Abstract
Sodium-glucose cotransporter 2 (SGLT2) inhibitors, dapagliflozin, and empagliflozin, first developed as glucose-lowering agents for the treatment of Type 2 diabetes, have been demonstrated to improve prognosis in patients with heart failure and reduced ejection fraction (HFrEF) regardless of the presence of diabetes. Since these drugs have only recently been included among the four pillars of HFrEF treatment, cardiologists are still unfamiliar with their use in this setting. This article provides an up-to-date practical guide for the initiation and monitoring of patients treated with SGLT2 inhibitors.
Keywords: Heart failure, SGLT2 inhibitors, Pharmacotherapy
Introduction
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a novel class of therapeutic agents known as glifozines that act by inhibiting SGLT2 located in the proximal convoluted tubule of the nephron. Initially developed as glucose-lowering drugs for the treatment of patients with Type 2 diabetes mellitus (T2DM), clinical trials have shown dapagliflozin and empagliflozin to reduce the risk of death from cardiovascular events and the rate of hospitalizations due to heart failure (HF), even in the absence of diabetes.1,2 One of the established mechanisms underlying clinical outcome improvement in patients with HF and reduced ejection fraction (HFrEF) is osmotic diuresis, with a consequent improvement in haemodynamics and neurohormonal activation. However, weight reduction, improved glycaemic control, and other incompletely defined mechanisms are postulated to contribute to the cardioprotective effects of these drugs.3
Based on the significantly favourable effects on HF outcomes, national and international cardiology societies have recently updated recommendations for HF management and have included these two gliflozins among the therapies recommended for patients with HFrEF to reduce cardiovascular death and HF hospitalization risk [class of recommendation I, level of evidence A in European Society of Cardiology (ESC) guidelines].4–6 According to ESC guidelines on HFrEF management, the use of dapagliflozin or empagliflozin is recommended in combination with optimal medical therapy (OMT) with angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin receptor antagonists (AR) or sacubitril + valsartan (ARNI) and β blockers and mineralocorticoid receptor antagonists.
Since delayed OMT initiation has been demonstrated to be ‘associated with never initiating’,5 and time-to-treatment is an established modifiable risk factor of a worse prognosis,7 implementation of this treatment must be prioritized in clinical practice. However, due to the recent market approval of SGLT2 inhibitors for HFrEF management, physicians may still be uncertain about their use in this specific setting in daily practice. The aim of this statement is to report practical indications for the use of SGLT2 inhibitors (in this article, SGLT2 inhibitors specifically refer to dapagliflozin and empagliflozin) in the growing therapeutic armamentarium for patients with HF, with a focus on precautions to take into account to benefit the most from these medications. A greater familiarity with SGLT2 inhibitors in HF management will allow broader and earlier use.
The use of SGLT2 inhibitors for heart failure management
On the basis of significant clinical benefits shown in large trials, international regulatory authorities have approved dapagliflozin and empagliflozin use in adults (≥18 years) for the treatment of symptomatic patients with chronic HF with reduced left ventricular ejection fraction (≤40%).8–11 For both drugs, pharmacokinetic drug interactions are limited, and the recommended dose is 10 mg once daily regardless of the presence of diabetes. Unlike other medications for HFrEF, dapagliflozin and empagliflozin do not need dosage titration. Clinical trials investigated these drugs as add-on treatment to established OMT, and therefore, their use is recommended together with renin-angiotensin-aldosterone system blockers (ACE-I or AR or ARNI and aldosterone antagonists) and β blockers.1,2 However, post hoc analyses have shown a consistent clinical benefit of dapagliflozin regardless of background HF treatment.12 A significant reduction in adverse outcomes was found even in patients treated with doses of renin–angiotensin blockers, β-blockers, and aldosterone antagonists <50% of guideline targets and in patients with and without cardiac resynchronization therapy.12 Similar dapagliflozin efficacy and safety were also observed between patients treated and untreated with ARNI.13 Overall, current evidence supports the combined use of triple neurohormonal blockade therapy, including ARNI, and SGLT2 inhibitors as a default strategy.14 In view of the fact that in the real-world dose-related side effects of HFrEF treatments often do not allow patients to reach guideline-recommended target doses, in clinical practice the achievement of target doses of OMT should not delay the initiation of SGLT2 inhibitor treatment in HFrEF.15
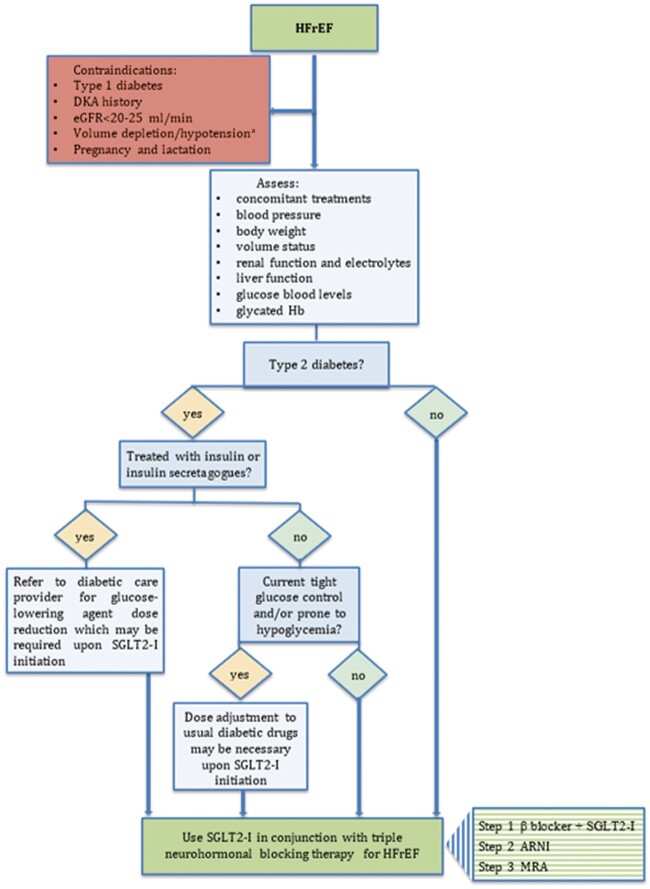
A recently proposed sequencing treatment strategy that maximizes the likelihood that disease-modifying therapies will be implemented within 4 weeks includes the first step with simultaneous initiation of a β-blocker and an SGLT2 inhibitor (Figure 1, box with horizontal green strips).16 According to this strategy, ARNI may be added after 1–2 weeks from the first step, and mineralocorticoid receptor antagonists after a further 1–2 weeks, subject to renal function and serum potassium assessment.16 Following the implementation of all drugs recommended for HFrEF treatment, physicians are advised to gradually up-titrate β-blockers and ACE-I/AR/ARNI and mineralocorticoid receptor antagonists to the maximally tolerated dose as recommended by guidelines.
Figure 1.
A flowchart for the use of SGLT2 inhibitors in HFrEF. The box with horizontal stripes reports a stepwise strategy proposal for early implementation of HFrEF treatments as suggested by McMurray et al.16 The transition from one step to the next should occur within 1–2 weeks, subject to renal function, and serum potassium assessment. aReconsider SGLT2 inhibitor treatment after adequate volume repletion. DKA, diabetic ketoacidosis; eGFR, estimated glomerular fraction rate; Hb, haemoglobin; HFrEF, heart failure with reduced ejection fraction; SGLT2-I, sodium-glucose cotransporter 2 inhibitors.
Since the presence of unstable haemodynamics may increase the risk of SGLT2 inhibitor adverse effects and since the main Phase III trials recruited outpatients with chronic HFrEF and data on the safety and efficacy in the acute phase of cardiovascular diseases are limited,17 the initiation of these agents is currently recommended in the outpatient setting in patients with stable conditions. Overall, all patients with HFrEF should be considered for SGLT2 inhibitor treatment, but patient comorbidity and the risk of potential adverse effects must be evaluated before drug prescription (Figure 1).
Heart failure and reduced ejection fraction management with SGLT2 inhibitor use should include educating patients by informing them and their caregivers of the bioparameters to monitor, potential adverse effects of these drugs, and problem-solving/self-care advice (Table 1).
Table 1.
Counselling for patients initiating/treated with SGLT2 inhibitors
|
|
|
|
|
|
|
Precautions before initiation and during treatment
Renal function must be assessed before starting SGLT2 inhibitor therapy and monitored periodically thereafter. A small dip in glomerular filtration rate (GFR) is common in the first weeks after SGLT2 inhibitor initiation (reduction in GFR of ∼3–5 mL/min over the first weeks), but this effect is transient and should not prompt drug withdrawal.18 Indeed, SGLT2 inhibitors have been demonstrated to lower the risk of renal function decline in the mid/long term and the risk of serious renal adverse events.2,19
The osmotic diuresis induced by SGLT2 inhibitors may lead to a reduction in intravascular volume. Sodium-glucose cotransporter 2 inhibitors are associated with a mild reduction in systolic and diastolic blood pressure (4–6 and 1–2 mmHg, respectively).20 This effect may be more pronounced in the elderly, in patients with severe hyperglycaemia, and in those treated with diuretics. The patient’s volume status must be assessed before initiating SGLT2 inhibitor treatment, and hypovolaemia must be corrected. It should be taken into account that volume depletion may also cause electrolyte imbalance. In some cases, the reduction in loop diuretic and other antihypertensive agent dose should be considered at the time of SGLT2 inhibitor initiation or during treatment. Patients should be instructed to monitor their blood pressure and body weight daily, especially during the first weeks of treatment. Patients should also be educated to recognize orthostatic hypotension symptoms.
Before initiation of SGLT2 inhibitors, the patient’s blood-glucose levels must be evaluated. When SGLT2 inhibitors are prescribed in patients with T2DM already on glucose-lowering treatments, current glycaemic control and potential risk factors for hypoglycaemia and diabetic ketoacidosis must be carefully assessed. Further indications on the management of diabetic patients treated with SGLT2 inhibitors are reported in the ‘Diabetes’ section.
Use in specific clinical settings
Renal impairment
Dapagliflozin and empagliflozin are approved for use in patients with HF and reduced GFR. Indeed, in patients with HF, clinical trials showed a consistent safety profile and persistent significant clinical benefit in patients with renal function impairment (estimated GFR < 60 mL/min/1.73 m2),1,2 and no dose adjustment is necessary. However, due to limited data, it is not recommended to start treatment with dapagliflozin in patients with an eGFR <25 mL/min or to start treatment with empagliflozin in patients with an eGFR <20 mL/min.4 In clinical practice, pre-initiation and periodic assessment of renal function are recommended, especially in patients with chronic kidney disease.
Elderly
No dose adjustment is needed in the elderly. However, an increased risk of volume depletion and/or hypotension should be considered, and more frequent renal function and electrolyte level assessment is advisable. Furthermore, the European Medicines Agency does not recommend empagliflozin use in patients aged ≥85 years due to insufficient data in this group of patients.8
Diabetes
Sodium-glucose cotransporter 2 inhibitor use is not recommended for the treatment of patients with Type 1 diabetes.5 Since the glucose-lowering mechanism of these drugs is glycaemia dependent, the risk of hypoglycaemia is low in patients with T2DM unless these agents are used concomitantly with insulin or insulin secretagogue agents (e.g. sulfonylureas). Patients treated with metformin, dipeptidyl peptidase-4 inhibitors, and glitazones with glucose levels above the glycaemic targets and with no history of hypoglycaemia do not require dose adjustment. However, the patient’s primary care physician must be informed of the SGLT2 inhibitor initiation and should follow-up with patients. Prompt reduction in the dose of sulfonylurea and insulin at the time of SGLT2 inhibitor initiation is usually required. Due to the complexity of the dose management of these latter glucose-lowering agents when used concomitantly with SGLT2 inhibitors, close collaboration with diabetologists is advisable to appropriately manage and monitor these patients. Though the risk of ketoacidosis is greater in Type 1 diabetes, the risk of this adverse event is also present in patients with T2DM. The section dedicated to diabetic ketoacidosis reports further details regarding the management of this complication.
Adverse effects
Although generally well tolerated, SGLT2 inhibitors may have a number of adverse effects that must be recognized and promptly managed in clinical practice. An increased risk of lower limb amputation was observed among patients treated with canagliflozin.21 This adverse event was reported in the CANVAS but not CREDENCE22 study and was not reported in other studies with empagliflozin or dapagliflozin.1,2 A recent real-world study found no differences in the risk of amputation in patients treated with SGLT2 inhibitors compared to those treated with other anti-diabetic drugs.23 Regular foot examination and referral to a vascular surgeon in case of uncertainty about the presence and severity of peripheral artery disease is advised.
Volume depletion and hypotension
Polyurea associated with SGLT2 inhibitors may lead to intravascular volume contraction and hypotension. The risk of these adverse effects is higher in elderly patients and in those treated with anti-hypertensive drugs or diuretics. Patient must be informed about the need to maintain adequate fluid intake. Of note, the magnitude of polyurea is mainly glycaemia-dependent and optimal glucose control may reduce polyurea. Blood pressure and body weight should be closely monitored, particularly in the first weeks after SGLT2 inhibitor initiation. Healthcare providers should be notified in case of an excessive reduction in body weight (>1 kg over 24 h or 2 kg in 1 week)20 or blood pressure lowering associated with symptoms like dizziness.
Hypoglycaemia
The risk of hypoglycaemia during SGLT2 inhibitor treatment is increased in diabetic patients with a history of hypoglycaemia or tight glycaemic control or in those treated with sulfonylureas or insulin. These patients should receive SGLT2 is under specialized diabetic care. In these patients, regular home blood glucose monitoring is recommended. A decrease in the latter antidiabetic agent dose is often necessary at the time of SGLT2 inhibitor initiation. Patients should be counselled about hypoglycaemia symptoms and how to manage this adverse effect.
Genital tract infection
Genital mycotic infections are the most common adverse effect reported in clinical trials, mostly vaginitis or balanitis. These infections are generally mild and occur during early treatment. If treatment is needed, topical antifungals are usually sufficient to resolve the infection, whereas oral fluconazole may be required to treat more severe cases. Proper counselling is paramount and should focus on daily genital hygienic measures to avoid urinary glucose remaining in contact with the skin or underwear. Some cases of Fournier’s gangrene, a necrotizing fasciitis of the perineum, have been reported in patients taking SGLT2 inhibitors. This is a rare but serious perineum infection that may be life-threatening and require urgent antibiotic treatment and surgical debridement.
Diabetic ketoacidosis
Patients with T2DM treated with SGLT2 inhibitors have an increased risk of ketoacidosis, which is usually associated with stressor events. Potential precipitating factors of ketoacidosis include dehydration, hyperglycaemia, limited food intake, alcohol abuse, and ketogenic dietary pattern. Therefore, diabetic subjects with a very thin build should be considered with caution. These conditions require blood glucose and ketone level monitoring. Of note, diabetic ketoacidosis can occur even if glucose levels are normal.20 Therefore, if symptoms, such as nausea, vomiting, abdominal pain, excessive thirst, difficulty breathing, confusion, or unusual fatigue occur, patients should be assessed for ketoacidosis by measuring gap-anions and blood ketone levels, regardless of blood glucose level. Treatment with SGLT2 inhibitors must be withheld immediately if diabetic ketoacidosis is suspected or diagnosed. SGLT2 inhibitors should also be discontinued in case of hospitalization due to major surgery or acute serious medical illnesses, such as severe infections (so-called sick-day rule).
Conclusions
Over recent years, the continuous expansion of evidence-based treatments for HFrEF has made patient management more complex. Sodium-glucose cotransporter 2 inhibitors are the most recent additions to the therapeutic armamentarium for HFrEF. Although SGLT2 inhibitors are a generally well-tolerated drug class, the appropriate implementation of their use in HF requires an awareness of the parameters to be assessed before initiation and monitored during treatment as well as of possible adverse effects. This practical guide is aimed at informing cardiologists about the use of these novel drugs that have proven to be effective in improving HFrEF outcomes so that more patients can benefit from the early use of these drugs.
Conflict of interest: none declared.
Data availability
There are no new data associated with this article.
References
- 1. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang C-E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde A-M; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 2. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi D-J, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca H-P, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde M-F, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 3. Joshi SS, Singh T, Newby DE, Singh J.. Sodium-glucose co-transporter 2 inhibitor therapy: mechanisms of action in heart failure. Heart 2021;107:1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, de Boer RA, Christian Schulze P, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes‐Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen M‐L, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen J‐C, Neubeck L, Noutsias M, Petersen SE, Sonia Petronio A, Ponikowski P, Prescott E, Rakisheva A, Richter DJ, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa‐Uva M, Tocchetti CG, Touyz RM, Tschoepe C, Waltenberger J; Authors/Task Force Members, ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 5. Maddox TM, Januzzi JL, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld JAnn, Masoudi FA, Motiwala SR, Oliveros E, Patterson JH, Walsh MN, Wasserman A, Yancy CW, Youmans QR; Writing Committee. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2021;77:772–810. [DOI] [PubMed] [Google Scholar]
- 6. Gronda E, Napoli C, Iacoviello M, Urbinati S, Caldarola P, Mannucci E, Colivicchi F, Gabrielli D. ANMCO Position Paper: sodium-glucose co-transporter 2 inhibitors for the prevention of heart failure in diabetic patients and for the treatment of heart failure patients with and without diabetes. G Ital Cardiol (Rome) 2021;22:675–687. [DOI] [PubMed] [Google Scholar]
- 7. Abdin A, Anker SD, Butler J, Coats AJS, Kindermann I, Lainscak M, Lund LH, Metra M, Mullens W, Rosano G, Slawik J, Wintrich J, Böhm M.. ‘Time is prognosis’ in heart failure: time-to-treatment initiation as a modifiable risk factor. ESC Heart Fail 2021;8:4444–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jardiance Summary of Product Characteristics 2021 Jardiance, INN-Empagliflozin. europa.eu (21 February 2022).
- 9.JARDIANCE® (Empagliflozin Tablets), Prescribing Information.2021 Label. fda.gov (21 February 2022).
- 10.Forxiga Summary of Product Characteristics Forxiga, INN-Dapagliflozin. europa.eu (21 February 2022).
- 11.Forxiga Full Prescribing Information 2021 Label. fda.gov (21 February 2022).
- 12. Docherty KF, Jhund PS, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, DeMets DL, Sabatine MS, Bengtsson O, Sjöstrand M, Langkilde AM, Desai AS, Diez M, Howlett JG, Katova T, Ljungman CEA, O’Meara E, Petrie MC, Schou M, Verma S, Vinh PN, Solomon SD, McMurray JJV.. Effects of dapagliflozin in DAPA-HF according to background heart failure therapy. Eur Heart J 2020;41:2379–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Solomon SD, Jhund PS, Claggett BL, Dewan P, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Inzucchi SE, Desai AS, Bengtsson O, Lindholm D, Sjostrand M, Langkilde AM, McMurray JJV.. Effect of dapagliflozin in patients with HFrEF treated with sacubitril/valsartan: the DAPA-HF trial. JACC Heart Fail 2020;8:811–818. [DOI] [PubMed] [Google Scholar]
- 14. Bauersachs J. Heart failure drug treatment: the fantastic four. Eur Heart J 2021;42:681–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Warden BA, Steiner J, Camacho A, Nguyen K, Purnell JQ, Barton Duell P, Craigan C, Osborn D, Fazio S.. Optimizing sodium-glucose co-transporter 2 inhibitor use in patients with heart failure with reduced ejection fraction: a collaborative clinical practice statement. Am J Prev Cardiol 2021;6:100183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McMurray JJV, Packer M.. How should we sequence the treatments for heart failure and a reduced ejection fraction? A redefinition of evidence-based medicine. Circulation 2021;143:875–877. [DOI] [PubMed] [Google Scholar]
- 17. Koufakis T, Mustafa OG, Ajjan RA, Garcia-Moll X, Zebekakis P, Dimitriadis G, Kotsa K.. The use of sodium-glucose co-transporter 2 inhibitors in the inpatient setting: is the risk worth taking? J Clin Pharm Ther 2020;45:883–891. [DOI] [PubMed] [Google Scholar]
- 18. Kraus BJ, Weir MR, Bakris GL, Mattheus M, Cherney DZI, Sattar N, Heerspink HJL, Ritter I, von Eynatten M, Zinman B, Inzucchi SE, Wanner C, Koitka-Weber A.. Characterization and implications of the initial estimated glomerular filtration rate ‘dip’ upon sodium-glucose cotransporter-2 inhibition with empagliflozin in the EMPA-REG OUTCOME trial. Kidney Int 2021;99:750–762. [DOI] [PubMed] [Google Scholar]
- 19. Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde A-M, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446. [DOI] [PubMed] [Google Scholar]
- 20. Vardeny O, Vaduganathan M.. Practical guide to prescribing sodium-glucose cotransporter 2 inhibitors for cardiologists. JACC Heart Fail 2019;7:169–172. [DOI] [PubMed] [Google Scholar]
- 21. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 22. Perkovic V, , JardineMJ, , NealB, , BompointS, , HeerspinkHJ, , CharytanDM, , EdwardsR, , AgarwalR, , BakrisG, , BullS, , CannonCP, , CapuanoG, , ChuP-L, , De ZeeuwD, , GreeneT, , LevinA, , PollockC, , WheelerDC, , YavinY, , ZhangH, , ZinmanB, , MeiningerG, , BrennerBM, , Mahaffey KW.. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. New England Journal of Medicine 2019;380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 23. Paul SK, Bhatt DL, Montvida O.. The association of amputations and peripheral artery disease in patients with type 2 diabetes mellitus receiving sodium-glucose cotransporter type-2 inhibitors: real-world study. Eur Heart J 2021;42:1728–1738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no new data associated with this article.