Abstract
MicroRNAs (miRNAs) regulate gene expression post-transcriptionally and circulate in the blood, making them attractive biomarkers of disease state for tissues like bone that are challenging to interrogate directly. Here we report on five miRNAs – miR-197–3p, miR-320a, miR-320b, miR-331–5p, and miR-423–5p – associated with bone mineral density (BMD) in 147 healthy adult baboons. These baboons ranged in age from 15 to 25 years (45 to 75 human equivalent years) and 65% were female with a broad range of BMD values including a minority of osteopenic animals. miRNAs were generated via RNA sequencing from buffy coats collected at necropsy and areal BMD (aBMD) measured post-mortem via dual-energy x-ray absorptiometry (DXA) of the lumbar vertebrae. Differential expression analysis controlled for the underlying pedigree structure of these animals to account for genetic variation which may drive miRNA abundance and aBMD values. While many of these miRNAs have been associated with risk of osteoporosis in humans, this finding is of interest because the cohort represents a model of normal aging and bone metabolism rather than a disease cohort. The replication of miRNA associations with osteoporosis or other bone metabolic disorders in animals with healthy aBMD suggests an overlap in normal variation and disease states. We suggest that these miRNAs are involved in the regulation of cellular proliferation, apoptosis, and protein composition in the extracellular matrix throughout life; and age-related dysregulation of these systems may lead to disease. These miRNAs may be early indicators of progression to disease in advance of clinically detectible osteoporosis.
Keywords: bone mineral density, miRNA, non-human primate
Introduction
Research interest in microRNAs (miRNAs) has exploded in the past decade alongside recognition of the potential for these small circulating RNAs to act as biomarkers of disease state or cellular messengers. As post-transcriptional regulators, these small non-coding RNAs are uniquely able to regulate gene expression in the cytoplasm and may travel among cells to do so. This unique ability to travel between cells – sometimes in membrane-bound extracellular vesicles1 – allows for the detection of potentially functional miRNAs in the peripheral blood in contrast to other omic technologies like transcriptomics and proteomics which require access to the relevant tissues. The relatively non-invasive nature of peripheral blood miRNA assessments makes them attractive biomarkers for clinical applications. Indeed, a panel of miRNA biomarkers aimed at osteoporosis diagnosis in postmenopausal women is now commercially available2. In human studies, circulating miRNAs have been implicated in bone formation, turnover and homeostatic maintenance, osteoporosis, and fracture risk3–7.
While a number of studies have focused on the potential of miRNAs as biomarkers for osteoporosis and fracture risk, little is understood about miRNAs in the context of normal variation in spinal bone mineral density (BMD) among healthy adults. Here we report on five circulating miRNAs that are associated with variation in BMD within the healthy range for middle-aged and older baboons.
The baboon is a unique model for age-related bone loss, as it is closely related phylogenetically to humans, is relatively large bodied, and exhibits intracortical remodeling throughout life, unlike rodent models of skeletal aging. The adult bone remodeling and skeletal fracture properties of baboons are also more similar to humans than are other mid- to large-sized mammals 8,9. Like humans, female baboons undergo bone loss associated with late-in-life hormonal shifts and sex and age influence BMD 10 leading to osteopenia in approximately 25% of older females 11. Furthermore, biomechanical properties directly relevant to fracture, including vertebral trabecular bone mechanical properties 12, femoral cortical bone microstructure 13, and femoral bone shape 14, are strongly heritable in the baboon, making pedigreed animals ideal for assessing the effects of genetic and non-genetic factors related to bone biomarkers and bone fragility 15. We leveraged the unique anatomical and genetic advantages of the baboon as a model of aging bone to identify circulating miRNAs associated with BMD across a broad range of mildly osteopenic and healthy adult baboons while controlling for underlying genetic factors.
Methods
Study Population
We generated miRNA and BMD data for 147 middle-aged and older baboons (hybrid Papio hamadryas species) drawn from a larger pedigree of baboons housed at the Southwest National Primate Center (SNPRC) at Texas Biomedical Research Institute 16. The sample is 65% female and ranged in age from 15 to 25 years of age at necropsy which is the equivalent of approximately 45 to 75 human years (Figure 1).
Figure 1.
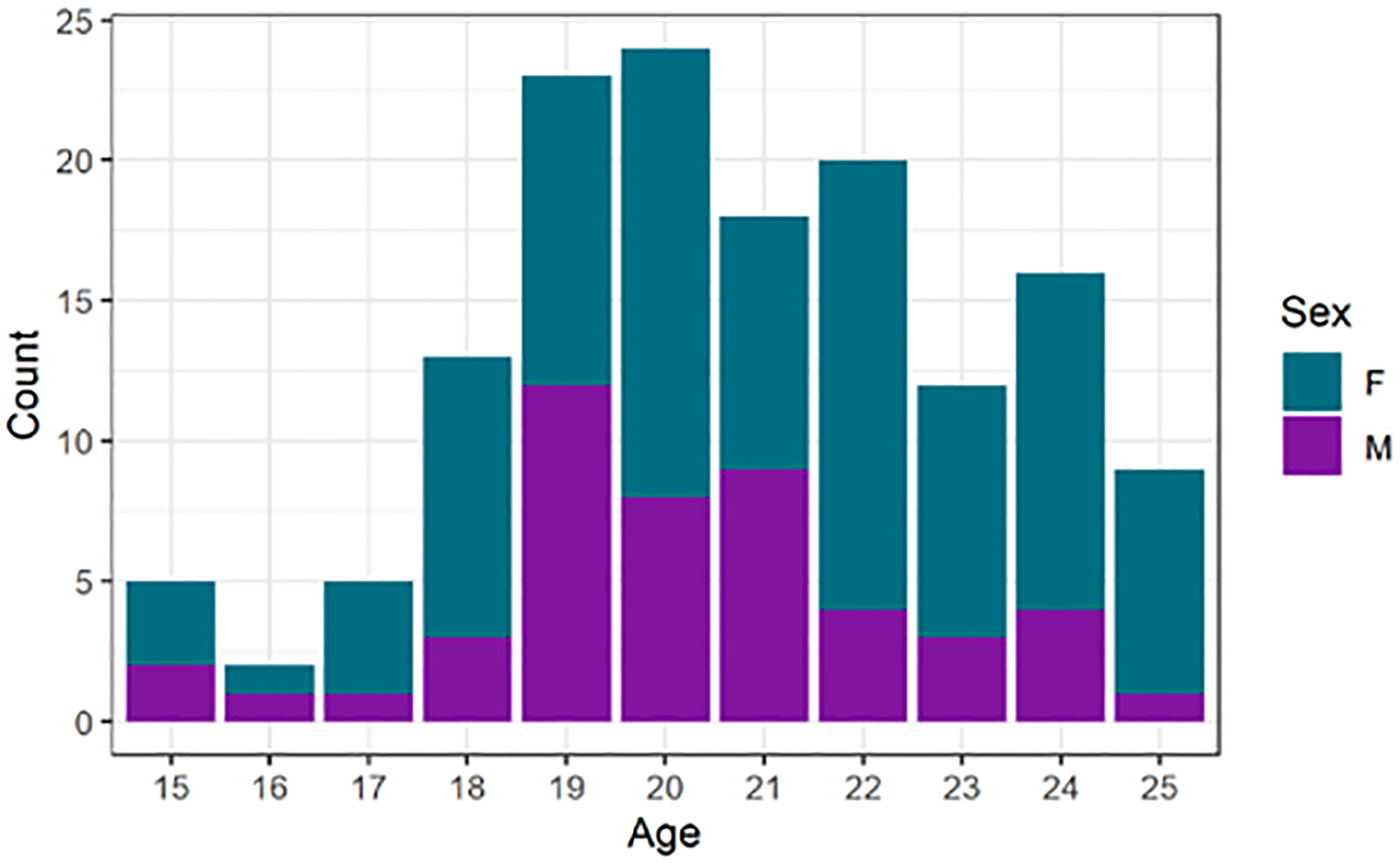
Distribution of animals in sample by age and sex.
During life, all animals were housed outdoors in large social group cages and maintained on commercial monkey chow to which they had ad libitum access. Animals with medical conditions known to influence bone metabolism (e.g. diabetes, chronic renal disease) or a history of traumatic fracture were excluded. No animals were sacrificed for this study; all were euthanized for other reasons. All animal procedures were approved by the Institutional Animal Care and Use Committee at Texas Biomedical Research Institute and conducted in Association for Assessment and Accreditation of Laboratory Animal Care approved facilities at the SNPRC. Blood samples were drawn in EDTA tubes at necropsy and processed buffy coat layers stored at -80̊C until RNA was extracted. Lumbar spines were frozen immediately after necropsy and stored at -20̊C until thawed for analysis.
DXA
Dual-energy x-ray absorptiometry (DXA) scans were performed post-mortem on thawed lumbar vertebrae17,18 using a Lunar DPX 6529 (General Electric) scanner and areal BMD (aBMD) was determined in the anterior-posterior (AP) direction for L4-L2 using the manufacturer’s software for adults. To account for sexual dimorphism in aBMD, we performed z-scoring on male and female aBMD values separately and combined the scaled values for analysis.
miRNA Sequencing and Analysis
Total RNA was isolated from buffy coat using TRIzol (Invitrogen) and the Qiagen miRNeasy Mini Kit as described previously 19. RNA quality was assessed using an Agilent Bioanalyzer 2100 with RNA Integrity Number (RIN) values greater than or equal to 8.0 for all samples. RNA was enriched for small non-coding RNAs (sncRNAs) by using the Ambion mirVana miRNA Isolation Kit. Complementary DNA (cDNA) libraries were generated with the Illumina Small RNA Prep Kit v1.5 following the manufacturer’s protocol and sequenced in Illumina’s Genome Analyzer (GAIIx) 20. mirDeep2 21 was used to align reads to the known human miRbase version 21 22,23 mature and hairpin miRNAs and to identify novel miRNAs. We aligned to the human miRNAs because miRNAs remain relatively poorly annotated in all non-human primate species. We required a log odds score of 4 or greater for sequence matching to ensure that miRNAs with low homology between humans and baboons were not included in the analysis.
Raw miRNA counts were analyzed using the R statistical package DESeq2 to identify individual miRNAs associated with aBMD 24,25. DESeq2 has the advantage of incorporating shrinkage estimators for dispersion and fold change which better handles low-abundance miRNAs compared to other methods. After z-scoring, weight and age were no longer associated with aBMD so these values were not included as covariates. miRNAs significantly associated with aBMD at a false discovery rate (FDR) < 0.05 were further tested to determine if this association was driven by underlying genetic variation in the pedigree. A likelihood ratio test was calculated to compare linear mixed effects models for z-scored aBMD as the outcome variable fit with lmekin in the coxme R package 26 with and without miRNA abundance as a fixed effect while including pedigree-based kinship as a random effect.
miRNA Target Prediction
A major challenge in understanding the potential functional role of a circulating miRNA is that most are computationally predicted to bind dozens of different mRNAs. To better evaluate roles these circulating miRNAs could play in bone biology, we used the R package multiMiR 27 to query three experimentally validated miRNA target databases – miRecords 28, miRTarBase 29, and TarBase 30. To be considered a potential target we required experimental validation of miRNA-mRNA interaction via luciferase, qRT-PCR, or Western blot experiments as well as the presence of the target protein in baboon bone (Supplementary Table 1).
Results
Bone Mineral Density
Baboons exhibit high levels of sexual dimorphism in weight and body size leading to significant differences in mean aBMD (0.22 g/cm2, p = 1×10−6, Figure 2). The distribution of aBMD in these animals is consistent with previously published reference standards from more than 650 baboons 10. Within the 10-year agespan of our study cohort, there is no significant decline in aBMD. Nevertheless, the distribution of aBMD across the sample is broad, ranging from 4.3 standard deviations below to 8.4 standard deviations above the healthy, young adult mean for females and −3.5 to +9.3 standard deviation for males.
Figure 2.
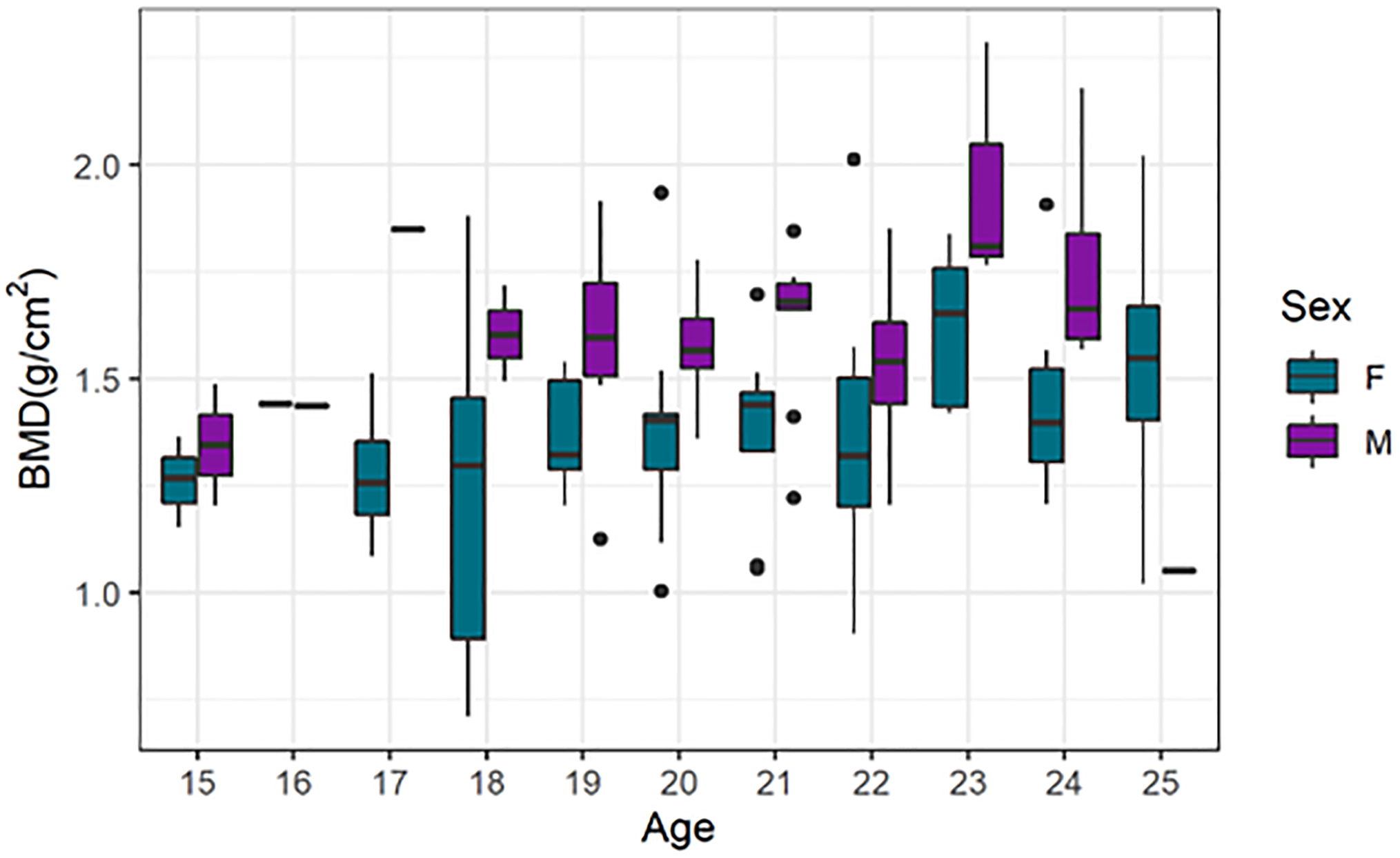
Distribution of aBMD in study sample by age and sex.
miRNAs Associations
After FDR correction, 15 miRNAs were significantly associated with aBMD in the DESeq2 analysis of which five were associated (likelihood ratio test p < 0.1) after correcting for the genetic relatedness of the animals (Table 1). All five of these highly associated miRNAs– miR-197–3p, miR-320a, miR-320b, miR-331–5p, and miR-423–5p – are negatively correlated with aBMD (Figure 3), suggesting that increased levels of these miRNAs, which would be expected to decrease mRNA expression, are indicative of a decline in aBMD. Individually, these miRNAs do not account for a large proportion of the overall variance in aBMD as highlighted by the broad distribution of aBMD among individuals with similar miRNA counts. However, these miRNAs are part of broader patterns of differential miRNA expression (Figure 4).
Table 1.
miRNAs significantly associated with aBMD after FDR correction in DESeq2 analysis. Bolded miRNAs remained significantly associated after correction for pedigree structure.
| β | Mean Expression | p | LRT p | |
|---|---|---|---|---|
|
| ||||
| hsa-miR-423–5p | −0.28 | 886 | 2.4X10 −4 | 0.03 |
| hsa-miR-320a | −0.22 | 2,561 | 3.9X10 −3 | 0.04 |
| hsa-miR-339–5p | −0.19 | 1,697 | 6.2X10−3 | 0.22 |
| hsa-miR-3173–5p | −0.38 | 16 | 3.0X10−2 | 0.14 |
| hsa-miR-371a-5p | 0.6 | 7 | 3.0X10−2 | 0.40 |
| hsa-miR-16–2-3p | −0.44 | 137 | 3.3X10−2 | 0.27 |
| hsa-miR-197–3p | −0.18 | 577 | 3.5X10 −2 | 0.09 |
| hsa-miR-21–5p | 0.16 | 30,037 | 3.5X10−2 | 0.68 |
| hsa-miR-331–5p | −0.09 | 808 | 3.5X10 −2 | 0.07 |
| hsa-miR-374a-3p | 0.26 | 231 | 3.5X10−2 | 0.88 |
| hsa-miR-374a-5p | 0.19 | 4,255 | 3.5X10−2 | 0.92 |
| hsa-miR-424–3p | −0.16 | 86 | 3.5X10−2 | 0.10 |
| hsa-miR-484 | −0.13 | 2,334 | 3.5X10−2 | 0.19 |
| hsa-miR-320b | −0.21 | 28 | 3.6X10 −2 | 0.05 |
| hsa-miR-574–3p | −0.16 | 94 | 4.1X10−2 | 0.25 |
Figure 3.
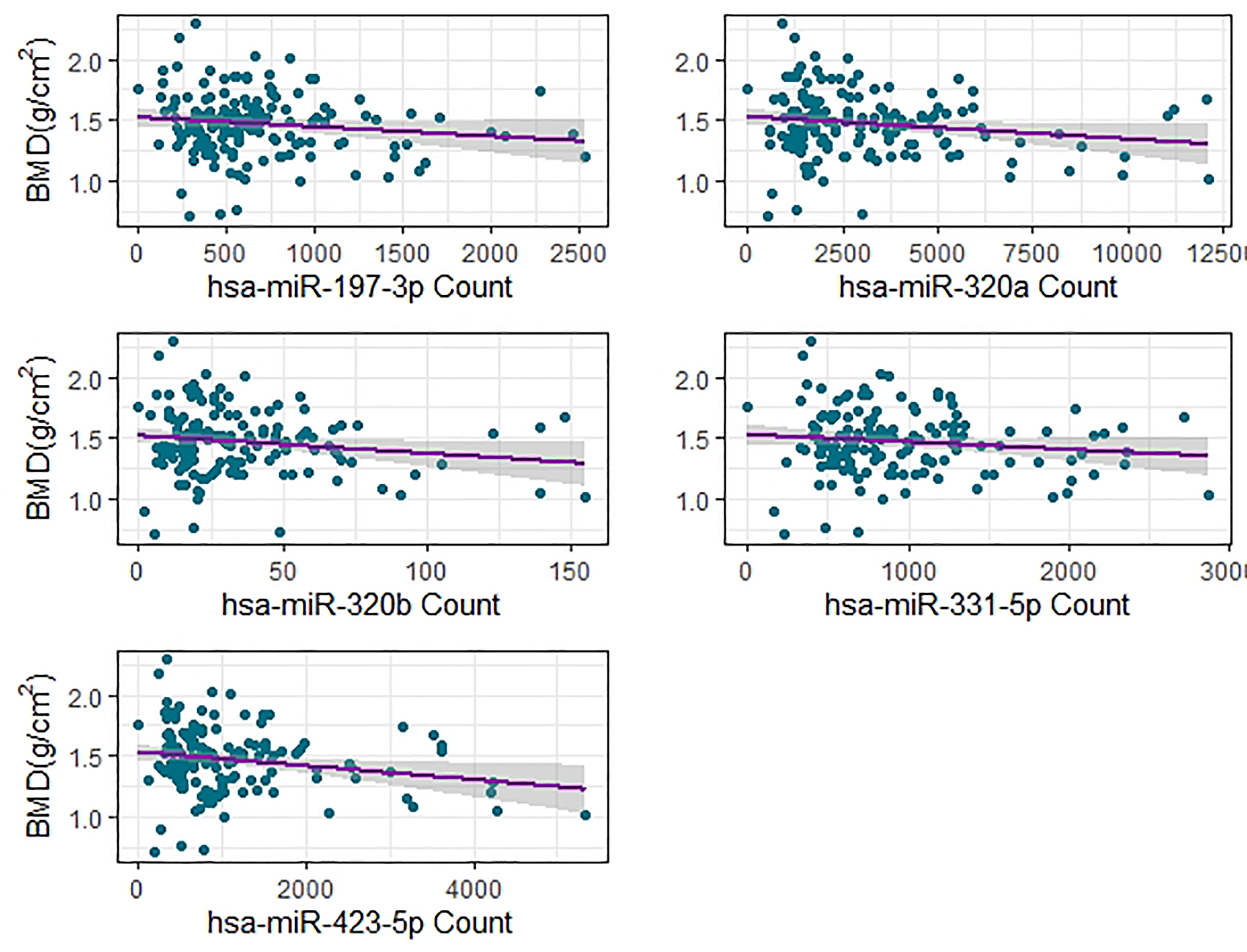
miRNA abundance versus aBMD for significantly associated miRNAs.
Figure 4.
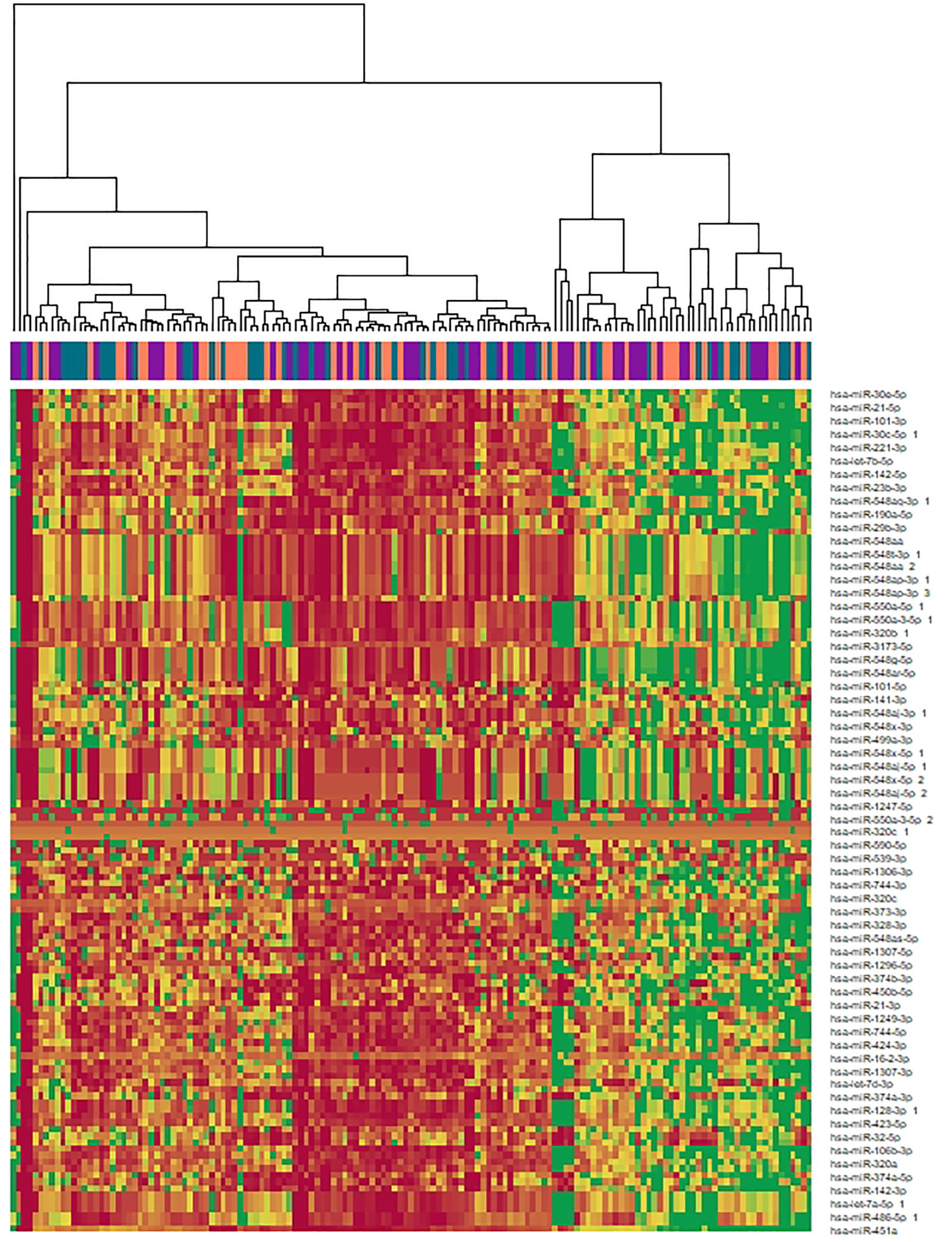
Heatmap of all miRNAs with nominal p < 0.05 in DeSeq2. Columns are color-coded by z-scored aBMD tertile with purple indicating the lowest tertile and teal the highest.
Predicted miRNA targets
Based on our screening criteria, we identified 60 genes present in bone and experimentally validated as potential targets of our five significant miRNAs (Supplementary Table 2). Analysis in DAVID 31 identified several significantly enriched functional annotations within the 60 genes. The top five enriched gene ontology terms representing more than 10% of the genes on the list are myelin sheath (26.4 fold enrichment, FDR = 6.1×10−10), focal adhesion (11.2 fold enrichment, FDR = 2.9×10−7), extracellular matrix (11.1 fold enrichment, FDR = 3.6×10−5), cadherin binding involved in cell-cell adhesion (9.5 fold enrichment, FDR = 9.0×10−4), and cell-cell adherens junction (9.0 fold enrichment, FDR = 5.4×10−4). Additionally, these putative targets include seven genes previously linked to osteoporosis in genome wide association studies based on data drawn from the Public Health Genomics and Precision Health Knowledge Base (v7.1) 32 (Table 2).
Table 2.
miRNA targets linked to osteoporosis in genome wide association studies
| GENE NAME | MIRNA |
|---|---|
|
| |
| FGB | hsa-miR-197–3p |
| IGFBP5 | hsa-miR-197–3p |
| SOD1 | hsa-miR-197–3p |
| SOD2 | hsa-miR-197–3p hsa-miR-331–5p |
| GAPDH | hsa-miR-320a hsa-miR-423–5p |
| MIF | hsa-miR-320a |
| MMP9 | hsa-miR-320a |
Discussion
Most previous research on bone density and miRNAs has focused on patients with osteoporosis or other disorders of bone metabolism rather than aBMD variation among healthy individuals. This bias in the literature is apparent in the fact that many of our healthy aBMD-associated miRNAs have been suggested as potential clinical biomarkers in humans.
Of the miRNAs we identified, literature linking the miRNA-320 family to bone fragility is the most robust. miR-320b is more common in women with a recent fracture compared to age-matched controls 33. This may be due to its role in osteoblast differentiation. miR-320b over-expression prevents osteoblast differentiation, while inhibition promotes bone matrix mineralization and differentiation via BMP-234. Additionally, researchers studying low-energy fractures in premenopausal, postmenopausal, and idiopathic osteoporosis patients found miR-320a was upregulated in these patients compared to controls (Kocijan et al., 2016). Prior analysis of trabecular bone tissue showed significant dysregulation of miR-320a in patients with osteoporosis, possibly via regulation of osteogenesis by targeting bone-forming genes such as CTNNB1 (B-catenin) and RUNX2 35. These findings led to the inclusion of miR-320a on the commercially available OsteomiR panel (Tamirna).
Similarly, in a study of plasma miRNAs associated with fracture risk, miR-423–5p expression was significantly negatively associated with FRAX score, but not BMD, in osteoporosis patients 36. This finding was reinforced by research on facial bone atrophy where miR-423–5pwas shown to promote bone proliferation via prevention of apoptosis in mesenchymal stem cells 37. While there is little additional evidence of a role for this miRNA in bone, it has been linked to regulation of apoptosis in cardiomyocytes 38, kidney cells 39, retinal pigment epithelial cells 40, and colon cancer cells 41.
Circulating miR-331–5p levels have been identified as a potential biomarker for osteoporosis and subsequent bone fracture 42, although little is known about the role of this miRNA in bone. Hints to its function come from studies of vascular smooth muscle cells, where miR-331–5p is induced by BMP2-PPARγ signaling to regulate cellular proliferation 43. We predict a role for miR-331–5p in regulating SOD2 expression which directly induces a BMP2 response under hypoxic conditions 44.
While miR-197–3p has not previously been linked to adult bone metabolism, it was identified in the downregulation of osteogenesis in human amniotic membrane-derived mesenchymal stem cells due to its suppression of SMAD2 in the TGF-β pathway during osteoblast differentiation 45. We predict that miR-197–3p may target expression of both SOD1 and SOD2, antioxidative enzymes that respond to vitamin D levels 46 and are thought to play a critical role in the regulation of cellular senescence 47,48.
Taken together, our aBMD-associated miRNAs and their putative targets point towards regulation of extracellular matrix proteins, apoptosis, and cell proliferation – three key components in the maintenance of bone homeostasis. Our findings demonstrate the overlap in miRNAs associated with bone mineral density and related traits in humans and nonhuman primates, highlighting the utility of this model for understanding aging bone. The association of these miRNAs with variation in areal bone mineral density within the healthy, non-osteoporotic, range suggests they may be useful in identifying the earliest stages of preclinical metabolic shifts in bone. While the impact of epigenetic regulation of gene expression is evident in bone tissue, the use of miRNAs as biomarkers for low aBMD and high fracture risk is still relatively new. It is apparent more research is needed to better understand these molecular pathways. Future work should focus on identifying the presence and functional role of these miRNAs in bone tissue to solidify their promise as biomarkers.
Supplementary Material
Acknowledgements
We thank Lorena Havill for her role in the development of this project. This work was funded by R01 AR064244 to TLB and K01 AG056663 to EEQ. Care of animals in the SNPRC pedigree was supported by grant P51 OD011133.
References
- 1.Li Q, Huang QP, Wang YL & Huang QS Extracellular vesicle-mediated bone metabolism in the bone microenvironment. J. Bone Miner. Metab. 36, 1–11 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Kerschan-Schindl K et al. Diagnostic Performance of a Panel of miRNAs (OsteomiR) for Osteoporosis in a Cohort of Postmenopausal Women. Calcif. Tissue Int. 108, 725–737 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J et al. microRNA-Mediated Regulation of Bone Remodeling: A Brief Review. JBMR Plus 3, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellavia D et al. Deregulated miRNAs in bone health: Epigenetic roles in osteoporosis. Bone 122, 52–75 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Grillari J et al. Circulating miRNAs in bone health and disease. Bone 145, 115787 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Gao Y, Patil S & Qian A The role of micrornas in bone metabolism and disease. Int. J. Mol. Sci. 21, 1–23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAlinden A & Im G Il. MicroRNAs in orthopaedic research: Disease associations, potential therapeutic applications, and perspectives. J. Orthop. Res. 36, 33–51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Mabrey JD & Agrawal CM An interspecies comparison of bone fracture properties. Biomed. Mater. Eng. 8, 1–9 (1998). [PubMed] [Google Scholar]
- 9.Brommage R Perspectives on using nonhuman primates to understand the etiology and treatment of postmenopausal osteoporosis. J. Musculoskelet. Neuronal Interact. 1, 307–25 (2001). [PubMed] [Google Scholar]
- 10.Havill L et al. Bone mineral density reference standards in adult baboons (Papio hamadryas) by sex and age. Bone 33, 877–888 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Havill LM, Levine SM, Newman DE & Mahaney MC Osteopenia and osteoporosis in adult baboons (Papio hamadryas). J. Med. Primatol. 37, 146–153 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Havill L, Allen M & Bredbenner T Heritability of lumbar trabecular bone mechanical properties in baboons. Bone 46, 835–840 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Havill L, Harris J, Levine SM & Mahaney MC Strong and significant genetic effects on osteonal remodeling-associated cortical bone microstructure in pedigreed baboons. Bone 42, S53–54 (2008). [Google Scholar]
- 14.Hansen HL, Bredbenner TL, Nicolella DP, Mahaney MC & Havill LM Cross-sectional geometry of the femoral midshaft in baboons is heritable. Bone 45, 892–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox L a et al. Baboons as a model to study genetics and epigenetics of human disease. ILAR J. 54, 106–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vagtborg H The story of the southwest research center: a private, nonprofit, scientific research adventure. (University of Texas at Austin, 1973). [Google Scholar]
- 17.Wähnert D et al. Temperature influence on DXA measurements: bone mineral density acquisition in frozen and thawed human femora. BMC Musculoskelet. Disord. 10, 25 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hale AR & Ross AH The Impact of Freezing on Bone Mineral Density: Implications for Forensic Research. J. Forensic Sci. 62, 399–404 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Spradling KD, Glenn JP, Garcia R, Shade RE & Cox LA The baboon kidney transcriptome: analysis of transcript sequence, splice variants, and abundance. PLoS One 8, e57563 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karere GM, Glenn JP, VandeBerg JL & Cox LA Differential microRNA response to a high-cholesterol, high-fat diet in livers of low and high LDL-C baboons. BMC Genomics 13, 320 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackowiak SD Identification of Novel and Known miRNAs in Deep-Sequencing Data with miRDeep2. Curr. Protoc. Bioinforma. 12, 1–15 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Griffiths-Jones S, Saini HK, van Dongen S & Enright AJ miRBase: tools for microRNA genomics. Nucleic Acids Res. 36, D154–8 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A & Enright AJ miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 34, D140–4 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love MI, Huber W & Anders S Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team. R: A language and environment for statistical computing. (2019).
- 26.Therneau TM coxme: Mixed Effects Cox Models. (2020).
- 27.Ru Y et al. The multiMiR R package and database: integration of microRNA–target interactions along with their disease and drug associations. Nucleic Acids Res. 42, e133–e133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao F et al. miRecords: an integrated resource for microRNA-target interactions. Nucleic Acids Res. 37, D105–10 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu S-D et al. miRTarBase: a database curates experimentally validated microRNA-target interactions. Nucleic Acids Res. 39, D163–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karagkouni D et al. DIANA-TarBase v8: a decade-long collection of experimentally supported miRNA–gene interactions. Nucleic Acids Res. 46, D239–D245 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang DW, Sherman BT & Lempicki R a. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu W, Clyne M, Khoury MJ & Gwinn M Phenopedia and Genopedia: disease-centered and gene-centered views of the evolving knowledge of human genetic associations. Bioinformatics 26, 145–146 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weilner S et al. Differentially circulating miRNAs after recent osteoporotic fractures can influence osteogenic differentiation. Bone 79, 43–51 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Laxman N, Mallmin H, Nilsson O & Kindmark A miR-203 and miR-320 regulate bone morphogenetic protein-2-induced osteoblast differentiation by targeting distal-less homeobox 5 (Dlx5). Genes (Basel). 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De-Ugarte L et al. MiRNA profiling of whole trabecular bone: Identification of osteoporosis-related changes in MiRNAs in human hip bones. BMC Med. Genomics 8, 1–11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bedene A et al. MiR-148a the epigenetic regulator of bone homeostasis is increased in plasma of osteoporotic postmenopausal women. Wien. Klin. Wochenschr. 128, 519–526 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Yang S et al. Mir-148b-3p, mir-337–5p and mir-423–5p expression in alveolar ridge atrophy and their roles in the proliferation and apoptosis of ommscs. Exp. Ther. Med. 16, 5334–5342 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu X & Lu X MiR-423–5p inhibition alleviates cardiomyocyte apoptosis and mitochondrial dysfunction caused by hypoxia/reoxygenation through activation of the wnt/β-catenin signaling pathway via targeting MYBL2. J. Cell. Physiol. 234, 22034–22043 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Yuan X-P et al. MicroRNA-423–5p facilitates hypoxia/reoxygenation-induced apoptosis in renal proximal tubular epithelial cells by targeting GSTM1 via endoplasmic reticulum stress. Oncotarget 8, 82064–82077 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao Q et al. NFE2/miR-423–5p/TFF1 axis regulates high glucose-induced apoptosis in retinal pigment epithelial cells. BMC Mol. Cell Biol. 20, 39 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia W, Yu T, An Q, Cao X & Pan H MicroRNA-423–5p inhibits colon cancer growth by promoting caspase-dependent apoptosis. Exp. Ther. Med. (2018). doi: 10.3892/etm.2018.6288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y et al. In silico analysis of the molecular mechanism of postmenopausal osteoporosis. Mol. Med. Rep. 12, 6584–6590 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calvier L et al. PPARγ Links BMP2 and TGFβ1 Pathways in Vascular Smooth Muscle Cells, Regulating Cell Proliferation and Glucose Metabolism. Cell Metab. 25, 1118–1134.e7 (2017). [DOI] [PubMed] [Google Scholar]
- 44.Kamiya N et al. Acute BMP2 upregulation following induction of ischemic osteonecrosis in immature femoral head. Bone 53, 239–247 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Avendaño-Félix M et al. A Novel OsteomiRs Expression Signature for Osteoblast Differentiation of Human Amniotic Membrane-Derived Mesenchymal Stem Cells. Biomed Res. Int. 2019, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lisse TS Vitamin D Regulation of a SOD1-to-SOD2 Antioxidative Switch to Prevent Bone Cancer. Appl. Sci. 10, 2554 (2020). [Google Scholar]
- 47.Zhang Y et al. A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1− mice is correlated to increased cellular senescence. Redox Biol. 11, 30–37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeong S-G & Cho G-W Endogenous ROS levels are increased in replicative senescence in human bone marrow mesenchymal stromal cells. Biochem. Biophys. Res. Commun. 460, 971–976 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


