Abstract
Atherosclerosis is a cardiovascular disease, which is characterized by the interaction between carbohydrates, lipids, cells and various other molecules and genetic factors. Previous studies have demonstrated that resveratrol (RV) served protective roles in numerous types of human disease by regulating different signaling pathways. The aim of the present study was to investigate the therapeutic effects of RV and analyze the potential RV-mediated mechanism in umbilical vein endothelial cells (UVECS) in atherosclerosis model mice. Reverse transcription-quantitative PCR, western blotting and immunohistochemistry were used to analyze the therapeutic effects of RV both in vitro and in vivo. The results demonstrated that total cholesterol, triglycerides, low-density lipoprotein cholesterin and high-density lipoprotein cholesterin levels were significantly decreased in the RV group compared with the control group. RV demonstrated significant anti-atherosclerotic activity, which was determined through the atherogenic index, 3-hydroxy-3-methyl-glutaryl-Coa (HMG-CoA) reductase activity and marker enzymes, such as lactate dehydrogenase, creatine phosphokinase, aspartate transaminase, alanine transaminase and alkaline phosphatase. It was also observed that RV treatment significantly decreased the area of the arteriosclerotic lesion in the RV group compared with the control, as well as significantly decreasing the expression levels of matrix metalloproteinase-9 and CD40 ligand (CD40L) in arterial lesion tissue compared with the control group. Serum expression levels of tumor necrosis factor-α and C-reactive protein were also significantly decreased by RV treatment compared with the control group. Furthermore, RV treatment significantly decreased the expression levels of PI3K, AKT and mTOR in UVECS in vitro. In conclusion, these results suggested that the anti-atherosclerotic activity of RV may be due to its modulatory activity over the PI3K/AKT/mTOR signaling pathway. These findings suggested a potential novel treatment option for patients with atherosclerosis.
Keywords: resveratrol, atherosclerosis, inflammation, PI3K, AKT, mTOR
Introduction
Cardiovascular disease is the number one cause of death (~7.8%) worldwide, thus posing a significant threat to human health (1-3). Atherosclerosis is a cardiovascular disease that involves the biochemical narrowing of the diameter of blood vessels through the development of fatty streaks and plaques (4); and is characterized by the interaction between carbohydrates, lipids and genetic factors (5-7). Usai MV et al indicated that atherosclerosis is closely associated with the metabolic disorder of blood lipids (8); however, the mechanisms of atherosclerosis remain largely unknown. It is important to understand the mechanisms behind high fat diet-induced atherosclerosis to identify potential drugs that could relieve atherosclerosis.
Resveratrol (RV) is a multifunctional biological polyphenol that serves therapeutic roles in multiple types of human cardiovascular disease (9). A previous study demonstrated that RV exhibited neuroprotective effects in ischemic injury in rats through improving brain energy metabolism and alleviating oxidative stress (10). A study revealed that RV presented anti-oxidative and anti-inflammatory effects in the prevention or inhibition of age-related cardiovascular disease (11). In one study, the protective cardiovascular effects of RV were directly linked to improved heart contraction, which could be a potential target for the development of new clinical therapies for patients (12). Furthermore, a dietary and clinical perspective study observed that RV demonstrated cardioprotective benefits in the primary and secondary prevention of cardiovascular disease (13), with another study reporting that RV may be a potential agent for the prevention and treatment of cardiovascular disease (14); however, the potential RV-mediated mechanism remains largely understood.
Lipoprotein oxidation and oxidative processes in general serve important roles in the pathogenesis of atherosclerosis. Disorders of lipid metabolism manifest as elevations in the levels of plasma lipids and lipoprotein fractions, which in turn results in the development of cardiovascular disease (15). The Atherogenic Index of Plasma is a marker of cardiovascular disease (16). Several studies have demonstrated that total cholesterol (TC), triglyceride (TG), low density lipoprotein cholesterin (LDL-c) and high-density lipoprotein cholesterin (HDL-c) levels are responses to increased thickness of vessel walls in patients with atherosclerosis (17,18). A previous study indicated that RV may reduce serum cholesterol levels through downregulation of HMG-CoAreductase (HMGR) mRNA expression levels in hamsters fed a high-fat diet (HFD) (19). Total panax notoginsenosides are the main ingredients found in the root of Panax notoginseng (Burk) F.H. Chen, which belongs to the Araliaceae family. They are useful in preventing the development of atherosclerosis in apolipoprotein E-knockout mice by downregulating CD40 and matrix metalloproteinase-9 (MMP-9) expression (20). By contrast, it was demonstrated that increased expression levels of tumor necrosis factor (TNF)-α and C-reactive protein (CRP) promoted the development of early atherosclerosis by increasing the transcytosis of LDL across endothelial cells (21-23). In addition, activation of the mitochondrial-related AMP kinase/PI3K/AKT/endothelial nitric oxide synthase signaling pathway was observed to improve endothelial function and alleviate atherosclerosis (24). mTOR inhibitors have also been demonstrated to prevent lipid storage, increase LDL-c levels and activate lipolysis, which further lead to a decreased risk of developing atherosclerosis (25); however, the association between RV and the PI3K/AKT/mTOR signaling pathway have not been well investigated in umbilical vein endothelial cells (UVECS) in atherosclerosis.
Thus, the aim of the present study was to investigate the effects of RV treatment in atherosclerosis murine model. The regulatory effects of RV treatment on the expression of inflammatory markers, the AIP, 3-hydroxy-3-methyl-glutaryl-Coa (HMG-CoA) reductase activity and marker enzymes were analyzed. This study also indicated that RV treatment may mediate the PI3K/AKT/mTOR signaling pathway in UVECS through the administration of a PI3K inhibitor prior to RV treatment.
Materials and methods
Animal studies
The present study was carried out in strict accordance with the guidelines set by the Committee of the People's Hospital of Weifang. All animal experiments were approved by the Ethics Committee of the People's Hospital of Weifang (approval no. TPH20170812S08). A total of 36 Apolipoprotein E-deficient (ApoE-/-) mice (age, 6 weeks; sex, male; body weight, 18-23 g) were purchased from the Laboratory Animal Science Center at Peking University People's Hospital (Beijing, China). The mice were housed in a 12-h light/dark cycle at 24-26˚C and 60±10% humidity. All animals had access to food and water ad libitum.
To establish an atherosclerosis model, all ApoE-/- mice were fed a HFD, consisting of 21% fat from lard and 1.25% (wt/wt) cholesterol for 8 weeks. Subsequently, the ApoE-/- mice were randomly divided into 2 groups (n=12/group), a PBS and RV group and received an intraperitoneal injection of PBS or RV (50 mg/kg/day), respectively, for 5 weeks. At the end of experiment, all the mice were sacrificed following anesthesia with sodium pentobarbital (35 mg/kg) and cervical dislocation.
Immunohistochemistry (IHC) staining
Following euthanasia, the heart, aortic trunk and right apex of the left ventricular myocardium were dissected from the mice. The tissues were rinsed with pre-cooled 0.9% saline, fixed in 4% paraformaldehyde for 2 h at room temperature and embedded in paraffin. Paraffin-embedded tissue samples were cut into 5-µm serial sections. The tissue sections were subsequently deparaffinized in xylene at room temperature and rehydrated in a descending ethanol series. Sections were then blocked with 3% hydrogen peroxide for 10 min at 25˚C to inhibit endogenous peroxidase activity. Tissue sections were incubated with the following primary antibodies for 12 h at 4˚C: Anti-MMP-9 (1:4,000; cat. no. ab38898; Abcam) and anti-CD40L (1:4,000; cat. no. ab2391; Abcam). Following the primary antibody incubation, the sections were incubated with an Alexa Fluor 488-conjugated secondary antibody (1:2,000; ab150077; Abcam) at 25˚C for 2 h. The slides were observed under an Olympus IX73 microscope (magnification, x100; Olympus Corporation). The quantification of expression levels was performed using Quantity One version 3.2 software (Bio-Rad Laboratories, Inc.).
Oil Red O staining
To quantify the area of the atherosclerotic lesions in the aortic sinus, Oil Red O staining was used. Slices (5 µm) were fixed with formalin for 10 min, rinsed with 60% isopropanol for 5 min at room temperature and stained with freshly prepared Oil Red O (1.0%) at room temperature. Subsequently, slides were stained with alum hematoxylin at room temperature. The average area of the atherosclerotic lesions in the aortic sinus were quantified by determining the percentage of positively-stained Oil-Red O areas using Image-Pro Plus software Version 1.1 (Media Cybernetics, Inc.).
Biochemical analysis
Blood (5 ml) was obtained in week 1-5 and subsequently centrifuged at 2,000 x g for 10 min at 4˚C to obtain the serum. TC, TG, HDL-c, aspartate transaminase (AST; cat. no. EZ-0506; Assay Biotechnology Company, Inc.), alanine transaminase (ALT; cat. no. ab263882, Abcam), alkaline phosphatase (ALP, cat. no. KA1294 Amyjet Scientific, Inc.), lactate dehydrogenase (LDH; cat. no. A55954-050; EpiGentek Group, Inc.), creatine phosphokinase (CPK; cat. no. A55954-020; EpiGentek Group, Inc.), TNF-α (cat. no. ab208348; Abcam), and CRP cat. no. 256398; Abcam), expression levels were measured using commercial kits according to the manufacturer's protocol.
LDH and CPK activity measurement
Serum (200 µl) from mice was obtained as previously described above. LDH and CPK activity was estimated using a fully automated XL-640 clinical chemistry analyzer (Transasia Bio-Medicals).
Cell culture and reagents
UVECS were isolated from the ApoE-/- atherosclerosis model mice as described previously (26). UVECS were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.), supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and maintained in a humidified atmosphere at 37˚C and 5% CO2. For experiments, cells were treated with 1 mg/ml PI3K inhibitor (PI3KIR; cat. no. 526559; Sigma-Aldrich; Merck KGaA) and/or 1 mg/ml RV (cat. no. R5010; Sigma-Aldrich; Merck KGaA) for 6 h at 37˚C for further analysis. Cells treated with PBS were used as control.
Cell transfection
DNA sequences of PI3K were amplified and cloned into the pcDNA3.1 plasmid expression vector (Invitrogen; Thermo Fisher Scientific, Inc.) to generate PI3K overexpressing (PI3KOR) plasmids. Empty plasmids were used as the control. In brief, 1x106 UVECS were cultured in 6-well plates for 24 h at 37˚C and subsequently transfected with 100 nM PI3KOR or the empty plasmid using a Lipofectamine® 2000 transfection kit (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The cells were collected for subsequent experimentation following 72 h of transfection.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from 1x107-treated UVECS using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The purity of the RNA was determined using an RNA 6000 nano-bioanalyzer (Agilent Technologies, Inc.). Total RNA was reverse transcribed into cDNA using the SuperScript™ II Reverse Transcriptase kit (Invitrogen; Thermo Fisher Scientific, Inc.) for 2 h at 42˚C, according to the manufacturer's protocol. qPCR was subsequently performed using 12.5 µl 2X real-time PCR SYBR®-Green master mix, 1.5 µl cDNA template, 2 µl primers and 9 µl 0.1% DEPC H2O (Fast SYBR-Green Master Mix; Invitrogen; Thermo Fisher Scientific, Inc.). The following primer pairs were used for the qPCR: PI3K forward, 5'-ATTACGCTAGTTACACTGCA-3' and reverse, 5'-TGGACCTGGCCATCGACTGA-3'; AKT forward, 5'-GCCACCATGAATGAGGTGAAT-3' and reverse, 5'-GCGTATGACAAAGGTGTTGGG-3'; mTOR forward, 5'-ACTCGCTTCTATGACCAACTGA-3' and reverse, 5'-TTTCCATGACAACTGGGTCATTG-3'; and β-actin forward, 5'-CAACGAGCGGTTCAGGTGT-3' and reverse, 5'-TGGAGTTGAAGGTGGTCTCGT-3'. The following thermocycling conditions were used for the qPCR: Initial denaturation at 95˚C for 30 sec; 45 cycles of 95˚C for 30 sec, 56.8˚C for 30 sec and 72˚C for 30 sec. Expression levels were normalized to the internal reference gene β-actin. Expression levels were quantified using the 2-ΔΔCq method (27).
Western blot analysis
Total protein was extracted from arterial tissue (1.0 g) using RIPA lysis buffer (Thermo Fisher Scientific, Inc.). Total protein was quantified using a bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.) and 40 µg protein/lane was separated using 15% SDS-PAGE. The separated proteins were subsequently transferred onto a nitrocellulose membrane and blocked with 5% BSA (Sigma-Aldrich; Merck KGaA) for 1 h at 37˚C. The membranes were subsequently incubated with the following primary antibodies for 12 h at 4˚C: Anti-PI3K (1:2,000; cat. no. ab232997; Abcam), anti-phosphorylated (p)-PI3K (1:1,000; cat. no. ab182651; Abcam), anti-AKT (1:2,000; cat. no. ab185633; Abcam), anti-p-AKT (1:2,000; cat. no. ab133458; Abcam), anti-mTOR (1:2,000; cat. no. ab32028; Abcam) and anti-β-actin (1:1,000; cat. no. ab8226; Abcam). Following the primary antibody incubation, membranes were incubated with HRP-conjugated goat anti-rabbit IgG secondary antibody (1:5,000; ab6721; Abcam) for 24 h at 4˚C. Protein bands were visualized using the Pierce™ ECL Western Blotting Substrate (cat. no. 32209; Invitrogen; Thermo Fisher Scientific, Inc.). Protein expression was quantified using Quantity-One version 3.2 software (Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were repeated at least three times. Statistical analysis was performed using SPSS version 13.0 software (SPSS Inc.). Statistical differences among groups were determined using Student's t-test or one-way ANOVA followed by Tukey's post hoc analysis. All data are presented as the mean ± SEM. P<0.05 was considered to indicate a statistically significant difference.
Results
Pathological characteristics of ApoE-/-atherosclerosis model mice
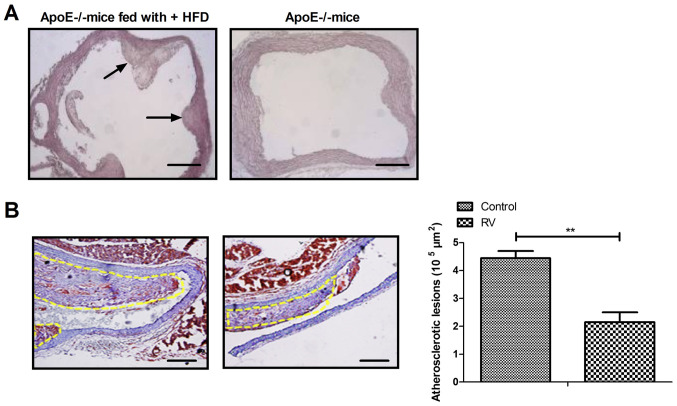
To verify the role of RV treatment in ApoE-/- mice, the characteristics of the arteriosclerotic lesions were investigated. Fibrous caps of atherosclerotic lesions were observed in the aortic sinus of ApoE-/- mice fed the high fat diet (Fig. 1A); however, no significant lesion was observed in the aortic sinus intima of ApoE-/- mice without high fat diet. RV treatment significantly decreased the formation of atherosclerotic plaques in atherosclerosis model mice compared to the control group (Fig. 1B). These results indicated that RV may improve the pathological characteristics of atherosclerosis in ApoE-/- mice.
Figure 1.
Characteristics of atherosclerotic lesions in ApoE-/- mice. (A) Sections of the aortic tunica intima in ApoE-/- mice fed a HFD or normal diet. (B) RV treatment decreased the area of atherosclerotic lesions in atherosclerosis model mice. HFD, high fat diet. Scale bars, 50 µm. **P<0.01. HFD, high fat diet; RV, resveratrol; ApoE-/-, Apolipoprotein E deficient.
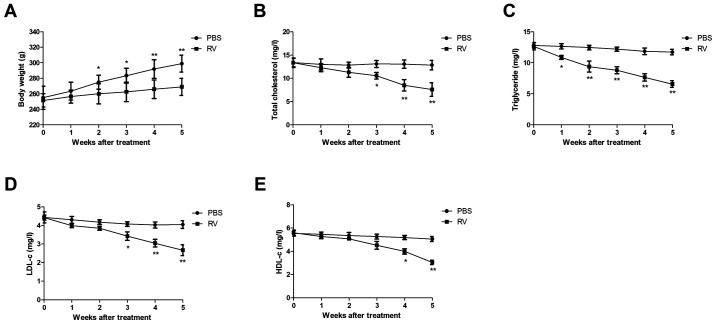
RV treatment increases body weight and improves blood lipid levels in atherosclerosis model mice
Changes in the body weight of atherosclerosis model mice were analyzed in the RV and control treatment group. The body weight of mice was significantly decreased from 2 weeks to the end of experiment in the RV group compared with the control group (Fig. 2A). Furthermore, RV treatment significantly reduced the TC, TG, LDL-c and HDL-c levels compared with the PBS group in atherosclerosis model mice following 5 weeks of treatment (Fig. 2B-E).
Figure 2.
RV treatment decreases the body weight and improves blood lipid levels in atherosclerosis model mice. (A) Effects of RV treatment on the body weight compared with the control. (B-E) Effects of RV treatment on (B) total cholesterol, (C) triglyceride, (D) LDL-c and (E) HDL-c levels compared with the control. *P<0.05 and **P<0.01. RV, resveratrol; LDL-c, low density lipoprotein cholesterin; HDL-c, high-density lipoprotein cholesterin.
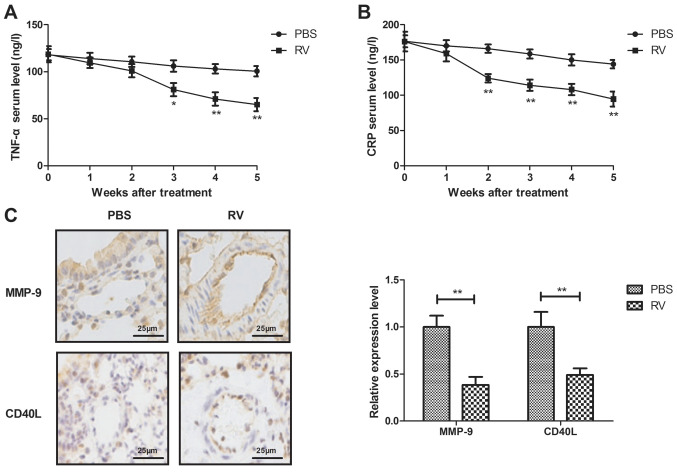
RV treatment decreases serum levels of inflammatory cytokines in atherosclerosis model mice
The anti-inflammatory effects of RV were investigated in atherosclerosis model mice. RV treatment significantly decreased the serum levels of TNF-α and CRP compared with the control group (Fig. 3A and B). In addition, RV treatment significantly decreased MMP-9 and CD40L expression levels in arterial lesion tissue compared with the control group (Fig. 3C).
Figure 3.
RV treatment decreases serum levels of inflammatory cytokines in atherosclerosis model mice. (A and B) Effects of RV treatment on the serum levels of (A) TNF-α and (B) CRP compared with the control. (C) Effects of RV treatment on MMP-9 and CD40L expression levels in arterial lesion tissue compared with the control. Scale bars, 25 µm. *P<0.05 and **P<0.01. RV, resveratrol; TNF-α, tumor necrosis factor-α; CRP, c-reactive protein; MMP-9, matrix metalloproteinase-9.
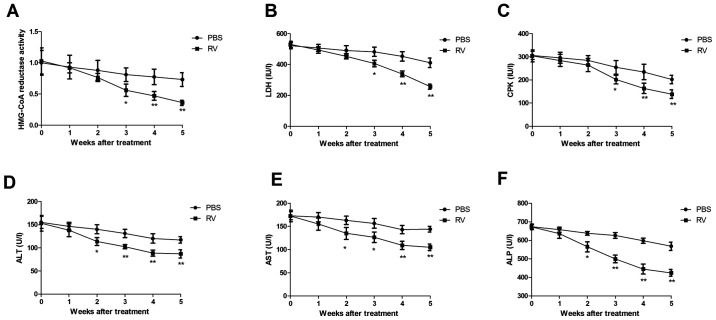
RV treatment demonstrates anti-atherosclerotic activity in atherosclerosis model mice
RV treatment significantly decreased HMG-CoA reductase activity compared with the control group (Fig. 4A). LDH and CPK activities were also significantly decreased by 5 weeks post-treatment in the RV group compared with the control group (Fig. 4B and C). In addition, serum levels of ALT, AST and ALP were significantly decreased in the RV group compared to the control group (Fig. 4D-F). These results indicated that RV treatment may exhibit anti-atherosclerotic activity in atherosclerosis model mice.
Figure 4.
RV treatment promotes anti-atherosclerotic activity in atherosclerosis model mice. (A-F) Effects of RV treatment on (A) HMG-CoA reductase activity, (B) LDH levels, (C) CPK activity, (D) serum ALT levels, (E) serum AST levels and (F) serum ALP levels compared with the control. *P<0.05 and **P<0.01. RV, resveratrol; LDH, lactate dehydrogenase; CPK, creatine phosphokinase; ALT, alanine transaminase; AST, aspartate transaminase; ALP, alkaline phosphatase.
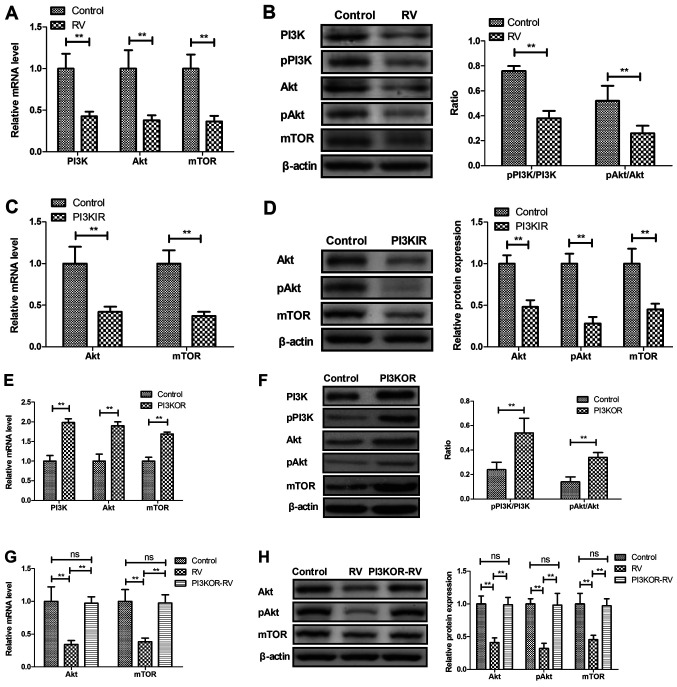
RV decreases PI3K/AKT/mTOR signaling in UVECs
The potential RV-mediated mechanism was investigated in UVECs isolated from atherosclerosis model mice. RV treatment significantly decreased the mRNA and protein expression levels of PI3K, AKT and mTOR compared with the control group (Fig. 5A and B). UVECs treated with the PI3KIR demonstrated significantly decreased mRNA and protein expression levels of AKT and mTOR compared with the control group (Fig. 5C and D). In addition, PI3K overexpression in UVECs using PI3KOR was observed to significantly increase the mRNA and protein expression levels of PI3K, AKT and mTOR compared with the control group (Fig. 5E and F), whereas PI3KOR-transfected UVECs significantly reversed the RV-mediated decrease in mRNA and protein expression levels of AKT and mTOR (Fig. 5G and H).
Figure 5.
RV treatment reduces the PI3K/AKT/mTOR signaling pathway in UVECs. (A and B) Effects of RV treatment on (A) mRNA and (B) protein expression levels of total and phosphorylated PI3K and AKT, and mTOR in UVECs compared with the control. (C and D) Effects of PI3KIR on (C) mRNA and (D) protein expression levels of total and phosphorylated AKT and mTOR in UVECs compared with the control. (E and F) Effects of PI3KOR on the (E) mRNA and (F) protein expression levels of total and phosphorylated AKT and PI3K, and mTOR in UVECs compared with the control. (G and H) Effects of PI3KOR on RV-regulated (G) mRNA and (H) protein expression levels of total and phosphorylated AKT, and mTOR in UVECs compared with the control. **P<0.01. UVECs, umbilical vein endothelial cells; PI3KIR, PI3K inhibitor; PI3KOR, PI3K overexpression; RV, resveratrol; p, phosphorylated.
Discussion
RV was previously demonstrated to reduce the expression levels of inflammatory cytokines in atherosclerosis model rabbits (28). In the present study, the therapeutic effects of RV on atherosclerosis model mice was analyzed and the relationship between RV and the PI3K/AKT/mTOR signaling pathway in UVECS obtained from atherosclerosis model mice was also investigated. The results demonstrated that RV treatment increased the expression levels of PI3K, AKT and mTOR, as well as the ratios between phosphorylated and total proteins in UVECS and increased the serum levels of TC, TG, LDL-c and HDL-c in atherosclerosis model mice. The study also observed that RV promoted anti-inflammatory effects, which may contribute to the anti-atherosclerotic activity of RV.
A previous study reported that RV treatment protected against TNF-α-induced injury in human UVECs through promoting sirtuin-1-induced repression of NF-kB and p38 MAPK (29). Verschuren et al (30) demonstrated that RV treatment decreased CRP expression levels, which improved lipid metabolism and reduced atherosclerotic lesion development in female transgenic mice. Results from the present study observed that RV treatment decreased TNF-α and CRP serum levels in atherosclerosis model mice. In addition, HMG-CoA reductase inhibitors reduced chronic subacute inflammation in atherosclerosis induced by dietary cholesterol (31). The present study found that RV treatment decreased the body weight of atherosclerotic mice by measuring pathological improvement of atherosclerosis. A previous study reported that the association between liver function and coronary atherosclerosis may be more complex than appreciated (32), and TG and LDL-c levels were found at higher levels in patients with atherosclerosis (33). In this study, RV treatment decreased the serum levels of TC, TG, LDL-c and HDL-c in atherosclerotic model mice compared to the control group. It was also reported that RV treatment decreased HMG-CoA reductase activity and marker enzymes, including LDH, CPK, AST, ALT and ALP in atherosclerosis model mice, which suggested that atherosclerosis may be associated with liver dysfunction.
RV has demonstrated numerous pharmacological effects, including acting as an antioxidant, an anti-inflammatory agent, eliminating free radicals, exhibiting an anti-tumorigenic role, regulating lipids and regulating glucose metabolism (34-36). Data from the current study observed that RV treatment improved lipid metabolism compared with the control group in atherosclerosis model mice. MMP-9 serum levels are consistently associated with markers of carotid atherosclerosis and lesion vulnerability (37); and the present study demonstrated that RV treatment could significantly decrease MMP-9 and CD40L expression levels in arterial lesion tissue compared to the control group. Notably, the in vivo experiments demonstrated the protective role of RV against atherosclerotic lesions in atherosclerosis model mice. Clinically, HDL-c is response to statin treatment by improving carotid intima-media thickness, which is closely related to a regression of atherosclerosis (38). Data have supported that serum levels of LDL-c can be used to predict the severity of coronary atherosclerosis (39). Consistently, our data found that RV treatment significantly reduced the degree of atherosclerosis. In addition, RV treatment increased the metabolism of hyperlipidemia in HFD-fed atherosclerosis model mice, which further led to an increase in anti-atherosclerotic activity and may prevent cardiovascular complications. Thus, it was hypothesized that decreasing HDL-c levels in the serum may have a negative effect of RV in the treatment of HFD-fed atherosclerosis; however, further studies should be performed to identify the anti-atherosclerotic mechanism of RV in oxidized (ox)-LDL-induced human endothelial cells.
The mTOR inhibitor, everolimus, has been proven to prevent the development of atherosclerosis in LDLR-/- mice, even in the presence of severe hypercholesterolemia (40). A previous study also suggested that the increased activation of the PI3K/AKT signaling pathway attenuated ox-LDL-induced endothelial cell apoptosis (41); and similarly, another study demonstrated that targeting the PI3K/AKT/mTOR signaling pathway in vascular endothelial cells represented a potential therapeutic target for the treatment of atherosclerosis (42). Notably, the activation of the PI3K/AKT/mTOR signaling pathway exerted a protective role against atherosclerosis (43). In addition, previous studies have reported that inhibiting the PI3K/AKT/mTOR pathway alleviated ox-LDL-induced apoptosis of human endothelial cells, which further prevented atherosclerosis development (44-46). Results in the present study were consistent with the majority of these previous studies; RV treatment downregulated the PI3K/AKT/mTOR signaling pathway in UVECS obtained from atherosclerosis model mice. In addition, it was demonstrated that PI3K overexpression increased and abolished RV-regulated AKT and mTOR expression in UVECS; however, further investigations are required to determine the therapeutic efficacy of RV in patients with atherosclerosis. In addition, future studies should aim to analyze the dissociation constant/inhibition constant (KD/KI) between downstream molecules of RV. In conclusion, the results from the present study indicated that RV may improve atherosclerosis through the PI3K/AKT/mTOR signaling pathway. This study provided evidence for the application of RV in the treatment of atherosclerosis.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The analyzed datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
WJ performed the experiments and data analysis. JS and ZH performed experiments and collected data. BS designed the experiments and wrote the original manuscript. All authors read and approved the final manuscript. WJ, JS, ZH and BS confirm the authenticity of all the raw data.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of The People's Hospital of Weifang.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gandhi S, Chen S, Hong L, Sun K, Gong E, Li C, Yan LL, Schwalm JD. Effect of mobile health interventions on the secondary prevention of cardiovascular disease: Systematic review and meta-analysis. Can J Cardiol. 2017;33:219–231. doi: 10.1016/j.cjca.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: Systematic review and meta-analysis. BMJ. 2016;355(i5953) doi: 10.1136/bmj.i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aziz M, Ali SS, Das S, Younus A, Malik R, Latif MA, Humayun C, Anugula D, Abbas G, Salami J, et al. Association of subjective and objective sleep duration as well as sleep quality with non-invasive markers of sub-clinical cardiovascular disease (CVD): A systematic review. J Atheroscler Thromb. 2017;24:208–226. doi: 10.5551/jat.36194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedighi M, Bahmani M, Asgary S, Beyranvand F, Rafieian-Kopaei M. A review of plant-based compounds and medicinal plants effective on atherosclerosis. J Res Med Sci. 2017;22(30) doi: 10.4103/1735-1995.202151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milic NM, Milin-Lazovic J, Weissgerber TL, Trajkovic G, White WM, Garovic VD. Preclinical atherosclerosis at the time of pre-eclamptic pregnancy and up to 10 years postpartum: Systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;49:110–115. doi: 10.1002/uog.17367. (In En, Spanish) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zanoli L, Signorelli SS, Inserra G, Castellino P. Subclinical atherosclerosis in patients with inflammatory bowel diseases: A systematic review and meta-analysis. Angiology. 2017;68(463) doi: 10.1177/0003319716675076. [DOI] [PubMed] [Google Scholar]
- 7.Wu GC, Leng RX, Lu Q, Fan YG, Wang DG, Ye DQ. Subclinical atherosclerosis in patients with inflammatory bowel diseases: A systematic review and meta-analysis. Angiology. 2017;68:447–461. doi: 10.1177/0003319716652031. [DOI] [PubMed] [Google Scholar]
- 8.Usai MV, Bosiers MJ, Bisdas T, Torsello G, Beropoulis E, Kasprzak B, Stachmann A, Stavroulakis K. Surgical versus endovascular revascularization of subclavian artery arteriosclerotic disease. J Cardiovasc Surg (Torino) 2020;61:53–59. doi: 10.23736/S0021-9509.18.10144-3. [DOI] [PubMed] [Google Scholar]
- 9.Petrovski G, Gurusamy N, Das DK. Resveratrol in cardiovascular health and disease. Ann N Y Acad Sci. 2011;1215:22–33. doi: 10.1111/j.1749-6632.2010.05843.x. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Yan Z, Zhu J, Yang J, He J. Neuroprotective effects of resveratrol on ischemic injury mediated by improving brain energy metabolism and alleviating oxidative stress in rats. Neuropharmacology. 2011;60:252–258. doi: 10.1016/j.neuropharm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Csiszar A. Anti-inflammatory effects of resveratrol: Possible role in prevention of age-related cardiovascular disease. Ann N Y Acad Sci. 2011;1215:117–122. doi: 10.1111/j.1749-6632.2010.05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Yang YJ, Qian HY, Zhang Q, Xu H, Li JJ. Resveratrol in cardiovascular disease: What is known from current research? Heart Fail Rev. 2012;17:437–448. doi: 10.1007/s10741-011-9260-4. [DOI] [PubMed] [Google Scholar]
- 13.Tomé-Carneiro J, Gonzálvez M, Larrosa M, Yáñez-Gascón MJ, García-Almagro FJ, Ruiz-Ros JA, Tomás-Barberán FA, García-Conesa MT, Espín JC. Resveratrol in primary and secondary prevention of cardiovascular disease: A dietary and clinical perspective. Ann NY Acad Sci. 2013;1290:37–51. doi: 10.1111/nyas.12150. [DOI] [PubMed] [Google Scholar]
- 14.Ruan BF, Lu XQ, Song J, Zhu HL. Derivatives of resveratrol: Potential agents in prevention and treatment of cardiovascular disease. Curr Med Chem. 2012;19:4175–4183. doi: 10.2174/092986712802430054. [DOI] [PubMed] [Google Scholar]
- 15.Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19(42) doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 16.Niroumand S, Khajedaluee M, Khadem-Rezaiyan M, Abrishami M, Juya M, Khodaee G, Dadgarmoghaddam M. Atherogenic index of plasma (AIP): A marker of cardiovascular disease. Med J Islam Repub Iran. 2015;29(240) [PMC free article] [PubMed] [Google Scholar]
- 17.Henrot P, Foret J, Barnetche T, Lazaro E, Duffau P, Seneschal J, Schaeverbeke T, Truchetet ME, Richez C. Assessment of subclinical atherosclerosis in systemic lupus erythematosus: A systematic review and meta-analysis. Joint Bone Spine. 2018;85:155–163. doi: 10.1016/j.jbspin.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Yong WC, Sanguankeo A, Upala S. Association between sarcoidosis, pulse wave velocity, and other measures of subclinical atherosclerosis: A systematic review and meta-analysis. Clin Rheumatol. 2018;37:2825–2832. doi: 10.1007/s10067-017-3926-9. [DOI] [PubMed] [Google Scholar]
- 19.Cho IJ, Ahn JY, Kim S, Choi MS, Ha TY. Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem Biophys Res Commun. 2008;367:190–194. doi: 10.1016/j.bbrc.2007.12.140. [DOI] [PubMed] [Google Scholar]
- 20.Liu G, Wang B, Zhang J, Jiang H, Liu F. Total panax notoginsenosides prevent atherosclerosis in apolipoprotein E-knockout mice: Role of downregulation of CD40 and MMP-9 expression. J Ethnopharmacol. 2009;126:350–354. doi: 10.1016/j.jep.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Yang X, Bian F, Wu P, Xing S, Xu G, Li W, Chi J, Ouyang C, Zheng T, et al. TNF-α promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: Crosstalk between NF-κB and PPAR-γ. J Mol Cell Cardiol. 2014;72:85–94. doi: 10.1016/j.yjmcc.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Piechota W. Correlation of high-sensitivity CRP concentration with the extent of coronary atherosclerosis in men with symptoms of ischemic heart disease. Pol Merkur Lekarski. 2005;18:511–515. (In Polish) [PubMed] [Google Scholar]
- 23.Egorova MO. Increased serum level of the acute inflammation phase parameter CRP and the high level of low density lipoprotein cholesterol-factors of increased risk of development of atherosclerosis and its complications (a literature review) Klin Lab Diagn. 2002;3-6 (In Russian) [PubMed] [Google Scholar]
- 24.Xing SS, Yang XY, Zheng T, Li WJ, Wu D, Chi JY, Bian F, Bai XL, Wu GJ, Zhang YZ, et al. Salidroside improves endothelial function and alleviates atherosclerosis by activating a mitochondria-related AMPK/PI3K/Akt/eNOS pathway. Vascul Pharmacol. 2015;72:141–152. doi: 10.1016/j.vph.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Kurdi A, Martinet W, De Meyer GRY. mTOR inhibition and cardiovascular diseases: Dyslipidemia and atherosclerosis. Transplantation. 2018;102 (Suppl 1):S44–S46. doi: 10.1097/TP.0000000000001693. [DOI] [PubMed] [Google Scholar]
- 26.Hu Q, Chai J, Liu L, Hou Y, Wang Y, Li B, Yang H. Isolation, culture, and identification of canine umbilical vein vascular endothelial cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013;27:460–463. (In Chinese) [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Song R, Li WQ, Dou JL, Li L, Hu Y, Guo J, Lu D, Zhang G, Sun L. Resveratrol reduces inflammatory cytokines via inhibiting nuclear factor-κB and mitogen-activated protein kinase signal pathway in a rabbit atherosclerosis model. Zhonghua Xin Xue Guan Bing Za Zhi. 2013;41:866–869. (In Chinese) [PubMed] [Google Scholar]
- 29.Pan W, Yu H, Huang S, Zhu P. Resveratrol protects against TNF-α-induced injury in human umbilical endothelial cells through promoting sirtuin-1-induced repression of NF-KB and p38 MAPK. PLoS One. 2016;11(e0147034) doi: 10.1371/journal.pone.0147034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verschuren L, Wielinga PY, van Duyvenvoorde W, Tijani S, Toet K, van Ommen B, Kooistra T, Kleemann R. A dietary mixture containing fish oil, resveratrol, lycopene, catechins, and vitamins E and C reduces atherosclerosis in transgenic mice. J Nutr. 2011;141:863–869. doi: 10.3945/jn.110.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleemann R, Kooistra T. HMG-CoA reductase inhibitors: Effects on chronic subacute inflammation and onset of atherosclerosis induced by dietary cholesterol. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:441–453. doi: 10.2174/156800605774962077. [DOI] [PubMed] [Google Scholar]
- 32.Doganer YC, Rohrer JE, Aydogan U, Agerter DC, Cayci T, Barcin C. Atherosclerosis and liver function tests in coronary angiography patients. West Indian Med J. 2015;64:333–337. doi: 10.7727/wimj.2014.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin JH, Lin YF, Wang WJ, Lin YF, Chueh SJ, Wu VC, Chu TS, Wu KD. Plasma aldosterone concentration as a determinant for statin use among middle-aged hypertensive patients for atherosclerotic cardiovascular disease. J Clin Med. 2018;7(382) doi: 10.3390/jcm7110382. Taiwan Primary Aldosteronism Investigation (TAIPAI) Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal R, Agarwal P. Targeting extracellular matrix remodeling in disease: Could resveratrol be a potential candidate? Exp Biol Med (Maywood) 2017;242:374–383. doi: 10.1177/1535370216675065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vilar-Pereira G, Carneiro VC, Mata-Santos H, Vicentino ARR, Ramos IP, Giarola NLL, Feijó DF, Meyer-Fernandes JR, Paula-Neto HA, Medei E, et al. Resveratrol reverses functional chagas heart disease in mice. PLoS Pathog. 2016;12(e1005947) doi: 10.1371/journal.ppat.1005947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naia L, Rosenstock TR, Oliveira AM, Oliveira-Sousa SI, Caldeira GL, Carmo C, Laço MN, Hayden MR, Oliveira CR, Rego AC. Comparative mitochondrial-based protective effects of resveratrol and nicotinamide in Huntington's disease models. Mol Neurobiol. 2017;54:5385–5399. doi: 10.1007/s12035-016-0048-3. [DOI] [PubMed] [Google Scholar]
- 37.Silvello D, Narvaes LB, Albuquerque LC, Forgiarini LF, Meurer L, Martinelli NC, Andrades ME, Clausell N, dos Santos KG, Rohde LE. Serum levels and polymorphisms of matrix metalloproteinases (MMPs) in carotid artery atherosclerosis: Higher MMP-9 levels are associated with plaque vulnerability. Biomarkers. 2014;19:49–55. doi: 10.3109/1354750X.2013.866165. [DOI] [PubMed] [Google Scholar]
- 38.Ishigaki Y, Kono S, Katagiri H, Oka Y, Oikawa S. Elevation of HDL-C in response to statin treatment is involved in the regression of carotid atherosclerosis. J Atheroscler Thromb. 2014;21:1055–1065. doi: 10.5551/jat.22095. NTTP investigators. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Wu NQ, Li S, Zhu CG, Guo YL, Qing P, Gao Y, Li XL, Liu G, Dong Q, Li JJ. Non-HDL-C is a better predictor for the severity of coronary atherosclerosis compared with LDL-C. Heart Lung Circ. 2016;25:975–981. doi: 10.1016/j.hlc.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 40.Mueller MA, Beutner F, Teupser D, Ceglarek U, Thiery J. Prevention of atherosclerosis by the mTOR inhibitor everolimus in LDLR-/-mice despite severe hypercholesterolemia. Atherosclerosis. 2008;198:39–48. doi: 10.1016/j.atherosclerosis.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 41.Lu XL, Zhao CH, Yao XL, Zhang H. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway. Biomed Pharmacother. 2017;85:658–671. doi: 10.1016/j.biopha.2016.11.077. [DOI] [PubMed] [Google Scholar]
- 42.Lv J, Yang L, Guo R, Shi Y, Zhang Z, Ye J. Ox-LDL-induced microRNA-155 promotes autophagy in human endothelial cells via repressing the Rheb/mTOR pathway. Cell Physiol Biochem. 2017;43:1436–1448. doi: 10.1159/000481875. [DOI] [PubMed] [Google Scholar]
- 43.Yao X, Yan C, Zhang L, Li Y, Wan Q. LncRNA ENST00113 promotes proliferation, survival, and migration by activating PI3K/Akt/mTOR signaling pathway in atherosclerosis. Medicine (Baltimore) 2018;97(e0473) doi: 10.1097/MD.0000000000010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Che J, Liang B, Zhang Y, Wang Y, Tang J, Shi G. Kaempferol alleviates ox-LDL-induced apoptosis by up-regulation of autophagy via inhibiting PI3K/Akt/mTOR pathway in human endothelial cells. Cardiovasc Pathol. 2017;31:57–62. doi: 10.1016/j.carpath.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Zhai C, Cheng J, Mujahid H, Wang H, Kong J, Yin Y, Li J, Zhang Y, Ji X, Chen W. Selective inhibition of PI3K/Akt/mTOR signaling pathway regulates autophagy of macrophage and vulnerability of atherosclerotic plaque. PLoS One. 2014;9(e90563) doi: 10.1371/journal.pone.0090563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang F, Yang TL. MicroRNA-126 alleviates endothelial cells injury in atherosclerosis by restoring autophagic flux via inhibiting of PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun. 2018;495:1482–1489. doi: 10.1016/j.bbrc.2017.12.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.