Highlights
-
•
Lactic fermentation was used as unconventional way to valorise black soldier fly prepupae and by-products.
-
•
Black soldier fly biomasses (prepupae, pupuria and adult flies) were characterized before and after fermentation.
-
•
LAB fermentation modified insect protein and lipid but not chitin fraction.
-
•
Fermentation improved BSF biomasses in terms of unsaturated fatty acids and essential amino acids.
Keywords: Black soldier flies, Insect by-products, Puparia, Fermentation, Lactic acid bacteria, Nutritional composition
Abstract
Black soldier fly (BSF) is being increasingly used for agro-food by-products valorisation. Adult flies, puparia, and excess of prepupae are the by-products of this process, which could be further valorised. Lactic fermentation of BSF biomasses with two different strains (L. rhamnosus and L. plantarum) has been used for this purpose.
Deep changes in the molecular composition were observed, without significant differences related to the different strains used. The lipid and protein fractions were the most impacted. Fermentation enriched the biomass in monounsaturated and polyunsaturated fatty acids and essential amino acids, significantly improving the nutritional properties of the substrates. Although not particularly marked, a proteolytic activity of lactobacilli was observed on the BSF muscular and cuticular proteins, especially in the samples of adult flies and puparia, where fermentation resulted more effective. Conversely, there was no evidence of chitinolytic activity.
1. Introduction
Insects have been demonstrated to be important bioconversion tools to valorise agro-industrial by-products (Barbi et al., 2020, Salomone et al., 2017). They grow and reproduce easily on many organic wastes, and numerous species are able to implement a bioconversion mechanism on organic residues to obtain a biomass rich in particular of proteins and lipids (Oonincx & de Boer, 2012). Hermetia illucens, also called black soldier flies (BSF), is one of the insect species most commonly employed in bioconversion of underutilized agro-food by-products into high nutritional biomasses. BSF can be used as feed ingredients thanks to the optimal nutritional composition, particularly abundant in lipids and essential amino acids, which make it very similar to common feeds as soybean and fishmeal (De Smet et al., 2018). Processed proteins from farmed edible insects, including BSF, were already permitted by the European Commission for aquaculture (Reg. 2017/893/UE). Commission Regulation (UE) 2021/1372 also allowed proteins derived from insects for feeding chickens and pigs. BSF larvae are also used in pet food (Wang & Shelomi, 2017). BSF is also considered one of the insects’ species with the greatest potential to be used also as food according to the Novel Foods Regulation (European Council Regulations No. 2015/2283, 2015).
As a result of the wide spectrum of potential applications, an intensification of BSF rearing on a large scale in the last years has been observed. Despite BSF bioconversion ability has been the object of a lot of studies (Rumpold & Schlüter, 2013), there is a lack of information related to the potential environmental impact that a large-scale insect-rearing might cause. These data would be of particular importance to correctly quantify the environmental benefits of intensive insect rearing as alternative solutions for food waste management and as response to the rising global demand of food and feed. It is estimated that from 10 tonnes of food-waste, approximately 300 kg of dried larvae and 3,346 kg of compost (frass) are produced (Salomone et al., 2017). However, in an industrial mass rearing of BSF, besides frass, other by-products are generated as puparia, which are the shells left after the adult emergence, and the bodies of the adult flies after mating and egg laying. In this sense, there is the need of specific studies to better understand the molecular composition of insect by-products and to exploit further strategies to valorise also these biomasses, in the optic of a perfect circular economy. Moreover, due to the very high growth factor and the small life cycle of BSF, its large-scale breeding can generate an excess of larval/prepupal biomasses compared to the market demand that need to be further transformed and valorised.
Several studies reported that one possible strategy to add value to food by-products is solid state fermentation (SSF) (Hadj Saadoun et al., 2021, Sadh et al., 2018, Wang and Shelomi, 2017, Thomas et al., 2013). Among the bacterial species, Lactic Acid Bacteria (LAB) are the most used for organic biomass fermentation, and thus exploited for industrial bioprocesses (Koutsoumanis et al., 2020). Their adaptation to specific substrates leads to the production of organic acids, volatile fatty acids, antibiotics, bioactive peptides, etc. The acid environment generated during the fermentation facilitates the LAB endogenous hydrolytic enzymes, acting on lipids, proteins, polysaccharides of the starting food matrix, increasing their bioavailability and generating new bioactive compounds. The ability of LAB to produce organic acids and microbial and fungal inhibitory metabolites brings tangible advantages, among others the possibility of using the fermented biomasses as natural antimicrobials (Chelule et al., 2010). As a consequence, LAB fermentation can potentially give an industrial-scale interest to an unutilized biomass, such as BSF bioconversion waste, supporting the circular economy strategy (Sabater et al., 2020).
In a previous work, a LAB fermentation by using two different strains, Lacticaseiobacillus rhamnosus (1473) and Lactiplantiobacillus plantarum (285), was applied as a method to confer antimicrobial activity to BSF prepupae and derived biomasses. A preliminary compositional analysis of the BSF biomasses after fermentation suggested some nutrients modifications (Hadj Saadoun et al., 2020). In this work the molecular composition of BSF and their derived waste before and after fermentation was deeply explored and fermentation was proposed as a method to further enhance these unconventional biomasses.
2. Materials and methods
2.1. Prepupae and derived by-products samples
The BSF prepupae and derived biomasses (puparia and adult flies) were kindly provided by the Laboratory of Applied Entomology – BIOGEST-SITEIA, Department of Life Science, University of Modena and Reggio Emilia, Reggio Emilia (RE), Italy. The breeding conditions of BSF larvae until they reach the prepupae stage have been previously reported (Hadj Saadoun et al., 2020). Briefly, larvae were reared at 27 °C and 70% relative humidity and fed at libitum with the Gainesville Housefly diet (50% wheat bran, 30% alfalfa meal and 20% corn meal). Prepupae were killed by freezing and kept at −20 °C until chemical analysis. Puparia and dead flies were also collected and kept in falcon vials at −20 °C.
2.2. Fermentation
Fermentation of BSF samples was carried out as reported by Hadj Saadoun et al. (2020). Briefly, Lacticaseibacillus rhamnosus 1473 isolated from Parmigiano Reggiano cheese, and Lactiplantibacillus plantarum 285, isolated from Brazilian cheese, were chosen for fermentation. Both the strains belonged to the collection of the Department of Food and Drug (University of Parma, Parma, Italy). The strains were maintained at −80 °C in De Man, Rogosa, and Sharpe (MRS) broth (Oxoid, Basingstoke, UK) supplemented with 12.5% glycerol (v/v). Finely ground prepupae were combined with sugar (8.5% w/w), while BSF Puparia and adults’ powders with sugar (8.5% w/w) and water (70% v/w). All the samples were then sterilized in autoclave at 121° for 20 min in a glass jar. Then, 30 g of each BSF substrates were incubated with a final concentration of 7 Log CFU/g of each bacterial suspension for 72 h at 30 °C for L. plantarum 285 and at 37 °C for L. rhamnosus 1473. The sample obtained and the sample codes used thorough the manuscript are schematized below:
| Unfermented | fermented with L. rhamnosus 1473 |
fermented with L. plantarum 285 |
|
|---|---|---|---|
| BSF Adult | AD_Unf | AD_1473 | AD_285 |
| BSF Puparia | PUP_Unf | PUP_1473 | PUP_285 |
| BSF Prepupa | PRE_Unf | PRE_1473 | PRE_285 |
2.3. Experimental setup and statistical analysis
In this work both unfermented and fermented BSF prepupae and derived biomasses were analysed in terms of proximate composition and more detailed analysis on protein (total amino acids, free amino acids, peptides, degree of hydrolysis), lipid (fatty acid profile, lipid classes distribution) and chitin fractions (total chitin and chitooligosaccharides). Each fermentation was performed in duplicate and each samples was analysed in duplicate. Results are as a consequence expressed as mean ± standard deviation calculated on four determinations. Only for the peptides identification the analysis was carried out in duplicate. Data were subjected to one-way analysis of variance (ANOVA) followed by a Tukey post hoc test using IBM SPSS software version 21.0 (SPSS Inc., Chicago, IL, USA) to determine differences between unfermented and fermented samples. Significant differences were compared at a level of p < 0.05.
2.4. Proximate composition
Standard procedures (AOAC, 2002) were used to test moisture, lipid and ash composition of BSF biomasses before and after fermentation. Moisture was determined in the oven at 105 °C for 24 h. An automatized Soxhlet extractor (SER 148/3 VELP SCIENTIFICA, Usmate Velate, Italy) was used to determine crude fat, extracted using diethyl ether as solvent. Total ash was determined after mineralization at 550 °C for a total time of 10 h (5 h + 5 h). Due to the contemporary presence of chitin and protein, total proteins cannot be determined by Kjeldhal standard method, and chitin cannot be determined by official gravimetric methods for dietary fibre. As a consequence, they were determined simultaneously as reported in Paragraph 2.5, from the sum of total amino acids and glucosamine (GlcN) released from these two macromolecules after acid hydrolysis, Multiplying the moles of each amino acid and glucosamine for their residual molecular mass (molecular mass of amino acids and glucosamine subtracted by the molecular mass of water).
2.5. Proteins and chitin fractions characterization
2.5.1. Total amino acids and chitin determination
Samples (0.5 g) were hydrolysed with 6 mL of HCl 6 N at 110 °C for 23 h. At the end of hydrolysis, 7.5 mL of 5 mM Norleucine in HCL 0.1 N, used as internal standard for total amino acid determination, was added. After a filtration in Buckner, the samples were brought up to a volume of 100 mL with deionized water. Therefore, 8 µl Galactosamine 46 mM, used as internal standard for glucosamine determination, were added to 450 µl of hydrolysate solution. The solution was adjusted to 500 µl with deionized water.
Only for the analysis of cysteine, determined as cysteic acid, the acid hydrolysis was preceded by performic acid oxidation, following the procedure described by Caligiani et al. (2018). Tryptophan analysis was carried out separately by alkaline hydrolysis, according to the method described by Caligiani et al. (2018).
All the hydrolysed samples were analysed by UPLC/ESI-MS after derivatization with reconstituted AccQ Tag reagent (Waters Co., Milford, U.S.A.) according to the method described by Leni et al. (2020). UPLC/ESI-MS analysis was performed by using an ACQUITY UPLC separation system with an Acquity BEH C18 column (1.7 µm, 2.1 × 150 mm). The mobile phase was composed by H2O + 0.2% CH3CN + 0.1% HCOOH (eluent A) and CH3CN + 0.1% HCOOH (eluent B). Gradient elution was performed: isocratic 100% A for 1.8 min, from 100% A to 50% A by a linear gradient in 11.4 min and 0.8 min at 50% A plus washing step at 0% A (100% B) and reconditioning. The flow rate was set at 0.25 mL/min, injection volume 2 μl, column temperature 35 °C and sample temperature 23 °C. Detection was performed by using Waters SQ mass spectrometer: ESI source in positive ionization mode, capillary voltage 3.2 kV, cone voltage 30 V, source temperature 150 °C, desolvation temperature 300 °C, cone gas flow (N2): 100 L/h, desolvation gas flow (N2): 650 L/h, full scan acquisition (100–2000 m/z), scan duration 1 s.
Calibration for total amino acids was performed with a standard solution prepared mixing 133.3 µl of Norleucine (5 mM), 133.3 µl of amino acids hydrolysate standard mixture (2.5 mM), 133.3 µl of cysteic acid in HCL 0.1 N (2.5 mM), and 100 µl of deionized water. For chitin determination, calibration was performed by using five different concentrations, ranging from 1.5 mM to 0,1 mM of a standard solution prepared by mixing a commercial chitin (external standard) subjected to hydrolysis with the selected internal standard (galactosamine), both at 2,5 mM as final concentration.
2.5.2. Free amino acids profile determination
In addition to the total amino acid analysis, free amino acids profile was evaluated following the procedure described by Leni et al. (2020). Samples were analysed by UPLC/ESI-MS after derivatization following the conditions described in paragraph 2.5.1. Quantification was performed against a set of standard solutions.
2.5.3. Determination of the protein degree of hydrolysis
The percentage of peptide bonds cleaved with respect to the total number of peptide bonds is defined as degree of hydrolysis (DH). The protein DH was calculated using o-phthaldialdehyde (OPA) method described by Spellman McEvoy, O’Cuinn, and FitzGerald (2003), with some modification (Spellman et al., 2003). It exploits the formation of an isoindole spectroscopically quantifiable at 340 nm. 100 mL of OPA/NAC (N-acetylcysteine) reagent was prepared by combining 10 mL of 50 mM OPA (in methanol) and 10 mL of NAC 50 mM (in methanol), 5 mL of 20% (w/v) SDS, and 75 mL of borate buffer (0.1 M, pH 9.5). The reagent was kept in dark and stirred for at least 1 h before use. The OPA assay was carried out by the addition of 20 μl of sample (or standard) to 2.4 mL of OPA/NAC reagent in a 5 mL of a plastic Eppendorf. Before the analysis, the samples were centrifuged at room temperature for 10 min at 1000 rpm. The absorbance of this solution was measured at 340 nm with JASCO B-530 UV–Vis-spectrophotometer (JASCO, Oklahoma City, OK, U.S.A) against a control cell containing the reagent plus 20 μl of borate buffer. The intrinsic absorbance of the sample was measured before OPA addition and subtracted. A standard curve was prepared using isoleucine (0–2 mg mL − 1). The degree of hydrolysis (DH) was calculated as the ration between the free nitrogen groups after hydrolysis and the total nitrogen groups: DH% = (N free/N total) × 100.
2.5.4. Determination of oligopeptides by UPLC-ESI/MS
To evaluate the integrity of the proteins and to identify the peptides potentially generated after fermentation, an extraction of the water-soluble compounds was performed following the method described by Bottari et al. (2017), with some modifications. 4,5 mL of HCl 0.1 M were added to one gram of grinded samples of BSF prepupae, puparia and adults. Samples were homogenized for 1 min using an UltraTurrax (IKA T50 basic, Staufen, Germany). 0.25 mL of phenylalanyl-phenylalanine (Phe-Phe) 1 mM in water was added to each sample and used as internal standard. Acid conditions generated a precipitation of the insoluble proteins, removed by centrifugation (30 min at 4 °C at 3900 rpm). The supernatant containing the water-soluble compounds was filtered through paper filter and extracted three times with diethyl ether to remove the fat component. Ether residues were removed with a rotavapor, and the residual solutions were centrifuged again for 15 min at 4 °C at 3900 rpm. Subsequently 1,5 mL of supernatant was collected and stored at −20 °C until use. Samples were separated by a reverse phase column (Acquity UPLC BEH 300 C18, 1.7 μm, 2.1 × 150 mm equipped with a Acquity UPLC BEH C18 VanGuard Pre-column, 300 Å, 1.7 μm, 2.1 × 5 mm, Waters) in an UPLC system coupled with ESI- MS (UPLC Acquity with a single quadrupole detector SQD, Waters). Elution was performed with the following gradient of eluent A (water with 0.1% formic acid and 0.2% acetonitrile) and eluent B (acetonitrile with 0.1% formic acid): 0–7 min, 100% A; 7–50 min, from 100% A to 50% A; 50–52.6 min, 50% A; 52.6–53 min, from 50% A to 0% A; 53–58.2 min, 0% A; 58.2–59 min, from 0% A to 100% A; 59–72 min, 100% A. The UPLC-ESI/MS parameters were: flow 0.2 mL/min; analysis time 72 min; column temperature 35 °C; sample temperature 18 °C; injection volume 10 μl for water soluble extracts; acquisition time 0–58.2 min; ionization type: positive ion mode; capillary voltage 3.2 kV; cone voltage 30 V; source temperature 150 °C; desolvation temperature 350 °C; cone gas flow 100 L/h; desolvation gas flow 650 L/h. Samples were analyzed in the Full Scan mode, with a scan range of 100–2000 m/z. The ions of interest, corresponding to oligopeptides were integrated using MassLynx software (4.0) and semi-quantified using Phe-Phe area. The ratio between the chromatographic peak of the peptide and that of Phe-Phe did not yield absolute peptide concentration but allowed the comparison of the same peptide in different samples.
2.5.5. Peptides identification by high resolution mass spectrometry on LTQ-Orbitrap instrument
The same peptides extracts obtained in paragraph 2.5.5 were dried and reconstituted with 50 μl of 0.2% formic acid solution for HRMS analysis, using a μHPLC DIONEX Ultimate3000 interfaced with an LTQ-Orbitrap XL Thermo Fisher Scientific. Column: Jupiter C18 4 μ, Proteo 90 Å 150 × 0.30 mm, Phenomenex; eluent A: water + 0.1% formic acid; eluent B: acetonitrile + 0.1% formic acid; flow: 5 μl/min, gradient: 0–4 min from 100% A to 95% A, 4–60 min from 95% A to 50% A, 60–62 min from 50% A to 10% A, 62–72 min 10% A, 72–74 min from 10% A to 95% A, 74–90 min 95% A; analysis time (min): 90; column temperature (°C): 30; injection volume (μL): 5; acquisition time (min): 0–75; ionization mode: ESI+; scan range (m/z): 200–1800; source voltage (kV): 3.5; capillary voltage (kV): 35; source temperature (°C): 275. Scan event details: (Fourier transform) FTMS + p res = 30,000 or (250.0–2000.0); (ion trap) ITMS+c Dep MS/MS Most intense ion form; activation type: CID; isolation width: 2.00; normalized coll. energy: 35.0; default charge state: 2; activation Q: 0.250; activation time: 30.000; dynamic exclusion enabled; repeat count: 2; repeat duration (s): 10.00; exclusion duration (s): 30.00. Charge state rejection: enabled; unassigned charge states: rejected; charge state 1: rejected; charge state 2: not rejected; charge state 3: not rejected; charge states 4+: not rejected; ion signal threshold: 10,000. Protein identification was performed by using PEAKS software (Bioinformatics Solutions Inc) and INSECTA (UniProt) database. Positive hits for protein identification were arbitrarily set for all those proteins identified by the program with a score (expressed as −10lgP) > 50, all those peptides with a score (−10lgP) > 20 and ppm in the range ±6, since such value should reduce the risk of false positives to zero.
2.5.6. Determination of chitooligosaccharides by UPLC-ESI/MS
A deeper study of the integrity of chitin fraction related to the possible formation of chitin oligosaccharides in BSF insect samples from fermentation process was carried out following an extraction procedure based on the protocol described by Kim et al (2003), with some modifications. Briefly, 10 mL of 10% aqueous solution of ethanol was added to 1 g of BSF samples. The mixture was stirred at 50 °C for 1 h and centrifuged at 3900 rpm for 30 min at 20 °C. Finally, 1 mL of the supernatant was collected, evaporated under nitrogen, and redissolved in a solution of distilled water/acetonitrile 1:1. 400 µl were sampled for the analysis of the potentially extracted oligosaccharides from insect material. Chitin oligomers content in the extracts were checked by using an UPLC/ESI-MS analysis (ACQUITY UPLC® BEH Amide column (2.1 × 100 mm, 1.7 µm). The mobile phase was composed by 80% CH3CN + 20% H2O + 0.1% NH4OH (eluent A) and 30% CH3CN + 70% H2O + 0.1% NH4OH (eluent B). Gradient elution was performed: from 100% A to 40% A and 60% B by linear gradient in the first 10 min, from 40% to 100% A in 0.02 min, isocratic 100% A from 10.02 to 30 min. Flow rate was set at 0.17 mL/min, injection volume 2 μl, strong needle wash 20% CH3CN + 80% H2O, weak needle wash 75% CH3CN + 25% H2O, seal wash 50% CH3CN + 50% H2O, column temperature 35 °C and sample temperature 18 °C. Detection was performed by using Waters SQ mass spectrometer: ESI source in negative ionization mode, capillary voltage 2.8 kV, cone voltage 25 V, source temperature 120 °C, desolvation temperature 350 °C, cone gas flow (N2) 50 L/hr, desolvation gas flow (N2) 500 L/hr, full scan acquisition (100–2000 m/z).
2.6. Characterization of the lipid fraction
2.6.1. Determination of fatty acids profile by GC–MS
The determination of fatty acids profile was carried out on the crude fat extracted using the Soxhlet extraction technique (Section 2.4), as described in the article of Hadj Saadoun et al. (2020), by using a Thermo Scientific Trace 1300 gas-chromatograph (Thermo Scientific, Waltham, Massachusetts, USA) and a Supelcowax ms capillary column (30 m, i.d 25 mm, Supelco, Bellafonte, USA) coupled to a Thermo Scientific Trace ISQ mass spectrometer (Thermo Scientific, Waltham, Massachusetts, USA). The carrier gas was helium (1 mL/min), injector and detector temperatures were kept at 250 °C, while oven temperature was programmed from 80 to 240 °C at 20 °C/min. The content of every single fatty acid was calculated against the concentration of the internal standard, after calculating the response factors using the Supelco® 37 Component FAME Mix (Sigma Aldrich, Saint Louis, MO, USA). Results were expressed as percentage of each fatty acid respect to the sum of total fatty acids (g/100 g).
2.6.2. 1H NMR lipidomics
Distribution of the lipid classes was performed by 1H NMR according to Caligiani et al. (2019). NMR spectra were registered on a Bruker Avance III 400 MHz NMR Spectrometer (Bruker BioSpin, Rheinstetten, Karlsruhe, Germany) operating at a magnetic field-strength of 9.4 T.
2.6.3. Determination of monoglycerides of lauric acid by GC–MS
30 mg of BSF oil were mixed with 0,5 mL of DMF, 0,5 mL of hexamethyldisilazane and 0,3 mL of chlorotrimethylsilane. The mixture was heated for 30 min and then extracted with 4 mL of hexane. Hexane solutions were split injected (1 µl) on an Agilent 7820A gas-chromatograph (Agilent Technologies, Inc., Shanghai, China) carrying a Supelco SLB-5 capillary column (30 m, i.d 25 mm, Supelco, Bellafonte, USA) coupled to an Agilent 5977B single quadrupole mass spectrometer (Agilent Technologies, Inc., Shanghai, China). The carrier gas was helium (1 mL/min), injector and detector temperatures were kept at 280 °C, while oven temperature was programmed from 60 to 280 °C at 20 °C/min. The qualitative presence of monoglyceride of lauric acid was determined by extracting its specific molecular mass.
3. Results
Fermentation of the BSF prepupae and derived biomasses was carried out maintaining the same conditions used by Hadj Saadoun et al. (2020). In the present work, LAB strains confirmed the growth abilities on the different substrates previously observed. L. plantarum (285) was able to grow on all the insect substrates. L. rhamnosus (1473) showed the best growth capacity on puparia and on adult sample and in both cases, after 72 h of incubation, an average increase of 2.5 Log CFU/g was achieved. On the contrary, the use of BSF prepupae as fermentation substrate induced a reduction of average 3 Log CFU/g for L. rhamnosus.
3.1. Proximate composition
The molecular changes induced by the fermentation were firstly studied in terms of proximate composition. Results of moisture, protein, crude fat, chitin, and ash analyses are reported in Table 1. The starting insect biomasses (unfermented samples) have different composition, being BSF adults and prepupae richer in lipids and protein as compared to puparia and the latter the richest in chitin. Focusing on the differences before and after fermentation, in general no substantial modifications in the proximate composition occurred. Regarding lipid fraction, the only significative difference is related to the adult sample fermented with L. plantarum, where the lipid content resulted slightly lower respect to the unfermented sample. For the protein fraction no significant differences were found in any samples before and after fermentation, independently by the LAB strain utilized. Chitin content remains generally constant after fermentation, with the only significant difference in the chitin value found in puparia fermented by L. rhamnosus (22.0 ± 0.3%) respect that one in the unfermented puparia (23.8 ± 0.3%). These three main fractions were the object of more detailed molecular analysis, as reported in the following paragraphs.
Table 1.
Proximate composition of unfermented and fermented BSF adults, puparia, and prepupae with two different LAB strains *Significance (<0,05) of each fermented sample respect its correspondent unfermented material (t-test comparison). **Values are expressed on dry matter basis (sugar free) and are the result of four replicate analysis.
| Composition (%) | AD_Unf | AD_1473 | AD_285 | PUP_Unf | PUP_1473 | PUP_285 | PRE_Unf | PRE_1473 | PRE_285 |
|---|---|---|---|---|---|---|---|---|---|
| Lipid (Soxhlet)** | 25,8 ± 0,5 | 26,2 ± 0,4 | 24,2 ± 0,6 * | 12,5 ± 1,8 | 12,9 ± 0,2 | 8,8 ± 0,7 | 23,6 ± 0,4 | 17,9 ± 0,3 | 25,6 ± 0,9 |
| Proteins, from | 59,6 ± 1,8 | 59,9 ± 0,5 | 61,0 ± 0,2 | 39,5 ± 2,1 | 38,5 ± 0,1 | 36,4 ± 0,5 | 59,6 ± 1,0 | 61,5 ± 0,3 | 55,4 ± 0,6 |
| total AA(UPLC/ESI-MS)** | |||||||||
| Chitin (UPLC/ESI-MS | 8,3 ± 0,2 | 8,7 ± 0,1 | 8,17 ± 0,01 | 23,8 ± 0,3 | 22,0 ± 0,3* | 26,0 ± 0,5 | 10,0 ± 0,4 | 9,3 ± 0,3 | 8,9 ± 0,6 |
| Determination of Glucosamine)** | |||||||||
| Ash (Oven 550 °C)** | 6,1 ± 0,1 | 4,9 ± 0,1 * | 6,5 ± 0,1 | 23,9 ± 0,1 | 26,4 ± 0,3 | 28,5 ± 0,1 | 6,6 ± 0,0 | 11,1 ± 0,1 * | 9,8 ± 0,1 * |
3.2. BSF protein and chitin fraction
Despite the substantially unchanged total protein content before and after fermentation, preliminary results suggested some modifications in amino acid profile (Hadj Saadoun et al., 2020). To confirm these data here the complete amino acid profile of BSF samples before and after fermentation was determined. In addition, to better understand the modification of the protein fraction at molecular level, a detailed evaluation of the proteolytic activity by means of determination of free amino acids, degree of hydrolysis and oligopeptides was performed. Moreover, chitinolytic activity was also determined through the quantification of chitooligosaccharides.
3.2.1. Variations of total amino acid profile after fermentation
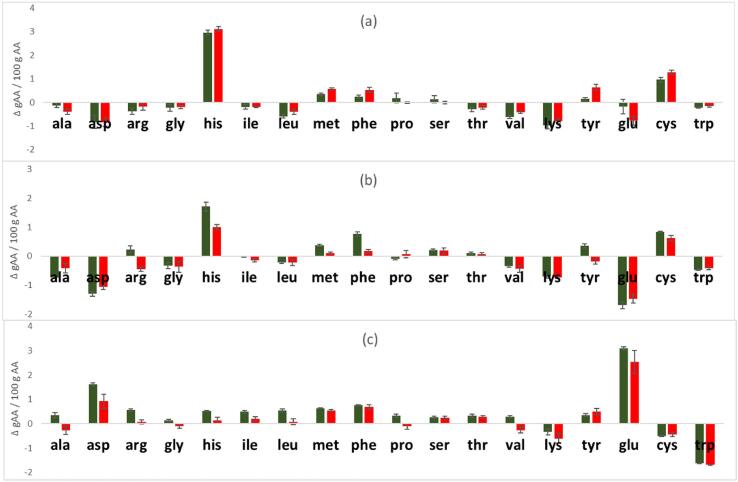
Complete data on the total amino acid composition of insect samples are reported in Table S2 of the supplementary material. The variation in the total amino acid distribution before and after fermentation is reported in Fig. 1, where the graph baseline represents the amino acid content of the unfermented samples expressed in g AA/100 g AA and the bars indicated how total amino acid composition changes after fermentation with the two different LAB strains. Many changes in amino acid distribution were observed depending primarily on the substrate of fermentation, with only slight effects that can be attributed to the different LAB strains. In fact, as it is possible to see from the Fig. 1, the L. rhamnosus and L. plantarum, exhibit the same behaviour on each insect substrate, suggesting a similar protein metabolism. In particular, the effect of the LAB growth was very similar on the fermented BSF adults and puparia. Both presented, respect to the corresponding unfermented biomasses, an enhanced amount of some essential amino acids such as histidine, cysteine, phenylalanine (only for AD_1473) and methionine (only for PUP_1473), and a significant reduction of other amino acids (alanine, asparagine, glycine, and glutamic acid) including the essential amino acids isoleucine, leucine, lysine, valine and tryptophan. Different changes were observed in BSF prepupae: in this case lysine, cysteine and tryptophan, and valine (only for L. plantarum strain) were the only ones which undergo a significative decrease after fermentation. All the others amino acids, with just few exceptions (as in the case of arginine), significantly increase after fermentation.
Fig. 1.
Variations of distribution of total Amino Acids (UPLC/ESI-MS) in BSF adults (a), puparia (b), and prepupae (c) following fermentation. Each graph baseline represent the unfermented BSF substrate and the bars indicate how AA composition change after fermentation with L. rhamnosus (first line) and L. plantarum (second line).
3.2.2. Proteolytic and chitinolytic effects of fermentation on BSF biomasses
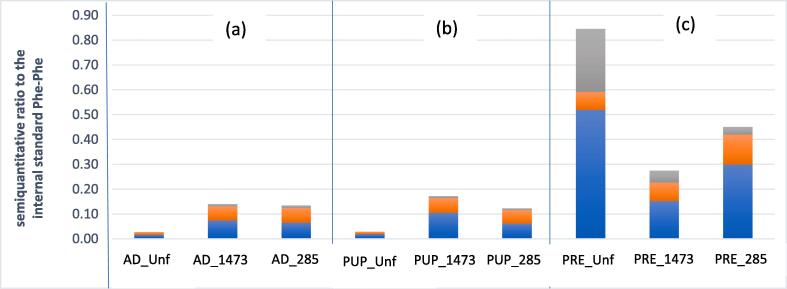
To deeply investigate the proteolytic ability of LABs, a comparison between the oligopeptide’s profiles of the fermented substrates with the unfermented was done. Peptides have been individuated based on their mass spectra and especially on their molecular weights. A semiquantitative evaluation of three classes of peptides was made, corresponding to molecular weights <300 m/z, 300–500 m/z, and > 500 m/z, respectively. The full data are reported in supplementary materials, Table S4. The results are summarised in Fig. 2. The unfermented prepupae is clearly the richest in peptides, that are instead reduced after fermentation with both strains, suggesting that LABs consume the natural prepupae peptides as a source of nitrogen for their metabolism. The peptide consumption seems not to be balanced by LAB proteolytic activity and this might relate to the lower microbial growth on prepupae substrates, as previously mentioned. On the other hand, the other insect biomasses (adults and puparia) were at the beginning very poor in peptides, while after fermentation their amount was increased, suggesting a proteolytic activity, which can be linked to the better fermentation that occurred on these insect biomasses with respect to prepupaee. As a whole, the proteolytic activity found is not marked. The results obtained by other proteolytic indices, measured to better define the proteolytic activity of the LAB on the BSF biomasses, such as the free amino acids amount and the degree of hydrolysis (data not shown), confirmed this evidence. Analysing the evolution of free amino acids during fermentation, in fact, it turned out that the fermented BSF biomasses were characterised by a very low amount of free amino acids, ranging from 0.87 to 2.28%, and very low degree of hydrolysis (not more than 1.7%).
Fig. 2.
Semiquantitative percentage of <300 m/z (blu), 300–500 m/z (orange), and >500 m/z (grey) peptides (UPLC/ESI-MS) in BSF adults (a), puparia(b), and prepupae (c) unfermented and. fermented by L. rhamnosus and L. plantarum. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The potential hydrolytic activity on the insect chitin with consequent formation of chitooligosaccharides has been also evaluated, however HPLC- ESI MS analysis did not show the presence of chitooligomers for any insect samples.
3.2.3. Peptides identification by high resolution mass spectrometry
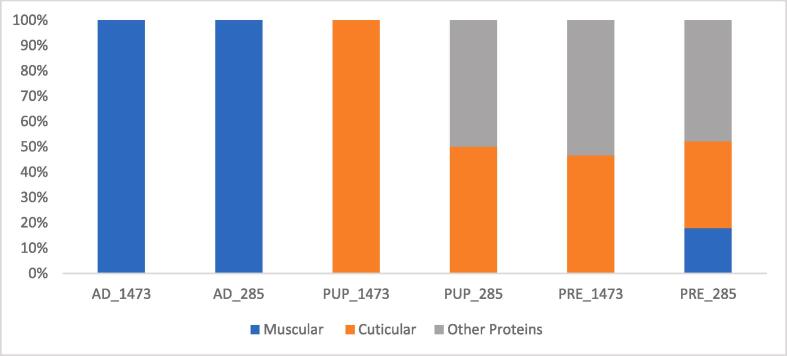
In order to understand the insect protein classes susceptible to the limited LAB proteolytic activity, as a further step, a high-resolution mass spectrometry identification of peptides present in fermented biomasses was carried out. In this way it was possible to identify the protein of origins and as a consequence to understand which proteins are the preferential substrate of LABs during their growth. Fig. 3 reports the identified peptide distribution, based on their origin protein in the different fermented samples. The peptides identified in the fermented BSF adults were all from muscular protein, in particular myosin and actin derived peptides. On the other hand, most of the peptides present in the fermented prepupae and puparia were respectively from cuticular (such as pupal/larval cuticle proteins) and metabolic proteins.
Fig. 3.
The protein origin (muscular, cuticular or other) of peptides extracted from BSF fermented substrates, identified by High Resolution Mass Spectrometry on LTQ-Orbitrap instrument and expressed in relative percentage.
3.3. BSF lipid fraction
Preliminary data on fatty acid composition of BSF prepupae and derived biomasses before and after LAB fermentation indicated a clear shift, after fermentation, from a typical lipid composition of insects to a mixed composition of both insects and lactic acid bacteria (Hadj Saadoun et al., 2020). In the present work data on fatty acid before and after fermentation were assessed from a nutritional point of view. Moreover, to better understand the LAB lipolytic activity, the lipid classes distribution, especially in terms of mono-, di- and tri-acylglycerols was determined, with a particular focus on the presence of monoaylglycerol of lauric acid.
3.3.1. Distribution of lipid classes determined by 1H NMR
1H NMR lipidomic approach was applied on BSF prepupae and derived biomasses, before and after fermentation, to quantify the relative molar percentages of the different lipid classes, as shown in Table 3. 1H NMR spectra related to the unfermented and fermented with the two LABs BSF adult are reported in supplementary (Fig. S1), as an example. Even if from the gross composition no significant variations were observed in the total lipid content of insect samples before and after fermentation, the lipidomic approach highlighted some compositional changes. The greatest effect of the fermentation was found on the content of triglycerides, higher in the fermented samples. In this case also differences among the LAB strains used were observed. For instance, the content of triglycerides in the BSF adults and puparia reaches higher values when fermented respectively with L. plantarum (6.52%) and L. rhamnosus (1.51%) respect to the corresponding unfermented samples (2% for AD_Unf and 0.71% for PUP_Unf). Also monoglyceride and diglyceride content was affected by the fermentation treatment and their content was in general higher in the fermented samples rather than in the unfermented ones. A GC–MS targeted analysis on the same lipid extracts used for the NMR was performed aimed at identifying monoglyceride of lauric acid, known antimicrobial compounds. However, no signals associated with the presence of the monoglyceride of lauric acid were recognized (data not shown), probably due to its low abundance in the samples. Unfermented samples resulted on the contrary richest in free fatty acids. The free fatty acids molar percentage maintained high levels (higher than 80%) also after fermentation processes. The high free fatty acids amount in BSF samples found explanation in the strong lipase activity generated inside the insect biomass during insect killing by freezing, already demonstrated in other studies (Caligiani et al., 2018).
Table 3.
Relative molar percentages determined by 1H NMR of the different glycerides and free fatty acids present in BSF oil from BSF adults, puparia and prepupae, unfermented and fermented with two LAB strains. CV% < 5%. Abbreviations: TG: triglyceride; 1,2-DG: 1,2-diglyceride; 1,3-DG: 1,3-diglyceride; 2-MG: 2-monoglyceride; 1-MG: 1-monoglyceride; FFA: free fatty acid.
| Lipid class | AD_Unf | AD_1473 | AD_285 | PUP_Unf | PUP_1473 | PUP_285 | PRE_Unf | PRE_1473 | PRE_285 |
|---|---|---|---|---|---|---|---|---|---|
| 1,2DG | 3.32 | 2.20 | 2.11 | 2.17 | 7.56 | 5.41 | 0.26 | 0.00 | 0.72 |
| 2MG | 0.31 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 |
| TG | 2.00 | 4.99 | 6.52 | 0.71 | 1.51 | 0.64 | 0.15 | 0.00 | 0.57 |
| 1.3DG | 1.23 | 1.27 | 1.43 | 0.71 | 0.85 | 0.55 | 0.06 | 0.15 | 0.64 |
| 1MG | 3.71 | 4.78 | 5.57 | 4.91 | 8.56 | 6.35 | 0.43 | 0.63 | 2.96 |
| free fatty acids | 89.44 | 86.74 | 84.36 | 91.50 | 81.52 | 87.05 | 99.10 | 99.22 | 95.03 |
3.3.2. Variations of fatty acid profile after fermentation
The differences in the fatty acid composition before and after fermentation in terms of nutritional value are reported in Table 2. Here the classic indices generally used to assess the nutritional value of food lipid fraction were reported, in particular the sum of saturated fatty acids (SFA), of monounsaturated fatty acid (MUFA), of polyunsaturated fatty acid (PUFA), the PUFA/SFA ratio, and the oleic and the linoleic acid amount, in addition with the most common fatty acid in BSF matrix (i.e. Lauric acid) (Chen & Liu, 2020).
Table 2.
Major fatty acid composition, fatty acid partial sums and nutritional ratio of BSF adults, puparia and prepupae unfermented and fermented by L. rhamnosus (1473) and L. plantarum (285). Values are expressed as mean ± SD of relative percentages on total fat. SFA: Saturated fatty acids; MUFA: monounsaturated fatty acids; PUFA: Polyunsaturated fatty acids; LA: Linoleic acid; CLA: sum of conjugated linoleic acid isomers. *Significance (<0,05) of each fermented sample respect its correspondent unfermented material (t-test).
| AD_Unf | AD_1473 | AD_285 | PUP_Unf | PUP_1473 | PUP_285 | PRE_Unf | PRE_1473 | PRE_285 | |
|---|---|---|---|---|---|---|---|---|---|
| Lauric acid | 44.1 ± 0.4 | 31.6 ± 0.1* | 33.7 ± 0.9* | 37.2 ± 0.1 | 33.7 ± 1.3 | 30.2 ± 0.5* | 47.1 ± 0.4 | 28.6 ± 1.2* | 27.2 ± 0.6* |
| Palmitic acid | 11.5 ± 0.3 | 12.5 ± 0.2 | 13.7 ± 0.3* | 13.7 ± 0.2 | 17.9 ± 0.8 | 24.3 ± 1.3* | 14.3 ± 0.2 | 12.9 ± 0.4 | 12.7 ± 0.3 |
| SFA | 73.4 ± 0.1 | 64.1 ± 0.1* | 67.1 ± 0.2* | 75.1 ± 0.1 | 76.5 ± 0.3 | 78.3 ± 0.3 | 80.9 ± 0.1 | 62.3 ± 0.3* | 59.9 ± 0.1* |
| OLEIC ACID | 10.4 ± 0.1 | 8.7 ± 0.0* | 6.6 ± 0.2* | 11.7 ± 0.0 | 6.5 ± 0.3* | 6.4 ± 0.1* | 6.8 ± 0.0 | 9.1 ± 0.1* | 8.6 ± 0.1* |
| MUFA | 13.7 ± 0.0 | 14.5 ± 0.0* | 14.4 ± 0.0 | 15.4 ± 0.0 | 10.9 ± 0.0* | 10 ± 0.1* | 9.3 ± 0.0 | 15.9 ± 0.1* | 15.8 ± 0.0* |
| LA | 12.1 ± 0.2 | 20.9 ± 0.3* | 18.2 ± 0.2* | 8 ± 0.3 | 11.7 ± 0.1* | 11.3 ± 1.6 | 8.9 ± 0.3 | 20.1 ± 1.1* | 22.6 ± 0.2* |
| CLA | 0 ± 0.1 | 0.3 ± 0.1 | 0. ± 0.1 | 0 ± 0.1 | 0.8 ± 0.0* | 0.3 ± 0.1 | 0.2 ± 0.1 | 1.7 ± 0.2* | 1.6 ± 0.0* |
| PUFA | 12.1 ± 0.2 | 21.2 ± 0.2* | 18.4 ± 0.2* | 8 ± 0.2 | 12.5 ± 0.1* | 11.6 ± 0.9 | 9.1 ± 0.2 | 21.8 ± 0.6* | 24.2 ± 0.1* |
| PUFA/SFA | 0.2 | 0.3* | 0.3* | 0.1 | 0.2* | 0.1 | 0.1 | 0.4* | 0.4* |
A significant reduction in the total saturated fatty acids after fermentation with both LAB strains can be noted in BSF adult and prepupae samples. At the same time, significantly increased levels of monounsaturated fatty acids in general were observed in BSF prepupae and derived by-products after LAB incubation. More specifically, among monounsaturated fats, oleic acid (C18:1 ω9) increases significantly after fermentation in prepupae samples, while greatly decreases in fermented BSF adults and puparia. The sum of polyunsaturated fatty acids as well was significantly enhanced after fermentation, with the only exception of PUP_285. In particular, the percentages of linoleic acid (C18:2 ω6) is almost double in all cases after fermentation, while the conjugated linoleic acid (CLA) showed a significative increment especially in fermented prepupae samples and in puparia fermented with L. rhamnosus.
4. Discussion
Lactic acid bacteria fermentation was recently proposed as a possible strategy of management of insect biomasses (Hadj Saadoun et al., 2020), suggesting the possibility to modify insect molecular composition and to confer specific features, as antimicrobial properties. In this work, detailed molecular variations following fermentation by L. rhamnosus and L. plantarum strains in insect biomasses were studied, which is the prerequisite to find the possible end applications. Molecular composition was studied at different levels, from gross composition to specific evaluation of proteolytic, chitinolytic and lipolytic activities of fermenting strains.
Proximate composition showed only slight modifications of insect biomasses after fermentation, being the main compositional differences related to the different insect materials used as substrates of fermentation: prepupae and adult flies are richer in protein and lipid, while chitin and ashes were higher in puparia, the chitinous shell of insects. This is coherent with the known distribution of macromolecules in insect body (Oonincx et al., 2015). However, it is generally known that solid state fermentation is a biotechnological process able to change the nutritional properties of food beyond the proximate composition (Giacometti & Buretić-Tomljanović, 2017). Thus, the fermented lipid, protein and chitin fractions were further analysed to explore the modifications induced by fermentation to a deeper molecular level, also using -omics approaches.
Results of protein fraction showed that total amount of proteins remained the same before and after fermentation (Table 1), while a clear redistribution of amino acids occurred, suggesting a balance between insect proteins utilized by LAB and new protein produced. Hadj Saadoun et al. (2020) reported a variation of the amino acids redistribution also depending on the different LAB strain used for the fermentation of BSF biomasses, while the data obtained in the present work suggested a more pronounced effect of the type of insect biomass. Interestingly, the samples presenting a similar variation in amino acid profile, namely BSF adults and puparia, were also those ones better fermented, while BSF prepupae (less fermented) have a different aminoacidic redistribution. This is in line with previous studies reporting that microorganisms can modify the concentration of amino acids by protein synthesis or catabolism of amino acids into different products, for example volatile compounds affecting food flavour (Pozo-Bayón et al., 2005). This therefore can explain why, where LAB grew better, similar changes in the amino acidic composition of the substrates were observed. Other studies have been previously reported a total amount of protein unchanged but a positive effect of fermentation on essential amino acid composition of food (Khan et al., 2018).
From a nutritional perspective, many essential amino acids were significatively increased following the bacterial growth in insect biomasses. This is a clear benefit of fermenting BSF prepupae and derived biomasses, suggesting that insects as future human food could be nutritionally enriched using fermentation. Of course the increase in some essential amino acids is associated with a decrease of others. Nonetheless this result does not compromise the nutritional value of the BSF protein fraction. In fact, Table S3 of supplementary material shows that all BSF samples after fermentation maintained a positive nutritional profile in essential amino acids, which in any case satisfied the reference values suggested by FAO/WHO for human nutrition.
Despite amino acid redistribution clearly indicate a LAB activity on insect protein fraction, the determination of other proteolytic indexes as free amino acid, degree of hydrolysis and oligopeptides indicates globally a very slight proteolytic activity of LAB strains in insects. Even if Lactobacilli are documented to exert a proteolytic activity on different food sources, this finding could be coherent with some detailed studies that have highlighted how their proteolytic system is weakest compared with other species, as Lactococcus, Pediococcus or Enterococcus genus (Fadda et al., 2010, García-Cano et al., 2019, Lim et al., 2019).
Interestingly, the sequences of oligopeptides identified indicate that the protein fractions mostly utilized by LABs mirror the most abundant classes of proteins in BSF biomasses, namely cuticular proteins in puparia, muscular protein in adults and metabolic proteins in prepupae. In fact, it is known that puparium, the cuticle which cover the larval and prepupae stage before the fly metamorphosis, is particularly rich of chitin closely linked to cuticular proteins, both derived from the epidermis layer below (Tajiri et al., 2017). Although no specific studies were found in the literature that investigates the most abundant protein classes present in the BSF adults, it is also clear that removing the exoskeleton during the transition to the adult stage of BSF prepupae will result in an enrichment of muscle proteins compared to the prepupae stage. As a whole, from the results obtained for the peptides present after fermentation, it is evident that LABs consume the most abundant proteins present in the insect biomass, suggesting good flexibility to grow on different kinds of insect nitrogen sources.
Beside proteolytic, also chitinolytic activity was checked in fermented insect biomasses, through determination of chitooligosaccharides. The absence of the latter in every fermented insect biomass suggests the absence in the LAB metabolism of chitinase and/or low hydrolytic activity on chitin fraction, probably due to its insoluble crystalline structure. In a previous work on chitinase activity in Lactobacillus strains, no chitinolytic activity was observed, although the presence of chitinase coding gene in the microorganisms was confirmed (Horwh-Szanics et al., 2020). It is therefore possible that, even if the LAB selected for the experiment showed in their genomes the presence of different chitinases (data not shown), they were not able to express them. Still few works in literature on the Lactobacillus strains chitinase are present and further studies on the issue are encouraged, in view of the growing interest on the utilization of insect chitin.
Regarding the lipid fraction, the differences found after fermentation suggest that LABs are able to carry out a biosynthesis of glycerol esters using as starting material the free fatty acids naturally present in the lipid fraction of BSF. In particular, an increased amount of mono-, di- and tri-acylglycerols was observed, suggesting the possible presence of lauric acid monoglyceride. Lauric acid, of which BSF is particularly rich (Caligiani et al., 2018), among all fatty acids is the strongest in the growth inactivation of Gram-positive and Gram-negative such as respectively Staphylococcus aureus and E. coli bacteria (Khoramnia et al., 2013). Many studies showed the same antimicrobial effect for monoglycerides and diglycerides of medium chain fatty acids (including C12:0) (Bergsson et al., 2001, Schiavone et al., 2017) and these are the most studied among all the fatty acid esters for its known bactericidal and antifungal activity (Dayrit, 2015). The targeted determination of this compounds was performed specifically to explain the antimicrobial activity evidenced on our previous work for fermented insect biomasses (Hadj Saadoun et al., 2020). However no significant amount of this compounds was observed in fermented insect biomasses, suggesting that lactic acid bacteria fermentation generates in raw substrates other compounds exerting antimicrobial activity (Sadh, Kumar, et al., 2018).
One of the most important biotransformation operated by LAB on BSF biomasses is on fatty acid profile. Black soldier fly is one of the most highly rated insect species for animal and future human nutrition due to the high nutritional composition (Meneguz et al., 2018). However, it is also known that BSF fat is particularly rich in saturated fatty acids. Among these the most representative are lauric acid (C12:0) and palmitic acid (C16:0), that generally correspond respectively to the 40% and 15% of total fatty acids (Kim et al., 2020). Total monounsaturated fatty acids are instead present in lower concentrations, followed by the polyunsaturated. Although it has been demonstrated that BSF lipid fraction, if part of a animal diet, has a positive influence on intestinal functions, antioxidant and immunomodulatory powers (Kim et al., 2020), it is not able to provide high concentrations of unsaturated fatty acids that are highly recommended also in human diet for their protective effects against cardiovascular diseases, atherosclerosis and modulatory properties on lipid metabolism (Lada & Rudel, 2003). Based on the results obtained in this work, fermentation leads to a redistribution of fatty acids, resulting in a significantly increased PUFA/SFA ratio, enriching the nutritional profile of BSF oil. Changes observed here in the nutritional profile of BSF fats are in accordance with many works that show how LAB fermentation could modify the positional distribution and proportions of fatty acids on different food sources, leading to enhanced lipid compositions (Vieira et al., 2015). The ability of the LAB to increase the content of long chain fatty acids and to create unsaturations has also previously been observed (Ravyts et al., 2012). Vieira, Álvares, Gomes, Torres, Paschoalin and Conte (2015) showed that fermentation of matrices rich in lipids might lead to higher production of polyunsaturated fatty acids. This point can explain why the puparia, poor in lipids, is the sample with the lowest production of unsaturated fatty acids. As a whole, fermentation is able to enrich even more the BSF biomasses with nutritional benefits in terms of fatty acid composition.
5. Conclusion
Insect fermentation is still very poorly explored, but it can be considered an interesting and alternative way to produce insect-based materials with unique properties for food and feed. From the current investigation it was found that fermentation actually induces a deep modification of the molecular composition of the biomasses, even if not visible in the bulk composition. The fermented samples presented a fatty acid profile enhanced in monounsaturated and polyunsaturated fatty acids and a modified aminoacidic profile rich of essential amino acids. A proteolytic activity, although not pronounced, has been found in both LAB strains.
Looking beyond the nutritional value, fermented insect biomasses can acquire also biofunctional properties including antioxidant, health-promoting, antimicrobial activities. In particular, the latter has been previously demonstrated in fermented BSF insect material but a further step aimed at identifying the molecules exerting the antimicrobial activity and understand their origin is needed. In this perspective, our investigation and molecular approach constitutes an excellent starting point giving us a precise overall view of the main molecular dynamic changes that the substrate undergoes after fermentation. This knowledge at the molecular level can be further improved to elucidate the origin of antimicrobial activity and other biofunctional properties of fermented insect biomasses.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100327.
Contributor Information
Anna Valentina Luparelli, Email: annavalentina.luparelli@unipr.it.
Jasmine Hadj Saadoun, Email: jasmine.hadjsaadoun@unipr.it.
Veronica Lolli, Email: veronica.lolli@unipr.it.
Camilla Lazzi, Email: camilla.lazzi@unipr.it.
Stefano Sforza, Email: stefano.sforza@unipr.it.
Augusta Caligiani, Email: augusta.caligiani@unipr.it.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Barbi S., Macavei L.I., Fuso A., Luparelli A.V., Caligiani A., Ferrari A.M., Maistrello L., Montorsi M. Valorization of seasonal agri-food leftovers through insects. Science of the Total Environment. 2020;709 doi: 10.1016/j.scitotenv.2019.136209. [DOI] [PubMed] [Google Scholar]
- Bergsson G., Arnfinnsson J., Steingrímsson Ó., Thormar H. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrobial Agents and Chemotherapy. 2001;45(11):3209–3212. doi: 10.1128/AAC.45.11.3209-3212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottari B., Quartieri A., Prandi B., Raimondi S., Leonardi A., Rossi M., Ulrici A., Gatti M., Sforza S., Nocetti M., Amaretti A. Characterization of the peptide fraction from digested Parmigiano Reggiano cheese and its effect on growth of lactobacilli and bifidobacteria. International Journal of Food Microbiology. 2017;255:32–41. doi: 10.1016/j.ijfoodmicro.2017.05.015. [DOI] [PubMed] [Google Scholar]
- Caligiani A., Marseglia A., Leni G., Baldassarre S., Maistrello L., Dossena A., Sforza S. Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Research International. 2018;105:812–820. doi: 10.1016/j.foodres.2017.12.012. [DOI] [PubMed] [Google Scholar]
- Caligiani A., Marseglia A., Sorci A., Bonzanini F., Lolli V., Maistrello L., Sforza S. Influence of the killing method of the black soldier fly on its lipid composition. Food Research International. 2019;116:276–282. doi: 10.1016/j.foodres.2018.08.033. [DOI] [PubMed] [Google Scholar]
- Chelule P.K., Mbongwa H.P., Carries S., Gqaleni N. Lactic acid fermentation improves the quality of amahewu, a traditional South African maize-based porridge. Food Chemistry. 2010;122(3):656–661. doi: 10.1016/j.foodchem.2010.03.026. [DOI] [Google Scholar]
- Chen J., Liu H. Nutritional indices for assessing fatty acids: A mini-review. International Journal of Molecular Sciences. 2020;21(16):5695. doi: 10.3390/ijms21165695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayrit F.M. The properties of lauric acid and their significance in coconut oil. Journal of the American Oil Chemists’ Society. 2015;92(1):1–15. doi: 10.1007/s11746-014-2562-7. [DOI] [Google Scholar]
- De Smet J., Wynants E., Cos P., Van Campenhout L. Microbial community dynamics during rearing of black soldier fly larvae (Hermetia illucens) and impact on exploitation potential. Applied and Environmental Microbiology. 2018;84(9):e02722–17. doi: 10.1128/AEM.02722-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda S., López C., Vignolo G. Role of lactic acid bacteria during meat conditioning and fermentation: Peptides generated as sensorial and hygienic biomarkers. Meat Science. 2010;86(1):66–79. doi: 10.1016/j.meatsci.2010.04.023. [DOI] [PubMed] [Google Scholar]
- García-Cano I., Rocha-Mendoza D., Ortega-Anaya J., Wang K., Kosmerl E., Jiménez-Flores R. Lactic acid bacteria isolated from dairy products as potential producers of lipolytic, proteolytic and antibacterial proteins. Applied Microbiology and Biotechnology. 2019;103(13):5243–5257. doi: 10.1007/s00253-019-09844-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacometti J., Buretić-Tomljanović A. Peptidomics as a tool for characterizing bioactive milk peptides. Food Chemistry. 2017;230:91–98. doi: 10.1016/j.foodchem.2017.03.016. [DOI] [PubMed] [Google Scholar]
- Hadj Saadoun J., Luparelli A.V., Caligiani A., Macavei L.I., Maistrello L., Neviani E., Galaverna G., Sforza S., Lazzi C. Antimicrobial biomasses from lactic acid fermentation of black soldier fly prepupae and related by-products. Microorganisms. 2020;8(11):1785. doi: 10.3390/microorganisms8111785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadj Saadoun J., Ricci A., Cirlini M., Bancalari E., Bernini V., Galaverna G., Neviani E., Lazzi C. Production and recovery of volatile compounds from fermented fruit by-products with Lacticaseibacillus rhamnosus. Food and Bioproducts Processing. 2021 doi: 10.1016/j.fbp.2021.06.002. [DOI] [Google Scholar]
- Horwh-Szanics E., Perjessy J., Klupacs A., Takacs K., Nagy A., Koppany-Szabo E., Hegyi F., Nemeth-Szerdahelyi E., Du M.Y., Wang Z.R., Kan J.Q., Zalan Z. Study of chitinase and chitinolytic activity of Lactobacillus strains. Acta Alimentaria. 2020;49(2):214–224. doi: 10.1556/066.2020.49.2.11. [DOI] [Google Scholar]
- Khan S.A., Liu L., Lai T., Zhang R., Wei Z., Xiao J., Deng Y., Zhang M. Phenolic profile, free amino acids composition and antioxidant potential of dried longan fermented by lactic acid bacteria. Journal of Food Science and Technology. 2018;55(12):4782–4791. doi: 10.1007/s13197-018-3411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoramnia A., Ebrahimpour A., Ghanbari R., Ajdari Z., Lai O.M. Improvement of medium chain fatty acid content and antimicrobial activity of coconut oil via solid-state fermentation using a malaysian geotrichum candidum. BioMed Research International. 2013 doi: 10.1155/2013/954542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kim W., Hwang I.K. Optimization of the extraction and purification of oligosaccharides from defatted soybean meal. International Journal of Food Science and Technology. 2003;38(3):337–342. doi: 10.1046/j.1365-2621.2003.00679.x. [DOI] [Google Scholar]
- Kim Y.B., Kim D.H., Jeong S.B., Lee J.W., Kim T.H., Lee H.G., Lee K.W. Black soldier fly larvae oil as an alternative fat source in broiler nutrition. Poultry Science. 2020 doi: 10.1016/j.psj.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsoumanis K., Allende A., Alvarez-Ordóñez A., Bolton D., Bover-Cid S., Chemaly M.…Herman L. Scientific opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA (2017–2019) EFSA Journal. 2020;18(2) doi: 10.2903/j.efsa.2020.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lada A.T., Rudel L.L. Dietary monounsaturated versus polyunsaturated fatty acids: Which is really better for protection from coronary heart disease? Current Opinion in Lipidology. 2003;14(1):41–46. doi: 10.1097/00041433-200302000-00008. [DOI] [PubMed] [Google Scholar]
- Leni G., Soetemans L., Jacobs J., Depraetere S., Gianotten N., Bastiaens L., Caligiani A., Sforza S. Protein hydrolysates from alphitobius diaperinus and hermetia illucens larvae treated with commercial proteases. Journal of Insects as Food and Feed. 2020;6(4):393–404. doi: 10.3920/JIFF2019.0037. [DOI] [Google Scholar]
- Lim Y.H., Foo H.L., Loh T.C., Mohamad R., Abdullah N. Comparative studies of versatile extracellular proteolytic activities of lactic acid bacteria and their potential for extracellular amino acid productions as feed supplements. Journal of Animal Science and Biotechnology. 2019;10(1):1–13. doi: 10.1186/s40104-019-0323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneguz M., Schiavone A., Gai F., Dama A., Lussiana C., Renna M., Gasco L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. Journal of the Science of Food and Agriculture. 2018;98:5776–5784. doi: 10.1002/jsfa.9127. [DOI] [PubMed] [Google Scholar]
- Oonincx D.G.A.B., van Huis A., van Loon J.J.A. Nutrient utilisation by black soldier flies fed with chicken, pig, or cow manure. Journal of Insects as Food and Feed. 2015;1:131–139. doi: 10.3920/jiff2014.0023. [DOI] [Google Scholar]
- Oonincx D.G.A.B., de Boer I.J.M. Environmental impact of the production of mealworms as a protein source for humans - A life cycle assessment. PLoS ONE. 2012;7(12) doi: 10.1371/journal.pone.0051145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo-Bayón M.A., G-Alegría E., Polo M.C., Tenorio C., Martín-Álvarez P.J., Calvo De La Banda M.T., Ruiz-Larrea F., Moreno-Arribas M.V. Wine volatile and amino acid composition after malolactic fermentation: Effect of Oenococcus oeni and Lactobacillus plantarum starter cultures. Journal of Agricultural and Food Chemistry. 2005;53(22):8729–8735. doi: 10.1021/jf050739y. [DOI] [PubMed] [Google Scholar]
- Ravyts F., Vuyst L.D., Leroy F. Bacterial diversity and functionalities in food fermentations. Engineering in Life Sciences. 2012;12(4):356–367. doi: 10.1002/elsc.201100119. [DOI] [Google Scholar]
- Rumpold B.A., Schlüter O.K. Potential and challenges of insects as an innovative source for food and feed production. Innovative Food Science and Emerging Technologies. 2013;17:1–11. doi: 10.1016/j.ifset.2012.11.005. [DOI] [Google Scholar]
- Sabater C., Ruiz L., Delgado S., Ruas-Madiedo P., Margolles A. Valorization of vegetable food waste and by-products through fermentation processes. Frontiers in Microbiology. 2020;11:2604. doi: 10.3389/fmicb.2020.581997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadh P.K., Duhan S., Duhan J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresources and Bioprocessing. 2018;5(1):1–15. doi: 10.1186/s40643-017-0187-z. [DOI] [Google Scholar]
- Sadh P.K., Kumar S., Chawla P., Duhan J.S. Fermentation: A boon for production of bioactive compounds by processing of food industries wastes (By-Products) Molecules. 2018;23(10):2560. doi: 10.3390/molecules23102560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomone R., Saija G., Mondello G., Giannetto A., Fasulo S., Savastano D. Environmental impact of food waste bioconversion by insects: Application of Life Cycle Assessment to process using Hermetia illucens. Journal of Cleaner Production. 2017;140:890–905. doi: 10.1016/j.jclepro.2016.06.154. [DOI] [Google Scholar]
- Schiavone A., Cullere M., De Marco M., Meneguz M., Biasato I., Bergagna S., Dezzutto D., Gai F., Dabbou S., Gasco L., Zotte A.D. Partial or total replacement of soybean oil by black soldier fly larvae (Hermetia illucens L.) fat in broiler diets: Effect on growth performances, feed-choice, blood traits, carcass characteristics and meat quality. Italian Journal of Animal Science. 2017;16(1):93–100. doi: 10.1080/1828051X.2016.1249968. [DOI] [Google Scholar]
- Spellman D., McEvoy E., O’Cuinn G., FitzGerald R.J. Proteinase and exopeptidase hydrolysis of whey protein: Comparison of the TNBS, OPA and pH stat methods for quantification of degree of hydrolysis. International Dairy Journal. 2003;13(6):447–453. doi: 10.1016/S0958-6946(03)00053-0. [DOI] [Google Scholar]
- Tajiri R., Ogawa N., Fujiwara H., Kojima T. Mechanical control of whole body shape by a single cuticular protein obstructor-E in Drosophila melanogaster. PLoS Genetics. 2017;13(1) doi: 10.1371/journal.pgen.1006548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L., Larroche C., Pandey A. Current developments in solid-state fermentation. Biochemical Engineering Journal. 2013:146–161. doi: 10.1016/j.bej.2013.10.013. [DOI] [Google Scholar]
- Vieira C.P., Álvares T.S., Gomes L.S., Torres A.G., Paschoalin V.M.F., Conte C.A. Kefir grains change fatty acid profile of milk during fermentation and storage. PLoS ONE. 2015;10(10) doi: 10.1371/journal.pone.0139910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.S., Shelomi M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods. 2017;6(10):91. doi: 10.3390/foods6100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.