Abstract
This study was conducted to investigate the effects of dietary supplementation of polysaccharides derived from Astragalus membranaceus and Glycyrrhiza uralensis on growth performance, intestinal health, and gut microbiota composition in broilers. A total of 480 one-day-old male Arbor Acres broilers were randomly divided into 4 treatments with 6 replicates comprising 20 broilers each. Treatments included: basal diet without antibiotics (CON); basal diet supplemented with 500 mg/kg terramycin calcium (ANT); basal diet supplemented with 300 mg/kg Astragalus membranaceus polysaccharides (APS); and basal diet supplemented with 150 mg/kg Glycyrrhiza uralensis polysaccharides (GPS). The results showed that ANT, AP,S and GPS supplementation significantly increased average daily gain (ADG) and decreased feed conversion ratio (FCR) of broilers from 1 to 42 d of age. At 42 d, serum immunoglobulin A (IgA), immunoglobulin M (IgM) and immunoglobulin G (IgG) levels of the APS and GPS group were notably higher than those of the CON group, while serum levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) as well as diamine oxidase (DAO) activity in the APS and GPS group were obviously decreased. Moreover, diets supplemented with APS and GPS could significantly increase villus height (VH) and the ratio of villus height to crypt depth (VH/CD) and remarkably upregulated occludin, claudin-1 and mucin-2 (MUC2) mRNA expression in duodenum, jejunum, and ileum of broilers. In addition, 16S rRNA gene sequencing revealed that APS and GPS supplementation altered cecal microbial diversity and composition in broilers. Higher Shannon index was observed in the APS and GPS group compared with the CON group, while GPS supplementation could also increase Chao1 index and Observed species. The result of Principal coordinate analysis (PCoA) showed that microbial community in the CON, ANT, APS, and GPS group clustered separately. Notably, both APS and GPS supplementation significantly decreased the abundance of Bacteroidetes, Bacteroides, Faecalibacterium, Desulfovibrio, and Butyricicoccus, while increased the abundance of Firmicutes, Prevotella, Parabacteroides, Ruminococcus, and Alistipes. The correlation analysis showed that the changes in cecal microbial composition induced by dietary APS and GPS supplementation were closely associated with the alteration of the phenotype of broilers including ADG, FCR, TNF-α, IL-1β, IL-6, IgA, IgG, DAO, Occludin, Claudin-1, ZO-1, and MUC2. In conclusion, polysaccharides derived from Astragalus membranaceus and Glycyrrhiza uralensis could improve growth performance of broilers by enhancing intestinal health and modulating gut microbiota.
Key words: broiler, polysaccharides, astragalus membranaceus, Glycyrrhiza uralensis, intestine health
INTRODUCTION
Broilers are susceptible to external factors including diseases, nutritional, and environmental challenges due to their imperfect intestinal function development and low immunity, often resulting in poor health and high rates of mortality and morbidity. In the last several decades, antibiotic growth promoters were commonly used in the intensive poultry industry to control diseases and improve growth performance (Castanon, 2007). However, the residues of antibiotics in animal derived foods pose health risks to consumers (Suresh et al., 2018). More seriously, the overuse of antibiotics has participated to the development of drug-resistant bacteria (Witte, 2000). Currently, the use of antibiotics as feed additives has been banned in many countries including China. Given the high demand for high-quality poultry products, it is therefore of great importance to develop antibiotic alternatives that can both increase productive potential and maintain broiler health.
There is growing evidence that polysaccharides from natural plants possess the potential to replace antibiotic growth promoters in poultry production (Ao and Kim, 2020; Long et al.,2020; Wang et al., 2021). Astragalus polysaccharide (APS) is the main component of water-soluble heteropolysaccharide extracted from the stems or dried roots of traditional Chinese medicine Astragalus membranaceus, which exerts multiple biological activities, including antioxidant, anti-inflammatory, antibacterial, and antiviral, as well as immunomodulatory properties (Zheng et al., 2020). Dietary supplementation with APS has been found to improve the body weight gain of juvenile broilers, potentially due to enhanced digestive enzyme activity and antioxidant capacity (Wu, 2018). Moreover, APS intake by drinking water could effectively stimulate mucosal immune function and improve intestinal morphology of Muscovy ducklings infected with Muscovy duck reovirus (Liao et al., 2021). Glycyrrhiza polysaccharides (GPS) is one of the major active components of Glycyrrhiza uralensis, and its biological activity is closely connected with the spatial structure of alpha-D-pyran polysaccharide (Shen et al., 2015). GPS has drawn increasing attention due to its beneficial effects in immunity regulation, phagocytosis, anti-fungal and stimulating macrophages de novo (Mutaillifu et al., 2020). In broilers, it has been reported that the addition of GPS in diet could mitigate lipopolysaccharide-induced inflammatory response (Zhang et al., 2021). Moreover, a recent study showed that oral administration of GPS could improve and prolong the response of chickens to the Newcastle disease vaccine (Wu et al., 2022).
Intestinal barrier function and intestinal bacterial community play a critical role in maintaining the health of broilers. Intestinal mucosal barrier not only preserves the ability to digest and absorb nutrients but also acts as the first-line for the prevention of pathogen invasion (Yang et al., 2019a). Intestinal microbiota participates in nutrient metabolism, development of innate immunity, and clearance of pathogens (Kogut, 2019). Although there are several reports on the growth-promoting effects of APS and GPS in broilers, their potential influences on intestinal barrier and intestinal microbiota of broilers are not yet fully understood. Thus, the purpose of the present study was to investigate the effects of APS and GPS supplementation on growth performance, intestinal morphology and barrier function, and cecal gut microflora of broilers. The finding of this study would provide new insights into the maintenance of growth performance and health in broiler chickens fed antibiotic-free diets.
MATERIALS AND METHODS
Ethics Statement
The experimental protocol used in the present study was approved by the Animal Care and Use Committee of the College of Henan Agricultural University. Every effort was made to minimize animal pain, suffering, and distress.
Experimental Birds, Diet, and Management
A total of 480 one-day-old male Arbor Acres broiler chickens were randomly assigned to 4 dietary treatments with 6 replications and 20 broilers per replication. The four dietary treatments were as follows: 1) CON group (basal diet), 2) ANT group (basal diet supplemented with 500 mg/kg terramycin calcium), 3) APS group (basal diet supplemented with 300 mg/kg Astragalus polysaccharides), and 4) GPS group (basal diet supplemented with 150 mg/kg Glycyrrhiza polysaccharides). APS and GPS were purchased from Inner Mongolia Evergrand Pharmaceutical Co. LTD (Inner Mongolia, China). The content of polysaccharides in APS and GPS is 70.23 and 61.36%, respectively. APS is composed of mannose, rhamnose, galacturonic acid, glucose, galactose, and arabinose with a molar ratio of 1.00: 1.32: 1.97: 13.25: 3.86: 6.76. GPS consisted of mannose, rhamnose, glucose, galactose, and arabinose with a molar ratio of 1.00: 1.23: 14.59: 24.57: 16.71. The experimental diets were fed for 42 d, including starter (d 1 to 21) and finisher (d 22 to 42) phases. The ingredients and nutrient levels of basal diets were formulated to meet the NRC (1994) nutrient requirements of broiler chickens (Table 1). The broilers were vaccinated with Newcastle disease vaccine and the infectious bursal vaccine on d 7 and 14 of the experiment, respectively. All broilers had free access to feed and clean water during the experiment. The temperature of chicken coop was maintained at 33°C at the age of 1 to 4 d and then reduced by 2°C per week to a final temperature of around 24°C.
Table 1.
Composition and nutrient levels of basal diet (air-dry basis, %).
| Items | 1 to 21 d of age | 22 to 42 d of age |
|---|---|---|
| Ingredients | ||
| Corn | 54.50 | 55.42 |
| Soybean meal | 29.70 | 25.30 |
| Corn gluten meal | 8.00 | 8.00 |
| CaHPO4 | 1.30 | 1.20 |
| Limestone | 1.40 | 1.40 |
| NaCl | 0.30 | 0.30 |
| Soybean oil | 2.80 | 6.50 |
| Soda | 0.15 | 0.15 |
| L-Lysine | 0.87 | 0.80 |
| DL-Methionine | 0.25 | 0.21 |
| Threonine | 0.13 | 0.12 |
| Premix1 | 0.60 | 0.60 |
| Total | 100.00 | 100.00 |
| Nutrient levels2 | ||
| Metabolizable energy (MJ/kg) | 12.61 | 13.59 |
| Crude protein | 23.39 | 21.19 |
| Calcium | 0.77 | 0.72 |
| Total phosphorus | 0.56 | 0.54 |
| Lysine | 1.50 | 1.34 |
| Methionine | 0.62 | 0.55 |
| Threonine | 0.97 | 0.88 |
Supplied per kilogram of diet: for 1 to 21 d, 12,000 IU vitamin A; 4,500 IU vitamin D3; 30 IU vitamin E; 4.5 mg vitamin K; 2.8 mg vitamin B1; 9.6 mg vitamin B2; 3.75 mg vitamin B6; 30 μg vitamin B12; 49.5 mg niacin; 20 mg calcium pantothenate; 1.5 mg folic acid; 0.18 mg biotin; 500 mg choline; 100 mg Zn; 110 mg Fe; 20 mg Cu; 120 mg Mn; 0.7 mg I; 0.3 mg Se. For 22 to 42 d, 10,000 IU vitamin A; 3,750 IU vitamin D3; 25 IU vitamin E; 3.75 mg vitamin K; 2.3 mg vitamin B1; 8 mg vitamin B2; VB6, 3.1 mg vitamin B6; 25 μg vitamin B12; 41.2 mg niacin; 20 mg calcium pantothenate; 1.25 mg folic acid; 0.12 mg biotin; 400 mg choline; 100 mg Zn; 110 mg Fe; 20 mg Cu; 120 mg Mn; 0.7mg I; 0.3 mg Se.
Metabolizable energy was calculated, while the others were measured.
Growth Performance Determination
On d 1, 21, and 42, body weight and daily feed intake of birds in each pen were recorded to calculate the average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR; feed: gain, g: g) of each group. Chicken mortality was recorded daily after which performance parameters were corrected for mortality.
Sample Collection
At 42 d of age, one broiler was randomly selected from each replicate. Blood samples were collected from the wing vein and allowed to clot at 37°C for 2 h. Serum samples were obtained after centrifugation (3,000 × g for 15 min at 4°C) and stored at −20°C for analysis. The selected birds were sacrificed by cervical dislocation and exsanguinated. After dissection, the middle of duodenum, jejunum, and ileum (approximately 1 cm) were collected, rinsed, and fixed in 10% neutral formaldehyde. The mucosa from duodenum, jejunum, and ileum was scraped with sterilized slides, put into RNAase-free tubes, snap-frozen in liquid nitrogen, and stored at −80°C for mRNA extraction. The cecum contents were carefully collected, immediately placed in cryogenic vials, and stored at −80°C until they were processed for microbial DNA analysis.
Serum Sample Analysis
The levels of immunoglobulin A (IgA), immunoglobulin M (IgM), immunoglobulin G (IgG), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), diamine oxidase (DAO), and D-lactic acid (D-LA) in serum were determined using commercially available assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). All steps were carried out according to the manufacturer's instructions.
Intestinal Morphology Analysis
The intestinal segments were dehydrated in ethanol, equilibrated in xylene, and embedded in paraffin wax. A 5-μm section of each tissue was procured and stained with hematoxylin and eosin. To determine villus height (VH) and crypt depth (CD), the stained samples were observed using a digital camera microscope (BA400Digital, McAudi Industrial Group Co., Ltd.) and analyzed by the ImagePro Plus 6.0 software (Media Cybernetics, Bethesda, MD). A total of nine slides were prepared and analyzed per sample, and 3 observations were randomly taken per slide. The VH was measured from the tip of the villi to the villus-crypt junction, and CD referred to depth from this junction to the base of the crypt. The ratio of villus height to crypt depth (VH/CD) was calculated from the obtained VH and CD values.
Total RNA Extraction and mRNA Quantification
Total RNA was extracted from the intestinal mucosa samples using TRIzol reagent (Invitrogen, Carlsbad, CA), followed by quality measurement on 1.0% denaturing agarose gel and yield determination on a NanoDrop-2000 spectrophotometer (ThermoFisher Scientific, Waltham, MA). Total cDNA was synthesized using SuperScript III First-Strand Synthesis SuperMix (Invitrogen, 11752-050) according to the manufacturer's instructions. Real-time quantitative PCR were carried out on a CFX 96 real-time PCR Detection System (Bio-Rad, Hercules, CA). Sequences of the oligonucleotide primers used for quantitative real-time PCR are listed in Table 2. Amplification was performed in a total volume of 20 μL containing 10 μL of SYBR Green PCR Master Mix (TakaRa, Beijing, China), 1 μL of each primer, 1 μL of cDNA, and 7 μL of sterilized double-distilled water. The reaction conditions were as follows: denaturation at 95°C for 5 min; followed by 40 cycles of 95°C for 10 s, 58°C for 30 s, and 72°C for 30 s; and 72°C for 5 min. The relative levels of mRNA expression were calculated using the 2−△△CT method. GAPDH was used as an endogenous control to normalize the expression of the targeted genes.
Table 2.
Sequences of the oligonucleotide primers used for quantitative real-time PCR.
| Gene | Accession number | Sequence (5’-3’) | Amplicon size (bp) |
|---|---|---|---|
| GAPDH | NM_204305 | Forward: AGCCATTCCTCCACCTTTGAT Reverse: AGTCCACAACACGGTTGCTGTAT |
112 |
| OCLN | NM_205128.1 | Forward: GATGGACAGCATCAACGACC Reverse: CTTGCTTTGGTAGTCTGGGC |
142 |
| CLDN-1 | NM_001013611.2 | Forward: ACACCCGTTAACACCAGATTT Reverse: GCATTTTTGGGGTAGCCTCG |
152 |
| ZO-1 | XM_040680624.1 | Forward:TCGTCGCATTGTTGAGTCTGA Reverse: TATAGCGTGTCCACAACCCG |
129 |
| MUC2 | NM_001318434 | Forward: ATTGAAGCCAGCAATGGTGT Reverse: TGACATCAGGGCACACAGAT |
214 |
Abbreviations: CLDN-1, Claudin-1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MUC2, Mucin-2; OCLN, Occludin; ZO-1, Zonula occludens-1.
16S rRNA-Based Microbiota Analysis
Microbial DNA was extracted from cecum contents using the QIAamp DNA Stool Mini Kit (QIAGEN, CA, Hamburg, Germany) according to the manufacturer's protocol. The V3–V4 region of the 16S rRNA gene were amplified with primer pairs 338F (5’-ACTCCTACGGGAGGCACAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’). The 16S rRNA genes were sequenced on the Illumina MiSeq platform as per the standard protocols by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The raw reads were demultiplexed, quality-filtered by Trimmomatic, and merged by FLASH to obtain the effective reads. The obtained high-quality reads were assigned to operational taxonomic units (OTUs) with a 97% similarity using UPARSE (version 7.11), and chimera sequences were removed by comparing with the Silva database using UCHIME algorithm. The taxonomy of representative sequence for each OTU was analyzed by RDP Classifier. The diversity, composition and difference of the bacterial communities were analyzed on the I-Sanger Cloud Platform provided by Majorbio Bio-Pharm Technology Co., Ltd.
Statistical Analysis
Data were analyzed by one-way ANOVA using the GLM procedure of the statistical software SPSS 20.0 (SPSS Inc., Chicago, IL). Differences among means were assessed using Duncan's multiple range tests at P < 0.05 probability levels. Variability in the data was expressed as the pooled standard error of mean. To assess the correlation between the differential cecal microbial community of genera and the phenotype of broilers, we performed the Spearman's test in GraphPad Prism 8.0. P < 0.05 was considered as statistical significance.
RESULTS
Growth Performance
The effects of dietary APS and GPS on the growth performance of broilers are presented in Table 3. The addition of ANT, APS, and GPS in diets improved BW of broilers at 42 d of age in comparison with the control group (P < 0.05). During d 1 to 42, birds in ANT, APS, and GPS groups had increased (P < 0.05) ADG than those fed with basal diet. No significant difference was observed on ADFI among the 4 dietary treatments. The FCR of birds during d 1 to 42 in ANT, APS, and GPS groups was lower (P < 0.05) than that in control group.
Table 3.
Effects of dietary supplementation of ANT, APS, and GPS on growth performance in broilers (n = 6).
| Treatments |
||||||
|---|---|---|---|---|---|---|
| Items | CON | ANT | APS | GPS | SEM | P-value |
| BW, g | ||||||
| 1 d | 46.18 | 46.27 | 46.63 | 46.07 | 0.17 | 0.679 |
| 21 d | 841.67 | 866.27 | 853.42 | 874.12 | 5.43 | 0.155 |
| 42 d | 2,488.77b | 2,578.15a | 2,580.35a | 2,567.63a | 13.21 | 0.029 |
| ADG, (g/d) | ||||||
| 1–21 d | 37.88 | 39.05 | 38.42 | 39.43 | 0.26 | 0.145 |
| 22–42 d | 78.43 | 81.52 | 82.23 | 80.64 | 0.54 | 0.060 |
| 1–42 d | 58.16b | 60.28a | 60.33a | 60.04a | 0.31 | 0.030 |
| ADFI, g | ||||||
| 1–21 d | 48.10 | 50.19 | 47.17 | 47.29 | 0.71 | 0.428 |
| 22–42 d | 142.08 | 138.68 | 141.80 | 139.14 | 1.52 | 0.821 |
| 1–42 d | 95.09 | 94.43 | 94.48 | 93.22 | 0.69 | 0.829 |
| FCR | ||||||
| 1–21 d | 1.27 | 1.29 | 1.23 | 1.20 | 0.02 | 0.565 |
| 22–42d | 1.81 | 1.70 | 1.72 | 1.73 | 0.02 | 0.216 |
| 1–42 d | 1.64a | 1.57b | 1.57b | 1.55b | 0.01 | 0.014 |
Means within a row with no common superscripts differ (P < 0.05).
Abbreviations: ADG, average daily gain; ADFI, average daily feed intake; ANT, basal diet supplemented with 500 mg/kg terramycin calcium; APS, basal diet supplemented with 300 mg/kg Astragalus membranaceus polysaccharides; BW, body weight; CON, control group with basal diet; FCR, feed conversion ratio; GPS, basal diet supplemented with 150 mg/kg Glycyrrhiza uralensis polysaccharides; SEM, pooled standard error of the mean.
Serum Immunoglobulins and Inflammatory Factors
The results of the effects of APS and GPS on serum immunoglobulins and inflammatory factors of broilers at 42 d of age are displayed in Table 4. Serum IgA and IgM contents in ANT, APS, and GPS groups were remarkably elevated (P < 0.05) compared with the control group, while serum IgG level in APS and GPS groups was higher than that in the control group (P < 0.05). Furthermore, broilers fed diets supplemented with ANT, APS, and GPS had lower serum levels of TNF-α, IL-1β, and IL-6 than those of control group.
Table 4.
Effects of dietary supplementation of ANT, APS, and GPS on serum immunoglobulins and inflammatory factors in broilers (n = 6).
| Treatments |
||||||
|---|---|---|---|---|---|---|
| Items | CON | ANT | APS | GPS | SEM | P-value |
| IgA (ng/mL) | 2.88b | 4.49a | 4.83a | 3.98a | 0.23 | 0.007 |
| IgM (ng/mL) | 1.97b | 2.82a | 2.59a | 2.59a | 0.11 | 0.036 |
| IgG (ng/mL) | 41.86c | 53.40bc | 79.72a | 65.03ab | 3.77 | <0.001 |
| TNF-α (pg/mL) | 39.72a | 26.94b | 24.19b | 21.25b | 1.71 | <0.001 |
| IL-1β (pg/mL) | 290.42a | 195.3b | 173.51b | 170.25b | 13.1 | <0.001 |
| IL-6 (pg/mL) | 134.05a | 64.85b | 55.18b | 46.23b | 8.67 | <0.001 |
Means within a row with no common superscripts differ (P < 0.05).
Abbreviations: ANT, basal diet supplemented with 500 mg/kg terramycin calcium; APS, basal diet supplemented with 300 mg/kg Astragalus membranaceus polysaccharides; CON, control group with basal diet; GPS, basal diet supplemented with 150 mg/kg Glycyrrhiza uralensis polysaccharides; IgA, immunoglobulin A; IgM, immunoglobulin M; IgG, immunoglobulin G; TNF-α, tumor necrosis factor-α; IL-β, interleukin-1β; IL-6, interleukin-6; SEM, pooled standard error of the mean.
Intestinal Morphology
The influence of dietary APS and GPS addition on intestinal morphology of broilers at 42 d is shown in Table 5. The VH of duodenum, jejunum, and ileum in APS and GPS group was higher (P < 0.05) than that in control group, while dietary ANT supplementation resulted in higher (P < 0.05) VH in ileum. However, there were no statistically significant differences (P > 0.05) among the 4 dietary treatments regarding to CD of duodenum, jejunum, and ileum. In addition, the ratio of VH to CD of duodenum and jejunum was increased (P < 0.05) with dietary APS and GPS addition, and the value of ileum was increased (P < 0.05) with ANT, APS, and GPS supplementation.
Table 5.
Effects of dietary supplementation of ANT, APS, and GPS on intestine morphology in broilers (n = 6).
| Treatments |
||||||
|---|---|---|---|---|---|---|
| Items | CON | ANT | APS | GPS | SEM | P-value |
| Duodenum | ||||||
| VH, μm | 1,354.90b | 1,397.11b | 1,484.59a | 1,471.39a | 14.30 | <0.001 |
| CD, μm | 389.61 | 384.56 | 375.00 | 373.74 | 3.44 | 0.307 |
| VH/CD | 3.49b | 3.64b | 3.96a | 3.94a | 0.05 | <0.001 |
| Jejunum | ||||||
| VH, μm | 1,181.67b | 1,249.00ab | 1,285.65a | 1,320.09a | 18.05 | 0.031 |
| CD, μm | 284.25 | 282.78 | 259.57 | 263.32 | 6.67 | 0.450 |
| VH/CD | 4.22b | 4.48ab | 4.97a | 5.03a | 0.11 | 0.022 |
| Ileum | ||||||
| VH, μm | 925.63b | 1,094.92a | 1,053.82a | 1,052.37a | 16.74 | <0.001 |
| CD, μm | 262.15 | 253.26 | 241.01 | 239.16 | 4.06 | 0.144 |
| VH/CD | 3.56b | 4.35a | 4.38a | 4.42a | 0.11 | 0.003 |
Means within a row with no common superscripts differ (P < 0.05).
Abbreviations: ANT, basal diet supplemented with 500 mg/kg terramycin calcium; APS, basal diet supplemented with 300 mg/kg Astragalus membranaceus polysaccharides; CD, crypt depth; CON, control group with basal diet; GPS, basal diet supplemented with 150 mg/kg Glycyrrhiza uralensis polysaccharides; SEM, pooled standard error of the mean; VH, villus height; VH/CD, the ratio of villus height to crypt depth.
Serum DAO Activity and D-LA Concentration
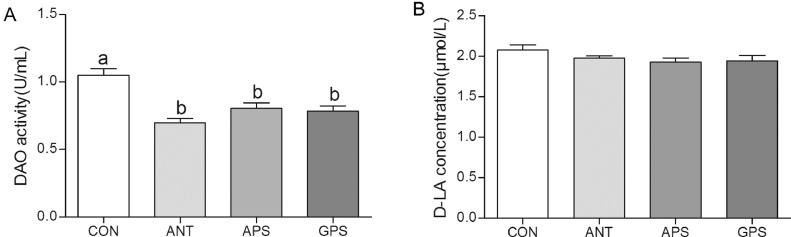
As shown in Figure 1A, serum DAO activity is reduced (P < 0.05) in the ANT, APS, and GPS group compared with the control group. However, dietary treatments did not affect (P > 0.05) the serum D-LA level (Figure 1B).
Figure 1.
Effects of ANT, APS, and GPS on serum diamine oxidase (DAO) (A) and D-lactic acid (D-LA) (B) level in broilers. Data are means ± SEM, n = 6. Means with different letters indicate significant difference (P < 0.05). Abbreviations: ANT, basal diet supplemented with 500 mg/kg terramycin calcium; APS, basal diet supplemented with 300 mg/kg Astragalus membranaceus polysaccharides; CON, control group with basal diet; GPS, basal diet supplemented with 150 mg/kg Glycyrrhiza uralensis polysaccharides.
Relative mRNA Expression of Occludin, Claudin-1, ZO-1, and MUC2 in Small Intestine
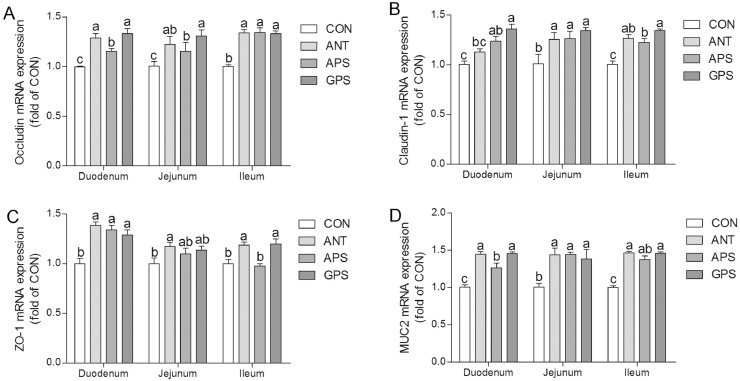
As indicated in Figure 2A, dietary ANT, APS, and GPS addition increase (P < 0.05) the mRNA expression of occludin in duodenum, jejunum, and ileum. Moreover, the GPS group showed higher (P < 0.05) occludin mRNA expression in duodenum and jejunum in comparison with the APS group. The mRNA expression of claudin-1 in duodenum was improved (P < 0.05) with dietary APS and GPS supplementation but was not significantly affected (P > 0.05) by dietary ANT supplementation (Figure 2B). Furthermore, birds from the ANT, APS, and GPS group showed higher (P < 0.05) claudin-1 mRNA expression level in jejunum and ileum than that from the control group. The ZO-1 mRNA expression levels in duodenum and ileum were upregulated (P < 0.05) with dietary ANT and GPS addition, while ZO-1 mRNA expression in duodenum was increased (P < 0.05) with APS supplementation (Figure 2C). Compared with control diet, supplementation of diets with ANT, APS,and GPS resulted in increased (P < 0.05) level of MUC2 mRNA expresion in duodenum, jejunum, and ileum (Figure 2D). In addition, duodenal MUC2 mRNA expression in APS group was less (P < 0.05) than that in the ANT and GPS group.
Figure 2.
Effects of ANT, APS, and GPS on mRNA expression of occludin (A), claudin-1 (B), zonula occludens-1(ZO-1) (C), and mucin-2(MUC2)(D) in small intestine. Data are means ± SEM, n = 6. Means with different letters indicate significant difference (P < 0.05). Abbreviations: ANT, basal diet supplemented with 500 mg/kg terramycin calcium; APS, basal diet supplemented with 300 mg/kg Astragalus membranaceus polysaccharides; CON, control group with basal diet; GPS, basal diet supplemented with 150 mg/kg Glycyrrhiza uralensis polysaccharides.
Cecal Microbial Diversity and Composition
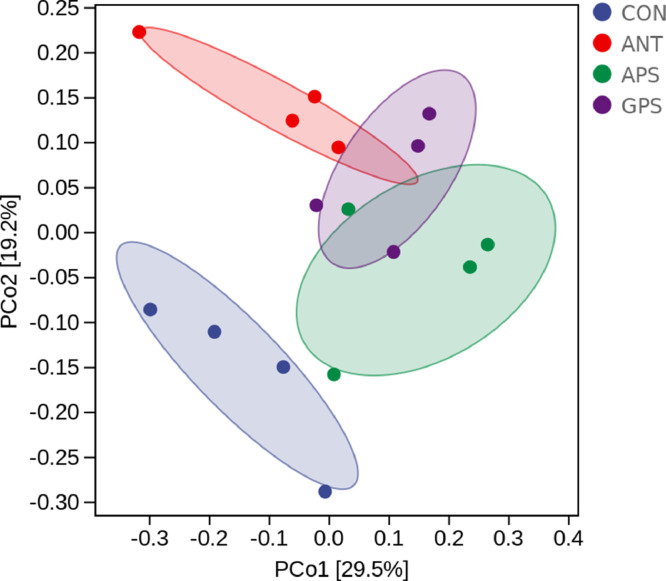
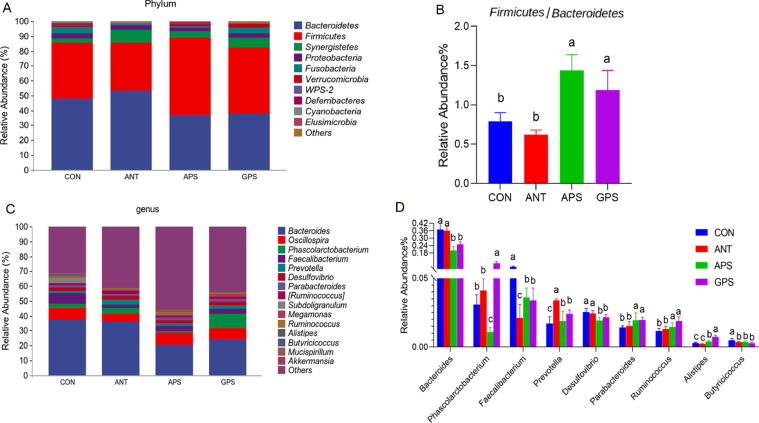
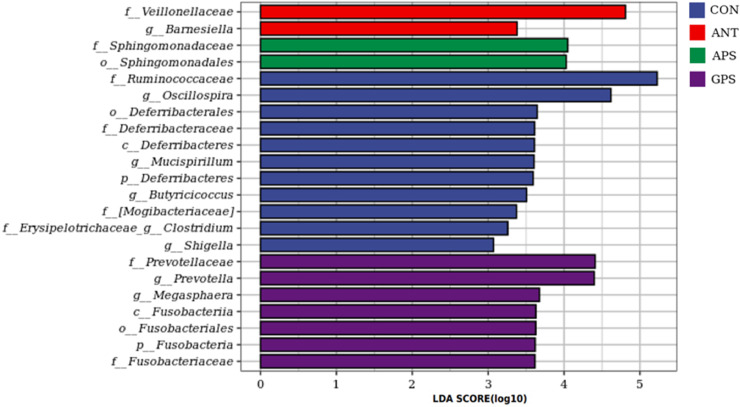
The 16S rRNA gene sequencing was performed to investigate the cecal microbiota of broilers from the 4 dietary treatments. After size filtering, quality control, and chimera removal, a total of 1,081,442 high-quality sequences were obtained with an average length of 416 bp. Based on the 97% identity level, these sequences were decomposed into 13,963 operational taxonomic units (OTUs). The data of alpha diversity indexes are presented in Table 6. The GPS group had higher (P < 0.05) Chao1 and Observed species compared with the control and ANT group. The Shannon index in the ANT, APS, and GPS group was greater (P < 0.05) than that in the control. There was no significant difference in the Simpson index among all groups (P > 0.05). Beta diversity is illustrated by Principal coordinate analysis (PCoA) in Figure 3, which revealed that cecal microbial community among the four groups formed distinct clusters. The treatment groups were well separated with 29.5 and 19.2% variation by the principal components PC1 and PC2, respectively. The relative abundance at phylum and genus levels was studied. At the phylum level, Bacteroidetes, Firmicutes, Synergistetes, Proteobacteria, and Fusobacteria were the 5 major bacteria in the cecum of broilers, accounting for more than 96% of the total cecum bacterial community (Figure 4A). Compared to the control and ANT group, the relative abundance of Bacteroidetes decreased while that of Firmicutes and the proportion of Firmicutes to Bacteroidetes increased in the cecal microbiota of the APS and GPS group (Figures 4A and 4B). At the genus level, Bacteroides (20–37%) was the dominant genera across all groups (Figure 4C). There was notable difference in the relative abundance of 9 of the top 15 genera among the 4 treatment groups (Figure 4D). The characteristics of bacterial taxonomic abundance in the collected samples were further analyzed using LEfSe algorithm (Figure 5). In the aggregate, 22 genera were detected with a LDA threshold > 2. f_Ruminococcaceae, g_Oscillospira, o_Deferribacterales, f_Deferribacteraceae, c_Deferribacteres, g_Mucispirillum, p_Deferribacteres, g_Butyricicoccus, f_Mogibacteriaceae, f_Erysipelotrichaceae_ g_Clostridium, and g_Shigella were enhanced in CON group. f_Veillonellaceae and g_Barnesiella were enhanced in ANT group. f_Sphingomonadaceae and o_Sphingomonadaceae were enriched in APS group, while f_Prevotellaceae, g_Prevotella, g_Megasphaera, c_Fusobacteriia, o_Fusobacteriales, p_Fusobacteria, and p_Fusobacteriaceae were enriched in GPS group.
Table 6.
Effects of dietary supplementation of ANT, APS, and GPS on alpha diversity index of cecal microbiota in broilers (n = 4).
| Treatments |
||||||
|---|---|---|---|---|---|---|
| Items | CON | ANT | APS | GPS | SEM | P-value |
| Chao1 | 1,758.65b | 1,838.56b | 1,898.76ab | 2,099.48a | 47.22 | 0.046 |
| Shannon | 7.88b | 8.10a | 8.21a | 8.09a | 0.040 | 0.009 |
| Simpson | 0.98 | 0.99 | 0.98 | 0.98 | 0.003 | 0.479 |
| Observed species | 1613.98b | 1597.35b | 1775.28ab | 1926.23a | 46.17 | 0.017 |
Means within a row with no common superscripts differ (P < 0.05).
Abbreviations: ANT, basal diet supplemented with 500 mg/kg terramycin calcium; APS, basal diet supplemented with 300 mg/kg Astragalus membranaceus polysaccharides; CON, control group with basal diet; GPS, basal diet supplemented with 150 mg/kg Glycyrrhiza uralensis polysaccharides; SEM, pooled standard error of the mean.
Figure 3.
Principal coordinate analysis (PCoA) of the cecal microbiota based on Weighted UniFrac distance (n = 4). The scatterplot of the PCoA scores shows four different clusters. Abbreviations: ANT, basal diet supplemented with 500 mg/kg terramycin calcium; APS, basal diet supplemented with 300 mg/kg Astragalus membranaceus polysaccharides; CON, control group with basal diet; GPS, basal diet supplemented with 150 mg/kg Glycyrrhiza uralensis polysaccharides.
Figure 4.
Effects of ANT, APS, and GPS on microbial composition in the cecum of broilers. (A) Relative abundance of cecal microbiota at the phylum level. (B) The proportion of Firmicutes to Bacteroidetes. (C) Relative abundance of cecal microbiota at the genus level. (D) Relative abundance difference analysis of cecal bacterial species at the genus level. Abbreviations: ANT, basal diet supplemented with 500 mg/kg terramycin calcium; APS, basal diet supplemented with 300 mg/kg Astragalus membranaceus polysaccharides; CON, control group with basal diet; GPS, basal diet supplemented with 150 mg/kg Glycyrrhiza uralensis polysaccharides.
Figure 5.
Linear discriminant analysis (LDA) effect size (LEfSe) analysis of cecal microbiota. LDA scores generated for the differentially abundant microbiota (LDA > 2, P < 0.05). Abbreviations: ANT, basal diet supplemented with 500 mg/kg terramycin calcium; APS, basal diet supplemented with 300 mg/kg Astragalus membranaceus polysaccharides; CON, control group with basal diet; GPS, basal diet supplemented with 150 mg/kg Glycyrrhiza uralensis polysaccharides.
Correlation Between the Differential Microbes and Main Parameters
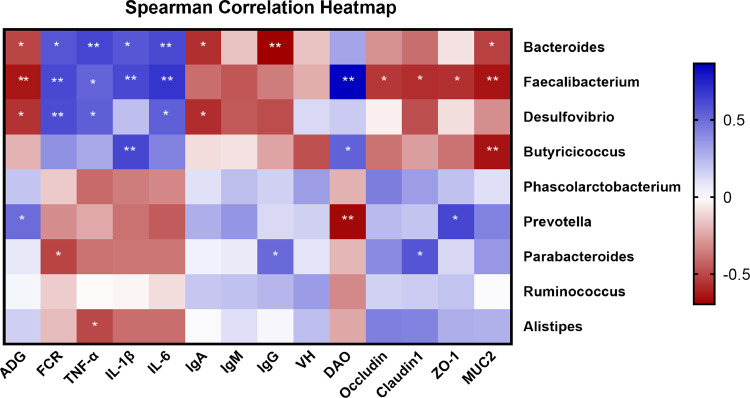
In order to predict the correlation between the differential cecal microbial community of genera and the phenotypes of broilers, a Spearman correlation matrix was conducted. As shown in Figure 6, Bacteroides is positively correlated with FCR, TNF-α, IL-1β, and IL-6, but negatively correlated with ADG, IgA, IgG, and MUC2 (P < 0.05). Similarly, Faecalibacterium was significantly positively correlated with FCR, TNF-α, IL-1β, IL-6, and DAO, but negatively correlated with ADG, and jejunal mRNA expression of occludin, claudin-1, ZO-1, and MUC2 (P < 0.05). Desulfovibrio was positively correlated with FCR, TNF-α, and IL-6, but negatively correlated with ADG and IgA (P < 0.05). Butyricicoccus was positively correlated with IL-1β and DAO, but negatively correlated with MUC2 (P < 0.05). Prevotella was positively correlated with ADG and ZO-1, but negatively correlated with DAO (P < 0.05). Parabacteroides was negatively correlated with FCR, but positively correlated with IgG and claudin-1 (P < 0.05). The serum TNF-α content was negatively correlated with the relative abundance of Alistipes (P < 0.05). There were no correlations for Phascolarctobacterium and Ruminococcus.
Figure 6.
Correlation between the differential cecal microbial community of genera and the phenotype of broilers by Spearman correlation analysis. Asterisks indicate significant correlations (* P ≤ 0.05, ** P ≤ 0.01.). Blue indicates a positive correlation, while red indicates a negative correlation. The deeper color means the greater correlation. VH, Occludin, Claudin-1, ZO-1, and MUC2 are the jejunal values. Abbreviations: ADG, average daily gain; DAO, diamine oxidase; FCR, feed conversion ratio; IL-1β, interleukin-1β; IL-6, interleukin-6; IgA, immunoglobulin A; IgM, immunoglobulin M; IgG, immunoglobulin G; MUC-2, mucin-2; VH, villus height; TNF-α, tumor necrosis factor-α; ZO-1, zonula occludens-1.
DISCUSSION
Several recent studies have verified that Chinese herbal polysaccharides can improve poultry growth performance (Ao and Kim, 2020; Long et al., 2020; Liu et al., 2020; Long et al., 2021). In the present study, it was shown that dietary supplementation of APS and GPS improved ADG and lowered FCR of broilers from 1 to 42 d. The growth-promoting effects of APS and GPS were equal to that of ANT, suggesting that APS and GPS could be alternatives to antibiotic growth promoters in poultry diets. Similar results were reported by Wu (2018), who observed the improvement of body weight gain and FCR of juvenile broilers fed diet containing 1 g kg−1 APS. Besides, Zhang et al. (2021) found that supplementation of the diet with 200 mg kg−1 GCP resulted in lowered FCR of broilers from 22 to 42 d. The growth-promoting effect of APS and GPS may be directly due to the improvement of digestibility. Wu (2018) found that dietary APS administration increased the activities of digestive enzymes including amylase, lipase, and protease in juvenile broilers. In addition, Yin et al. (2009) reported that the inclusion of APS to diet enhanced ileal digestibilities and serum concentrations of amino acids of early weaned pigs.
Immunoglobulins are produced by B lymphocytes and have key roles in immune function in poultry. It has been well-known that plant polysaccharides possess immunomodulatory properties (Long et al., 2020; Zhao et al., 2020; Liu et al., 2021a). Liao et al. (2021) reported that oral administration of APS could promote the production of secretory IgA in ileal mucosa of Muscovy ducklings infected with Muscovy duck reovirus. Wu et al. (2022) verified GPS as an immune booster capable of enhancing the antibody potency of the Newcastle disease vaccine in chickens. Consistent with these findings, we observed in this study that dietary APS and GPS supplementation promoted the humoral immune response of broilers, leading to elevation in serum IgA, IgM, and IgG concentrations. Proinflammatory cytokines are produced predominantly by activated macrophages and are involved in the upregulation of inflammatory reactions. Wang et al. (2013) previously found that APS could mitigate the LPS-induced increase of proinflammatory factors TNF-α, IL-1β, and IL-8 in Caco2 cells. And the authors further evidenced that APS possessed anti-inflammatory activities by down-regulating the expression of jejunal TNF-α and IL-1β in LPS-stimulated broilers (Wang et al., 2014). Similarly, dietary GPS has been shown to be effective in reducing IL-1β expression in the liver of broilers challenged with LPS. Our current results indicated that feeding of APS and GPS decreased serum TNF-α, IL-1β, and IL-6 levels in broilers. Taken together, these findings suggest that APS and GPS improve immunity function and exert anti-inflammatory activity in broilers.
The morphological indexes like villus length, crypt depth, and VH/CD ratio are important indicators of the maturity and functional capacity of small intestine. In the current study, dietary APS and GPS supplementation promoted the growth of the villi, thus resulting in an increase of VH/CD ratio in small intestine (duodenum, jejunum, and ileum). These results were in agreement with those of Wang et al. (2021) who showed that dietary APS increased VH of 3 small intestine segments and VH/CD ratio of the jejunum and ileum. Liao et al. (2021) revealed that APS supplementation could maintain intestinal integrity of Muscovy ducklings infected with Muscovy duck reovirus by increasing VH, VH/CD ratio and wall thickness of the small intestine. The study on weaned piglets also showed that dietary APS could improve jejunal VH and V/C ratio (Yang et al., 2019a). However, to the best of our knowledge, no available data have evaluated the impact of GPS inclusion in diets on intestinal morphology of broilers. Mohammed et al. (2020) reported that dietary Glycyrrhiza glabra extract supplementation maintained the intestinal and gill morphology of Nile tilapia fingerlings exposed to heavy metals. The increase of VH and VH/ CD ratio may increase absorptive surface area and thus improve the digestive and absorptive function of small intestine (Boguslawska et al., 2012).
Diamine oxidase is an intracellular enzyme secreted by intestinal epithelia, and it exists only in the intestinal mucosa and ciliated cells, while D-lactic acid is a metabolic product of intestinal bacterial fermentation (Nieto et al., 2000). A complete intestinal mucosal barrier can prevent DAO and D-LA from entering the blood circulation. Thus, serum DAO activity and D-LA concentration are biomarkers for monitoring intestinal barrier function. The present study showed that neither dietary supplementation with APS nor GPS affected serum D-LA concentration. However, birds in the APS and GPS group had lower serum DAO activity. The data suggested that dietary APS and GPS may be beneficial to the intestinal mucosal barrier function of broilers. We further evaluated the effects of APS and GPS on tight junctions (TJs) of small intestine, which played a key role in the regulation of intestinal permeability by maintaining intestinal barrier integrity and ensuring normal barrier function (Turner, 2009). The integral membrane proteins (such as occludin and claudins) and cytoplasmic plaque protein ZO-1 are the major components of tight junctions. In this study, dietary APS supplementation upregulated the mRNA expression of occludin and claudin-1 in the 3 small intestine segments, while birds from the GPS group had higher mRNA expression of occludin and claudin-1 in the 4 small intestine segments as well as ZO-1 in duodenum and ileum than birds from the control. Similar with our present findings, it was reported that APS could elevate the mRNA expression of occludin and ZO-1 and the number of goblet cells in jejunum of broilers (Wang et al., 2021). Moreover, Wang et al. (2020) evidenced the protective effect of dietary APS in LPS-challenged weaned piglets by enhancing jejunal occludin and claudin expression. The effect of GPS on intestinal barrier function has not been described. However, dose-dependent increase of jejunal occludin in broilers was observed when the birds fed diets supplemented with Glycyrrhiza glabra extract (Ibrahim et al., 2020). Apart from occludin, claudin-1, and ZO-1, the increase of MUC-2 mRNA expression in small intestine of birds in the APS and GPS group was also documented in our study. MUC-2, secreted by goblet cells of the intestine, is involved in maintaining the thickness of the intestinal mucus layer. These findings suggested that dietary APS and GPS supplementation benefits intestinal health of broilers by improving intestinal morphology and barrier function.
The gut microflora exists in a symbiotic relationship with its host through the regulation of digestion, intestine development, nutrients absorption, and metabolism, as well as the innate and adaptive immune system (Mahmood and Guo, 2020). The findings of the present study showed that dietary supplementation with APS and GPS improved the richness and diversity of cecal microbiota of broilers as indicated by increased Chao 1 index, Shannon index and Observed species. The result of beta diversity revealed that microbiota structure in the APS and GPS group was clearly distinguished from that of the control group. The taxonomical composition analysis showed that Firmicutes and Bacteroidetes were the 2 most dominant phyla of the cecum in broilers, which was in line with previous studies (Xiang et al., 2020; Liu et al., 2021b). The proportion of Firmicutes to Bacteroidetes is significantly associated with the capacity for energy harvest by host (Kasai et al., 2015). It has been noticed that Firmicutes and Bacteroidetes account for 71.36 and 23.40% in fat chickens and 53.44 and 41.09% in lean chickens, respectively (Hou et al., 2016). In the present study, we found that dietary APS and GPS supplementation increased the ratio of Firmicutes to Bacteroidetes, which was consistent with the higher body weight gain in the 2 groups compared with the control group.
Next, we explored the links of differential cecal microbes in genus level and the phenotypes of broilers. It has been reported that Bacteroides and Desulfovibrio are the main producers of LPS (Diling et al., 2017). Our results revealed that Bacteroides and Desulfovibrio were positively related to serum inflammatory cytokines TNF-α, IL-1β, and IL-6. The abundances of Bacteroides and Desulfovibrio decreased in the APS and GPS group, indicating that APS and GPS might inhibit the proliferation of these bacteria and hence reduce the secretion of LPS, which resulted in the decrease of serum inflammatory cytokines. APS and GPS intake could notably increase the levels of Prevotella, Parabacteroides, and Ruminococcus, which were reported to be involved in the degradation of polysaccharides and the production of short-chain fatty acids (Zhang et al., 2017; Chen et al., 2019). Prevotella is a beneficial bacterium that exhibits a growth promoting effect because it can reduce inflammation by decreasing intestinal permeability (Zhang et al., 2018). In this study, our data also showed that the abundance of Prevotella correlated positively with ADG of birds, but correlated negatively with serum DAO activity. Moreover, Prevotella and Bacteroides are the two main genera of Bacteroidetes, and these 2 genera compete for the same niche in the gut (Kovatcheva et al., 2015). The decrease in Bacteroidetes after APS and GPS consumption was attributed to downregulation of the abundance of Bacteroides. According to the study of Guo et al.(2021), the genus Parabacteroides could modulate host immune response by increasing the sIgA and butyric acid levels in the intestine. Zhang et al. (2017) found that Longan polysaccharides intake increased the abundance of Parabacteroides accompanied with the elevation of serum immunoglobulins IgA, IgG, and IgM. Similarly, the results of this study indicated that Parabacteroides was positively correlated with IgG. Faecalibacterium, belonging to Phylum Firmicutes, have limited ability to utilize polysaccharides including arabinogalactan, xylan, and soluble starch (Ferreira-Halder et al., 2017). Liu et al. (2021b) figured out that APS administration significantly decreased the abundance of Faecalibacterium in broilers, and our results were in agreement with this finding. Genus Alistipes from phylum Bacteroidales can produce succinic acid and other long-chain fatty acids such as C15, which is generally considered beneficial to the host gut (Chen et al. 2020). In this trial, the relative abundance of Alistipes was elevated in the APS and GPS treatment. This was similar to the result found by Chen et al. (2019), who exhibited that feeding diets supplemented with pectic heteropolysaccharides increased the population of Alistipes in cyclophosphamide-treated mice.
In conclusion, polysaccharides derived from Astragalus membranaceus and Glycyrrhiza uralensis could increase the body weight gain and decrease feed conversion ratio of broilers, and benefit intestinal health by improving intestinal morphology and mucosal barrier function. Moreover, dietary supplementation with APS and GPS increased the richness and diversity of cecal microbiota of broilers, and significantly altered microbial community composition. These findings provide better understanding of the mechanism of growth-promoting effects of APS and GPS, which could further provide useful information for developing effective and safe antibiotic alternatives in poultry industry.
DISCLOSURES
The authors have no conflicts of interest to report.
ACKNOWLEDGMENTS
This work was supported by Zhongyuan high level talents special support plan (Grant No. 204200510010)
REFERENCES
- Ao X., Kim I.H. Effects of Achyranthes bidentata polysaccharides on performance, immunity, antioxidant capacity, and meat quality in Pekin ducks. Poult. Sci. 2020;99:4884–4891. doi: 10.1016/j.psj.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguslawska M., Otrowska A., Burlikowska K. Dietary fructans and their potential beneficial influence on health and performance parametrs in broiler chickens. J. Cent. Eur. Agric. 2012;13:272–291. [Google Scholar]
- Castanon J.I.R. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Chen D., Chen G., Ding Y., Wan P., Peng Y., Chen C., Ye H., Zeng X., Ran L. Polysaccharides from the flowers of tea (Camellia sinensis L.) modulate gut health and ameliorate cyclophosphamide-induced immunosuppression. J. Funct. Foods. 2019;61 [Google Scholar]
- Chen Y., Wang J., Yu L., Xu T., Zhu N. Microbiota and metabolome responses in the cecum and serum of broiler chickens fed with plant essential oils or virginiamycin. Sci. Rep. 2020;10:5382. doi: 10.1038/s41598-020-60135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diling C., Chaoqun Z., Jian Y., Jian L., Jiyan S., Yizhen X., Guoxiao L. Immunomodulatory activities of a fungal protein extracted from Hericium erinaceus through regulating the gut microbiota. Front. Immunol. 2017;8:666. doi: 10.3389/fimmu.2017.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Halder C.V., Faria A.V.d.S., Andrade S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017;31:643–648. doi: 10.1016/j.bpg.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Guo C.E., Cui Q., Cheng J., Chen J., Zhao Z., Guo R., Dai X., Wei Z., Li W. Probiotic-fermented Chinese dwarf cherry [Cerasus humilis (Bge.) Sok.] juice modulates the intestinal mucosal barrier and increases the abundance of Akkermansia in the gut in association with polyphenols. J. Funct. Foods. 2021;80 [Google Scholar]
- Hou Q., Kwok L.-Y., Zheng Y., Wang L., Guo Z., Zhang J., Huang W., Wang Y., Leng L., Li H., Zhang H. Differential fecal microbiota are retained in broiler chicken lines divergently selected for fatness traits. Sci. Rep. 2016;6:37376. doi: 10.1038/srep37376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim D., Sewid A.H., Arisha A.H., abd El-fattah A.H., Abdelaziz A.M., Al-Jabr O.A., Kishawy A.T.Y. Influence of Glycyrrhiza glabra extract on growth, gene expression of gut integrity, and Campylobacter jejuni colonization in broiler chickens. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.612063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai C., Sugimoto K., Moritani I., Tanaka J., Oya Y., Inoue H., Tameda M., Shiraki K., Ito M., Takei Y., Takase K. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015;15:100. doi: 10.1186/s12876-015-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Sci Tech. 2019;250:32–40. [Google Scholar]
- Kovatcheva D.P., Nilsson A., Akrami R., Lee Y.S., De V.F., Arora T., Hallen A., Martens E., Bjorck I., Backhed F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell. Metab. 2015;22:971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Liao L., Li J., Li J., Huang Y., Wu Y. Effects of Astragalus polysaccharides on intestinal morphology and intestinal immune cells of Muscovy ducklings infected with Muscovy duck reovirus. Poult. Sci. 2021;100:64–72. doi: 10.1016/j.psj.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.C., Guo Y., Zhao Z.H., Jha R., Balasubramanian B. Algae-derived polysaccharides promote growth performance by improving antioxidant capacity and intestinal barrier function in broiler chickens. Front. Vet. Sci. 2020;7:990. doi: 10.3389/fvets.2020.601336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.C., Ou B.H., Liang Z.L., Zhang R., Zhao Z.H. Algae-derived polysaccharides supplementation ameliorates heat stress-induced impairment of bursa of Fabricius via modulating NF-κB signaling pathway in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.S., Li S., Wang X.F., Xing T., Li J.L., Zhu X.D., Zhang L., Gao F. Microbiota populations and short-chain fatty acids production in cecum of immunosuppressed broilers consuming diets containing γ-irradiated Astragalus polysaccharides. Poult. Sci. 2021;100:273–282. doi: 10.1016/j.psj.2020.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L.N., Kang B.J., Jiang Q., Chen J.S. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult. Sci. 2020;99:744–751. doi: 10.1016/j.psj.2019.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L.N., Zhang H.H., Wang F., Yin Y.X., Yang L.Y., Chen J.S. Effects of polysaccharide-enriched Acanthopanax senticosus extract on growth performance, immune function, antioxidation, and ileal microbial populations in broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood T., Guo Y. Dietary fiber and chicken microbiome interaction: where will it lead to? Anim. Nutr. 2020;6:1–8. doi: 10.1016/j.aninu.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed E., Kamel M., El Iraqi K., Tawfik A.M., Khattab M.S., Elsabagh M. Zingiber officinale and Glycyrrhiza glabra, individually or in combination, reduce heavy metal accumulation and improve growth performance and immune status in Nile tilapia, Oreochromis niloticus. Aquacult. Res. 2020;51:1933–1941. [Google Scholar]
- Mutaillifu P., Bobakulov K., Abuduwaili A., Huojiaaihemaiti H., Nuerxiati R., Aisa H.A., Yili A. Structural characterization and antioxidant activities of a water soluble polysaccharide isolated from Glycyrrhiza glabra. Int. J. Biol. Macromol. 2020;144:751–759. doi: 10.1016/j.ijbiomac.2019.11.245. [DOI] [PubMed] [Google Scholar]
- Nieto N., Torres M.I., Fernández M.I., Girón M.D., Ríos A., Suárez M.D., Gil A. Experimental ulcerative colitis impairs antioxidant defense system in rat intestine. Digest. Dis. Sci. 2000;45:1820–1827. doi: 10.1023/a:1005565708038. [DOI] [PubMed] [Google Scholar]
- Shen H., Zeng G., Sun B., Cai X., Bi L., Tang G., Yang Y. A polysaccharide from Glycyrrhiza inflata Licorice inhibits proliferation of human oral cancer cells by inducing apoptosis via mitochondrial pathway. Tumor Biol. 2015;36:4825–4831. doi: 10.1007/s13277-015-3135-6. [DOI] [PubMed] [Google Scholar]
- Suresh G., Das R.K., Kaur Brar S., Rouissi T., Avalos Ramirez A., Chorfi Y., Godbout S. Alternatives to antibiotics in poultry feed: molecular perspectives. Crit. Rev. Microbiol. 2018;44:318–335. doi: 10.1080/1040841X.2017.1373062. [DOI] [PubMed] [Google Scholar]
- Turner J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Wang K., Zhang H., Han Q., Lan J., Chen G., Cao G., Yang C. Effects of Astragalus and Ginseng polysaccharides on growth performance, immune function and intestinal barrier in weaned piglets challenged with lipopolysaccharide. J. Anim. Physiol Anim. Nutr. 2020;104:1096–1105. doi: 10.1111/jpn.13244. [DOI] [PubMed] [Google Scholar]
- Wang Q., Wang X.F., Xing T., Li J.L., Zhu X.D., Zhang L., Gao F. The combined impact of xylo-oligosaccharides and gamma-irradiated Astragalus polysaccharides on growth performance and intestinal mucosal barrier function of broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Li Y., Yang X., Yao J. Astragalus polysaccharide reduces inflammatory response by decreasing permeability of LPS-infected Caco2 cells. Int. J. Biol. Macromol. 2013;61:347–352. doi: 10.1016/j.ijbiomac.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Wang X., Shen J., Li S., Zhi L., Yang X., Yao J. Sulfated Astragalus polysaccharide regulates the inflammatory reaction in LPS-infected broiler chicks. Int. J. Biol. Macromol. 2014;69:146–150. doi: 10.1016/j.ijbiomac.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Witte W. Selective pressure by antibiotic use in livestock. Int. J. Antimicrob Ag. 2000;16:19–24. doi: 10.1016/s0924-8579(00)00301-0. [DOI] [PubMed] [Google Scholar]
- Wu S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018;97:3489–3493. doi: 10.3382/ps/pey220. [DOI] [PubMed] [Google Scholar]
- Wu Y., Li N., Zhang T., Che Y., Duan K., Wang Y., Zhou H., Wan X., Lei H., Nguyễn A.D., De Souza C., Li K., Wu Y., Liu J., Wang D. Glycyrrhiza polysaccharides can improve and prolong the response of chickens to the Newcastle disease vaccine. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.M., Han Q.J., Wang K.L., Xu Y.L., Lan J.H., Cao G.T. Astragalus and Ginseng polysaccharides improve developmental, intestinal morphological, and immune functional characters of weaned piglets. Front. Physiol. 2019;10:418. doi: 10.3389/fphys.2019.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F.G., Liu Y.L., Yin Y.L., Kong X.F., Huang R.L., Li T.J., Wu G.Y., Hou Y. Dietary supplementation with Astragalus polysaccharide enhances ileal digestibilities and serum concentrations of amino acids in early weaned piglets. Amino Acids. 2009;37:263–270. doi: 10.1007/s00726-008-0142-6. [DOI] [PubMed] [Google Scholar]
- Zhang C., Li C.X., Shao Q., Chen W.B., Ma L., Xu W.H., Li Y.X., Huang S.C., Ma Y.B. Effects of Glycyrrhiza polysaccharide in diet on growth performance, serum antioxidant capacity, and biochemistry of broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yang G., Wen Y., Liu S., Li C., Yang R., Li W. Intestinal microbiota are involved in the immunomodulatory activities of Longan polysaccharide. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201700466. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kang C., Wang X.L., Zhou M., Chen M.T., Zhu X.H., Liu K., Wang B., Zhang Q.Y., Zhu J.D., Mi M.T. Dietary factors modulate colonic tumorigenesis through the interaction of gut microbiota and host chloride channels. Mol. Nutr. Food Res. 2018;62 doi: 10.1002/mnfr.201700554. [DOI] [PubMed] [Google Scholar]
- Zhao Z.T., Ye X.M., Ouyang K.H., Wang W.J. Effects of polysaccharides from Yingshan Yunwu tea on meat quality, immune status and intestinal microflora in chickens. Int. J. Biol. Macromol. 2020;155:61–70. doi: 10.1016/j.ijbiomac.2020.03.198. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Ren W., Zhang L., Zhang Y., Liu D., Liu Y. A review of the pharmacological action of Astragalus polysaccharide. Front. Pharmacol. 2020;11:349. doi: 10.3389/fphar.2020.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]