Abstract
Objective:
Numerous commercial pharmacogenetics panels are now widely available for clinical use in psychiatric practice. However, there is a paucity of literature evaluating the use of combinatorial pharmacogenetics panels to enhance outcomes in the treatment of adolescents with depression. This study sought to prospectively evaluate the clinical impact of combinatorial pharmacogenetics testing in a double-blind, randomized, controlled effectiveness study for the pharmacologic treatment of adolescents with depression.
Method:
Adolescents aged 13–18 years (N=176) with moderate to severe major depressive disorder (MDD) were randomized to treatment arm guided by testing in which pharmacogenetic testing results were available at the baseline visit (GENE arm, n=84) or a treatment as usual arm [(TAU) arm, n=92] in which testing results were not available until an 8 week visit. Raters, participants, and families were blinded to group allocation. Symptom improvement, side effects, and satisfaction were assessed throughout the study at 4 weeks, 8 weeks and 6 months.
Results:
There were no differences between the GENE and TAU arms at 8 weeks or 6 months for symptom improvement, side effect burden or satisfaction. Selective serotonin reuptake inhibitors were prescribed at higher rates in the TAU arm compared to the GENE arm (p=0.024).
Conclusion:
Combinatorial pharmacogenetics guided treatment did not demonstrate improved outcomes compared to TAU in adolescents with MDD. Future research should examine how specific medication-gene pairs may impact clinical outcomes in the treatment of adolescents with depression and how best to integrate pharmacogenetics into clinical practice.
Keywords: Pharmacogenetics testing, randomized, double-blind, adolescent, depression
Lay Summary:
Pharmacogenetics testing has been at the forefront of potential promise for personalized medicine in psychiatry. This double-blind, randomized, controlled effectiveness study evaluated the clinical impact of pharmacogenetics testing in adolescents with depression. There were no differences in symptom improvement, side effect burden or satisfaction for those adolescents whose psychiatrists had the testing results at the beginning of the study compared to those that had access to the testing results at 8 weeks. Future research should examine how medication-gene pairs may impact clinical outcomes and best integrate into clinical practice.
INTRODUCTION
Major depressive disorder (MDD) is a common and serious public health problem for youth in the United States. Lifetime and 12-month prevalence rates for adolescents aged 13–18 years have been shown to be 11.0% and 7.5%, respectively.1 Finding the right treatment at the right time and dose for each individual is critical in this population given black box warnings for antidepressants as a class. Moreover, for many young patients seeking treatment early in the course of their illness, ineffective treatments can lead to residual or progressing depressive symptoms, which disrupts social, emotional, and academic developmental trajectories.2 Additionally, the timely treatment of depression is critical given that it is a risk factor for suicide, the second leading cause of death in young people ages 10–24 years in the United States.3,4 Unfortunately, approximately 40% of youth will not respond to a trial of an antidepressant.5–7 The “trial and error” approach to finding the right treatment can be a major burden for patients and families. For this reason, the concept of precision and personalized medicine has garnered a great deal of attention over the last decade, and pharmacogenetics testing has been at the forefront of potential promise in personalized medicine approaches for the pharmacological treatment of depression.8–11
Clinicians are increasingly considering and using combinatorial pharmacogenetics panels in clinical practice.12,13 These combinatorial panels provide genetic testing results for gene variations that may influence a patient’s response to a medication by evaluating genes that effect metabolic enzymes such as the cytochrome P450 system (pharmacokinetics) and genes that effect neuronal function (pharmacodynamics). Many of the commercially available combinatorial panels present decision support tools, based on proprietary algorithms, by combining the predicted impact of multiple polymorphisms of multiple genes for psychotropic medications. On these panels, medications are often listed in one of three categories, which are usually associated with a color. For example, medications may be listed in a “use as directed” (i.e., green) category, “use with caution” (i.e., yellow) category, or “use with increased caution and more frequent monitoring” (i.e., red) category.8,12,14 It is common for medications to be metabolized through multiple cytochrome P450 pathways, and the proprietary algorithms will weigh the polymorphisms at the various genes a medication is metabolized through to determine a combined effect of all gene-drug interactions for that particular medication in that particular patient.14 However, experts in clinical psychiatry have cautioned against the widespread adoption of combinatorial pharmacogenetics testing in clinical practice due to insufficient data.13
Despite great initial enthusiasm and a surge of companies offering pharmacogenetics testing for psychiatric medications, controversy within the psychiatric field regarding the utility of pharmacogenetics testing is widespread.15,16 In this context, there is a paucity of literature evaluating the use of commercialized, combinatorial pharmacogenetics panels in youth with depression despite widespread use in clinical practice.17 There are no published, prospective, randomized, double blind studies examining adolescents with depression to date. In fact, in October 2018, the United States Food and Drug Administration (FDA) issued a warning emphasizing the lack of clinical evidence for genetic tests that made claims of predicting response or risk of side effects to medications.18 Furthermore, in March 2020, the American Academy of Child and Adolescent Psychiatry (AACAP) released a policy statement highlighting the lack of randomized controlled trials in the pediatric population, as well as the limitations of the current research studies. AACAP’s policy recommends against using pharmacogenetics testing to select a treatment course for pediatric patients and calls for prospective studies to evaluate the clinical significance of pharmacodynamic and combinatorial pharmacogenetics testing in children and adolescents.19
Much of the extant literature for youth with depression is based on data focusing on genetic variants for pharmacokinetic and pharmacodynamic genes that may influence tolerability and response to a single medication, such as sertraline, escitalopram, and citalopram.20–22 For example, the Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines report evidence for evaluating specific pharmacokinetic gene-medication pairs (e.g., CYP2C19 metabolizer status and es/citalopram) and recommend dose adjustments to help reduce side effects and increase efficacy.23 There are also emerging data to suggest that pharmacodynamic gene-medication pairs (e.g., HTR2A genotype and fluoxetine) may influence time to response.24 It is possible that reviewing pharmacogenetics results gene by gene may be a preferred approach to prescribing as the field moves forward.25(in press) Nonetheless, there remains a dearth of literature to address the utility of whether combinatorial pharmacogenetics panels with decision support tools impact clinical outcomes for youth with depression. The clinical implementation of combinatorial pharmacogenetics may be outpacing the relevant evidence base.
To address this knowledge gap, we conducted a randomized trial that examined outcomes in a sample of adolescents with depression randomized to treatment as usual [(TAU) (pharmacogenetic testing results were not available to the treating psychiatrist until after the 8 week visit rating scales were completed)] or treatment guided with a combinatorial pharmacogenetics panel [(GENE) (pharmacogenetic testing results were available to the treating psychiatrist at the first medication visit)]. Patients, parents, and raters were blinded to the treatment arm and testing results until after rating scales were completed at week 8. We hypothesized that guided treatment would demonstrate superior outcomes with respect to efficacy assessed with the Children’s Depression Rating Scale-Revised (CDRS-R)26 and side effect burden. The primary outcome measure was continuous CDRS-R score improvement from baseline to week 8.
METHODS
Participants
All study procedures were reviewed and approved by the Institutional Review Board prior to any research activity. Parents and 18-year-old patients provided informed consent, and patients 13–17 years old provided informed assent. The research team recruited adolescents ages 13–18 with major depressive disorder with moderate to severe symptom severity from outpatient psychiatric clinics, community primary care clinics, and a psychiatric inpatient unit between 2015–2018 in Rochester, Minnesota and the surrounding communities.
Inclusion criteria were intentionally broad to obtain a real-world sample of adolescents with depression and enhance generalizability. Inclusion criteria included: aged 13–18 years and the presence of a current, moderate to severe major depressive episode as assessed with the Kiddie Schedule for Affective Disorders and Schizophrenia-Past and Lifetime (K-SADS-PL) (score ≥40 on the CDRS-R). Additionally, the treating clinician, patient, and family had to agree that pharmacotherapy was indicated as part of a comprehensive treatment plan. Exclusion criteria included the following: Young Mania Rating Scale (YMRS)27 score ≥15; diagnosis of autism spectrum disorder, anorexia nervosa, bipolar disorder, and schizophrenic spectrum disorders. Psychotropic medication change (including dosage) between the screening visit and physician baseline visit; meeting criteria in the Diagnostic and Statistical Manual of Mental Disorders-5th Edition (DSM-5)28 for significant and current substance use disorder other than nicotine, caffeine, or cannabis; serious suicidal risk as judged by the investigator; unstable medical condition; pregnancy; or pharmacogenetic testing within the last 5 years were also exclusionary.
Procedure
Screening, Randomization and Blinding
All participants were screened using the K-SADS-PL to confirm the diagnosis of a major depressive episode. Initial rating scales were completed at screening to ensure appropriate inclusion. A buccal swab was obtained and sent for pharmacogenetics testing, and randomization occurred. The K-SADS-PL and all rating scales were conducted by blinded raters. A board certified child and adolescent psychiatrist (PEC) trained all raters, provided ongoing supervision, performed quality control (supervised rating interviews), and reviewed every clinical assessment.
Participants were randomized to one of two groups: pharmacogenetics testing results were available at the physician baseline visit (GENE arm) or pharmacogenetics testing results were not available until after ratings were completed at the 8 week visit (TAU arm). The treating clinician was notified which arm each participant was assigned to after randomization. The study was conducted under double blind (rater and patient/parent blind) conditions so that neither the patient, his/her family, nor the rater would know if the participant was in the GENE or TAU group. In both arms, the treating physician was not allowed to disclose or refer to the testing results with the patient in the room as to ensure that participants, families and raters remained blinded.
Post-Enrollment
After a minimum of 3 days from the screening/consent/randomization visit, which allowed enough time for the pharmacogenetics testing results to return, participants and their families participated in a baseline visit with a board-certified child and adolescent psychiatrist (JLVV, SSO, JS). Additionally, the study included a 4 week, 8 week and 6 month visit. During this study, the treating psychiatrist had the option to use any and all medications they felt were clinically indicated for the patient. The psychiatrist recorded the reason for their decision, such as whether the decision was based on pharmacogenetics testing or clinical judgment. See Figure 1 for the flow of participants during the study.
Figure 1.
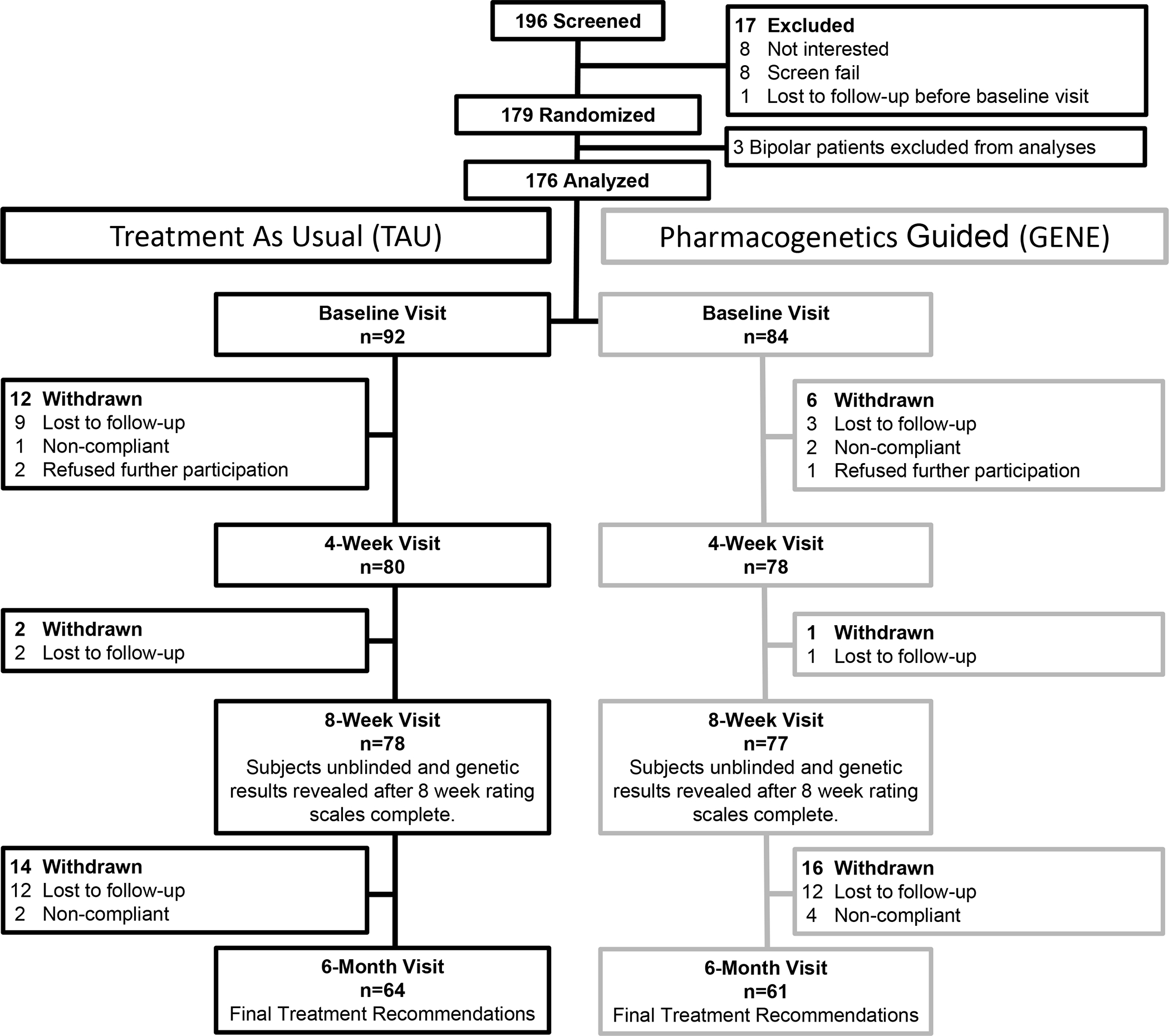
CONSORT Diagram for Adolescents Randomized to Treatment As Usual (TAU) or Treatment Guided by Combinatorial Pharmacogenetics Testing (GENE)
In addition to a physician visit, patients and families met with a study coordinator to complete numerous assessments at the baseline rating scale visit, 4 week, 8 week and 6 month visits. These assessments were overseen by a separate board-certified child psychiatrist (PEC). Assessments included the following: K-SADS-PL; CDRS-R; Quick Inventory of Depressive Symptomatology-Clinician Rated, Adolescent Self Report, and Parent Report (QIDS-A17-CR, QIDS-A17-SR, QIDS-A17-SR(P), respectively)29; Children’s Global Assessment Scale (CGAS)30; YMRS; Frequency, Intensity, and Burden of Side Effects Ratings (FIBSER) and Safety Monitoring Uniform Research Form-Modified for this study (SMURF-M)31 to track number of adverse events/side effects (AEs/SE); Satisfaction Survey Baseline-Patient and Parent; and Satisfaction Survey 8 Week-Patient and Parent. The satisfaction survey asked patients and parents their “overall satisfaction with the clinical visit.” At the end of the 8 week visit, after all assessments for that visit were complete, all participants were given their pharmacogenetic testing results, which were reviewed with them by the treating child and adolescent psychiatrist.
Pharmacogenetics Testing
The pharmacogenetics testing was completed by Assurex Health, Inc. (Mason, OH) as described in previous publications.32 The combinatorial pharmacogenetics panel used in this study included 8 genes (CYP2D6, CYP2C19, CYP2C9, CYP3A4, CYP2B6, CYP1A2, SLC6A4, and HTR2A) and evaluated for 51 genetic variants. The genetic variants that are included in this pharmacogenetics test are found across the globe, including Europeans/Caucasians, Africans, East Asians, South Asians, Hispanics, African Americans, and Admixed Americans.33,34 The testing results reported the participant’s genotype and phenotype for each tested gene. The testing technology integrated these genetic data with the pharmacology for each medication on the panel, incorporating data from the existing literature, FDA-approved labels, and published literature. The combinatorial pharmacogenetics panel included 22 medications classified as antidepressants, 16 classified as antipsychotics, and 8 classified as anticonvulsants/mood stabilizers. All medications were categorized into three color-coded bins: “Use As Directed” (green), “Use With Caution” (yellow), and “Use With Increased Caution And With More Frequent Monitoring” (red). Medications in the green bin are considered likely to be unaffected by any tested genetic polymorphisms the individual may possess. Medications in the yellow bin are purportedly at risk for a gene-drug interaction that may necessitate alternative dosing. Medications in the red bin are classified as high risk for a gene-drug interaction that may necessitate alternative dosing or medication selection.
Statistical Analysis
Demographic, clinical, and genetic characteristics are presented as mean (SD) for continuous variables and frequency (%) for categorical variables. Comparisons were made between randomized treatment groups using t-tests or Wilcoxon rank sum tests for continuous variables, chi-square or fisher’s exact test for categorical variables. Changes in parent and patient satisfaction were compared by treatment group using ordinal logistic regression models. Analyses outcome measures were tested as raw continuous scores, change in raw scores from baseline, and as dichotomized remission and response variables using baseline score-adjusted linear and logistic regression models, respectively. Patients and parents who completed the 8 week and 6 month assessments were used in all analyses. Patients who did not complete their 8 week visit, 6 month visit, or respond to visit requests by phone were included in the analyses for intention-to-treat analyses (ITT) only. For ITT analyses, patients who dropped out of the study before their appointment were included as non-responders, non-remitters, and as having decreased satisfaction. Bonferroni corrections were applied for multiple comparisons within the treatment arm analyses of change in satisfaction. Analyses were conducted using SAS (version 9.4; Cary, NC).
Power and Sample Size
This study was powered (90%) to find a group (GENE vs TAU) difference of 4 or more on the CDRS, if such a difference existed between the groups. The final group sample size of 84 per arm, which accounts for a dropout rate of 10%,35 provides 90% power to reject the null hypothesis with a significance level (alpha) of 0.050 using a two-sided two-sample equal-variance t-test. This power analysis is based on prior literature suggesting that a minimal clinically important difference (MCID) for pharmacologic treatments of depression in adolescents is 9 ± 2.24 on the CDRS.36
RESULTS
Participant Description
In this study, 196 patients were screened, 179 were consented and randomized, and 176 were included in this analysis (n=84 GENE, n=92 TAU). In terms of completion, 155 completed the 8 week visit and 125 completed the 6 month visit (Figure 1). In the full sample, 43 were male patients (24.4%), 145 were white (82.3%), and the mean age was 15.4 years (SD 1.5, range 13–18).
Anxiety disorders were a common comorbidity present in approximately 41% of the sample for GENE and TAU arms. Attention-deficit/hyperactivity disorder (ADHD) was the second most prevalent comorbidity at approximately 17% and 19% in the GENE and TAU arms, respectively. See Table 1 for additional rates of comorbidities in each treatment arm. The two arms did not statistically differ from one another regarding the prevalence of psychiatric comorbidities.
Table 1.
Summary of Demographics, Clinical Characteristics and Medications Prescribed During the Study by Treatment Arm (N=176)
| GENE |
TAU |
||||
|---|---|---|---|---|---|
| Variable | n | Mean(SD) | n | Mean(SD) | p-value |
|
| |||||
| Age | 84 | 15.5 (1.5) | 92 | 15.3 (1.5) | 0.501 |
| Gender (Male) | 84 | 19 (22.6%) | 92 | 24 (26.1%) | 0.593 |
| Race (white) | 83 | 70 (84.3%) | 87 | 75 (86.2%) | 0.731 |
| Anxiety | 84 | 35 (41.7%) | 91 | 37 (40.7%) | 0.892 |
| PTSD | 84 | 5 (6.0%) | 91 | 5 (5.5%) | 0.896 |
| ADHD | 84 | 14 (16.7%) | 90 | 17 (18.9%) | 0.702 |
| Dysthymia | 84 | 7 (8.3%) | 90 | 6 (6.7%) | 0.676 |
| Cannabis use | 83 | 11 (13.3%) | 91 | 5 (5.5%) | 0.077 |
| Substance use | 84 | 4 (4.8%) | 90 | 6 (6.7%) | 0.748 |
|
| |||||
| Number of past AD trials | 81 | 1.21 (1.16) | 87 | 1.08 (0.96) | 0.637 |
|
| |||||
| Duration of illness (years) | 82 | 1.00 (1.03) | 89 | 1.05 (1.27) | 0.814 |
|
| |||||
| Age of onset | 65 | 13.43 (2.24) | 86 | 13.73 (2.12) | 0.503 |
|
| |||||
| SSRI | 84 | 56 (66.7%) | 92 | 75 (81.5%) | 0.024a |
| SNRI | 15 (17.9%) | 10 (10.9%) | |||
| Atypical Antidepressant | 10 (11.9%) | 3 (3.3%) | |||
| Antipsychotic | 1 (1.2%) | 0 (0%) | |||
| Other | 2 (2.4%) | 4 (5.4%) | |||
|
| |||||
| Fluoxetine | 84 | 28 (33.3%) | 92 | 44 (47.8%) | 0.051 |
| Escitalopram | 84 | 16 (19.1%) | 92 | 22 (23.9%) | 0.433 |
Note:
Compares the prescription of SSRIs vs any other medication; AD = Antidepressant; ADHD = Attention-Deficit/Hyperactivity Disorder; GENE = Pharmacogenetics Guided Treatment; PTSD = Posttraumatic Stress Disorder; SNRI = Serotonin-Norepinephrine Reuptake Inhibitor; SSRI = Selective Serotonin Reuptake Inhibitor; TAU = Treatment As Usual
The majority of patients in the sample were prescribed a selective serotonin reuptake inhibitor (SSRI). Participants in the GENE arm were prescribed the following: 66.7% SSRI, 17.9% serotonin-norepinephrine reuptake inhibitor (SNRI), 11.9% atypical antidepressant, and 1.2% antipsychotic. Participants in the TAU arm were prescribed the following: 81.5% SSRI, 10.9% SNRI, 3.3% atypical antidepressant, and 0% antipsychotic. The SSRI prescribed rates were higher in the TAU group (p=0.024). The most prevalent medication prescribed in both treatment arms was fluoxetine with escitalopram being second.
Symptom Improvement
At baseline, the mean CDRS scores for the GENE and TAU arms were 57.8 (SD 9.2) and 58.0 (SD 7.4), respectively (p=0.843). At week 8, the mean CDRS-R scores for the GENE and TAU arms were 40.0 (SD 11.0) and 41.1 (SD 9.7), respectively. There were no statistical differences in the change from baseline in this primary outcome measure between the two treatment arms at 8 weeks (p=0.889) or 6 months (p=0.558) (Table 2). When evaluating response and remission rates based on the CDRS-R and QIDS for the GENE and TAU arm, there were no statistically significant differences between the GENE and TAU arms at any time point in the completer (Table 3) or the ITT sample (Table S1, available online).
Table 2.
Outcomes at Baseline, Week 8, and 6 Months and Comparison of Changes from Baseline by Treatment Arm
| GENE | TAU | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Outcome measure | Timepoint | n | Mean(SD) | n | Mean(SD) | Δ p-valuea |
|
| ||||||
| CDRS-R composite | Baseline | 84 | 57.8 (9.2) | 92 | 58.0 (7.4) | - |
| 8 week | 78 | 40.0 (11.0) | 80 | 41.1 (9.7) | 0.889 | |
| 6 month | 61 | 31.8 (11.5) | 64 | 30.3 (9.2) | 0.558 | |
|
| ||||||
| QIDS Composite total | Baseline | 84 | 16.4 (3.5) | 90 | 16.4 (3.4) | - |
| 8 week | 77 | 10.0 (4.8) | 78 | 10.0 (4.4) | 0.798 | |
| 6 month | 61 | 8.7 (4.6) | 64 | 8.4 (4.0) | 0.496 | |
|
| ||||||
| CGAS | Baseline | 83 | 47.1 (6.0) | 92 | 46.2 (5.8) | - |
| 8 week | 76 | 69.9 (12.9) | 77 | 69.2 (12.9) | 0.805 | |
| 6 month | 61 | 75.5 (14.7) | 65 | 78.3 (12.3) | 0.130 | |
|
| ||||||
| Total number of AE/SEs | Baseline | 79 | 0.92 (1.97) | 84 | 0.93 (1.97) | - |
| 8 week | 77 | 0.88 (1.74) | 75 | 1.01 (1.61) | 0.285b | |
| 6 month | 61 | 0.46 (1.18) | 64 | 0.59 (1.27) | 0.663b | |
|
| ||||||
| YMRS | Baseline | 84 | 0.55 (1.03) | 91 | 0.60 (1.38) | - |
| 8 week | 77 | 0.34 (0.91) | 78 | 0.29 (0.94) | 0.676 | |
| 6 month | 61 | 0.18 (0.56) | 65 | 0.20 (0.73) | 0.624 | |
Note:
All p-values were >0.05 before and after multiple comparisons
p-values represent comparison at each time point; Δ p-value = comparison of change from baseline between treatment arms; AE/SE = Adverse Events/Side Effects; CDRS-R = Children’s Depression Rating Scale, Revised; CGAS = Children’s Global Assessment Scale; GENE = Pharmacogenetics Guided Treatment; QIDS = Quick Inventory of Depressive Symptomatology; TAU = Treatment As Usual; YMRS = Young Mania Rating Scale
Table 3.
CDRS-R Composite and QIDS Total Composite Response and Remission by Treatment Group
| n | Total | TAU | GENE | p-value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| QIDS response | 8 week | 155 | 56 (36.1%) | 27 (34.6%) | 29 (37.7%) | 0.693 |
| 6 month | 125 | 66 (52.8%) | 36 (56.3%) | 30 (49.2%) | 0.429 | |
|
| ||||||
| QIDS remission | 8 week | 155 | 20 (12.9%) | 8 (10.3%) | 12 (15.6%) | 0.323 |
| 6 month | 125 | 30 (24.0%) | 15 (23.4%) | 15 (24.6%) | 0.880 | |
|
| ||||||
| CDRS-R response | 8 week | 155 | 110 (71.0%) | 56 (71.8%) | 54 (70.1%) | 0.819 |
| 6 month | 125 | 100 (80.0%) | 55 (86.9%) | 45 (73.8%) | 0.089 | |
|
| ||||||
| CDRS-R remission | 8 week | 155 | 50 (32.3%) | 22 (28.2%) | 28 (36.4%) | 0.277 |
| 6 month | 125 | 69 (47.2%) | 29 (46.9%) | 29 (47.5%) | 0.941 | |
Note: CDRS-R = Children’s Depression Rating Scale, Revised; GENE = Pharmacogenetics Guided Treatment; QIDS = Quick Inventory of Depressive Symptomatology; TAU = Treatment As Usual
Additional measures, including the continuous score improvement for QIDS and CGAS were also analyzed. Results showed no statistical differences between the GENE and TAU arms regarding symptom improvement on any of these measures at any time point during the study (Table 2).
The power analyses for depressive symptom reduction assessed by the CDRS-R was based on a clinically relevant difference on the CDRS-R of 9 ± 2.24 from prior work.36 This clinically relevant difference was based on expert consensus. The standard deviation of the prior study was 2.2436 and this is much lower than the standard deviation (SD) observed in the present study (the mean in CDRS-R SD in the two arms was 10.35 at week 8, Table 2). Therefore, a power analysis using the observed standard deviation and mean difference on the CDRS-R between the two treatment arms provides more meaningful information for a power estimation. In addition, the sample size available at week 8 differed from baseline and was considered for a subsequent power analysis, as the difference in CDRS-R scores between the two treatment arms was considered at week 8. Given a mean difference between the two groups of 4.7, a standard deviation of 10.35, a power of 0.80, 78 individuals per arm were included, yielding 0.80 power of detecting a difference on the CDRS-R of 4.7 ± 10.3, which corresponds to Cohen’s d=0.45, at the two-sided ɑ=0.05 significance level.
Adverse Events/Side Effects
The total number of adverse events/side effects, which were gathered from the FIBSER and SMURF-M, did not differ between the GENE arm and TAU arm at 8 weeks (p=0.28) or 6 months (p=0.66). Additionally, mean YMRS scores remained extremely low throughout the study in both treatment arms with no statistical differences seen in the change in YMRS between GENE and TAU arms at 8 weeks (p=0.676) or 6 months (p=0.624) (Table 2). There was no significant difference in the loss to follow-up at 8 weeks (GENE 8.3%, TAU 15.2%; p=0.16) or 6 months (GENE 27.4%, TAU 30.4%; p=0.66) between the treatment groups.
Patient/Parent Satisfaction
In completers analyses, there was a significant change in patient and parent satisfaction scores from baseline to week 8 in the total sample (p≤0.014), and in each treatment group after adjusting for multiple comparisons (patient TAU p=0.034, GENE p=0.028; parent TAU p=0.011, GENE p=0.001). There was not a statistically significant improvement in satisfaction from baseline to week 8 between the treatment groups (patient p-value=0.143, parent p-value=0.468) (Table 4). ITT analyses, assuming patients and parents lost to follow up experienced a decrease in satisfaction, showed no differences in patient satisfaction by between groups, or changes in patient satisfaction. However, there were higher rates of decreased parental satisfaction at 8 weeks overall (p=0.015) and in the TAU group specifically (p=0.024, Table S2, available online).
Table 4.
Change in Patient and Parent Satisfaction Scores from Baseline to Week 8
| Patient satisfaction 8 weeks | Total | TAU | GENE | Treatment difference p-value |
|---|---|---|---|---|
|
| ||||
| Improved | 39 (28.1%) | 17 (23.6%) | 22 (32.8%) | 0.143 |
| Did not change | 86 (61.9%) | 50 (69.4%) | 36 (53.7%) | |
| Decreased | 14 (10.1%) | 5 (6.9%) | 9 (13.4%) | |
|
| ||||
| Over-all change p-value | 0.014 | 0.034a | 0.028a | |
|
| ||||
| Parent satisfaction 8 weeks | ||||
|
| ||||
| Improved | 45 (40.2%) | 22 (39.3%) | 23 (41.1%) | 0.468 |
| Did not change | 55 (49.1%) | 26 (46.4%) | 29 (51.8%) | |
| Decreased | 12 (10.7%) | 8 (14.3%) | 4 (7.1%) | |
|
| ||||
| Over-all change p-value | 0.0003 | 0.011a | 0.001a | |
Note:
adjusted for multiple comparisons; GENE = Pharmacogenetics guided treatment; TAU = Treatment as usual
Use of Pharmacogenetics Testing Report
During the blinding period (baseline and week 4 visits) for patients randomized to the GENE arm, the pharmacogenetics testing report was used to inform clinician decision making for 77 out of 84 patients (91.7%). Reasons for not using the testing report included patient preference (n=6) and medications discontinued and started were not on the testing report (i.e., clinician relied on their own judgement) (n=1).
DISCUSSION
This was the first, double-blind, randomized, controlled trial evaluating the clinical utility of combinatorial pharmacogenetics testing in adolescents with depression. This study found that participants in both the GENE and TAU arms improved throughout the duration of the study, and there was no statistical difference in improvement between the two arms on the CDRS-R, QIDS, and CGAS. Additionally, there was no statistical difference in YMRS scores or the number of adverse events/side effects between the GENE and TAU arms throughout the duration of the study.
The results of this study may reflect an actual outcome that pharmacogenetics testing has no clinical impact on the measured outcomes. However, it is also possible that the results of pharmacogenetics testing in this study are diluted by patients in the TAU arm who were incidentally prescribed a medication with no gene-drug interactions. Pharmacogenetics testing may be valuable for a subset of patients in the population who are prescribed a medication with significant gene-drug interactions, but these interactions would only be known after the testing is ordered. A large, prospective study with 1167 adult outpatients, referred to as the GUIDED trial which has a similar design as the study presented here, found no improvement in depressive symptomatology between the pharmacogenetic guided treatment arm and TAU arm.14 However, a post-hoc analysis including patients who were only taking medications subject to gene-drug interactions found that pharmacogenetics testing significantly improved depressive symptoms and response and remission rates.37 Similar analyses in the adolescent population are needed.
A statistically significant finding that did emerge in this study was the difference in prescribing practices for medication classes between the GENE and TAU arms. The pharmacogenetics testing reports do include medications that are not FDA approved for children and adolescents, and the psychiatric providers were allowed to use any and all medication they felt were in the patient’s best interest as a means to generalize this study to real-world practice. The two FDA approved medications for adolescents with depression include fluoxetine and escitalopram, both SSRIs. Although SSRIs were the most frequently prescribed medications in both treatment arms, the TAU arm had nearly 15% more SSRIs prescribed than the GENE arm. The GENE arm had a greater proportion of SNRI and atypical antidepressant use. This finding is somewhat unexpected and suggests that combinatorial pharmacogenetics testing does influence physician decision making. In this study, it may reflect the tendency of the treating psychiatrist to prescribe outside of the SSRI medication class more frequently if there are testing results that suggest fewer gene-drug interactions with non-SSRIs, and this may suggest that physicians are more willing to deviate from prior evidence based treatment algorithms based on testing results. However, it is also noteworthy that no tricyclic antidepressants or monoamine oxidase inhibitors were prescribed during this study. These medications are often last-line medications in adolescents38,39 and arguably have a limited clinical trial evidence base for use in adolescents.40 Even though these medications are listed within the three color-coded columns in the pharmacogenetics testing results, the treating psychiatrists appeared to stay within practice parameters and not simply prescribe a medication that was listed in the “use as directed” (i.e. green bin) column. It is also important to recognize that the medication desvenlafaxine is not metabolized by any of the pharmacokinetic genes on the panel and does not have relevance to the pharmacodynamic genes, but was consistently listed in the “use as directed” (i.e., green bin) in all reports. An important area for future work includes how to reconcile evidence from clinical trial data and pharmacogenetic findings on a patient level. Although combinatorial pharmacogenetics testing is frequently available in clinical practice, there are remaining knowledge gaps related to how youth respond to many of the medications that combinatorial decision support tools may recommend. Further, it is unknown if combinatorial pharmacogenetics testing improves patient outcomes in the treatment of adolescents with major depressive disorder.
There are several strengths and limitations to this study. In terms of strengths, this was designed to be representative of real-world clinical practice, and as a result there were minimal exclusions for youth struggling with depression. Patients were recruited from academic and community settings that included both inpatients and outpatients. Second, this is the first and largest, randomized, patient-, parent-, rater-blinded study for pharmacogenetics testing in adolescents with depression. Third, providing active treatment in both the GENE and TAU arms reflected standard clinical practice, which helps evaluate the real-world, clinical utility of combinatorial pharmacogenetics testing with decision support tools. Fourth, the sample size provided had at least 80% power to find a difference of 4 or more on the CDRS between the treatment arms, suggesting that the present effort was a negative trial rather than an underpowered or failed trial.
In terms of limitations, the majority of our sample was white, and it may be difficult to generalize these results to other ethnic groups. Additional studies in more diverse populations are needed. Second, this study did not control for psychotherapy, and any improvement in depressive symptoms cannot be directly linked to medication management alone. Lastly, the prescribing psychiatrist was not blinded to treatment arm given the ethical limitations of managing medications in adolescent patients. An ideal future study would have a triple blind but the pragmatic, ethical, and regulatory aspects of such an endeavor would be challenging. As noted in the results, the initial power analysis was problematic as a clinically relevant difference on the CDRS-R of 9 ± 2.24 was referenced from a prior study.36 The standard deviation of CDRS-R scores in this prior study by Suresh and colleagues was much lower than the standard deviation in the present study. Also, the original power analyses considered the sample size at baseline rather than the slightly smaller sample observed at week 8. In some respects it is difficult to understand the large difference in the reported standard deviations across the prior36 and the present study. The prior study focused on a large explanatory clinical trial.36 The present study was by design a pragmatic clinical trial conducted in a setting as close to clinical practice as possible.41 It is expected that the present trial would have a more heterogeneous sample and variability in depressive symptom severity scores. The prior clinically relevant difference referenced was also based on expert consensus.36 Regardless, it is also evident with a subsequent power analysis that the present study had an adequate sample size at 8 weeks to detect a clinically meaningful difference.
Depression is a heterogeneous and complex disorder with potentially many underlying mechanisms of illness, and treatment response may be dependent upon those mechanisms of illness.16 Antidepressant response is not dictated solely by pharmacokinetic and pharmacodynamic properties42 as age, gender, renal and hepatic functioning, medical and psychiatric comorbidity, and nutritional status may all impact treatment response.8 For these reasons alone, it is only rational to view pharmacogenetics testing as a potential guide, not a guarantee, to medication selection. Educating patients and parents regarding this notion is not only ethical but critical and essential.
Pharmacogenetics testing held a great deal of promise as the gateway to personalized medicine. In 2001, Phillips et al. suggested it may be considered standard of care in 2020,43 but unfortunately, the field is not there yet and clear clinical utility for commercial combinatorial platforms in adolescents with depression remains elusive. Future research will evaluate whether concordance (defined as when the clinician’s treatment recommendation matches the pharmacogenetics testing report of “use as directed”) is associated with greater response/remission rates or lower side effect burden when compared to non-concordant treatment. Additional research with larger, prospective, randomized studies across more diverse ethnic groups is needed, and a more honed focus for evaluating treatment outcomes based on medication/dosing decisions related to specific medication-gene pairs needs further exploration as this could prove more useful than reliance on decision support tools associated with commercial combinatorial platforms.
Supplementary Material
Clinical Guidance:
Combinatorial pharmacogenetics panels should be interpreted carefully.
Future research is needed to evaluate the clinical utility of medication-gene pairs.
Acknowledgements:
The authors gratefully thank the participants and their families who participated in this study.
Funding and Disclosures:
Funding for this study was primarily provided by the Mayo Clinic Center for Individualized Medicine. Additionally, Dr. Vande Voort and Dr. Croarkin were co-primary investigators on this investigator initiated study that had a grant-in-kind for supplies and genotyping only through Assurex Health, Inc. Assurex Health, Inc. did not have any role in the development or design of the study, collection of data, statistical analysis, interpretation of data, writing of the manuscript, or decision for publication of this study.
Dr. Vande Voort is a site primary investigator for a multicenter study funded by the National Networks of Depression Centers. Dr. Croarkin receives research support from the National Institutes of Health (NIH). Dr. Croarkin has received research grant support from Pfizer, Inc. and equipment support from Neuronetics, Inc., and MagVenture, Inc. He is the primary investigator for a multicenter study funded by Neuronetics, Inc. and a site primary investigator for a study funded by NeoSync, Inc. Dr. Croarkin serves as a paid consultant for Procter & Gamble Company and Myriad Neuroscience. Dr. Leibman receives grant funding through the National Institute of General Medical Sciences of the National Institutes of Health (NIH) (T32 GM008685). Dr. Frye has received grant funding through Mayo Foundation, Medibio, Assurex Health, Inc. He has been a consultant for Actify Neurotherapies, Allergan, Intra-Cellular Therapies, Inc., Janssen, Myriad, Neuralstem Inc., Sanofi, Takeda, and Teva Pharmaceuticals. He has received CME/Travel/Honoraria through American Physician Institute, CME Outfitters, and Global Academy for Medical Education. All authors are currently employed or have been employed by Mayo Clinic, which had a prior financial interest in Assurex Health, Inc and currently has a financial interest in OneOme and the technology referenced with this publication. Dr. Orth, Dr. Shekunov, Dr. Romanowicz, Ms. Geske and Ms. Ward report no biomedical financial interests or potential conflicts of interest.
Footnotes
Presentation Information:
American Academy of Child and Adolescent Psychiatry 66th Annual Meeting; October 14–19, 2019; Chicago, Illinois
This study has been registered on www.clinicaltrials.gov (Identifier NCT02286440).
Contributor Information
Dr. Jennifer L. Vande Voort, Mayo Clinic in Rochester, MN, USA.
Dr. Scott S. Orth, Olmsted Medical Center in Rochester, MN, USA.
Dr. Julia Shekunov, Mayo Clinic in Rochester, MN, USA.
Dr. Magdalena Romanowicz, Mayo Clinic in Rochester, MN, USA.
Ms. Jennifer R. Geske, Mayo Clinic in Rochester, MN, USA.
Ms. Jessica A. Ward, Mayo Clinic in Rochester, MN, USA.
Dr. Nicole I. Leibman, Mayo Clinic in Rochester, MN, USA.
Dr. Mark A. Frye, Mayo Clinic in Rochester, MN, USA.
Dr. Paul E. Croarkin, Mayo Clinic in Rochester, MN, USA.
REFERENCES
- 1.Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry. 2015;54(1):37–44 e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heron M Deaths: Leading Causes for 2016. Natl Vital Stat Rep. 2018;67(6):1–77. [PubMed] [Google Scholar]
- 4.Maruf AA, Greenslade A, Arnold PD, Bousman C. Antidepressant pharmacogenetics in children and young adults: A systematic review. J Affect Disord. 2019;254:98–108. [DOI] [PubMed] [Google Scholar]
- 5.Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683–1696. [DOI] [PubMed] [Google Scholar]
- 7.Emslie GJ. The psychopharmacology of adolescent depression. J Child Adolesc Psychopharmacol. 2012;22(1):2–4. [DOI] [PubMed] [Google Scholar]
- 8.Hall-Flavin DK, Winner JG, Allen JD, et al. Using a pharmacogenomic algorithm to guide the treatment of depression. Transl Psychiatry. 2012;2:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller D, Kennedy JL, Himmerich H. Future roles of pharmacogenomic testing and biomarkers in psychiatry. Int Rev Psychiatry. 2013;25(5):493. [DOI] [PubMed] [Google Scholar]
- 10.Schildcrout JS, Denny JC, Bowton E, et al. Optimizing drug outcomes through pharmacogenetics: a case for preemptive genotyping. Clin Pharmacol Ther. 2012;92(2):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winner JG, Carhart JM, Altar CA, Allen JD, Dechairo BM. A prospective, randomized, double-blind study assessing the clinical impact of integrated pharmacogenomic testing for major depressive disorder. Discov Med. 2013;16(89):219–227. [PubMed] [Google Scholar]
- 12.Tanner JA, Davies PE, Voudouris NC, et al. Combinatorial pharmacogenomics and improved patient outcomes in depression: Treatment by primary care physicians or psychiatrists. J Psychiatr Res. 2018;104:157–162. [DOI] [PubMed] [Google Scholar]
- 13.Zeier Z, Carpenter LL, Kalin NH, et al. Clinical Implementation of Pharmacogenetic Decision Support Tools for Antidepressant Drug Prescribing. Am J Psychiatry. 2018;175(9):873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greden JF, Parikh SV, Rothschild AJ, et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: A large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. 2019;111:59–67. [DOI] [PubMed] [Google Scholar]
- 15.Hicks JK, Bishop JR, Gammal RS, et al. A Call for Clear and Consistent Communications Regarding the Role of Pharmacogenetics in Antidepressant Pharmacotherapy. Clin Pharmacol Ther. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zubenko GS, Sommer BR, Cohen BM. On the Marketing and Use of Pharmacogenetic Tests for Psychiatric Treatment. JAMA Psychiatry. 2018;75(8):769–770. [DOI] [PubMed] [Google Scholar]
- 17.Ramsey LB, Bishop JR, Strawn JR. Pharmacogenetics of treating pediatric anxiety and depression. Pharmacogenomics. 2019;20(12):867–870. [DOI] [PubMed] [Google Scholar]
- 18.United States Food and Drug Administration. The FDA Warns Against the Use of Many Genetic Tests with Unapproved Claims to Predict Patient Response to Specific Medications: FDA Safety Communication. https://www.fda.gov/medical-devices/safety-communications/fda-warns-against-use-many-genetic-tests-unapproved-claims-predict-patient-response-specific. Published 2018. Updated Novermber 1, 2018. Accessed November 3, 2019, 2019.
- 19.American Academy of Child and Adolescent Psychiatry. Clinical Use of Pharmacogenetic Tests in Prescribing Psychotropic Medications for Children and Adolescents https://www.aacap.org/AACAP/Policy_Statements/2020/Clinical-Use-Pharmacogenetic-Tests-Prescribing-Psychotropic-Medications-for-Children-Adolescents.aspx. Published 2020. Accessed April 12, 2020.
- 20.Aldrich SL, Poweleit EA, Prows CA, Martin LJ, Strawn JR, Ramsey LB. Influence of CYP2C19 Metabolizer Status on Escitalopram/Citalopram Tolerability and Response in Youth With Anxiety and Depressive Disorders. Front Pharmacol. 2019;10:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poweleit EA, Aldrich SL, Martin LJ, Hahn D, Strawn JR, Ramsey LB. Pharmacogenetics of Sertraline Tolerability and Response in Pediatric Anxiety and Depressive Disorders. J Child Adolesc Psychopharmacol. 2019;29(5):348–361. [DOI] [PubMed] [Google Scholar]
- 22.Ramsey LB, Prows CA, Zhang K, et al. Implementation of Pharmacogenetics at Cincinnati Children’s Hospital Medical Center: Lessons Learned Over 14 Years of Personalizing Medicine. Clin Pharmacol Ther. 2019;105(1):49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clin Pharmacol Ther. 2015;98(2):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troy TF, Poweleit EA, Strawn JR, Martin LJ, Ramsey LB. The Influence of Pharmacodynamic Genes on Fluoxetine Response in Pediatric Anxiety and Depressive Disorders. J Child Adolesc Psychopharmacol. 2020;30(4):276–277. [DOI] [PubMed] [Google Scholar]
- 25.Ramsey LB, Namerow LB, Bishop JR, et al. Thoughtful Clinical Use of Pharmacogenetics in Child and Adolescent Psychopharmacology. J Am Acad Child Adolesc Psychiatry. 2020;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. J Am Acad Child Psychiatry. 1984;23(2):191–197. [DOI] [PubMed] [Google Scholar]
- 27.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 29.Bernstein IH, Rush AJ, Trivedi MH, et al. Psychometric properties of the Quick Inventory of Depressive Symptomatology in adolescents. Int J Methods Psychiatr Res. 2010;19(4):185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS). Arch Gen Psychiatry. 1983;40(11):1228–1231. [DOI] [PubMed] [Google Scholar]
- 31.Greenhill LL, Vitiello B, Fisher P, et al. Comparison of increasingly detailed elicitation methods for the assessment of adverse events in pediatric psychopharmacology. J Am Acad Child Adolesc Psychiatry. 2004;43(12):1488–1496. [DOI] [PubMed] [Google Scholar]
- 32.Jablonski MR, King N, Wang Y, et al. Analytical validation of a psychiatric pharmacogenomic test. Per Med. 2018;15(3):189–197. [DOI] [PubMed] [Google Scholar]
- 33.McGraw J, Waller D. Cytochrome P450 variations in different ethnic populations. Expert Opin Drug Metab Toxicol. 2012;8(3):371–382. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Ingelman-Sundberg M, Lauschke VM. Worldwide Distribution of Cytochrome P450 Alleles: A Meta-analysis of Population-scale Sequencing Projects. Clin Pharmacol Ther. 2017;102(4):688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emslie GJ, Ventura D, Korotzer A, Tourkodimitris S. Escitalopram in the treatment of adolescent depression: a randomized placebo-controlled multisite trial. J Am Acad Child Adolesc Psychiatry. 2009;48(7):721–729. [DOI] [PubMed] [Google Scholar]
- 36.Suresh V, Mills JA, Croarkin PE, Strawn JR. What next? A Bayesian hierarchical modeling re-examination of treatments for adolescents with selective serotonin reuptake inhibitor-resistant depression. Depress Anxiety. 2020;37(9):926–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thase ME, Parikh SV, Rothschild AJ, et al. Impact of Pharmacogenomics on Clinical Outcomes for Patients Taking Medications With Gene-Drug Interactions in a Randomized Controlled Trial. J Clin Psychiatry. 2019;80(6). [DOI] [PubMed] [Google Scholar]
- 38.Practice parameters for the assessment and treatment of children and adolescents with depressive disorders. AACAP. J Am Acad Child Adolesc Psychiatry. 1998;37(10 Suppl):63S–83S. [DOI] [PubMed] [Google Scholar]
- 39.Hughes CW, Emslie GJ, Crismon ML, et al. Texas Children’s Medication Algorithm Project: update from Texas Consensus Conference Panel on Medication Treatment of Childhood Major Depressive Disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(6):667–686. [DOI] [PubMed] [Google Scholar]
- 40.Qin B, Zhang Y, Zhou X, et al. Selective serotonin reuptake inhibitors versus tricyclic antidepressants in young patients: a meta-analysis of efficacy and acceptability. Clin Ther. 2014;36(7):1087–1095 e1084. [DOI] [PubMed] [Google Scholar]
- 41.Ford I, Norrie J. Pragmatic Trials. N Engl J Med. 2016;375(5):454–463. [DOI] [PubMed] [Google Scholar]
- 42.Tansey KE, Guipponi M, Hu X, et al. Contribution of common genetic variants to antidepressant response. Biol Psychiatry. 2013;73(7):679–682. [DOI] [PubMed] [Google Scholar]
- 43.Phillips KA, Veenstra DL, Oren E, Lee JK, Sadee W. Potential role of pharmacogenomics in reducing adverse drug reactions: a systematic review. JAMA. 2001;286(18):2270–2279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


