Abstract
Radiotherapy (RT) is a cornerstone treatment strategy for brain tumours. Besides cytotoxicity, RT can cause disruption of the blood–brain barrier (BBB), resulting in an increased permeability into the surrounding brain parenchyma. Although this effect is generally acknowledged, it remains unclear how and to what extent different radiation schemes affect BBB integrity. The aim of this systematic review and meta-analysis is to investigate the effect of photon RT regimens on BBB permeability, including its reversibility, in clinical and preclinical studies. We systematically reviewed relevant clinical and preclinical literature in PubMed, Embase, and Cochrane search engines. A total of 69 included studies (20 clinical, 49 preclinical) were qualitatively and quantitatively analysed by meta-analysis and evaluated on key determinants of RT-induced BBB permeability in different disease types and RT protocols. Qualitative data synthesis showed that 35% of the included clinical studies reported BBB disruption following RT, whereas 30% were inconclusive. Interestingly, no compelling differences were observed between studies with different calculated biological effective doses based on the fractionation schemes and cumulative doses; however, increased BBB disruption was noted during patient follow-up after treatment. Qualitative analysis of preclinical studies showed RT BBB disruption in 78% of the included studies, which was significantly confirmed by meta-analysis (p < 0.01). Of note, a high risk of bias, publication bias and a high heterogeneity across the studies was observed. This systematic review and meta-analysis sheds light on the impact of RT protocols on BBB integrity and opens the discussion for integrating this factor in the decision-making process of future RT, with better study of its occurrence and influence on concomitant or adjuvant therapies.
Abbreviations: BBB, Blood-brain barrier; RT, Radiotherapy; MRI, Magnetic resonance imaging (MRI); LC-MS, Liquid chromatography-mass spectrometry; CT, Computed tomography; PET, Positron emission tomography; NMR, Nuclear magnetic resonance; NSCLC, Non-small cell lung cancer; AVM, Arteriovenous malformations; WBRT, Whole-brain RT; SRS, Stereotactic radiosurgery; EB, Evans Blue
Keywords: Blood-brain barrier, Radiotherapy, Permeability, Dose Fractionation, Radiotherapy Dosage
Introduction
Homeostasis of the central nervous system is sustained by the blood–brain barrier (BBB), also known as the neurovascular unit, that protects the brain tissue from potential harmful pathogens or substances. The BBB has a restricted permeability due to being both (1) a physical barrier formed by tight junctions between the endothelial cells that are surrounded by pericytes and a basal membrane and (2) a functional barrier where ATP-binding cassette efflux transporters have the potential to pump a large spectrum of molecules from the extravascular interstitium back into the blood stream [1]. These anatomical and functional features result in the exclusion of large substances (greater than 500 Da) [2] and over 98% of all small molecules from the brain, amongst which are chemotherapeutics and targeted therapies [3]. Hence, the BBB limits the overall treatment efficacy in brain malignancies because of the reduced, if not absent, drug delivery into the brain parenchyma [4], [5].
Meanwhile, radiotherapy (RT), after maximal safe surgery, is still a cornerstone for the treatment of brain tumours such as high-grade gliomas and diffuse midline gliomas. While conventional photon radiation therapy has been applied as a treatment modality for roughly 50% of all cancer patients [6], technological advances, like image-guided RT or different particle radiations (electron, proton, neutron beams) have improved the specificity of the treatment modality and enabled better and precise radiation treatment of the tumours while sparing the healthy tissue [7]. Despite this technical progress, large volumes of the functioning brain issue have to be radiated due to the highly infiltrative nature of most primary brain tumours [8]. Due to low radio-sensitivity of certain tumours [9], often high dosages are needed to achieve the maximal anti-tumour effect, which also cause damage to the surrounding normal tissue. Vascular endothelial cells are one of the most radiosensitive cells and consequently the brain vasculature is prone to be affected by radiation [10].
There is evidence that BBB integrity is altered after the application of RT leading to both reversible and irreversible tissue damage for the patient. Whereas early brain damage caused by radiation is mostly reversible, later, more chronic injuries, manifesting at the earliest three months after treatment, can cause (sometimes severe) problems for the patient [11]. It is assumed that cellular and vascular responses of the BBB upon RT are mediated by astrogliosis and endothelial ultrastructural changes [12]. These changes to the BBB can eventually lead to seizures, brain inflammation and leaky vessels causing haemorrhage and/or stroke [13], [14], [15]. Furthermore, it has been postulated that mostly RT with cumulative doses between 20 and 30 Gy increases BBB permeability [16], however the actual impact of RT protocols (fractions, frequency) on BBB integrity remains to be elucidated, in order to support decision-making with regard to the prevention of toxicity and the use of concomitant chemotherapeutic therapies. To the best of our knowledge, there is no clear consensus on to what extent radiation doses and fractionation schemes affect BBB integrity. Subsequently, it is unknown to what extent confounding factors, such as the patients’ clinical picture, interplay in the evaluation of RT-induced effects on BBB permeability. In addition, ascertainment of the kinetics of BBB opening can be helpful to decide on dosing and timing for drugs that are not expected to cross an intact BBB.
The aim of this study is therefore to provide a thorough review of clinical and preclinical studies that have ascertained the effect of conventional photon RT on BBB permeability and its reversibility following different RT regimens. A systematic review of all available clinical and preclinical literature was performed, in three different search engines. Data were processed by qualitative analysis and meta-analysis to statistically assess the extent of BBB disruption following photon radiation in comparison to a non-irradiated control group.
Methodology
Data sources and literature search
A literature search was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)-statement [17]. To identify all relevant publications, systematic searches in the bibliographic databases PubMed, Embase, and The Cochrane Library (via Wiley) were performed on April 24th, 2020, without any restrictions on publication date. Search terms were based on two key words; “Radiotherapy” and “Blood-brain barrier” and included controlled terms (MeSH in PubMed and Emtree in Embase), as well as free text terms. The full search strategies for all databases can be found in the supplementary data, Table S1, S2, and S3.
Study selection and in- and exclusion criteria
All abstracts from the search were screened and assessed for their relevance in this study. Upon inclusion and abstract screening, full articles were examined based on the in- and exclusion criteria, see Table S4. To emphasize, the articles were screened for conventional photon RT, indicated as “RT” in the rest of the article. For both screening levels all studies were evaluated by two independent investigators.
Risk of bias of individual studies and publication bias assessment
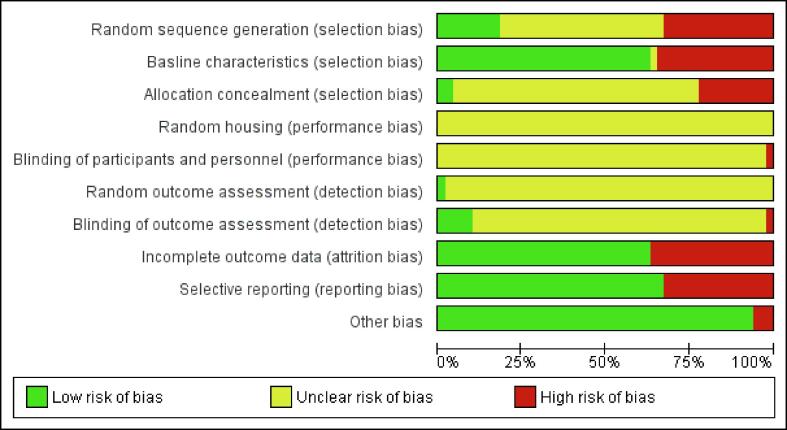
Risk of bias was determined by the Cochrane Risk of Bias tool for the clinical studies [18] and by the SYRCLE Risk of Bias tool for the preclinical studies [19]. Parameters chosen were based on the objectives of this review and the characteristics of all included studies. “Other bias” includes all other potential sources of bias, not included in the predefined parameters. Scoring of the studies was performed by two independent investigators until a unanimous result was achieved. Risk of bias graphs were established by Review Manager 5.3 (The Cochrane Community, the Nordic Cochrane Centre: Copenhagen, 2014) [20]. To estimate publication bias, a funnel plot was created in Rstudios [21] with the Metaviz package [22] and an Eggers test was performed [23]. Possible missing studies were imputed using the trim-and-fill method [24].
Data collection
For the qualitative and quantitative analyses, studies were classified based on 1) the disease type of the subjects (clinical only), 2) the preclinical model (preclinical only), 3) the type of radiation used, 4) the biological effective dose (BED) (≤ 50 Gy, 50–100 Gy and ≤ 100 Gy), 5) the readout technique that measured BBB disruption, and 6) the timepoint(s) which BBB disruption was measured (follow-up time). To allow for better interstudy comparison and analysis, per study both the RT fractionation scheme and cumulative radiation dose were used to calculate the BEDbased on the linear-quadratic formula by Fowler et al (1989) [25] with an α-β of 3 (patients with solid tumours and animals) or 10 (patients with AVM and leukaemia). The timing of the occurrence of BBB disruption by RT, described in the clinical studies was classified based on the radiation injury classification of Greene-Schloesser et al (2012) [26], as follows: 1) acute effects (within one month), 2) early delayed effects (within 1–6 months), and 3) late delayed effects (after 6 months of radiation) [26]. For preclinical studies the classification of Wei et al (2016) and Collins et al (2017) was used: 1) acute effects (within 4 weeks), 2) early delayed effects (within 4–12 weeks), and (3) late delayed effects (after 12 weeks) [27], [28]. For the quantitative analyses, data was extracted from the studies either directly or using ImageJ [29] as a digital ruler for the figures.
Statistical analysis
Analyses were conducted using Review Manager 5.3 software (The Cochrane Community) [20]. The effect of RT on BBB permeability was assessed based on continuous variables found in the included studies, as described in Table S4. Only studies containing a treatment and control group were eligible for meta-analysis. In the meta-analysis, random-effect models were applied because of anticipated heterogeneity [30] between studies with inverse-variance weighting to obtain the summary effect size. The summary effect measurement was calculated as the standard mean difference (SMD) with the corresponding 95% confidence interval (CI). Because most studies were composed of multiple experimental groups, the effect measurement was calculated at group level (instead of study level). Forest plots were generated based on the following parameters: 1) animal model, 2) BED, 3) read-out technique of BBB disruption and 4) follow-up time after radiation for all included quantitative preclinical studies. Subgroup analysis was applied if groups contained at least five studies or more. Heterogeneity was calculated by means of the dispersion index of effect sizes I2. Publication bias was studied using Funnel plots, the Egger method and trim-and-fill analysis.
Results
Search results
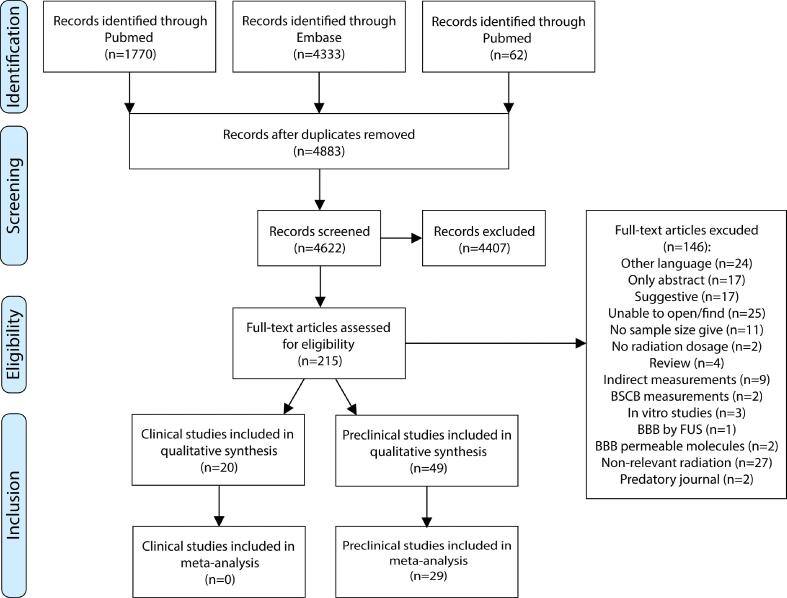
A total of 4883 unique studies were screened, of which 215 studies deemed eligible (Fig. 1). After full text assessment, 20 clinical studies and 49 preclinical studies could be included for qualitative analysis. The meta-analysis encompassed 29 preclinical studies. The remaining 20 preclinical studies were excluded because of missing information regarding the effect size, group size, or any procedural information. None of the clinical studies could be included in the meta-analysis, since none of these studies included randomized control groups.
Fig. 1.
PRISM flow chart of study selection. After selection and filtering of a total of 4883 studies, 215 studies were included in this study, of which 20 clinical studies were evaluated for inclusion in the subsequent qualitative analysis and no clinical study was suited for meta-analysis. 49 preclinical studies were qualitatively analysed, of which 29 studies were included in the meta-analysis.
Description of the included studies
The 20 relevant clinical studies were published between 1979 and 2018 (Table 1). Of these 20 studies, four included patients diagnosed with arteriovenous malformations (AVM) [31], [32], [33], [34], four described patients suffering from haematological cancers [35], [36], [37], [38], one focused on nasopharyngeal cancer [39], six included primary brain tumour patients [40], [41], [42], [43], [44], [45], [46] and four studies included patients diagnosed with brain metastases of other cancer types [47], [48], [49], [50]. The 49 preclinical studies were published between 1964 and 2019, consisting of different animal models (Table 2). The majority of the studies, i.e., 27 (55%), investigate BBB disruption in rats [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77]. In addition, eleven (22%) studies use mice [12], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], five involve rabbits [88], [89], [90], [91], [92], two include dogs [93], [94], two describe monkeys [95], [96], one article studied the effects of RT on the BBB in sharks [97] and one in pigs [98].
Table 1.
Clinical studies included in the qualitative synthesis and the key parameters of interest in this review article.
| Author, year | Disease type | Type of radiotherapy | Number of fractions | Fraction dose | Total dose | Biological Effective Dose | Readout technique | Age | Time | BBB disruption | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lim et al, 2018 | Supratentorial glioblastoma | RT | ? | ? | 45 or 61.1 Gy | ? | MRI | Adults | 4.2 months | Yes |
| 2 | Okawa et al, 2018 | Brain metastases (NSCLC) | WBRT | ? | ? | 30 Gy | ? | LC-MS | Adults | 3 weeks | Yes |
| 3 | Teng et al, 2017 | Brain metastases | WBRT/SRS | Single, 10 or 15 | 3 or 2.5 Gy | 15, 18, 24, 30, or 37.5 Gy | 37.5, 39, 46.9, 50.4 or 81.6 | MRI | Adults | 1 month | Yes |
| 4 | Fang et al, 2015 | Brain metastases (NSCLC) | WBRT | 10 | 3 Gy | 30 Gy | 39 Gy | LC-MS | Adults | 29 days | No |
| 5 | Farjam et al, 2015 | Low-grade glioma | Conformal or intensity-modulated RT | 28 – 33 | 1.8 Gy | 50.4–59.4 Gy | 59.5–70.1 Gy | MRI | Adults | 18 months | Yes |
| 6 | Moraes et al, 2015 | AVM | RT/SRS | ? | ? | 10–22.5 Gy | ? | MRI/CT | Adults | greater than 6 months | Unclear |
| 7 | Parkhutik et al, 2012 | AVM | SRS | Single dose | – | 24 Gy | 216 Gy | MRI | Adults | 63 months | Unclear |
| 8 | Cao et al, 2009 | Low-grade glioma | Conformal RT | 28 – 33 | 1.8 Gy | 50.4–59.4 Gy | 59.5–70.1 Gy | MRI | Adults | 6 months | Unclear |
| 9 | Matulewicz et al, 2006 | Glioma (high and low grade) | RT | 30 | 2 Gy | 60 Gy | 72 Gy | NMR spectroscopy | Adults | 24 months | Unclear |
| 10 | Tu et al, 2006 | AVM | SRS | Single dose | – | 18–20 Gy | 126–153.3 Gy | Electron microscopy | Adults/children | 64 months | Yes |
| 11 | Wu et al, 2006 | Glioblastoma | RT | 28 | 1.6 Gy | 45 Gy | 52.2 Gy | MRI/CT | ? | 5–10 days | No |
| 12 | Cao et al, 2005 | High grade glioma | Conformal RT | 35 | 2 Gy | 70 Gy | 84 Gy | MRI | Adults | 6 months | Unclear |
| 13 | Levegrün et al, 2004 | AVM | SRS | Single dose | – | 19 Gy | 139.3 Gy | MRI | Adults/children | 26.8 months | Yes |
| 14 | Chan et al, 1999 | Nasopharyngeal carcinoma | RT | ? | ? | 66–71.2 Gy | ? | MRI | Adults | 4.4 years | Yes |
| 15 | Riccardi et al, 1998 | Acute leukemia | RT | 10 or 12 | 1.8 Gy | 18 or 24 Gy | 28.8 or 38.4 Gy | LC-MS | Children | 24 h | No |
| 16 | Ott et al, 1991 | Intracranial lymphoma | RT | ? | ? | 30 or 40 Gy | ? | PET | Adults | 21 weeks | No |
| 17 | Riccardi et al, 1991 | Acute leukemia | RT | ? | ? | 18 or 24 Gy | ? | LC-MS | Children | 1 year | No |
| 18 | Qin et al, 1990 | Intracranial tumors | RT | 15 or 20 | 2 Gy | 30 or 40 Gy | 36 or 48 Gy | CT | ? | 8 months | Unclear |
| 19 | Jarden et al, 1985 | Brain metastases | WBRT | 6 or 10 | 2, 3, or 4/6 Gy | 20, or 30 Gy | 24, 34 or 44.4 Gy | PET | Adults | 72 h | No |
| 20 | Seshadri et al, 1979 | Acute leukemia/ non-hodgkin’s lymphoma | RT | 12 or 16 | 1.5 or 2 Gy | 24 Gy | 36 or 40 Gy | LC-MS | Adults/children | 48 h | No |
Abbreviations: NSCLC, non-small cell lung cancer; AVM, arteriovenous malformations; RT, radiotherapy; WBRT, whole-brain RT; SRS, stereotactic radiosurgery; MRI, magnetic resonance imaging; LC-MS, liquid chromatography mass spectrometry; NMR, nuclear magnetic resonance; CT, computed tomography; PET, positron emission tomography.
Table 2.
Preclinical studies included in the qualitative synthesis and the key parameters of interest in this review article.
| Author, year | Preclinical model | Type of radiotherapy | Number of fractions | Fraction dose | Total dose | Biological Effective Dose | Readout technique | Time | BBB disruption | meta-analysis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Jost et al, 2019 | Rats | RT | 6 fractions | 5 Gy | 30 Gy | 45 Gy | MRI | 2 days | Yes | Yes |
| 2 | Yoshida et al, 2018 | Mice | RT | Single dose | – | 60 Gy | 420 Gy | EB extravasation | 1 week | Unclear | Yes |
| 3 | Constanzo et al, 2017 | Rats | Gamma knife RT | Single dose | – | 10, 37, or 100 Gy | 20, 173.9, 1100 Gy | MRI | 140 days | Yes | Yes |
| 4 | Kalm et al, 2017 | Mice | RT | Single dose | – | 8 Gy | 14.4 Gy | Radioactive brain uptake | 72 h | Yes | Yes |
| 5 | Zhou et al, 2017 | Rats | RT | Single dose | – | 6 Gy | 9.6 Gy | Immunocytochemistry | 24 h | Yes | Yes |
| 6 | Murrell et al, 2016 | Mice | WBRT | 2 | 10 Gy | 20 Gy | 40 Gy | MRI | 36 days | No | Yes |
| 7 | Ngen et al, 2016 | Mice | RT | Single dose | – | 80 Gy | 720 Gy | MRI | 2 weeks | Yes | Yes |
| 8 | Tamborini et al, 2016 | Mice | WBRT | Single dose | – | 2 Gy | 2.4 Gy | Immunohistochemistry | 48 h | Yes | Yes |
| 9 | Tong et al, 2016 | Mice | RT | Single dose | – | 10 Gy | 20 Gy | EB extravasation | 48 h | Yes | yes |
| 10 | Fan et al, 2015 | Rats | WBRT | Single dose | – | 22 Gy | 70.4 Gy | MRI or EB extravasation | 2 h | Yes | Yes |
| 11 | Zhang et al, 2015 | Mice | RT | Single dose | – | 20 Gy | 60 Gy | EB extravasation | 4 weeks | Yes | Yes |
| 12 | Cheng et al, 2014 | Rats | Gamma knife RT | Single dose | – | 60 Gy | 420 Gy | EB extravasation | 24 weeks | Yes | Yes |
| 13 | Jin et al, 2014 | Rats | RT | 2 | 3 Gy | 6 Gy | 7.8 Gy | EB extravasation | 28 days | Yes | Yes |
| 14 | Lampron et al, 2012 | Mice | WBRT | Single dose | – | 10 Gy | 20 Gy | Immunohistochemistry | 7 days | No | No |
| 15 | Guan et al, 2011 | Rats | X-knife RT | Single dose | – | 20 Gy | 60 Gy | CT perfusion imaging | 5 days | Yes | Yes |
| 16 | Khatri et al, 201 | Rats | RT | Single dose | – | 10 or 20 Gy | 20 or 60 Gy | LC-MS | 6 h | Yes | No |
| 17 | Zhou et al, 2011 | Rats | RT | 4 or 8 | 5 Gy | 20 or 40 Gy | 30 or 60 Gy | EB extravasation | 12 weeks | Yes | Yes |
| 18 | Liu et al, 2010 | Rats | WBRT | Single dose | – | 15 Gy | 37.5 Gy | EB extravasation | 24 h | Yes | Yes |
| 19 | Wilson et al, 2009 | Mice | RT | Single dose | – | 20 Gy | 60 Gy | Intravital microscopy | 48 h | Yes | Yes |
| 20 | Ernst-Stecken et al, 2007 | Rats | SRS | 2–4 | 10 Gy | 20, 30, or 40 Gy | 40, 60 or 80 Gy | MRI/CT | 16 weeks | Yes | No |
| 21 | Yuan et al, 2006 | Mice | RT | 20 | 2 Gy | 40 Gy | 48 Gy | Intravital microscopy | 180 days | Yes | Yes |
| 22 | Kaya et al, 2004 | Rats | WBRT | Single dose | – | 18 Gy | 50.4 Gy | EB extravasation | 24 h | Yes | Yes |
| 23 | Yuan et al, 2003 | Rats | RT | Single dose | – | 20 Gy | 60 Gy | Intravital microscopy | 96 h | Yes | Yes |
| 24 | Mima et al 1999 | Rats | RT | Single dose | – | 25 Gy | 87.5 Gy | Immunohistochemistry | 5 days | Yes | No |
| 25 | Fike et al, 1998 | Dogs | Interstitial RT | Single dose | – | 20 Gy | 60 Gy | CT | 2–8 weeks | Yes | No |
| 26 | Karger et al, 1997 | Rats | SRS | Single dose | – | 20, 30, 40, 50, or 100 Gy | 60, 120, 200, 300, 1100 Gy | MRI | 19 months | Unclear | No |
| 27 | Kamiryo et al, 1996 | Rats | Gamma knife RT | Single dose | – | 50, 75, or 125 Gy | 300, 637.5, 1687.5 Gy | EB extravasation | 12 months | Unclear | No |
| 28 | Miot et al, 1995 | Pigs | RT | Single dose | – | 40 or 60 Gy | 200, 420 Gy | MRI/EB extravasation | 180 days | Yes | No |
| 29 | Nakata et al, 1995 | Rats | RT | Single dose | – | 20, 40, or 80 Gy | 60, 200, 720 Gy | Immunohistochemistry | 30 days | Unclear | No |
| 30 | Omary et al, 1995 | Rats | Gamma knife RT | Single dose | – | 120 Gy | 1560 Gy | MRI | 4 weeks | Yes | No |
| 31 | Krueck et al, 1994 | Rats | WBRT | Single dose | – | 15 or 25 Gy | 37.5 or 87.5 Gy | MRI | 48 h | Yes | Yes |
| 32 | Rubin et al, 1994 | Rats | RT | Single dose | – | 60 Gy | 420 Gy | MRI | 24 weeks | Yes | Yes |
| 33 | d’Avella et al, 1992 | Rats | WBRT | 20 | 2 Gy | 40 Gy | 48 Gy | Radioactive brain uptake | 3 weeks | Yes | Yes |
| 34 | Lo et al, 1992 | Rabbits | RT | Single dose | – | 60 Gy | 420 Gy | MRI | 10 weeks | Unclear | Yes |
| 35 | Gobbel et al, 1991 | Dogs | Interstitial RT | Single dose | – | 20 Gy | 60 Gy | CT | 6 weeks | Yes | No |
| 36 | Lo et al, 1991 | Rabbits | RT | Single dose | – | 15 or 30 Gy | 37.5 or 120 Gy | MRI | 8 months | Yes | No |
| 37 | Bezek et al, 1990 | Rats | RT | Single dose | – | 25 Gy | 87.5 Gy | Radioactive brain uptake | 7 days | Unclear | No |
| 38 | Delattre et al, 1989 | Rats | RT | Single dose | – | 3 Gy | 3.9 Gy | Radioactive brain uptake | 3 h | Yes | Yes |
| 39 | Spence et al, 1987 | Rats | Whole body RT | Single dose | – | 20 Gy | 60 Gy | Radioactive brain uptake | 2 days | No | Yes |
| 40 | Kourtopouios et al, 1983 | Rabbits | RT | Single dose | – | 10 Gy | 20 Gy | Chemical brain uptake | 90 min | Yes | Yes |
| 41 | Levin et al, 1979 | Rats | RT | Single dose, 3, 5, 10, or 25 | 2 or 4 Gy | 2, 4, 7, 10, 12, 20, 25, 30 Gy | 2.4, 5.6, 11.9, 12, 16.8, 20, 24, 36 or 87.5 Gy, | Radioactive brain uptake | 24 h | Unclear | No |
| 42 | O’neill et al, 1977 | Monkeys | RT | Single dose | – | 35 Gy | 157.5 Gy | EB extravasation | 22 weeks | Yes | No |
| 43 | Blomstrand et al, 1975 (1) | Rabbits | RT | Single dose | – | 30 Gy | 120 Gy | EB extravasation | 4 months | Yes | No |
| 44 | Blomstrand et al, 1975 (2) | Rabbits | RT | Single dose | – | 30 Gy | 120 Gy | EB extravasation | 1 week | Yes | No |
| 45 | Olsson et al, 1975 | Rats | RT | Single dose | – | 300 Gy | 9300 Gy | EB extravasation | 9 days | Yes | No |
| 46 | Tanaka et al, 1975 | Monkeys | RT | Single dose | – | 35 Gy | 157.5 Gy | Radioactive brain uptake | 40 weeks | Yes | Yes |
| 47 | Olsson et al, 1972 | Sharks | RT | Single dose | – | 10, 35, 50, 107, 200, 250 or 300 Gy | 20, 157.5, 300, 1251.9, 4200, 6500, 9300 Gy | EB extravasation | 28 months | No | No |
| 48 | Bulat et al, 1966 | Rats | WBRT | Single dose | – | 9 or 80 Gy | 17.1, 720 Gy | Chemical brain uptake | 24 h | No | Yes |
| 49 | Nair and Roth, 1964 | Mice | RT | Single dose | – | 80 or 115 Gy | 720, 1437.5 Gy | Radioactive brain uptake | 120 h | Yes | No |
Abbreviations: EB, Evans blue.
Risk of bias and reported quality
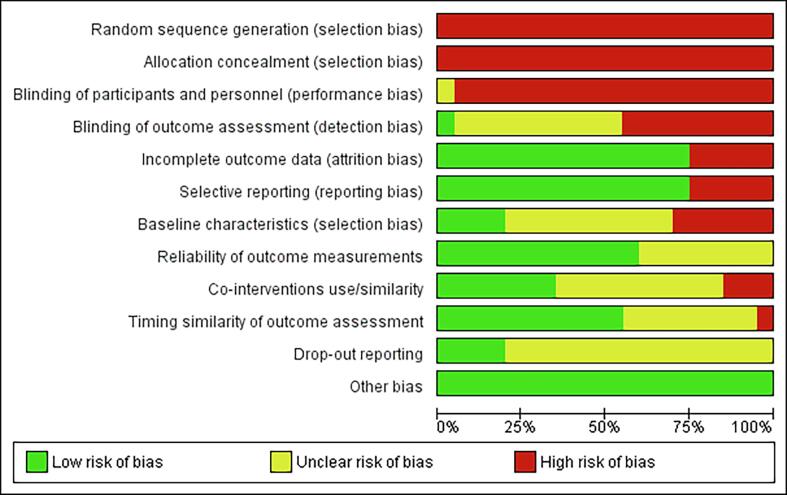
The risk of bias for individual studies was assessed separately for clinical and preclinical studies. For clinical studies, a high or unclear risk of bias was found for seven of the twelve scoring criteria (Fig. S1). Conversely, a low risk of 75%, 75%, 60% and 55% was scored for the categories “Incomplete outcome data”, “Selective reporting”, “Reliability of outcome measurements” and “Timing similarity of outcome assessment” respectively, while no other bias is found. Preclinical studies were assessed with a high or unclear risk of bias for six of the ten scoring criteria (Fig. S2). A low risk of bias was assigned in 63%, 63% and 67% of the studies for the scoring criteria: “Baseline characteristics”, “Incomplete outcome data” and “Selective outcome reporting”, respectively. Three studies were found to have a high risk of “Other bias” for the following reasons: 1) “Missing statistics”, 2) “Missing group sizes”, and 3) “Fluctuating follow-up times”.
Effect of RT on BBB permeability
Clinical data - qualitative analysis
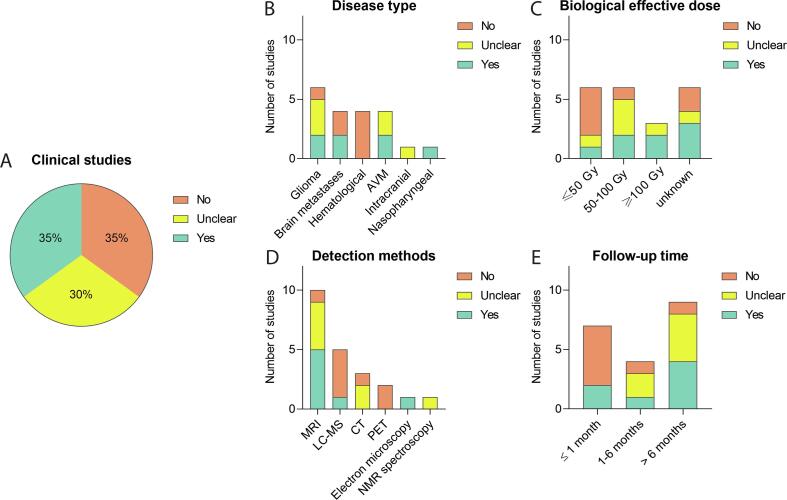
Of the 20 clinical studies that investigated BBB integrity after RT, fifteen (75%) were performed in adults, three [32], [33], [38] (15%) in both adult and paediatric patients, and two [35], [36] (10%) studied the effect of RT on the BBB in children only (Table 1). Seven out of the 20 studies (35%) reported alterations of BBB permeability [32], [33], [39], [40], [41], [47], [50], six studies (30%) observed a shallow but unclear effect [31], [34], [42], [43], [44], [46], whereas seven (35%) do not detect any effect [35], [36], [37], [38], [45], [48], [49] (Fig. 2A).
Fig. 2.
Effect of RT on BBB permeability in clinical studies - qualitative analysis. Analysing clinical studies, the absence or presence of RT-induced BBB permeability was evaluated (A) and subgrouped by disease type (B), Biological effective dose (C), BBB disruption detection method D), and duration of patient follow-up (E), showing differential effects.
Disease type
Two of the six studies including glioma and two of the four studies with patients suffering brain metastases observed BBB permeability after treatment (Fig. 2B). None of the studies in which patients were treated with RT for a haematological disease reported any alteration of BBB integrity after treatment. Causal links between the application of radiation and the impact on the BBB in brain tumours and exposed non-tumour tissue, could not be established because of the design of the clinical studies.
Biological effective dose
In only one of the six studies [47], in which patients were treated with a BED of ≤ 50 Gy [47], and two of the six studies [41], [47] with a BED of 50–100 Gy, an increase in BBB permeability was observed (Fig. 2C). Two of these studies, Farjam et al (2015) [41] (yes) and Cao et al (2009) [44] (unclear), noticed a peak in permeability at 1–1.5 months, which was reversed over time. Interestingly, in both studies patients with low-grade glioma were irradiated, with the same fractionation scheme, cumulative dose, BED, and the same read-out technique was applied. The three studies with a BED of ≥ 100 Gy used a single-dose radiation in patients suffering from AVM where RT is used for stereotactic radiosurgery; Tu et al (2006) [32] and Levegrün et al (2004) [33] reported a clear BBB disruption, while Parkhutik et al (2012) [31] only observed this in part of the patients. For the remaining six studies [34], [35], [37], [39], [40], [50] no BED could be calculated, because details on the fractionation scheme applied were not reported. Of these six studies, three did observe an increased BBB permeability. Two of these studies, Chan et al (1999) [39] and Lim et al (2018) [40] used a cumulative dose between 61 and 80 Gy observed increased BBB breakdown after radiation. Looking at the fractionation scheme Farjam et al (2015) [41] was the only one of five studies with a fraction dose below 2 Gy that observed a significant increase in BBB permeability after 1 month of radiation, while Cao et al (2009) [44] reported temporal changes in the vascular volumes and Gd-DTPA signal in the cerebral tissue. One of the six studies with a 2 Gy fraction scheme observed a clear increase BBB permeability after RT, in contrast to Cao et al (2005) [43] who noticed only a BBB permeability difference close to the tumour. Qin et al (1990) [46] noted a change but also observed recovery 8 months after radiation; the authors indicated that acute effects can be reversible and do not necessarily result in permanent damage. Remarkably, Jarden et al (1985) described 6 Gy per fraction without any BBB alterations, albeit in combination with dexamethasone [49].
Detection method
Five [33], [39], [40], [41], [47] of the ten studies using magnetic resonance imaging (MRI) measuring the enhancement/extravasation of gadolinium-DTPA observed a clear change in BBB permeability. Other studies used a more indirect technique to detect BBB disruption. One [50] of the five studies using liquid chromatography–mass spectrometry (LC-MS) to detect drugs in cerebrospinal fluid as a surrogate marker of BBB disruption noticed a change (Fig. 2D). Fang et al (2015) [48] treated patients with Gefitinib but did not observe an effect despite a high cumulative radiation dose (40 Gy vs 30 Gy). Tu et al (2006) [32] detected an alteration in BBB integrity by electron microscopy, while studies using computed tomography (CT) or positron emission tomography (PET) imaging did not detect any effect. Even with a high cumulative dose of 60 Gy, Matulewicz et al (2006) [42], who used nuclear magnetic resonance (NMR) spectroscopy, could not draw any clear conclusion but observed oscillations of choline-containing compounds over time, which might be indicative of BBB disruption and repair processes.
Follow-up time
Two of the seven clinical studies (29%) investigating an acute effect in BBB permeability after radiotherapy (Fig. 2E) reported changes after three and four weeks [47], [50]. Of the four studies studying early delayed effects, only Lim et al (2018) observed a clear increase in BBB permeability [40], while Cao et al (2009) [44] and Cao et al (2005) [43] were unclear In their conclusion but both documented a peak of BBB permeability for radiation doses greater than 40 and in the range 20 to 40 Gy respectively. Late delayed effects of radiation on the BBB is found in four of nine studies [32], [33], [39], [41].
Preclinical data – Qualitative analysis
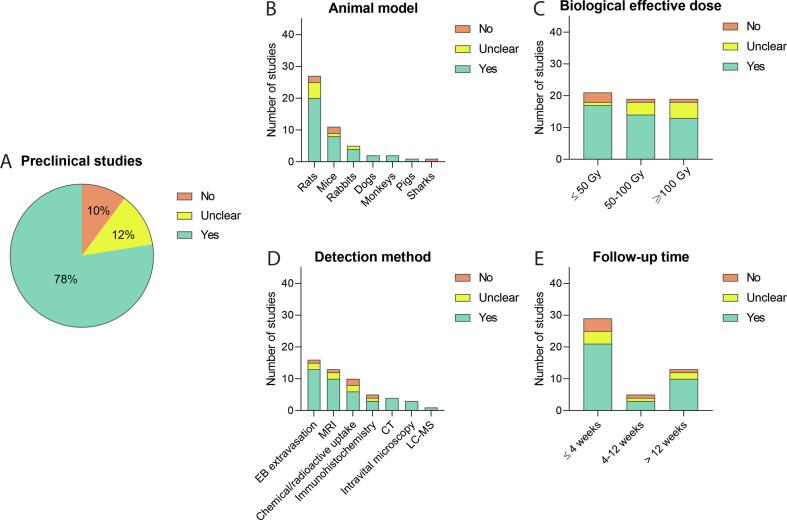
With respect to the qualitative analysis of the 49 preclinical studies, 38 (78%) reported a clear difference in BBB permeability after RT, six (12%) detect an unclear effect, and five (10%) did not observe an effect (Fig. 3A).
Fig. 3.
Effect of RT on BBB permeability in preclinical studies - qualitative analysis. Analysing preclinical studies, the absence or presence of RT-induced BBB permeability was evaluated (A), and subgrouped by animal model used (B), Biological effective dose (C), BBB disruption detection method (D), and duration of follow-up (E), showing differential effects.
Animal model
In 55% of the preclinical studies, rats were examined as animal model. Of these rat studies 74% reported that radiotherapy influenced the BBB permeability (Fig. 3B). Other animal species were less frequently used, but permeability changes were also observed in mice, rabbits, dogs, monkeys, and pigs, but not for sharks. Olsson et al (1972) concluded that the shark brain is not a suitable model because of its radio-resistant properties [97]. Of note, Spence et al (1987) [66] and Bulat (1966) [67] reported an absence of effect on BBB integrity disturbances in rats after a short follow-up time of 24 and 48 h. In addition, in two mouse studies, no changes were observed in BBB permeability; Murrell et al (2016) [83] and Lampron et al (2012) [78] noticed no changes in BBB permeability with a cumulative dose of 20 and 10 Gy respectively.
Biological effective dose
Most of the preclinical studies (81%) with a BED of ≤ 50 Gy reported an increase of BBB permeability. Comparable effects were observed in studies using a BED of 50–100 Gy (74%) and ≥ 100 Gy (68%), see Fig. 3C. Although an overall comparable BED was used, clinical studies relatively used a higher cumulative dose compared to preclinical studies, whereas in clinical studies BBB permeability was observed to lesser extent than in preclinical studies (35% vs 78%). Additionally, the bulk of the preclinical studies (84%) used a single dose fraction for the irradiation of the animals, with 76% of these studies observing an enhanced BBB permeability. When multiple fractionations were applied, the animals received 2 or up to 10 Gy per fraction, with a cumulative dose of 6 or up to 40 Gy. Levin et al (1979) [75], applied fractions of 2 or 4 Gy with a cumulative dose up to 30 Gy and detected some permeability changes, whereas Murrell et al (2016) [83] did not observe any change after two fractions of 10 Gy with a cumulative dose of 20 Gy.
Detection method
Unlike clinical studies, animal research more easily allowed for post-mortem observations and the usage of multiple detection methods (Fig. 3D). Interestingly, two of the five studies that did not observe a BBB integrity issue were analysed by Evans blue extravasation or immunohistochemistry [78], [97], in which brain surgery and processing was necessary to acquire the results. When comparing clinical and preclinical studies that use MRI, the results are relatively similar.
Follow-up time
For each follow-up time (acute, early delayed, and late delayed) most preclinical studies observed a relatively equal occurrence of increased BBB permeability: 72%, 60% and 77%, respectively (Fig. 3E). In contrast, in clinical studies an increase in BBB permeability was predominantly reported as late delayed effect.
Preclinical data – Meta-analysis
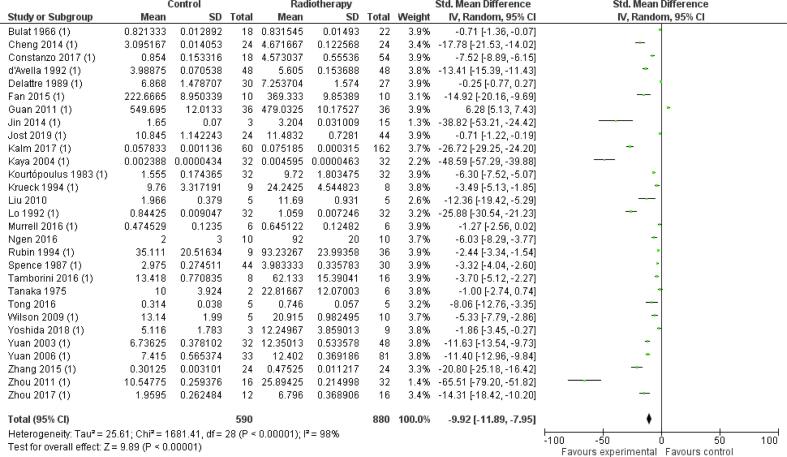
The 29 preclinical studies included in the meta-analysis showed a significant effect of radiation on BBB permeability between irradiated animals (radiotherapy group) and non-irradiated animals (control group): −9.92 [-11.89, −7.95] (n = 29, p < 0.01) (Fig. S3). However, heterogeneity was high (I2 = 98%).
Preclinical model
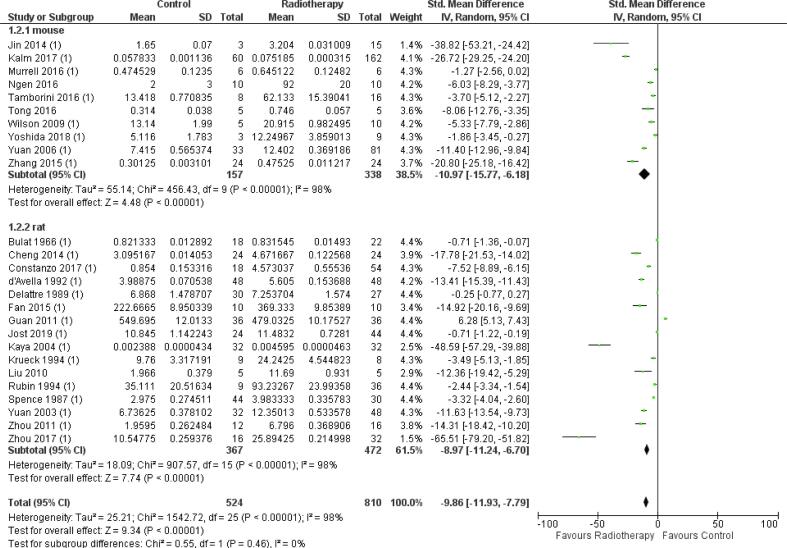
In the studies included in the meta-analysis, in both mice (34%) and rats (55%), RT significantly increased BBB permeability (mice SMD −10.97 [-15.77, −6.18], n = 10, p < 0.01; rats- SMD 8.79 [-11.24, −6.70], n = 16, p < 0.01) (Fig. S4). Subgroup analysis did not show any significant difference between effect estimates in mice and rats (p = 0.26). Heterogeneity in both subgroups was high (I2 = 98% and I2 = 98%) in mice and rats, respectively. Moreover, the studies using monkeys and rabbits were excluded from the meta-analysis because data was insufficient to create a subgroup, i.e., group size lower than five.
Biological effective dose
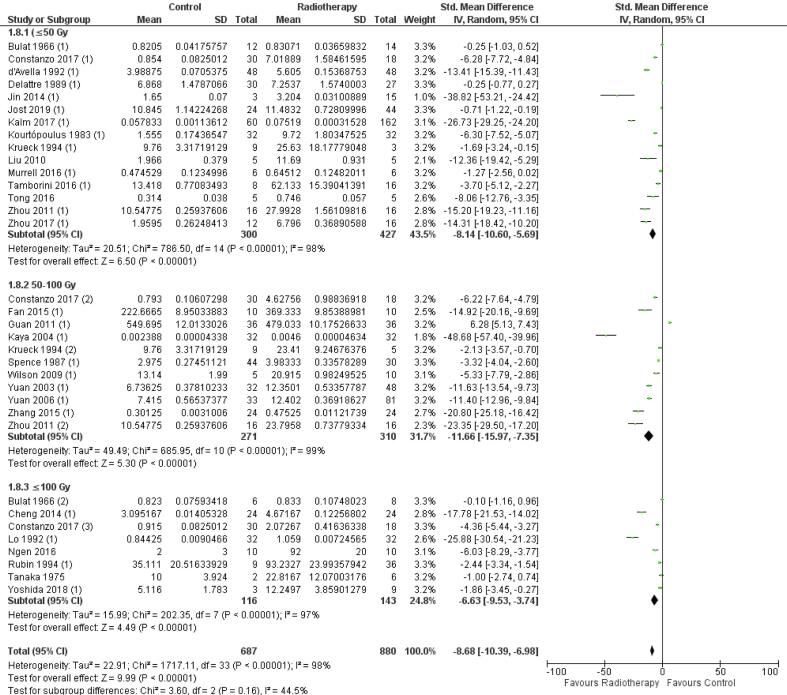
A significant effect of radiation on BBB permeability was found in all the three subgroups of the BED, ≤ 50 Gy, 50–100 Gy and ≥ 100 Gy: −8.14 [-10.60, −5.69] (n = 15, p < 0.01), −11.66 [-15.97, −7.35] (n = 11, p < 0.01), and −6.63 [-9.53, −3.74] (n = 8, p < 0.01) (Fig. S5). No significant difference of these effects is found between the three subgroups (Chi2, p = 0.16). In addition, a substantial heterogeneity was found in all three subgroups: I2 = 98%, I2 = 99%, and I2 = 97%, indicating a low similarity between studies with similar BEDs.
Detection method
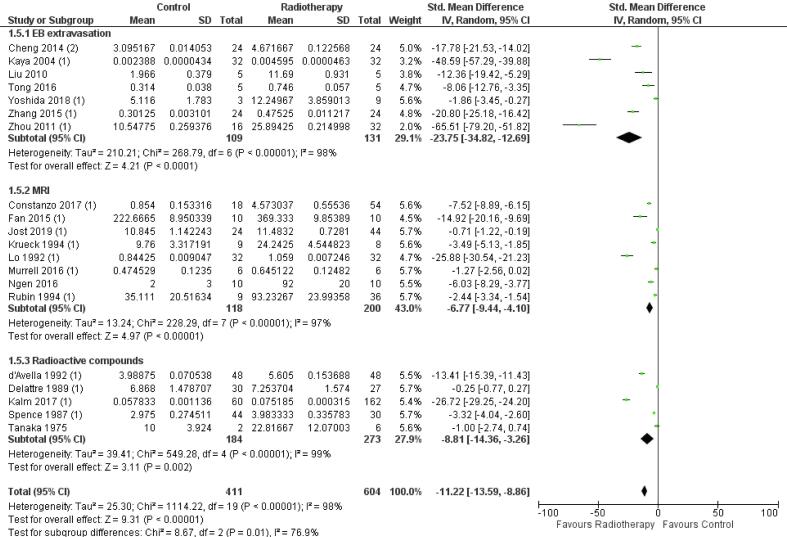
Subgroup analysis of the detection methods: Evans blue extravasation, MRI (gadolinium-DTPA) and extravasation of radioactive tracers (Fig. S6), showed a significant increase in BBB permeability after radiation: –23.75 [-34.82, −12.69] (n = 7, p < 0.01), −6.77 [-9.44, −4.10] (n = 8, p < 0.01) and −8.81 [-14.36, −3.26] (n = 5, p < 0.01), respectively. Comparison of the effect estimates between subgroups showed a significant difference in BBB permeability (p = 0.01), which could be ascribed to a difference between the sensitivity of Evans blue extravasation and MRI (ΔSMD = 16.98, 95% CI −25.38, −8.59) as a detection method. Heterogeneity was high in each subgroup: I2 = 98 %, I2 = 97% and I2 = 98% for Evans blue, MRI, or radioactive tracers, respectively.
Follow-up time
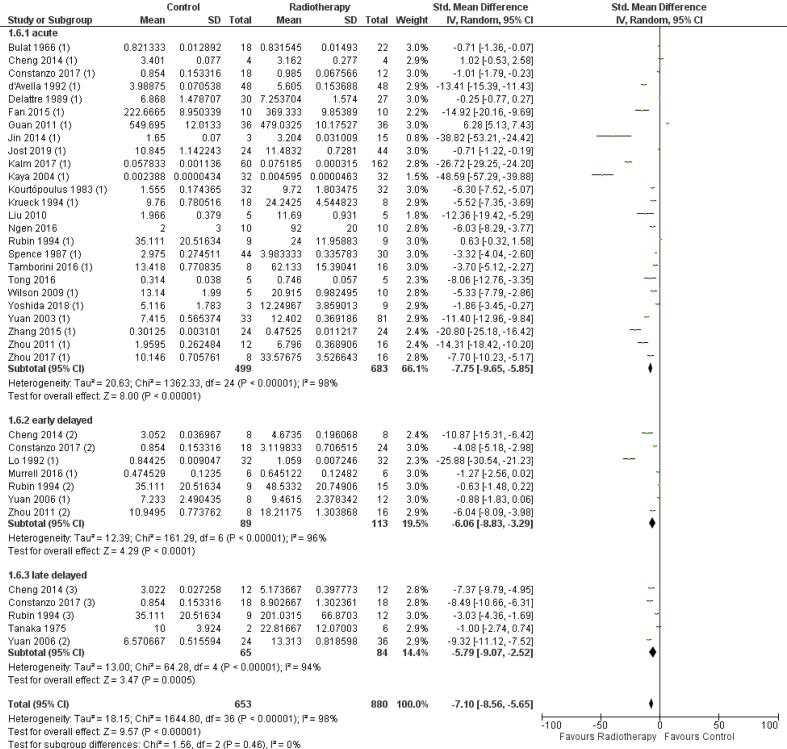
The onset of BBB permeability after radiation therapy was explored with acute, early delayed, and late delayed categories and a significant increase in BBB permeability is observed in all follow-up time categories: −7.75 [-9.65, −5.85] (n = 25, p < 0.01), −6.06 [-8.83, −3.29] (n = 7, p < 0.01) and −5.79 [-9.07, −2.52] (n = 5, p < 0.01), respectively (Fig. S7). For each category, a high heterogeneity was found: I2 = 98% (, I2 = 97%, and I2 = 94%, respectively. No significant difference between these follow-up time categories was found (p = 0.46).
Publication bias
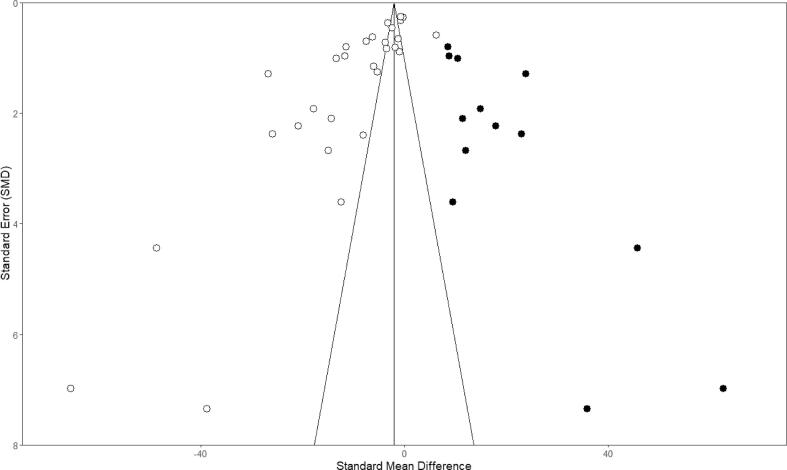
Potential publication bias was assessed for the outcome of BBB permeability upon RT in the 29 preclinical studies included in the meta-analysis (Fig. S8). Asymmetry observed in the funnel plot suggests the presence of publication bias, which was confirmed by Egger’s regression line. The funnel plot indicates that studies with small cohorts favouring negative results were missing in the publication record. Trim-and-fill analysis resulted in the addition of 13 extra predicted studies (black dots), with a new total calculated effect), indicating an overestimation of the effect size. Despite this overestimation, the effect of radiation on BBB permeability remains significant.
Discussion
Conventional photon RT is a therapeutic cornerstone in brain cancer and it is commonly postulated that this treatment modality alters BBB permeability [16], a key protective component to maintain brain tissue homeostasis. The downside of a dysfunctional BBB is that the brain tissue is more exposed to blood-borne proteins, waste products and pathogens, potentially resulting in a variation of neurological disorders such as neuroinflammatory reactions and neurodegenerative diseases [99], [100], [101]. On the other hand, in the context of neurological diseases and brain cancer, an increased BBB permeability can have therapeutic advantages, whereby drugs that normally have limited access to the brain parenchyma are able to better reach the diseased brain [4]. To illustrate, new upcoming techniques such as focused ultrasound, aim to increase the BBB permeability locally and transiently for the extravasation of drugs into the brain parenchyma for the treatment of neurodegenerative diseases such as Alzheimer’s Diseases and primary brain tumours as well as brain metastases [102], [103], [104]. In scope of the safety of patients and their (concomitant and adjuvant) treatment, it is crucial to monitor, evaluate and control the extent of BBB permeability caused by RT. However, the factors leading to BBB alteration remain to be better understood and a thorough analysis of the evidence on RT-induced BBB disruption thus far has been lacking. This systematic review and meta-analysis therefore explored the contribution of these determinants in the state-of-the-art literature. Based on a qualitative analysis of relevant literature and by performing a meta-analysis, we conclude from preclinical and clinical studies that photon radiotherapy indeed enhances the permeability of the BBB, although the low level of data-reporting and likely occurrence of publication bias of the included studies, limits the strength of these conclusions.
For better comparison between studies, the BED was calculated. Whereas clinical studies mostly observed BBB permeability upon RT at ≥ 100 Gy, preclinical studies display an overall effect in each of the BED categories. Most of the preclinical studies used a single dose instead of a multiple fractionation scheme which is mainly used in the clinical studies. The three included clinical studies using a single dose (18–24 Gy) observed an increase in BBB permeability, while this effect was observed in only 20% of the preclinical studies applying fractionation protocols at a respectively equal cumulative dose. Another point of interest: laboratory animals are generally given a higher BED given compared to the patients in the clinical studies. Recently, the use of proton therapy as an alternative to conventional photon therapy is gaining popularity. Proton beam RT may possibly overcome the effects of RT at the BBB to some extent, as proton therapy is characterized by the highest energy deposition at the point of interest without an exit dose, hereby lowering the dose in the surrounding healthy tissue [105]. Now an emerging option for paediatric patients, it may herewith reduce long term side effects at the developing brain. However, to our knowledge, there is still a limited data on the effects of proton therapy on BBB permeability, we were unable to analyse this in this systematic review and meta-analysis. Concerning the follow-up time, in clinical studies, increased BBB permeability was often observed after six-months, which could be explained by radio-necrosis [106]. In meantime a significant effect was reported in all post-RT time subcategories in the pre-clinical studies. Both clinical and preclinical studies mentioned a peak in permeability a few months after radiation, followed by BBB restoration afterwards [41], [56]. Furthermore, a correlation between longer permeability and increased radiation doses was observed [44], [58], [72]. Over the years, the techniques to measure BBB permeability have improved and are more refined in their measurements. Older clinical studies mostly did not detect any BBB permeability change while over the years MRI became more the standard and studies using this technique found the opposite effect. Older preclinical studies mostly relied only on the extravasation of Evans blue for visible conformation of increase permeability and reported mostly negative results, but on the other hand more recent studies using Evans blue measured that BBB permeability is increased after RT.
The disease type, stage and/or use of different pharmacological agents are also thought to cause alterations of the BBB and are therefore likely to be confounding factors in the assessment of RT-induced BBB alteration. For instance, glioblastoma often exhibits areas of increased BBB permeability at diagnosis, which is progressive in advanced stages of the disease [107]. This permeability not only occurs along the disease course, but is also often characterized, at a specific disease stage, by a spatial intra-patient heterogeneity owing to the anarchic formation of a blood-tumour barrier in the case of certain brain cancers [108]. Noteworthy, also inter-patient heterogeneity induces a significant variability in the interpretation of permeability data in BBB studies. For example, clinical studies exploring haematological malignancies did not observe any permeability indicating the fact that underlying diseases may influence the extent of BBB permeability, confounded that the cumulative dose of RT in these studies was lower. Moreover, most of the patients in the included clinical studies were treated with additional medication, which could have further compromised or restored BBB integrity [37], [38]. There is evidence that certain pharmacological agents exert an effect on BBB functioning and structure, for example inducing BBB permeability by efflux transporter inhibition [109], or by reinforcement, as reported in studies including dexamethasone [49]. Dexamethasone is often prescribed to reduce cerebral edema [110] in brain malignancies by initiating the glucocorticoid receptor-mediated signalling, ultimately leading to strengthening and restoration of BBB integrity [111]. It is often assumed that the juvenile brain (especially that of infants) is more permeable than the adult brain, even though animal and clinical studies [112], [113], [114] observed well-developed tight junctions and similar activity of transporters. As the juvenile brain is still in development, it can be hypothesized that it is more prone to damage and collateral effects [115]. Of the five clinical studies included, three enrolled paediatric patients but observed no effect on BBB permeability after radiation [35], [36], [38].
Our systematic review indicates that preclinical studies reported more RT-induced BBB permeability than clinical studies: 78% vs 35%, respectively. Besides the parameters investigated in this review several more other reasons can explain more the discrepancy: first, preclinical studies are designed and performed in a more controlled fashion, thus potentially reducing group variability and, in turn, increase statistical power and ultimately find significant differences. Second, preclinical studies give access to more readout modalities, which allows for multiparametric ascertainment and cross-validation of disease hallmarks, e.g., albumin extravasation into the brain parenchyma. In contrast, clinical studies mostly use MRI and/or LC-MS of CSF. However, preclinical protocols often require animal anaesthesia using agents that induce haemodynamic changes, e.g., isoflurane-induced vasodilation and increase in blood flow, which is directly sensed by the endothelial barrier, and may activate pathways that potentially modify BBB integrity likely to generate experimental biases [116]. Nonetheless, clinical modalities, such as MRI, CT and PET, can be performed in patients without resorting to the administration of anaesthetics. The ascertainment of BBB leakiness in clinical MRI protocols mainly uses gadolinium chelates whose extravasation, according to their high molecular weights, is mediated by tight junctions at a specific disease stage, thus only reflecting the status of the physical BBB; however, BBB leakiness for smaller sized molecules is also mediated by its functional counterpart, mainly including transcytosis, which may be upregulated in the course of specific diseases but cannot be measured by conventional MRI protocols. Drug-PET imaging after RT, using radioisotopes such as 11C or 18F for small molecule drugs and 89Zr for monoclonal antibodies allows for visualization of enhanced brain uptake of these compounds. Based on subgroup analysis of the pre-clinical studies, Evans blue extravasation shows a significant increase in BBB permeability compared to MRI and radioactive compounds, which have a similar effect size and seem to be more related. Conversely, in case of Evans blue extravasation the results are obtained from post-mortem tissues, while MRI and radioactive compounds are acquired in real-time, explaining this discrepancy. The (pre)clinical studies using MRI show similar results, which might indicate that this technique is more reliable to determine BBB disruption in both humans and animals. More preclinical research is therefore needed to study the effect of RT on BBB disruption for small to large sized molecules.
In our meta-analysis, no clinical subgroup was eligible for further processing owing to the absence of non-irradiated control groups. All the eligible subgroups, i.e., including at least five studies, showed a significant RT-induced BBB permeability. One of the excluded subgroups concerns monkeys, which are particular interesting due to their intracranial vessel structure close to humans [117]. However, the only non-human primate study exploring RT-induced BBB permeability reported non-significant differences in BBB permeability. The allocation of the follow-up time for the animal models was solely based on rats [27], [28], which might have influenced the outcome of all the other models and was not specifically established for radiation effects.
Importantly, the majority of the clinical and preclinical studies scored either a high or unclear risk of bias, affecting the reliability of the data, but also can cause an over- or underestimation of the results [118]. In case of the clinical data, bias can be caused by certain ethical considerations, for example patients can be excluded due to deviant baseline characteristics, which can cause an overestimation of the results. Lastly, a potential publication bias was detected, and may also explain the difference found between clinical and preclinical studies in our qualitative analyses where the percentage of studies reporting an effect of RT on BBB permeability was higher for the preclinical studies. Nevertheless, the trim-and-fill analysis confirmed RT-induced BBB disruption.
Conclusion and future perspectives
This systematic review and meta-analysis of the literature demonstrates that RT influences BBB permeability, although our findings show that suboptimal study designs and a publication bias in the selected studies may be the source of an overestimation of the extent of BBB permeability induced by RT. Worth mentioning, the robust comparison of the variables between the studies for qualitative and quantitative analysis makes it even more difficult for any hard conclusions.
Future preclinical and clinical studies using novel readout modalities should therefore be focused on fully elucidating the extent and timing of BBB opening induced by RT. These considerations will be key to adjust and guide treatment planning in treatment regimens that include RT to the brain. The effect of RT on the BBB in patients can be studied in more detail and longitudinally during and after radiotherapy, using advanced MRI and PET studies. Drug imaging with PET after RT, will provide more insight on possible RT-induced enhancement of drug delivery to the brain, avoiding toxicity and optimizing concomitant and adjuvant treatment strategies for an optimal therapeutic index [119].
Source of support
The review has been supported by the KWF Young Investigator Award (KWF 10911, Dr. D.G. van Vuurden.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2022.04.013.
Contributor Information
Elvin't Hart, Email: e.thart-6@prinsesmaximacentrum.nl.
Lucianne Groenink, Email: l.groenink@uu.nl.
Martijn W. Heymans, Email: mw.heymans@amsterdamumc.nl.
Maarten H. Lequin, Email: m.h.lequin@umcutrecht.nl.
Geert O.R. Janssens, Email: g.o.r.janssens@umcutrecht.nl.
Eelco W. Hoving, Email: e.w.hoving-3@prinsesmaximacentrum.nl.
Dannis G. van Vuurden, Email: d.g.vanvuurden@prinsesmaximacentrum.nl.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. S1.
Supplementary Fig. S2.
Supplementary Fig. S3.
Supplementary Fig. S4.
Supplementary Fig. S5.
Supplementary Fig. S6.
Supplementary Fig. S7.
Supplementary Fig. S8.
References
- 1.Abbott N.J., Patabendige A.A.K., Dolman D.E.M., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997;23(1-3):3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 3.Pardridge W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Tellingen O., Yetkin-Arik B., De Gooijer M.C., Wesseling P., Wurdinger T., De Vries H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 5.De Vries N.A., Beijnen J.H., Boogerd W., Van Tellingen O. Blood-brain barrier and chemotherapeutic treatment of brain tumors. Expert Rev Neurother. 2006;6:1199–1209. doi: 10.1586/14737175.6.8.1199. [DOI] [PubMed] [Google Scholar]
- 6.Delaney G., Jacob S., Featherstone C., Barton M. The role of radiotherapy in cancer treatment: estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer. 2005;104:1129–1137. doi: 10.1002/cncr.21324. [DOI] [PubMed] [Google Scholar]
- 7.Baskar R., Lee K.A., Yeo R., Yeoh K.-W. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9:193–199. doi: 10.7150/ijms.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claes A., Idema A.J., Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007;114:443–458. doi: 10.1007/s00401-007-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen-Jonathan-Moyal É., Vendrely V., Motte L., Balosso J., Thariat J. Radioresistant tumours: From identification to targeting. Cancer Radiother. 2020;24:699–705. doi: 10.1016/j.canrad.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor M.M., Mayberg M.R. Effects of radiation on cerebral vasculature: A review. Neurosurgery. 2000;46:138–151. doi: 10.1093/neurosurgery/46.1.138. [DOI] [PubMed] [Google Scholar]
- 11.Bentzen S.M. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6(9):702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 12.Yuan H., Gaber M.W., Boyd K., Wilson C.M., Kiani M.F., Merchant T.E. Effects of fractionated radiation on the brain vasculature in a murine model: Blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes. Int J Radiat Oncol Biol Phys. 2006;66:860–866. doi: 10.1016/j.ijrobp.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 13.Marchi N., Angelov L., Masaryk T., Fazio V., Granata T., Hernandez N., et al. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 2007;48(4):732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obermeier B., Daneman R., Ransohoff R.M. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng K.M., Chan C.M., Fu Y.T., Ho L.C., Cheung F.C., Law C.K. Acute hemorrhage in late radiation necrosis of the temporal lobe: report of five cases and review of the literature. J Neurooncol. 2001;51:143–150. doi: 10.1023/a:1010631112015. [DOI] [PubMed] [Google Scholar]
- 16.Van Vulpen M., Kal H.B., Taphoorn M.J.B., El Sharouni S.Y. Changes in blood-brain barrier permeability induced by radiotherapy: Implications for timing of chemotherapy? (Review) Oncol Rep. 2002;9:683–688. doi: 10.3892/or.9.4.683. [DOI] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:1–9. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooijmans C.R., Rovers M.M., De V.R., Leenaars M., Ritskes-hoitinga M., Langendam M.W. SYRCLE ’ s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:1–9. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. n.d.
- 21.RStudio Team. RStudio: Integrated Development for R. RStudio 2020.
- 22.Kossmeier M., Tran U.S., Voracek M. Visualizing Meta-Analytic Data with R Package Metaviz. R Package Version. 2020;(3):1. [Google Scholar]
- 23.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duval S., Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 25.Fowler J.F. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 26.Greene-Schloesser D., Robbins M.E., Peiffer A.M., Shaw E.G., Wheeler K.T., Chan M.D. Radiation-induced brain injury: A review. Front Oncol. 2012;2:1–18. doi: 10.3389/fonc.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei T., Guo T.Z., Li W.W., Kingery W.S., Clark J.D. Acute versus chronic phase mechanisms in a rat model of CRPS. J Neuroinflammation. 2016;13:1–15. doi: 10.1186/s12974-015-0472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins K.H., Hart D.A., Smith I.C., Issler A.M., Reimer R.A., Seerattan R.A., et al. Acute and chronic changes in rat soleus muscle after high-fat high-sucrose diet. Physiol Rep. 2017;5(10):e13270. doi: 10.14814/phy2.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997-2018. n.d.
- 30.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkhutik V., Lago A., Aparici F., Vazquez J.F., Tembl J.I., Guillen L., et al. Late clinical and radiological complications of stereotactical radiosurgery of arteriovenous malformations of the brain. Neuroradiology. 2013;55(4):405–412. doi: 10.1007/s00234-012-1115-8. [DOI] [PubMed] [Google Scholar]
- 32.Tu J., Stoodley M.A., Morgan M.K., Storer K.P. Responses of arteriovenous malformations to radiosurgery: Ultrastructural changes. Neurosurgery. 2006;58:749–757. doi: 10.1227/01.NEU.0000192360.87083.90. [DOI] [PubMed] [Google Scholar]
- 33.Levegrün S., Hof H., Essig M., Schlegel W., Debus J. Radiation-induced changes of brain tissue after radiosurgery in patients with arteriovenous malformations: Correlation with dose distribution parameters. Int J Radiat Oncol Biol Phys. 2004;59:796–808. doi: 10.1016/j.ijrobp.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 34.Moraes PauloL, Dias RodrigoS, Weltman E., Giordani AdelmoJ, Benabou S., Segreto H.C., et al. Outcome of cerebral arteriovenous malformations after linear accelerator reirradiation. Surg Neurol Int. 2015;6(1):96. doi: 10.4103/2152-7806.158205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riccardi R., Lasorella A., Tartaglia R.L., Riccardi A., Servidei T., Mastrangelo R. Cranial irradiation and cerebrospinal fluid levels of 6-mercaptopurine in children with acute leukemia. Med Oncol Tumor Pharmacother. 1991;8(2):95–98. doi: 10.1007/BF02988860. [DOI] [PubMed] [Google Scholar]
- 36.Riccardi R., Riccardi A., Lasorella A., Servidei T., Mastrangelo S. Cranial irradiation and permeability of blood-brain barrier to cytosine arabinoside in children with acute leukemia. Clin Cancer Res. 1998;4:69–73. [PubMed] [Google Scholar]
- 37.Ott R.J., Brada M., Flower M.A., Babich J.W., Cherry S.R., Deehan B.J. Measurements of blood-brain barrier permeability in patients undergoing radiotherapy and chemotherapy for primary cerebral lymphoma. Eur J Cancer Clin Oncol. 1991;27:1356–1361. doi: 10.1016/0277-5379(91)90009-3. [DOI] [PubMed] [Google Scholar]
- 38.Seshadri R.S., Ryall R.G., Rice M.S., Leahy M., Ellis R. The Effect of Cranial Irradiation on Blood-Brain Barrier Permeability to Methotrexate. J Paediatr Child Health. 1979;15:184–186. doi: 10.1111/j.1440-1754.1979.tb01223.x. [DOI] [PubMed] [Google Scholar]
- 39.Chan Y.-L., Leung S.-F., King A.D., Choi P.H.K., Metreweli C. Late radiation injury to the temporal lobes: morphologic evaluation at MR imaging. Radiology. 1999;213(3):800–807. doi: 10.1148/radiology.213.3.r99dc07800. [DOI] [PubMed] [Google Scholar]
- 40.Lim W.H., Choi S.H., Yoo R.-E., Kang K.M., Yun T.J., Kim J.-H., et al. Does radiation therapy increase gadolinium accumulation in the brain?: Quantitative analysis of T1 shortening using R1 relaxometry in glioblastoma multiforme patients. PLoS One. 2018;13(2):e0192838. doi: 10.1371/journal.pone.0192838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farjam R, Pramanik P, Aryal MP, Srinivasan A, Chapman CH, Tsien CI, et al. A radiation-induced hippocampal vascular injury surrogate marker predicts late neurocognitive dysfunction 2015;93:908–15. https://doi.org/10.1016/j.ijrobp.2015.08.014.A. [DOI] [PMC free article] [PubMed]
- 42.Matulewicz ł., Sokół M., Michnik A., Wydmański J. Long-term normal-appearing brain tissue monitoring after irradiation using proton magnetic resonance spectroscopy in vivo: Statistical analysis of a large group of patients. Int J Radiat Oncol Biol Phys. 2006;66(3):825–832. doi: 10.1016/j.ijrobp.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Cao Y., Tsien C.I., Shen Z., Tatro D.S., Ten Haken R., Kessler M.L., et al. Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy. J Clin Oncol. 2005;23(18):4127–4136. doi: 10.1200/JCO.2005.07.144. [DOI] [PubMed] [Google Scholar]
- 44.Cao Y., Tsien C.I., Sundgren P.C., Nagesh V., Normolle D., Buchtel H., et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for prediction of radiation-induced neurocognitive dysfunction. Clin Cancer Res. 2009;15(5):1747–1754. doi: 10.1158/1078-0432.CCR-08-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu G.N., Ford J.M., Alger J.R. MRI measurement of the uptake and retention of motexafin gadolinium in glioblastoma multiforme and uninvolved normal human brain. J Neurooncol. 2006;77:95–103. doi: 10.1007/s11060-005-9101-1. [DOI] [PubMed] [Google Scholar]
- 46.Qin D.-X., Zheng R., Tang J., Li J.-X., Hu Y.-H. Influence of Radiation on the Blood-Brain Barrier and Optimum Time of Chemotherapy. Radiat Oncol. 1990;19(6):1507–1510. doi: 10.1016/0360-3016(90)90364-p. [DOI] [PubMed] [Google Scholar]
- 47.Teng F., Tsien C.I., Lawrence T.S., Cao Y. Blood–tumor barrier opening changes in brain metastases from pre to one-month post radiation therapy. Radiother Oncol. 2017;125:89–93. doi: 10.1016/j.radonc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Fang L., Sun X., Song Y.u., Zhang Y., Li F., Xu Y., et al. Whole-brain radiation fails to boost intracerebral gefitinib concentration in patients with brain metastatic non-small cell lung cancer: A self-controlled, pilot study. Cancer Chemother Pharmacol. 2015;76(4):873–877. doi: 10.1007/s00280-015-2847-z. [DOI] [PubMed] [Google Scholar]
- 49.Jarden J.O., Dhawan V., Poltorak A., Posner J.B., Rottenberg D.A. Positron emission tomographic measurement of blood-to-brain and blood-to-tumor transport of 82Rb: The effect of dexamethasone and whole-brain radiation therapy. Ann Neurol. 1985;18:636–646. doi: 10.1002/ana.410180603. [DOI] [PubMed] [Google Scholar]
- 50.Okawa S., Shibayama T., Shimonishi A., Nishimura J., Ozeki T., Takada K., et al. Success of Crizotinib Combined with Whole-Brain Radiotherapy for Brain Metastases in a Patient with Anaplastic Lymphoma Kinase Rearrangement-Positive Non-Small-Cell Lung Cancer. Case Rep Oncol. 2018;11(3):777–783. doi: 10.1159/000492150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khatri A., Gaber M.W., Brundage R.C., Naimark M.D., Hanna S.K., Stewart C.F., et al. Effect of radiation on the penetration of irinotecan in rat cerebrospinal fluid. Cancer Chemother Pharmacol. 2011;68(3):721–731. doi: 10.1007/s00280-010-1542-3. [DOI] [PubMed] [Google Scholar]
- 52.Ernst-Stecken A., Jeske I., Hess A., Rödel F., Ganslandt O., Grabenbauer G., et al. Hypofractionated stereotactic radiotherapy to the rat hippocampus: Determination of dose response and toleranceHypofraktionierte stereotaktische Radiotherapie des Hippocampus der Ratte. Normalgewebstoleranz und Dosis-Wirkungs-Beziehung. Strahlentherapie Und Onkol. 2007;183(8):440–446. doi: 10.1007/s00066-007-1715-0. [DOI] [PubMed] [Google Scholar]
- 53.Zhou K., Boström M., Ek C.J., Li T., Xie C., Xu Y., et al. Radiation induces progenitor cell death, microglia activation, and blood-brain barrier damage in the juvenile rat cerebellum. Sci Rep. 2017;7(1) doi: 10.1038/srep46181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan X.-W., Chen F.u., Chen Y., Chen G.-H., Liu H.-H., Guan S.-K., et al. Electroacupuncture prevents cognitive impairments by regulating the early changes after brain irradiation in rats. PLoS One. 2015;10(4):e0122087. doi: 10.1371/journal.pone.0122087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng L., Ma L., Ren H., Zhao H., Pang Y., Wang Y., et al. Alterations in the expression of vascular endothelial growth factor in the rat brain following gamma knife surgery. Mol Med Rep. 2014;10:2263–2270. doi: 10.3892/mmr.2014.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin X., Liang B., Chen Z., Liu X., Zhang Z. The dynamic changes of capillary permeability and upregulation of VEGF in rats following radiation-induced brain injury. Microcirculation. 2014;21:171–177. doi: 10.1111/micc.12103. [DOI] [PubMed] [Google Scholar]
- 57.Guan L., Xia B., Qi X.-X., Xu K., Li Z., Zhao Y. Early changes measured by CT perfusion imaging in tumor microcirculation following radiosurgery in rat C6 brain gliomas. J Neurosurg. 2011;114:1672–1680. doi: 10.3171/2011.1.jns101513. [DOI] [PubMed] [Google Scholar]
- 58.Zhou H., Liu Z., Liu J., Wang J., Zhou D., Zhao Z., et al. Fractionated radiation-induced acute encephalopathy in a young rat model: Cognitive dysfunction and histologic findings. Am J Neuroradiol. 2011;32(10):1795–1800. doi: 10.3174/ajnr.A2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J.-L., Tian D.-S., Li Z.-W., Qu W.-S., Zhan Y., Xie M.-J., et al. Tamoxifen alleviates irradiation-induced brain injury by attenuating microglial inflammatory response in vitro and in vivo. Brain Res. 2010;1316:101–111. doi: 10.1016/j.brainres.2009.12.055. [DOI] [PubMed] [Google Scholar]
- 60.Kaya M., Palanduz A., Kalayci R., Kemikler G., Simsek G., Bilgic B., et al. Effects of lipopolysaccharide on the radiation-induced changes in the blood-brain barrier and the astrocytes. Brain Res. 2004;1019(1-2):105–112. doi: 10.1016/j.brainres.2004.05.102. [DOI] [PubMed] [Google Scholar]
- 61.Yuan H., Gaber M.W., McColgan T., Naimark M.D., Kiani M.F., Merchant T.E. Radiation-induced permeability and leukocyte adhesion in the rat blood-brain barrier: Modulation with anti-ICAM-1 antibodies. Brain Res. 2003;969:59–69. doi: 10.1016/S0006-8993(03)02278-9. [DOI] [PubMed] [Google Scholar]
- 62.Krueck W.G., Schmiedl U.P., Maravilla K.R., Spence A.M., Starr F.L., Kenney J. MR assessment of radiation-induced blood-brain barrier permeability changes in a rat glioma model. Am J Neuroradiol. 1994;15:625–632. [PMC free article] [PubMed] [Google Scholar]
- 63.Mima T., Ogawa Y., Taniguchi T., Toyonaga S., Mori K. Early decrease of P-glycoprotein in the endothelium of the rat brain capillaries after moderate dose of irradiation. Neurol Res. 1999;21:209–215. doi: 10.1080/01616412.1999.11740920. [DOI] [PubMed] [Google Scholar]
- 64.Rubin P., Gash D.M., Hansen J.T., Nelson D.F., Williams J.P. Disruption of the blood-brain barrier as the primary effect of CNS irradiation. Radiother Oncol. 1994;31:51–60. doi: 10.1016/0167-8140(94)90413-8. [DOI] [PubMed] [Google Scholar]
- 65.D’Avella D, Cicciarello R, Albiero F, Mesiti M, Gagliardi ME, Russi E, et al. Quantitative Study of Blood-Brain Barrier Permeability Changes after Experimental Whole- Brain Radiation Experimental 1992;30:1992–8. [DOI] [PubMed]
- 66.Spence A.M., Graham M.M., O'Gorman L.A., Muzi M., Abbott G.L., Lewellen T.K. Regional Blood-to-Tissue Transport in an Irradiated Rat Glioma Model. Radiat Res. 1987;111(2):225. [PubMed] [Google Scholar]
- 67.Bulat M., Supek Z., Deanović Ž. Effect of x-irradiation on the permeability of the blood-brain barrier for 5-hydroxytryptamine in normal and adrenalectomized rats. Int J Radiat Biol. 1966;11:307–310. doi: 10.1080/09553006614551141. [DOI] [PubMed] [Google Scholar]
- 68.Bezek Š., Trnovec T., Ščasnár V., Ďurišová M., Kukan M., Kállay Z., et al. Irradiation of the head by60Co opens the blood-brain barrier for drugs in rats. Experientia. 1990;46(10):1017–1020. doi: 10.1007/BF01940660. [DOI] [PubMed] [Google Scholar]
- 69.Jost G., Frenzel T., Boyken J., Pietsch H. Impact of brain tumors and radiotherapy on the presence of gadolinium in the brain after repeated administration of gadolinium-based contrast agents: an experimental study in rats. Neuroradiology. 2019;61:1273–1280. doi: 10.1007/s00234-019-02256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Delattre J.Y., Shapiro W.R., Posner J.B. Acute effects of low-dose cranial irradiation on regional capillary permeability in experimental brain tumors. J Neurol Sci. 1989;90:147–153. doi: 10.1016/0022-510x(89)90097-x. [DOI] [PubMed] [Google Scholar]
- 71.Karger C.P., Hartmann G.H., Peschke P., Debus J., Hoffmann U., Brix G., et al. Dose-response relationship for late functional changes in the rat brain after radiosurgery evaluated by magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 1997;39(5):1163–1172. doi: 10.1016/s0360-3016(97)00387-8. [DOI] [PubMed] [Google Scholar]
- 72.Kamiryo T., Kassell N.F., Thai Q.A., Lopes M.B.S., Lee K.S., Steiner L. Histological changes in the normal rat brain after gamma irradiation. Acta Neurochir (Wien) 1996;138:451–459. doi: 10.1007/BF01420308. [DOI] [PubMed] [Google Scholar]
- 73.Nakata H., Yoshimine T., Murasawa A., Kumura E., Harada K., Ushio Y., et al. Early blood-brain barrier disruption after high-dose single-fraction irradiation in rats. Acta Neurochir (Wien) 1995;136(1-2):82–87. doi: 10.1007/BF01411440. [DOI] [PubMed] [Google Scholar]
- 74.Omary R.A., Berr S.S., Kamiryo T., Lanzino G., Kassell N.F., Lee K.S., et al. Gamma knife irradition-Induced changes in the normal rat brain studied with 1H magnetic resonance spectroscopy and imaging. Acad Radiol. 1995;2(12):1043–1051. doi: 10.1016/s1076-6332(05)80511-2. [DOI] [PubMed] [Google Scholar]
- 75.Levin V.A., Edwards M.S., Byrd A. Quantitative Observations of the Acute Effects of X-Irradiation on Brain Capillary Permeability: Part 1. Radiat Oncol. 1979;5:1627–1631. doi: 10.1016/0360-3016(79)90786-7. [DOI] [PubMed] [Google Scholar]
- 76.Olsson Y., Klatzo I., Carsten A. The effect of acute radiation injury on the permeability and ultrastructure of intracerebral capillaries. Neuropathol Appl Neurobiol. 1975;1(1):59–68. [Google Scholar]
- 77.Constanzo J., Masson-Côté L., Tremblay L., Fouquet J.P., Sarret P., Geha S., et al. Understanding the continuum of radionecrosis and vascular disorders in the brain following gamma knife irradiation: An MRI study. Magn Reson Med. 2017;78(4):1420–1431. doi: 10.1002/mrm.26546. [DOI] [PubMed] [Google Scholar]
- 78.Lampron A., Lessard M., Rivest S. Effects of Myeloablation, Peripheral Chimerism, and Whole-Body Irradiation on the Entry of Bone Marrow-Derived Cells into the Brain. Cell Transplant. 2012;21:1149–1159. doi: 10.3727/096368911X593154. [DOI] [PubMed] [Google Scholar]
- 79.Wilson C.M., Gaber M.W., Sabek O.M., Zawaski J.A., Merchant T.E. Radiation-Induced Astrogliosis and Blood-Brain Barrier Damage Can Be Abrogated Using Anti-TNF Treatment. Int J Radiat Oncol Biol Phys. 2009;74:934–941. doi: 10.1016/j.ijrobp.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 80.Nair V., Roth L.J. Effect of X-Irradiation and Certain Other Treatments on Blood Brain Barrier Permeability. Radiat Res. 1964;23:249–264. doi: 10.2307/3571606. [DOI] [PubMed] [Google Scholar]
- 81.Yoshida Y., Sejimo Y., Kurachi M., Ishizaki Y., Nakano T., Takahashi A. X-ray irradiation induces disruption of the blood–brain barrier with localized changes in claudin-5 and activation of microglia in the mouse brain. Neurochem Int. 2018;119:199–206. doi: 10.1016/j.neuint.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 82.Kalm M., Boström M., Sandelius Å., Eriksson Y., Ek C.J., Blennow K., et al. Serum concentrations of the axonal injury marker neurofilament light protein are not influenced by blood-brain barrier permeability. Brain Res. 2017;1668:12–19. doi: 10.1016/j.brainres.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 83.Murrell D.H., Wong E., Jensen M.D., Zarghami N., Foster P.J., Chambers A.F. Evaluating Changes to Blood-Brain Barrier Integrity in Brain Metastasis over Time and after Radiation Treatment. Transl Oncol. 2016;9:219–227. doi: 10.1016/j.tranon.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ngen E.J., Wang L., Gandhi N., Kato Y., Armour M., Zhu W., et al. A preclinical murine model for the early detection of radiation-induced brain injury using magnetic resonance imaging and behavioral tests for learning and memory: with applications for the evaluation of possible stem cell imaging agents and therapies. J Neurooncol. 2016;128(2):225–233. doi: 10.1007/s11060-016-2111-3. [DOI] [PubMed] [Google Scholar]
- 85.Tamborini M., Locatelli E., Rasile M., Monaco I., Rodighiero S., Corradini I., et al. A Combined Approach Employing Chlorotoxin-Nanovectors and Low Dose Radiation to Reach Infiltrating Tumor Niches in Glioblastoma. ACS Nano. 2016;10(2):2509–2520. doi: 10.1021/acsnano.5b07375. [DOI] [PubMed] [Google Scholar]
- 86.Tong F., Zhang J., Liu L.i., Gao X., Cai Q., Wei C., et al. Corilagin Attenuates Radiation-Induced Brain Injury in Mice. Mol Neurobiol. 2016;53(10):6982–6996. doi: 10.1007/s12035-015-9591-6. [DOI] [PubMed] [Google Scholar]
- 87.Zhang J., Tong F., Cai Q., Chen L.-J., Dong J.-H., Wu G., et al. Shenqi Fuzheng Injection attenuates irradiation-induced brain injury in mice via inhibition of the NF-κB signaling pathway and microglial activation. Acta Pharmacol Sin. 2015;36(11):1288–1299. doi: 10.1038/aps.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lo EH, Frankel KA, Steinberg GK, Delapaz RL, Fabrikant JI. High-dose Single-Fraction Brain Irradiation: MRI, Cerebral Blood Flow, Electrophysiological, and Histological Studies 1992;22:47–55. [DOI] [PubMed]
- 89.Lo E.H., Delapaz R.L., Frankel K.A., Poljak A., Phillips M.H., Brennan K.M., et al. MRI and PET of Delayed Heavy-Ion Radiation Injury in the Rabbit Brain. Radiat Oncol. 1991;20:689–696. doi: 10.1016/0360-3016(91)90010-2. [DOI] [PubMed] [Google Scholar]
- 90.Blomstrand C. Dexamethasone effect on blood-brain barrier damage caused by acute hypertension in x-irradiated rabbits 1975:331–4. [DOI] [PubMed]
- 91.Blomstrand C., Johansson B., Rosengren B. Blood-brain barrier lesions in acute hypertension in rabbits after unilateral X-ray exposure of brain. Acta Neuropathol. 1975;31:97–102. doi: 10.1007/BF00688143. [DOI] [PubMed] [Google Scholar]
- 92.Kourtópouios H., Holm S.E., Norrby S.R. The effects or irradiation and probenecid on cerebrospinal fluid transport of penicillin. J Antimicrob Chemother. 1983;11:251–255. doi: 10.1093/jac/11.3.251. [DOI] [PubMed] [Google Scholar]
- 93.Fike J.R., Gobbel G.T., Mesiwala A.H., Shin H.J., Nakagawa M., Lamborn K.R., et al. Cerebrovascular effects of the bradykinin analog RMP-7 in normal and irradiated dog brain. J Neurooncol. 1998;37:199–215. doi: 10.1023/a:1005874206814. [DOI] [PubMed] [Google Scholar]
- 94.Gobbel G.T., Marton L.J., Lamborn K., Seilhan T.M., Fike J.R. Modification of Radiation-Induced Brain Injury by a-Difluoromethylornithine. Radiat Re. 1991;128:306–315. [PubMed] [Google Scholar]
- 95.Tanaka A., Ueno H., Yamashita Y., Caveness W.F. Regional Cerebral Blood Flow in Delayed Brain Swelling Following X-Irradiation of the Right Occipital Lobe in the Monkey. Brain Res. 1975;96(2):233–246. doi: 10.1016/0006-8993(75)90733-7. [DOI] [PubMed] [Google Scholar]
- 96.O'Neill R.R., Wakisaka S., Malamut B.L. Computer Assisted Tomography of Focal Cerebral Radiation Necrosis in the Monkey. J Neuropathol Exp Neurol. 1977;36(6):950–955. doi: 10.1097/00005072-197711000-00006. [DOI] [PubMed] [Google Scholar]
- 97.Olsson Y., Carsten A.L., Klatzo I. Effects of Gamma Radiation on the Shark Brain. Acta Neuropathol. 1972;21:1–10. doi: 10.1007/978-3-319-23162-4_2. [DOI] [PubMed] [Google Scholar]
- 98.Miot E., Hoffschir D., Pontvert D., Gaboriaud G., Alapetite C., Masse R., et al. Quantitative Magnetic Resonance and Isotopic Imaging: Early Evaluation of Radiation Injury to the Braini. Radiat Oncol. 1995;32(1):121–128. doi: 10.1016/0360-3016(94)00413-F. [DOI] [PubMed] [Google Scholar]
- 99.Takata F., Nakagawa S., Matsumoto J., Dohgu S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front Cell Neurosci. 2021;15 doi: 10.3389/fncel.2021.661838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Erickson M.A., Banks W.A. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2013;33:1500–1513. doi: 10.1038/jcbfm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sweeney M.D., Kisler K., Montagne A., Toga A.W., Zlokovic B.V. The role of brain vasculature in neurodegenerative disorders. Nat Neurosci. 2018;21:1318–1331. doi: 10.1038/s41593-018-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lipsman N., Meng Y., Bethune A.J., Huang Y., Lam B., Masellis M., et al. Blood-brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat Commun. 2018;9(1) doi: 10.1038/s41467-018-04529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ishida J., Alli S., Bondoc A., Golbourn B., Sabha N., Mikloska K., et al. MRI-guided focused ultrasound enhances drug delivery in experimental diffuse intrinsic pontine glioma. J Control Release. 2021;330:1034–1045. doi: 10.1016/j.jconrel.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 104.Englander Z.K., Wei H.-J., Pouliopoulos A.N., Bendau E., Upadhyayula P., Jan C.-I., et al. Focused ultrasound mediated blood-brain barrier opening is safe and feasible in a murine pontine glioma model. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-85180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kirsch D.G., Tarbell N.J. New technologies in radiation therapy for pediatric brain tumors: the rationale for proton radiation therapy. Pediatr Blood Cancer. 2004;42:461–464. doi: 10.1002/pbc.10471. [DOI] [PubMed] [Google Scholar]
- 106.Walker A.J., Ruzevick J., Malayeri A.A., Rigamonti D., Lim M., Redmond K.J., et al. Postradiation imaging changes in the CNS: how can we differentiate between treatment effect and disease progression? Future Oncol. 2014;10(7):1277–1297. doi: 10.2217/fon.13.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sarkaria J.N., Hu L.S., Parney I.F., Pafundi D.H., Brinkmann D.H., Laack N.N., et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018;20(2):184–191. doi: 10.1093/neuonc/nox175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arvanitis C.D., Ferraro G.B., Jain R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20:26–41. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wagner C.C., Bauer M., Karch R., Feurstein T., Kopp S., Chiba P., et al. A pilot study to assess the efficacy of tariquidar to inhibit P-glycoprotein at the human blood-brain barrier with (R)-11C-verapamil and PET. J Nucl Med. 2009;50(12):1954–1961. doi: 10.2967/jnumed.109.063289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kostaras X., Cusano F., Kline G.A., Roa W., Easaw J. Use of dexamethasone in patients with high-grade glioma: a clinical practice guideline. Curr Oncol. 2014;21:e493–e503. doi: 10.3747/co.21.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hue C.D., Cho F.S., Cao S., Dale Bass C.R., Meaney D.F., Morrison B., 3rd. Dexamethasone potentiates in vitro blood-brain barrier recovery after primary blast injury by glucocorticoid receptor-mediated upregulation of ZO-1 tight junction protein. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2015;35:1191–1198. doi: 10.1038/jcbfm.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nitta T., Hata M., Gotoh S., Seo Y., Sasaki H., Hashimoto N., et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161(3):653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Johansson P.A., Dziegielewska K.M., Ek C.J., Habgood M.D., Liddelow S.A., Potter A.M., et al. Blood-CSF barrier function in the rat embryo. Eur J Neurosci. 2006;24(1):65–76. doi: 10.1111/j.1460-9568.2006.04904.x. [DOI] [PubMed] [Google Scholar]
- 114.Møllgård K., Dziegielewska K.M., Holst C.B., Habgood M.D., Saunders N.R. Brain barriers and functional interfaces with sequential appearance of ABC efflux transporters during human development. Sci Rep. 2017;7:11603. doi: 10.1038/s41598-017-11596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saunders N.R., Liddelow S.A., Dziegielewska K.M. Barrier mechanisms in the developing brain. Front Pharmacol. 2012;3:46. doi: 10.3389/fphar.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang S., Gu C., Mandeville E.T., Dong Y., Esposito E., Zhang Y., et al. Anesthesia and surgery impair blood–brain barrier and cognitive function in mice. Front Immunol. 2017;8 doi: 10.3389/fimmu.2017.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Casals J.B., Pieri N.C., Feitosa M.L., Ercolin A.C., Roballo K.C., Barreto R.S., et al. The Use of Animal Models for Stroke Research: A Review. Comp Med. 2011;61:305–313. doi: 10.1109/TAES.1968.5408692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wood L., Egger M., Gluud L.L., Schulz K.F., Jüni P., Altman D.G., et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336(7644):601–605. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ghosh K.K., Padmanabhan P., Yang C.-T., Ng D.C.E., Palanivel M., Mishra S., et al. Positron emission tomographic imaging in drug discovery. Drug Discov Today. 2022;27(1):280–291. doi: 10.1016/j.drudis.2021.07.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.