Abstract
Half-saturation constants (Km), maximum uptake rates (Vmax), and threshold concentrations for sulfate and hydrogen were determined for two thermophilic sulfate-reducing bacteria (SRB) in an incubation system without headspace. Km values determined for the thermophilic SRB were similar to the constants described for mesophilic SRB isolated from environments with low sulfate concentrations.
Dissimilatory sulfate reduction and methanogenesis are the main terminal processes in the anaerobic food chain. Both the sulfate-reducing bacteria (SRB) and the methane-producing archaea (MPA) use acetate and hydrogen as substrates and, therefore, compete for common electron donors in sulfate-containing natural environments (22, 24). Due to a higher affinity for the electron donors acetate and hydrogen, SRB outcompete MPA for these compounds whenever sulfate is present in sufficient concentrations (1, 12, 13, 17).
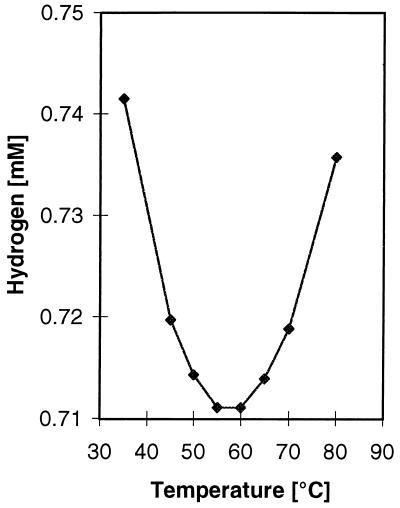
The solubility of H2 is generally considered to decrease with increasing temperature. Thermophilic H2-utilizing microorganisms could circumvent this challenge by an increased affinity and a lower threshold concentration for H2. The solubility of H2 calculated from Bunsen absorption coefficients (this study), however, varies less than 5% from 35 to 80°C (Fig. 1). The necessity for affinity compensation should therefore be minimal. Since no previous determination of kinetic constants or threshold concentrations for thermophilic SRB are available in the literature, we wanted to test how the higher growth temperature of two recently isolated thermophilic SRB affects these constants.
FIG. 1.
Solubility of H2 in water at different temperatures and at a partial pressure of 1 atm. The solubility Bunsen coefficients were calculated as described by Crozier and Yamamoto (5). The coefficients were transformed to millimoles per liter by division by RT, where R is the gas constant and T is the temperature in degrees kelvin.
The SRB studied were isolated from microbial mat samples collected downstream of a slightly alkaline (pH 8.7) hot spring in Hveragerdi, Iceland (19). Strain JSP was isolated from a microbial mat sample collected at approximately 75°C. Strain R1Ha3 was isolated from a microbial mat collected from a 55°C hot-spring-simulating reactor, which originally was inoculated with microbial mat samples collected at 55°C from the same hot spring in Hveragerdi. Analysis of the 16S rRNA sequences of strain JSP showed that this bacterium belongs to the genus Thermodesulfobacterium, while strain R1Ha3 belongs to the genus Thermodesulfovibrio (unpublished results). (The EMBL accession numbers for these strains are X96725 [strain JSP] and X96726 [strain R1Ha3]).
A modification of Postgate 3 medium (14) was used for cultivation of the bacteria and for the kinetic experiments. The medium contained the following: KH2PO4, 0.50 g liter−1; NH4Cl, 1.0 g liter−1; yeast extract, 1.0 g liter−1; ascorbic acid, 0.10 g liter−1; sodium thioglycolate, 0.114 g liter−1; NaCl, 0.10 g liter−1; sodium resazurin (Sigma), 0.00050 g liter−1; and a trace element solution, 1.0 ml liter−1 (3). The medium was adjusted to pH 7.0 with NaOH, autoclaved, and supplemented with sterile anaerobic solutions of sodium sulfate and sodium lactate to final concentrations of 20 and 25 mM, respectively, and with CaCl2 and MgCl2 to final concentrations of 0.68 and 0.6 mM, respectively. All experiments were performed at the optimal temperatures for growth, which are 65 and 70°C for strains R1Ha3 and JSP, respectively.
Prior to sulfate uptake kinetic experiments, late-exponential-phase cells (confirmed at an optical density of 578 [OD578]) were harvested by centrifugation (4,068 × g for 30 min) and washed twice in medium without sulfate under aseptic and anaerobic conditions. The sulfate uptake kinetics were measured as [35S]sulfate uptake as described previously (8) with the exception that the experiments were done in 30 ml of the modified Postgate medium. The experiments were repeated twice, each time with five replicates. Vials with autoclaved inoculum were used as controls. Cultures for hydrogen kinetic experiments were grown in medium with 2 mM sodium acetate and 101.3 kPa of hydrogen as the only energy source.
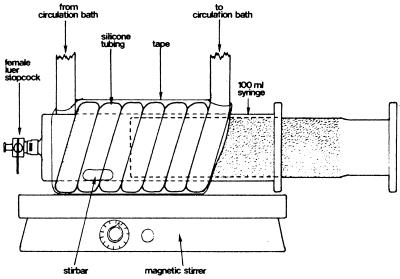
Hydrogen kinetics were measured in a headspace-free thermostated syringe system (Fig. 2). Preheated medium and the appropriate amounts of an exponentially growing culture were transferred to the syringe, which was left on the magnetic stirrer for 1 h to allow temperature equilibration and to release any surplus of dissolved gases. One milliliter of sterile H2 was then injected and allowed to equilibrate within the medium. The gas bubble was ejected, and the experiment was started. The depletion of H2 was measured by sampling 1 ml of culture from the syringe with a 1-ml N2-flushed pressure lock syringe (Dynatech, Boca Raton, Fla.) at appropriate intervals. Each time, the first 0.5 ml was discharged to avoid interference from the dead volume in the stopcock. The 1-ml sample was injected into an 8- or 1.8-ml precooled vial closed with a butyl rubber stopper and filled with H2-free argon. After vigorous shaking to release dissolved H2, 1 ml of the headspace gas was injected into a mercury vapor reduction gas analyzer (Trace Analytical, Menlo Park, Calif.). The dry weight of the cells was determined by use of a membrane filter (7). The kinetic constants Vmax and Km were determined from the depletion curves by the integrated solution of the Michaelis-Menten equation (2) or by velocity-substrate concentration curves (18).
FIG. 2.
The headspace-free thermostated syringe system used for hydrogen uptake kinetic measurements.
Strain JSP and R1Ha3 were able to grow with hydrogen as an electron donor when small amounts of acetate were added as a carbon source, but growth on hydrogen was poor compared to growth on lactate. After 5 days of growth on hydrogen, the OD578 increased from 0.037 to 0.044 for strain JSP and from 0.046 to 0.184 for strain R1Ha3. Use of Widdel’s bicarbonate-buffered medium (23) did not improve the growth yield (data not shown). Before the hydrogen kinetic experiments were performed, the cultures were transferred several times in medium with hydrogen as an energy source. A typical hydrogen progress curve of the kinetic experiments is shown in Fig. 3, while the results of the calculations are presented in Table 1. Due to a low cell production by strain JSP when grown on hydrogen, it was impossible to obtain reliable measurements of the dry weight. Instead, a standard curve (number of cells versus OD578) was made from a dilution series of cells which had grown on lactate, and the number of cells in the hydrogen experiments was estimated from that standard curve. Therefore, the maximum velocity, Vmax, is reported as nanomoles of dissolved hydrogen · 106 cells−1 · h−1 for strain JSP. The threshold values for hydrogen uptake were calculated as an average of the lower plateau of the progress curves and were found to be very low for both strains. The threshold values converted to micromoles liter−1 were 0.18 and 0.42, respectively, for strain JSP and R1Ha3. These values correspond to ΔG values of −22.65 kJ/reaction for strain JSP and −35.49 kJ/reaction for strain R1Ha3 when calculated as described by Westermann (21). Similar results were obtained when the experiments were run with different culture batches and with different initial hydrogen concentrations. No depletion of hydrogen was detected in experiments without cells added to the syringe system. The hydrogen concentrations in sample vials were found to be constant over time, showing that the use of an ice bath was sufficient to stop any further metabolic reactions. Sulfate uptake kinetics were measured with lactate as a substrate. A typical sulfate consumption curve is shown in Fig. 4, and the results of the experiments are shown in Table 1. The threshold level for sulfate consumption was approximately 1.8 μM for each strain. No sulfate consumption was measured in the autoclaved controls.
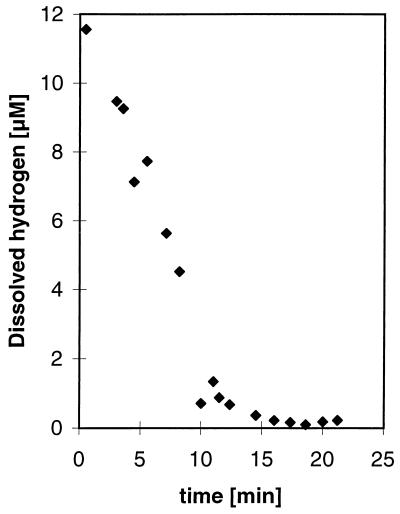
FIG. 3.
A representative time course of hydrogen consumption for a diluted culture of Thermodesulfobacterium sp. strain JSP. The experiment was performed at pH 7.0 and 70°C, Km and Vmax were found to be 1.08 μM and 1.5 nmol of dissolved hydrogen · 106 cells−1 · h−1, respectively, in this experiment.
TABLE 1.
Kinetic constants for hydrogen and sulfate uptake of Thermodesulfovibrio sp. strain R1Ha3 and Thermodesulfobacterium sp. strain JSP
| Strain | Sulfate kinetics (mean ± SD)
|
Hydrogen kinetics (mean ± SD)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Km (μM) | Vmaxa | Threshold (μM) | nd | Km (μM) | Vmax | Threshold (atm) (10−5) | nd | |
| R1Ha3 | 3.0 ± 1.1 | 99 ± 63 | 1.8 ± 0.1 | 5 | 2.4 ± 1.9 | 157 ± 43b | 1.2 ± 0.7 | 3 |
| JSP | 3.1 ± 1.3 | 59 ± 7 | 1.9 ± 0.1 | 4 | 1.9 ± 0.7 | 1.9 ± 0.4c | 0.49 ± 0.3 | 3 |
Expressed as micromoles of sulfate per milligram (dry weight) of cells per hour.
Expressed as micromoles of hydrogen per milligram (dry weight) of cells per hour.
Expressed as nanomoles of hydrogen per 106 cells per hour.
n, number of experiments.
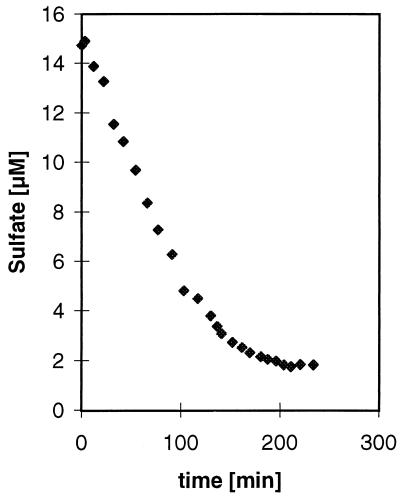
FIG. 4.
Time course of sulfate consumption by Thermodesulfobacterium sp. strain JSP. The experiment was performed at pH 7.0 and 70°C. Km and Vmax values were found to be 2.69 μM and 56.4 μmol of sulfate · mg (dry weight) of cells−1 · h−1, respectively, in this experiment.
The present work is to our knowledge the first kinetic study of thermophilic SRB. The headspace-free syringe method of measuring H2 uptake kinetics that was used in our study has several advantages in comparison to two-phase systems. (i) Due to the low Bunsen solubility coefficient of H2, the headspace of two-phase systems will constitute a much larger reservoir of H2 than the liquid phase. Conrad et al. (4) showed that the steady-state H2 pool size of sediment and sludge samples changed markedly when incubated with a headspace while the H2 pool size remained constant in a headspace-free incubation system. (ii) Measurements of microbial H2 consumption rates in two-phase systems based on the gas-phase partial pressure presupposes that H2 diffuses across the gas-liquid interphase at a rate similar to the microbial consumption rate. According to Robinson and Tiedje (15), the transfer of gases of low solubility such as H2 from the gaseous phase to an H2-consuming liquid culture phase follows approximately first-order kinetics regardless of the kinetic nature of substrate consumption by cells in the liquid phase. Whether phase transfer limitation occurs can be assessed by a combination of mathematical and experimental analyses (15). The element of uncertainty about phase transfer limitation can, however, be avoided by the use of a headspace-free incubation system. (iii) One way of circumventing phase transfer limitation is to reduce the H2 uptake rate by lowering the cell density. A prerequisite for carrying out a Michaelis-Menten kinetic study is, however, that the enzyme concentration is constant during the experiment. Prolonging the incubation time significantly by dilution of the cells might lead to growth, cell division, and, hence, an increase in enzyme concentration, leading to erroneous results. The avoidance of mass transfer limitation in the headspace-free syringe system allows the use of a relatively large cell mass and hence a short incubation time.
A headspace-free syringe has previously been used by Conrad et al. (4) to measure turnover rates of H2 in sludge and sediments but not for kinetic studies of axenic cultures. The syringe used in their study was incubated in a water bath and had to be removed from the water bath when sampling was carried out. When experiments are performed at temperatures far from room temperature, as in our case, a high sampling rate might lead to temperature fluctuations in the syringe affecting the metabolism of the bacteria under investigation. This is avoided in our system by water-jacketing the syringe. Furthermore, our system is completely mixed by a magnetic stirring bar to ensure that no sedimentation or uneven H2 consumption occurs.
The half-saturation constants for hydrogen and sulfate of strain JSP and R1Ha3 are in agreement with values obtained for mesophilic SRB isolated from freshwater environments. This is in accordance with the data shown in Fig. 1 in which the solubility of H2 at 65 and 70°C is almost similar to the solubility at the optimum temperatures of mesophilic sulfate reducers. Kms for sulfate have been reported to be 4.8 and 7.3 μM for Desulfovibrio vulgaris (Marburg) and Desulfovibrio sapovorans, respectively (8), while the Km for hydrogen of Desulfovibrio vulgaris (Marburg) has been reported to be 1 μM (11).
Kinetic constants measured in laboratory studies can be different from the situation in natural environments, where cells often are associated in a biofilm and where different gradients are present. Fukui and Takii (6) found that the Km for sulfate was much lower for particle-associated cells than for free-living cells of Desulfovibrio desulfuricans.
Even if the half-saturation constants measured in this study are higher than those under natural conditions, strains JSP and R1Ha3 are fully capable of functioning in the hot spring environment from which they were isolated; this is because the sulfate concentration has been determined to be approximately 290 μM and no steep sulfate gradient exists in the biofilm at the high temperature of this environment since the mat is very thin (19). Finally, low threshold values for sulfate were found for our thermophilic SRB. Low threshold concentrations for sulfate have also been reported for mesophilic SRB. Ingvorsen et al. (9) found a threshold concentration for Desulfobacter postgatei between 2 and 36 μM.
The kinetic constants for strain R1Ha3 were measured at 65°C, the optimum growth temperature for the bacterium (unpublished results). However, the bacterium was isolated at 55°C, a temperature at which a thicker biomat consisting of Chloroflexus and cyanobacteria is present, especially in the summertime (10). No reports concerning hydrogen concentrations in hot springs have been published, but in the hot spring, at 55°C, in which the strain R1Ha3 originates, methane production has been measured and hydrogenotrophic MPA have been shown to be present (19). Therefore, SRB are assumed to compete with MPA for substrates, including H2 in hot spring microbial mats at 55°C, especially in periods when sulfate becomes limiting due to an intensive degradation of organic material. In contrast, it is unknown whether competition for hydrogen occurs at 75°C since no methanogenic activity has been reported in alkaline hot springs at this temperature (16, 19, 20, 25).
Acknowledgments
This work was supported by grant 9400581 program from the Danish Natural Science Research Council.
REFERENCES
- 1.Abram J W, Nedwell D B. Inhibition of methanogenesis by sulphate reducing bacteria competing for transferred hydrogen. Arch Microbiol. 1978;117:89–92. doi: 10.1007/BF00689356. [DOI] [PubMed] [Google Scholar]
- 2.Ahring B K, Westermann P. Kinetics of butyrate, acetate, and hydrogen metabolism in a thermophilic anaerobic butyrate-degrading triculture. Appl Environ Microbiol. 1987;53:434–439. doi: 10.1128/aem.53.2.434-439.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelidaki I, Petersen S P, Ahring B K. Effects of lipids on thermophilic anaerobic digestion and reduction of lipid inhibition upon addition of bentonite. Appl Microbiol Biotechnol. 1990;33:469–472. doi: 10.1007/BF00176668. [DOI] [PubMed] [Google Scholar]
- 4.Conrad R, Phelps T J, Zeikus J G. Gas metabolism evidence in support of juxtapositioning of hydrogen-producing and methanogenic bacteria in sewage sludge and lake sediments. Appl Environ Microbiol. 1985;50:595–601. doi: 10.1128/aem.50.3.595-601.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crozier T E, Yamamoto S. Solubility of hydrogen in water, seawater, and NaCl solutions. J Chem Eng Data. 1974;19:242–244. [Google Scholar]
- 6.Fukui M, Takii S. Kinetics of sulfate respiration by free-living and particle-associated sulfate-reducing bacteria. FEMS Microbiol Ecol. 1994;13:241–248. [Google Scholar]
- 7.Greenberg A E, Clesceri L S, Eaton A D. Standard methods for the examination of water and wastewater. Washington, D.C: American Public Health Association; 1992. [Google Scholar]
- 8.Ingvorsen K, Jørgensen B B. Kinetics of sulfate uptake by freshwater and marine species of Desulfovibrio. Arch Microbiol. 1984;139:61–66. [Google Scholar]
- 9.Ingvorsen K, Zehnder A J B, Jørgensen B B. Kinetics of sulfate and acetate uptake by Desulfobacter postgatei. Appl Environ Microbiol. 1984;47:403–408. doi: 10.1128/aem.47.2.403-408.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jørgensen B B, Nelson D C. Bacterial zonation, photosynthesis, and spectral light distribution in hot spring microbial mats of Iceland. Microb Ecol. 1988;16:133–147. doi: 10.1007/BF02018909. [DOI] [PubMed] [Google Scholar]
- 11.Kristjansson J K, Schönheit P, Thauer R K. Different Ks values for hydrogen of methanogenic bacteria and sulfate reducing bacteria: an explanation for the apparent inhibition of methanogenesis by sulfate. Arch Microbiol. 1982;131:278–282. [Google Scholar]
- 12.Lovley D R, Dwyer D F, Klug M J. Kinetic analysis of competition between sulfate reducers and methanogens for hydrogen in sediments. Appl Environ Microbiol. 1982;43:1373–1379. doi: 10.1128/aem.43.6.1373-1379.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oude Elferink S J W H, Visser A, Hulshoff Pol L W, Stams A J M. Sulfate reduction in methanogenic bioreactors. FEMS Microbiol Rev. 1994;15:119–136. [Google Scholar]
- 14.Postgate J R. Versatile medium for the enumeration of sulfate-reducing bacteria. Appl Microbiol. 1963;11:265–267. doi: 10.1128/am.11.3.265-267.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson J A, Tiedje J M. Kinetics of hydrogen consumption by rumen fluid, anaerobic digestor sludge, and sediments. Appl Environ Microbiol. 1982;44:1374–1384. doi: 10.1128/aem.44.6.1374-1384.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandbeck K A, Ward D M. Temperature adaptations in the terminal processes of anaerobic decomposition of Yellowstone National Park and Icelandic hot spring microbial mats. Appl Environ Microbiol. 1982;44:844–851. doi: 10.1128/aem.44.4.844-851.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schönheit P, Kristjansson J K, Thauer R K. Kinetic mechanism for the ability of sulfate reducers to out-compete methanogens for acetate. Arch Microbiol. 1982;132:285–288. [Google Scholar]
- 18.Segel I H. Biochemical calculations. How to solve mathematical problems in general biochemistry. New York, N.Y: John Wiley & Sons, Inc.; 1968. [Google Scholar]
- 19.Sonne-Hansen J, Ahring B K. Anaerobic microbiology of a slightly alkaline Icelandic hot-spring. FEMS Microbiol Ecol. 1997;23:31–38. [Google Scholar]
- 20.Ward D M. Thermophilic methanogenesis in a hot-spring algal-mat (71 to 30°C) Appl Environ Microbiol. 1978;35:1019–1026. doi: 10.1128/aem.35.6.1019-1026.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westermann P. Temperature regulation of anaerobic degradation of organic matter. World J Microbiol Biotechnol. 1996;12:497–503. doi: 10.1007/BF00419463. [DOI] [PubMed] [Google Scholar]
- 22.Whitman W B, Bowen T L, Boone D R. The methanogenic bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 719–767. [Google Scholar]
- 23.Widdel F, Bak F. Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 3352–3378. [Google Scholar]
- 24.Widdel F, Hansen T A. The dissimilatory sulfate- and sulfur-reducing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 583–624. [Google Scholar]
- 25.Zeikus J G, Ben-Bassat A, Hegge P W. Microbiology of methanogenesis in thermal, volcanic environments. J Bacteriol. 1980;143:432–440. doi: 10.1128/jb.143.1.432-440.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]