In Brief
The objective of this study was to characterize a noninvasive, nonablative strategy to focally destroy neurons in the brain parenchyma with the goal of limiting damage to nontarget structures. The authors were able to produce focal neuronal lesions in a variety of brain regions while sparing nonneuronal cells and axons of passage in the targeted area. This strategy could provide a noninvasive means of disconnecting aberrant circuitry with limited collateral damage.
Keywords: noninvasive, axon sparing, vascular sparing, neurotoxic, neurosurgery, focused ultrasound
ABBREVIATIONS : BBB = blood-brain barrier; DCE = dynamic contrast-enhanced; FUS = focused ultrasound; LITT = laser interstitial thermal therapy; MBP = myelin basic protein; MRgFUS = MR-guided FUS; PING = precise, intracerebral, noninvasive, guided surgery; QA = quinolinic acid; ROI = region of interest
Abstract
OBJECTIVE
Surgery can be highly effective for the treatment of medically intractable, neurological disorders, such as drug-resistant focal epilepsy. However, despite its benefits, surgery remains substantially underutilized due to both surgical concerns and nonsurgical impediments. In this work, the authors characterized a noninvasive, nonablative strategy to focally destroy neurons in the brain parenchyma with the goal of limiting collateral damage to nontarget structures, such as axons of passage.
METHODS
Low-intensity MR-guided focused ultrasound (MRgFUS), together with intravenous microbubbles, was used to open the blood-brain barrier (BBB) in a transient and focal manner in rats. The period of BBB opening was exploited to focally deliver to the brain parenchyma a systemically administered neurotoxin (quinolinic acid) that is well tolerated peripherally and otherwise impermeable to the BBB.
RESULTS
Focal neuronal loss was observed in targeted areas of BBB opening, including brain regions that are prime objectives for epilepsy surgery. Notably, other structures in the area of neuronal loss, including axons of passage, glial cells, vasculature, and the ventricular wall, were spared with this procedure.
CONCLUSIONS
These findings identify a noninvasive, nonablative approach capable of disconnecting neural circuitry while limiting the neuropathological consequences that attend other surgical procedures. Moreover, this strategy allows conformal targeting, which could enhance the precision and expand the treatment envelope for treating irregularly shaped surgical objectives located in difficult-to-reach sites. Finally, if this strategy translates to the clinic, the noninvasive nature and specificity of the procedure could positively influence both physician referrals for and patient confidence in surgery for medically intractable neurological disorders.
One-third or more of individuals afflicted with epilepsy are unresponsive to available seizure-suppressing medications.1,2 When antiepileptic drugs prove ineffective for treating focal epilepsy, surgery can be highly effective for reducing or eliminating seizures,3–5 improving quality of life,6,7 and reducing mortality.8 However, epilepsy surgery remains substantially underutilized, despite extensive evidence demonstrating its benefits.9–13 The reasons underlying this lack of implementation include contraindications for surgery, complications during surgery, and deficits resulting from surgery.13–17 Nonsurgical issues contributing to underutilization include poor referral rates, costs, access to treatment, patient concerns, and socioeconomic status.11,12,18–22 Together, these obstacles conspire to limit the utilization of an effective treatment for a medically intractable, neurological disorder.
Refinements in resective procedures and advances using minimally invasive and noninvasive procedures offer substantial promise for limiting surgical complications and for providing more precise targeting. Among these procedures are laser interstitial thermal therapy (LITT), radiosurgery, high-intensity focused ultrasound, and radiofrequency thermocoagulation. A comprehensive review of the advantages and disadvantages of each of these procedures is outside of the scope of this paper, but the improvements provided by LITT are illustrative. This minimally invasive approach is effective in reducing or eliminating seizures23–27 and is generally associated with shorter hospitalization and lower morbidity. Moreover, a major benefit of LITT is that it creates a selective thermal ablation that may lessen functional deficits28,29 by limiting collateral structural damage. However, LITT is an invasive procedure, and treatment of irregularly shaped targets can require multiple penetrations with a laser probe to provide adequate target removal. Finally, LITT is an ablative procedure that can destroy neuronal, glial, vascular, and ventricular elements alike. This pannecrotic impact, which can lead to collateral tissue damage, is a shared feature of all the aforementioned minimally invasive and noninvasive ablative procedures.
Limiting collateral damage is a primary goal for any surgical procedure. Such injury may derive from any of a variety of causes, including 1) disruption of vascular supply to tissue neighboring a surgical objective, 2) disturbance of ventricular patency, 3) dissection of axons of passage, 4) direct damage to neighboring tissue, and/or 5) damage to tissue intervening between a site of surgical access and the surgical objective. In an effort to further refine surgical precision and reduce neuropathological consequences, we have recently developed a noninvasive strategy to produce focal loss of targeted neurons in the brain.30 Low-intensity MR-guided focused ultrasound (MRgFUS) has been used to transiently open the blood-brain barrier (BBB) to focally deliver a neurotoxin (quinolinic acid [QA]) to a targeted region of the brain parenchyma. QA is a natural metabolite of tryptophan produced via the kynurenine pathway. The selection of this neurotoxin was based on previous evidence demonstrating that QA is poorly permeable through the BBB31 but, when directly applied to brain tissue, produces perikaryal neuronal damage while sparing axons of passage.32 Peripheral administration of a systemically tolerated BBB-impermeable neurotoxin produces focal neuronal damage in the targeted area of BBB opening. Importantly, systemic administration of saline instead of the neurotoxin does not produce damage, indicating that the loss of neurons is not a direct effect of the low-intensity FUS treatment itself. However, a key remaining question is whether this strategy can be implemented in a manner that limits collateral damage to nontarget cellular elements. The present study tests whether this strategy is safe and capable of targeting appropriate brain regions and can limit collateral neuropathology. The study defines the time frame over which the BBB remains open after FUS, assesses the potential vulnerability of CNS regions that possess restricted BBB function but are not sonicated (i.e., circumventricular nuclei), delineates the regions of the brain in which the procedure is effective, and defines the impact on nontarget cellular structures (e.g., axons of passage) in a sonicated area. For the convenience of presentation, this approach will herein be termed precise, intracerebral, noninvasive, guided surgery (PING).
Methods
PING Procedure
Procedures were approved by the University of Virginia Animal Care and Use Committee. Male Sprague Dawley rats (130–170 g; Taconic, n = 24) and weight-matched tish rats (local colony, n = 7) were anesthetized with 4% isoflurane (induction) and 2% isoflurane (maintenance). Tish rats were included in this study because they possess prominent cortical dysplasias, and dysplastic cortical tissue is a common target for epilepsy surgery.33 Details of the PING procedure have been reported previously.30,34 Briefly, an animal was placed in an MR-compatible FUS system (Image Guided Therapy) for targeting and delivering sonication. A transducer was positioned on the skull, and T2-weighted images were used to localize the target. Just prior to sonication, microbubbles were injected via an intravenous tail vein catheter. Microbubbles were prepared in-house using probe sonication from decafluorobutane gas and stabilization with 1,2-distearoyl-sn-glycero-3-phosphocholine/polyethylene glycol stearate monolayer shell, as described previously by Klibanov.35 The mean microbubble size was approximately 2 μm. Sonication parameters were as follows: 1.5 MHz, 20-msec wave packet, 2% duty cycle, 1-Hz burst repetition frequency, and 120-second duration per sonication, producing an estimated 0.69 MPa in the target area. Immediately after sonication, gadodiamide contrast was injected intravenously for contrast-enhanced T1-weighted scans to confirm opening of the BBB and accuracy of targeting. Starting 30 minutes after MRgFUS, QA was infused intravenously for 1 hour, resulting in a total dose of 225 mg/kg per animal. MRI was performed using a 7T ClinScan system (Bruker BioSpin).
Kinetics of BBB Opening
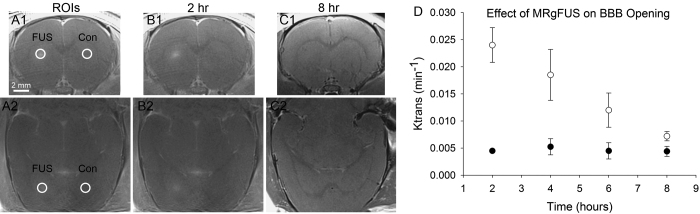
Previous studies have characterized the dynamics and safety of FUS-induced BBB opening.36,37 Nonetheless, it remains important to define the time course of BBB opening using the current experimental protocol because variations in specific protocols can affect the dynamics of BBB opening and closing. The duration of BBB opening was assessed using dynamic contrast-enhanced (DCE) MRI to calculate Ktrans coefficients. Ktrans values have previously been used to evaluate the kinetics of BBB opening, including openings produced by FUS.38–42 Sprague Dawley rats received an intravenous injection of microbubbles, followed immediately by FUS targeting the striatum (Fig. 1A and B). Immediately after FUS, the contrast agent gadodiamide (Omniscan, 0.2 ml/kg, GE Healthcare AS) was administered intravenously, and contrast-enhanced T1-weighted MRI was performed to confirm successful opening of the BBB and accurate targeting. Gadodiamide was again administered at later postsonication time points (2, 4, 6, and/or 8 hours), and DCE imaging was performed. A given animal was assessed at only two of the postsonication time points separated by 4 hours to avoid the presence of residual gadodiamide on the follow-up MR images. DCE MRI analyses were performed in two regions of interest (ROIs) in each animal: one region was the area targeted by FUS, and the other was the untreated homotopic area in the contralateral hemisphere (Fig. 1). The mean Ktrans values were calculated at each postsonication time point, and T2-weighted images were obtained 8 hours postsonication to assess possible injury to the tissue.
FIG. 1.
Time course of BBB opening in response to MRgFUS. A1–C2: Contrast-enhanced T1-weighted MR images, all obtained in the same animal (row 1, coronal plane; row 2, axial [horizontal] plane). The locations of the ROIs (white circles) in the striatum are depicted (A1 and A2). These areas were used for calculating Ktrans coefficients on the side of the brain receiving FUS and on the contralateral control side (Con). At 2 hours postsonication, an area of hyperintensity is observed in the FUS target area (B1 and B2). This is evidence of the extravasation of gadodiamide into the target area of the striatum and opening of the BBB. At 8 hours postsonication, hyperintensity in the area of sonication is no longer observed, indicative of closing of the BBB (C1 and C2). D: The time course of BBB opening is quantified in a graph presenting the Ktrans values (mean ± SEM) over the 8-hour postsonication time course. Open circles are from the FUS-side ROI and filled circles are from the control-side ROI (n = 8 animals total, with n = 4 animals per time point).
Tissue Preparation and Staining
Animals were deeply anesthetized with isoflurane and transcardially perfused with saline followed by 4% paraformaldehyde in 0.1-M phosphate buffer (pH 7.4). The brains were removed, postfixed, and sectioned with a cryostat in preparation for immunohistochemical, Fluoro-Jade, Luxol fast blue, and/or Nissl staining. The primary antibodies used for immunohistochemical analysis were rabbit anti–myelin basic protein (MBP; 1:100, Abcam), mouse anti-NeuN (1:1000, Millipore), rabbit anti-Iba1 (1:1000, Wako), rabbit anti-FoxJ1 (1:1000, Abcam), and rabbit anti-GFAP (1:1000, DakoCytomation). Primary antibodies were detected using species-specific Alexa Fluor 488 and 594 secondary antibodies (1:1000, Invitrogen Molecular Probes).
Results
Reversible, Focal Opening of the BBB After FUS
The time course of BBB opening was defined by calculating Ktrans values at 2, 4, 6, and 8 hours postsonication (Fig. 1). Initial contrast-enhanced T1-weighted MRI showed that MRgFUS successfully and precisely opened the BBB in the targeted area, after which the BBB closed with a half-life of approximately 5 hours. T2-weighted images obtained at 8 hours postsonication (i.e., a time at which Ktrans coefficients had returned to baseline levels) showed no evidence of injury to the tissue (data not shown). These findings demonstrate precise temporal and spatial control over the opening of the BBB and that the window for delivering a systemically administered drug (e.g., QA) is well defined and consistent across animals. The absence of injury in this experiment, which did not involve the injection of QA, is consistent with previous observations indicating that the FUS procedure by itself does not produce injury.36 Moreover, the general time frame over which the BBB remained open is consistent with previous studies.36,37
PING Is Effective in Producing Focal Neuronal Injury in Various Brain Regions
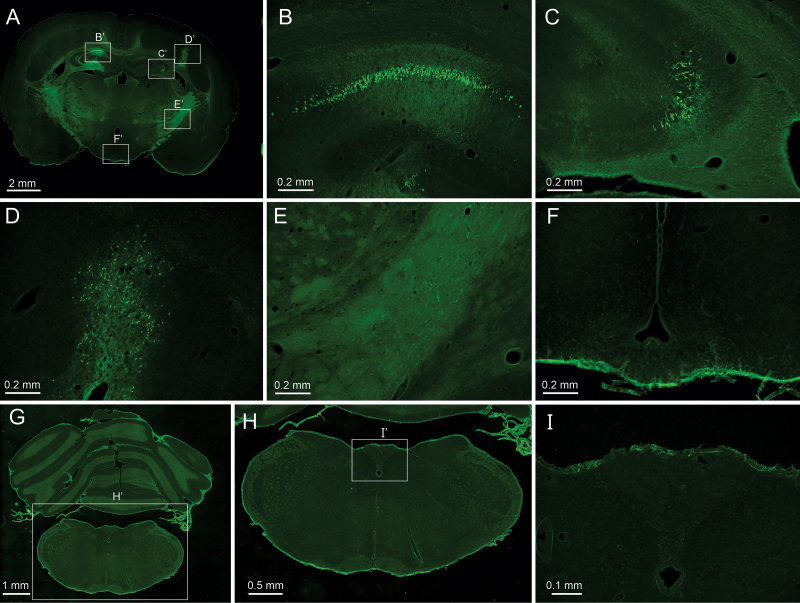
PING was capable of producing neuronal loss in all brain regions that were targeted, including the striatum, thalamus, septum, nucleus accumbens, hypothalamus, and multiple neocortical areas. Findings were consistent across animals targeted in the same areas. Figure 2 demonstrates focal neuronal loss of dysplastic neocortical neurons in the tish rat brain (Fig. 2D), as well as in the CA1 and CA3 regions of the hippocampus (Fig. 2B and C). These observations are of note because neocortical dysplasias and the hippocampus are common targets for epilepsy surgery. Notably, in the same brain, neuronal loss was not observed in nonsonicated areas, including circumventricular nuclei neighboring the third ventricle (Fig. 2E and F).
FIG. 2.
PING produces neuronal loss restricted to targeted areas of sonication. Fluoro-Jade staining of degenerating neurons (bright green) in coronal brain sections after PING. A–F: Fluoro-Jade staining in a tish rat that survived 5 days after being administered PING at three sites. Low-magnification image of a coronal section of a tish brain in which the CA1 region of the hippocampus (B’), CA3 region of the hippocampus (C’), and dysplastic neocortex (D’) were targeted (A). The rectangles labeled B’–F’ correspond to the areas enlarged in panels B–F of the figure. Layer of degenerating CA1 pyramidal cells (B). Degenerating CA3 pyramidal cells (C). Degenerating neocortical neurons in cortical heterotopia (D). Internal capsule, striatum, and neighboring neocortex, which were not sonicated, do not exhibit degenerating neurons (E). Areas surrounding the third ventricle, including the arcuate nucleus and median eminence, which were not sonicated, do not exhibit degenerating neurons (F). G–I: Fluoro-Jade staining in a Sprague Dawley rat that was administered QA but did not receive FUS (4-day survival). Low-magnification image of a coronal section at the level of the caudal medulla (G). The rectangle labeled H’ indicates the region magnified in panel H. Degenerating neurons were not observed in the caudal medulla (H). The rectangle labeled I’ indicates the region magnified in panel I. The area postrema does not exhibit degenerating neurons (I).
The issue of whether circumventricular nuclei are vulnerable to circulating QA in the absence of sonication was further investigated in three animals in which QA was injected intravenously but no FUS was administered. Semiserial coronal sections extending from the caudal medulla through the frontal cortex were stained with Fluoro-Jade and evaluated for degenerating neurons 4 days after QA injection. Only rare and occasional degenerating neurons were observed in the brain, and degenerating neurons were not observed in circumventricular nuclei. Notably, the area postrema of the caudal medulla, a region known to have differential barrier function in comparison with most brain regions, did not exhibit degenerating neurons (Fig. 2G–I). These findings indicate that intravenous injection of QA, by itself, does not produce neuronal loss.
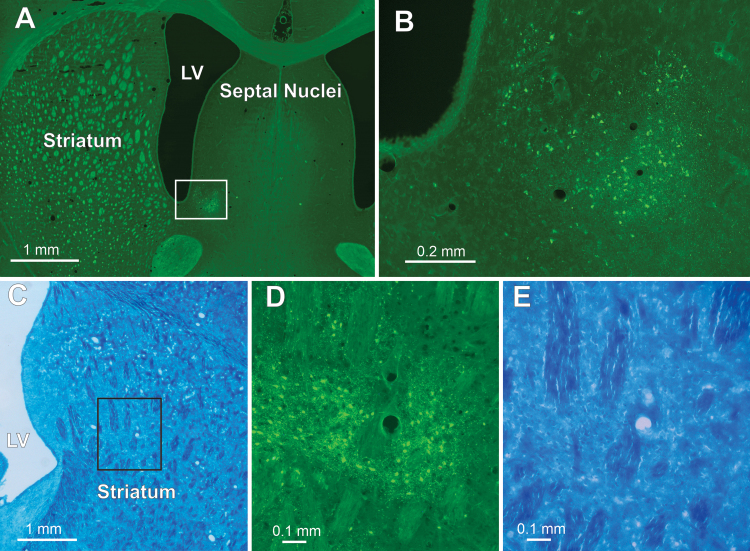
Figure 3 presents two more examples of targeted neuronal loss in Sprague Dawley rats. Two subcortical nuclei, the nucleus accumbens and the striatum, were targeted, and both exhibited focal neuronal degeneration in the region of sonication. Notably, blood vessels in the areas of neuronal loss in both of these nuclei appeared patent.
FIG. 3.
Degenerating neurons in the nucleus accumbens and striatum after PING. A and B: PING targeting the nucleus accumbens. Low-magnification image of a coronal section stained with Fluoro-Jade 5 days post-PING (A). The white rectangle indicates the area magnified in panel B. Degenerating neurons are seen in the targeted nucleus accumbens (B). C–E: PING targeting the striatum. Low-magnification image of a Luxol fast blue–stained (myelinated fibers) coronal section from a different animal in which PING targeted the striatum (C). The black rectangle in the striatum indicates the area magnified in panels D and E; the blood vessel in the middle of each of these panels can be used as a landmark for orientation. Fluoro-Jade staining showing degenerating neurons in the targeted area of the striatum (D). Luxol fast blue–stained fibers are still present in the area of sonication (E). LV = lateral ventricle.
Together, these findings demonstrate that a range of brain regions can be targeted effectively by PING, that intravenous administration of QA restricted to the postsonication period is effective in producing neuronal loss, that brain regions with differential barrier function to the circulation are not vulnerable to systemically administered QA in the absence of sonication, and that local vasculature is not destroyed.
Nonneuronal Cells Are Spared in Areas Targeted by PING
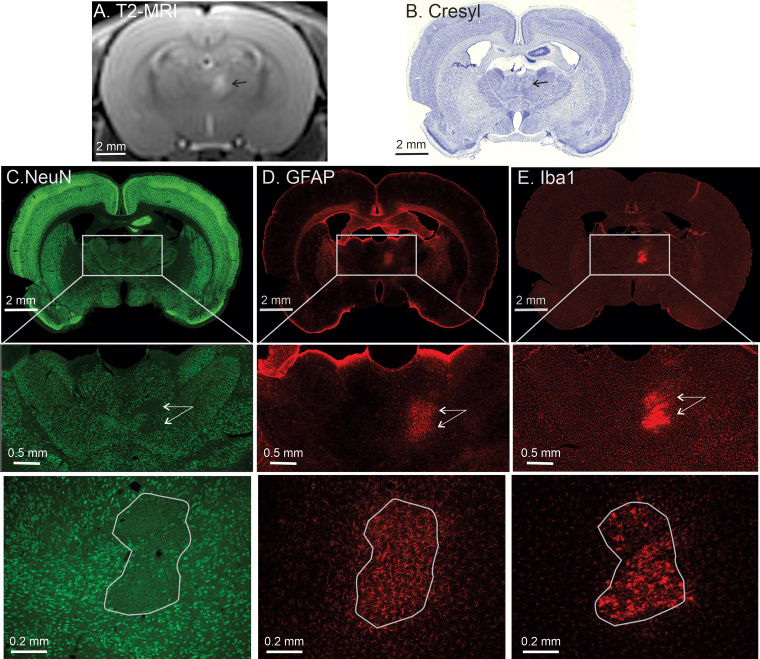
The issue of whether glial cells are destroyed by PING was examined by staining for astroglia and microglia in an area of PING-induced neuronal loss. Figure 4 shows tissue sections from an animal in which PING targeted the anteromedial thalamus. As with other regions of the brain, neuronal loss was observed in the targeted region. However, astroglia and microglia were not destroyed. Rather, both of these populations of cells are present in the area of the neuronal lesion and are undergoing typical injury responses to the loss of neighboring neurons. One caveat to the observations using anti-Iba1 immunohistochemistry is that this antibody recognizes macrophages of noncentral origin, as well as microglia. Thus, it is possible that some of the Iba1-positive cells in the area of neuronal loss are derived peripherally.
FIG. 4.
PING spares astroglia and microglia in the area of a neuronal lesion. A: Coronal T2-weighted MR image obtained 1 day post-PING showing an area of hyperintensity (black arrow) in the target area of the thalamus. B: A corresponding cresyl violet–stained tissue section from the same animal. One day post-PING, an area of hyperintensity (black arrow) is seen on the MR image. The same area is indicated (black arrow) on the cresyl violet–stained tissue section from the same animal prepared after a 5-day post-PING survival period. C–E: Immunohistochemical staining for healthy neurons (anti-NeuN; C), astroglia (anti-GFAP; D), and microglia/macrophages (anti-Iba1; E). The upper row shows a full coronal section containing the target area of PING. The rectangle in each of those sections denotes the magnified area in the center row of each column. Neuronal loss (i.e., an area sparse for NeuN-positive cells) is observed in the target area (white arrows). Astrogliosis and microgliosis are observed in the same area in the center row of panels D and E (white arrows). The lower row shows higher magnifications of the target region, containing an outline of the area of neuronal loss or gliosis.
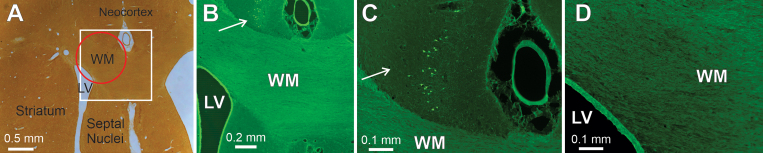
The issue of whether fibers of passage are spared in areas targeted by PING was evaluated in three brain regions. The first target, the striatum, contains large fiber bundles of axons of passage, representing a major component of the internal capsule. PING produced an area of degenerating neurons in the striatum but did not destroy fiber bundles passing through the same area (Fig. 3C–E). The next target, subcortical white matter, carries a variety of efferent and afferent pathways connecting the neocortex with subcortical regions and other neocortical regions. PING treatment did not appear to damage this major axon bundle (Fig. 5). Moreover, in this experiment, the size and targeting of the sonication were adjusted, such that the outer portion of the sonicated zone included two other structures: the inferior aspect of the overlying neocortex and the dorsal wall of the lateral ventricle. The lateral ventricle wall appeared intact after PING, but the limited portion of the overlying neocortex receiving sonication did exhibit neuronal loss (Fig. 5). Thus, neither the major axonal pathway nor the underlying ventricular wall appeared damaged, while the neighboring neocortex provided a positive control for the effect of the PING in creating neuronal loss.
FIG. 5.
PING targeting of the subcortical white matter (WM), lateral ventricle (LV), and neocortex. Coronal sections from the brain of a Sprague Dawley rat 7 days after PING are shown. A: An uncounterstained section photographed in bright field shows the location of the subcortical white matter, lateral ventricle, neocortex, striatum, and septal nuclei. The white square indicates the region magnified in panel B, and the red circle depicts the area of sonication. B–D: Fluoro-Jade staining in the same section as shown in panel A. A small number of degenerating neurons were observed in the neocortex overlying the white matter target (arrows; B and C). This area was within the dorsal aspect of the zone of sonication and indicates that sufficient QA reached the tissue to damage neurons. The white matter in the area of sonication does not appear shrunken compared with the neighboring unsonicated white matter (B). The dorsal wall of the lateral ventricle, which was also included in the area of sonication, appears intact (B and D).
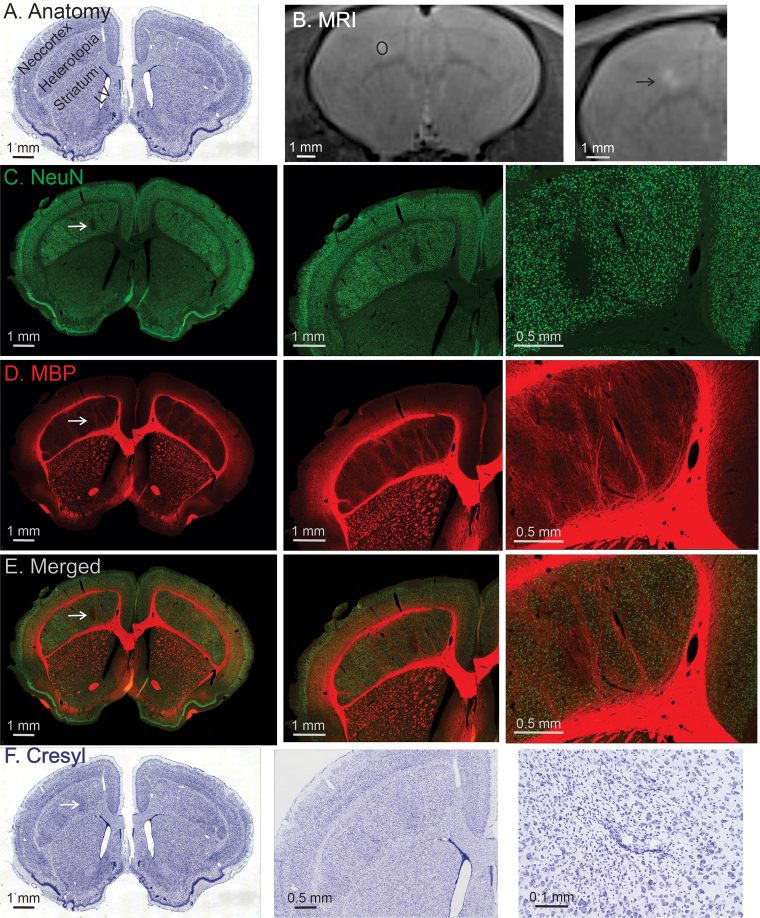
One issue regarding the assessment of intact versus degenerating axons at the two preceding targets is that the post-PING survival period used in these experiments (4–7 days) was optimized to observe degenerating neurons. While this survival period is effective for demonstrating degenerating neurons, it may have been too brief to unequivocally identify slower degeneration in myelinated pathways. We therefore addressed the issue of axon sparing at a longer survival time point by targeting a third region, a neocortical dysplasia (subcortical band heterotopia) in the tish rat brain.33 In this experiment, animals were allowed to survive for 2 weeks postsonication. This survival time is too long for utilizing Fluoro-Jade to identify degenerating neurons. Consequently, neuronal nuclei-specific staining for intact neurons was performed in conjunction with staining for MBP. This allowed for the assessment of where neurons had been lost and whether myelinated axons were spared in the same area, using a survival period sufficient for axonal degeneration to have occurred. Consistent with our findings shown in Fig. 2, focal neuronal loss was observed in the targeted area of the cortical dysplasia (Fig. 6). However, surviving MBP-positive axons were clearly present in the area of neuronal loss (Fig. 6). Nissl staining in the region of neuronal loss also demonstrated patent blood vessels and numerous small cells, presumably representing microglia/macrophages responding to the neuronal injury. Notably, no seizure activity was observed after any PING treatment.
FIG. 6.
PING produces axon-sparing neuronal lesions in dysplastic neocortex. A: The anatomical organization of the tish brain is depicted using a cresyl violet–stained coronal section. The brain contains a normally positioned neocortex, under which large heterotopia containing dysplastic neocortical neurons are located. The striatum and lateral ventricle are labeled for the purpose of orientation. B: Two magnifications of a T2-weighted MR image obtained 2 days postsonication, showing a region of hyperintensity consistent with damage to the target (black ellipse shows the targeted area). The black arrow indicates the site of sonication. C–F: Each row contains a sequence of the same image presented at increasing magnifications from the animal, which survived 2 weeks post-PING. White arrows indicate the site of sonication. NeuN staining labels healthy neurons, and a clear loss of neurons is observed in the area of sonication (C). MBP staining labels myelinated axons (D). MBP staining is retained in the area of sonication indicating a sparing of myelinated axons with PING. Merging the NeuN and MBP images shows that the area of neuronal loss contains myelinated axons (E). Cresyl violet is a general cell body stain (F). This Nissl-stained section demonstrates that the area of sonication has numerous small cells, presumed to be microglia and invading macrophages, while blood vessels exhibit intact walls.
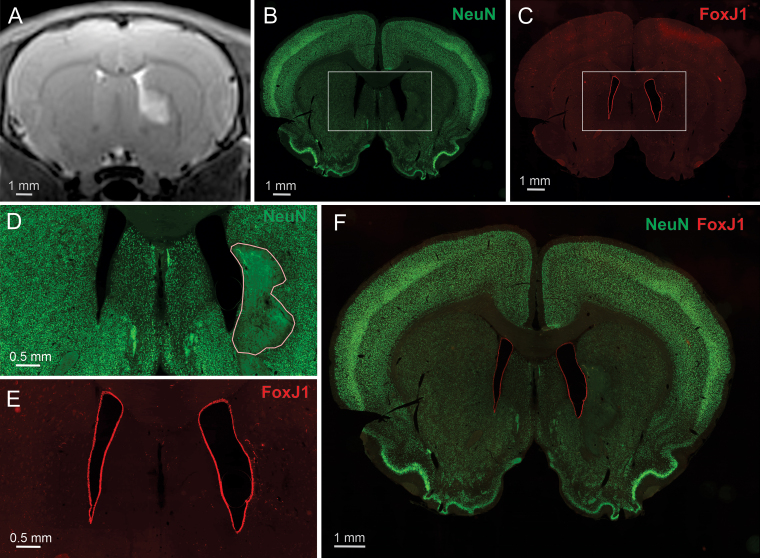
Cerebral ventricles are also a potential site of collateral damage during surgery. Consequently, the impact of PING was evaluated by targeting the medial striatum with an area of sonication that included the wall of the lateral ventricle. Figure 7 shows a loss of neurons in the parenchymal area targeted by PING, but a sparing of the wall of the lateral ventricle.
FIG. 7.
PING produces neuronal lesions that spare the ventricular wall. A: A coronal T2-weighted MR image taken 1 day postsonication that unilaterally targeted the lateral ventricle and adjacent medial striatum. Hyperintensity on the T2-weighted image is present in the targeted area of the striatum. B–F: Immunohistochemical staining of a coronal section taken from the same animal, which was euthanized 7 days postsonication. The rectangles shown in B and C depict the regions enlarged in D and E, respectively. Anti-NeuN, which stains healthy neurons (bright green), shows typical staining of neurons in most of the brain (B and D). Neuronal loss (outlined) is observed unilaterally in the area that exhibited hyperintensity in the T2 image (D). Anti-FoxJ1, which stains ventricular ependymal cells (red), exhibits continuous staining along the walls of the lateral ventricles bilaterally (C and E). F: An overlay image of both neuronal staining (green) and ependymal cell staining (red).
These findings demonstrate that PING can produce neuronal loss in normal and dysplastic neurons, while sparing key nonneuronal targets, including glia, axons of passage, blood vessels, and the ventricular wall.
Discussion
Targeted removal of aberrant neural tissue is a highly effective strategy for treating certain medically intractable neurological disorders. In the case of drug-resistant focal epilepsy, surgical intervention can reduce or eliminate seizures in appropriately selected candidates. However, a key remaining challenge for such procedures, be they invasive, minimally invasive, or noninvasive, is how to limit the risk of unintended neuropathological consequences in nontarget tissue. The complexity of CNS structure renders the surgical removal of a targeted neuronal pathway virtually impossible without affecting nontarget cellular elements at the site of removal and/or nontarget tissue neighboring the site of removal. The current study characterized a strategy designed to circumvent these critical challenges.
PING Strategy
We have recently developed a noninvasive approach for producing focal neuronal damage in the brain.30 Taking advantage of the ability of low-intensity MRgFUS to transiently and focally open the BBB, it was possible to provide targeted delivery of an otherwise BBB-impermeable neurotoxin to a restricted area of the brain parenchyma. The systemically administered neurotoxin, QA, then produced degeneration of neuronal cell bodies in the area of BBB opening. However, several important issues remained regarding the potential utility and safety of such an approach. First, could the neurotoxin be administered without risking injury to brain regions that have less-than-complete BBB function? In other words, would an area with restricted barrier function, such as the area postrema, be at risk for direct injury from the systemic neurotoxin? Second, is the strategy selective to vulnerable brain regions, or can it be effective in a broader range of brain areas? The original description of this approach focused on the hippocampus, which, under many conditions, is a selectively vulnerable area of the brain. The breadth of regions that can be effectively targeted would dictate the general utility of this strategy. Third, can this strategy produce neuronal injury that spares nontarget cellular elements in the area of treatment? Surgical approaches using resection, thermal injury, or radiation produce pannecrotic damage that can result in collateral damage to nontarget cells in the area of treatment and surrounding tissue. Finally, can the protocol for administering the neurotoxin be streamlined to better accommodate implementation in a clinical setting? Our original approach utilized intraperitoneal injections over a few days, which would be less-than-optimal for a clinical procedure. Is there a more efficient treatment protocol that produces desired outcomes?
Safety and Specificity
Two sets of findings address the issue of whether systemic neurotoxin administration by itself represents a risk to brain regions with specialized barrier function between the circulation and brain parenchyma. Circumventricular nuclei near the third ventricle did not exhibit degenerating neurons after systemic QA administration, even when sonication was shown to be effective in producing neuronal damage in other regions in the same brain (Fig. 2). In addition, assessments across the longitudinal axis of the brain, from the medulla to the frontal cortex, revealed only occasional degenerating neurons in the brain. Such cells were extremely rare, were not preferentially located in any particular area of the CNS, and are assumed to reflect spontaneous cell loss. Notably, degenerating neurons were not observed in what might be considered the most vulnerable brain region in terms of barrier function between the circulation and brain parenchyma (i.e., the area postrema; Fig. 2). The issue of whether PING is effective only in selectively vulnerable regions of the brain, such as the hippocampus, was addressed in multiple experiments. PING was demonstrated to produce focal neuronal loss when targeting a variety of cortical and subcortical regions (Figs. 2–7). These findings support the concept that PING can effectively target a wide range of brain areas. This is important for the potential utility of PING because its application would not be restricted to a limited number of vulnerable structures. The question of whether PING can produce neuronal loss but spare nonneuronal structures in an area of sonication is a key issue for the strategy. Perikaryal-specific neuronal degeneration produced by QA is generally thought to occur via an overactivation of NMDA receptors;32 however, additional mechanisms of injury have also been implicated.43 The findings herein indicate that PING spares axons of passage, glia, vasculature, and ventricular structures. Unlike comprehensive thermal lesions, which produce glial scaring surrounding the lesion site, reactive glial cells are located in the midst of the degenerating neurons after PING. As discussed above, this is an unmet challenge for surgical interventions treating medically intractable neurological disorders. None of the currently available surgical strategies for treating epilepsy would be able to produce an outcome of this type because they are pannecrotic in nature and would have destroyed the other nontarget structures. Thus, PING could broaden the treatment envelope to areas currently deemed inoperable. The issue of whether a more efficient protocol for administering the neurotoxin can be effective was addressed in all of the experiments in this study. Each of the animals received a single-phase administration of QA during the peak postsonication period of BBB opening. This was highly effective in producing neuronal loss while sparing nonneuronal structures. This is important in the context of the possible translation of the PING strategy because of the relative simplicity of a single-phase versus multiday administration protocol. Although systemic administration of QA in humans has not been examined, it is noteworthy that QA is well tolerated when injected systemically in mice and rats,30,31,44,45 presumably because of its negligible BBB permeability.31 Our data show that the area of BBB opening correlates well with the area of neuronal loss. An important goal for future investigation will be to define quantitatively the relation between volumes of BBB opening and extent of neuronal loss.
Several key issues remain for future studies characterizing the impact of PING. Central among these issues is the need to define the long-term effect of PING on the physiological function of surviving cells in a cellular milieu devoid of neurons. In addition to providing the substrate for communication among brain regions, neurons play important roles in the local regulation of glial and vascular function. For instance, the neurovascular unit is a fundamental component in the control of local blood flow in the CNS. It will be important to define how local blood flow is affected in an area retaining vascular and glial components but lacking neurons. In this context, it is also possible that newly generated neurons could colonize a depopulated area that retains the structural substrates for sustaining neurons. Thus, the long-term fate of an area in which selective neuronal loss has been created will be an important area for future research.
Conclusions
The current findings characterize a noninvasive strategy for producing neuronal lesions that spare axons of passage and nonneuronal structures. The approach is effective in a wide range of brain structures, including hippocampal neurons and dysplastic neocortical neurons, which are prime targets in cases of drug-resistant epilepsy. An additional advantage of this approach is that multiple sonications can be administered to a given target, allowing for contouring of the sonication pattern to treat irregularly shaped targets. Serial surgery at different targets with PING should also be feasible. Moreover, if a restricted lesion or series of lesions with PING were to prove ineffective, then standard-of-care resective or ablative surgery would remain an option. The nature of the PING approach could also improve the underutilization of epilepsy surgery. FUS treatments do not involve an invasive procedure or prolonged hospitalization, which could render PING more amenable to prospective patients. Notably, we have recently demonstrated that PING can reduce or eliminate seizures in animal models of temporal lobe epilepsy,44,45 and it will be important to define the efficacy of this approach in the presence of antiseizure drugs. Clearly, substantial additional investigation remains to be done to define the long-term functional impact of PING and its effects in models of neurological disorders, such as epilepsy. The hope for such studies is to move this strategy forward to augment currently available procedures used for the surgical treatment of medically intractable neurological disorders.
Acknowledgments
This work was supported by the National Institutes of Health (R01 NS102194 to K.S.L. and R01 CA217953-01 to M.W.), the Chester Fund (K.S.L.), and the Focused Ultrasound Foundation (K.S.L. and J.W.).
We thank Rene Roy, Department of Radiology, University of Virginia, for outstanding MRI technical support.
Disclosures
Drs. Lee, Wintermark, and Bertram: coinventors on a pending patent for the PING procedure. Dr. Klibanov: shareholder in Targeson (now dissolved) and non–study-related clinical or research effort from SoundPipe Therapeutics. Dr. Dumont: ownership in Image Guided Therapy.
Author Contributions
Conception and design: Lee, Bertram, Wintermark. Acquisition of data: Wang, Anzivino, Zhang, Woznak, Klibanov, Dumont. Analysis and interpretation of data: Lee, Wang, Anzivino, Woznak. Drafting the article: Lee, Woznak. Critically revising the article: Lee, Woznak. Reviewed submitted version of manuscript: Lee, Anzivino. Approved the final version of the manuscript on behalf of all authors: Lee.
References
- 1. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 2. Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 2018;75(3):279–286. doi: 10.1001/jamaneurol.2017.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 4. Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7(6):525–537. doi: 10.1016/S1474-4422(08)70109-1. [DOI] [PubMed] [Google Scholar]
- 5. Engel J, Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307(9):922–930. doi: 10.1001/jama.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi H, Sell RL, Lenert L, Muennig P, Goodman RR, Gilliam FG, Wong JB. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy: a decision analysis. JAMA. 2008;300(21):2497–2505. doi: 10.1001/jama.2008.771. [DOI] [PubMed] [Google Scholar]
- 7. Sheikh S, Thompson N, Bingaman W, Gonzalez-Martinez J, Najm I, Jehi L. (Re)Defining success in epilepsy surgery: the importance of relative seizure reduction in patient-reported quality of life. Epilepsia. 2019;60(10):2078–2085. doi: 10.1111/epi.16327. [DOI] [PubMed] [Google Scholar]
- 8. Sperling MR, Barshow S, Nei M, Asadi-Pooya AA. A reappraisal of mortality after epilepsy surgery. Neurology. 2016;86(21):1938–1944. doi: 10.1212/WNL.0000000000002700. [DOI] [PubMed] [Google Scholar]
- 9. Engel J., Jr The timing of surgical intervention for mesial temporal lobe epilepsy: a plan for a randomized clinical trial. Arch Neurol. 1999;56(11):1338–1341. doi: 10.1001/archneur.56.11.1338. [DOI] [PubMed] [Google Scholar]
- 10. Engel J., Jr Surgical treatment for epilepsy: too little, too late? JAMA. 2008;300(21):2548–2550. doi: 10.1001/jama.2008.756. [DOI] [PubMed] [Google Scholar]
- 11. de Flon P, Kumlien E, Reuterwall C, Mattsson P. Empirical evidence of underutilization of referrals for epilepsy surgery evaluation. Eur J Neurol. 2010;17(4):619–625. doi: 10.1111/j.1468-1331.2009.02891.x. [DOI] [PubMed] [Google Scholar]
- 12. Englot DJ, Ouyang D, Garcia PA, Barbaro NM, Chang EF. Epilepsy surgery trends in the United States, 1990-2008. Neurology. 2012;78(16):1200–1206. doi: 10.1212/WNL.0b013e318250d7ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wiebe S, Jette N. Pharmacoresistance and the role of surgery in difficult to treat epilepsy. Nat Rev Neurol. 2012;8(12):669–677. doi: 10.1038/nrneurol.2012.181. [DOI] [PubMed] [Google Scholar]
- 14. Rydenhag B, Silander HC. Complications of epilepsy surgery after 654 procedures in Sweden, September 1990-1995: a multicenter study based on the Swedish National Epilepsy Surgery Register. Neurosurgery. 2001;49(1):51–57. doi: 10.1097/00006123-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 15. Helmstaedter C, Van Roost D, Clusmann H, Urbach H, Elger CE, Schramm J. Collateral brain damage, a potential source of cognitive impairment after selective surgery for control of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2004;75(2):323–326. [PMC free article] [PubMed] [Google Scholar]
- 16. McClelland S, III, Guo H, Okuyemi KS. Population-based analysis of morbidity and mortality following surgery for intractable temporal lobe epilepsy in the United States. Arch Neurol. 2011;68(6):725–729. doi: 10.1001/archneurol.2011.7. [DOI] [PubMed] [Google Scholar]
- 17. Hader WJ, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, Wiebe S, Kwon CS, Jette N. Complications of epilepsy surgery: a systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia. 2013;54(5):840–847. doi: 10.1111/epi.12161. [DOI] [PubMed] [Google Scholar]
- 18. Benbadis SR, Heriaud L, Tatum WO, Vale FL. Epilepsy surgery, delays and referral patterns-are all your epilepsy patients controlled? Seizure. 2003;12(3):167–170. doi: 10.1016/s1059-1311(02)00320-5. [DOI] [PubMed] [Google Scholar]
- 19. Hakimi AS, Spanaki MV, Schuh LA, Smith BJ, Schultz L. A survey of neurologists’ views on epilepsy surgery and medically refractory epilepsy. Epilepsy Behav. 2008;13(1):96–101. doi: 10.1016/j.yebeh.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 20. Erba G, Moja L, Beghi E, Messina P, Pupillo E. Barriers toward epilepsy surgery. A survey among practicing neurologists. Epilepsia. 2012;53(1):35–43. doi: 10.1111/j.1528-1167.2011.03282.x. [DOI] [PubMed] [Google Scholar]
- 21. Burneo JG, Shariff SZ, Liu K, Leonard S, Saposnik G, Garg AX. Disparities in surgery among patients with intractable epilepsy in a universal health system. Neurology. 2016;86(1):72–78. doi: 10.1212/WNL.0000000000002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steinbrenner M, Kowski AB, Holtkamp M. Referral to evaluation for epilepsy surgery: reluctance by epileptologists and patients. Epilepsia. 2019;60(2):211–219. doi: 10.1111/epi.14641. [DOI] [PubMed] [Google Scholar]
- 23. Willie JT, Laxpati NG, Drane DL, Gowda A, Appin C, Hao C, et al. Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery. 2014;74(6):569–585. doi: 10.1227/NEU.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang JY, Wu C, Tracy J, Lorenzo M, Evans J, Nei M, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. 2016;57(2):325–334. doi: 10.1111/epi.13284. [DOI] [PubMed] [Google Scholar]
- 25. Hoppe C, Witt JA, Helmstaedter C, Gasser T, Vatter H, Elger CE. Laser interstitial thermotherapy (LiTT) in epilepsy surgery. Seizure. 2017;48:45–52. doi: 10.1016/j.seizure.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 26. Jermakowicz WJ, Kanner AM, Sur S, Bermudez C, D’Haese PF, Kolcun JPG, et al. Laser thermal ablation for mesiotemporal epilepsy: analysis of ablation volumes and trajectories. Epilepsia. 2017;58(5):801–810. doi: 10.1111/epi.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu C, Jermakowicz WJ, Chakravorti S, Cajigas I, Sharan AD, Jagid JR, et al. Effects of surgical targeting in laser interstitial thermal therapy for mesial temporal lobe epilepsy: a multicenter study of 234 patients. Epilepsia. 2019;60(6):1171–1183. doi: 10.1111/epi.15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Drane DL, Loring DW, Voets NL, Price M, Ojemann JG, Willie JT, et al. Better object recognition and naming outcome with MRI-guided stereotactic laser amygdalohippocampotomy for temporal lobe epilepsy. Epilepsia. 2015;56(1):101–113. doi: 10.1111/epi.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Drane DL. MRI-guided stereotactic laser ablation for epilepsy surgery: promising preliminary results for cognitive outcome. Epilepsy Res. 2018;142:170–175. doi: 10.1016/j.eplepsyres.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Tan H, Bertram EH, Aubry JF, Lopes MB, Roy J, et al. Non-invasive, focal disconnection of brain circuitry using magnetic resonance-guided low-intensity focused ultrasound to deliver a neurotoxin. Ultrasound Med Biol. 2016;42(9):2261–2269. doi: 10.1016/j.ultrasmedbio.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 31. Foster AC, Miller LP, Oldendorf WH, Schwarcz R. Studies on the disposition of quinolinic acid after intracerebral or systemic administration in the rat. Exp Neurol. 1984;84(2):428–440. doi: 10.1016/0014-4886(84)90239-5. [DOI] [PubMed] [Google Scholar]
- 32. Schwarcz R, Whetsell WO, Jr, Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219(4582):316–318. doi: 10.1126/science.6849138. [DOI] [PubMed] [Google Scholar]
- 33. Lee KS, Schottler F, Collins JL, Lanzino G, Couture D, Rao A, et al. A genetic animal model of human neocortical heterotopia associated with seizures. J Neurosci. 1997;17(16):6236–6242. doi: 10.1523/JNEUROSCI.17-16-06236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang W, Zhang Y, Anzivino MJ, Bertram EH, Woznak J, Klibanov A, et al. Targeted neuronal injury for the non-invasive disconnection of brain circuitry. J Vis Exp. 2020;163(163). doi: 10.3791/61271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klibanov AL. Microbubble contrast agents: targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Invest Radiol. 2006;41(3):354–362. doi: 10.1097/01.rli.0000199292.88189.0f. [DOI] [PubMed] [Google Scholar]
- 36. Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220(3):640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 37. McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits. Ultrasound Med Biol. 2005;31(11):1527–1537. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 38. Vlachos F, Tung YS, Konofagou EE. Permeability assessment of the focused ultrasound-induced blood-brain barrier opening using dynamic contrast-enhanced MRI. Phys Med Biol. 2010;55(18):5451–5466. doi: 10.1088/0031-9155/55/18/012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park J, Zhang Y, Vykhodtseva N, Jolesz FA, McDannold NJ. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J Control Release. 2012;162(1):134–142. doi: 10.1016/j.jconrel.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chai WY, Chu PC, Tsai MY, Lin YC, Wang JJ, Wei KC, et al. Magnetic-resonance imaging for kinetic analysis of permeability changes during focused ultrasound-induced blood-brain barrier opening and brain drug delivery. J Control Release. 2014;192:1–9. doi: 10.1016/j.jconrel.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 41. Heye AK, Culling RD, Valdés Hernández MC, Thrippleton MJ, Wardlaw JM. Assessment of blood-brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. Neuroimage Clin. 2014;6(6):262–274. doi: 10.1016/j.nicl.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chu PC, Chai WY, Tsai CH, Kang ST, et al. Focused ultrasound-induced blood-brain barrier opening: association with mechanical index and cavitation index analyzed by dynamic contrast-enhanced magnetic-resonance. Imaging Sci Rep. 2016;6:33264. doi: 10.1038/srep33264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lugo-Huitrón R, Ugalde Muñiz P, Pineda B, Pedraza-Chaverrí J, Ríos C, Pérez-de la Cruz V. Quinolinic acid: an endogenous neurotoxin with multiple targets. Oxid Med Cell Longev. 2013;2013:104024. doi: 10.1155/2013/104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Y, Zhou H, Qu H, Jiang H, Huang S, Ghobadi SN, et al. Effects of non-invasive, targeted, neuronal lesions on seizures in a mouse model of temporal lobe epilepsy. Ultrasound Med Biol. 2020;46(5):1224–1234. doi: 10.1016/j.ultrasmedbio.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang Y, Buckmaster PS, Qiu L, Wang J, Keunen O, Ghobadi SN, et al. Non-invasive, neurotoxic surgery reduces seizures in a rat model of temporal lobe epilepsy. Exp Neurol. 2021;343:113761. doi: 10.1016/j.expneurol.2021.113761. [DOI] [PMC free article] [PubMed] [Google Scholar]