Abstract
Introduction: Fluid stewardship targets optimal fluid management to improve patient outcomes. Intravenous (IV) medications, flushes, and blood products, collectively referred to as hidden fluids, contribute to fluid intake in the intensive care unit (ICU). The impact of specific IV medications on fluid intake is unknown. Objective: Characterize IV medication classes based on contribution to ICU fluid intake by frequency of administration and total volume infused to identify targets for fluid stewardship. Methods: This multi-center, retrospective nested cohort study included patients admitted to a medical or surgical ICU between January 2017 and December 2018. The primary outcome was to identify the volume contribution of specific IV medication classes administered over the first 3 ICU days. Secondary outcomes were the administration frequency of these medications and their proportion of total daily volume intake over the first 3 ICU days. Results: The study included 210 patients. The largest mean administration volumes over the course of the first 3 ICU days were attributed to antibacterials (968 ± 846 mL), vitamins/minerals/electrolytes (416 ± 935 mL), pain/agitation/delirium agents (310 ± 512 mL), and vasoactive agents (282 ± 744 mL). The highest frequencies over the course of the first 3 ICU days were attributed to antibacterials (n = 180; 86%), pain/agitation/delirium agents (n = 143; 68%), vitamins/minerals/electrolytes (n = 123; 59%), and vasoactive agents (n = 96; 46%). IV medications contributed 2601 ± 2573 mL of fluid volume per patient over the first 3 ICU days, accounting for 42% ± 29% of overall volume. Conclusion: IV medications contribute over 40% of total fluid intake within the first 3 days of ICU admission, with antibacterials as top contributors by administration volume and frequency. Future research implementing fluid stewardship to ICU fluid sources, such as concentrating IV medications, switching IV medications to oral formulations, de-escalation of antibacterials, and reduction of maintenance fluids, should be performed to minimize hidden fluids from IV medications.
Keywords: critical care, fluid and electrolyte disorders, intravenous therapy
Background
Fluid stewardship promotes the proper administration of intravenous fluids (IVF) to prevent complications of fluid overload (FO) and improve patient outcomes. 1 Notably, this initiative can be pharmacist-driven. Adverse outcomes resulting from FO may arise from any organ system resulting in increased mortality, length of intensive care unit (ICU) stay, illness severity, need for invasive procedures, and acute kidney injury. 2 Approximately 20% of all patients inappropriately receive IVF, and the use of the 4 rights construct of fluid stewardship (patient, drug, dose, and route) has the potential to optimize patient outcomes.1,3,4 In fact, achieving negative fluid balance either actively or passively has been shown to shorten the duration of complications and reduce mortality. 5 Hidden fluids, which consist of IV medications, blood products, and flushes, may be a significant contributor to the “right dose.” 1 Hidden fluids are not always considered when evaluating fluid intake at the bedside, as their volumes are not specifically prescribed, but recent data indicate that IV medication diluents may provide the largest volume of fluid sources, especially when administered intermittently. 6 Data have demonstrated that IV medications contribute 61% of total fluid intake on the first day of ICU admission and 40% over the first 7 days.6,7 However, literature generalizes this data to all IV medications without specifying fluid contribution by medication class.
Identifying such classes may highlight fluid sources that can be minimized to reduce cumulative fluid balance and complications of FO as well as improve patient outcomes. The purpose of this study was to characterize which individual IV medication classes contribute most toward the fluid intake of critically ill patients during the first 3 days of ICU admission.
Methods
This retrospective, nested cohort study was conducted at 3 sites across the state of Georgia, including a large academic medical center and 2 community hospitals. All sites implemented medication barcode scanning for nurse charting, stocked products similar in concentration (Supplemental Table 1), and used a decentralized pharmacy model involving rounding clinical pharmacists. No formal fluid stewardship efforts were performed at any site at the time of this study. The study was approved by a central institutional review board at the University of Georgia and was acknowledged and/or approved separately by the institutional review board at each site.
Patients were eligible for inclusion if they were at least 18 years old, admitted into a medical or surgical ICU between January 2017 and December 2018, and had an ICU length of stay of at least 4 days. Exclusion criteria included patients who were pregnant, received total parenteral nutrition, had end stage renal disease or any form of renal replacement therapy, were admitted to the ICU under a “do not resuscitate” or “do not intubate” status, were transferred from an external hospital, or had a specific indication for maintenance intravenous fluids (mIVF). Indications for mIVF included diabetic ketoacidosis, diabetes insipidus, cerebral salt wasting, high output fistula (volume >500 mL/day), high output drains (volume >500 mL/day), tumor lysis syndrome, or rhabdomyolysis.
The primary outcome was the volume of specific IV medications administered over the first 3 ICU days. The cumulative mean volume was defined as the mean of the total volume captured per patient over the first 3 ICU days. Secondary outcomes included the frequency of specific IV medications, which was defined as the proportion of patients who received each medication, and the proportion of total fluid intake made up by IV medications over the first 3 ICU days.
Hidden fluids were defined as blood products, enteral nutrition, flushes, and IV medications, the volumes of which are not specifically prescribed. IV medications were categorized as albumin, antiarrhythmics, antibacterials, anticoagulants, anticonvulsants, antifungals, antivirals, dextrose, diuretics, insulin infusions, pain/agitation/delirium (PAD) agents, proton pump inhibitor (PPI) boluses and infusions, sodium bicarbonate, vasoactive agents, and vitamins/minerals/electrolytes (VME). Antihypertensive medications were included within the vasoactive agent category. Complete fluid sources were defined as resuscitation, maintenance, and hidden fluids. Fluid data were collected daily over the first 3 calendar ICU days from the time of admission, not a 24-hour period. Volumes on Day 1 that were administered on a previous floor or in the emergency department were excluded.
All statistical analyses were performed using SPSS® Statistics Version 26. All results were reported using descriptive statistics. Data followed a parametric distribution. As such, continuous variables were reported as mean and standard deviation and categorical variables were reported as number and percentage.
Results
A total of 210 patients were included in this study. The cohort was predominantly male (54%) with a mean age of 62 ± 14.8 years with the most common co-morbidities being congestive heart failure (47%) and chronic lung disease (33%). The majority of patients were admitted to a medical ICU (95%). Baseline characteristics of all patients are summarized in Table 1.
Table 1.
Baseline Characteristics.
| n = 210 | |
|---|---|
| Age, years | 62 ± 14.8 |
| Male | 114 (54) |
| Race | |
| Caucasian | 121 (58) |
| African American | 80 (38) |
| Other/unknown | 9 (4) |
| Weight, kg | 84.4 ± 29.4 |
| Height, cm | 170.1 ± 10.6 |
| Past medical history | |
| Congestive heart failure | 98 (47) |
| Chronic lung disease | 69 (33) |
| Cancer | 31 (15) |
| Chronic kidney disease | 32 (15) |
| Atrial fibrillation | 19 (9) |
| Chronic liver disease | 17 (8) |
| LVEF ≤40% | 13 (6) |
| SOFA score on Day 1 | 5.9 ± 3.4 |
| ICU types | |
| Medical | 200 (95) |
| Surgical | 10 (5) |
Note. Values represented as number (%) or mean ± standard deviation.
LVEF = left ventricular ejection fraction; SOFA = sequential organ failure assessment; ICU = intensive care unit.
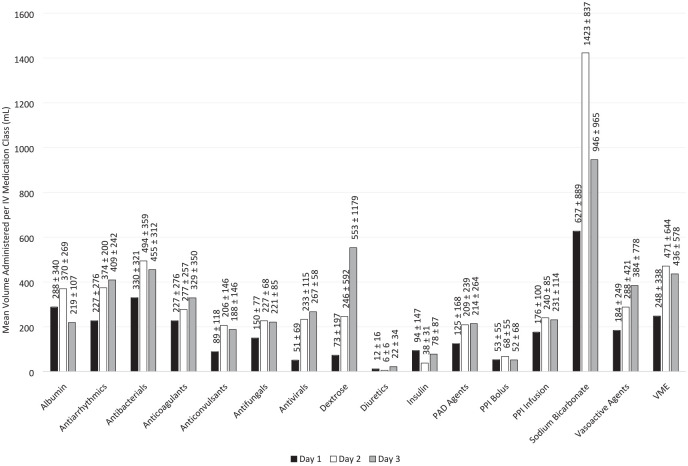
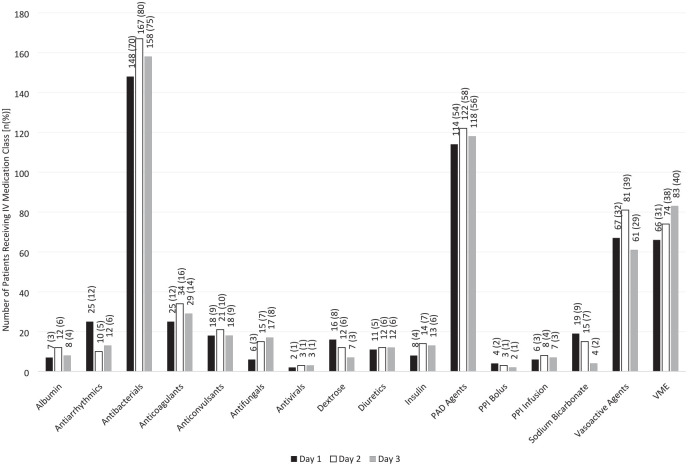
The IV medication classes with the largest cumulative mean volumes and highest administration frequencies over the first 3 ICU days (n = 210) include antibacterials, VME, PAD agents, and vasoactive agents. Complete cumulative mean volumes and administration frequencies are reported in Table 2. Complete daily administration volume, among only patients who received each medication, is reported in Figure 1. The proportion of patients who received each medication is reported in Figure 2.
Table 2.
Cumulative Mean Volume and Administration Frequency by IV Medication Class over the First 3 ICU Days, among All Included Patients (n = 210).
| Fluid | Volume (mL) | Frequency |
|---|---|---|
| Albumin | 39 ± 166 | 16 (8) |
| Antiarrhythmic | 56 ± 231 | 18 (9) |
| Antibacterials | 968 ± 846 | 180 (86) |
| Anticoagulants | 117 ± 407 | 39 (19) |
| Anticonvulsants | 44 ± 170 | 22 (10) |
| Antifungals | 38 ± 141 | 17 (8) |
| Antivirals | 8 ± 62 | 4 (2) |
| Dextrose | 38 ± 426 | 20 (10) |
| Diuretics | 2 ± 11 | 24 (11) |
| Insulin | 11 ± 51 | 19 (9) |
| PAD agents | 310 ± 512 | 143 (68) |
| PPI Bolus | 2 ± 16 | 7 (3) |
| PPI Infusion | 22 ± 118 | 8 (4) |
| Sodium bicarbonate | 176 ± 675 | 23 (11) |
| Vasoactive agents | 282 ± 744 | 96 (46) |
| VME | 416 ± 935 | 123 (59) |
Note. Values represented as number (%) or mean ± standard deviation.
PAD = pain/agitation/delirium; PPI = proton pump inhibitor; VME = vitamins/minerals/electrolytes.
Figure 1.
Mean IV medication administration volume, among patients who received each medication (n = 210)*.
*Day 1 represents a calendar day, not a 24-hour period. Day 1 data exclude administrations from a previous floor location or in the emergency department prior to ICU admission, which may result in skewed values.
Figure 2.
Proportion of patients who received specific IV medications (n = 210)*.
*Day 1 represents a calendar day, not a 24-hour period. Day 1 data exclude administrations from a previous floor location or in the emergency department prior to ICU admission, which may result in skewed values.
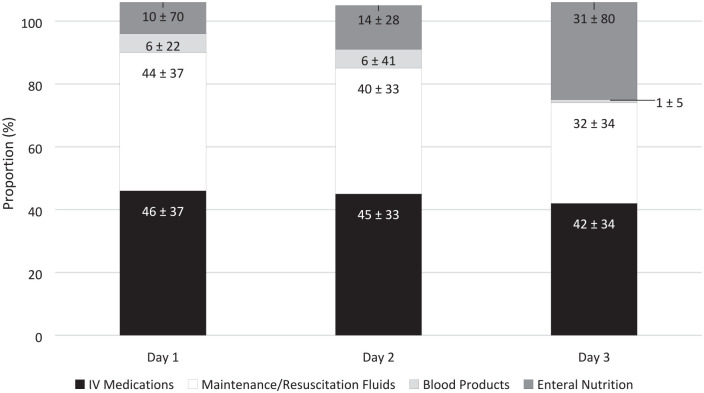
The proportion of daily fluid intake of maintenance/resuscitation, IV medications, and other sources of hidden fluids per patient over the first 3 ICU days (n = 135) were 43% ± 30%, 42% ± 29%, and 14% ± 18%, respectively. These proportions correspond to mean volumes of 3271 ± 2942 mL, 2601 ± 2573 mL, and 1027 ± 1337 mL, respectively. Hidden fluids collectively contributed 56% ± 30% of fluid intake per patient over the course of the first 3 ICU days, which corresponds to 3629 ± 2913 mL. Complete daily proportion of fluid intake results are reported in Figure 3, among all patients included in the study.
Figure 3.
Mean proportion of fluid type by daily administration (n = 135)*.
Day 1 represents a calendar day, not a 24-hour period. Day 1 data exclude administrations from a previous floor location or in the emergency department prior to ICU admission, which may result in skewed values.
*Cumulative proportion of each fluid type does not necessarily equal 100% because the proportions were calculated at the patient level as opposed to the cohort level.
Discussion
Antibacterials, VME, PAD agents, and vasoactive agents may act as key targets for fluid stewardship interventions due to their large volume and high frequency of administration. In particular, antibacterials contribute the most to fluid intake over the first 3 ICU days with both the highest frequency of administration and cumulative largest volume.
Antibacterial agents present strong targets for stewardship both from a volume and antimicrobial perspective within an ICU setting. The high rate of prescribing observed in this study is similar to the reported literature, with nearly 70% of all patients receiving an antibacterial agent. 8 Empiric antibacterial therapy often consists of 2 or more agents and is traditionally de-escalated to fewer agents in the effort to direct therapy toward microbiologic data.9,10 Empiric therapy commonly includes vancomycin, which is a strong target for antimicrobial stewardship and fluid stewardship. A common way to de-escalate empiric broad spectrum antibiotic therapy, especially for respiratory and diabetic foot infections, is through a polymerase chain reaction nares test for methicillin resistant Staphylococcus aureus, which can lead to early discontinuation of vancomycin when negative.11,12 Another effective de-escalation method is switching from IV to oral administration of antibiotics, when appropriate. Overall, the efforts of antimicrobial stewardship that have been wildly utilized indeed parallel the goals of fluid stewardship.
This study’s results regarding hidden fluids and more specifically IV medications are comparable with previous literature. Van Regenmortel et al quantified volume intake by maintenance fluids and “fluid creep,” which was defined as volumes attributed to electrolytes, flushes, and diluents. Bashir et al evaluated “hidden obligatory” fluids contribition to fluid intake. The hidden fluids data from Van Regenmortel et al, Bashir et al, and this study resulted in hidden fluids contributing 68.6%, 55.7%, and 56% to total fluid intake, respectively.13,14 In the Hawkins and colleagues study, fluid sources were quantified to highlight areas for volume minimization with 61.4% of total volume intake on the first ICU day and 40% over the first 7 days sourced from IV medications. 7 These results suggest a discrepancy with the findings from Day 1 (46%) of this study but are comparable for Days 2 (45%) and 3 (42%). This discrepancy could be explained by the study design, in which Day 1 data represented the first calendar day in the ICU as opposed to the 24-hour period following ICU admission. Overall, the data show that the contribution of IV medications remains relatively constant over the course of an ICU stay and contributes substantial volumes of hidden fluids, which do increase over the indexed ICU admission.
The large impact of IV medications and other sources of hidden fluids administration on total fluid intake should influence how fluids are prescribed and administered. Bisher et al concluded that hidden fluids should be incorporated in the evaluation of volume intake when managing fluid administration in critically ill patients. 14 Van Regenmortel et al more specifically suggested that maintenance fluids may be avoided and electrolytes may be concentrated to reduce “fluid creep” contribution. 13 Furthermore, the results from this study highlight the need to utilize fluid minimization. Maintenance and resuscitation fluids can be targeted through conservative dosing strategies, and all sources of hidden fluids should be considered when assessing fluid balance.1,15
Focusing on the reduction of volumes associated with IV medications, many methods many be considered. The most effective approach is to discontinue these medications when clinically appropriate. This could include the weaning or cessation of IV infusions as well as discontinuing orders that have been unused to avoid accidental administration. Switching from IV to an enteral route would also avoid the administration of these volumes altogether. However, this requires the patient to be stable enough to tolerate oral intake and to have appropriate diet orders, which may not be possible for this target ICU patient population. This may be achievable for patient with enteral tubes, such as nasogastric. Changing from IV infusion to IV push would significantly reduce the administered volumes but may be restricted due to lack of stability data or short half-lives of the medications. Specifically for antibacterials, implementing extended or continuous infusions may result in fewer required doses and respectively reduce the volumes associated with these agents. When these IV medications are necessary and the patient is at risk for fluid overload or already has a positive fluid balance, concentrating infusions is an alternative option. Although this would reduce the volumes administered, this method is also associated with risks. Concentrated infusions may require a central line, which puts the patient at additional risk for infection as well as the potential difficulty to remove this access. Specific products, such as propofol, may not be available commercially for sterile compounding and would not be candidates for concentrating. Additionally, data confirming the stability of these concentrated medications may be lacking and therefore would be unsafe to administer to a patient. The American Society of Health-System Pharmacists (ASHP) acknowledges this approach when clinically possible with respect to feasibility and fluid status. 16 However, it also encourages the use of premixed products or standardized compounded concentrations as they have proven stability and reduce medication errors. 16
Although hidden fluids have been associated with the development of fluid overload, future research should be conducted to further confirm these trends over a longer ICU stay. 17 Other areas for further investigation include identifying specific IV agents that contribute the most to intake as targets for fluid stewardship, identifying admission diagnoses that may clinically benefit from targeted IV medication volume minimization, evaluating the effects of stewardship focused on hidden fluids on patient centered outcomes, and evaluating the association between IV medication administration and fluid balance. Prescribing practices may also be analyzed proceeding provider education and formal efforts to minimize IV medication volumes.
Previous literature has identified IV medications as a source of fluid intake in numerous instances, but this study is the first to identify by medication class. Furthermore, the multi-center design and incorporation of patients from both academic and community hospitals, which increases generalizability, are additional strengths. Limitations include the observational design and predominant medical ICU population. Data collection was limited by the accuracy of charting and abstraction of data from the medical record; thus, it is possible that fluids were administered and not captured in our study due to incomplete documentation. Daily portion results regarding resuscitation/maintenance and hidden fluids were calculated from 135 patients, as these data were collected from 2 of the 3 sites (2 community hospitals). Data were collected over the course of the first 3 calendar days after admission to the ICU and not over the course of 72 hours, which may have under-represented true volumes and frequencies on day 1. Day 1 data exclude administrations from a previous floor location or in the emergency department prior to ICU admission, which may result in skewed values. Although a detailed data dictionary was used to standardize the data collection process across study sites, differences in data collection among sites, especially compounded by different medical record software, cannot be ruled out. Additionally, admission diagnosis was not included within data collection.
Conclusion
IV medications provide a significant proportion of total fluid intake in the first 3 days in the ICU, with antibacterials as the leading contributor. Overall, percent volume from hidden fluids increases daily and contributes over half of total fluid intake in this timeframe. Fluid stewardship initiatives may significantly reduce total ICU volume intake by targeting IV medications.
Supplemental Material
Supplemental material, sj-pdf-1-hpx-10.1177_00185787211016339 for Hidden Fluids in Plain Sight: Identifying Intravenous Medication Classes as Contributors to Intensive Care Unit Fluid Intake by Kelly C. Gamble, Susan E. Smith, Christopher M. Bland, Andrea Sikora Newsome, Trisha N. Branan and William Anthony Hawkins in Hospital Pharmacy
Acknowledgments
We appreciate Aanand Patel, PharmD; Nathan Allen, PharmD; Bernard Hsia, PharmD; and William J. Olney, PharmD for their aid in data collection. REDCap was used for all data collection and management.
Footnotes
Authors’ Note: On behalf of The University of Georgia College of Pharmacy Critical Care Collaborative (UGAC3). Previous affiliations: At the time of writing, Dr. Gamble was a PharmD candidate at the University of Georgia College of Pharmacy in Savannah, GA.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Christopher M. Bland is a consultant and has received grant funding from Merck Pharmaceuticals. Andrea Sikora Newsome is supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under Award Numbers UL1TR002378 and KL2TR002381. All other authors declare no relevant conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Kelly C. Gamble  https://orcid.org/0000-0002-7463-2364
https://orcid.org/0000-0002-7463-2364
Susan E. Smith  https://orcid.org/0000-0002-5171-8405
https://orcid.org/0000-0002-5171-8405
Christopher M. Bland  https://orcid.org/0000-0001-8806-4583
https://orcid.org/0000-0001-8806-4583
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Hawkins WA, Smith SE, Newsome AS, Carr JR, Bland CM, Branan TN. Fluid stewardship during critical illness: a call to action. J Pharm Pract. 2020;33(6):863-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Claure-Del Granado R, Mehta RL. Fluid overload in the ICU: evaluation and management. BMC Nephrol. 2016;17(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoste EA, Maitland K, Brudney CS, et al. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malbrain M, Van Regenmortel N, Saugel B, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care. 2018;8(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Silversides JA, Fitzgerald E, Manickavasagam US, et al. Deresuscitation of patients with iatrogenic fluid overload is associated with reduced mortality in critical illness. Crit Care Med. 2018;46(10):1600-1607. [DOI] [PubMed] [Google Scholar]
- 6. Magee CA, Bastin MLT, Laine ME, et al. Insidious harm of medication diluents as a contributor to cumulative volume and hyperchloremia: a prospective, open-label, sequential period pilot study. Crit Care Med. 2018;46(8):1217-1223. [DOI] [PubMed] [Google Scholar]
- 7. Hawkins A, Sams J, Jozefczyk H, Kumar G. 367: Fluid stewardship identifying opportunities for targeted fluid minimization. Crit Care Med. 2016;44(12):168. [Google Scholar]
- 8. Vincent J-L, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323-2329. [DOI] [PubMed] [Google Scholar]
- 9. Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev. 2012;25(3):450-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Waele JJ, Schouten J, Beovic B, Tabah A, Leone M. Antimicrobial de-escalation as part of antimicrobial stewardship in intensive care: no simple answers to simple questions—a viewpoint of experts. Intensive Care Med. 2020;46(2):236-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garnacho-Montero J, Escoresca-Ortega A, Fernández-Delgado EJ. Antibiotic de-escalation in the ICU: how is it best done? Curr Opin Infect Dis. 2015;28(2):193-198. [DOI] [PubMed] [Google Scholar]
- 12. Mergenhagen KA, Croix M, Starr KE, Sellick JA, Lesse AJ. The utility of methicillin-resistant Staphylococcus aureus nares screening for patients with a diabetic foot infection. Antimicrob Agents Chemother. 2020;64(4):e02213-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Regenmortel N, Verbrugghe W, Roelant E, Van den Wyngaert T, Jorens PG. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018;44(4):409-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bashir MU, Tawil A, Mani VR, Farooq U, A. DeVita M. Hidden obligatory fluid intake in critical care patients. J Intensive Care Med. 2017;32(3):223-227. [DOI] [PubMed] [Google Scholar]
- 15. Carr J, Hawkins AW, Sikora Newsome A, Smith SE, Bland C, Branan T. Fluid stewardship of maintenance intravenous fluids. J Pharm Pract. Published online April 8, 2021. doi: 10.1177/08971900211008261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Society of Health-System Pharmacists. Standardized concentrations: adult continuous IV infusions version 1.01. In: Standardize 4 Safety. ASHP; 2016. [Google Scholar]
- 17. Branan TN, Smith SE, Phan R, Sikora Newsome A, Hawkins WA. Association of hidden fluid administration with development of fluid overload reveals opportunities for targeted fluid minimization. Sage Open Med. 2020;8:2050312120979464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-hpx-10.1177_00185787211016339 for Hidden Fluids in Plain Sight: Identifying Intravenous Medication Classes as Contributors to Intensive Care Unit Fluid Intake by Kelly C. Gamble, Susan E. Smith, Christopher M. Bland, Andrea Sikora Newsome, Trisha N. Branan and William Anthony Hawkins in Hospital Pharmacy