Abstract
This study evaluated and compared the overall equipment effectiveness (OEE), sensitivity, specificity, and efficiency of the high-end hematology analyzers, Yumizen H2500, DxH 800, DxH 900 and XN-9000 (XN-10). A total of 400 anonymized left over’s K2 EDTA whole blood samples were analyzed for complete blood count. Of 400 samples, 200 were tested on Yumizen H2500; DxH 800 & DxH 900 while the other 200 were tested on Yumizen H2500 & XN-9000 (XN-10), respectively. The OEE was good and comparable for all the hematology analyzers except DxH 800 showing an average status. The sensitivity (%), specificity (%) and turnaround time (in minutes) for Yumizen H2500, DxH 800, DxH 900 and XN-9000 (XN-10) were 91.67, 61.11 & 103; 66.67, 54.55, & 149; 83.33, 27.27 & 136; 83.33, 28.57 & 122, respectively. Confusion matrix highlights the difficulty for DxH 800 and DxH 900 to discriminate left shift or blasts with large hyper-segmented neutrophils. The flags triggered by Yumizen H2500 were markedly changed to large hyper-segmented neutrophils. Lymphoblast caused more confusion for XN-9000 (XN-10), as it came out to be atypical lymphocytes, or hypersegmented neutrophils. Although comparable in OEE index to other analyzers, the Yumizen H2500 seems to be more reliable in detecting the abnormal cells as it has high sensitivity, specificity and less turnaround time. Thus, analysis adding specificity, sensitivity, and efficiency parameters to the OEE index provides more reliable information of the analyzers.
Keywords: Overall equipment effectiveness, High-end hematology analyzers, Yumizen H2500, Sensitivity, Specificity, Efficiency
1. Introduction
In clinical laboratories with a heavy patient load, the testing quality of the automated hematology analyzers depends on their accuracy, reliability, and performance as well as efficiency. Continuous evaluation of the equipment with the appropriate methods is the key to ensure sustained quality of reporting in clinical laboratories with a heavy patient load. However, the approaches used for maintaining quality control (QC) include equipment calibration every 6 months, the daily measurement of QC standards, and the inter-instrument comparison within the 6-month interval using patient samples (>40 samples) [1,2].
Although the performance of the automated hematology analyzers expressed as turn-around time is one of the important criteria in high-volume testing labs to deliver patient quality results on time, it may not be comparable for the analyzers from different companies. Therefore, these analyzers should be compared and evaluated in real-time, using overall equipment effectiveness (OEE) method [3]. The metrics behind OEE utilizes three factors such as availability, performance and quality to measure the percentage of the truly productive time [4].
Only few studies have compared different hematology analyzers in parallel [[5], [6], [7], [8]]. None of them has compared the OEE for different hematology analyzers as the definition of OEE does not include all variables that decrease the capacity utilization of clinical laboratory equipment. Arguments are made that sensitivity, specificity, and efficiency should usually be applied in the context of comparing the clinical equipment used for the patient's specimen. Therefore, technology that reduced interference or flagging rates by increasing specificity and sensitivity of equipments have the potential to significantly improve workload and turn-around times without endangering patients by reporting false or misleading results. Thus, the objective of the present study is to evaluate and compare the OEE, sensitivity, specificity, and efficiency for four contemporary high-end hematology analyzers in the setting of a large tertiary care hospital from Southern India.
2. Materials and methods
2.1. Study design, center and hematology analyzers
This was a prospective cross-sectional comparative study of 4 hematology analyzers [Yumizen H2500 (Horiba, Kyoto, Japan), the DxH 800 and DxH 900 (Beckman Coulter, Miami, USA), and the XN-9000 (XN-10) (Sysmex Corporation, Japan)] available as a routine instrument in the central laboratory of Christian Medical College, Vellore, India. As of 2019, the center has the capacity to process 3500–4000 samples per day of various pathologies. Each hematology analyzer had its maintenance record having all pertinent information, which made it possible to assess the condition of equipment and perform necessary maintenance.
The Yumizen H2500 (Horiba, Kyoto, Japan) uses the double hydrodynamic sequential system and the impedance method. The Yumizen H2500 mixes the samples at 360° to ensure their homogeneity. The analyzer capacity is 120 samples per hour and the sample volume needed for analysis is 110 μl [9].
Both DxH 800 and DxH 900 use impedance, and five light scatter and absorption measurements (volume, conductivity, and scatter [VCS] technology) method for counting white blood cells (WBCs) differential, red blood cells (RBCs), platelets (PLT), and nucleated red blood cells (NRBC). The VCS technology allows improved data acquisition per sample. The capacity is twenty separate five-tube cassettes with a maximal automated throughput of 100 samples per hour [[10], [11], [12]].
This Sysmex XN-10 uses fluorescence and flow cytometry technology with a semiconductor laser to categorize WBCs. In case of additional fluorescence interference, it uses a recently developed pRBC flag to correct WBC counts on the WBC differential scattergram [13].
2.2. K2 EDTA whole blood samples
To permit unbiased testing of the four instruments, a total of 400 anonymized left over’s K2 EDTA whole blood samples, out of routine diagnostics and without prior knowledge of blood count analysis or clinical background, were collected in 2 ml tube, Samples with inadequate quantity, inappropriate blood to anticoagulant proportion, or tiny clots were excluded from the study.
2.3. Specimen analysis
Under aseptic precautions, whole blood samples were mixed well by gentle inversion. CBC analysis of all blood samples was performed using the different automated hematology analyzers. Of 400 samples, 200 were processed on Yumizen H2500; DxH 800 & DxH 900 (Set 1) while the other 200 were processed on Yumizen H2500 & XN-9000 (XN-10) (Set 2), respectively. During the entire period of study, the quality assurance or quality control procedures were followed.
2.3.1. Criteria for flagging
Each sample was reviewed according to the laboratory auto-validation criteria for hematology analyzers. If a specified flag appeared and/or output was beyond the specified range, a rule in the criteria would be triggered. Thin blood smears were prepared for all the flagged samples and were stained with Leishman stain. Manual peripheral smear review was performed to identify morphological abnormalities, immature cells and to confirm results produced by the analyzer.
2.4. Comparative assessment of equipment
The comparisons of flagging quality, manual microscopy and inter-instrument efficiency were done as per Clinical and Laboratory Standards Institute (CLSI) guidelines [1]. Blood smear analysis was done by two experts, performing a 200-cell manual differential. Left shift was defined as a band count ≥8% in blood smear.
2.5. Statistical analysis
All statistical analyses were performed using Microsoft Excel software (Microsoft Corp., Redmond, WA).
2.5.1. Overall equipment effectiveness
The OEE was calculated for each hematology analyzer using the following formula.
| Overall equipment effectiveness (OEE) = Availability × Performance × Quality |
According to the OEE index, 100% effectiveness is an ideal and only theoretical performance. However, analyzers that are above 90% are more effective and come under the good category. 80–90% of OEE analyzers are defined as the average OEE category.
2.5.1.1. Availability
Availability is the ratio of the runtime to the planned testing time. It takes into consideration the availability losses. Availability losses are all the downtimes that the process faces during the time that it is supposed to be running. Also, there are planned stops that may occur because of changeover and unplanned stops that may be caused by lack of samples or equipment failure, and then the remaining time from the whole testing time, deducting the availability loss, is called the run time. The availability is calculated by the following equation:
| Availability = Runtime/Planned testing time |
2.5.1.2. Performance
Performance is the ratio of the net run time to the practical run time, and this factor takes into consideration anything that may reduce maximum speed of testing, including minor stops and slow cycles. Mathematical calculation of the performance is done by the following equation:
Performance = Ideal one CBC time × Total CBC count/Practical run time.
Where the ideal one CBC time is the theoretical time to perform one CBC claimed by the manufacturer, total CBC count is the total number of samples performed by the hematology analyzer without consideration of quality and practical run time is the period on the hematology analyzers is required for an operator to perform total counts.
2.5.1.3. Quality
The quality factor takes into consideration that whether or not all the results that occurred during the testing process met the quality standards. To calculate the quality the following equation was used:
Quality: auto-validated count/total CBC count
Where the auto-validated count is the number of the results that met the quality standards (reference) and the total is the number of all CBC count. A sample was classified as true positive (TP) if it was selected to review by certain screening criteria (SC) and the microscopic analysis produced some positive smear findings (PSF). A sample was classified as false positive (FP) if it was selected to review by SC with no PSF in microscopy. A sample was classified as true negative (TN) if it was not selected to review by any SC and the manual blood smear review (MBSR) did not show any PSF. Finally, a sample was classified as false negative (FN) if it was not selected to review by any SC and the MBSR contained some PSF. To compare the hematology analyzers, a confusion matrix was calculated for slide reviews triggered by flag. The accuracy, sensitivity, specificity, precision, and F1-Measure of the analyzers were also calculated using the following equations [14].
| Accuracy (%) = (TP + TN)/TP + FP + TN + FN) x 100 |
| Sensitivity (%) = TP/ (TP + FN) x 100 |
| Specificity (%) = TN/ (TN + FP) x 100 |
| Precision (%) = TP/ (TP + FP) x 100 |
| F1-measures (%) = 2 x (Sensitivity x precision)/ (Sensitivity + Precision) |
3. Results
3.1. Overall equipment effectiveness
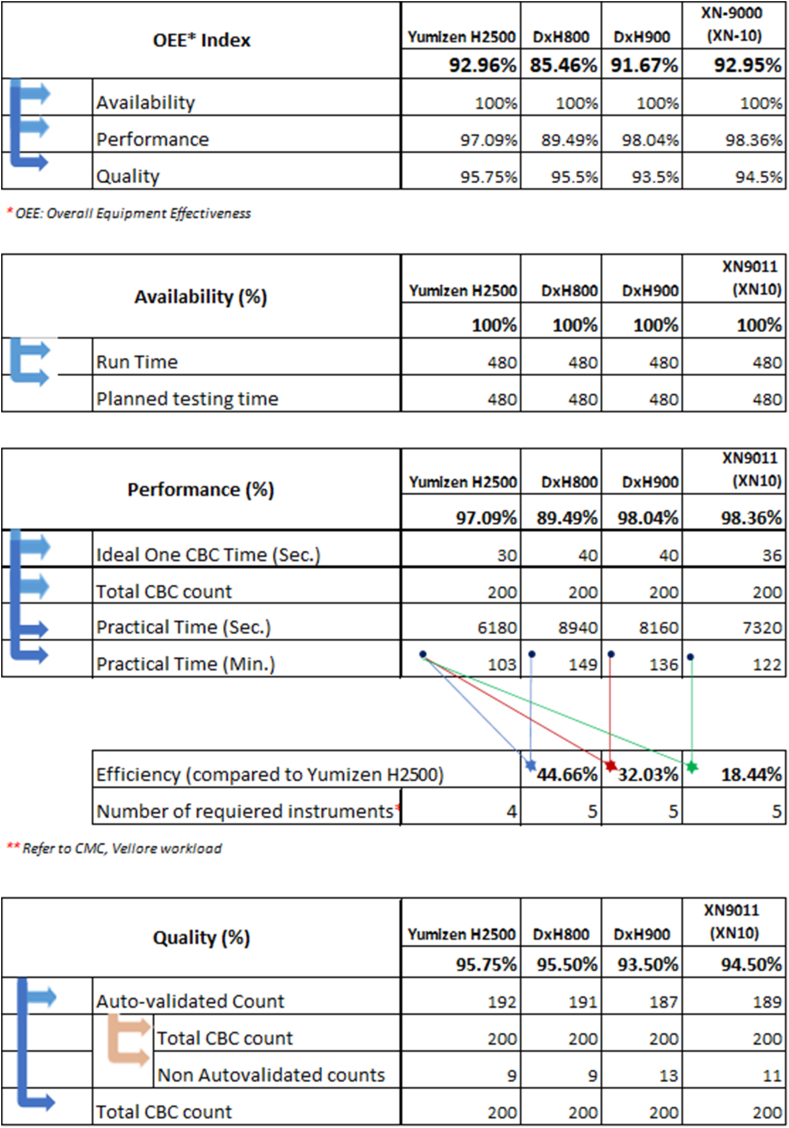
The OEE of Yumizen H2500, the DxH 800 and DxH 900, and the XN-9000 (XN-10) were 92.96%, 85.46%, 91.67%, and 92.95%, respectively. OEE was observed to be good with the Yumizen H2500, DxH 900, and XN-9000 (XN-10), while the DxH 800 showed its average value based on the established OEE index. The availability was 100% for all the instruments, as there was no breakdown and instruments worked well during the analysis as shown in Fig. 1.
Fig. 1.
Overall equipment effectiveness of 4 hematology analyzers.
3.2. Performance of hematology analyzers
The performance, as measured by total CBC count, of Yumizen H2500, DxH 800, DxH 900 and XN-9000 (XN-10) was 97.09%, 89.49%, 98.04% and 98.36% respectively. Of the 200 samples processed on different hematology analyzers, the total number of flags generated by Yumizen H2500, DxH 800, and DxH 900 were 9, 9, and 13 respectively. Similarly, 200 samples were processed on Yumizen H2500 and XN-9000 (XN-10). Total flags generated by the Yumizen H2500 and XN-9000 (XN-10) were 8 and 11 respectively.
The Yumizen H2500 required approximately 103 min for total 200 samples analysis in comparison to 149, 136, and 122 min for DxH 800, DxH 900 & XN-9000 (XN-10) hematology analyzers, respectively. This time was analyzed on practical laboratory scenario where samples were selected randomly. The DxH 800, DxH 900, and XN-9000 (XN-10) analyzers were about 44.66%, 32.03%, and 18.44% less efficient compared with Yumizen H2500.
3.3. Output quality
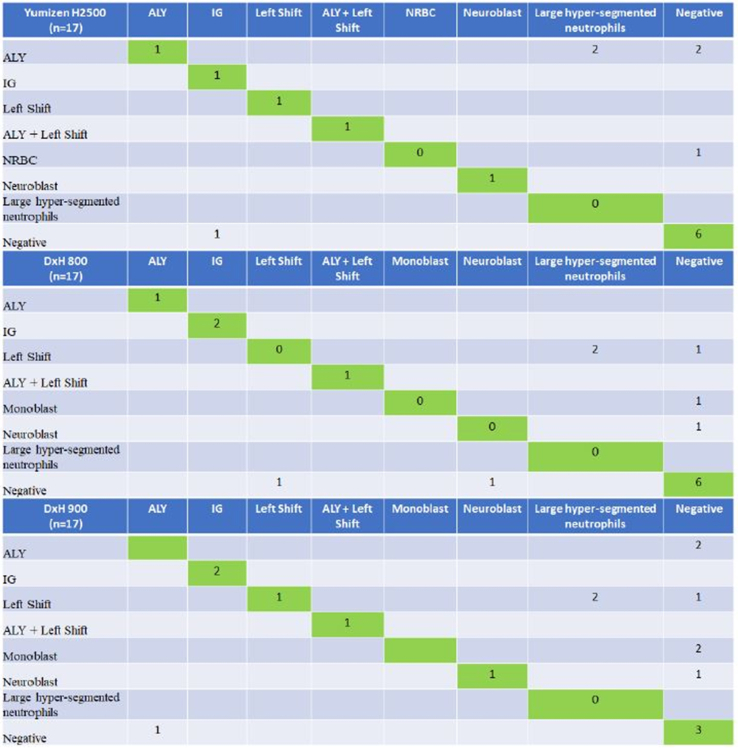
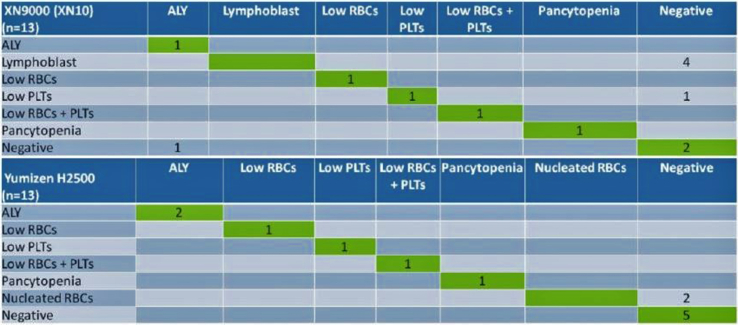
The output quality of Yumizen H2500, DxH 800, DxH 900 and XN-9000 (XN-10) was 95.75%, 95.50%, 93.50% and 94.50%, respectively. The slides of flagged samples by all analyzers were prepared and verified with the expert microscopic cell counts. False-negative output on each of the instruments (Yumizen H2500, DxH 800, DxH 900) was comparable, but false-positive output for Yumizen H2500 was lower than others (Table 2, Table 3). This has important implications for slide review rates. In set 1, the DxH 900 had the highest false-positive rate [4% (8/200)], followed by the DxH 800 [2.5% (5/200)] and the Yumizen H2500 [2.5% (5/200)]. The rates of false-positive results in set 2 were 1% (2/200) and 2.5% (5/200) for Yumizen H2500 analyzer and XN-9000 (XN-10), respectively. Table 1 shows the accuracy, sensitivity, specificity, precision, and F1-Measure of Yumizen H2500, DxH 800, DxH 900 and XN-9000 (XN-10) analyzers respectively. Fig. 2 presents the confusion matrix for Yumizen H2500, DxH 800, and DxH 900 hematology analyzers. Left shift/blasts and large hyper-segmented neutrophils cause more confusion for DxH 800, and DxH 900; 6 samples (3 on DxH 800 and 3 on DxH 900) of left shift and 4 samples of blasts (2 on DxH 800 and 2 on DxH 900) were classified as large hyper-segmented neutrophils. Fig. 3 presents the confusion matrix for Yumizen H2500, and XN-9000 (XN-10) hematology analyzers. Lymphoblast/NRBC and negative results cause more confusion. 4 samples of lymphoblast on XN-9000 (XN-10) were classified as negative, and 2 samples of NRBC on Yumizen H2500 were classified as negative.
Table 2.
Comparison regarding reason for non-autovalidation between Yumizen H2500, DxH 800 and DxH 900 hematology analyzers (n = 17).
| Yumizen H2500 | Conclusion | DxH 800 | Conclusion | DxH 900 | Conclusion | Slide review comments |
|---|---|---|---|---|---|---|
| – | True Negative | NE blast | False Positive | NE blast | False Positive | Large hypersegmented neutrophils falling in flag zone. |
| – | True Negative | Monoblast | False positive | Monoblast | False positive | Large hypersegmented neutrophils falling in flag zone. |
| ALY | False positive | Left shift | False positive | Left shift | False positive | Large Monocytes falling in flag zone Some of the large neutrophils with hypersegmentation overlapped the ALY flag system |
| False Negative | IG | True positive | IG | True positive | Higher proportion of IG was present in blood smear | |
| ALY | True positive | ALY | True positive | – | False negative | Monocytosis with reactive & large lymphocytes causing the ALY flag |
| ALY + Left shift | True positive | ALY + Left shift | True positive | ALY + Left shift | True positive | Lymphoid crisis, ALY, Band count ≥8% in blood smear |
| IG | True Positive | IG | True positive | IG | True positive | Higher proportion of IG was present in blood smear |
| ALY | False positive | Left shift | False positive | Left shift | False positive | Large neutrophils with hypersegmentation falling in flagging zone and overlapping into ALY/LY |
| – | True Negative | Left shift | False positive | Left shift | False positive | Large hypersegmented neutrophils falling in IG flag zone |
| ALY | False Positive | – | True negative | – | True negative | Large neutrophils with hypersegmentation falling in flagging zone and overlapping into ALY/LY |
| NRBC | False Positive | – | True negative | – | True negative | Negative for NRBC |
| ALY | False Positive | – | True negative | – | True negative | Large neutrophils with hypersegmentation falling in flagging zone and overlapping into ALY/LY |
| NE blast | True Positive | – | False negative | NE blast | True positive | Positive for NE blast |
| – | True Negative | – | True negative | ALY | False positive | Large neutrophils with hypersegmentation falling in flagging zone and overlapping into ALY/LY |
| – | True Negative | – | True negative | ALY | False positive | Large neutrophils with hypersegmentation falling in flagging zone and overlapping into ALY/LY |
| – | True Negative | – | True negative | Monoblast | False positive | Large hypersegmented neutrophils falling in flag zone. |
| Left shift | True Positive | – | False negative | Left shift | True positive | Band count ≥8% in blood smear |
Abbreviations: ALY- Atypical Lymphocytes, IG – Immature Granulocytes, NRBC – Nucleated Red Blood Cells, NE blast – Neutroblast; CML – Chronic myeloid leukemia.
Table 3.
Comparison regarding reason for non-autovalidation between Yumizen H2500 and XN-9000 (XN-10) hematology analyzers (n = 13).
| S. No | Yumizen H2500 | Conclusion for slide | XN-9000 (XN-10) | Conclusion for slide | Slide review comments |
|---|---|---|---|---|---|
| 1 | True negative | Lymphoblast | False Positive | Occasional reactive lymphocytes seen which agrees with ALY but negative for lymphoblast. | |
| 2 | True negative | Low PLTs | False Positive | Slide review came because MPV was not reported in XN in case of thrombocytopenia but in Yumizen H2500, MPV was reported. | |
| 3 | Pancytopenia | True Positive | Pancytopenia | True Positive | Positive for pancytopenia |
| 4 | Low RBCs/Low platelets | True Positive | Low RBCs/Low platelets | True Positive | Positive for low RBCs/low platelets |
| 5 | Low RBCs | True Positive | Low RBCs | True Positive | Positive for low RBCs |
| 6 | ALY | True Positive | ALY | True Positive | Positive for ALY |
| 7 | Low Platelets | True Positive | Low Platelets | True Positive | Slide review showed low platelet count |
| 8 | True negative | Lymphoblast | False Positive | Negative for lymphoblasts | |
| 9 | True negative | Lymphoblast | False Positive | Smear showed smudge cells. These could be reactive lymphocyte and large hypersegmented neutrophils. | |
| 10 | True negative | Lymphoblast | False Positive | Proportion of ALY is less. But Slide reveals hypogranular or pseudo-Pelger-Huët neutrophils | |
| 11 | NRBC | False Positive | Slide was not required | True negative | Negative for NRBC |
| 12 | ALY | True Positive | False negative | Positive for ALY. More smudge cells | |
| 13 | NRBC | False Positive | PLT aggregates | True negative | NRBC were not there but PLT aggregates and Large PLT were seen. |
Abbreviations: ALY- Atypical Lymphocytes, IG – Immature Granulocytes, NRBC – Nucleated Red Blood Cells, PLT – Platelets, MPV – Mean Platelet Volume.
Table 1.
Comparison between Yumizen H2500, DxH 800, DxH 900 and XN-9000 (XN-10) hematology analyzers.
| Parameters | Yumizen H2500 (Aggregate) | DxH 800 (Set 1) | DxH 900 (Set 1) | Yumizen H2500 (Set 1) | XN-9000 (XN-10) (Set 2) |
Yumizen H2500 (Set 2) |
|---|---|---|---|---|---|---|
| Accuracy, (%) | 73.33 | 58.82 | 47.06 | 64.71 | 53.85 | 84.62 |
| Sensitivity, (%) | 91.67 | 66.67 | 83.33 | 83.33 | 83.33 | 100.00 |
| Specificity, (%) | 61.11 | 54.55 | 27.27 | 54.55 | 28.57 | 71.43 |
| Precision, (%) | 61.11 | 44.44 | 38.46 | 50.00 | 50.00 | 75.00 |
| F1-measure, (%) | 73.04 | 52.80 | 52.13 | 62.41 | 62.41 | 85.71 |
| True positive, (n) | 11 | 4 | 5 | 5 | 5 | 6 |
| True negative, (n) | 11 | 6 | 3 | 6 | 2 | 5 |
| False positive, (n) | 7 | 5 | 8 | 5 | 5 | 2 |
| False negative, (n) | 1 | 2 | 1 | 1 | 1 | 0 |
Fig. 2.
Confusion matrix of Yumizen H2500, DxH 800, and DxH 900 hematology analyzers (ALY – Atypical Lymphocytes, IG – Immature Granulocytes, NRBC – Nucleated Red Blood Cells). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Confusion matrix of Yumizen H2500 and XN-9000 (XN-10) hematology analyzers (ALY – Atypical Lymphocytes, RBC – Red Blood Cells, PLTs – Platelets). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Timely and accurate reporting of blood cell count and differentials is the primary goal of clinical hematology laboratories. The key factors that influence hematology analyzer selection include reliability of the analyzer, cost of analyzer, availability, and turnaround time. Since manual verification of the obtained result is labor-intensive and time-consuming, there is a need to use a more accurate and sensitive hematology analyzer to effectively handle many samples with minimum mistakes and slide review. The currently used inter-instrument comparison approach includes a periodic regression analysis that uses at least 40 CBC samples of patients [1,2]. When the laboratory uses this strategy, the consistency of multiple instruments cannot be confirmed. Hence, in this study the performance, maintenance, and reporting quality of 4 different hematology analyzers were compared using OEE method and the reliability was assessed using manual microscopy as the gold standard. In this study, turnaround time was also measured as an indicator of performance because this improved the workflow rate in large volume laboratories.
In this study, the OEE of different automated analyzers was compared in terms of the total number of CBC parameters performed at a particular point within the shift, day, or production run. The OEE was good and comparable for all the hematology analyzers except DxH 800 showing an average status. A study by Chabert et al. comparing the performance of Yumizen H2500 hematology analyzer to other blood counter models in terms of precision and linearity reported that the Yumizen H1500 and H2500 instruments are as safe, as effective, and perform as well or better than the Advia 2120, Sysmex XE2100 and XN10 instruments for all the usual CBC parameters [15].
Cellular interference may influence the WBC, PLT, and NRBC counts that lead not only reduction in operational efficiency of the hematology laboratory, but also inaccurate clinical decision making [[16], [17], [18], [19], [20], [21], [22]]. The presence of interfering particles in automated blood cell enumeration often triggers a ‘flag’ for manual review. A ‘flag’ from the hematology analyzer is a notification that a reported result might be incorrect. This means that either a result is outside the established normal ranges programmed into the instrument (resulting in false negatives) or the hematology analyzer misidentifies other cellular types (resulting in false positives) [[23], [24], [25], [26], [27]]. In 20%–25% of CBCs, abnormal cell flags are generated, which needs manual film reviews [28,29]. However, manual film reviews range varies from 10% to 50% in different laboratories depending on the local guidelines and clinical population [29,30]. In this study, the manual blood smear review of all blood samples that cause flagging was performed by two experts for increasing the results credibility. Two levels were evaluated: the first level was to check the presence of a flag (independently of its accuracy) and the second level was to check the accuracy of the flag.
In 2016, Eldanasoury et al. reported that the suspect flags were accountable for 60.2% of their false-positive results [31]. They indicated that the hematology analyzers were responsible for an increase in unnecessary manual smear review due to over flagging. Similarly, several other studies have also indicated higher number of manual film reviews triggered by abnormal cell flags [14,31,32]. In contrast, our findings indicate lower number (about 4%–7%) of manual film reviews for each of the analyzers triggered by abnormal cell flags. Kim et al. stated that the slide review rate might vary among the different analyzers studied and that only most appropriate analyzers should be selected by individual laboratories based on clinical characteristics such as clinic size and patient population [33]. In this study, the efficiency evaluation of all the four hematology analyzers was obtained by assessment of the turnaround time for complete blood count in the laboratory. Our findings indicate that the use of the Yumizen H2500 in the routine laboratory would reduce the turnaround time from about 149 min, 136 min, and 122 min on the DxH 800, DxH 900, and XN-9000 (XN-10), to about 103 min. With a daily workload of about 3500 samples in CMC Vellore, this difference represents a reduction of about one Yumizen H2500 (from about 5 instruments to 4 in numbers). The low turnaround time and number [about 4% (17/400)] of manual film reviews for Yumizen H2500 highlights the importance of ongoing software appraisal and optimization.
Studies have shown highly sensitivity, specificity and accuracy of flags on DxH 800 [11,34,35]. However, the high flagging sensitivity for the XN series and markedly lower sensitivity for the DxH 800 have also been indicated in other studies [7,8,36]. Our findings indicate high flagging sensitivity for the Yumizen H2500, DxH 900 and XN-9000 (XN-10) compared with DxH 800. Beside high flagging sensitivity, low false-positive alerts and high specificity are the other important parameters to reduce unnecessary manual smear reviews. Studies have shown that the flagging parameters most contributing to the number of false-positive samples were IGs followed by left shift and PLT clumping [[5], [6], [7],33].
Combining flagging alerts for presence of blasts, left shift and atypical/abnormal lymphocytes, DxH 900 revealed the lowest specificity (27.27%) among all investigated instruments in this study primarily due to a relatively high number of false-positive blasts and left shift warnings. In this study, XN-9000 (XN-10) was the second instrument with less specificity (28.57%) after the DxH 900 due to a relatively high number of false-positive monoblasts. In contrast, a previous study by Jones et al. comparing XN-1000 with XE-500 for sensitivity and specificity of flagging for abnormal WBC on pediatric samples reported XN-1000 superiority over XE-5000, with greater reduction in blood films for review [37]. However, the flag specificity for blood samples collected from infants between 8 days and 2 years of age on XN-1000 was <35%, which increased up to 67% thereafter [38]. Yumizen H2500 analyzer with low false positive rate in this study exhibited a high flagging sensitivity (91.67%) and specificity (61.11%). The high sensitivity, precision and specificity of Yumizen H2500 can be explained owing to improved cell separation (360°), ongoing software appraisal and optimization. This results in significant decrease in unnecessary morphology reviewing by microscopy, thus saving significant time in the laboratory.
Confusion matrix (Fig. 2) highlights the difficulty for DxH 800 and DxH 900 to discriminate left shift or blasts with large hyper-segmented neutrophils. The negative flags triggered by Yumizen H2500 were markedly changed after manual slide review to large hyper-segmented neutrophils. Lymphoblast caused more confusion for XN-9000 (XN-10), as it came out to be atypical lymphocytes, hypersegmented neutrophils, or hypogranular neutrophils.
Overall, Yumizen H2500 appears to be a satisfactory analyzer for the common clinical laboratory use; though comparable to DxH 900 & XN-9000 (XN-10) hematology analyzers in terms of OEE index. This index measures machine effectiveness based on the availability and performance rate element to obtain the actual maintenance performance level, without considering problems related to reliability (specificity and sensitivity) and turnaround time (efficiency) parameters. Based on this study, the structured technique of the modified OEE index can be proposed [SnSr index named after author’s names Sukesh Nair (Sn) and Shubham Rastogi (Sr)]. This index uses specificity, sensitivity, and efficiency parameters along with the OEE index for identifying the usefulness of the laboratory instrumentations. According to SnSr index, 100% effectiveness, efficiency, sensitivity, and specificity are ideal and only theoretical performance. However, analyzers that are above 90% are more effective and come under the good category. 80–90% analyzers are defined as average on the SnSr index.
The authors want to highlight few limitations present in the study: (i) the sensitivity, specificity and efficiency analysis of Yumizen H2500 is based on blood smear evaluation of a small number of samples. (ii) In this study the observers were not blinded and had the access to reports of hematology analyzers (though not referred to), which might have resulted into morphological changes over-reporting, particularly those marked by suspect flags. (iii) The data presented are generated from a single population with a specific profile in a CMC Vellore that uses a certain type of hematology analyzer.
5. Conclusion
All the 4 hematology analyzers showed comparable OEE in technical evaluation. However, the Yumizen H2500 analyzer seems to be more reliable in detecting the abnormal cells as it has high sensitivity, specificity and less turnaround time compared to other analyzers. Thus, analysis adding specificity, sensitivity, and efficiency of the laboratory instrumentations to the OEE index provides more reliable information on accuracy, productive time and turnaround time.
Author's contribution
SCN, SR, CF, JJM designed the study; SM, PM and ASS performed laboratory experiment, while SCN performed blood smear analysis and SR was involved in data compilation and analysis. All the authors approved the final version of the article submitted.
Declaration of competing interest
Shubham Rastogi is the employee of HORIBA ABX SAS. SCN, JJM, SM, PM and ASS declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.plabm.2022.e00275.
Contributor Information
Shubham Rastogi, Email: shubham.rastogi@horiba.com.
Sukesh Chandran Nair, Email: scnair@cmcvellore.ac.in.
Pandiyan Murugan, Email: muruganmr2011@gmail.com.
Asady Sukanya Sukumar, Email: asadysukanya1993@gmail.com.
Joy J. Mammen, Email: joymammen@cmcvellore.ac.in.
Saravanan Mullai, Email: saravananmullai14@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Clinical and Laboratory Standards Institute . Validation V, and Quality Assurance of Automatic Hematology Analyzers: Approved Standard—Second Edition. Clinical and Laboratory Standards Institute; Wayne, PA: 2010. CLSI Guideline H26-A2. [Google Scholar]
- 2.College of American Pathologists (CAP) CAP; Northfield, IL: 2013. Hematology and Coagulation Checklist: HEM.28000 H, HEM.29332. [Google Scholar]
- 3.Nakajima S. ProductivityPress. Inc Cambridge Massachusetts; 1998. Introduction to TPM Total Productive Maintenance. [Google Scholar]
- 4.Puvanasvaran P., Teoh Y., Tay C. Consideration of demand rate in Overall Equipment Effectiveness (OEE) on equipment with constant process time. J. Ind. Eng. Manag. 2013;6:507–524. [Google Scholar]
- 5.Tan B.T., Nava A.J., George T.I. Evaluation of the beckman coulter UniCel DxH 800, beckman coulter LH 780, and abbott diagnostics cell-dyn sapphire hematology analyzers on adult specimens in a tertiary care hospital. Am. J. Clin. Pathol. 2011;135:939–951. doi: 10.1309/AJCP1V3UXEIQTSLE. [DOI] [PubMed] [Google Scholar]
- 6.Meintker L., Ringwald J., Rauh M., Krause S.W. Comparison of automated differential blood cell counts from abbott sapphire, siemens Advia 120, beckman coulter DxH 800, and Sysmex XE-2100 in normal and pathologic samples. Am. J. Clin. Pathol. 2013;139:641–650. doi: 10.1309/AJCP7D8ECZRXGWCG. [DOI] [PubMed] [Google Scholar]
- 7.Hotton J., Broothaers J., Swaelens C., Cantinieaux B. Performance and abnormal cell flagging comparisons of three automated blood cell counters: cell-Dyn Sapphire, DxH-800, and XN-2000. Am. J. Clin. Pathol. 2013;140:845–852. doi: 10.1309/AJCPE5R4SOQBUULZ. [DOI] [PubMed] [Google Scholar]
- 8.Bruegel M., Nagel D., Funk M., Fuhrmann P., Zander J., Teupser D. Comparison of five automated hematology analyzers in a university hospital setting: abbott CELL-DYN sapphire, beckman coulter DxH 800, siemens ADVIA 2120i, Sysmex XE-5000, and Sysmex XN-2000. Clin. Chem. Lab. Med. 2015;53:1057–1071. doi: 10.1515/cclm-2014-0945. [DOI] [PubMed] [Google Scholar]
- 9.Małecka M., Ciepiela O. A comparison of Sysmex-XN 2000 and Yumizen H2500 automated hematology analyzers. Pract. Lab. Med. 2020;22 doi: 10.1016/j.plabm.2020.e00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genc S., Dervisoglu E., Erdem S., Arslan O., Aktan M., Omer B. Comparison of performance and abnormal cell flagging of two automated hematology analyzers: Sysmex XN 3000 and Beckman Coulter DxH 800. Int. J. Lit. Humanit. 2017;39:633–640. doi: 10.1111/ijlh.12717. [DOI] [PubMed] [Google Scholar]
- 11.Hedley B.D., Keeney M., Chin-Yee I., Brown W. Initial performance evaluation of the UniCel® DxH 800 Coulter® cellular analysis system. Int. J. Lit. Humanit. 2011;33:45–56. doi: 10.1111/j.1751-553X.2010.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DxH900 hematology anlayzer. https://www.beckmancoulter.com/products/hematology/dxh-900
- 13.Dumas C., Tirard-Collet P., Mestrallet F., Girard S., Jallades L., Picot S., et al. Flagging performance of Sysmex XN-10 haematology analyser for malaria detection. J. Clin. Pathol. 2020;73:676–677. doi: 10.1136/jclinpath-2019-206382. [DOI] [PubMed] [Google Scholar]
- 14.Comar S.R., Malvezzi M., Pasquini R. Evaluation of criteria of manual blood smear review following automated complete blood counts in a large university hospital. Rev. Bras. Hematol. Hemoter. 2017;39:306–317. doi: 10.1016/j.bjhh.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chabert C., Gougaud J.-R., Davis B. Performance validation for Horiba Medical Yumizen H2500 and H1500 blood cell counters, components for horiba evolution laboratory organization (HELO) solution. Int. J. Lit. Humanit.: WILEY 111 RIVER ST, HOBOKEN 07030-5774, NJ USA. 2017:27. [Google Scholar]
- 16.Schwartz S.O., Stansbury F. Significance of nucleated red blood cells in peripheral blood; analysis of 1,496 cases. J. Am. Med. Assoc. 1954;154:1339–1340. doi: 10.1001/jama.1954.02940500019007. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez T., Domack L.B., Montes D., Pineiro R., Landrum E., Vital E. Performance evaluation of the Coulter LH 750 hematology analyzer. Lab. Hematol. 2001;7:217–228. [Google Scholar]
- 18.La Porta A.D., Bowden A.S., Barr S. Impact of the coulter LH 750 on productivity, turnaround time, and work flow in a core laboratory. Lab. Hematol. 2002;8:218–224. [Google Scholar]
- 19.Bourner G., Dhaliwal J., Sumner J. Performance evaluation of the latest fully automated hematology analyzers in a large, commercial laboratory setting: a 4-way, side-by-side study. Lab. Hematol. Off. Publ. Int. Soc. Lab. Hematol. 2005;11:285–297. doi: 10.1532/lh96.05036. [DOI] [PubMed] [Google Scholar]
- 20.Harrison P., Segal H., Briggs C., Murphy M., Machin S. Impact of immunological platelet counting (by the platelet/RBC ratio) on haematological practice. Cytometry B Clin. Cytometry. 2005;67:1–5. doi: 10.1002/cyto.b.20058. [DOI] [PubMed] [Google Scholar]
- 21.Segal H.C., Briggs C., Kunka S., Casbard A., Harrison P., Machin S.J., et al. Accuracy of platelet counting haematology analysers in severe thrombocytopenia and potential impact on platelet transfusion. Br. J. Haematol. 2005;128:520–525. doi: 10.1111/j.1365-2141.2004.05352.x. [DOI] [PubMed] [Google Scholar]
- 22.Bodey G.P. The changing face of febrile neutropenia-from monotherapy to moulds to mucositis. Fever and neutropenia: the early years. J. Antimicrob. Chemother. 2009;63(Suppl 1):i3–13. doi: 10.1093/jac/dkp074. [DOI] [PubMed] [Google Scholar]
- 23.Brown W., Kaplan S., Keeney M., Johnson K., Wolfe N., Yee I. Workflow improvement with random access and enhanced flagging on the New Coulter LH 750 Hematology Analyzer. Lab. Hematol. 2001;7:229–235. [Google Scholar]
- 24.Yee I.C., Keeney M., Johnson K., Brown W., Wolfe N., Kaplan S. White blood cell flagging rates of the coulter LH 750 analyzer compared with the Coulter Gen• S Hematology Analyzer. Lab. Hematol. 2001;7:211–216. [Google Scholar]
- 25.Chin-Yee I., Brown W., Johnson K., Keeney M., Steele B., Wolfe N., et al. Identification and enumeration of nucleated red blood cells on the coulter LH 750. Lab. Hematol. 2002;8:210–217. [Google Scholar]
- 26.Ghys T., Malfait R., VANdB J. Performance evaluation of the Sysmex XS-1000i automated haematology analyser. Int. J. Lit. Humanit. 2009;31:560–566. doi: 10.1111/j.1751-553X.2008.01081.x. [DOI] [PubMed] [Google Scholar]
- 27.Müller R., Mellors I., Johannessen B., Aarsand A.K., Kiefer P., Hardy J., et al. European multi-center evaluation of the Abbott Cell-Dyn sapphire hematology analyzer. Lab. Hematol. Off. Publ. Int. Soc. Lab. Hematol. 2006;12:15–31. doi: 10.1532/LH96.05041. [DOI] [PubMed] [Google Scholar]
- 28.Novis D.A., Walsh M., Wilkinson D., St Louis M., Ben-Ezra J. Laboratory productivity and the rate of manual peripheral blood smear review: a College of American Pathologists Q-Probes study of 95,141 complete blood count determinations performed in 263 institutions. Arch. Pathol. Lab Med. 2006;130:596–601. doi: 10.5858/2006-130-596-LPATRO. [DOI] [PubMed] [Google Scholar]
- 29.Barnes P.W., McFadden S.L., Machin S.J., Simson E. The international consensus group for hematology review: suggested criteria for action following automated CBC and WBC differential analysis. Lab. Hematol. Off. Publ. Int. Soc. Lab. Hematol. 2005;11:83–90. doi: 10.1532/LH96.05019. [DOI] [PubMed] [Google Scholar]
- 30.Briggs C., Longair I., Slavik M., Thwaite K., Mills R., Thavaraja V., et al. Can automated blood film analysis replace the manual differential? An evaluation of the CellaVision DM96 automated image analysis system. Int. J. Lit. Humanit. 2009;31:48–60. doi: 10.1111/j.1751-553X.2007.01002.x. [DOI] [PubMed] [Google Scholar]
- 31.Eldanasoury A.S., Boshnak N.H., Abd El-Monem R.E. Validation of criteria for smear review following automated blood cell analysis in ain shams university laboratory. Int. J. Sci. Res. 2016;5:484–493. [Google Scholar]
- 32.Xing Y., Wang J.Z., Pu C.W., Shang K., Yan Z.L., Bai Y.Z., et al. [Establishment and evaluation of review criteria for ADVIA 120/2120 and different series of hematology analyzers] Zhonghua Yixue Zazhi. 2010;90:1526–1530. [PubMed] [Google Scholar]
- 33.Kim S.J., Kim Y., Shin S., Song J., Choi J.R. Comparison study of the rates of manual peripheral blood smear review from 3 automated hematology analyzers, Unicel DxH 800, ADVIA 2120i, and XE 2100, using international consensus group guidelines. Arch. Pathol. Lab Med. 2012;136:1408–1413. doi: 10.5858/arpa.2010-0757-OA. [DOI] [PubMed] [Google Scholar]
- 34.Jean A., Boutet C., Lenormand B., Callat M.P., Buchonnet G., Barbay V., et al. The new haematology analyzer DxH 800: an evaluation of the analytical performances and leucocyte flags, comparison with the LH 755. Int. J. Lit. Humanit. 2011;33:138–145. doi: 10.1111/j.1751-553X.2010.01257.x. [DOI] [PubMed] [Google Scholar]
- 35.Barnes P.W., Eby C.S., Shimer G. Blast flagging with the UniCel DxH 800 coulter cellular analysis system. Lab. Hematol. Off. Publ. Int. Soc. Lab. Hematol. 2010;16:23–25. doi: 10.1532/LH96.09015. [DOI] [PubMed] [Google Scholar]
- 36.Depoorter M., Goletti S., Latinne D., Defour J. Optimal flagging combinations for best performance of five blood cell analyzers. Int. J. Lit. Humanit. 2015;37:63–70. doi: 10.1111/ijlh.12238. [DOI] [PubMed] [Google Scholar]
- 37.Jones A.S., Tailor H., Liesner R., Machin S.J., Briggs C.J. The value of the white precursor cell channel (WPC) on the Sysmex XN-1000 analyser in a specialist paediatric hospital. J. Clin. Pathol. 2015;68:161–165. doi: 10.1136/jclinpath-2014-202640. [DOI] [PubMed] [Google Scholar]
- 38.Becker P.H., Fenneteau O., Da Costa L. Performance evaluation of the Sysmex XN-1000 hematology analyzer in assessment of the white blood cell count differential in pediatric specimens. Int. J. Lit. Humanit. 2016;38:54–63. doi: 10.1111/ijlh.12436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.