Abstract
Certain pathogenic genetic variants impact neurodevelopment and cause deviations from typical cognitive trajectories. Understanding variant-specific cognitive trajectories is clinically important for informed monitoring and identifying patients at risk for comorbid conditions. Here, we demonstrate a variant-specific normative chart for cognitive development for individuals with 22q11.2 deletion syndrome (22q11DS). We used IQ data from 1365 individuals with 22q11DS to construct variant-specific normative charts for cognitive development (Full Scale, Verbal, and Performance IQ). This allowed us to calculate Z-scores for each IQ datapoint. Then, we calculated the change between first and last available IQ assessments (delta Z-IQ-scores) for each individual with longitudinal IQ data (n = 708). We subsequently investigated whether using the variant-specific IQ-Z-scores would decrease required sample size to detect an effect with schizophrenia risk, as compared to standard IQ-scores. The mean Z-IQ-scores for FSIQ, VIQ, and PIQ were close to 0, indicating that participants had IQ-scores as predicted by the normative chart. The mean delta-Z-IQ-scores were equally close to 0, demonstrating a good fit of the normative chart and indicating that, as a group, individuals with 22q11DS show a decline in IQ-scores as they grow into adulthood. Using variant-specific IQ-Z-scores resulted in 30% decrease of required sample size, as compared to the standard IQ-based approach, to detect the association between IQ-decline and schizophrenia (p < 0.01). Our findings suggest that using variant-specific normative IQ data significantly reduces required sample size in a research context, and may facilitate a more clinically informative interpretation of IQ data. This approach allows identification of individuals that deviate from their expected, variant-specific, trajectory. This group may be at increased risk for comorbid conditions, such as schizophrenia in the case of 22q11DS.
Subject terms: Psychology, Neurodevelopmental disorders
Introduction
Over the past two decades, a growing list of genetic variants associated with clinical phenotypic outcomes has emerged, including cognitive trajectories that deviate from what is typical in the general population [1–3]. In the general population, the age-adjusted level of cognitive functioning is generally stable over the lifespan; i.e., the IQ curve, where obtained scores are age-adjusted, is expected to be a virtually constant line over the years [4]. A divergent trajectory may be part of the developmental impact of an underlying pathogenic genetic variant. Examples include early cognitive decline and loss of acquired skills in the case of Rett’s syndrome [5, 6], or early onset dementia in the case of Down’s syndrome [7–10]. General cognitive functioning is the term we use in this article to reflect the important human quantitative trait that accounts for much of the variation in diverse cognitive abilities, including intellectual functioning, and can be operationalized as the commonly used Intelligence Quotient (IQ) [11–13].
Populations of individuals with pathogenic variants that impact neurodevelopment would benefit from a better understanding of variant-specific cognitive trajectories. To that end, ideally variant-specific (age-) normative reference data are obtained, allowing for the comparison of an individual’s performance to the group’s indices over time and potentially helpful in setting realistic expectations regarding (future) performance. This is analogous to the significantly improved accuracy and clinical relevance of monitoring physical growth in an individual with Down syndrome when using normative physical growth data from studies of individuals with Down syndrome [14, 15]. When using norm data obtained from the general population, a child with Down syndrome may be considered growth-delayed, whereas in reality their growth trajectory may be as expected for someone with this genetic condition.
In a similar way, genetic subgroup-specific normative data on cognitive development may be highly informative. Such cognitive norm charts may be relevant for both research and clinical purposes as they allow the identification of individuals who deviate from what is a typical trajectory given the genetic variant and potentially, monitoring effects of interventions over time. For example, when an individual does not follow their expected IQ trajectory; i.e., deviates from their IQ curve, this may indicate underlying brain-related pathology, warranting additional examinations. A parallel may be drawn to how in a child who deviates from their expected physical growth curve a diagnostic work-up is warranted that could help identify the cause (e.g., endocrine problems), and potentially inform treatment strategies (e.g., growth hormones) [16].
The 22q11.2 deletion syndrome (22q11DS) is a genetic condition associated with aberrant neurodevelopmental outcomes [17]. It is the most common chromosomal microdeletion disorder, estimated to result from (in ~85% of cases de novo) nonhomologous meiotic recombination events occurring in approximately 1 in every 1000 fetuses [18]. 22q11DS has a highly variable phenotypic expression [19–22], including various levels of cognitive functioning with differing developmental trajectories that, on average, appear to display a mild downward trend [17, 23]. Individuals with 22q11DS also have a 25-fold increased risk for developing schizophrenia, making it the strongest single molecular genetic risk variant for psychotic disorders [24]. We have previously reported that the subgroup of individuals with a cognitive decline steeper than average in this population had an even further elevated risk for schizophrenia [25]. Here, we aim to generate a 22q11DS-specific normative chart for IQ to be used as a reference in both clinical and research settings. We will demonstrate how a normative chart for cognitive development in a genetically defined population can be reliably established and provide potential directions for its future utility.
Methods
Participants and instruments
Data on 1789 individuals with a confirmed 22q11.2 microdeletion were collected from 22 different sites as part of the international Brain and Behavior Consortium on 22q11DS [26, 27]. For this study, we included individuals who had at least one Wechsler IQ assessment available and were between the ages of 6 and 38 years, resulting in a total number of participants of 1365 (76.3%). Above the age of 38 years, the number of participants in each age-year was too small (n < 10) to obtain a reliable normative value for that particular year. Below the age of 6 years, the number of participants in each age-year was also small and scores showed disproportionally greater variability (consistent with greater testing effects observed in IQ-tests in younger children). 657 individuals (48.1%) had one assessment available, and we refer to this subgroup as the baseline sample. 708 (51.9%) individuals had two or more IQ assessments available, and we refer to this subgroup as the longitudinal sample (see also Fig. 1). All individuals, and when appropriate their legal guardians, provided informed consent and the study was approved by the local institutional research ethics boards of each site.
Fig. 1. Flowchart of participants with 22q11DS for inclusion in the current study.
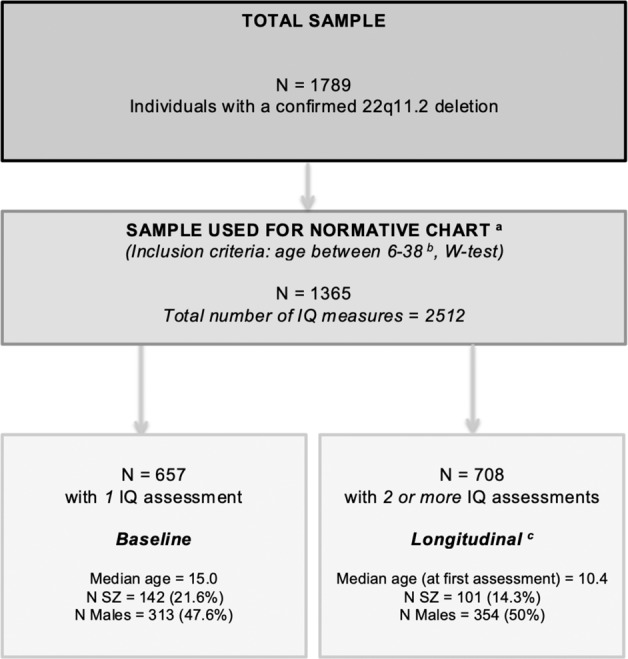
N SZ = individuals diagnosed with a schizophrenia spectrum disorder. aData (cross-sectional and longitudinal) from these 1365 individuals were used for the construction of the normative chart (Methods section 2.2.i). bFor the current study we limited the age range to 6–38 years, as the main purpose of the study is to create one easily applicable normative chart. Above the age of 38 years, the number of participants in each age-year was too small (n < 10) to obtain a reliable normative value for that particular year. Below the age of 6 years, the number of participants in each age-year was also small and scores showed disproportionally greater variability (consistent with greater testing effects observed in IQ-tests in younger children). cData (longitudinal) from these 708 individuals were used for calculating the delta-z-scores (“Methods” section 2.2.ii).
Level of overall intellectual functioning (IQ) was assessed using age-appropriate Wechsler scales (see also Table 1) [28–34] and all IQ-data underwent extensive quality control (Supplemental Note 1). Clinical diagnoses of schizophrenia spectrum disorders were made by experienced clinicians in accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fourth edition [35].
Table 1.
Sample descriptives for total sample (n = 1789) of individuals with a 22q11.2 deletion, baseline subset (n = 657) and longitudinal subset (n = 708).
| Total sample | Subset: baseline | Subset: longitudinal | p a | |
|---|---|---|---|---|
| N = 1789 | N = 657 | N = 708 | ||
| Age in years at first assessment | – | |||
| Mean (SD) | 17.1 (8.0) | 11.6 (4.8) | ||
| Median (Range) | 15.0 (6.0–37.8) | 10.4 (6.0–35.2) | <0.001 | |
| Age in years at last assessment | – | – | ||
| Mean (SD) | 17.9 (5.8) | |||
| Median (Range) | 17.1 (7.4–38) | – | ||
| Sex (% males) | 868 (48.5%) | 313 (47.6%) | 354 (50%) | 0.4139 |
| Psychotic illness expression | <0.001 | |||
| Psychotic illness | 332 (18.6%) | 142 (21.6%) | 101 (14.3%) | |
| Control (age > 25 y) | 295 (16.5%) | 99 (15.1%) | 63 (8.9%) | |
| Putative control | 850 (47.5%) | 323 (49.2%) | 385 (54.4%) | |
| Control combined | 1145 (64%) | 422 (64.2%) | 448 (63.3%) | |
| Putative subthreshold | 268 (15%) | 74 (11.3%) | 146 (20.6%) | |
| Affective psychosis | 33 (1.8%) | 14 (2.1%) | 13 (1.8%) | |
| Unknown | 11 (0.6%) | 5 (0.8%) | 0 | |
| Age in years at last psychiatric assessment | ||||
| Mean (SD) | 21.3 (11.4) | 19.9 (10.0) | 19.0 (6.8) | 0.05692 |
| Median (Range) | 18.0 (2–71) | 17.0 (5–56) | 18.0 (7–61) | |
| IQ-test used (first assessment) | <0.001 | |||
| WPPSI | – | 49 (7.5%) | 32 (4.5%) | |
| WPPSI-R | – | 5 (0.8%) | 18 (2.5%) | |
| WISC-III | – | 153 (23.3%) | 323 (45.6%) | |
| WISC-IV | – | 96 (11.6%) | 100 (14.1%) | |
| WISC-R | – | 24 (3.7%) | 56 (7.9%) | |
| WAIS-III | – | 139 (21.2%) | 49 (6.9%) | |
| WAIS-IV | – | 50 (7.6%) | 9 (1.3%) | |
| WAIS-R | – | 61 (9.3%) | 7 (1.0%) | |
| WASI | – | 80 (12.2%) | 114 (16.1%) | |
| Mean baseline FSIQ (SD) | – | 72.0 (14.3) | 73.3 (13.1) | 0.1048 |
| Mean baseline VIQ (SD) | – | 76.3 (14.5) | 76.9 (14.6) | 0.4236 |
| Mean baseline PIQ (SD) | – | 73.2 (14.9) | 74.0 (13.4) | 0.2741 |
ap value of difference statistic between baseline and longitudinal subsets. T-tests were used for age and IQ-score variables, chi-square tests were used for variables of sex, psychotic illness expression, and IQ-test measures.
Data analysis
The data analysis for this study consisted of two steps. First, we constructed the normative charts for IQ and second, we used the available longitudinal data to calculate the difference (delta) scores between the first and last available IQ assessments (see also Fig. 1). All data quality control and statistical analyses were conducted in R 3.6.2 GUI 1.70.
Normative chart
To construct the normative chart for IQ, we used all available IQ datapoints (n = 2512) from all participants with at least one IQ-assessment available (n = 1365). We used polynomial regression models of the 1st, 2nd, 3rd, and 4th order and we used the Akaike and the Bayesian Information Criterion (AIC and BIC) to determine the best fit. Furthermore, we checked basic assumptions for polynomial regressions, including multivariate normality and homoscedasticity, by examining the distribution of the residuals and the residual variance of the final model.
Subsequently, we used the coefficients derived from the best fit to determine the normative IQ-chart. This normative chart enabled us to calculate a (standardized) Z-score for each individual IQ-point, and thereby identify how much individuals deviated from the average IQ in this population at a certain time point, given their age. We applied the same strategy for all basic summary IQ-scores: Full Scale IQ (FSIQ), Verbal IQ (VIQ), and Performance IQ (PIQ).
Delta Z-scores
For those individuals with more than one IQ assessment available (n = 708, 51.9%), we determined delta-Z-scores by calculating the difference between the Z-score corresponding to the first available IQ measurement and that of the last IQ-measurement (Last IQ Z-score – first IQ Z-score = delta-Z-score). The average delta-Z score across all participants provides an indication of the extent to which individuals follow, on average, their expected trajectory as predicted by the normative chart. In addition, we examined the distribution of the delta-Z-scores.
Post-hoc
Post-hoc, we investigated whether using the delta-Z-score, as compared to the standard (population-normed) IQ-scores would result in a decrease of required sample size to detect the previously reported association between IQ-decline and schizophrenia risk [25]. To this end, we compared two regression models: both models had schizophrenia status (yes/no) as the dependent variable and baseline VIQ (we focused on VIQ and VIQ-decline as this component of IQ had the strongest association with schizophrenia risk [25]), sex, age, and time-interval as covariates. In the (variant-specific) Z-based model the main (binary) independent variable was VIQ-Z-decline (yes/no; based on a cut-off of −0.5 SD in delta Z-score). In the parallel model the main independent variable was VIQ-decline (yes/no; based on a cut-off of −7.5 IQ-points (i.e., −0.5 SD) in absolute (population normed) VIQ-difference scores). To compare both strategies, we calculated sample sizes needed in both models to obtain sufficient power to detect the association with increased schizophrenia risk.
In addition, at two points we performed bootstrap procedures: first for the normative charts for FSIQ, VIQ, and PIQ (to obtain further estimates of the robustness of the results), and second, as an additional estimate of the maximum reduction of sample sizes.
Results
Participants and instruments
Figure 1 provides a schematic depiction of the participants included in this study. Table 1 provides descriptives for all participants, as well as separately for those with only one IQ-assessment (baseline) and those with two or more IQ-assessments available (longitudinal). Importantly, there were no differences in mean FSIQ, VIQ, and PIQ scores (on the first available assessment) between the baseline- and longitudinal samples (Table 1).
Normative chart statistics
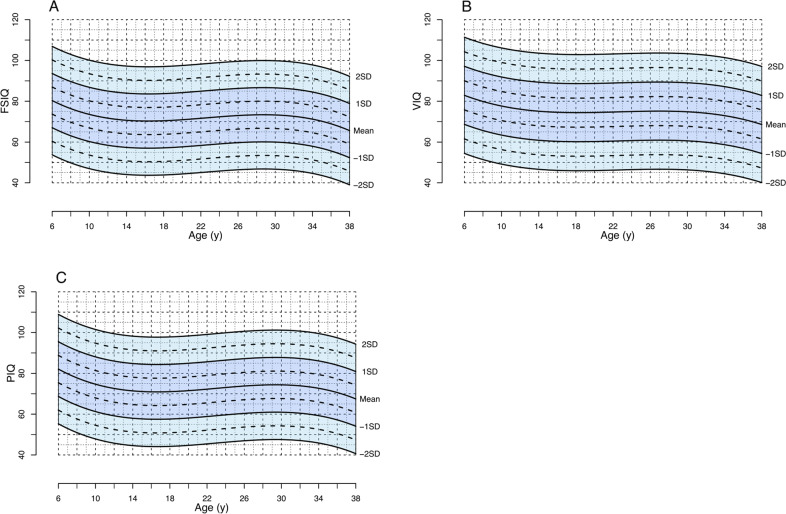
The 3rd order polynomial regression provided the best fit for the FSIQ, VIQ, and PIQ data, as indicated by the AIC and BIC (Supplementary Table S1), and the normative charts were constructed based on this. The parameters for the model for FSIQ were R2 = 0.03, F (3,2508) = 24.01, p < 0.001; for VIQ R2 = 0.03, F(3,2439) = 19.19, p < 0.001; and for PIQ R2 = 0.03, F(3,2336) = 26.35, p < 0.001. Supplementary Table S2 provides the coefficients of the regressions. The residuals of the model were normally distributed and constant over the age range, indicating accurate prediction of the trajectory by the normative chart. Further, the distribution of the Z-scores confirmed that the normative chart provided a good fit for the data. The mean Z-scores were close to 0 for FSIQ (−0.03), VIQ (−0.02), and PIQ (−0.03), indicating that on average, individuals with 22q11DS had an IQ-score as predicted by the model considering their age. In addition, there was no difference of the mean Z-scores between the baseline- and the longitudinal-samples. The standard deviations (SD) of the z-scores were close to 1 for FSIQ (1.02), VIQ (1.01), and PIQ (1.04) (an SD of 1 is the equivalent of 15 IQ-points). Figure 2 displays the normative growth chart for FSIQ, VIQ, and PIQ (including data points in Supplementary Fig. S1).
Fig. 2. 22q11DS-specific normative chart for FSIQ (A), VIQ (B), and PIQ (C) over time.
These figures represent the normative charts for IQ development in individuals with the 22q11.2 deletion (A: FSIQ, B: VIQ, C: PIQ). The lines represent the observed average IQ trajectories (“Mean”), and the observed trajectories that deviate ±1 or 2 SDs from the mean. The normcharts are derived from 2512 IQ assessments in 1365 individuals with the 22q11.2 deletion between the ages of 6 and 38 years. These figures represent the normative charts for IQ development in individuals with the 22q11.2 deletion (A: FSIQ, B: VIQ, C: PIQ). The lines represent the observed average IQ trajectories (“Mean”), and the observed trajectories that deviate ±1 or 2 SDs from the mean. The normcharts are derived from 2512 IQ assessments in 1365 individuals with the 22q11.2 deletion between the ages of 6 and 38 years.
In addition, as an illustration to aid in understanding the IQ decline observed on average in individuals with 22q11DS, Supplementary Fig. S2 represents the approximate corresponding raw score trajectory for 22q11DS, compared to raw IQ score change in the general population.
Delta Z-scores statistics
For the 708 individuals with longitudinal IQ-data were, we calculated delta-Z-scores; i.e., the difference between the Z-scores corresponding to the first and last available IQ-measurements. A model with a good fit would be expected to result in mean delta-Z-scores of around 0, as this would indicate that, on average, individuals stay on their trajectory. Supplementary Fig. S3 displays the distribution of the delta-Z-scores for FSIQ, VIQ, and PIQ. The means were close to 0 (0.064, 0.069, and 0.089, respectively) and the standard deviations were 0.637, 0.679, and 0.720, respectively. Of the 708 individuals, 58% (FSIQ and VIQ) and 55% (PIQ) were between −0.5 and 0.5 SD. This indicates that on average, individuals stay on their trajectories as predicted by the normative IQ charts. Figure 3, presenting IQ data of two hypothetical individuals, serves to illustrate the enhanced impact of using delta-Z-IQ-scores (referenced to 22q11DS-specific norms) compared to general-population delta-IQ-values.
Fig. 3. Comparing two hypothetical cases.
Two hypothetical cases that illustrate the advantage of 22q11DS-specific normative IQ-data over only (general population-based) IQ-data.
Supplemental Materials 1 provides the calculator which allows for obtaining the expected IQ-score given a certain age, and hence the corresponding Z-score for an individual given their age and observed IQ-score. When multiple IQ-assessments for one individual are available, the delta-Z-scores can be calculated. This can be done for FSIQ, VIQ, and PIQ.
Post-hoc analyses
Post-hoc, we compared the Z-IQ-scores model to the population-based IQ model in terms of required sample size to detect the previously reported association between IQ-decline and schizophrenia risk [25]. As expected, both models reveal a significant association between VIQ-decline and schizophrenia. Based on the OR’s (Z-based OR = 2.84, 95% CI = 1.595–5.025, p = 3.405e−04; versus IQ-based OR = 2.09, 95% CI = 1.231–3.533, p = 5.862e−03) we calculated sample sizes needed to obtain sufficient power to detect the association. To obtain 80% power, the Z-based model required a sample size of 64, while a sample size of 91 was needed using the untransformed IQ values (Fig. 4). Hence, using the Z-scores-based approach resulted in a 30% decrease of needed sample size, as compared to the untransformed IQ-based approach, to detect the association between IQ-decline and schizophrenia illness expression with 80% power.
Fig. 4. Comparison of sample sizes.
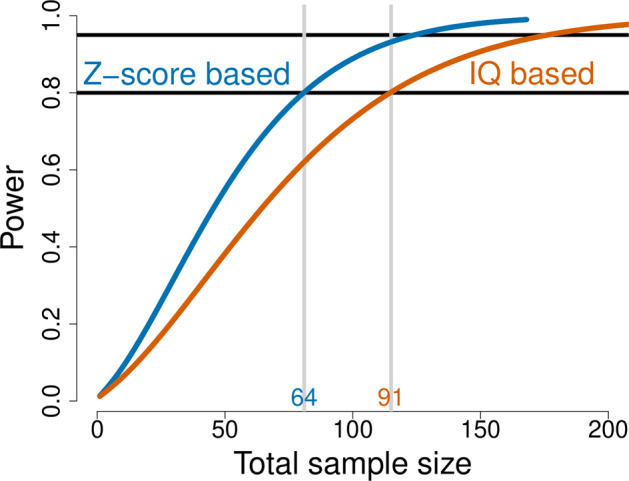
Sample sizes of individuals with 22q11DS required to detect a significant effect (between VIQ-decline and schizophrenia) with 80% power: Z-score based approach versus IQ-based approach.
Lastly, results from both bootstrapping procedures supported the robustness of the normative charts for IQ (Supplementary Fig. S4) and the advance of using the z-based model over the untransformed model with respect to sample size (Supplementary Fig. S5).
Discussion
In this study we constructed a variant-specific normative chart for cognitive development from the largest sample of individuals with the 22q11.2 deletion available to date. Our findings suggest that in this population, a variant-specific normative IQ-chart can be reliably constructed and our discussion offers the rationale for how other pathogenic variants may benefit from a similar strategy. We propose that this approach allows for more accurate and informative interpretation of individual IQ-scores and trajectories, compared to using (untransformed) population-based IQ norms.
The findings further demonstrate that using variant-specific normative IQ data can significantly reduce the sample size needed to detect a certain effect (i.e., VIQ-decline and schizophrenia risk in 22q11DS), compared to population-based normative IQ data. From a research perspective, this is an important discovery. It is challenging to assemble adequately large datasets to provide sufficient power for phenotype–phenotype, or genotype–phenotype analyses, in particular with respect to longitudinal (deep-phenotyping) data. In populations with high-impact variants associated with neurodevelopmental outcome this challenge is even further magnified, given the low population-wide prevalence rates of such conditions.
22q11DS, IQ, and schizophrenia
Using data from 1365 individuals with the 22q11.2 deletion, our findings corroborate several important observations regarding IQ in this population. First, the data confirm the previously reported lower baseline IQ in individuals with 22q11DS [36, 37], and show that the deletion appears to shift the IQ-distribution to the left (~−2 SD) as compared to the general population, but does not alter the characteristics of the distribution. This is in line with a recent study which reported that while FSIQ, VIQ, and PIQ were ~30 IQ-points lower in 22q11DS patients compared to their unaffected parents, the distribution was normal and significantly associated to the parental distribution [38].
Second, our data reiterate that in individuals with 22q11DS there is, on average, a decline in IQ over the lifespan [25, 23]. This observation underscores the impetus for regular and comprehensive cognitive assessments in individuals with 22q11DS [39–41]. We posit that in childhood and adolescence, the observed typical decline in 22q11DS mostly reflects a slower pace in cognitive development in individuals with 22q11DS, compared to their typically developing peers [42] (see also Supplementary Fig. S2). In adulthood, however, this decline in age-adjusted IQ-scores suggests that individuals with 22q11DS may be losing cognitive capacities at a faster pace compared to the general population [31]. Future studies including raw IQ-data will be helpful in elucidating such mechanisms.
Third, as previously reported [25], individuals with 22q11DS who show a VIQ-decline that is steeper than what is expected based on the variant-specific trajectory are at a further increased risk for subsequently developing a psychotic disorder. This is in contrast to individuals with 22q11DS who do not deviate from their expected trajectory, but may still show a VIQ-decline when compared to general population norms. These findings corroborate longitudinal studies in the general population, which report that individuals who later developed a psychotic disorder or are at high risk for psychosis showed increasing cognitive impairments over time, especially during adolescence [13, 43–46].
Implications for this and other pathogenic variants
The often atypical and complex cognitive profile in individuals with pathogenic variants, such as the 22q11.2 deletion, adds to the challenge of finding equilibrium between an individual’s profile of strengths and weaknesses on the one hand, and environmental demands on the other [39]. Realistic daily-life expectations given an individual’s capabilities are key in optimizing the fit between their individual profile and demands, and this is particularly important in populations with increased neurodevelopmental and psychiatric vulnerability [39, 47].
Variant-specific normative IQ-data allow for “plotting” an individual’s IQ-score against norms given their specific variant, and, by extension, a likely projection into future performance. In other words, they may allow setting more realistic expectations and more informative monitoring of individual with pathogenic variants. For example, our data suggest that for a child with 22q11DS, a decline of 7 IQ-points between the ages of 7 and 13 is not unlikely. Such knowledge is relevant as it may help to adjust environmental demands such as in school as to match the current and likely future abilities of the child. Moreover, such knowledge may be helpful in avoiding unnecessary concern as the child’s cognitive development, while showing a decline in IQ-scores, is in line with the phenotypic performance for their genetic condition.
Further, variant-specific normative IQ data enable the identification of those individuals who deviate more than what can be expected given the genetic variant; i.e., who deviate from their (adjusted) curve. This may be helpful in interpreting the observed IQ-decline and distinguishing between individuals who cannot keep up with increasing environmental (social, academic) expectations, and those who display an actual loss of abilities. While in both scenarios a decline in absolute IQ-scores can be observed, the underlying mechanisms and clinical implications may be very different [17, 23, 25, 48]. Future studies should include raw IQ data (i.e., not standardized and norm-referenced) to further elucidate these underlying mechanisms of IQ-decline, and to allow for further improved specificity and greater variance at the extremes end of the IQ-distribution [49].
Variant-specific normative IQ-data may also enable improved risk stratification for comorbid conditions. This strategy applied to 22q11DS facilitates the identification of those individuals with 22q11DS with a VIQ-decline in excess of what is typical for this population and that may be a significant risk factor for developing schizophrenia [25]. The clinical implication is that increased (early) monitoring for signs of psychotic development may be warranted in this subgroup. Vice versa, while still at increased risk of psychosis compared to the general population, the individuals who do not deviate from their expected trajectory (but may still show an IQ-decline when compared to general population norms) could receive care as usual for 22q11DS patients [40, 41]. Pending replication and further specification of our findings, an additional consequence could be that the stress experienced by patients and caregivers due to this genetically determined a priori risk for schizophrenia [50] may be somewhat mitigated in this group.
As is the case for 22q11DS, the variability in (degree of) expressed phenotypes with any rare pathogenic variant can still only be described in terms of group prevalence rates. Our current inability to provide individualized outcome prediction causes uncertainty for caregivers with respect to individual needs and daily life expectations [51, 52], and undermines the potential for prevention or early intervention strategies. Although variant-specific normative IQ-data provide an important step towards improved outcome prediction at a group level, the identification of factors influencing individualized outcome prediction is needed. Recent studies are making progress in this regard in individuals with various high-impact genetic variants including 22q11DS, for example by investigating the impact of parental functioning on patient functioning on several phenotypes [53, 54].
Similarly, more research is needed to further improve individualized risk stratification with respect to comorbid conditions and, subsequently, to elucidate how to potentially implement this in clinical practice. A recent IBBC study shows promising progress in this area by demonstrating that the use of polygenic scores, in the context of a population with an a priori increased risk (22q11DS), can significantly improve the positive predictive value with respect to a particular phenotype; in this case schizophrenia [55].
Strengths and limitations
The main strength of this study is that we used IQ-data from the largest database of individuals with 22q11DS currently available. The multi-site collected data underwent extensive quality control, as described elsewhere [26]. We provide an easy-to-use normative IQ chart for the three main IQ constructs, which is readily accessible both to the clinical and research communities.
Limitations are that the available data did not allow for using independent samples in the two main parts of the analyses: creating the normative IQ chart (using all available data), and calculating the z-scores (using only longitudinal data), which would have been methodologically preferable. The results, however, provided confidence that our data are not affected in any major way by biases due to selection or ascertainment, and can therefore be considered normative. The data revealed no differences in IQ-parameters between the subsets with longitudinal data available and the subset with only cross-sectional IQ-data (see also Table 1).
Our normative IQ-chart is limited to individuals with the 22q11.2 deletion between the ages of 6 and 38 (see also Footnote Fig. 1), with decreasing availability of IQ-measures with increasing age, and the sample was not stratified for other key variables typically used in the development of normative tables (e.g., socioeconomic status, region of country). Future studies could include both younger and older individuals to expand coverage of the normchart, and in particular focus on the inclusion of adult subjects to further increase robustness.
Finally, it is important to note that at the time of the current study, available data were limited to VIQ and PIQ, in addition to FSIQ, while there are four key components of overall IQ that formally or informally permeate all versions of the Wechsler scales. Working Memory and Processing Speed are the two other subscales, assessed independently from VIQ and PIQ in most Wechsler scales, and reflect key neuropsychological processes. Specific abnormalities in these domains may be associated with specific psychiatric or neurodevelopmental outcomes [31]. Thus, future studies that aim to elucidate Working Memory and Processing Speed data and trajectories in individuals with 22q11DS are warranted to further our understanding of the complete cognitive profile in individuals with this high-impact variant.
Conclusion
Here, we have discussed the rationale and methodology for using a normative chart for IQ and IQ-development specific to a population with a particular pathogenic variant. Using the 22q11.2 deletion as a model, we demonstrate that a variant-specific normative IQ-chart can be reliably constructed and offers advantages over using only standard (general population) IQ-norms. It allows for more informed interpretation and monitoring of cognitive performance in individuals with the pathogenic variant. It also contributes to the identification of individuals who deviate from their expected trajectory and may be at increased risk for clinically relevant comorbid conditions; e.g., in individuals with 22q11DS and a VIQ-decline steeper than what is expected in this population, the risk of schizophrenia is further elevated. We also demonstrated that using variant-specific normative IQ-data significantly reduces required sample size to detect relevant effects in a research context. The development of this normative chart, based on the largest sample of individuals with 22q11.2DS in the world, should provide additional opportunities to study the cognitive phenotypic presentation of this population specifically, but also provides a proof of principle regarding the identification of cognitive developmental trajectories in groups of individuals affected by other pathogenic variants. We expect that such knowledge will be valuable for clinical researchers and, ultimately, facilitate advances in clinical practice for these individuals and their families.
Funding and disclosure
This study was supported in part by the NIMH International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome (U01MH101719 to RG); the Ter Meulen Grant of the Royal Netherlands Academy of Arts and Sciences and the UMCU Strategic Network Development Grant (AF); the Brain and Behavior Research Foundation (formerly NARSAD; Young Investigator Award to JV); the Academic Scholars Award from the Department of Psychiatry, University of Toronto; and the O’Brien Scholars Fund, Hospital for Sick Children Toronto, Ontario (both EJB). All authors, including the members of the 22q11DS International Consortium on Brain and Behavior, declare that there are no conflicts of interest in relation to the subject of this study.
Supplementary information
Supplement1: Z-score Calculator Normative Chart 22q11DS
Supplement2: Group Authorship IBBC Normative Chart 22q11DS
Acknowledgements
The authors wish to thank all participants and their families for their participation in this study.
Author contributions
Authors AMF, JASV, and EJB had full access to the data and take responsibility for the accuracy of the data and analyses. Authors JASV and EJB contributed equally to this manuscript. All authors, including the members of the 22q11DS International Consortium on Brain and Behavior, contributed important intellectual content and reviewed the manuscript. A complete list of contributing authors, including the members of the 22q11DS International Consortium on Brain and Behavior, is listed in Supplementary Materials 2.
Footnotes
The original online version of this article was revised due to errors in the author byline and in supplementary material.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jacob Vorstman, Elemi J. Breetvelt
Stephen Warren passed away during the preparation of this manuscript.
A full list of members and their affiliations appears in the Supplementary Information
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-00988-6.
References
- 1.Steele A, Scerif G, Cornish K, Karmiloff-Smith A. Learning to read in Williams syndrome and Down syndrome: syndrome-specific precursors and developmental trajectories. J Child Psychol Psychiatry. 2013;54:754–62. doi: 10.1111/jcpp.12070. [DOI] [PubMed] [Google Scholar]
- 2.Bernier R, Hudac CM, Chen Q, Zeng C, Wallace AS, Gerdts J, et al. Developmental trajectories for young children with 16p11.2 copy number variation. Am J Med Genet B Neuropsychiatr Genet. 2017;174:367–80. doi: 10.1002/ajmg.b.32525. [DOI] [PubMed] [Google Scholar]
- 3.Hall SS, Burns DD, Lightbody AA, Reiss AL. Longitudinal changes in intellectual development in children with Fragile X syndrome. J Abnorm Child Psychol. 2008;36:927–39. doi: 10.1007/s10802-008-9223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franic S, Dolan CV, van Beijsterveldt CE, Hulshoff Pol HE, Bartels M, Boomsma DI. Genetic and environmental stability of intelligence in childhood and adolescence. Twin Res Hum Genet. 2014;17:151–63. doi: 10.1017/thg.2014.26. [DOI] [PubMed] [Google Scholar]
- 5.Smeets EE, Townend GS, Curfs LMG. Rett syndrome and developmental regression. Neurosci Biobehav Rev. 2019;104:100–01. doi: 10.1016/j.neubiorev.2019.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Einspieler C, Marschik PB. Regression in Rett syndrome: developmental pathways to its onset. Neurosci Biobehav Rev. 2019;98:320–32. doi: 10.1016/j.neubiorev.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 7.Ballard C, Mobley W, Hardy J, Williams G, Corbett A. Dementia in Down’s syndrome. Lancet Neurol. 2016;15:622–36. doi: 10.1016/S1474-4422(16)00063-6. [DOI] [PubMed] [Google Scholar]
- 8.Startin CM, D’Souza H, Ball G, Hamburg S, Hithersay R, Hughes KMO, et al. Health comorbidities and cognitive abilities across the lifespan in Down syndrome. J Neurodev Disord. 2020;12:4. doi: 10.1186/s11689-019-9306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grieco J, Pulsifer M, Seligsohn K, Skotko B, Schwartz A. Down syndrome: Cognitive and behavioral functioning across the lifespan. Am J Med Genet C Semin Med Genet. 2015;169:135–49. doi: 10.1002/ajmg.c.31439. [DOI] [PubMed] [Google Scholar]
- 10.Patterson T, Rapsey CM, Glue P. Systematic review of cognitive development across childhood in Down syndrome: implications for treatment interventions. J Intellect Disabil Res. 2013;57:306–18. doi: 10.1111/j.1365-2788.2012.01536.x. [DOI] [PubMed] [Google Scholar]
- 11.Davies G, Tenesa A, Payton A, Yang J, Harris SE, Liewald D, et al. Genome-wide association studies establish that human intelligence is highly heritable and polygenic. Mol Psychiatry. 2011;16:996–1005. doi: 10.1038/mp.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batty GD, Deary IJ, Gottfredson LS. Premorbid (early life) IQ and later mortality risk: systematic review. Ann Epidemiol. 2007;17:278–88. doi: 10.1016/j.annepidem.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Kahn RS, Keefe RS. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013;70:1107–12. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- 14.Zemel BS, Pipan M, Stallings VA, Hall W, Schadt K, Freedman DS, et al. Growth charts for children with Down syndrome in the United States. Pediatrics. 2015;136:e1204–11. doi: 10.1542/peds.2015-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mircher C, Briceno LG, Toulas J, Conte M, Tanguy ML, Cieuta-Walti C, et al. Growth curves for French people with Down syndrome from birth to 20 years of age. Am J Med Genet A. 2018;176:2685–94. doi: 10.1002/ajmg.a.40639. [DOI] [PubMed] [Google Scholar]
- 16.Growth Hormone Research S. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. GH Research Society. J Clin Endocrinol Metab. 2000;85:3990–3. doi: 10.1210/jcem.85.11.6984. [DOI] [PubMed] [Google Scholar]
- 17.Swillen A, McDonald-McGinn D. Developmental trajectories in 22q11.2 deletion. Am J Med Genet C Semin Med Genet. 2015;169:172–81. doi: 10.1002/ajmg.c.31435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JA, et al. 22q11.2 deletion syndrome. Nat Rev Dis Prim. 2015;1:15071. doi: 10.1038/nrdp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van L, Boot E, Bassett AS. Update on the 22q11.2 deletion syndrome and its relevance to schizophrenia. Curr Opin Psychiatry. 2017;30:191–96. doi: 10.1097/YCO.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 20.Schneider M, Debbane M, Bassett AS, Chow EW, Fung WL, van den Bree M, et al. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry. 2014;171:627–39. doi: 10.1176/appi.ajp.2013.13070864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiksinski AM, Breetvelt EJ, Duijff SN, Bassett AS, Kahn RS, Vorstman JA. Autism spectrum and psychosis risk in the 22q11.2 deletion syndrome. Findings from a prospective longitudinal study. Schizophr Res. 2017;188:59–62. doi: 10.1016/j.schres.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vorstman JA, Morcus ME, Duijff SN, Klaassen PW. Heineman-de Boer JA, Beemer FA, et al. The 22q11.2 deletion in children: high rate of autistic disorders and early onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry. 2006;45:1104–13. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- 23.Duijff SN, Klaassen PW, de Veye HF, Beemer FA, Sinnema G, Vorstman JA. Cognitive development in children with 22q11.2 deletion syndrome. Br J Psychiatry. 2012;200:462–8. doi: 10.1192/bjp.bp.111.097139. [DOI] [PubMed] [Google Scholar]
- 24.Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11:402–16. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vorstman JA, Breetvelt EJ, Duijff SN, Eliez S, Schneider M, Jalbrzikowski M, et al. Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiatry. 2015;72:377–85. doi: 10.1001/jamapsychiatry.2014.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cleynen I, Engchuan W, Hestand MS, Heung T, Holleman AM, Johnston HR, et al. Genetic contributors to risk of schizophrenia in the presence of a 22q11.2 deletion. Mol Psychiatry. 10.1038/s41380-020-0654-3 2020. [DOI] [PMC free article] [PubMed]
- 27.Gur RE, Bassett AS, McDonald-McGinn DM, Bearden CE, Chow E, Emanuel BS, et al. A neurogenetic model for the study of schizophrenia spectrum disorders: the International 22q11.2 Deletion Syndrome Brain Behavior Consortium. Mol Psychiatry. 2017;22:1664–72. doi: 10.1038/mp.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wechsler D. Wechsler Adult Intelligence Scale—revised. The Psychological Corporation: San Antonio, TX; 1981.
- 29.Wechsler D. Wechsler Adult Intelligence Scale-III. The Psychological Corporation: San Antonio, TX; 1997.
- 30.Wechsler D. Wechsler Abbreviated Scale of Intelligence—Second Edition (WASI-II). NCS Pearson: San Antonio, TX; 2011.
- 31.Wechsler D. Wechsler Adult Intelligence Scale–Fourth Edition. Pearson: San Antonio, TX; 2008.
- 32.Wechsler D. The Wechsler preschool and primary scale of intelligence, Third Edition (WPPSI-III). The Psychological Corporation: San Antonio, TX; 2002
- 33.Wechsler D. The Wechsler intelligence scale for children—third edition. The Psychological Corporation: San Antonio, Texas; 1991.
- 34.Wechsler D. Wechsler Intelligence Scale for Children–Fourth Edition. The Psychological Corporation: San Antonio, TX; 2003.
- 35.Association AP. Diagnostic and statistical manual of mental disorders (4th ed., Text Revision). Author: Washington, DC; 2000.
- 36.Chow EW, Watson M, Young DA, Bassett AS. Neurocognitive profile in 22q11 deletion syndrome and schizophrenia. Schizophr Res. 2006;87:270–8. doi: 10.1016/j.schres.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Smedt B, Devriendt K, Fryns JP, Vogels A, Gewillig M, Swillen A. Intellectual abilities in a large sample of children with Velo-Cardio-Facial Syndrome: an update. J Intellect Disabil Res. 2007;51:666–70. doi: 10.1111/j.1365-2788.2007.00955.x. [DOI] [PubMed] [Google Scholar]
- 38.Fiksinski A, Heung T, Corral M, Breetvelt E, Costain G, Marshall C, et al. Within-family influences on dimensional neurobehavioral traits in a high-risk genetic model. Psychol Med. 2021:1–9. [DOI] [PMC free article] [PubMed]
- 39.Fiksinski AM, Schneider M, Murphy CM, Armando M, Vicari S, Canyelles JM, et al. Understanding the pediatric psychiatric phenotype of 22q11.2 deletion syndrome. Am J Med Genet A. 2018:1–10. [DOI] [PMC free article] [PubMed]
- 40.Bassett AS, McDonald-McGinn DM, Devriendt K, Digilio MC, Goldenberg P, Habel A, et al. Practical guidelines for managing patients with 22q11.2 deletion syndrome. J Pediatr. 2011;159:332–9 e1. doi: 10.1016/j.jpeds.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fung WL, Butcher NJ, Costain G, Andrade DM, Boot E, Chow EW, et al. Practical guidelines for managing adults with 22q11.2 deletion syndrome. Genet Med. 2015;17:599–609. doi: 10.1038/gim.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swillen A, McDonald-McGinn D. Developmental trajectories in 22q11.2 deletion. Am J Med Genet Part C: Semin Med Genet. 2015;169:172–81. doi: 10.1002/ajmg.c.31435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacCabe JH, Wicks S, Lofving S, David AS, Berndtsson A, Gustafsson JE, et al. Decline in cognitive performance between ages 13 and 18 years and the risk for psychosis in adulthood: a Swedish longitudinal cohort study in males. JAMA Psychiatry. 2013;70:261–70. doi: 10.1001/2013.jamapsychiatry.43. [DOI] [PubMed] [Google Scholar]
- 44.Mollon J, David AS, Zammit S, Lewis G, Reichenberg A. Course of cognitive development from infancy to early adulthood in the psychosis spectrum. JAMA Psychiatry. 2018;75:270–79. doi: 10.1001/jamapsychiatry.2017.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harvey PD, Bowie CR, Friedman JI. Cognition in schizophrenia. Curr Psychiatry Rep. 2001;3:423–8. doi: 10.1007/s11920-996-0038-7. [DOI] [PubMed] [Google Scholar]
- 46.Keefe RSE, Kahn RS. Cognitive decline and disrupted cognitive trajectory in schizophrenia. JAMA Psychiatry. 2017;74:535–36. doi: 10.1001/jamapsychiatry.2017.0312. [DOI] [PubMed] [Google Scholar]
- 47.Sullivan PF, Owen MJ. Increasing the clinical psychiatric knowledge base about pathogenic copy number variation. Am J Psychiatry. 2020;177:204–09. doi: 10.1176/appi.ajp.2019.19040335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chawner S, Doherty JL, Moss H, Niarchou M, Walters JTR, Owen MJ, et al. Childhood cognitive development in 22q11.2 deletion syndrome: case-control study. Br J Psychiatry. 2017;211:223–30. doi: 10.1192/bjp.bp.116.195651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sansone SM, Schneider A, Bickel E, Berry-Kravis E, Prescott C, Hessl D. Improving IQ measurement in intellectual disabilities using true deviation from population norms. J Neurodev Disord. 2014;6:16. doi: 10.1186/1866-1955-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Briegel W, Schneider M, Schwab KO. 22q11.2 deletion syndrome: behaviour problems of children and adolescents and parental stress. Child Care Health Dev. 2008;34:795–800. doi: 10.1111/j.1365-2214.2008.00850.x. [DOI] [PubMed] [Google Scholar]
- 51.Fiksinski AM, Breetvelt EJ, Lee YJ, Boot E, Butcher N, Palmer L, et al. Neurocognition and adaptive functioning in a genetic high risk model of schizophrenia. Psychol Med. 2018;49:1047–54. doi: 10.1017/S0033291718001824. [DOI] [PubMed] [Google Scholar]
- 52.Butcher NJ, Chow EW, Costain G, Karas D, Ho A, Bassett AS. Functional outcomes of adults with 22q11.2 deletion syndrome. Genet Med. 2012;14:836–43. doi: 10.1038/gim.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moreno-De-Luca A, Evans DW, Boomer KB, Hanson E, Bernier R, Goin-Kochel RP, et al. The role of parental cognitive, behavioral, and motor profiles in clinical variability in individuals with chromosome 16p11.2 deletions. JAMA Psychiatry. 2015;72:119–26. doi: 10.1001/jamapsychiatry.2014.2147. [DOI] [PubMed] [Google Scholar]
- 54.Huguet G, Schramm C, Douard E, Jiang L, Labbe A, Tihy F, et al. Measuring and estimating the effect sizes of copy number variants on general intelligence in community-based samples. JAMA Psychiatry. 2018;75:447–57. doi: 10.1001/jamapsychiatry.2018.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davies RW, Fiksinski AM, Breetvelt EJ, Williams NM, Hooper SR, Monfeuga T, et al. Using common genetic variation to examine phenotypic expression and risk prediction in 22q11.2 deletion syndrome. Nat Med. 2020;26:1912–18. doi: 10.1038/s41591-020-1103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement1: Z-score Calculator Normative Chart 22q11DS
Supplement2: Group Authorship IBBC Normative Chart 22q11DS