-tract digestibility of beef heifers across three contrasting diets. J Anim Sci. 2016;94:1179–1193. doi: 10.2527/jas.2015-0080. [DOI] [PubMed] [Google Scholar]
- Mead L.J., Jones G. Isolation and presumptive identification of adherent epithelial bacteria (“epimural” bacteria) from the ovine rumen wall. Appl Environ Microbiol. 1981;41:1020–1028. doi: 10.1128/aem.41.4.1020-1028.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer W., Schoennagel B., Kacza J., Busche R., Hornickel I.N., Hewicker-Trautwein M., et al. Keratinization of the esophageal epithelium of domesticated mammals. Acta Histochem. 2014;116:235–242. doi: 10.1016/j.acthis.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Montanholi Y., Fontoura A., Swanson K., Coomber B., Yamashiro S., Miller S. Small intestine histomorphometry of beef cattle with divergent feed efficiency. Acta Vet Scand. 2013;55:1–6. doi: 10.1186/1751-0147-55-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanholi Y.R., Haas L.S., Swanson K.C., Coomber B.L., Yamashiro S., Miller S.P. Liver morphometrics and metabolic blood profile across divergent phenotypes for feed efficiency in the bovine. Acta Vet Scand. 2017;59:1–11. doi: 10.1186/s13028-017-0292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer P.R., Smith T.P., Wells J.E., Kuehn L.A., Freetly H.C. Rumen microbiome from steers differing in feed efficiency. PLoS One. 2015;10:e0129174. doi: 10.1371/journal.pone.0129174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na S.W., Chun B.H., Beak S.-H., Khan S.A., Haque M.N., Lee J.S., et al. Pseudoprevotella muciniphila gen. Nov., sp. Nov., a mucin-degrading bacterium attached to the bovine rumen epithelium. PLoS One. 2021;16:e0251791. doi: 10.1371/journal.pone.0251791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento C., Branco R., Bonilha S., Cyrillo J., Negrão J., Mercadante M. Residual feed intake and blood variables in young nellore cattle. J Anim Sci. 2015;93:1318–1326. doi: 10.2527/jas.2014-8368. [DOI] [PubMed] [Google Scholar]
- Nkrumah J., Okine E., Mathison G., Schmid K., Li C., Basarab J., et al. Relationships of feedlot feed efficiency, performance, and feeding behavior with metabolic rate, methane production, and energy partitioning in beef cattle. J Anim Sci. 2006;84:145–153. doi: 10.2527/2006.841145x. [DOI] [PubMed] [Google Scholar]
- Nwaogu I., Ezeasor D. Morphological studies on rumen development in west african dwarf goats (capra hircus) Sokoto J Vet Sci. 2008;7(2):1–6. [Google Scholar]
- Paz H.A., Hales K.E., Wells J.E., Kuehn L.A., Freetly H.C., Berry E.D., et al. Rumen bacterial community structure impacts feed efficiency in beef cattle. J Anim Sci. 2018;96:1045–1058. doi: 10.1093/jas/skx081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner G.B., Aschenbach JrR., Gabel G., Rackwitz R., Oba M. Epithelial capacity for apical uptake of short chain fatty acids is a key determinant for intraruminal ph and the susceptibility to subacute ruminal acidosis in sheep. J Nutr. 2009;139:1714–1720. doi: 10.3945/jn.109.108506. [DOI] [PubMed] [Google Scholar]
- Penner G.B. 25th Florida ruminant nutrition symposium. 2014. Mechanisms of volatile fatty acid absorption and metabolism and maintenance of a stable rumen environment.https://animal.ifas.ufl.edu/apps/dairymedia/rns/2014/2014_proceedings.pdf [Google Scholar]
- Pérez–Barbería F., Elston D., Gordon I., Illius A. The evolution of phylogenetic differences in the efficiency of digestion in ruminants. Proc R Soc Lond B Biol Sci. 2004;271:1081–1090. doi: 10.1098/rspb.2004.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri R., Schwaiger T., Penner G., Beauchemin K., Forster R., McKinnon J., et al. Changes in the rumen epimural bacterial diversity of beef cattle as affected by diet and induced ruminal acidosis. Appl Environ Microbiol. 2013;79:3744–3755. doi: 10.1128/AEM.03983-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri R.M., Schwaiger T., Penner G.B., Beauchemin K.A., Forster R.J., McKinnon J.J., et al. Characterization of the core rumen microbiome in cattle during transition from forage to concentrate as well as during and after an acidotic challenge. PLoS One. 2013;8:e83424. doi: 10.1371/journal.pone.0083424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri R., Kleefisch M., Metzler-Zebeli B., Zebeli Q., Klevenhusen F. Changes in the rumen epithelial microbiota of cattle and host gene expression in response to alterations in dietary carbohydrate composition. Appl Environ Microbiol. 2018;84 doi: 10.1128/AEM.00384-18. e00384-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri R., Wetzels S., Qumar M., Khiaosa-Ard R., Zebeli Q. Adaptive responses in short-chain fatty acid absorption, gene expression, and bacterial community of the bovine rumen epithelium recovered from a continuous or transient high-grain feeding. J Dairy Sci. 2019;102:5361–5378. doi: 10.3168/jds.2018-15691. [DOI] [PubMed] [Google Scholar]
- Petri R.M., Neubauer V., Humer E., Kröger I., Reisinger N., Zebeli Q. Feed additives differentially impact the epimural microbiota and host epithelial gene expression of the bovine rumen fed diets rich in concentrates. Front Microbiol. 2020;11:119. doi: 10.3389/fmicb.2020.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S.A., Decker P. Comparative study of the urease in the rumen wall and rumen content. Nature. 1966;209:618–619. doi: 10.1038/209618b0. [DOI] [PubMed] [Google Scholar]
- Reisinger N., Wendner D., Schauerhuber N., Mayer E. Effect of lipopolysaccharides (lps) and lipoteichoic acid (lta) on the inflammatory response in rumen epithelial cells (rec) and the impact of lps on claw explants. Animals. 2021;11:2058. doi: 10.3390/ani11072058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remond D., Ortigues I., Jouany J.-P. Energy substrates for the rumen epithelium. Proc Nutr Soc. 1995;54:95–105. doi: 10.1079/pns19950040. [DOI] [PubMed] [Google Scholar]
- Richardson ECa, Herd R. Biological basis for variation in residual feed intake in beef cattle. 2. Synthesis of results following divergent selection. Aust J Exp Agric. 2004;44:431–440. [Google Scholar]
- Ritzhaupt A., Breves G., Schörder B., Winckler C.G., Shirazi-Beechey S.P. Urea transport in gastrointestinal tract of ruminants: effect of dietary nitrogen. Biochem Soc Trans. 1997;25:490S. doi: 10.1042/bst025490s. [DOI] [PubMed] [Google Scholar]
- Ritzhaupt A., Wood I.S., Jackson A.A., Moran B.J., Shirazi-Beechey S.P. Isolation of a rt-pcr fragment from human colon and sheep rumen rna with nucleotide sequence similarity to human and rat urea transporter isoforms. Biochem Soc Trans. 1998;26:S122. doi: 10.1042/bst026s122. [DOI] [PubMed] [Google Scholar]
- Røjen B., Poulsen S., Theil P., Fenton R., Kristensen N. Effects of dietary nitrogen concentration on messenger rna expression and protein abundance of urea transporter-b and aquaporins in ruminal papillae from lactating holstein cows. J Dairy Sci. 2011;94:2587–2591. doi: 10.3168/jds.2010-4073. [DOI] [PubMed] [Google Scholar]
- Sadet S., Martin C., Meunier B., Morgavi D. Pcr-dgge analysis reveals a distinct diversity in the bacterial population attached to the rumen epithelium. Animal. 2007;1:939–944. doi: 10.1017/S1751731107000304. [DOI] [PubMed] [Google Scholar]
- Sasaki N., Miyamoto K., Maslowski K.M., Ohno H., Kanai T., Sato T. Development of a scalable coculture system for gut anaerobes and human colon epithelium. Gastroenterology. 2020;159:388–390. doi: 10.1053/j.gastro.2020.03.021. [DOI] [PubMed] [Google Scholar]
- Sbardellati D., Fischer A., Cox M., Li W., Kalscheur K., Suen G. The bovine epimural microbiota displays compositional and structural heterogeneity across different ruminal locations. J Dairy Sci. 2020;103:3636–3647. doi: 10.3168/jds.2019-17649. [DOI] [PubMed] [Google Scholar]
- Schurmann B. University of Saskatchewan; 2013. Functional adaptation of the rumen epithelium. [Master degree thesis dissertation] [Google Scholar]
- Seddik H., Xu L., Wang Y., Mao S. A rapid shift to high-grain diet results in dynamic changes in rumen epimural microbiome in sheep. Animal. 2019;13:1614–1622. doi: 10.1017/S1751731118003269. [DOI] [PubMed] [Google Scholar]
- Semjén G., Gálfi P. Factors influencing the adherence of strains of streptococcus bovis and escherichia coli isolated from ruminal epithelium. Vet Res Commun. 1990;14:181–191. doi: 10.1007/BF00347736. [DOI] [PubMed] [Google Scholar]
- Sharma V., Kundu S., Datt C., Prusty S., Kumar M., Sontakke U. Buffalo heifers selected for lower residual feed intake have lower feed intake, better dietary nitrogen utilisation and reduced enteric methane production. J Anim Physiol Anim Nutr. 2018;102:e607–e614. doi: 10.1111/jpn.12802. [DOI] [PubMed] [Google Scholar]
- Steven D., Marshall A. In: Physiology of digestion and metabolism in the ruminant. Phillipson A.T., editor. Oriel Press; Newcastle upon Tyne: 1970. Organization of the rumen epithelium. [Google Scholar]
- Stewart G., Graham C., Cattell S., Smith T., Simmons N., Smith C. UT-B is expressed in bovine rumen: potential role in ruminal urea transport. Am J Physiol Regul Integr Comp Physiol. 2005;289:R605–R612. doi: 10.1152/ajpregu.00127.2005. [DOI] [PubMed] [Google Scholar]
- Stewart R.D., Auffret M.D., Warr A., Walker A.W., Roehe R., Watson M. Compendium of 4,941 rumen metagenome-assembled genomes for rumen microbiome biology and enzyme discovery. Nat Biotechnol. 2019;37:953–961. doi: 10.1038/s41587-019-0202-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stick D., Davis M., Loerch S., Simmen R. Relationship between blood serum insulin-like growth factor i concentration and postweaning feed efficiency of crossbred cattle at three levels of dietary intake. J Anim Sci. 1998;76:498–505. doi: 10.2527/1998.762498x. [DOI] [PubMed] [Google Scholar]
- Stumpff F., Georgi M.-I., Mundhenk L., Rabbani I., Fromm M., Martens H., et al. Sheep rumen and omasum primary cultures and source epithelia: barrier function aligns with expression of tight junction proteins. J Exp Biol. 2011;214:2871–2882. doi: 10.1242/jeb.055582. [DOI] [PubMed] [Google Scholar]
- Styriak I., Gálfi P., Kmet V. Adherence of ruminal streptococcus bovis and lactobacillus strains to primary and secondary cultures of rumen epithelium. Acta Microbiol Hung. 1992;39:323–325. [PubMed] [Google Scholar]
- Štyriak I., Galfi P., Kmet V. The adherence of three streptococcus bovis strains to cells of rumen epithelium primoculture under various conditions. Arch Anim Nutr. 1994;46:357–365. doi: 10.1080/17450399409381786. [DOI] [PubMed] [Google Scholar]
- Suárez B., Van Reenen C., Stockhofe N., Dijkstra J., Gerrits W. Effect of roughage source and roughage to concentrate ratio on animal performance and rumen development in veal calves. J Dairy Sci. 2007;90:2390–2403. doi: 10.3168/jds.2006-524. [DOI] [PubMed] [Google Scholar]
- Sun D., Mao S., Zhu W., Liu J. Effect of starter diet supplementation on rumen epithelial morphology and expression of genes involved in cell proliferation and metabolism in pre-weaned lambs. Animal. 2018;12:2274–2283. doi: 10.1017/S1751731118000290. [DOI] [PubMed] [Google Scholar]
- Sun D., Yin Y., Guo C., Liu L., Mao S., Zhu W., et al. Transcriptomic analysis reveals the molecular mechanisms of rumen wall morphological and functional development induced by different solid diet introduction in a lamb model. J Anim Sci Biotechnol. 2021;12:1–15. doi: 10.1186/s40104-021-00556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan R.S., Zhou M., Li F. Identifying active rumen epithelial associated bacteria and archaea in beef cattle divergent in feed efficiency using total rna-seq. Curr Res Microb Sci. 2021;2:100064. doi: 10.1016/j.crmicr.2021.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J., Kerley M., Schnabel R., Pomp D., Garrick D., Hansen S.L., et al. National program for genetic improvement of feed efficiency in beef cattle. 2016. https://digitalcommons.unl.edu/animalscifacpub/907/
- Trabi E.B., Seddik H-e, Xie F., Wang X., Liu J., Mao S. Effect of pelleted high-grain total mixed ration on rumen morphology, epithelium-associated microbiota and gene expression of proinflammatory cytokines and tight junction proteins in hu sheep. Anim Feed Sci Technol. 2020;263:114453. [Google Scholar]
- Usda F. United States Department of Agriculture. Foreign Agriculture Service; July 2021. Livestock and poultry: world markets and trade.https://usda.library.cornell.edu/concern/publications/73666448x?locale=en [Google Scholar]
- Vijay J., Gauthier M.-F., Biswell R.L., Louiselle D.A., Johnston J.J., Cheung W.A., et al. Single-cell analysis of human adipose tissue identifies depot-and disease-specific cell types. Nat Metab. 2020;2:97–109. doi: 10.1038/s42255-019-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. The mechanism of passage of endogenous urea through the rumen wall and the role of ureolytic epithelial bacteria in the urea flux. Br J Nutr. 1979;42:553–557. doi: 10.1079/bjn19790147. [DOI] [PubMed] [Google Scholar]
- Wallace R., Cheng K.-J., Dinsdale D., Ørskov E. An independent microbial flora of the epithelium and its role in the ecomicrobiology of the rumen. Nature. 1979;279:424–426. doi: 10.1038/279424a0. [DOI] [PubMed] [Google Scholar]
- Walpole M., Schurmann B., Górka P., Penner G., Loewen M., Mutsvangwa T. Serosal-to-mucosal urea flux across the isolated ruminal epithelium is mediated via urea transporter-b and aquaporins when holstein calves are abruptly changed to a moderately fermentable diet. J Dairy Sci. 2015;98:1204–1213. doi: 10.3168/jds.2014-8757. [DOI] [PubMed] [Google Scholar]
- Wetzels S., Mann E., Metzler-Zebeli B., Wagner M., Klevenhusen F., Zebeli Q., et al. Pyrosequencing reveals shifts in the bacterial epimural community relative to dietary concentrate amount in goats. J Dairy Sci. 2015;98:5572–5587. doi: 10.3168/jds.2014-9166. [DOI] [PubMed] [Google Scholar]
- Wozny M., Bryant M., Holdeman Lt, Moore W. Urease assay and urease-producing species of anaerobes in the bovine rumen and human feces. Appl Environ Microbiol. 1977;33:1097–1104. doi: 10.1128/aem.33.5.1097-1104.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J.Y., Groer M., Dutra S.V.O., Sarkar A., McSkimming D.I. Gut microbiota and immune system interactions. Microorganisms. 2020;8:1587. doi: 10.3390/microorganisms8101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan K., Gong X., Chen Y., Jiang M., Yang T., Zhao G. Short-chain fatty acids regulate the immune responses via g protein-coupled receptor 41 in bovine rumen epithelial cells. Front Immunol. 2019;10:2042. doi: 10.3389/fimmu.2019.02042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Ye H., Liu J., Mao S. High-grain diets altered rumen fermentation and epithelial bacterial community and resulted in rumen epithelial injuries of goats. Appl Microbiol Biotechnol. 2017;101:6981–6992. doi: 10.1007/s00253-017-8427-x. [DOI] [PubMed] [Google Scholar]
- Zhao K., Chen Y., Penner G., Oba M., Guan L. Transcriptome analysis of ruminal epithelia revealed potential regulatory mechanisms involved in host adaptation to gradual high fermentable dietary transition in beef cattle. BMC Genom. 2017;18:1–17. doi: 10.1186/s12864-017-4317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]