Abstract
In epithelial tumors, oncoprotein E6 binds with the ubiquitin ligase E6AP to form E6/E6AP heterodimer; then this heterodimer recruits p53 to form E6/E6AP/p53 heterotrimer and induces p53 degradation. Recent experiments demonstrated that three E6 single-site mutants (F47R, R102A, and L50E) can inhibit the E6/E6AP/p53 heterotrimer formation and rescue p53 from the degradation pathway. However, the molecular mechanism underlying mutation-induced heterotrimer inhibition remains largely elusive. Herein, we performed extensive molecular dynamics simulations (totally ∼13 μs) on both heterodimer and heterotrimer to elucidate at an atomic level how each p53-degradation-defective HPV16 E6 mutant reduces the structural stabilities of the two complexes. Our simulations reveal that the three E6 mutations destabilize the structure of E6/E6AP/p53 complex through distinct mechanisms. Although F47RE6 mutation has no effect on the structure of E6/E6AP heterodimer, it results in an electrostatic repulsion between R47E6 and R290p53, which is unfavorable for E6-p53 binding. R102AE6 mutation destabilizes the structure of E6/E6AP heterodimer and significantly disrupts hydrophobic and cation-π interactions between F47E6 and E286p53/L298p53/R290p53. L50EE6 mutation impairs both E6 interdomain interactions (especially F47-K108 cation-π interaction) and E6-E6AP intermolecular interactions important for the stabilization of E6/E6AP heterodimer. This study identifies the intra- and intermolecular interactions crucial for the complex stability, which may provide mechanistic insights into the inhibition of complex formation by the three HPV16 E6 mutations.
Significance
The most studied function of high-risk HPV E6 is the E6-ubiquitin-mediated degradation of tumor suppressor p53 by forming E6/E6AP/p53 heterotrimer. Recent experiments demonstrated that three E6 single-site mutants (F47R, R102A, and L50E) can inhibit the E6/E6AP/p53 heterotrimer formation and rescue p53 from the degradation pathway. The underlying molecular mechanism is yet poorly understood. Using all-atom molecular dynamics simulations, we investigate the mechanism of the three HPV16 E6 mutants (F47R, R102A, and L50E) in the destabilization of E6/E6AP and E6/E6AP/p53 complex and identify the molecular interactions (E6 interdomain interactions, E6-E6AP and E6-p53 intermolecular interactions) crucial for the formation of heterodimer/heterotrimer. Our results may provide possible target sites for disrupting the HPV16 E6-mediated p53 degradation.
Introduction
Tumor suppressor p53, as a “guardian of the genome” (1), regulates a series of gene expression patterns involved in cell cycle arrest, damaged DNA repair, and cell apoptosis in response to the cell stress (2). According to the International Agency for Research on Cancer (IARC) TP53 Database, more than 50% of human cancers are linked to the inactivation of p53 (3). In the normal cells, the level of p53 is stable via a negative feedback loop with oncoprotein MDM2 that degrades p53 by the ubiquitin-mediated protein degradation pathway (4). In response to cell stress, the MDM2-ubiquitin-mediated degradation of p53 is inhibited, and then the level of p53 increases in normal cells (5). However, the level of p53 in cervical carcinoma cells infected by high-risk human papillomaviruses (HPVs) is lower than that in normal cells because the product oncoprotein E6 of high-risk HPVs can degrade p53 via the ubiquitin-mediated degradation and decrease the level of p53 (6). More specifically, E6 is a viral oncoprotein of high-risk HPV. HPVs are double-stranded DNA viruses and have over 180 subtypes. Except for high-risk HPVs, low-risk HPVs also exist (7,8). High-risk HPVs cause more than 90% of cervical carcinomas and 20% of head-neck squamous carcinomas (9,10), whereas low-risk HPVs are associated with benign cellular proliferations (11). HPV-16 and -18 are the most prevalent high-risk HPVs and they cause over 50% and 20% of cervical carcinomas respectively (12,13).
Earlier experimental studies reported the high-risk HPV E6-mediated p53 degradation pathway: E6 firstly binds to the acidic leucine-rich LxxLL motif of E6-association protein (E6AP) to form E6/E6AP heterodimer (14); subsequently, E6/E6AP heterodimer recruits p53 to form E6/E6AP/p53 heterotrimer, and then p53 is degraded through the ubiquitin-mediated protein degradation pathway (15). E6AP is an E3 ubiquitin ligase and is unable to degrade p53 when E6 is absent (16). E6 also targets other cellular proteins (17), such as interferon regulatory factor-3 (18), co-activators p300 and CREB binding protein (19), the human homolog of the Drosophila discs-large tumor suppressor protein (20), and the membrane-associated guanylate kinases (21,22). Therefore, oncoprotein E6 is a critical factor responsible for the cellular transformation and tumorigenesis (23).
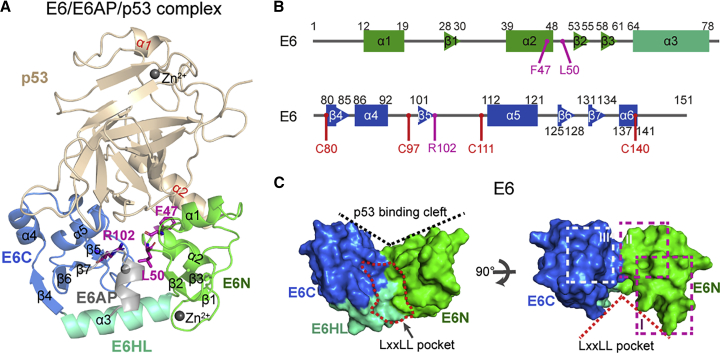
The x-ray crystal structure of E6/E6AP/p53 heterotrimer (PDB: 4XR8) (Fig. 1 A) encoded by high-risk HPV-16 was resolved in 2016 (24) by Travé, Zanier, and their co-workers. This complex consists of full-length E6 (residues 1–151), the LxxLL motif (372ELTLQELLGEER383) of E6AP, and p53 core domain (p53C, residues 94–292). The full-length E6 contains two zinc-binding domains E6N (residues 1–61) and E6C (residues 79–151) that are linked by E6 helix linker (abbreviated E6HL, residues 62–78) (Figs. 1 A and B). In the crystal structure, the four cysteines (4C: C80, C97, C111, and C140) of E6 are mutated to four serines (4S) to prevent disulfide-mediated aggregation (Fig. 2 B) (24). It was reported that the efficiency of E6 4C/4S mutant to degrade p53 is almost equal to wild-type (WT) E6 (24). The LxxLL motif of E6AP is embedded in the hydrophobic cavity (also called LxxLL pocket) that is formed by E6N, E6C, and E6HL (Figs. 1 A and C). Fig. S1 A shows the dominant interactions between the LxxLL motif of E6AP and E6 (25). p53C binds to the E6 cleft that is formed by E6N and E6C (Figs. 1 A and C). This cleft is called the p53 binding cleft. The E6-p53C interface is divided into three subinterfaces (right panel of Fig. 1 C), and the E6-p53C interactions at the three interfaces are shown in Fig. S1 B (24).
Figure 1.
E6/E6AP/p53 heterotrimer in the x-ray crystal structure (PDB: 4XR8). (A) The cartoon representation of E6/E6AP/p53 heterotrimer. E6N and E6C: N- and C-terminal zinc-binding domains; E6HL: E6 helix linker. (B) The sequence of E6, where four C-to-S mutation sites and three p53-degradation-defective mutation sites (F47, L50, and R102) are labeled. (C) The surface representation of E6 with different views. E6 in the left panel of (C) is shown in the same view as that in (A). In (A)–(C), wheat: p53; green: E6N; cyan: E6HL; blue: E6C; gray: LxxLL motif of E6AP. To see figure in color, go online.
Figure 2.
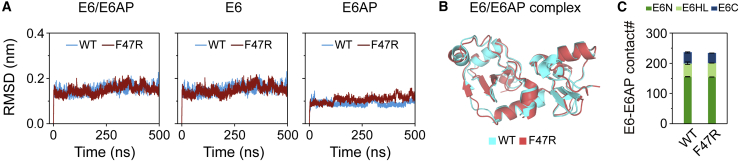
Analysis of structural stability of E6/E6AP and E6F47R/E6AP heterodimers. (A) The time evolution of backbone RMSD values of E6/E6AP, E6, and E6AP relative to their initial conformations in WT and F47R mutant heterodimers. The RMSD values are averaged over three individual MD simulations. (B) The final structure of E6F47R/E6AP heterodimer (pink) superposed with the final structure of WT E6/E6AP heterodimer (cyan). (C) Number of native contacts between E6 and E6AP in WT and F47R mutant heterodimers, residues 1–8 of E6 and 381–383 of E6AP. This result demonstrates that F47RE6 mutation has no effect on the structure of E6/E6AP heterodimer. To see figure in color, go online.
Neither E6 nor E6AP is separately able to recruit p53 (14,26), revealing that the formation of E6/E6AP heterodimer is a prerequisite for p53 binding. Formation of E6/E6AP/p53 heterotrimer is required for high-risk HPV-mediated degradation of p53 (24). Therefore, the inhibition of formation of E6/E6AP/p53 complex is a potential strategy to impair the p53 degradation activity of E6. The LxxLL pocket and the p53 binding cleft of E6 are used as ideal targets for the screening of some peptides and small molecules (27, 28, 29, 30, 31). In addition, some E6 mutations at the p53 binding cleft of E6 (F47R) or the LxxLL pocket of E6 (R102A and L50E) exhibit the ability to impede the formation of E6/E6AP/p53 heterotrimer and to destroy the p53 degradation activity of E6 (24,25,32,33). F47RE6 mutation does not impair the binding of E6 with E6AP (33), and the x-ray crystal structure of E6F47R in complex with the LxxLL motif of E6AP has been resolved (25). This mutation hinders the binding between E6F47R/E6AP heterodimer and p53, blocks the formation of E6/E6AP/p53 heterotrimer, and then rescues p53 from degradation pathway (24,32, 33, 34). R102AE6 mutation located in the LxxLL pocket significantly reduces the binding affinity of E6 with E6AP and almost completely abolishes p53 degradation activity of E6 (25). Unlike F47RE6 and R102AE6 mutations, L50EE6 mutation completely blocks the binding of E6AP-E6 and abolishes p53 degradation activity of E6 (25). Those experimental results demonstrate that the three single-site E6 mutations (F47R, R102A, and L50E) display different inhibitory effects on the formation of E6/E6AP heterodimer, but suggest that they all abolish the formation of E6/E6AP/p53 heterotrimer. In spite of extensive experimental studies and the emergence of computational studies on conformational dynamics of E6/E6AP heterodimer (29), the atomic-level mechanisms underlying the different inhibitory effects of the three E6 mutations on the bindings of E6-E6AP and E6/E6AP-p53 are poorly understood.
Molecular dynamics (MD) simulation is an effective technique in simulating biomolecule systems (35, 36, 37), and it has been widely used to investigate the conformational dynamics of p53C (38, 39, 40, 41) and p53 isoforms (42), p53–DNA interaction (43), p53–MDM2 interaction (44,45), p53 peptide aggregation (46,47), and small molecule-p53C interaction (48). In this study, we utilized MD simulations to explore at the atomic level how the three individual mutations (F47R, R102A, and L50E) of E6 impede the stability of E6/E6AP/p53 complex. We performed three independent 500-ns MD simulations for each of the four heterodimers (WT E6/E6AP and the three E6/E6AP mutants) and three independent 800-ns MD simulations for each of the three heterotrimers (WT E6/E6AP/p53, E6F47R/E6AP/p53, and E6R102A/E6AP/p53). Here, E6AP refers to the LxxLL motif of E6AP. Our multiple MD simulations for the first time to our knowledge show how the three E6 mutations reduce the structural stabilities of the two complexes through different atomic-level mechanisms. This study may provide insights into the physical interactions crucial for the binding of E6-E6AP and E6-p53.
Materials and Methods
Four E6/E6AP heterodimer systems: WT E6/E6AP, E6F47R/E6AP, E6R102A/E6AP, and E6L50E/E6AP
The heterodimers of E6AP with WT E6 and three single-site E6 mutants (F47R, R102A, or L50E) are studied, and they are labeled as WT E6/E6AP, E6F47R/E6AP, E6R102A/E6AP, and E6L50E/E6AP heterodimers. The initial coordinate of WT E6/E6AP heterodimer is taken from the x-ray crystal structure (PDB: 4XR8 (24), the LxxLL motif of E6AP (chain A): residues 372–383, E6 (chain F): residues 1–143). The three heterodimers with E6 mutants are generated by mutating the residues at the corresponding sites of WT E6 in E6/E6AP heterodimer using Pymol (49).
Three E6/E6AP/p53 heterotrimer systems: WT E6/E6AP/p53, E6F47R/E6AP/p53, and E6R102A/E6AP/p53
Three heterodimer systems are studied, including WT E6/E6AP/p53, E6F47R/E6AP/p53, and E6R102A/E6AP/p53 heterotrimers. The initial coordinate of WT E6/E6AP/p53 heterotrimer is taken from the x-ray crystal structure (PDB: 4XR8 (24), the LxxLL motif of E6AP (chain A): residues 372–383, p53C (chain C): residues 94–292, E6 (chain F): residues 1–143). E6F47R/E6AP/p53 and E6R102A/E6AP/p53 heterotrimers are obtained using the same strategy as that for the heterodimers with E6 mutants.
To mimic the neutral experimental pH condition (24) (∼pH 7.0), in both heterodimers and heterotrimers, the side chains of Lys and Arg are protonated (Arg+ and Lys+), and the side chains of Glu and Asp are deprotonated (Glu− and Asp−). We predicted the residue pKa values of WT heterotrimer using Propka web server. The predicted result shows that all pKa values of His resides are less than pH 7.0, indicating that the total net charge of His residues is zero (i.e., in HID or HIE state). As ND atom of His179p53 is coordinated to Zn2+, this His179p53 was modeled as the HIE tautomer. For simplicity, we modeled all His residues as the HIE tautomer. Using this His tautomeric state, our simulations show that both WT dimer and trimer remain stable during the full period of three replicate MD runs, in good agreement with experimental results (25). The agreement between our simulations and the experiments established the suitability of the HIE tautomeric state of HIS modeling in our simulations, so we modeled all His residues as HIE tautomer for all three mutants.
Simulation details
We carried out three independent 500-ns MD simulations for each E6/E6AP heterodimer system and three independent 800-ns MD simulations for each E6/E6AP/p53 heterotrimer system at 310 K using GROMACS 5.1.4 software package (50) in combination with CHARMM36m force field (51). Each system is placed in a cubic box filled with TIP3P water, with a minimum distance of 1.2 nm between protein atoms and the box edges. Counterions (Cl− ions) were added to neutralize the charge of each system. As done in our recent study (41), the distances of Zn2+ ion with the four coordinated atoms are restrained using the piecewise harmonic/linear potential energy, where a flat bottom potential is used when the distances between Zn2+ ion and four coordinated atoms are within a reference distance of 0.2 nm. The force constants are adopted from the work by Luo et al. (52), and the partial charges of atoms in the four coordinated residues are taken from CHARMM36m force field (51). In each system, solvent molecules were energy-minimized by steepest descent method for 10,000 steps with position restraints on the proteins. The solvent minimization was followed by another energy-minimization for 20,000 steps without position restraints on the proteins. Subsequently, each system was equilibrated under a canonical ensemble (T = 310 K) and followed by an isothermal-isobaric ensemble (T = 310 K, p = 1 bar), both for 0.1 ns. During canonical ensemble and isothermal-isobaric ensemble equilibrations, all-bonds of proteins were constrained using LINCS method, and no restraints were applied for protein heavy atoms. After that, 500-ns and 800-ns production MD simulations were performed under an isothermal-isobaric ensemble. The pressure was kept at 1 bar using Parrinello-Rahman method (53). The temperature was maintained at 310 K by separately coupling the protein and nonprotein groups to an external heat bath with velocity rescaling method (54). Electrostatic interactions were calculated using the particle mesh Ewald method with a real space cutoff of 1.4 nm (55). The van der Waals interactions were treated using a cutoff of 1.4 nm.
Results and discussion
F47RE6 mutation leads to an electrostatic repulsion between R47E6 and R290p53, which disfavors the residue-residue interactions crucial for the stabilization of E6-p53 subinterface II and III.
The x-ray crystal structure of E6F47R/E6AP heterodimer (PDB: 4DIZ) was resolved by Zanier et al. (25), and it is superimposable with the x-ray crystal structure of WT E6/E6AP heterodimer in E6/E6AP/p53 heterotrimer (PDB: 4XR8) (with a backbone RMSD of 0.12 nm), except for residues 1–8 of E6 and 381–383 of E6AP (24). This result demonstrates that F47RE6 mutation has no effect on the structure of E6/E6AP heterodimer. In order to check whether CHARMM36m force field is suitable for our simulated systems, we first calculate the backbone RMSD values of E6/E6AP, E6, and E6AP averaged over the three MD runs for WT E6/E6AP or E6F47R/E6AP heterodimer relative to their initial conformations. The average RMSD values of E6/E6AP, E6, and E6AP in E6F47R/E6AP and WT E6/E6AP heterodimers are quite small (∼0.2 nm) (Fig. 2 A). Quantitatively similar results are observed in each simulation (Fig. S2). These data indicate that the structure of E6F47R/E6AP heterodimer is stable and highly similar to that of WT E6/E6AP heterodimer, which can also be clearly seen from the all-atom superposition of their final conformations (Fig. 2 B). The agreement between our simulations and the experiments established the suitability of CHARMM36m force field for E6/E6AP heterodimers. Moreover, we calculated the number of native contacts between E6 and E6AP in WT E6/E6AP and E6F47R/E6AP heterodimers (Fig. 2 C), and the number of E6-E6AP in E6F47R/E6AP heterodimer (234 ± 1) is almost the same as that in WT E6/E6AP heterodimer (236 ± 4). The average E6-E6AP binding energy over the three MD runs for F47RE6 heterodimer (−55.55 kcal mol−1) is similar to that for WT heterodimer (−55.81 kcal mol−1) (Table S1), which further supports the results of E6-E6AP contact number analyses. Taken together, the contact number and binding free energy analyses are in good agreement with previous biophysical experiments, showing that F47RE6 mutation has a negligible effect on the binding of E6 with E6AP (32).
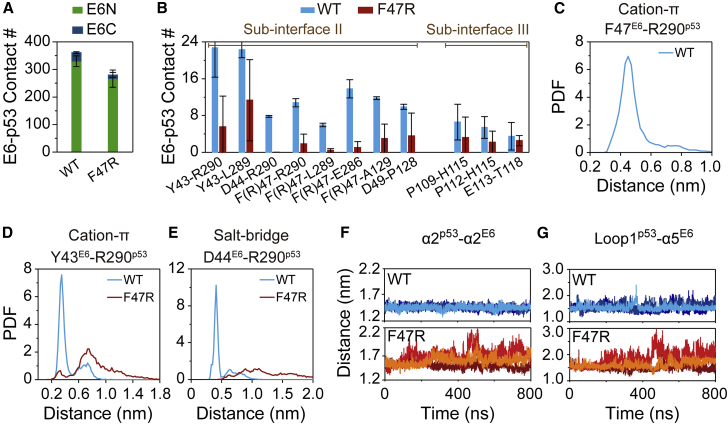
Having now established the suitability of CHARMM36m force field for E6/E6AP heterodimer and p53 (41), we turn to the exploration of the effects of F47RE6 mutation on interactions between E6/E6AP and p53, using the same protein force field. The RMSD values of E6/E6AP (Fig. S3 A) and native contact number of E6-E6AP (Fig. S3 B) in WT E6/E6AP/p53 heterotrimer are also similar to those in E6F47R/E6AP/p53 heterotrimer, indicating that E6/E6AP in both WT and F47RE6 heterotrimers is stable, and F47RE6 mutation does not affect the structural stability of E6/E6AP in heterotrimer. However, the number of native contacts between p53 and E6F47R/E6AP heterodimer is significantly decreased (Fig. 3 A), implying that F47RE6 mutation disrupts the interactions between p53 and the heterodimer. Our simulation result is in a way consistent with recent experiments showing that F47RE6 mutation can inhibit the association of p53 with E6/E6AP heterodimer (24,32,33). By calculating the number of native contacts of intermolecular residue pairs at E6-p53 subinterface, we find that F47RE6 mutation weakens the residue-residue interactions crucial for the stabilization of E6-p53 subinterface II and III (Fig. 3 B). Almost all E6-p53 residue pairs at subinterface II display reduced interactions, including Y43E6-L289p53/R290p53, D44E6-R290p53, F47E6-E286p53/L289p53/R290p53/A129p53, and D49E6-P128p53. Specifically, F47E6 in WT heterotrimer interacts with R290p53 by a cation-π interaction (Figs. 3 C and S4), whereas this cation-π interaction in the mutant heterotrimer is destroyed. Moreover, F47RE6 mutation results in an electrostatic repulsion between R47E6 and R290p53 (Fig. S4), which reduces the interactions of several residue pairs around R47E6 and R290p53, including hydrophobic interactions of F47E6-E286p53/L289p53/A129p53, Y43E6-L289p53, and D49E6-P128p53 (Fig. S4), Y43E6-R290p53 cation-π interaction (Figs. 3 D and S4), and D44E6-R290p53 salt bridge interaction (Figs. 3 E and S4). It is conceivable that these weakened intermolecular residue-residue interactions are crucial for the stability of the complex. Thus, we can reasonably infer that these interactions may be also important for the dimer/trimer formation. Our inference is supported by a recent study showing that a small-molecule inhibitor of E6-p53 interaction, identified by a structure-based virtual screening method using a druggable pseudocavity around F47E6 and D49E6, can reactivate p53 functions.
Figure 3.
The effect of F47RE6 mutation on the interactions between E6 and p53 in E6/E6AP/p53 heterotrimer. (A) Native contact number of E6-p53 in WT E6/E6AP/p53 and E6F47R/E6AP/p53 heterotrimers. (B) Native contact number of residue pairs at E6-p53 subinterface. Probability density function (PDF) of the minimum centroid distance between the ring of aromatic residues and ε-amino group (NH3+) in the side chain of Arg: (C) for F47E6-R290p53 and (D) for Y43E6-R290p53. (E) PDF of the distance between the side chain charge center of D44E6 and R290p53. (F and G) The time evolution of centroid distance (F) between α2-helixp53 and α2-helixE6 and (G) between loop1p53 and α5-helixE6. To see figure in color, go online.
As residues at subinterface II are mostly located within α2-helix of E6 (residues 39–48) and α2-helix of p53 (residues 278–290) (Fig. S4). F47RE6 mutation causes markedly increased distance between the two helixes (Figs. 3 F and S4), indicating that α2-helixp53 tends to dissociate from E6F47R/E6AP heterodimer. Due to the positive correlation between residues 278–282 within α2-helixp53 and residues 122–124 within loop1p53 (Fig. S5) in E6F47R/E6AP/p53 heterotrimer, loop1p53 also tends to dissociate from E6F47R/E6AP heterodimer (Figs. 3 G and S4), leading to reduced interactions at E6-p53 subinterface III (Fig. S4). Those decreased interactions include P109E6/P112E6-H115p53 hydrophobic interactions and E113E6-T118p53 H-bonding interaction (Fig. S4). The H-bond occupancy between E113E6 and T118p53 is 21% in WT heterotrimer, and it is 11% in mutant heterotrimer (an average of three MD runs). As shown in Table S2, the MM/GBSA binding energy between E6 and p53 in F47RE6 (−29.31 kcal mol−1) (Table S2) heterotrimer is higher than that in WT heterotrimer (−43.13 kcal mol−1), which further supports the results of E6-p53 contact number analyses. These data indicate that when F47 is mutated into positively charged residue (R), the electrostatic repulsion between R47E6 and R290p53 would block the residue-residue interactions at E6-p53 subinterfaces and the formation of the heterotrimer.
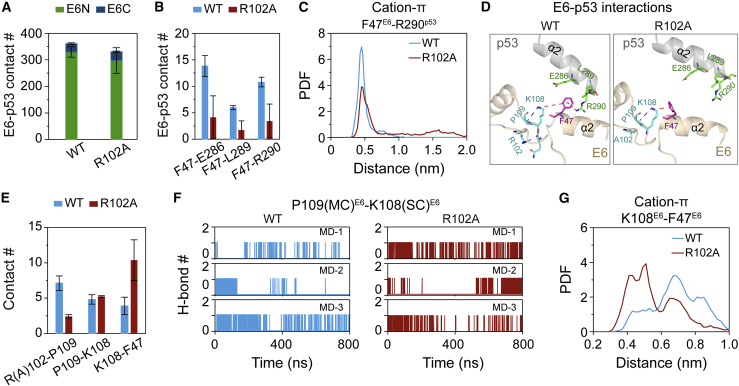
R102AE6 mutation disrupts the hydrophobic and cation-π interactions between F47 of E6 and E286/L298/R290 of p53, crucial for the stabilization of heterotrimer
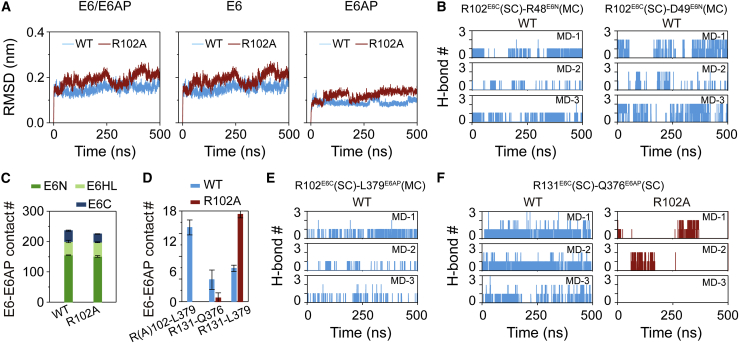
A pull-down experiment showed that R102A E6 mutant has a reduced binding ability with E6AP relative to WT E6 (25), indicating that E6R102A/E6AP heterodimer is less stable than WT E6/E6AP heterodimer. To elucidate at an atomic level how R102AE6 mutation decreases the E6AP-binding ability of E6 or the structural stability of the heterodimer, we first calculated the time evolution of backbone RMSD values of WT E6/E6AP and E6R102A/E6AP heterodimers (left panel in Fig. 4 A). It can be seen that R102A mutant heterodimer has a larger RMSD value than WT heterodimer (Figs. 4 A and S6), indicating that R102AE6 mutation decreases the structural stability of E6/E6AP heterodimer, in accordance with the pull-down experiments (25). In addition, R102AE6 mutation also destabilizes the structures of both E6 and E6AP (middle and right panels in Figs. 4 A and S6). We then analyzed the influence of R102AE6 mutation on the intramolecular residue-residue interactions crucial for the stabilization of E6. In WT E6, the side chain of R102E6C forms H-bonds with the main chains of two residues R48E6N/D49E6N (22% and 32% H-bond occupancy, respectively) (Figs. 4 B and S7), thus holding E6C and E6N together and stabilizing the structure of E6. However, R102AE6 mutation abolishes the H-bonding interactions (Fig. S7) and destabilizes E6.
Figure 4.
The influences of R102AE6 mutation on E6/E6AP heterodimer structure and crucial interactions stabilizing the heterodimer. (A) The time evolution of backbone RMSD values of E6/E6AP, E6, and E6AP relative to their initial conformations. The RMSD value is averaged over three individual MD simulations. (B) The time evolution of H-bond number between the side chain (SC) of R102E6 and the main chain (MC) of R48E6/D49E6 in WT heterodimer. (C) Number of native contacts between E6 and E6AP. (D) Contact number of R(A)102E6-L379E6AP, R131E6-Q376E6AP, and R131E6-L379E6AP. (E) The time evolution of H-bond number between the SC of R102E6 and the MC of L379E6AP in WT heterodimer. (F) The time evolution of H-bond number between the SCs of R131E6 and Q376E6AP. To see figure in color, go online.
The number of native contacts between E6 and E6AP in E6R102A/E6AP heterodimer (225 ± 3) is also smaller than that in WT E6/E6AP heterodimer (236 ± 4) (Fig. 4 C). Although the average E6-E6AP binding energy for R102AE6 dimer (−56.95 kcal mol−1) is slightly lower than that for WT heterodimer (−55.81 kcal mol−1) (Table S3), the E6-E6AP binding energy for R102AE6 dimer in two out of the three MD runs (−54.01 and −53.69 kcal mol−1) is higher than that for WT dimer (−55.81 kcal mol−1) (Table S4). The result is somehow consistent with the pull-down experiment showing that R102AE6 mutation led to an E6-E6AP binding probability of ∼25% relative to 100% of WT (inferred from Fig. 3 B in ref. 25). The decrease of E6-E6AP contact number is mostly contributed by two residue pairs between E6C and E6AP: R102E6C-L379E6AP and R131E6C-Q376E6AP (Fig. 4 D). An H-bond is formed between the side chain of R102E6 and the main chain of L379E6AP (with an 8% H-bond occupancy) (Figs. 4 D, E and S7), whereas R102AE6 mutation eliminates the H-bond formation. R131E6 that is close to R102E6 (Fig. S7) has hydrophobic interaction with L379E6AP (25). R102A mutation provides more space for the side chain of R131 (Fig. S7), thus facilitates the hydrophobic interaction between R131E6 and L379E6AP (Figs. 4 D and S7), but it abolishes R131E6-Q376E6AP H-bonding interaction (Figs. 4 D, F and S7). The H-bond occupancies of R131E6-Q376E6AP in WT and R102AE6 heterotrimers are 35% and 0, respectively. These results suggest that R102AE6 mutation has minor influence on the interactions between E6N/E6HL and E6AP, and it eliminates the H-bond formation of E6C-E6AP residue pairs (R(A)102E6C-L379E6AP, R131E6C-Q376E6AP), thus destabilizing the structure of E6/E6AP heterodimer.
The p53 degradation assay experiments demonstrated that R102AE6 mutation abolishes p53 degradation (25), implying that R102AE6 mutation impedes the formation of E6/E6AP/p53 heterotrimer. In order to get some insights into the molecular mechanism by which R102AE6 mutation inhibits the formation of stable E6/E6AP/p53 heterotrimer, we first calculated the number of native contacts between E6 and p53 in WT and mutant heterotrimers. Fig. 5 A shows that R102AE6 mutation causes a decrease in the native contact number of E6-p53, revealing a disruptive effect of R102AE6 mutation on the interactions between E6 and p53. Further residue-based E6-p53 interaction analysis shows that R102AE6 mutation reduces the contact number of F47E6N-E286p53/L289p53/R290p53 residue pairs (Fig. 5 B). F47E6N interacts with E286p53/L289p53 by hydrophobic interaction and with R290p53 by cation-π interaction in WT heterotrimer (24), whereas these interactions are greatly reduced in R102AE6 mutant heterotrimer (Figs. 5 B, C and D). The E6-p53 binding energy in R102AE6 heterotrimer (−40.17 kcal mol−1) is higher than that in WT heterotrimer (−43.13 kcal mol−1), which is consistent with the results of the contact number analysis.
Figure 5.
Mechanistic analyses of the inhibitory effect of R102E6 mutation on the binding of p53 with E6/E6AP heterodimer. (A) Native contact number of E6-p53 in heterotrimers. (B) Number of contacts between F47E6 and E286p53/L289p53/R290p53. (C) Probability density function (PDF) of the minimum centroid distance between aromatic ring of F47E6 and ε-amino group (NH3+) in the side chain of R290p53. (D) The snapshots (the center structure of the first cluster obtained by clustering analysis) showing the R102AE6 mutation-induced alteration of E6C-E6N and E6N-p53 interaction network. Thin red dashed line: cation-π interaction; thick blue dashed line: H-bond. (E) Side chain contact number of residue pairs: R(A)102E6C-P109E6C, P109E6C-K108E6C, and K108E6C-F47E6N. (F) The time evolution of H-bond number between the side chain (SC) of K108E6 and the main chain (MC) of P109E6 in WT and R102A mutant heterotrimers. (G) PDF of the centroid distance between the ring of aromatic residues F47E6N and ε-amino group (NH3+) in the side chain of K108E6C. To see figure in color, go online.
To elucidate how R102AE6C mutation results in a reduction in the interactions of those E6-p53 residue pairs, we calculated the side chain contact number of residue pairs within E6 in the two heterotrimers and find that R102AE6 mutation destroys R102E6C-P109E6C hydrophobic interaction (Figs. 5 D and E), which facilitates P109E6C-K108E6C H-bonding interaction (Figs. 5 D, E and F). The occupancies of P109E6C-K108E6C H-bond in WT and R102AE6 heterotrimers are 3% and 14%, respectively. This enhanced H-bonding interaction restrains the flexibility of the side chain of K108E6C, which facilitates the K108E6C-F47E6N cation-π interaction (Figs. 5 D, E and G), and in turn leads to a decrease in the aforementioned interactions (F47E6N-E286p53/L298p53/R290p53) between E6 and p53. Taken together, R102AE6 mutation markedly disrupts the hydrophobic and cation-π interactions between F47 of E6 and E286/L298/R290 of p53, which may disfavor the binding of p53 with E6/E6AP heterodimer.
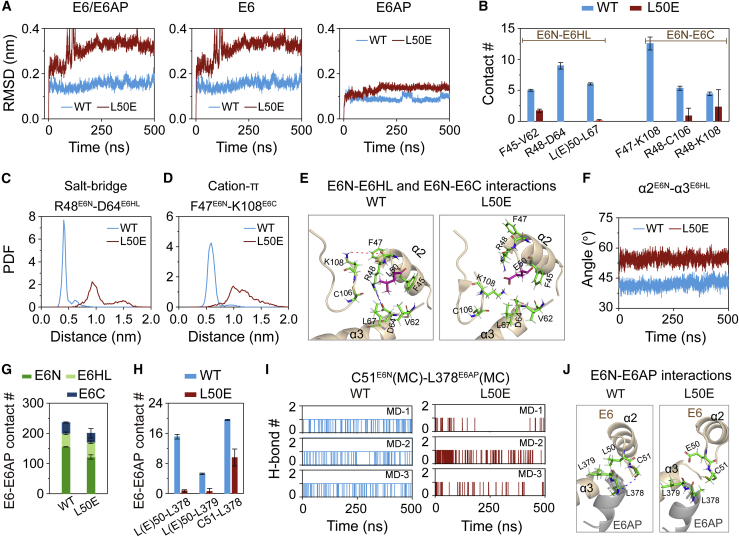
L50EE6mutation disrupts E6 interdomain hydrophobic, salt bridge, and cation-π interactions and E6-E6AP intermolecular hydrophobic interactions, thus destabilizes the structure of E6/E6AP heterodimer
In the pull-down experiments, L50EE6 mutation completely suppresses the assembly of E6/E6AP complex and thus abolishes the p53 degradation activity of E6 (25). To understand how L50EE6 mutation impedes the formation of E6/E6AP heterodimer, we first examined the effect of L50EE6 mutation on the structural stability of E6L50E/E6AP heterodimer by calculating the backbone RMSD values of E6/E6AP. It can be seen from Figs. 6 A and S8 that E6L50E/E6AP heterodimer has a much larger RMSD value than WT E6/E6AP heterodimer, indicating that L50EE6 mutation significantly disrupts the structure of E6/E6AP heterodimer. Both E6 and E6AP also display increased RMSD values (Figs. 6 A and S8), especially E6. We then explore how L50EE6 mutation damages the structure of E6 by calculating the native contact number within E6 in WT E6/E6AP and E6L50E/E6AP heterodimers (Fig. 6 B). The residue pairs stabilizing the E6N-E6HL and E6N-E6C interdomain interactions display distinctly decreased contact number (Fig. 6 B). At E6N-E6HL interface, the residue pairs with decreased interactions include F45E6N-V62E6HL, L50E6N-L67E6HL, and R48E6N-D64E6HL. L50EE6 mutation almost completely disrupts L50E6N-L67E6HL hydrophobic interaction and simultaneously leads to an electrostatic repulsion between E50E6N and D64E6HL, which in turn results in the disappearance of R48E6N-D64E6HL salt bridge and the reduction of F45E6N-V62E6HL hydrophobic interaction (Figs. 6 C and E). At E6N-E6C interface, three residue pairs exhibit decreased interactions. Specifically, F47E6N-K108E6C cation-π interaction (Figs. 6 D and E) is abolished, and hydrophobic interactions between the aliphatic groups of R48E6N and C106E6C/K108E6C (Fig. 6 E) are significantly reduced. The marked decrease of E6N-E6HL and E6N-E6C interdomain interactions destabilize the structure of E6, especially E6C and E6HL regions (Fig. S9). The structural disruption of E6C and E6HL can also be seen from the time evolution of the angle between α2E6N and α3E6HL (Figs. 6 F and S10 A), showing a drastic increase of the angle in E6L50E/E6AP heterodimer (Fig. S10 B).
Figure 6.
Mechanistic analyses of the inhibitory effect of L50EE6 mutation on E6/E6AP heterodimer formation. (A) The time evolution of backbone RMSD values of E6/E6AP, E6, and E6AP in heterodimers relative to their initial conformations. (B) Side chain contact number of E6N-E6HL and E6N-E6C. (C) Probability density function (PDF) of the distance of the side chain charge center of R48E6-D64E6. (D) PDF of the centroid distance between aromatic ring of F47E6 and ε-amino group (NH3+) in the side chain of K108E6. (E) The snapshots (the center structure of the first cluster obtained by clustering analysis) showing the L50EE6 mutation-induced alteration of E6 interdomain interactions. Thin red dashed line: cation-π interaction; thin blue dashed line: salt bridge interaction. (F) The time evolution of the angle between α2E6N and α3E6HL. (G and H) Native contact number of E6-E6AP (g) and of L(E)50E6-L378E6AP/L379E6AP and C51E6-L378E6AP residue pairs (H). (I) The time evolution of H-bond number between the main chain atoms of C51E6 and L378E6AP. (J) The snapshots (the center structure of the first cluster obtained by clustering analysis) showing the L50EE6 mutation-induced alteration of E6-E6AP interactions. Thick blue dashed line: H-bond. To see figure in color, go online.
In addition, we find that the E6-E6AP binding energy in L50EE6 heterodimer (−45.63 kcal mol−1) is significantly higher than that in WT heterodimer (−55.81 kcal mol−1) (Table S6), consistent with the significant reduction of the native contact number of E6-E6AP in L50EE6 heterodimer (Fig. 6 G). These data suggest that L50EE6 mutation reduces the E6-E6AP binding. In more detail, L50EE6 mutation abolishes the hydrophobic interactions between L50E6 and L378E6AP/L379E6AP (Figs. 6 H and J), which further results in significantly decreased C51E6–L378E6AP H-bonding interaction. The occupancies of C51E6–L378E6AP H-bond in WT and L50EE6 heterodimer are 99% and 2%, respectively (Figs. 6 I and J). Taken together, L50EE6 mutation disrupts E6 interdomain interactions (F45E6N-V62E6HL, L50E6N-L67E6HL, R48E6N-D64E6HL, and F47E6N-K108E6C) and intermolecular E6N-E6AP interactions (L50E6-L378E6AP/L379E6AP), and thus notably destabilizes E6/E6AP heterodimer. These results suggest that the aforementioned hydrophobic, salt bridge, and cation-π interactions are crucial for the heterodimer formation, and their disruption by L50EE6 mutation would block the binding of E6 with E6AP, thus impeding p53 recruitment.
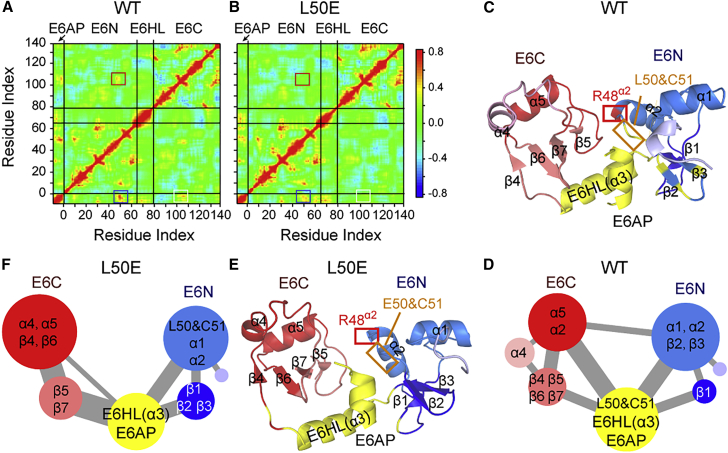
We also explore the effect of L50EE6 mutation on the dynamic network of E6/E6AP heterodimer by calculating the cross correlation matrix of WT E6/E6AP and E6L50E/E6AP heterodimers (Figs.7 A and B). To assess the convergence of the cross correlation values, we calculated the matrix of Cα-Cα cross correlation RMSDs using the data from the three replicate MD simulations of WT and L50EE6 heterodimers (Fig. S11) (56). Small differences are observed between the raw matrices of the systems (Fig. S11), suggesting the high convergence of the cross correlation values in our simulations. L50EE6 mutation has almost no effect on all the correlation values within E6N or within E6C, whereas it reduces the correlation values between F47/R48/D49/L50 in E6N and C106/Q107/K108 in E6C (marked by red rectangle in Figs. 7 A and B). In E6 of WT heterodimer, residue R48 of E6N belongs to the red community of E6C (Figs. 7 C and D), whereas in E6 of mutant heterodimer, it belongs to the light blue community of E6N (Figs. 7 E and F). Moreover, L50EE6 mutation results in the disappearance of the edge of E6N-E6C (Figs. 7 D and F), indicative of the significant disruption of E6N-E6C cross talk. For the cross-correlations between E6 and E6AP, two regions in the correlation matrix show significantly decreased correlation values (marked by white and blue rectangles in Figs. 7 A and B). The white rectangle denotes the correlation between C106/Q107/K108 in E6C and L378/L379/G380 in E6AP. The blue rectangle denotes the correlation between L50/C51 in E6N and L378/L379/G380 in E6AP. The community network in Figs. 7C and D shows that in WT heterodimer, L50E6N and C51E6N in the α2-β2 loop belong to the community containing E6HL and E6AP (yellow), whereas in L50E mutant heterodimer (Figs. 7 E and F), they belong to the community containing α1, α2, β2, and β3 of E6N (light blue). These results demonstrate that L50EE6 mutation disrupts the interdomain communication between E6N and E6C, the intermolecular communication between E6 and E6AP, and the global community network of E6/E6AP heterodimer.
Figure 7.
Correlation and community network analysis. The correlation matrix of (A) WT E6/E6AP and (B) E6L50E/E6AP heterodimers. The correlation values between F47/R48/D49/L50 in E6N and C106/Q107/K108 in E6C are marked by red rectangle. The white rectangle denotes the correlation between C106/Q107/K108 in E6C and L378/L379/G380 in E6AP, and the blue rectangle denotes the correlation between L50/C51 in E6N and L378/L379/G380 in E6AP. Cartoon representation of the community network of (C) WT and (E) mutant heterodimers. Schematic diagrams of the community networks of (D) WT and (F) mutant heterodimers. In (D) and (F), each circle represents a single community. The size of the circle and the width of the edges correspond respectively to the size of community and the connectivity strength between two communities. To see figure in color, go online.
The dissociation of the different complexes is not observed in our all-atom MD simulations. In order to explore the dissociation process, we also carried out 10-μs coarse-grained (CG) MD simulations using MARTINI force field. The CG model of E6/E6AP heterodimer, which is mapped from all-atom structure, is given in Fig. S12 A. It can be seen that the structure of the heterodimer collapses at the end of simulation (Figs. S12 B and C), and the dissociation of the E6/E6AP heterodimer is not observed in this MARTINI-based CG MD simulation. Therefore, a complete study of the mechanism of complex formation and how this is affected by the mutations remains to be performed using other CG models or enhanced MD simulations (such as replica exchange MD methods).
Conclusions
In summary, we have explored at an atomic-level the mechanisms of three single-site E6 mutations (F47R, R102A, and L50E) in destabilizing the structure of E6/E6AP heterodimer and E6/E6AP/p53 heterotrimer by performing multiple MD simulations on both WT and mutant heterodimer/heterotrimer with a total simulation time of ∼13 μs. We find that in heterotrimer, F47RE6 mutation leads to an electrostatic repulsion between R47E6 and R290p53, and in turn it disrupts the interresidue interactions important for the stabilization of E6-p53 subinterface II and III. Specifically, E6-p53 subinterface II is mostly stabilized by hydrophobic interactions (F47E6-E286p53/L289p53/A128p53, Y43E6-L289p53, and D49E6-P128p53), salt bridge interaction (D44E6-R290p53), and cation-π interactions (Y43E6/F47E6-R290p53). E6-p53 subinterface III is mostly stabilized by P109E6/P112E6-H115p53 hydrophobic interactions and E113E6-T118p53 H-bonding interaction. R102AE6 mutation abolishes the formation of interdomain H-bond between E6C and E6N (R102(SC)E6C-R48(MC)E6N/D49(MC)E6N) and intermolecular H-bond between E6C and E6AP (R102(SC)E6C-L379(MC)E6AP, R131(SC)E6C-Q376(SC)E6AP), which destabilizes the structure of E6/E6AP heterodimer and thus may disfavor heterotrimer formation. Moreover, R102AE6 mutation damages hydrophobic and cation-π interactions between F47 of E6 and E286/L298/R290 of p53, which is unfavorable for the binding of p53 with E6/E6AP heterodimer. L50EE6 mutation severely destabilizes the structural stability of E6 by impairing interdomain interactions of E6N-E6HL (F45E6N-V62E6HL, L50E6N-L67E6HL, and R48E6N-D64E6HL) and E6N-E6C (F47E6N-K108E6C and R48E6N-C106E6C/K108E6C), which may disfavor stable heterodimer formation. In addition, L50EE6 mutation abolishes L50E6-L378E6AP/L379E6AP hydrophobic interactions and weakens C51E6-L378E6AP H-bonding interaction, thus significantly disrupts the E6/E6AP heterodimer, suggesting their key roles in the heterodimer formation. A summary of our simulation results is illustrated in a schematic diagram (Fig. S13). This study reveals the molecular interactions crucial for the binding of E6-E6AP and E6/E6AP-p53, which may provide some insights into the disruption of E6/E6AP heterodimer and E6/E6AP/p53 heterotrimer formation by the three HPV16 E6 mutations.
Author contributions
L.L. and G.W. conceived the project and drafted the manuscript. L.L. performed the simulations. All authors analyzed the data, reviewed, and approved the final version of the manuscript.
Acknowledgments
This work has been supported by the National Key Research and Development Program of China (Grant No. 2016YFA0501702) and the National Natural Science Foundation of China (Grant No. 12074079). All simulations were performed using the GPU clusters of our group.
Editor: Diego U Ferreiro.
Footnotes
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2022.03.030.
Supporting material
References
- 1.Joerger A.C., Fersht A.R. Structural biology of the tumor suppressor p53. Annu. Rev. Biochem. 2008;77:557–582. doi: 10.1146/annurev.biochem.77.060806.091238. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B., Lane D., Levine A.J. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 3.Olivier M., Eeles R., et al. Hainaut P. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum. Mutat. 2002;19:607–614. doi: 10.1002/humu.10081. [DOI] [PubMed] [Google Scholar]
- 4.Honda R., Tanaka H., Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS. Lett. 1997;420:25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 5.Lahav G., Rosenfeld N., et al. Alon U. Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat. Genet. 2004;36:147–150. doi: 10.1038/ng1293. [DOI] [PubMed] [Google Scholar]
- 6.Thomas M., Pim D., Banks L. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene. 1999;18:7690–7700. doi: 10.1038/sj.onc.1202953. [DOI] [PubMed] [Google Scholar]
- 7.Bernard H.U., Burk R.D., et al. de Villiers E.-M. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 2010;401:70–79. doi: 10.1016/j.virol.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allison D.B., Maleki Z. HPV-related head and neck squamous cell carcinoma: an update and review. J. Am. Soc. Cytopathol. 2016;5:203–215. doi: 10.1016/j.jasc.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Syrjänen S. Human papillomavirus (HPV) in head and neck cancer. J. Clin. Virol. 2005;32:59–66. doi: 10.1016/j.jcv.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Marur S., D'Souza G., et al. Forastiere A.A. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–789. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burd E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003;16:1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monie A., Hung C.-F., et al. Wu T.C. Cervarix™: a vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biologics. 2008;2:107–113. [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler C.M., Hunt W.C., et al. New Mexico HPV Pap Registry Steering Committee A population-based study of human papillomavirus genotype prevalence in the United States: baseline measures prior to mass human papillomavirus vaccination. Int. J. Cancer. 2013;132:198–207. doi: 10.1002/ijc.27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huibregtse J.M., Scheffner M., Howley P.M. Localization of the E6-AP regions that direct human papillomavirus E6 binding, association with p53, and ubiquitination of associated proteins. Mol. Cell. Biol. 1993;13:4918–4927. doi: 10.1128/mcb.13.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheffner M., Nuber U., Huibregtse J.M. Protein ubiquitination involving an E1-E2-E3 enzyme ubiquitin thioester cascade. Nature. 1995;373:81–83. doi: 10.1038/373081a0. [DOI] [PubMed] [Google Scholar]
- 16.Cooper B., Schneider S., et al. Pol S.V. Requirement of E6AP and the features of human papillomavirus E6 necessary to support degradation of p53. Virology. 2003;306:87–99. doi: 10.1016/s0042-6822(02)00012-0. [DOI] [PubMed] [Google Scholar]
- 17.Manzo-Merino J., Thomas M., et al. Banks L. HPV E6 oncoprotein as a potential therapeutic target in HPV related cancers. Expert Opin. Ther. Targets. 2013;17:1357–1368. doi: 10.1517/14728222.2013.832204. [DOI] [PubMed] [Google Scholar]
- 18.Ronco L.V., Karpova A.Y., et al. Howley P.M. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes. Dev. 1998;12:2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmermann H., Degenkolbe R., et al. O’Connor M.J. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J. Virol. 1999;73:6209–6219. doi: 10.1128/jvi.73.8.6209-6219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiyono T., Hiraiwa A., et al. Ishibashi M. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc. Natl. Acad. Sci. U S A. 1997;94:11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glaunsinger B.A., Lee S.S., et al. Javier R. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene. 2000;19:5270–5280. doi: 10.1038/sj.onc.1203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas M., Laura R., et al. Banks L. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene. 2002;21:5088–5096. doi: 10.1038/sj.onc.1205668. [DOI] [PubMed] [Google Scholar]
- 23.Moody C.A., Laimins L.A. Human papillomavirus oncoproteins: pathways to transformation. Nat. Rev. Cancer. 2010;10:550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Zapien D., Ruiz F.X., et al. Zanier K. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature. 2016;529:541–545. doi: 10.1038/nature16481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanier K., Charbonnier S., et al. Travé G. Structural basis for hijacking of cellular LxxLL motifs by papillomavirus E6 oncoproteins. Science. 2013;339:694–698. doi: 10.1126/science.1229934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ansari T., Brimer N., Pol S.B.V. Peptide interactions stabilize and restructure human papillomavirus type 16 E6 to interact with p53. J. Virol. 2012;86:11386–11391. doi: 10.1128/JVI.01236-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanier K., Stutz C., et al. Hoppe-Seyler F. The E6AP binding pocket of the HPV16 E6 oncoprotein provides a docking site for a small inhibitory peptide unrelated to E6AP, indicating druggability of E6. PloS one. 2014;9:e112514. doi: 10.1371/journal.pone.0112514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malecka K.A., Fera D., et al. Marmorstein R. Identification and characterization of small molecule human papillomavirus E6 inhibitors. ACS. Chem. Biol. 2014;9:1603–1612. doi: 10.1021/cb500229d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricci-López J., Vidal-Limon A., et al. Aguila S. Molecular modeling simulation studies reveal new potential inhibitors against HPV E6 protein. PLoS One. 2019;14:e0213028. doi: 10.1371/journal.pone.0213028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celegato M., Messa L., et al. Banks L. A novel small-molecule inhibitor of the human papillomavirus E6-p53 interaction that reactivates p53 function and blocks cancer cells growth. Cancer Lett. 2020;470:115–125. doi: 10.1016/j.canlet.2019.10.046. [DOI] [PubMed] [Google Scholar]
- 31.Kumar A., Rathi E., Kini S.G. Identification of E6 inhibitors employing energetically optimized structure-based pharmacophore modelling, ligand docking and molecular dynamics simulations studies. ChemistrySelect. 2019;4:10701–10708. [Google Scholar]
- 32.Nominé Y., Masson M., et al. Weiss E. Structural and functional analysis of E6 oncoprotein: insights in the molecular pathways of human papillomavirus-mediated pathogenesis. Mol. Cell. 2006;21:665–678. doi: 10.1016/j.molcel.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Zanier K., ould M'hamed ould Sidi A., et al. Travé G. Solution structure analysis of the HPV16 E6 oncoprotein reveals a self-association mechanism required for E6-mediated degradation of p53. Structure. 2012;20:604–617. doi: 10.1016/j.str.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ristriani T., Fournane S., et al. Masson M. A single-codon mutation converts HPV16 E6 oncoprotein into a potential tumor suppressor, which induces p53-dependent senescence of HPV-positive HeLa cervical cancer cells. Oncogene. 2009;28:762–772. doi: 10.1038/onc.2008.422. [DOI] [PubMed] [Google Scholar]
- 35.Zhou R., Huang X., et al. Berne B.J. Hydrophobic collapse in multidomain protein folding. Science. 2004;305:1605–1609. doi: 10.1126/science.1101176. [DOI] [PubMed] [Google Scholar]
- 36.Krone M.G., Hua L., et al. Shea J.-E. Role of water in mediating the assembly of Alzheimer amyloid-β Aβ16-22 protofilaments. J. Am. Chem. Soc. 2008;130:11066–11072. doi: 10.1021/ja8017303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lei J., Sheng G., et al. Huang X. Two symmetric arginine residues play distinct roles in Thermus thermophilus Argonaute DNA guide strand-mediated DNA target cleavage. Proc. Natl. Acad. Sci. U S A. 2019;116:845–853. doi: 10.1073/pnas.1817041116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wassman C.D., Baronio R., et al. Hatfield G.W. Computational identification of a transiently open L1/S3 pocket for reactivation of mutant p53. Nat. Commun. 2013;4:1–9. doi: 10.1038/ncomms2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ng J.W., Lama D., et al. Sim A.Y. R248Q mutation—beyond p53-DNA binding. Proteins. 2015;83:2240–2250. doi: 10.1002/prot.24940. [DOI] [PubMed] [Google Scholar]
- 40.Pradhan M.R., Siau J.W., et al. Verma C.S. Simulations of mutant p53 DNA binding domains reveal a novel druggable pocket. Nucleic Acids Res. 2019;47:1637–1652. doi: 10.1093/nar/gky1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li L., Li X., et al. Wei G. Common cancer mutations R175H and R273H drive the p53 DNA-binding domain towards aggregation-prone conformations. Phys. Chem. Chem. Phys. 2020;22:9225–9232. doi: 10.1039/c9cp06671c. [DOI] [PubMed] [Google Scholar]
- 42.Lei J., Qi R., et al. Ma B. Conformational stability and dynamics of the cancer-associated isoform Δ133p53β are modulated by p53 peptides and p53-specific DNA. FASEB. J. 2019;33:4225–4235. doi: 10.1096/fj.201801973R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma B., Pan Y., et al. Nussinov R. Comparison of the protein-protein interfaces in the p53-DNA crystal structures: towards elucidation of the biological interface. Proc. Natl. Acad. Sci. U S A. 2005;102:3988–3993. doi: 10.1073/pnas.0500215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.ElSawy K.M., Lane D.P., et al. Caves L.S. Recognition dynamics of p53 and MDM2: implications for peptide design. J. Phys. Chem. B. 2016;120:320–328. doi: 10.1021/acs.jpcb.5b11162. [DOI] [PubMed] [Google Scholar]
- 45.Liu S.-X., Geng Y.-Z., Yan S.-W. Structural effects and competition mechanisms targeting the interactions between p53 and MDM2 for cancer therapy. Front. Phys. 2017;12:128908. [Google Scholar]
- 46.Lima I., Navalkar A., et al. Cino E.A. Biophysical characterization of p53 core domain aggregates. Biochem. J. 2020;477:111–120. doi: 10.1042/BCJ20190778. [DOI] [PubMed] [Google Scholar]
- 47.Lei J., Qi R., et al. Ma B. Self-aggregation and coaggregation of the p53 core fragment with its aggregation gatekeeper variant. Phys. Chem. Chem. Phys. 2016;18:8098–8107. doi: 10.1039/c5cp06538k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauer M.R., Krämer A., et al. Joerger A.C. Targeting cavity-creating p53 cancer mutations with small-molecule stabilizers: the Y220X paradigm. ACS. Chem. Biol. 2020;15:657–668. doi: 10.1021/acschembio.9b00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.The PyMOL Molecular Graphics System, Version 2.0. Schrödinger, LLC.
- 50.Van Der Spoel D., Lindahl E., et al. Berendsen H.J. GROMACS: fast, flexible, and free. J. Comput. Chem. 2005;26:1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- 51.Huang J., Rauscher S., et al. MacKerell A.D. CHARMM36m: an improved force field for folded and intrinsically disordered proteins. Nat. Methods. 2017;14:71–73. doi: 10.1038/nmeth.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu Q., Tan Y.-H., Luo R. Molecular dynamics simulations of p53 DNA-binding domain. J. Phys. Chem. B. 2007;111:11538–11545. doi: 10.1021/jp0742261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nosé S., Klein M. Constant pressure molecular dynamics for molecular systems. Mol. Phys. 1983;50:1055–1076. [Google Scholar]
- 54.Bussi G., Donadio D., Parrinello M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007;126:014101. doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- 55.Deserno M., Holm C. How to mesh up Ewald sums. I. A theoretical and numerical comparison of various particle mesh routines. J. Chem. Phys. 1998;109:7678–7693. [Google Scholar]
- 56.Hernandez Gonzalez J.E., Hernández Alvarez L., et al. Leite V.B. Prediction of noncompetitive inhibitor binding mode reveals promising site for allosteric modulation of Falcipain-2. J. Phys. Chem. B. 2019;123:7327–7342. doi: 10.1021/acs.jpcb.9b05021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.