Summary
Patient-specific airway basal stem cells (BSCs) can be derived from tracheal aspirate (TA) samples from intubated patients, thus providing an invaluable lung stem cell derivation method that bypasses the need for lung tissue. The primary culture of BSCs provides the ideal model to study the function and differentiation of the conducting lung epithelium. This protocol outlines the specific steps for isolation, culture maintenance, passaging, freezing, thawing, differentiation, and immunofluorescence characterization of human TA-derived airway BSCs. For complete details on the use and execution of this protocol, please refer to Lu et al. (2021).
Subject areas: Cell Biology, Cell culture, Cell isolation, Stem Cells
Graphical abstract
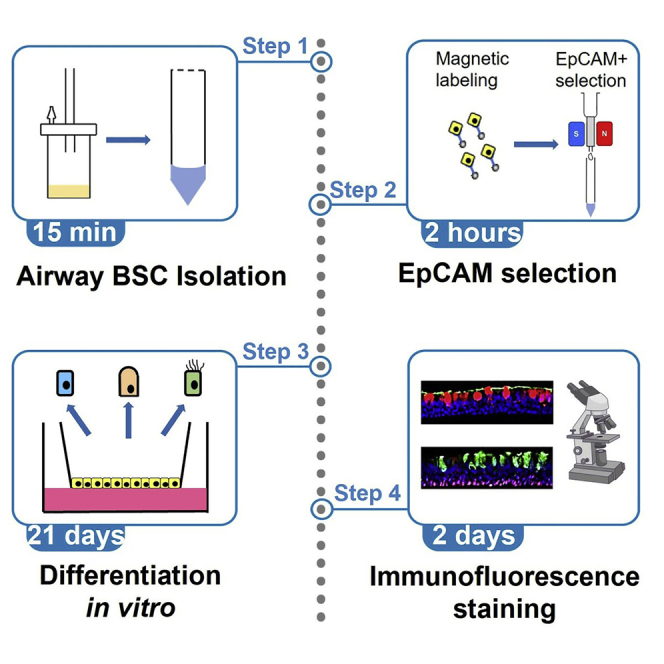
Highlights
-
•
Derivation of human airway basal stem cells from tracheal aspirates
-
•
In vitro culture expansion and maintenance (passaging, thawing, and freezing)
-
•
Differentiation on air liquid interface and immunofluorescence
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Patient-specific airway basal stem cells (BSCs) can be derived from tracheal aspirate (TA) samples from intubated patients, thus providing an invaluable lung stem cell derivation method that bypasses the need for lung tissue. The primary culture of BSCs provides the ideal model to study the function and differentiation of the conducting lung epithelium. This protocol outlines the specific steps for isolation, culture maintenance, passaging, freezing, thawing, differentiation, and immunofluorescence characterization of human TA-derived airway BSCs.
Before you begin
It is important for the researchers who follow this protocol to be proficient in cell culture and aseptic techniques.
Institutional permissions
All TA samples were collected using informed consent forms approved by the Institutional Review Board of Partners HealthCare (Protocol# 2019P003296).
Sterilization
Timing: 15–20 min
-
1.
Autoclave forceps and keep them sterile under the hood.
-
2.
Mix 350 mL ethanol with 150 mL of water to make 500 mL of 70% ethanol solution and store at 20°C–22°C.
-
3.
Turn on the UV light in the tissue culture hood for at least 15 min prior to use.
Preparation of cell culture medium and other reagents
Timing: 15–20 min
-
4.
Refer to the “materials and equipment” protocol for directions on how to prepare the required medium.
-
5.
Store the medium and buffers at 4°C for up to 1 month.
Pre-coat the cell culture vessel with 804G-conditioned medium
Timing: 4–6 h
-
6.
It is important to coat the cell culture with 804G-conditioned medium at least 4 h in a 37°C incubator with 5% CO2 before seeding human airway BSCs.
Note: Cell culture vessels should only be incubated with 804G-conditioned medium for up to 2 weeks for optimal cell adhesion.
-
7.
Refer to Table 1 for the exact measurements for the most common cell culture vessels.
Table 1.
Information on the most common cell culture vessels
| Cell culture vessel | Cell seeding density | Cells at confluency | Medium (mL) | Trypsin (mL) | 804G-conditioned medium (mL) |
|---|---|---|---|---|---|
| 35 mm dish | 0.3 × 106 | 1.2 × 106 | 2 | 1 | 1 |
| 100 mm dish | 2.2 × 106 | 8.8 × 106 | 10 | 3 | 3 |
| 6-well plate | 0.3 × 106 | 1.2 × 106 | 3–5 | 2 | 2 |
| 12-well plate | 0.1 × 106 | 0.4 × 106 | 2 | 1 | 1 |
| 24-well plate | 0.05 × 106 | 0.2 × 106 | 1 | 0.5 | 0.5 |
| T25 flask | 0.7 × 106 | 2.8 × 106 | 3–5 | 3 | 3 |
| T75 flask | 2.1 × 106 | 8.4 × 106 | 10–15 | 5 | 5 |
| T175 flask | 4.6 × 106 | 18.4 × 106 | 20–25 | 10 | 10 |
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-FOXA2 antibody (rabbit) (1:100) | Abcam | Cat# ab23630 |
| NKX2.1 antibody (rabbit) (1:100) | Santa Cruz Biotechnology | Cat# sc-13040 |
| CC10 antibody (E-11) (mouse) (1:100) | Sigma-Aldrich | Cat# HPA031828 |
| Muc5ac Monoclonal antibody (45M1) (mouse) (1:100) | Thermo Scientific | Cat# MA5-12178 |
| p63-α (D2K8X) XP® (rabbit) antibody (1:100) | Cell Signaling Technology | Cat# 13109S |
| p63 (D-9) (mouse) antibody (1:100) | Santa Cruz Biotechnology | Cat# sc-25268 |
| KRT8 antibody (mouse) (1:100) | BioLegend | Cat# 904804 |
| KRT5 antibody (rabbit) (1:100) | Abcam | Cat# ab53121 |
| Biotin anti-human CD326 antibody (EpCAM) (mouse) (1:100) | BioLegend | Cat# 369812 |
| Anti-RFX3 antibody (rabbit) (1:100) | Sigma-Aldrich | Cat# HPA035689 |
| Goat anti Rabbit IgG (H+L) Secondary antibody, Alexa Fluor 594, Invitrogen (1:200) | Thermo Scientific | Cat# A11037 |
| Goat anti Mouse IgM Heavy Chain Secondary antibody, Alexa Fluor 488, Invitrogen (1:200) | Thermo Scientific | Cat# A-21042 |
| Monoclonal Anti-Tubulin, antibody (mouse) (1:100) | Sigma-Aldrich | Cat# T6793 |
| DAPI Solution (1 mg/mL) | Thermo Scientific | Cat# 62248 |
| Anti-Biotin Microbeads | Miltenyi Biotec | Cat# 130-090-485 |
| Sucrose, for molecular biology, ≥99.5% (GC) | Sigma-Aldrich | Cat# S0389 |
| Chemicals, peptides, and recombinant proteins | ||
| Ethanol | Fisher Scientific | Cat# BP2818-4 |
| Bovine serum albumin (BSA) | Fisher Scientific | Cat# BP1605100 |
| Roswell Park Memorial Institute (RPMI) 1640 with L-glutamine | Gibco | Cat# 11875-119 |
| Fetal bovine serum (FBS) | Gibco | Cat# 10437-028 |
| DPBS without Calcium and Magnesium | Fisher Scientific | Cat# 14-190-250 |
| Small Airway Epithelial Cell Growth Medium (SAGM) | PromoCell | Cat# C-21070 |
| PneumaCult-ALI Medium | STEMCELL Technologies | Cat# 05001 |
| Y-27632 2HCl | Selleck Chemicals | Cat# S1049 |
| RAPAMYCIN 99% | Fisher Scientific | Cat# R-5000 |
| CHIR 99021 | Tocris Bioscience | Cat# 4423 |
| A 83-01 | Tocris Bioscience | Cat# 2939 |
| Normocin | InvivoGen | Cat# ant-nr-1 |
| Iscove’s Modified Dulbecco’s Medium (IMDM) w/ L-glutamine and HEPES | Lonza | Cat# 12-722F |
| EDTA | Sigma-Aldrich | Cat# 03690 |
| Trypsin-EDTA (0.5%), phenol red | Thermo Scientific | Cat# 25200114 |
| Triton X-100 | Sigma-Aldrich | Cat# T8787 |
| Critical commercial assays | ||
| Red blood cell lysis buffer | Sigma-Aldrich | Cat# R7757 |
| Dithiothreitol (DTT) | Cytiva | Cat# 45-000-232 |
| Paraformaldehyde solution 4% in PBS | Santa Cruz Biotechnology | Cat# sc-281692 |
| Experimental models: Cell lines | ||
| 804G Rat Urinary System Neoplasms∗ | ATCC | CRL-11555 |
| Other | ||
| 15 mL conical tubes | Corning | Cat# 352196 |
| 50 mL conical tubes | Corning | Cat# 430291 |
| 70 μm cell strainer | CELLTREAT | Cat# 229483 |
| Hemocytometer | Bulldog Bio | Cat# DHC-N51 |
| Centrifuge | Thermo Scientific | CL2 |
| Cell culture CO2 incubator | Thermo Fisher Scientific | HEPA Class100 |
| 0.22 μm filter 500 mL vacuum filter | Corning | Cat# 430769 |
| T25 flasks | Thermo Scientific | Cat# 156367 |
| T75 flasks | Thermo Scientific | Cat# 156499 |
| Cell Culture Microscope | Nikon | Eclipse TS100 |
| Cryostar CS10 (freezing medium) | BioLife Solutions | Cat# 210102 |
| 24-well 0.4 μm insert | Corning | Cat# 3470 |
| 12-well 0.4 μm insert | Corning | Cat# 3460 |
| Thermanox Plastic Coverslip, Round | Thermo Scientific | Cat# 174950 |
| CryoPlus Storage System Liquid Nitrogen Tank | Thermo Scientific | 8207 |
| MACS column | Miltenyi Biotec | Cat# 130-042-201 |
| Microcentrifuge tubes | MTC Bio | Cat# C2000 |
| 10 mm × 10 mm × 5 mm Cryomold | VWR | Cat# 25608-922 |
| OCT medium | Sakura | Cat# 4583 |
| Fluoromount-G | SouthernBiotech | Cat# 0100-01 |
| ColorFrost Plus Microscope Slides | Thermo Fisher Scientific | Cat# 12-550-17 |
| Mr. Frosty Freezing Container | Thermo Fisher Scientific | Cat# 5100-0001 |
∗804G cells are a critical reagent. They cannot be replaced with another cell line.
Note: Although we have not tested reagents from other companies, most reagents should be able to be exchanged for similar products from other suppliers.
Materials and equipment
More useful information about cell culture vessels can be found at: https://www.thermofisher.com/us/en/home/references/gibco-cell-culture-basics/cell-culture-protocols/cell-culture-useful-numbers.html.
Neutralization Medium
| Component | Final concentration | Amount |
|---|---|---|
| IMDM Medium | n/a | 500 mL |
| FBS | 10% | 50 mL |
| Normocin | n/a | 1 mL |
| Total | n/a | 551 mL |
Sterilize with 0.22 μm vacuum filter and store at 4°C for up to 1 month.
Roswell Park Memorial Institute (RPMI) Growth Medium
| Component | Final concentration | Amount |
|---|---|---|
| RPMI Medium | n/a | 500 mL |
| FBS | 10% | 50 mL |
| Normocin | n/a | 1 mL |
| Total | n/a | 551 mL |
Small Airway Epithelial Growth Medium (SAGM) Medium
| Component | Final concentration | Amount |
|---|---|---|
| SAGM Medium | n/a | 500 mL |
| SAGM Supplement Mix | n/a | 22.5 mL |
| Y-27632 2HCl | 5 μM | 250 μL |
| A 83-01 | 1 μM | 50 μL |
| CHIR 99021 | 0.4 μM | 20 μL |
| RAPAMYCIN 99% | 5 nM | 125 μL |
| Normocin | n/a | 1 mL |
| Total | n/a | 523.95 mL |
See Figure 2 in Lu et al. (2021) for representative images and proliferation quantification comparing BSC cultures with and without rapamycin.
Magnetic-Activated Cell Sorting (MACS) Buffer
| Component | Final concentration | Amount |
|---|---|---|
| 1× PBS | n/a | 500 mL |
| EDTA | 2 mM | 372.24 mg |
| BSA | 0.5% | 2.5 g |
| Total | n/a | 500 mL |
PneumaCult Medium with Inhibitors (first 7 days of differentiation)
| Component | Final concentration | Amount |
|---|---|---|
| PneumaCult-ALI Basal Medium | n/a | 500 mL |
| PneumaCult-ALI 10× Supplement | n/a | 50 mL |
| PneumaCult-ALI Maintenance Supplement | n/a | 5 × 1 mL |
| Y-27632 | 5 μM | 250 μL |
| A 83-01 | 0.5 μM | 25 μL |
| Normocin | n/a | 1 mL |
| Total | n/a | 556.3 mL |
PneumaCult Medium without Inhibitors (last 14 days of differentiation)
| Component | Final concentration | Amount |
|---|---|---|
| PneumaCult-ALI Basal Medium | n/a | 500 mL |
| PneumaCult-ALI 10× Supplement | n/a | 50 mL |
| PneumaCult-ALI Maintenance Supplement | n/a | 5 × 1 mL |
| Normocin | n/a | 1 mL |
| Total | n/a | 556 mL |
Immunofluorescence blocking buffer
| Component | Final concentration | Amount |
|---|---|---|
| Bovine Serum Albumin | 5% | 2.5 g |
| Triton X-100 | 0.1% | 50 μL |
| 1× PBS | n/a | 50 mL |
| Total | n/a | 50.05 mL |
Step-by-step method details
Preparation of 804G-conditioned medium
Timing: 10–15 days
This step contains information on how prepare 804G-conditioned medium from the 804G rat bladder epithelial cell line. The 804G-conditioned medium is used to coat cell culture vessels to promote airway BSC attachment (Levardon et al., 2018).
-
1.
Remove the freezing vial (2.1 × 106 cells) from liquid nitrogen and thaw at 20°C–22°C.
-
2.
Transfer the cell solution from the freezing vial to a 15 mL conical tube and add 5 mL neutralization medium (store in 4°C until expiration date) to the tube.
-
3.
Centrifuge the sample at 1,200 × g for 5 min at 20°C–22°C.
-
4.
Discard the supernatant.
-
5.
Resuspend the cell pellet with 10–15 mL RPMI medium (store in 4°C until expiration date) and plate into the T75 flask.
-
6.
Store the plate into a 37°C incubator with 5% CO2 and change SAGM medium daily.
-
7.Check the flask carefully for proper cuboidal cell shape and cell confluency, as well as any possible contamination.
-
a.If the cells have a proper cuboidal shape, the flask is 90% confluent, and there is no contamination, proceed to step 8.
-
b.If the cells are not confluent enough, change SAGM medium and check the flask in 24 h.
-
a.
-
8.Discard the SAGM medium from the T75 flask and add 5 mL 0.5% Trypsin to the T75 flask.
-
a.There is no need for a PBS wash.
-
b.Incubate the flask at 37°C until the cells dissociate from the flask (5–10 min).
-
c.If the cells have not dissociated after 5 min, lightly tap the side of the flask to force dissociation.
-
d.Check under the microscope to confirm that the cells have completely dissociated from the flask.
-
a.
-
9.
Transfer the cell suspension to a 15 mL conical tube and add 7 mL neutralization medium to the tube.
-
10.
Centrifuge the sample at 1,200 × g for 5 min at 20°C–22°C.
-
11.
Discard the supernatant, resuspend the cell pellet with 10–15 mL SAGM medium (store in 4°C until expiration date), plate into a T175 flask.
-
12.
Store the plate into a 37°C incubator with 5% CO2.
-
13.To grow and maintain the cells in culture, change SAGM medium 1 day after seeding and every other day thereafter.
-
a.Monitor the flask for proper cuboidal cell shape and any possible contamination every day.
-
b.Check the flask under a 5×, 10×, 20×, and 40× magnification to better visualize the cells and detect contamination.
-
a.
-
14.
Once confluency is reached, add 60 mL RPMI medium to the T175 flask and change media every other day for 8 days.
CRITICAL: After every 2 days, collect the supernatant — this is the 804G-conditioned medium. Sterilize the supernatant with 0.22 μm vacuum filter and store at 4°C for up to 1 month or −20°C for up to 1 year.
Isolation of airway BSCs from human TA samples
Timing: 15–20 min
This step contains information on how to isolate airway BSCs from human TA samples collected in mucus traps and maintain them in culture.
Note: This protocol yields best results when the TAs are freshly isolated. These samples can also be stored at 4°C for up to 24 h after collection, but efficiency decreases over time. If possible, use a separate hood from regular cell culture work for TA isolation to reduce the risk of contamination for the other samples in culture.
Optional: Before proceeding with the protocol, if the sample has an abundance of mucus, treat with 5 mL 0.1 mg/mL DTT in 1×PBS (from 10 mg/mL stock DTT solution). Incubate for 5 min at 20°C–22°C under the hood.
-
15.
Add 1×PBS to the mucus trap until the 5 mL mark to dilute the sample. Mix well using a 1 mL pipette.
-
16.
Using sterilized forceps, place the 70 μm cell strainer on top of a 50 mL conical tube and lubricate with 2 mL PBS.
-
17.
Carefully pipette the sample from the mucus trap through the 70 μm cell strainer to the 50 mL conical tube (1 mL at a time).
-
18.
Discard the cell strainer and centrifuge the sample at 1,500 × g for 5 min at 20°C–22°C.
-
19.
Discard the supernatant and the 804G-conditioned medium from the pre-coated T25 flask.
Optional: If the sample contains too many erythrocytes (indicated by a red cell pellet), a red blood cell lysis buffer may need to be used. Under the hood, add 1 mL of the red blood cell lysis buffer and incubate for 5 min at 20°C–22°C. Centrifuge the sample at 1,500 × g for 5 min at 20°C–22°C and discard the supernatant.
Optional: EpCAM selection of airway BSCs can be utilized to purify the cells that will be plated. Refer to the “EpCAM selection of airway BSCs from human TA samples” protocol for directions on purification.
-
20.
Resuspend the cell pellet with 5 mL SAGM medium and plate into the T25 flask.
Note: The pellet can also be frozen down directly and stored in liquid nitrogen until isolation, but this decreases efficiency of BSC derivation.
-
21.
Store the plate into a 37°C incubator with 5% CO2.
-
22.To grow and maintain the cells in culture, change SAGM medium 1 day after isolation and every other day thereafter.
-
a.Monitor the flask for proper cuboidal cell shape and any possible contamination every day.
-
b.Check the flask under a 5×, 10×, 20×, and 40× magnification to better visualize the cells and detect contamination.
- c.
-
a.
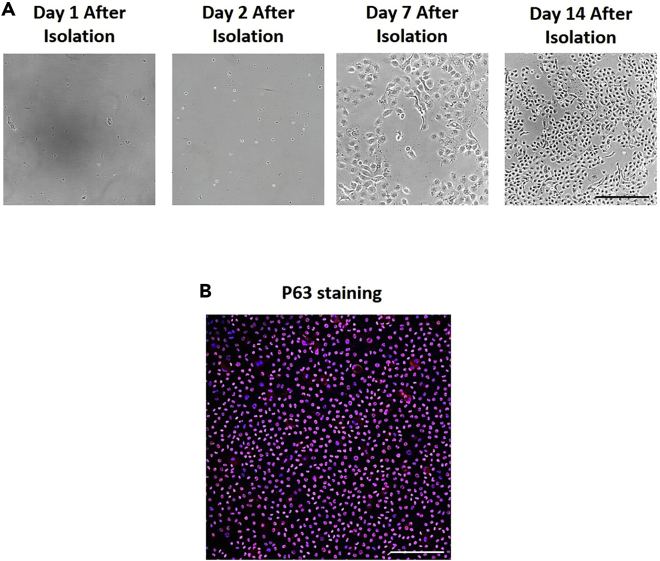
Figure 1.
Human airway BSCs isolated from TA samples
(A) Representative images of BSC cultures 1, 2, 7, and 14 days after isolation and EpCAM+ selection. Scale bar, 100 μm.
(B) P63 (basal stem cell marker) staining of patient-specific TA-derived BSCs in culture. Scale bar, 100 μm.
EpCAM selection of airway BSCs from human TA samples
Timing: 2 h
This step contains information on how to purify and enrich the human TA sample using EpCAM magnetic bead selection to target and isolate airway BSCs.
-
23.
After centrifugation, remove the supernatant and gently resuspend the cell pellet with 80 μL MACS buffer (store in 4°C until expiration date).
-
24.
Add 2 μL biotin-conjugated EpCAM antibody and mix the solution by horizontal hand shaking and incubate at 20°C–22°C under the hood for 30 min.
-
25.
Add 2 mL MACS buffer and centrifuge at 1,200 × g for 5 min.
-
26.
Discard the supernatant and gently resuspend the cell pellet with 80 μL MACS buffer.
-
27.
Add 20 μL anti-biotin magnetic microbeads and mix the solution by horizontal hand shaking and incubate at 20°C–22°C under the hood for 15 min.
-
28.
During the incubation period, set up the MACS column apparatus under the hood. Place a 15 mL conical tube underneath the MACS column for collection.
Note: Refer to Graphical Abstract step 2 to see the appropriate setup for the MACS column apparatus.
CRITICAL: Use sterilized forceps and careful aseptic technique as this is the step that has the most risk of contamination. Avoid contact with the plunger tip, as this is the region most susceptible to introducing contamination.
-
29.
Add 1.5 mL MACS buffer to the cells and mix gently.
-
30.
Add 500 μL MACS buffer to the column for initial lubrication.
-
31.
After about 80% of the initial lubrication MACS buffer has been filtered, add 500 μL cell mixture at a time to the MACS column and allow the solution to flow through to the 15 mL tube. Each time, wait until 80% of the solution is filtered before adding the next 500 μL. Repeat 2 more times until all 1.5 mL solution is filtered.
Note: The cells in the flowthrough are the EpCAM- cells.
-
32.
After all the solution has flowed through, quickly detach the column from the magnet and place it on top of a new 15 mL conical tube.
CRITICAL: In one motion, add 500 μL 1× PBS to the top of the column and immediately push the plunger down to release the solution into the 15 mL conical tube.
Note: The cells in the flowthrough are the EpCAM+ cells.
Passaging human TA-derived airway BSCs
Timing: 15–20 min
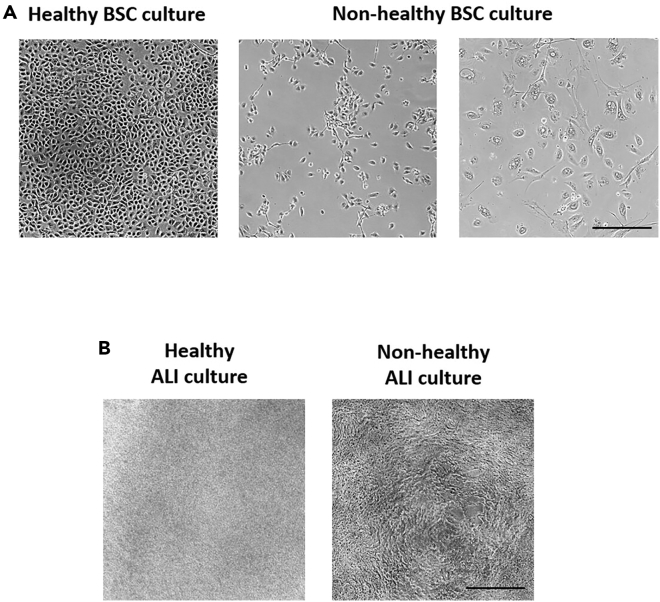
This step contains information on how to pass human TA-derived airway BSCs from a T25 flask to a T75 flask when 90% confluency is reached and maintain them in culture. See Figure 2A for examples of healthy vs. non-healthy BSC cultures.
Note: It is important to note that the proliferative capacity and differentiation efficiency of human TA-derived airway BSCs likely decreases after every passage. For functional assays and omics analysis, it is best to use airway BSCs before passage 5. Additionally, while this protocol is specific to passaging from a T25 flask to a T75 flask, this general concept can be applied to passaging between any two cell culture vessels. Refer to Table 1 for the exact measurements for the most common cell culture vessels.
-
33.Check the flask carefully for proper cuboidal cell shape and cell confluency, as well as any possible contamination.
-
a.If the cells have a proper cuboidal shape, the flask is 90% confluent, and there is no contamination, proceed to step 34.
-
b.If the cells are not quite confluent enough, check the flask in 24 h.
-
a.
-
34.Discard the SAGM medium from the T75 flask and add 2 mL 0.5% Trypsin.
-
a.There is no need for a PBS wash.
-
b.Incubate the flask at 37°C until the cells dissociate (5–10 min).
-
c.If the cells have not dissociated after 5 min, lightly tap the side of the flask to force dissociation.
-
d.Check under the microscope to confirm that the cells have completely dissociated from the flask.
-
a.
-
35.
Transfer the cell suspension to a 15 mL conical tube and add 4 mL neutralization medium to the tube.
-
36.
Centrifuge the sample at 1,200 × g for 5 min at 20°C–22°C.
-
37.
Discard the supernatant and the 804G-conditioned medium from the T75 flask.
-
38.
Resuspend the cell pellet with 10–15 mL SAGM medium and seed into the T75 flask.
-
39.
Store the plate into a 37°C incubator with 5% CO2.
-
40.To grow and maintain the cells in culture, change SAGM medium 1 day after seeding and every other day thereafter.
-
a.Monitor the flask for proper cuboidal cell shape and any possible contamination every day.
-
b.Check the flask under a 5×, 10×, 20×, and 40× magnification to better visualize the cells and detect contamination.
-
a.
Figure 2.
Human airway BSCs expanded in culture and seeded in ALI differentiation culture
(A) Representative images of healthy vs. non-healthy BSC cultures. Scale bar, 100 μm.
(B) Representative images of healthy vs. non-healthy BSC ALI differentiation cultures. Scale bar, 100 μm.
Differentiation of human airway BSCs using the air-liquid interface (ALI) and sample preparation for cryosectioning
Timing: 23–25 days
This step contains information on how to seed human TA-derived airway BSCs from a T75 flask into ALI culture for differentiation for 21 days, maintain them in culture, and prepare the sample for cryosectioning.
-
41.
Before beginning, coat the 24-well inserts with 400 μL 804G-conditioned medium.
-
42.Check the flask carefully for proper cuboidal cell shape and cell confluency, as well as any possible contamination.
-
a.If the cells have a proper cuboidal shape, the flask is 90% confluent, and there is no contamination, proceed to step 43.
-
b.If the cells are not quite confluent enough, change SAGM medium and check the flask in 24 h.
-
a.
-
43.Discard the SAGM medium from the T75 flask and add 5 mL 0.5% Trypsin.
-
a.There is no need for a PBS wash.
-
b.Incubate the flask at 37°C until the cells dissociate from the flask (5–10 min).
-
c.If the cells have not dissociated after 5 min, lightly tap the side of the flask to force dissociation.
-
d.Check under the microscope to confirm that the cells have completely dissociated from the flask.
-
a.
-
44.
Transfer the cell suspension to a 15 mL conical tube and add 7 mL neutralization medium to the tube.
-
45.
Centrifuge the sample at 1,200 × g for 5 min at 20°C–22°C.
-
46.
Discard the supernatant and resuspend the pellet with 1 mL SAGM medium.
-
47.
Aliquot 10 μL of the cell suspension and count the cells using a hemocytometer. Dilute the cells in SAGM medium to 1 × 106 cells/mL.
-
48.
Remove the 804G-conditioned medium from each pre-coated insert.
-
49.Seed 300 μL of the cell suspension (3 × 105 cells) to the insert. Add 150 μL SAGM medium to the insert and add 750 μL SAGM medium to the well.
-
a.The total volume in the insert should be 400 μL and the total volume in the well should be 750 μL.
-
b.Refer to Table 2 for an example of ALI Seeding Calculations.
-
a.
Note: For a 12-well insert, seed 500 μL of the cell suspension (5 × 105 cells) to the insert and add 1 mL SAGM medium to the well. The total volume in the insert should be 500 μL and the total volume in the well should be 1 mL.
-
50.
Store the plate into a 37°C incubator with 5% CO2.
-
51.
To freeze the remaining cells, refer to the “freezing human TA-derived airway BSCs” protocol.
-
52.The day after seeding, check under the microscope to see if the cells are confluent in the insert.
-
a.If they are not confluent, refeed the cells with SAGM medium (400 μL SAGM medium to the insert and add 750 μL SAGM medium to the well) and check again the next day.
-
b.If they are confluent, replace the SAGM medium with PneumaCult medium with inhibitors (400 μL to the insert, 750 μL to the well).
-
a.
Note: Add 500 μL PneumaCult medium with inhibitors to the insert and 1 mL to the well for a 12-well insert.
-
53.The day after the exchange of the medium to PneumaCult, remove the medium from the insert and from now on, only apply medium to the well.
-
a.Change medium every day for the first 7 days.
-
b.From days 7–21, change medium every other day.
-
a.
CRITICAL: For the first 7 days, use PneumaCult medium with inhibitors. For the last 14 days, use PneumaCult medium without inhibitors (store in 4°C until expiration date). This is more important for cilia functional analysis, but less important for differentiation, as the inhibitors compromise cilia motility if treated for an extended period of time in culture.
Optional: On days 14 and 21, perform an apical PBS wash to remove excess mucus. Add 400 μL sterile 1× PBS to the insert and incubate in a 37°C incubator with 5% CO2 for 30 min. After incubation, carefully remove the PBS from the insert without touching the membrane. Touching the membrane can result in cell detachment.
Note: See Figure 2B for examples of healthy vs. non-healthy BSC ALI cultures. Cell detachment is signified by holes in the membrane, as seen in the non-healthy BSC ALI culture.
-
54.
On day 21 of differentiation, remove the medium and add 400 μL 4% PFA in 1× PBS to the insert and 750 μL 4% PFA in 1× PBS to the well for cell fixation. Incubate for 20 min in the dark under the hood.
-
55.
Wash 3 times for 5 min with 1 mL 1× PBS in the insert and well.
Note: Proceed to step 56 for immediate sectioning. The inserts can also be stored in 1× PBS in 4°C for later processing.
-
56.
Using a scalpel and blunt forceps, carefully cut half of the membrane from the bottom of the insert transfer it into a 1.5 mL microcentrifuge tube.
Note: Only touch one corner of the membrane while handling to prevent cell detachment.
Pause point: The other half of the membrane can be stored in 1× PBS in 4°C for sectioning at a later time. These membranes can be stored for up to a year in the 4°C without any negative effects on sectioning and staining.
-
57.
Incubate the membrane in 10%, 15%, and 20% sucrose in 1× PBS on ice for 1 h each.
-
58.
Incubate the membrane in 1:1 20% sucrose:OCT in 4°C for 10 h.
-
59.
Add 1:3 20% sucrose:OCT to halfway fill a cryomold on dry ice.
Note: While loading the cryomold with 1:3 20% sucrose:OCT, it is important to avoid loading bubbles, as they can prevent quality sectioning.
-
60.
Once the solution turns opaque, place the membrane flat on top, noting its orientation.
Note: It is important for the cryomold to be completely flat on the dry ice to prevent sample misorientation while the OCT solidifies.
-
61.
Add 1:3 20% sucrose:OCT to fill the rest of the cryomold and incubate for 15 min or until solid.
-
62.
Perform cryosectioning.
Table 2.
Example of air-liquid interface (ALI) seeding calculations
| # Cells counted at confluency in T75 | # Cells required for seeding per 24-well insert | # Inserts to be seeded | # Total cells required | # Total cells remaining to freeze | # Cells required for T75 seeding |
|---|---|---|---|---|---|
| 5.5 × 106 | 3 × 105 | 4 | 1.2 × 106 | 4.3 × 106 | 2.1 × 106 |
Freezing human TA-derived airway BSCs
Timing: 15–20 min
This step contains information on how to freeze human TA-derived airway BSCs from a T75 flask when 90% confluency is reached.
-
63.Check the flask carefully for proper cuboidal cell shape and cell confluency, as well as any possible contamination.
-
a.If the cells have a proper cuboidal shape, the flask is 90% confluent, and there is no contamination, proceed to step 64.
-
b.If the cells are not quite confluent enough, change SAGM medium and check the flask in 24 h.
-
a.
-
64.Discard the SAGM medium form the T75 flask and add 5 mL 0.5% Trypsin.
-
a.There is no need for a PBS wash.
-
b.Incubate the flask 37°C until the cells dissociate from the flask (5–10 min).
-
c.If the cells have not dissociated after 5 min, lightly tap the side of the flask to force dissociation.
-
d.Check under the microscope to confirm that the cells have completely dissociated from the flask.
-
a.
-
65.
Transfer the cell suspension to a 15 mL conical tube and add 7 mL neutralization medium to the tube.
-
66.
Centrifuge the sample at 1,200 × g for 5 min at 20°C–22°C.
-
67.
Resuspend the pellet with 1 mL SAGM medium.
-
68.
Aliquot 10 μL of the resuspension and count the cells using a hemocytometer. Calculate the number of freezing vials required according to Table 2.
-
69.
Centrifuge the sample at 1,200 × g for 5 min at 20°C–22°C.
-
70.
Discard the supernatant and resuspend in the appropriate volume of freezing medium to reach 1 million cells per 1 mL.
-
71.
Aliquot 500 mL of the resuspended solution into each freezing vial.
-
72.
Place each freezing vial in the Mr. Frosty and into −80°C for 12 h.
-
73.
Transfer cells from the Mr. Frosty to liquid nitrogen the next day.
Pause point: TA BSCs can be stored in liquid nitrogen for long period of time.
Thawing human TA-derived airway BSCs
Timing: 5–10 min
This step contains information on how to thaw human TA-derived airway BSCs from liquid nitrogen to a T25 flask.
-
74.
Remove the freezing vial from liquid nitrogen and thaw at 20°C–22°C.
-
75.
Transfer the cell solution from the freezing vial to a 15 mL conical tube and add 5 mL neutralization medium to the tube.
-
76.
Centrifuge the sample at 1,200 × g for 5 min at 20°C–22°C.
-
77.
Discard the supernatant and the 804G-conditioned medium from the pre-coated T25 flask.
-
78.
Resuspend the cell pellet with 5 mL SAGM medium and plate into the T25 flask.
-
79.
Store the plate into a 37°C incubator with 5% CO2.
Immunofluorescence staining of human TA-derived airway BSCs
Timing: 2 days
This step contains information on how to perform immunofluorescent characterization of cultured human TA-derived airway BSCs on coverslips from a frozen vial in liquid nitrogen.
-
80.
Using sterilized forceps, gently place the coverslip to the 24-well plate.
Note: In most cases, there is a specific cell culture-treated side for the coverslip. Ensure that this is the side facing up when the coverslip is placed in the well.
-
81.
Remove the freezing vial from liquid nitrogen and hand-thaw.
-
82.
Transfer the cell solution from the freezing vial to a 15 mL conical tube and add 5 mL neutralization medium to the tube.
-
83.
Centrifuge the sample at 1,200 × g for 5 min at 20°C–22°C.
Discard the supernatant. And resuspend the pellet with 1 mL SAGM medium.
-
84.
Aliquot 10 μL of the resuspension and count the cells using a hemocytometer. Calculate the volume of suspension required for each well. Refer to Table 1 for exact seeding measurements.
-
85.
Aliquot the calculated volume of suspension to a separate 15 mL conical tube.
-
86.
Remove the 804G-conditioned medium from the pre-coated well.
-
87.
Aliquot the appropriate volume of the resuspension for each well and seed. Add enough SAGM medium to the well to make a total volume of 1 mL.
Note: To freeze the remaining cells, refer to the “freezing human TA-derived airway BSCs” protocol.
-
88.To grow and maintain the cells in culture, change SAGM medium 1 day after seeding and every other day thereafter.
-
a.Monitor the flask for proper cuboidal cell shape and any possible contamination every day.
-
b.Check the flask under a 5×, 10×, 20×, and 40× magnification to better visualize the cells and detect contamination.
-
a.
-
89.
Once the coverslip is 90% confluent, fix the cells on the insert using 1 mL 4% PFA in 1× PBS for 15 min.
-
90.
Wash with 1× PBS for 5 min twice.
Note: The plate can now be taken out of the cell culture hood, as it no longer needs to be handled under aseptic conditions.
-
91.
Continue with the standard protocol for immunofluorescence staining: blocking (blocking buffer can be stored in 4°C until expiration date), primary antibody, secondary antibody, and DAPI/any other nuclear stain.
-
92.
Once the staining protocol is complete, add a drop of Fluoromount-G to a microscope slide.
Note: Other mounting reagents, such as xylene-based reagents, can also be used.
-
93.
Use forceps and a pipette tip to carefully transfer the coverslip from the 24-well plate onto the microscope slide for imaging.
Note: Ensure that the cell culture-treated side is placed down onto the microscope slide.
-
94.
Allow the coverslip to dry completely before imaging.
Note: Before imaging, you may need to wipe the coverslip with a wet paper towel to eliminate any salt precipitate (as a result of the final PBS wash).
Expected outcomes
Upon the completion of this protocol, the researcher should have set up a robust protocol to isolate, culture, and differentiate human airway BSCs from clinical tracheal aspirations. Derivation and expansion of TA derived airway BSCs takes 2–4 weeks and differentiation takes 3 weeks. The expected population doubling time is about 48 h. Once the cells are passaged from the initial isolation flask, 100% of cells in the culture should be p63 positive. For optimal differentiation, use cells within 5 passages, as cells with high passage number may exhibit undesirable changes in proliferation and differentiation potentials. Deteriorating cultures can be identified by apparent holes in the membrane and/or dying cells. Once this method is set up, the researcher can use this approach to study different lung diseases, age-related phenotypes, and potential therapeutic interventions.
Limitations
The success of cell line derivation depends largely on the time between sample acquisition and cell culture and the quality of the initial sample. We have tried isolating cells from samples stored in 4°C for up to 5 days after collection but noticed a significant decrease in cell viability after 48 h of storage. Freezing of the initial sample after centrifugation as a “frozen pellet” for derivation at a later time also compromises cell viability. Therefore, we recommend direct isolation from a fresh TA-sample (within 48 h stored at 4°C). Another limitation is the potential of contamination in these patient derived samples, which makes cell line derivation impossible. If contamination of the sample is present, it can be detected during culture upon thorough daily examination as described above.
Troubleshooting
Problem 1
Step 22 of “isolation of airway BSCs from human TA samples”: The cell number after isolating from a frozen pellet is low.
Potential solution
One solution could be centrifuging at a higher speed to form a more robust pellet. Another solution could be to change SAGM medium more frequently to promote proliferation of the cells.
Problem 2
Step 53 of “differentiation of human airway BSCs using the air-liquid interface (ALI) and sample preparation for cryosectioning”: The cells in the ALI culture are unhealthy, dying, and/or detaching from the membrane.
Potential solution
First, ensure that the CO2 supply to the incubator in which these ALI cultures are stored is a constant 5%. Next, ensure that the incubator racks are level. If the racks are even slightly slanted, the ALI apparatus may tilt, leading to unequal coating and/or growth and differentiation of the cells. Lastly, more medium may be required in culture. Add 500 μL SAGM/PneumaCult medium when appropriate to the insert and 800 μL SAGM/PneumaCult medium to the well (steps 10 and 13 in the “differentiation of human airway BSCs using the air-liquid interface (ALI) and sample preparation for cryosectioning” section). Alternatively, next time, try adding 450 μL of 804G-conditioned medium to the insert for coating before seeding the cells.
Problem 3
Step 62 of “differentiation of human airway BSCs using the air-liquid interface (ALI) and sample preparation for cryosectioning”: The cells detach from the membrane after cryosectioning.
Potential solution
A potential explanation for this problem is that fixation was insufficient. Instead of 15 min, fix the cells on the membrane for 30 min.
Problem 4
Step 53 of “differentiation of human airway BSCs using the air-liquid interface (ALI) and sample preparation for cryosectioning”: The inserts leak during ALI culture, preventing the cells from differentiating.
Potential solution
Ensure that the 804G coating on the insert is fresh. Do not ever use an insert that has been incubated with 804G medium for more than 2 weeks. Other explanations could be that the cells possess intrinsic differentiation defects or there was an insufficient number of cells seeded on the insert.
Problem 5
Step 22 of “isolation of airway BSCs from human TA samples”: The cells are contaminated upon isolation from the TA sample.
Potential solution
Often, contamination in culture can be attributed to clinical contamination. If you see contamination in culture, check if the patient is suffering from an infection. If the patient’s respiratory cultures are contaminated, your culture will likely be contaminated as well. In this case, it is best to wait until the clinical infection is eliminated to resume BSC isolation.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Paul H. Lerou (plerou@mgh.harvard.edu).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
Research in the authors’ laboratory is supported by grants from NIH.
Author contributions
Conceptualization, G.M.A., R.W., K.B., J.E.S., X.A., and P.H.L.; methodology, G.M.A., R.W., K.B., and J.E.S.; writing – original draft, G.M.A.; writing – review & editing, G.M.A., R.W., X.A., and P.H.L.; funding acquisition, X.A. and P.H.L.; supervision, X.A. and P.H.L.
Declaration of interests
The authors declare no competing interests.
Data and code availability
This study did not generate or analyze any datasets.
References
- Levardon H., Yonker L., Hurley B., Mou H. Expansion of airway basal cells and generation of polarized epithelium. Bio Protoc. 2018;8:11. doi: 10.21769/BioProtoc.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Zhu X., Shui J.E., Xiong L., Gierahn T., Zhang C., Wood M., Hally S., Love J.C., Li H., et al. Rho/SMAD/mTOR triple inhibition enables long-term expansion of human neonatal tracheal aspirate-derived airway basal cell-like cells. Pediatr. Res. 2021;89:502–509. doi: 10.1038/s41390-020-0925-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate or analyze any datasets.