Abstract
Tape stripping is a minimally invasive, non-scarring method that can be utilized to assess gene expression in skin but is infrequently used given technical constraints. By comparing different tape stripping technologies and full thickness skin biopsy results of lesional and non-lesional psoriatic skin from the same patients, we demonstrate that tape stripping with optimized high-resolution transcriptomic profiling can be used to effectively assess and characterize inflammatory responses in skin. Upon comparison with single-cell RNA-seq data from psoriatic full thickness skin biopsies, we illustrate that tape-stripping efficiently captures the transcriptome of the upper layers of the epidermis with sufficient resolution to assess the molecular components of the feed-forward immune amplification pathway in psoriasis. Notably, non-lesional psoriatic skin sampled by tape stripping demonstrates activated, pro-inflammatory changes when compared to healthy control skin, suggesting a pre-psoriatic state, which is not captured on full-thickness skin biopsy transcriptome profiling. This work illustrates an approach to assess inflammatory response in the epidermis by combining non-invasive sampling with high throughput RNA sequencing, providing a foundation for biomarker discoveries and mechanism of action studies for inflammatory skin conditions.
Keywords: Psoriasis, tape-stripping, RNA-sequencing, single-cell sequencing, epidermis
INTRODUCTION
Psoriasis is a common chronic inflammatory disease of the skin characterized by keratinocyte hyperproliferation, altered epidermal differentiation, and thickening (acanthosis)(Gudjonsson and Elder, 2007). The majority of the changes detected by gene expression profiling of psoriatic skin are derived from the epidermal compartment(Li et al., 2014). Transcriptomic profiling can provide novel and important insights into disease biology(Lee et al., 2004; Zhou et al., 2003); it can facilitate elucidation of mechanism of action of therapeutic agents(Zaba et al., 2007) and identification of biomarkers for disease or therapeutic response. However, a major limitation of transcriptomic profiling of skin diseases is that it requires an invasive biopsy procedure, usually of both lesional and non-lesional skin, along with the use of an anesthetic agent. Due to the invasive nature of skin biopsies, some patient groups such as children or pregnant women, may be excluded and this may potentially limit the amount of information that can be gathered from larger studies and may preclude identification of robust biomarkers predicting treatment response. Tape-stripping, where an adhesive tape is used to sample the top layer of the epidermis, is a minimally invasive approach that can be used to profile various changes in the skin and has been used for characterization of proteins(Broccardo et al., 2009; Voegeli et al., 2009) and skin lipids(Jungersted et al., 2010). Several studies have used tape-stripping to analyze molecular changes in skin(Guttman-Yassky et al., 2019; He et al., 2020a; Wong et al., 2004), especially for atopic dermatitis(Goleva et al., 2020; He et al., 2020b; Lyubchenko et al., 2021; Olesen et al., 2020; Pavel et al., 2020), but a more comprehensive understanding is needed of how well transcriptomic profiling through tape stripping captures the anatomy and cytokine response of the underlying disease process in inflammatory skin conditions when compared to biopsy-based transcriptomic profiling.
The objective of this study was to provide a comprehensive evaluation of how well tape-stripping paired with high resolution RNA-sequencing captures the global transcriptomic changes in psoriasis. In addition, we examined whether tape-stripping captures elements of the inflammatory infiltrate. We determine from which cellular compartment the majority of the transcriptomic changes were captured and demonstrate that transcriptomic profiling of RNA isolated from tape-stripped lesional psoriatic skin efficiently captures genes expressed in the upper layers of the epidermis and accurately detects active immunological pathways and immune cell-generated cytokines in psoriasis. Furthermore, we demonstrate that tape-stripping is sensitive in capturing pre-psoriatic changes in uninvolved psoriatic skin and highlighting its keratinocyte inflammation, illustrating high sensitivity when coupled with RNA-seq technology. These data suggest that transcriptomic profiling using tape-stripping can be an effective and non-invasive method to assess gene expression and cytokine responses in psoriasis. Thus, as a less invasive and safer way of measuring transcriptomic changes in inflamed skin, tape stripping provides an opportunity to expand mechanism of action and biomarker studies in skin to much larger patient cohorts than has previously been feasible.
RESULTS
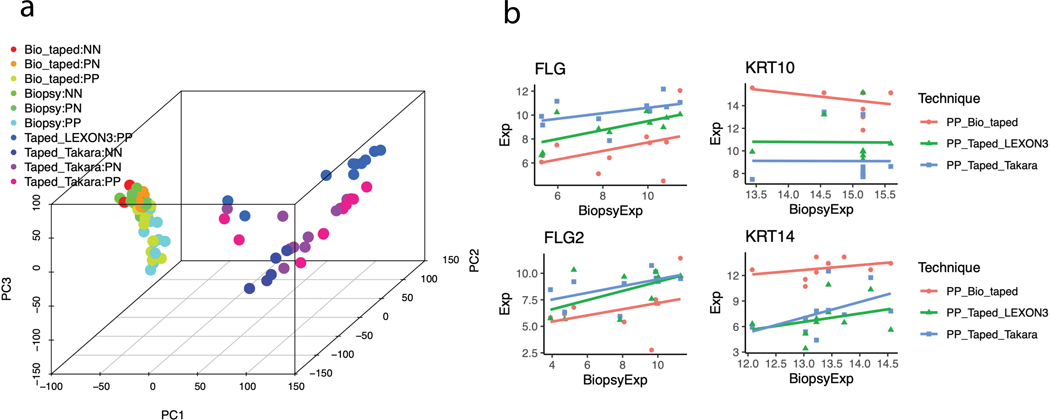
We collected lesional (PP), non-lesional (PN), and health control (NN) samples after quality control from: i) tape stripping (Takara: 5 NN, 10 PN, and 10 PP; Lexon3: 10 PP); ii) full-thickness intact skin biopsy in non-tape-stripped skin (5 NN, 9 PN, and 10 PP); iii) full-thickness biopsy from underneath the tape-stripped skin (5 NN, 10 PN, and 10 PP). Transcriptome-wide RNA-sequencing was performed on all samples collected. Principal component analysis (PCA) revealed clear separation between full-thickness skin biopsy samples and tape-stripped samples (Figure 1a). For protocol optimization, we compared two library preparation kits (Lexon3 vs. Takara); while the Lexon3 processing was only able to profile the lesional skin, the Takara library preparation provided consistent high-resolution data for both lesional, non-lesional, and healthy control skin. Significantly, we were able to distinguish the psoriatic skin from the control skin, but the contrast between NN and PN have higher contrast for the tape-stripped samples. Since we profiled psoriatic skin from the same patients with different techniques, we conducted correlation analysis between the expression profiles. Transcriptome-wide, the correlation was highest between those obtained between full-thickness biopsy in non-tape-stripped vs biopsy in tape-stripped areas (median spearman correlation = 0.27). A significantly lower degree of correlation was seen between the library prep samples for tape-stripping compared to intact full-thickness psoriatic skin (median spearman correlation ~ 0.03). However, when focusing on signature genes of the keratinized (FLG, FLG2), differentiated (KRT10), and basal (KRT14) compartments of the epidermal layer (Figure 1b) against full thickness biopsy data, we found that the tape-stripped data had stronger correlation with FLG and FLG2 expression (Supplementary Table 3).
Figure 1. Principal Component Analysis and Gene-gene correlation analyses.
(A) Principal component analysis of samples obtained from full-thickness biopsies and tape-stripped samples (representative from two types of library preparation kits - Takara and Lexon3). Lexon3 preparation only yielded usable RNA from a small proportion of lesional skin samples. The PC1 and PC2 explain 25% and 8% of the transcriptome variations, respectively. (B) Correlations of the normalized expression values for signature genes in the epidermal compartments between full-thickness biopsy (x-axis) versus other techniques (y-axis). “Bio_taped”, “Biopsy”, and “Taped” represent biopsy samples taken from tape-stripped area, biopsy samples, and tape- stripped samples, respectively.
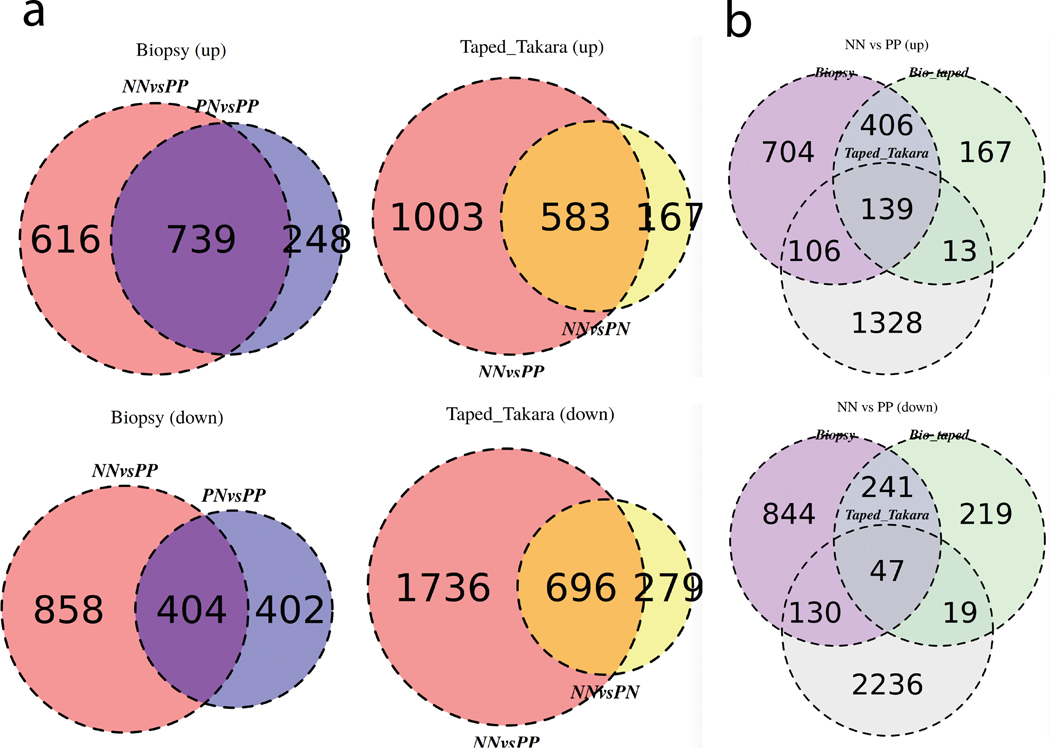
The criteria of 2-fold change and a false-discovery-rate (FDR) of ≤ 10% as criteria were used for comparison of the differential expression analysis. For the biopsy samples, we found 1,355 genes to be up-regulated in biopsies from full-thickness psoriatic skin, and 1,262 genes down-regulated, compared to healthy control skin. Consistent with previous studies(Tsoi et al., 2015; Tsoi et al., 2019a), there is less contrast between the non-lesional versus lesional psoriatic skin, with fewer up-(987) and down-(806) regulated genes. DEGs from tape-stripped, lesional psoriatic skin showed 1,586 genes upregulated and 2,432 down regulated compared to tape-stripped healthy control skin (Figure 2a). Interestingly, we identified significant number of differentially expressed genes between healthy control and non-lesional psoriatic skin, with 750 up- and 975 down-regulated genes (Figure 2a). Notably, no differentially expressed genes were observed when comparing healthy control vs. non-lesional skin in the biopsy sample nor in the non-lesional vs. lesional skin comparison from tape-stripping, suggesting that molecular profiles captured from tape-stripped skin samples are more similar between the non-lesional and the lesional skin. We then compared the DEGs across the technologies. Partial overlap in DEGs from healthy controls lesional skin was identified when comparing DEGs from the tape-stripping analysis with the DEGs from full-thickness biopsy (Figure 2a), indicating that tape-stripping can capture a proportion of both increased and decreased DEGs in psoriatic skin.
Figure 2. Venn diagram of upregulated and downregulated DEGs.
(A) (left) Up-regulated and down-regulated DEGs from RNA isolated from full-thickness biopsies comparing normal (NN) and uninvolved (PN) vs. lesional psoriatic skin (PP); (right) Up-regulated and down-regulated DEGs in RNA isolated from tape-stripped skin comparing normal (NN) vs. lesional (PP) and non-lesional psoriatic skin (PN). (B) Comparison of the DEGs obtained from RNA isolated from full-thickness biopsy, tape-stripping, and biopsies obtained underneath tape-stripped areas in normal (NN) vs. lesional psoriatic skin (PP). A criterion of 2-fold change and a false-discovery- rate (FDR) of < 10% as criteria were used for comparisons.
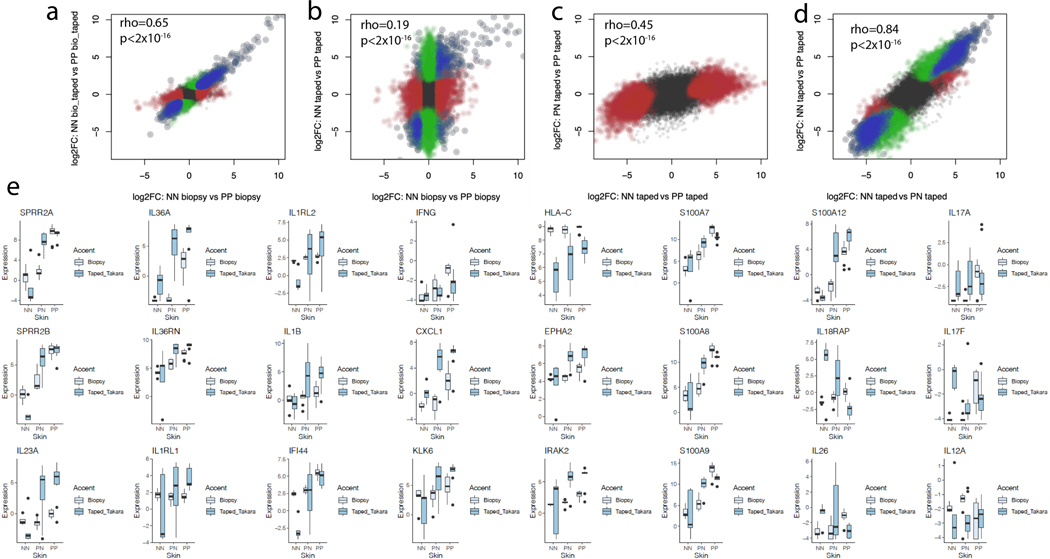
Several psoriasis-associated pro-inflammatory genes were detected to be more strongly up-regulated in the tape-stripped lesional skin samples, including S100A12 (600-fold increase, FDR=5.8×10−5), human beta defensin 2 (DEFB4; 376-fold, FDR=1.4×10−3), SPRR2B (1,311-fold increase, FDR=1.2×10−5), and SPRR2A (1,376-fold increase, FDR=1.4×10−4). DEGs captured in full-thickness biopsy and not in tape-stripped samples included WNT5A (5.4-fold, FDR=1.9×10−4 in biopsy), interferon response genes including OAS3 (6.0-fold, FDR=7.9×10−4), and KYNU (12.7- fold, FDR=6.4×10−4) (further examples of gene expression, see Table 1 and Supplementary Table 4). The correlation between the effect sizes of the NN vs PP comparison in intact lesional and that of from tape-stripping was most prominent for DEGs with the largest fold change magnitude. In stark contrast, there was much less correlation between the tape-stripping NN vs PP effect size with that from the tape-stripping PN vs PP comparison; however robust overlap was seen between the tape-stripping NN vs. PN and the NN vs. PP comparisons (Figure 3). This suggests that subtle pre-psoriatic changes in non-lesional psoriatic skin, previously described by our group(Gudjonsson et al., 2009), are efficiently picked up by the tape-stripping technique.
Table 1. Selected genes and expression across sample types.
Significant results are bolded.
| Biopsy NN vs. PP | Biopsy under taped area NN vs. PP | Taped NN vs. PP | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Gene | FC | FDR | FC | FDR | FC | FDR |
| IL36G | 54 | 5.1×10−05 | 28 | 6.1×10−04 | 775 | 2.1×10−04 |
|
| ||||||
| IL36A | 82 | 8.7×10−04 | 59 | 6.1×10−03 | 186 | 1.1×10−02 |
|
| ||||||
| DEFB4A | 134 | 5.4×10−04 | 83 | 3.4×10−03 | 376 | 1.4×10−03 |
|
| ||||||
| CCL20 | 11 | 7.8×10−03 | 15 | 6.0×10’04 | 7.3 | 2.63×10−01 |
|
| ||||||
| IL1B | 2.4 | 3.8×10−01 | 2.9 | 1.2×10−01 | 36 | 1.3×10−02 |
|
| ||||||
| IL17A | 7.5 | 1.8×10−02 | 6.7 | 1.2×10−02 | 1.9 | 7.2×10−01 |
|
| ||||||
| IFNG | 5.6 | 5.5×10−03 | 5.1 | 1.4×10−02 | 2.9 | 4.0×10−01 |
|
| ||||||
| S100A12 | 68 | 1.4×10−04 | 87 | 2.4×10−04 | 607 | 5.8×10−05 |
|
| ||||||
| SPRR2B | 156 | 1.4×10−05 | 157 | 1.3×10−04 | 1311 | 1.2×10−05 |
|
| ||||||
| SPRR2A | 417 | 9.4×10−05 | 725 | 1.7×10−05 | 1376 | 1.4×10−04 |
Figure 3. Correlation between effect sizes estimation in comparison from biopsy and tapestripping.
(A-D) Correlation between the effect sizes of gene dysregulation from full thickness intact lesional psoriatic (comparing against healthy control) versus full-thickness taped lesional psoriatic skin (left upper figure), and correlation between full-thickness intact lesional versus tape-stripped lesional (right upper figure). For the tape-stripped samples, correlation between lesional vs healthy skin and lesional vs non-lesional skin (left lower figure), and between gene expression in normal vs. uninvolved and normal vs lesional (right lower figure). Red color indicates significant DEGs for the label on the X-axis, green color indicates significant DEGs for the label on the Y-axis. Blue indicates significant DEGs from both. (E) Expression profiles for selected genes highlighting the values captured in different technologies. The lower and upper hinges correspond to the 25th and 75th percentiles; the upper whisker extends from the hinge to the largest value no further than 1.5x Inter-quartile Range (IQR) from the hinge, while the lower whisker extends from the hinge to the smallest value at most 1.5x of the IQR of the hinge.
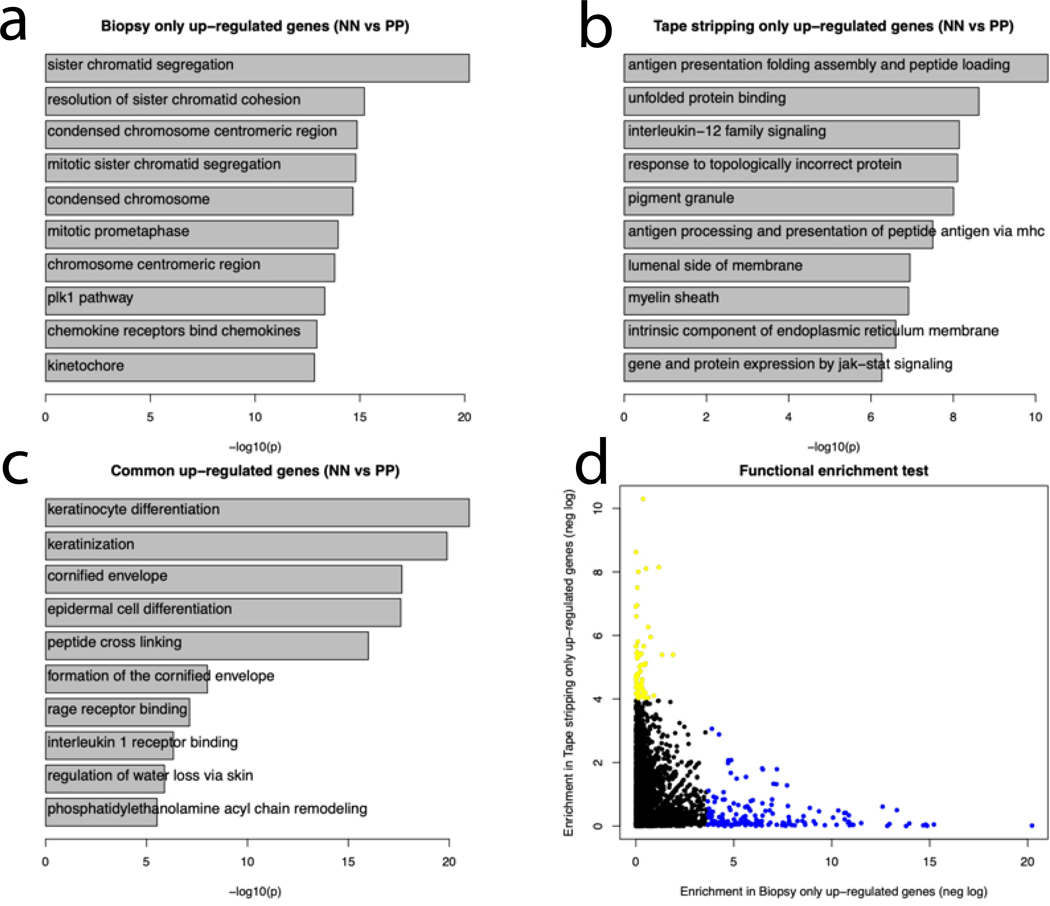
To reveal the biological functions captured by tape-stripping vs. full thickness biopsies from psoriatic and healthy control skin, we performed functional enrichment analyses for DEGs identified in NN vs. PP skin. The up-regulated genes found in both tape-stripped and biopsied psoriatic vs. normal skin were enriched in processes involving the epidermis, including “keratinocyte differentiation” (p<1×10−20), “keratinization” (p<1×10−15), “peptide cross-linking” (p<1×10−15), “interleukin 1 receptor binding” (p<1×10−5) and “regulation of water loss via skin” (p<1×10−5). Enriched functions in genes elevated only in full-thickness biopsy samples involved cell proliferation, such as: “sister chromatid segregation” (p<1×10−20), “condensed chromosome” (p<1×10−14), “mitotic prometaphase” (p<1×10−10), and other immune-related functions such as “chemokine receptors bind chemokines” (p<1×10−10). Notably, enriched G0 processes in DEGs only found in tape-stripped RNA samples included “antigen presentation folding assembly and peptide loading of class I MHC” (p<1×10−10), “interleukin-12 family signaling” (p<1×10−8), and “gene and protein expression by JAK-STAT signaling” (p<1×10−5) (Figure 4a–c; Supplementary Table 5). Comparison of the functional enrichment of tape-stripped only DEGs and full-thickness biopsy DEGs showed distinct enrichment patterns (Figure 4d). These results suggest that the tape-stripping approach is efficient in capturing biological processes that are active high up in the epidermis (i.e. cytokine response), but not at capturing gene expression changes associated with proliferative changes, which tend to occur in the basal and suprabasal layers of the psoriatic epidermis(Rijzewijk et al., 1989).
Figure 4. Enrichment of Biological Processes in tape stripped vs. biopsied skin.
(A,B,C) Genes identified in both full-thickness biopsy and tape-stripped skin RNA were enriched for biological functions primarily involving epidermal function such as keratinocyte differentiation, cornified envelop and interleukin-1 receptor binding. Genes only seen in the full-thickness biopsies were enriched for sister chromatic segregation, mitotic prometaphase and kinetochore. Genes only seen in the tape-stripping were enriched for antigen processing and presentation, IL-12 family signaling and JAK-STAT signaling. (D) There was no overlap in the significant functions for DEGs in biopsy only and tape-stripping only. Blue dots represent functions significantly enriched among genes up-regulated in the Biopsy samples only; yellow dots represent functions significantly enriched among genes up-regulated in Tape-stripping samples only.
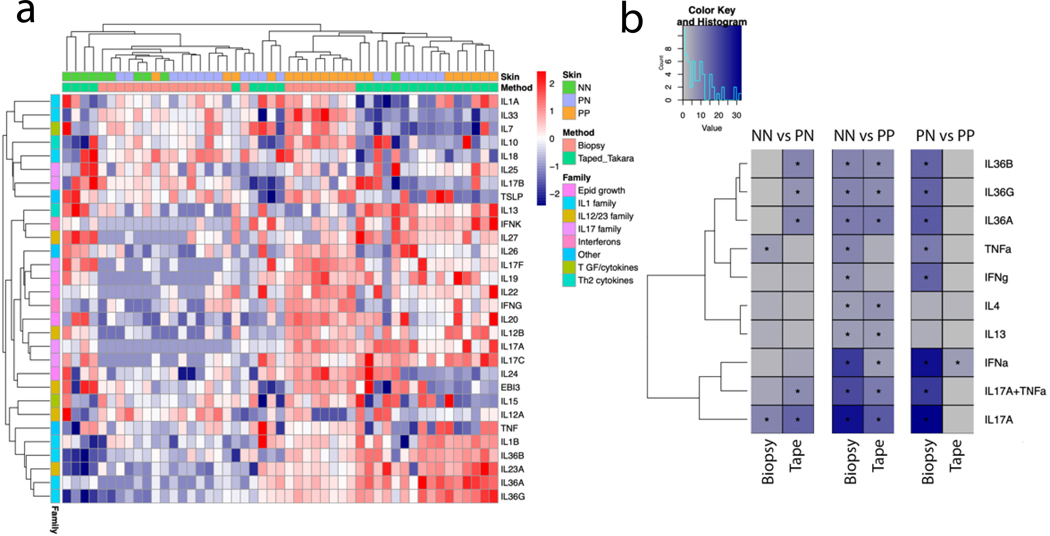
To determine how well tape-stripping captures cytokine-related genes, we assessed cytokine expression across several cytokine families in NN, PN and PP. Tape-stripping detected expression of several cytokines, although this sampling method was less sensitive than sampling intact full-thickness biopsies (Figure 5a; Figure 3e). However, tape-stripping effectively captured cytokine gene expression derived from the epidermis. This included the IL-36 family of cytokines (IL36G; FC=775 and FDR=2×10−4), IL23A (FC=188 and FDR=4.8×10−3), and IL1B (FC=36 and FDR=1.3×10−2), which have all been shown to be expressed by psoriatic keratinocytes(Buerger et al., 2012; Johnston et al., 2013; Li et al., 2018). We then studied the burden of cytokine response as described previously(Tsoi et al., 2019b; Tsoi et al., 2019c) and found prominent signatures of IL-17, TNF, IL-36, and type I and type II IFN in psoriatic skin from full-thickness biopsies as expected (Figure 5b). The IL17/IL36G/IFNα signatures were also revealed in genes elevated from the tape-stripped samples, particularly the IL-17 and IL-36 responses. Notably, these signatures were present in NN vs PN comparison for tape-stripped samples to the same extent as that in the NN vs PP comparison, with minimal to no difference between tape stripped DEGs from the PN vs PP comparison. Taken together, these data demonstrate that tape-stripping can help elucidate the inflammatory environment of psoriatic skin and can capture genes expressed in cell types other than keratinocytes. Interestingly, these data also suggest that tape-stripping can be more sensitive than full skin biopsy in detecting preclinical transcriptomic changes in non-lesional skin.
Figure 5. Cytokine expression and signatures in tape stripped vs. biopsied skin.
(A) The heatmap shows inverse normalized gene expression of various cytokines in normal (NN), uninvolved (PN) and lesional psoriatic skin (PP) from full thickness biopsies (pink) or from tape-stripped RNA (green). Hierarchical clustering was used in the dendrograms (B) Cytokine response signatures in full-thickness biopsy samples (first three columns) and from tape-stripped RNA compared against different groups (NN, PN and PP). Significant results are shown (*corrected p-value <= 0.05, and Observed/Expected ratio >=2)
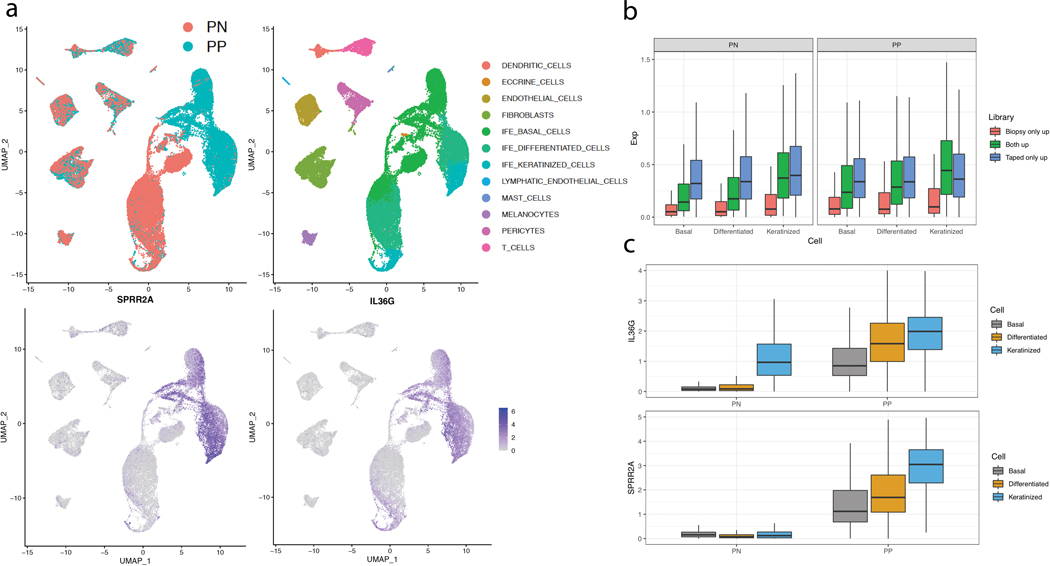
Finally, we compared the expression patterns of DEGs in the tape stripped samples against single cell RNA-seq data from epidermal keratinocytes, classified into basal (KRT5+/KRT14+), differentiated (KRT10+), or keratinized (FLG+) groups (Figure 6a). This allowed us to determine the expression of DEGs in each epidermal compartment from full-thickness biopsies or tapestripping method. Comparing the genes up-regulated only in full-thickness biopsy of lesional skin vs. those identified to be up-regulated in the tape-stripping samples (i.e. including genes commonly up-regulated in biopsy), the keratinized layer showed the most significant difference in the expression proportion (p=2.61×10−32 in PN and p=6.99×10−21 in PP), indicating that tape-stripping disproportionately captures genes expressed in the keratinized layer compared to those captured in full-thickness biopsies (Figure 6b). Two of the most highly expressed genes seen in the tape-stripped samples were SPRR2A (1375-fold, FDR=1.4×10−4) and IL36G (775-fold, FDR=2.1×10−4), with their expression being primarily found in the keratinized layer of psoriatic skin (Figure 6c; Supplementary Figure 1).
Figure 6. Tape-stripping captures gene expression signatures preferentially from the top layers of the epidermis.
(A) The UMAP shows cell clusters from scRNA-seq from psoriatic and non-psoriatic skin, along with the normalized expression of prominent genes (SPRR2A and IL36G) captures with tape-stripping. (B) The average normalized expression levels in scRNA-seq for genes identified to be up-regulated in PP skin from different techniques; the expression levels in different epithelial compartments (basal, differentiated/spinous, keratinized/granular) are shown. Genes captured by tape-stripping tend to be localized to the differentiated or keratinized layers of the epidermis. The lower and upper hinges correspond to the 25th and 75th percentiles; the upper whisker extends from the hinge to the largest value no further than 1.5x Inter-quartile Range (IQR) from the hinge, while the lower whisker extends from the hinge to the smallest value at most 1.5x of the IQR of the hinge. (C) Examples of the normalized expression levels of two genes (IL36G and SPRR2A) in different epithelial compartments.
DISCUSSION
Tape stripping is a minimally invasive, non-scarring method that can be utilized to assess gene expression in skin. Previous studies have used the tape-stripping approach to assess gene expression changes in atopic dermatitis(Dyjack et al., 2018; Guttman-Yassky et al., 2019; Leung et al., 2019). A challenge in using tape-stripping technique is the limited range of coverage, where gene expression changes might only be restricted to a small number of candidate genes. Therefore, it is critical to provide a comprehensive assessment on how effectively tapestripping captures the complexity of the underlying inflammatory disease process, as well as how far into the epidermis transcriptomic changes are captured, both in terms of keratinocytes and immune cell populations.
Here, we demonstrate that tape-stripping effectively captures transcriptomic changes occurring in differentiated and keratinized layers of the epidermis; however, it is less effective at capturing biological processes that are active in the basal layer of the epidermis, such as cellular proliferation (Figure 4a). While psoriatic skin has marked thinning of the suprapapillary plate, which brings the basal layer close to the surface of the skin, it is unlikely that the surface area of the dermal papilla (as a proportion of the surface captured by tape-stripping) is high enough, or that the tape-stripping goes deep enough, or through combination of the two, to capture substantial number of cells from the basal layer of the epidermis. Consistent with this, proliferative responses were underrepresented in the tape-stripped transcriptomic data, which aligns with proliferation primarily being observed in the basal and suprabasal layers of the psoriatic epidermis(Rijzewijk et al., 1989). In contrast, tape-stripping captures not only inflammatory response, but also up-regulation of genes involved in antigen processing and presentation by MHC class I in the epidermis; genetic analysis of psoriasis has shown these pathways to play a critical role in psoriasis susceptibility(Genetic Analysis of Psoriasis et al., 2010; Nair et al., 2009; Sun et al., 2010). In addition, tape-stripping also captures the feedforward amplification of immune responses in psoriatic skin, which are known to dominate in the upper layers of the epidermis(Hawkes et al., 2017). In this context, it is of interest that data from tape-stripping of non-lesional skin in psoriasis patients was more similar to that obtained from tape-stripped lesional skin. It has been demonstrated by our group(Gudjonsson et al., 2009), and others(Bata-Csorgo et al., 1998), that non-lesional psoriatic skin has subtle prepsoriatic gene expression changes. The data presented here suggest that these changes originate in the upper layers of the epidermis and are characterized by both upregulation of the antigen-presenting machinery as well as increased expression of pro-inflammatory cytokines such as IL-23 and IL-36 family cytokines (Figures 4 and 5).
It is worth noting that several of the cytokine genes shown to be detected, although not significantly increased in the tape-stripping dataset, are primarily expressed by T-cells, including IL17A and IL17F (Figure 5a). T-cells, particularly CD8+ T-cells are known to infiltrate the epidermis of psoriasis patients, whereas CD4+ T-cells are primarily localized to the upper dermis(Gudjonsson et al., 2004; Onuma, 1994). Consistent with the IL17A and IL17F being expressed in CD8 T cells within the psoriatic epidermis, expression of CD8B was increased by about 2.6-fold in the tape-stripped data from lesional psoriatic skin compared to healthy control, whereas its expression was unchanged in full-thickness biopsy of psoriatic samples relative to healthy control skin. In contrast, CD4 expression was decreased in the tape-stripped data (about 4-fold) and slightly increased in the full-thickness lesional psoriatic skin compared to healthy control. Thus, tape-stripping can capture elements of non-keratinocyte cell types but appears to be dependent upon those cells being located within the epidermal compartment.
Notably, IL-23 has been demonstrated to be a critical cytokine in psoriasis pathogenesis(Cargill et al., 2007; Kopp et al., 2015; Nair et al., 2009), and therapeutics targeting IL-23 are highly effective(Gordon et al., 2015; Kopp et al., 2015; Papp et al., 2017; Reich et al., 2017). Tape-stripping was very efficient in capturing expression of IL23A, which encodes the p19 subunit of the IL-23 protein(Oppmann et al., 2000). The increase in IL23A was much greater in the tape- stripped psoriatic skin (compared to healthy control), compared to its increase in full-thickness biopsies (188-fold vs. 2-fold). IL12B mRNA expression was also detectable in tape-stripped psoriatic skin compared to healthy control, but lower than that detected in full-thickness biopsies (2.8-fold vs. 6.5-fold). While dendritic cells and macrophages contribute likely the majority of IL-23 in psoriatic skin(Yawalkar et al., 2009), the epidermis has been demonstrated to be a source of IL-23 in psoriasis(Li et al., 2018; Piskin et al., 2006), and our data is consistent with this scenario and further demonstrates the role and contribution of the epidermis to psoriasis pathogenesis.
It is worth noting the large number of unique differentially expressed genes we find in tape-stripped skin, both increased and decreased (Figure 2c) are not well represented in data obtained from full-thickness biopsies. The likely reason for this is that many of the changes in psoriatic skin are most dramatic in the epidermis. While cellular hyperproliferation seen in psoriasis dominates in the lower epidermal compartments and is not captured by the tapestripping method (Figure 4a), other epidermal changes in psoriasis predominate in the upper layer of the epidermis. This includes the loss of the cornified layer, altered differentiation, dense parakeratosis with thick layer of superficial scale, and marked inflammatory response as part of the feed-forward amplification that is primarily active in the upper epidermal layer(Hawkes et al., 2017). Therefore, it is not unexpected that tape-stripping, which captures disproportionate amounts of RNA from the upper layers of the epidermis, has such marked differences from RNA obtained from full-thickness biopsies, where the transcriptome is diluted by transcripts from the dermal inflammatory infiltrate and dermal resident tissue cells such as fibroblasts and endothelial cells. We also acknowledge that the sequence read mapping rate in the tape-stripped samples is lower than that of skin biopsy (as low as only 13% high quality reads are mapped in tape-stripping vs ~75% for skin biopsy), likely driven by the lower quality of RNA captured on the skin-surface; thus, tape strip sampling itself can also drive the heterogeneity of the assay. However, we believe this limitation can be offset by the non-invasive nature of the technique to profile the transcriptome of psoriatic patients, especially its higher sensitivity on uninvolved skin to capture most of the gene signatures involved in the epidermal differentiation and cytokine signaling in keratinocytes.
In conclusion, our data, despite a small study cohort, demonstrate that tape-stripping can capture essential features of the inflammatory process in psoriasis. Perhaps, most strikingly, it effectively captures subclinical changes in uninvolved psoriatic skin and confirms that uninvolved psoriatic skin is in a state of low-grade inflammation. Our data also indicate the feasibility of using tape-stripping combined with high-resolution sequencing as a non-invasive way to measure changes in psoriatic skin, and suggest that tape-stripping, particularly when done in large cohorts of patients, could be used to identify biomarkers of disease, and possibly to track treatment response. Of note, patients were off all topical and systemic treatments for at least 2 weeks prior to enrollment, and while we cannot rule out residual effects of prior treatments, the study design and analyses with paired analyzes would minimize confounding effects. Whether transcriptomic analyses of tape-stripped skin are more sensitive or less sensitive to changes in psoriatic skin remains to be determined, but it is evident from the data presented here that tape-stripping can enable capture of high-quality data from patients and can open up the possibility for study of transcriptomic changes in skin in vulnerable patient populations, including children. Thus, tape-stripping in this setting could provide an avenue for future studies where larger samples can be collected in a non-invasive manner from an entire clinical study cohort to provide greater insight into how psoriasis behaves over time and with treatment.
MATERIALS AND METHODS
Patient population:
10 patients with chronic plaque psoriasis and 5 healthy controls were recruited for the tape stripping study. Demographics, including age, gender, ethnicity, were recorded (see Supplementary Table 1). Patients were off systemic treatment and any topical agents for at least 2 weeks prior to enrollment in the study. The study was approved by the University of Michigan Institutional Review Board (IRB). All patients provided written, informed consent. Tape stripping was performed on lesional and distal non-lesional psoriatic skin (buttocks) and on site-matched healthy control skin. Three 6mm punch biopsies were obtained from each patient: 1) an intact psoriatic plaque, 2) underneath the tape-stripped area, and 3) non-lesional skin. For the single-cell RNA-sequencing, 6 different patients with chronic plaque psoriasis were recruited and 6-mm punch biopsies were taken from both lesional and non-lesional skin. The studies were conducted according to the Declaration of Helsinki Principles.
Tape-stripping:
Skin was wiped clean by an alcohol swab. Standard adhesive disks (D100 D-Squame Standard Sampling Discs, CuDerm, Dallas Texas) with a 2.2cm diameter were placed on healthy, non-lesional, or lesional skin under a standard weight of 225 gm/cm2 over an area of 22.24mm using D500-D-Square Pressure Instrument for 10 seconds to obtain the stratum corneum. The adhesive disks were then removed from the skin using forceps and snap frozen in liquid nitrogen. Twenty sequential D-Squame disks were taken from each site.
Preparing and storing disks for processing:
At time of removal from the skin, the D-Squame disks were immediately placed inside sequentially labelled (#1–20) 1ml Eppendorf tubes with the adhesive side facing inside. 0nly one D-Squame disk was placed in each Eppendorf tube to avoid discs to stick to the tube or each other. Each tube was submerged into liquid nitrogen until processing.
Processing:
The processing of RNA was performed using the RNeasy kit (Qiagen, RNeasy plus mini kit, Cat.No.: 74136). 700μl of RLT Lysate buffer with 2% beta-mercaptoethanol was aliquoted into the tube containing the first D-Squame disk (tube#1). The tube was sonicated with Diagenode Picoraptor sonicator (cycle: 30seconds 0N, 30 seconds 0FF, 3 total cycles). The liquid was then transferred to the next tube (i.e. #2) and the process was repeated for all tubes.
Library prep:
Samples were processed for sequencing using the SMARTer Stranded Total RNA-seq Kit v2 - Pico Input Mammalian (Takara, Cat#634411). Parallel samples were processed using the Lexogen 3’ QuantSeq mRNA-seq Library Prep Kit FWD (Lexogen).
RNA-seq processing:
50-nucleotide single-end sequencing reads was generated using Illumina Hi-Seq 4000 sequencing system. 0ur in-house pipeline was used to perform adapter trimming and other quality control procedures for the RNA-seq samples. Reads were mapped using STAR, and gene expression levels were measured and normalized by HTSeq and DESeq2. The numbers of reads generated/aligned for each sample are listed in Supplementary Table 2. Differential expression analysis was conducted with DESeq2, which uses negative binomial, to model expression data and utilize generalized linear model for differential expression analysis. For analyzes comparing samples from same patient assayed by different techniques, or uninvolved vs involved skin comparison, we adjusted for the individual effect. Multiple testing corrections was performed to identify statistically significant results using a False Discovery Rate (FDR) of ≤ 10% and |log2FC| ≥ 1. We observed that mapping rate varied significantly among RNA-seq conducted for tape-stripping samples, therefore we focused only on the differential expression comparisons for skin conditions obtained from same method. Sequencing data is available on GEO: GSE178197
Cytokine responses.
We conducted RNA-seq experiments to profile the transcriptome of cytokine stimulated keratinocytes as described previously(Tsoi et al., 2019a; Tsoi et al., 2019c). For genes that were induced by cytokines, we conducted hypergeometric test to assess the enrichment against genes that are up regulated in different differential expression comparisons.
Single cell RNA-seq.
The sample preparation for scRNA-seq data was described as before(Gudjonsson et al., 2020): Skin was obtained from excisional samples from normal skin. Samples were incubated overnight in 0.4% dispase (Life Technologies) in Hank’s Balanced Saline Solution (Gibco) at 4°C. Epidermis and dermis were separated. Epidermis was digested in 0.25% Trypsin-EDTA (Gibco) with 10U/mL DNase I (Thermo Scientific) for 1 hour at 37°C, quenched with FBS (Atlanta Biologicals), and strained through a 70μm mesh. Dermis was minced, digested in 0.2% Collagenase II (Life Technologies) and 0.2% Collagenase V (Sigma) in plain medium for 1.5 hours at 37°C, and strained through a 70μm mesh. Epidermal and dermal cells were recombined and libraries were constructed by the University of Michigan Advanced Genomics Core on the 10X Chromium system. Libraries were then sequenced on the Illumina NovaSeq 6000 sequencer to generate 151-bp paired end reads. Raw reads from the single cell transcriptome analysis of skin was processed using the 10x Genomics Cell Ranger pipeline for quality control, alignment, and quantification. Further read filtering, normalization, and batch correction (by the Canonical Correlation analysis) were conducted using the R library Seurat(Butler et al., 2018). Top principal components were computed using the anchor genes, and in turn to conduct the uniform manifold approximation and project (UMAP) for visualization. We used 15 nearest neighbors and minimum distance=0.01 in the UMAP construction.
Supplementary Material
Supplementary Table 1. Demographics of Study Subjects
Supplementary Table 2. Read Coverage for Samples
Supplementary Table 3. Spearman expression correlation between different technologies of same lesional skin samples from psoriatic skin
Supplementary Table 4. Summary statistics for the different expression analyses. The NN vs PP comparisons for Biopsy (5 vs 10), Biopsy under tape-stripped area (5 vs 10), and Tape-stripping (5 vs 10) samples resulted in 1,355/1,262; 725/526; 1,586/2,432 up/down regulated genes, respectively, under the FDR<=10% and two-fold change criteria.
Supplementary Table 5. Functional enrichment results for gene up/down regulated
Supplementary Table 6. Quantity and quality of RNA isolated in biopsies vs. tape-stripped skin.
ACKNOWLEDGEMENT
This work was supported by a research grant from AbbVie to JEG. Further support was provided in part by the Babcock Endowment Fund (LCT, MKS, JT, JEG), by the National Institute of Health: R01-AR069071 and R01-AR073196 (JEG), P30-AR075043 (JEG), K01-AR072129 (LCT), and by the A. Alfred Taubman Medical Research Institute (JEG, JMK). National Psoriasis Foundation Psoriasis Prevention Initiative (JEG, LCT, JMK, EM), LCT is supported by the Dermatology Foundation, the Arthritis National Research Foundation, and the National Psoriasis Foundation.
CONFLICT OF INTEREST
This work is supported by AbbVie. JEG received research grants from AbbVie, AnaptysBio, Janssen, Pfizer, Novartis, Celgene/Bristol Myers Squibb (BMS), and Eli Lilly, and serves as an advisory board member for Novartis, AbbVie, Eli Lilly, MiRagen, and Almirall. YH reports grand support from Galderma and Abbvie. JMK has research grants from Celgene/BMS, Janssen, and Q32 Bio and has served on advisory boards for AstraZeneca, GlaxoSmithKlein, VielaBio, Admirex Pharmaceuticals, Eli Lilly, Bristol Myers Squibb, Avion Pharmaceuticals, Provention Bio, Aurinia Pharmaceuticals, Ventus Therapeutics, and Boehringer Ingleheim. KMS and VES are employees of AbbVie.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY STATEMENT
Datasets related to this article can be found at https://www.ncbi.nlm.nih.aov/aeo/query/acc.cai?acc=GSE178197. hosted at http://ncbi.nlm.nih.gov/gds on Gene Expression Omnibus (GEO) repository: GEO: GSE178197
REFERENCES
- Bata-Csorgo Z, Cooper KD, Ting KM, Voorhees JJ, Hammerberg C (1998) Fibronectin and alpha5 integrin regulate keratinocyte cell cycling. A mechanism for increased fibronectin potentiation of T cell lymphokine-driven keratinocyte hyperproliferation in psoriasis. J Clin Invest 101:1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broccardo CJ, Mahaffey SB, Strand M, Reisdorph NA, Leung DY (2009) Peeling off the layers: skin taping and a novel proteomics approach to study atopic dermatitis. J Allergy Clin Immunol 124:1113–5 e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger C, Richter B, Woth K, Salgo R, Malisiewicz B, Diehl S, et al. (2012) Interleukin-1beta interferes with epidermal homeostasis through induction of insulin resistance: implications for psoriasis pathogenesis. The Journal of investigative dermatology 132:2206–14. [DOI] [PubMed] [Google Scholar]
- Butler A, Hoffman P, Smibert P, Papalexi E, Satija R (2018) Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nature biotechnology 36:411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, et al. (2007) A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet 80:273–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyjack N, Goleva E, Rios C, Kim BE, Bin L, Taylor P, et al. (2018) Minimally invasive skin tape strip RNA sequencing identifies novel characteristics of the type 2-high atopic dermatitis disease endotype. J Allergy Clin Immunol 141:1298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetic Analysis of Psoriasis C, the Wellcome Trust Case Control C, Strange A, Capon F, Spencer CC, Knight J, et al. (2010) A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet 42:985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goleva E, Calatroni A, LeBeau P, Berdyshev E, Taylor P, Kreimer S, et al. (2020) Skin tape proteomics identifies pathways associated with transepidermal water loss and allergen polysensitization in atopic dermatitis. J Allergy Clin Immunol 146:1367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon KB, Duffin KC, Bissonnette R, Prinz JC, Wasfi Y, Li S, et al. (2015) A Phase 2 Trial of Guselkumab versus Adalimumab for Plaque Psoriasis. N Engl J Med 373:136–44. [DOI] [PubMed] [Google Scholar]
- Gudjonsson JE, Ding J, Li X, Nair RP, Tejasvi T, Qin ZS, et al. (2009) Global gene expression analysis reveals evidence for decreased lipid biosynthesis and increased innate immunity in uninvolved psoriatic skin. J Invest Dermatol 129:2795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson JE, Elder JT (2007) Psoriasis: epidemiology. Clinics in dermatology 25:535–46. [DOI] [PubMed] [Google Scholar]
- Gudjonsson JE, Johnston A, Sigmundsdottir H, Valdimarsson H (2004) Immunopathogenic mechanisms in psoriasis. Clin Exp Immunol 135:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson JE, Tsoi LC, Ma F, Billi AC, van Straalen KR, Vossen A, et al. (2020) Contribution of plasma cells and B cells to hidradenitis suppurativa pathogenesis. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman-Yassky E, Diaz A, Pavel AB, Fernandes M, Lefferdink R, Erickson T, et al. (2019) Use of Tape Strips to Detect Immune and Barrier Abnormalities in the Skin of Children With Early- Onset Atopic Dermatitis. JAMA Dermatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes JE, Chan TC, Krueger JG (2017) Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol 140:645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Bissonnette R, Wu J, Diaz A, Saint-Cyr Proulx E, Maari C, et al. (2020a) Tape strips detect distinct immune and barrier profiles in atopic dermatitis and psoriasis. J Allergy Clin Immunol. [DOI] [PubMed] [Google Scholar]
- He H, Olesen CM, Pavel AB, Clausen ML, Wu J, Estrada Y, et al. (2020b) Tape-Strip Proteomic Profiling of Atopic Dermatitis on Dupilumab Identifies Minimally Invasive Biomarkers. Front Immunol 11:1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston A, Fritz Y, Dawes SM, Diaconu D, Al-Attar PM, Guzman AM, et al. (2013) Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J Immunol 190:2252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungersted JM, Hogh JK, Hellgren LI, Jemec GB, Agner T (2010) Skin barrier response to occlusion of healthy and irritated skin: differences in trans-epidermal water loss, erythema and stratum corneum lipids. Contact Dermatitis 63:313–9. [DOI] [PubMed] [Google Scholar]
- Kopp T, Riedl E, Bangert C, Bowman EP, Greisenegger E, Horowitz A, et al. (2015) Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature 521:222–6. [DOI] [PubMed] [Google Scholar]
- Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, et al. (2004) Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med 199:125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DYM, Calatroni A, Zaramela LS, LeBeau PK, Dyjack N, Brar K, et al. (2019) The nonlesional skin surface distinguishes atopic dermatitis with food allergy as a unique endotype. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Tsoi LC, Swindell WR, Gudjonsson JE, Tejasvi T, Johnston A, et al. (2014) Transcriptome analysis of psoriasis in a large case-control sample: RNA-seq provides insights into disease mechanisms. J Invest Dermatol 134:1828–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Yao Q, Mariscal AG, Wu X, Hulse J, Pedersen E, et al. (2018) Epigenetic control of IL-23 expression in keratinocytes is important for chronic skin inflammation. Nat Commun 9:1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubchenko T, Collins HK, Goleva E, Leung DYM (2021) Skin tape sampling technique identifies proinflammatory cytokines in atopic dermatitis skin. Ann Allergy Asthma Immunol 126:46–53 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. (2009) Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nature genetics 41:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen CM, Pavel AB, Wu J, Mikhaylov D, Del Duca E, Estrada Y, et al. (2020) Tape-strips provide a minimally invasive approach to track therapeutic response to topical corticosteroids in atopic dermatitis patients. J Allergy Clin Immunol Pract. [DOI] [PubMed] [Google Scholar]
- Onuma S (1994) Immunohistochemical studies of infiltrating cells in early and chronic lesions of psoriasis. J Dermatol 21:223–32. [DOI] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. (2000) Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715–25. [DOI] [PubMed] [Google Scholar]
- Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger JG, Lacour JP, et al. (2017) Risankizumab versus Ustekinumab for Moderate-to-Severe Plaque Psoriasis. N Engl J Med 376:1551–60. [DOI] [PubMed] [Google Scholar]
- Pavel AB, Renert-Yuval Y, Wu J, Del Duca E, Diaz A, Lefferdink R, et al. (2020) Tape strips from early-onset pediatric atopic dermatitis highlight disease abnormalities in nonlesional skin. Allergy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB (2006) In vitro and in situ expression of IL- 23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol 176:1908–15. [DOI] [PubMed] [Google Scholar]
- Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaci D, et al. (2017) Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 390:276–88. [DOI] [PubMed] [Google Scholar]
- Rijzewijk JJ, Van Erp PE, Bauer FW (1989) Two binding sites for Ki67 related to quiescent and cycling cells in human epidermis. Acta Derm Venereol 69:512–5. [PubMed] [Google Scholar]
- Sun LD, Cheng H, Wang ZX, Zhang AP, Wang PG, Xu JH, et al. (2010) Association analyses identify six new psoriasis susceptibility loci in the Chinese population. Nature genetics 42:1005–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi LC, Iyer MK, Stuart PE, Swindell WR, Gudjonsson JE, Tejasvi T, et al. (2015) Analysis of long non-coding RNAs highlights tissue-specific expression patterns and epigenetic profiles in normal and psoriatic skin. Genome Biol 16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi LC, Rodriguez E, Degenhardt F, Baurecht H, Wehkamp U, Volks N, et al. (2019a) Atopic dermatitis is an IL-13 dominant disease with greater molecular heterogeneity compared to psoriasis. J Invest Dermatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi LC, Rodriguez E, Degenhardt F, Baurecht H, Wehkamp U, Volks N, et al. (2019b) Atopic Dermatitis Is an IL-13-Dominant Disease with Greater Molecular Heterogeneity Compared to Psoriasis. The Journal of investigative dermatology 139:1480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi LC, Rodriguez E, Stolzl D, Wehkamp U, Sun J, Gerdes S, et al. (2019c) Progression of acute- to-chronic atopic dermatitis is associated with quantitative rather than qualitative changes in cytokine responses. J Allergy Clin Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voegeli R, Rawlings AV, Breternitz M, Doppler S, Schreier T, Fluhr JW (2009) Increased stratum corneum serine protease activity in acute eczematous atopic skin. Br J Dermatol 161:70–7. [DOI] [PubMed] [Google Scholar]
- Wong R, Tran V, Morhenn V, Hung SP, Andersen B, Ito E, et al. (2004) Use of RT-PCR and DNA microarrays to characterize RNA recovered by non-invasive tape harvesting of normal and inflamed skin. The Journal of investigative dermatology 123:159–67. [DOI] [PubMed] [Google Scholar]
- Yawalkar N, Tscharner GG, Hunger RE, Hassan AS (2009) Increased expression of IL-12p70 and IL-23 by multiple dendritic cell and macrophage subsets in plaque psoriasis. J DermatolSci 54:99–105. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Cardinale I, Gilleaudeau P, Sullivan-Whalen M, Suarez-Farinas M, Fuentes-Duculan J, et al. (2007) Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med 204:3183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Krueger JG, Kao MC, Lee E, Du F, Menter A, et al. (2003) Novel mechanisms of T-cell and dendritic cell activation revealed by profiling of psoriasis on the 63,100-element oligonucleotide array. Physiol Genomics 13:69–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Demographics of Study Subjects
Supplementary Table 2. Read Coverage for Samples
Supplementary Table 3. Spearman expression correlation between different technologies of same lesional skin samples from psoriatic skin
Supplementary Table 4. Summary statistics for the different expression analyses. The NN vs PP comparisons for Biopsy (5 vs 10), Biopsy under tape-stripped area (5 vs 10), and Tape-stripping (5 vs 10) samples resulted in 1,355/1,262; 725/526; 1,586/2,432 up/down regulated genes, respectively, under the FDR<=10% and two-fold change criteria.
Supplementary Table 5. Functional enrichment results for gene up/down regulated
Supplementary Table 6. Quantity and quality of RNA isolated in biopsies vs. tape-stripped skin.
Data Availability Statement
Datasets related to this article can be found at https://www.ncbi.nlm.nih.aov/aeo/query/acc.cai?acc=GSE178197. hosted at http://ncbi.nlm.nih.gov/gds on Gene Expression Omnibus (GEO) repository: GEO: GSE178197