Abstract
As the population of cancer survivors has grown into the millions, there has been increasing emphasis on understanding how the late effects of treatment affect survivors’ ability to return to work/school, their capacity to function and live independently, and their overall quality of life. This review focuses on cognitive change associated with cancer and cancer treatments. Research in this area has progressed from a pharmacotoxicology perspective to a view of the cognitive change as a complex interaction of aspects of the treatment, vulnerability factors that increase risk for posttreatment cognitive decline, cancer biology, and the biology of aging. Methodological advances include the development of (a) measurement approaches that assess more fine-grained subcomponents of cognition based on cognitive neuroscience and (b) advanced statistical approaches. Conceptual issues that arise from this multidimensional perspective are described in relation to future directions, understanding of mechanisms, and development of innovative interventions.
Keywords: aging, biomarkers, cancer, cancer treatment, chemotherapy, cognition, endocrine therapy, genetics, neuroimaging, radiation therapy
OVERVIEW OF CANCER AND COGNITION
As the population of cancer survivors has grown into the millions (expected to reach 20 million in the United States by 2026; Am. Cancer Soc. 2017), there has been increasing emphasis on understanding how the late effects of treatment affect survivors’ ability to return to work/school, their capacity to function and live independently, and their overall quality of life. Cognitive changes are one of the most feared problems among cancer survivors (Ahles et al. 2012). Research examining the cognitive impact of brain tumors and treatments that directly affect the brain (cranial surgery and radiation therapy) has a long history (Correa 2010). Additionally, there is a substantial literature examining cognitive deficits in children treated for cancer (Winick 2011) and a growing literature on cognitive functioning in adult survivors of childhood cancers (Cheung & Krull 2015). However, evidence for cancer-associated cognitive decline (CACD) for the common non–central nervous system (non-CNS) cancers in adults (breast, colon, lymphoma, and prostate) has significantly broadened the scope of the problem and will be the focus of this review.
Research has examined cognitive change across a variety of cancer types (primarily breast cancer, but increasingly colon, prostate, and hematological cancers) and across a variety of treatments (standard and high-dose chemotherapy with stem cell transplant, endocrine/hormone ablation therapies, and local radiation). Cancer is frequently treated with multiple modalities, which complicates the study of CACD and the identification of the components of treatment responsible for cognitive change. Treatment for many cancers may consist of a combination of surgical resection, systemic chemotherapy, and local radiation therapy. Women with breast cancer may receive en-docrine therapy, and men with prostate cancer may have hormone ablation therapy included in the treatment regimen. Chemotherapy treatment may be neo-adjuvant, in which case chemotherapy is initiated prior to surgery to reduce the tumor size, or adjuvant, in which case chemotherapy fol lows surgery to address remaining cancer proliferation and occult cells that may have metastasized to lymph nodes or more remote sites. Radiation treatment may likewise be relatively localized, or the field may expose multiple organs (e.g., the lungs and heart). Endocrine therapy in breast cancer is used over longer periods of time post–primary therapy (up to 10 years) to control recurrence by limiting estrogen binding (tamoxifen) or estrogen production (aromatase inhibitors) in cases in which tumor cells are positive for either estrogen or progesterone receptors. In the case of some prostate cancers, tumor cells are sensitive to testosterone, and as a result, the goal of hormone ablation therapy is to reduce testosterone to nearly undetectable levels over longer periods of time. Surgery with general anesthesia can cause delirium and lasting cognitive change, particularly in older patients (Le Strat 2012). Even though radiation therapy is referred to as localized, it induces a systemic immune response, chronic fatigue, and potentially cognitive decline (Shibayama et al. 2014). Therapies that alter estrogen and testosterone levels have been shown to affect cognitive function in breast and prostate cancer (Schilder et al. 2010a). Therefore, even though many researchers have assumed to be studying the cognitive effects of chemotherapy, in reality most of the research has examined the cognitive impact of the entire package of treatment exposures.
Research suggests that most patients experience cognitive change (problems with attention, concentration, memory, and multitasking) during active treatment due to multiple factors, including feeling generally ill, anemia, nausea, disturbed sleep, use of sedating medications and/or steroids for control of side effects, stress/anxiety, etc. A significant number of patients report improvement in cognitive function following the end of primary treatment as other treatment side effects resolve, although improvement may be gradual, occurring over one to two years. The primary clinical concern is that a subgroup of survivors (estimates vary but are likely to be in the 20–30% range) demonstrates only partial recovery and report persistent cognitive changes up to 20 years after treatment (Koppelmans et al. 2012). One study also found no cognitive problems shortly after treatment in a subset of patients but reported decline at continued follow-up assessments, suggesting that there may be a subgroup of survivors who experience delayed cognitive decline (Wefel et al. 2010). The definition of end of treatment has had to change according to increasing evidence that endocrine treatment for breast cancer for up to 10 years after primary treatment, hormone ablation treatment for prostate cancer, and oral cancer treatments taken daily for years (e.g., tyrosine kinase inhibitors, TKIs) can also affect cognitive function (Phillips et al. 2013).
Initially, researchers in this area conceptualized the problem of chemotherapy-induced cognitive decline (known as chemobrain) from a pharmacotoxicology perspective, that is, assuming that patients diagnosed with cancer would have normal cognitive functioning prior to treatment, which would be adversely affected by exposure to certain chemotherapeutic agents. Given the above considerations, research has shifted toward the examination of the cognitive impact of multiple cancer treatments and of the factors that increase risk for long-term cognitive decline. Figure 1 provides a conceptual model that outlines the multiple factors that may contribute to posttreatment cognitive decline.
Figure 1.
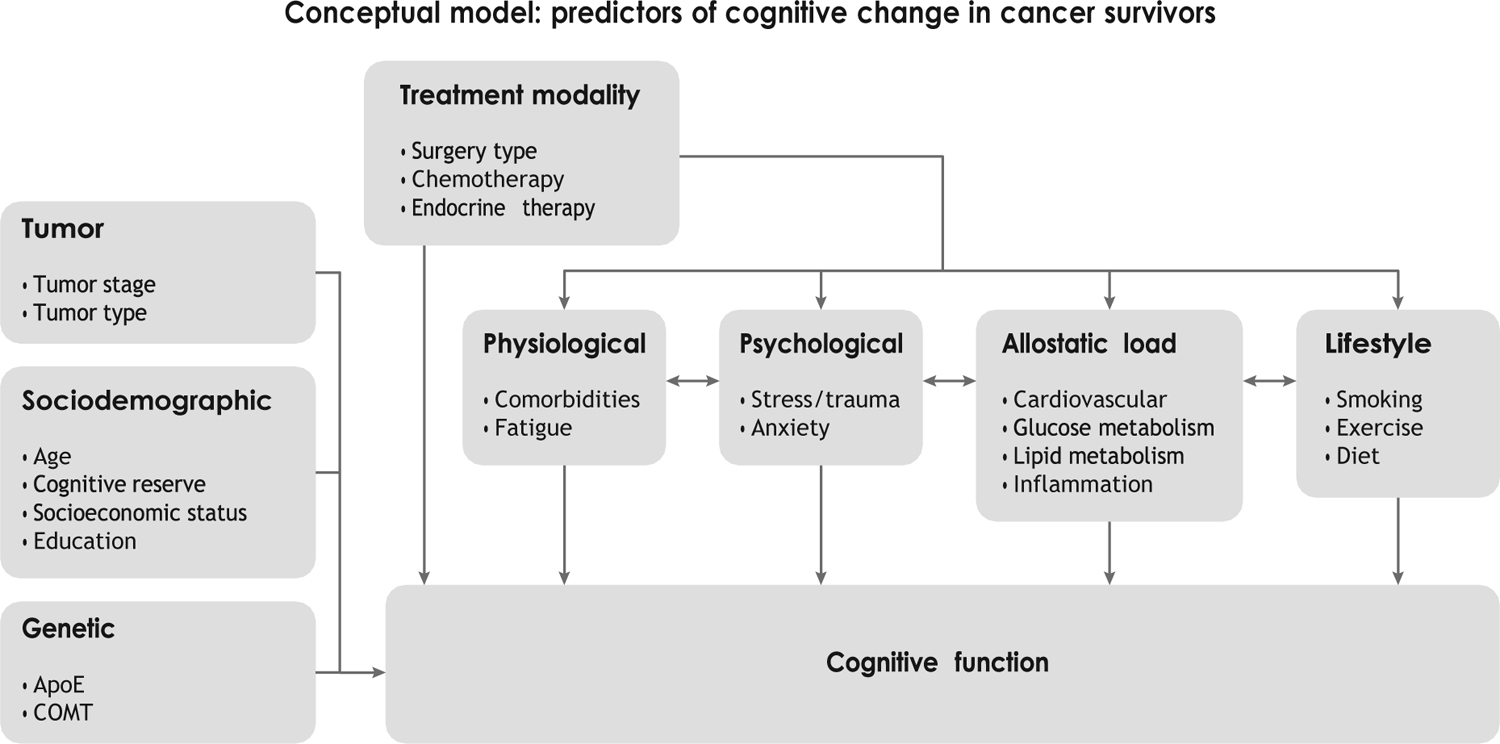
Cancer treatment can have a direct effect on cognition and can interact with various risk factors. The factors on the left side of the figure represent predisposing risk factors, whereas the remaining factors (physiological, psychological, allostatic load, and lifestyle) are both predisposing factors and factors that can be modified by treatment.
Cancer and cancer treatments clearly influence the emergence of cognitive decline. However, the risk for persistent cognitive decline is likely related to an interaction with sociodemographic, lifestyle, psychological, physiological, and genetic factors. The addition of biomarkers to cognitive studies that have focused on specific pathways (e.g., inflammation; Ganz et al. 2013) or on dysregulation across multiple biological systems (e.g., allostatic load; McEwen 2015) has contributed to the literature on potential mechanisms for cognitive decline. An emerging question, which will be addressed more completely below, is the extent to which cancer and cancer treatments have a direct effect on brain structure and function as well as the extent to which cancer treatments accelerate the aging process on a biological level, including cognitive aging (Ahles et al. 2012).
The goals of this review are to describe what is known about: (a) clinical presentation of cognitive deficits in cancer patients, (b) risk factors, (c) potential mechanisms (imaging, biomarkers, and animal studies), (d) clinical assessment, and (e) treatment. The review will end with a discussion of challenges and future directions for research.
CLINICAL PRESENTATION
The cognitive effects found in CACD are generally subtle compared to degenerative conditions and other CNS disorders, but they can significantly affect survivors’ quality of life. Although cancer is most commonly a disease of the elderly, there are many people diagnosed with cancer at younger ages, and given the changing demographic of the workforce, many people above age 65 continue to work and lead active lives. Therefore, most cancer survivors expect to recover and return to previous responsibilities. However, a subgroup of survivors consistently report difficulties returning to daily activities in both domestic life and work, increased stress, the need for more time and effort in their work, increased frustration, work conflicts, financial impact, and decreased quality of life (QOL) (Selamat et al. 2014, Von Ah et al. 2013).
Self-Report
The most commonly self-reported dysfunctions in cancer survivors include distraction, forgetfulness, and difficulties with attention, multitasking, and word finding. In early reports, half of cancer patients reported cognitive declines at some point in their treatment (Cull et al. 1995). Six months following treatment, 30% of lymphoma patients reported concentration difficulties and 52% reported forgetfulness (Cull et al. 1996). Up to 10 years after treatment in breast and lymphoma patients, Ahles et al. (2002) found reports of difficulties in concentration and complex attention in survivors who had been treated with chemotherapy. Other studies have found similar rates of self-reported difficulties (Castellon et al. 2004, Hermelink et al. 2007, Mehnert et al. 2007, Poppelreuter et al. 2004, Schagen et al. 2008, van Dam et al. 1998). More recent work on larger samples confirms earlier findings, with rates of self-reported cognitive dysfunction ranging between 37% and 58% six months following treatment (Janelsins et al. 2017a). Recent longitudinal studies in breast cancer suggest roles for both adjuvant chemotherapy treatment and endocrine therapy (Merriman et al. 2017).
Performance-Based Assessment
Research that utilizes objective, performance-based neuropsychological measures also finds significant effects of cancer treatments, although most suggest a more subtle dysfunction. Cross-sectional studies of patients with breast cancer and lymphoma have found significant differences between chemotherapy-treated patients, patients not exposed to chemotherapy (breast cancer), and healthy control groups (Ahles et al. 2002, Schagen et al. 1999, van Dam et al. 1998, Wouters et al. 2016, Yamada et al. 2010). A subset of studies revealed a chemotherapy dose-response re lationship (van Dam et al. 1998) as well as cognitive dysfunction at longer intervals (mean = 20 years) after treatment (Koppelmans et al. 2012). Longitudinal studies that control for baseline differences in cognition reveal subtle but significant effects (Fan et al. 2005, Hermelink et al. 2007, Schagen et al. 2006, Shilling et al. 2005). With regard to the specific domains affected, research has demonstrated changes in attention (Biglia et al. 2012, Hurria et al. 2006, Wefel et al. 2004), working memory (Collins et al. 2009, Stewart et al. 2008), processing speed (Ahles et al. 2010, Collins et al. 2009, Wefel et al. 2010), and learning and memory (Ahles et al. 2010; Bender et al. 2006; Collins et al. 2009; Hedayati et al. 2012; Hurria et al. 2006; Quesnel et al. 2009; Vearncombe et al. 2009; Wefel et al. 2004, 2010). In the most recently reported longitudinal study (Janelsins et al. 2017b), which included 580 breast cancer patients and 363 controls, patients’ performance on computerized measures of processing speed, working memory, and recognition memory, together with traditional measures of verbal fluency, declined when contrasted with control performance. Importantly, a subset of prospective studies of breast and colon cancer patients have found cognitive dysfunction in patients before chemotherapy treatment (Ahles et al. 2008, Hermelink et al. 2007, Schilder et al. 2010b, Vardy et al. 2014), before surgery (Hermelink et al. 2007), and before definitive diagnosis (Hedayati et al. 2011). These pretreatment or even prediagnosis effects have led to questions regarding the potential shared risk for cognitive decline and cancer (e.g., inefficient DNA repair mechanisms) (Ahles et al. 2012), the role of potential mood and adjustment reactions to diagnosis and treatment (Hermelink et al. 2015), and biological effects of stress and allostatic load (Andreotti et al. 2015). Despite numerous positive findings, a subset of studies failed to find any effect of treatment (Debess et al. 2010, Hermelink et al. 2008, Jenkins et al. 2006, Mehlsen et al. 2009). In a large study of colon cancer patients, Vardy et al. (2015) found that patients had substantially more cognitive impairment at the pretreatment and posttreatment assessments compared to healthy controls, but they found no significant effect of chemotherapy. In addition to baseline differences between patients and controls, we discuss other possible reasons for these inconsistencies below.
STRUCTURAL AND FUNCTIONAL NEUROANATOMY
Neuroimaging studies indicate prefrontal involvement, in addition to other regional changes, based on both structural and functional MRI. Cross-sectional and longitudinal studies of cancer patients have found reduced dorsolateral prefrontal cortex (DLPFC) volume (middle frontal gyrus, superior frontal gyrus, frontal poles) (Correa et al. 2013, Inagaki et al. 2007, McDonald & Saykin 2013, McDonald et al. 2010) and alterations in underlying white matter integrity in anterior/prefrontal regions (Correa et al. 2016, Deprez et al. 2011). These structural changes have been associated with functional alterations in survivors as well (McDonald et al. 2010, 2012), mainly in more distributed prefrontal cortical activation (Conroy et al. 2013, de Ruiter et al. 2011, Ferguson et al. 2007, Kesler et al. 2009, McDonald et al. 2012, Nudelman et al. 2014, Silverman et al. 2007) or in reduced activation (Wang et al. 2016). Recent work has also found differences in hippocampal structure, increased memory complaints, and decreased objective memory performance in breast cancer survivors following chemotherapy maintained on tamoxifen (Apple et al. 2017) and decreased hippocampal function during recognition memory (Wang et al. 2016). In addition to altered structure and function following treatment, pretreatment differences have also been reported that further contextualize the posttreatment findings. In a recent study, breast can cer patients, regardless of planned chemotherapy treatment, exhibited greater prefrontal activity on a working memory task together with decreased white matter integrity prior to adjuvant treat ment compared to controls, with fatigue being associated with both findings (Menning et al. 2015). Despite these structural and functional MRI differences, outcomes on cognitive paradigms administered as part of functional imaging are inconsistent in finding behavioral differences. Equivalent performance between groups despite structural and functional differences has been interpreted as compensatory and more effortful processing in patients (McDonald et al. 2012), as reflected in activation patterns that are more distributed and utilize additional cortical regions that support normatively expected performance.
A limited number of studies have used electroencephalography (EEG) to detect alterations in cancer survivors. Studies that have assessed the relative amplitude and latency of the P3b ERP component (Kreukels et al. 2006, 2008), a relatively later electrophysiological gauge of the salience and ease of classification of stimuli held in working memory, found altered amplitude and latency in chemotherapy-treated individuals relative to controls. These results suggest that chemotherapy undermines the speed and distinctiveness of stimulus encoding in working memory, leaving mechanisms of perceptual encoding intact. In preliminary work from our lab, we examined P50 suppression related to chemotherapy in breast cancer survivors several years after treatment and found that survivors exhibited a relatively weakened ability to inhibit redundant sensory stimulation in both a paired-click paradigm and in an oddball paradigm. The scalp topography of group differences in P50 suppression differed by paradigm, peaking over the right hemisphere in the paired-click paradigm. Dipole source analysis localized the survivors’ loss of P50 suppression to the hippocampus, with relative preservation of function in the gating-out mechanisms of the frontal lobe and auditory cortex. Survivors also showed a diminishment in the sensory memory processes needed to register novel or deviant information in an otherwise homogenous auditory environment. These findings suggest that chemotherapy is associated with a disruption of early mechanisms of sensory gating.
ANIMAL MODELS
Animal models have mainly focused on the effects of in vivo chemotherapy exposure on cognitive/behavioral and cellular changes in the brain. At a cellular/biological level, increased apoptosis, changes in cell morphology, reduced neurogenesis, and neuroinflammation have all been demonstrated in response to chemotherapy agents (see Seigers et al. 2013 for review). Behavioral and cognitive effects have been demonstrated as well, mainly in memory (Seigers et al. 2008, Winocur et al. 2012), with recent work suggesting a protective effect of exercise (Winocur et al. 2014). Particularly significant with regard to the role of poorer initial learning in cancer survivors (discussed below), the effects of chemotherapy exposure are magnified in high-interference conditions at the time of learning (Winocur et al. 2015). The finding of increased interference related to competing stimulation at the time of learning is consistent with EEG studies in humans, discussed above, as well as in a mouse model that found the P50 component, an index of sensory gating, to be altered following chemotherapy exposure (Gandal et al. 2008).
BIOMARKERS
Peripheral biomarkers associated with cognitive effects of cancer treatment have also been investigated. Direct and oxidative DNA damage, as measured by Comet assay techniques, has been found to be associated with chemotherapy exposure and cognition in a mouse model (Krynetskiy et al. 2013) and with brain structure and cognition in a cohort of breast cancer survivors (Conroy et al. 2013). Inflammation has been proposed as a potential mechanism for CACD. In breast cancer survivors, TNF (sTNF-RII) has been associated with self-reported cognitive dysfunction (Ganz et al. 2013), and both STNF-RII and IL-1ra have been associated with self-reported difficulties and cerebral metabolism (Pomykala et al. 2013). Serum interleukin-6 (IL-6) and TNF-α have also been associated with verbal memory performance and hippocampal volume (Kesler et al. 2013b), and IL-6 has been associated with increased self-reported cognitive difficulties (Amidi et al. 2015, Chae et al. 2016). In a recent study of testicular cancer patients treated with and without chemotherapy, poorer cognitive performance was associated with higher levels of TNF-α in the chemotherapy-treated group (Amidi et al. 2017). Cortisol and hypothalamic-pituitary-adrenal (HPA) axis function have also been hypothesized to play a role in cancer and cognition, as cortisol level has been associated with learning and memory performance in nonclinical samples (Andreotti et al. 2015). Altered cortisol response has been associated with fatigue in a sample of breast cancer patients (Bower et al. 2005), and serum cortisol was found to be associated with objective cognitive performance across multiple measures of verbal learning, memory, attention, and psychomotor speed in a sample of testicular cancer patients (Amidi et al. 2015).
GENETIC RISK FACTORS
Previous literature has generally demonstrated that only a subset of survivors may be at risk for CACD, either as assessed by self-report or by objective, performance-based assessment. We have previously suggested a number of potential moderating or mediating factors that may play a role in increasing risk for CACD in survivorship (Ahles & Saykin 2007), including biologic, demographic, educational, and lifestyle factors. However, only a subset of these factors has been investigated empirically.
Apolipoprotein E
The APOE gene is involved in the creation of apolipoprotein E, a protein, which, when combined with lipids, forms lipoproteins. Lipoproteins act to transport cholesterol throughout the vascula ture. Three common alleles consist of APOE e2, e3, e4, for a total of six genotype combinations. Beyond the increased risk of Alzheimer’s disease with APOE e4 carrier status (van der Flier et al. 2006), e4 status is also associated with higher risk of cognitive decline in normal aging (Schiepers et al. 2012) and in the presence of new-onset disease or insult (HIV, diabetes, stroke). Ahles et al. (2003) were the first to demonstrate a similar association of APOE e4 status with decreased performance in visual memory and spatial ability in chemotherapy-treated breast and lymphoma survivors. This finding was replicated in a prospective study of breast cancer patients (Ahles et al. 2014). Interestingly, a protective effect of smoking history for e4 carriers was found, presumably related to a correction for a deficit in nicotinic receptors in e4 carriers. Similar results have been reported by other groups in breast cancer samples (Koleck et al. 2014, Lengacher et al. 2015), in CNS cancer (Correa et al. 2014), and in a recently reported study on testicular cancer survivors (Amidi et al. 2017).
Catechol-O-Methyltransferase
The COMT gene encodes the protein catechol-O-methyltransferase, which in turn regulates the degradation of the catecholamines dopamine, epinephrine, and norepinephrine. The COMT polymorphism, Val158Met, has previously been identified as affecting neurotransmission via a three- to fourfold increase in rate of transmitter degradation in the Val/Val versus Met allele, with the effect of decreasing dopamine availability (Bastos et al. 2017). COMT gene polymorphisms have been associated with lower memory performance in normal aging and with altered prefrontal function and structure. Differences in cognitive functioning have been demonstrated in survivors of acute lymphocytic leukemia (Cole et al. 2015) and in adult breast cancer survivors (Small et al. 2011): Survivors with the COMT-Val allele exhibited decreased performance in attention, motor speed, and verbal fluency when compared to survivors with the COMT-Met allele.
Brain-Derived Neurotrophic Factor
BDNF, a protein implicated in neurogenesis, protection, regulation, and synaptic plasticity, is found in the hippocampus, caudate nucleus, and cerebral cortex, and has been associated with hippocampal volume and memory function in aging (Erickson et al. 2010). A recent study in early-stage breast cancer survivors suggests a protective effect for the BDNF Met/Met polymorphism on cognitive function over Val/Val carriers (Ng et al. 2015).
ADDITIONAL RISK FACTORS
Age
Advancing age has been found to be associated with changes in cognitive performance, with fluid abilities exhibiting relative declines, whereas crystallized abilities appear to improve or remain more stable in middle to older age. Decline in brain reserve—i.e., accrued structural and functional changes in the brain that decrease redundancy together with decreasing plasticity—may be one potential mechanism through which age is associated with declining cognition. Age has been found to moderate the effects of cancer treatment on cognition. Schilder et al. (2010a) found older age to interact with endocrine treatment in breast cancer survivors, resulting in more cognitive domains being affected by treatment in older versus younger survivors. Ahles et al. (2010) found that older-age patients exposed to chemotherapy had greater decreases in psychomotor speed as compared to patients not treated with chemotherapy and healthy controls.
A major gap in the field is that the majority of studies have been conducted with younger adults (<60 years), despite the fact that cancer is a disease of the elderly, with the majority of cancer patients (including breast, colon, prostate, and lung cancers) being diagnosed at ages 60 and above. However, results of studies focusing on older adults with cancer are beginning to emerge. Lange et al. (2017) evaluated older breast cancer patients (>65 years) prior to treatment and at the end of chemotherapy or radiation therapy. Although no overall differences were found over time between patients and matched healthy controls, the authors reported that patients 75 years or older who were exposed to chemotherapy were at the highest risk for cognitive decline. In a similarly designed, larger multicenter study, Mandelblatt et al. (2014b) found that comorbidity (primarily cardiovascular disease and diabetes) was associated with pretreatment cognitive impairment in patients but not in the healthy control group. This ongoing longitudinal study will be able to assess whether pretreatment comorbidity (along with other potential risk factors) predicts posttreatment cognitive decline in this older breast cancer population.
Cognitive Reserve
Cognitive reserve represents innate and developed cognitive capacity, which is influenced by genetics, education, occupation, lifestyle, cognitively stimulating activities, etc. (Barulli & Stern 2013). Low cognitive reserve has been associated with cognitive decline with aging, risk and severity of neurocognitive disorders (e.g., Alzheimer’s), and cognitive decline following insult to the brain (Barulli & Stern 2013). Ahles et al. (2010) found lower cognitive reserve, older age, and treatment with chemotherapy to be associated with greater posttreatment cognitive decline. Similarly, Mandelblatt et al. (2014b) found that older age, lower education (a proxy for cognitive reserve), and greater comorbidity were related to greater cognitive impairment prior to initiation of adjuvant treatment.
Pathologic Tumor Markers
Koleck et al. (2017) found a relationship between HER2 status, immunohistochemistry classifica tion (IHC), and performance on measures of memory prior to initiation of adjuvant treatment for breast cancer. Patients with HER2-positive tumors scored worse on measures of memory compared to patients with HER2-negative tumors, and as IHC scores increased, memory performance decreased. Although the biological explanation for the link between HER2 status and memory performance is not known, the investigators speculate that the relationship may be related to the role of the erb-b2 receptor tyrosine kinase 2 gene (ERBB2) in encoding HER2 and to neural development in both the central and peripheral nervous system.
Stress/Trauma
An understudied area is the impact of coping with stress/trauma history on brain structure and function and the potential for these changes to be related to increased risk for CACD (Andreotti et al. 2015). Exposure to chronic stress and traumatic events, particularly in childhood, can lead to biological changes such as alterations in the HPA axis (e.g., flattened cortisol response). Altered HPA axis function has been associated with structural and functional changes in frontal areas of the brain and hippocampus (similar to areas affected by cancer treatments) (McEwen 2016). Adaptive coping can reduce the negative impact of stress, whereas maladaptive patterns of coping (alcohol and drug use, smoking, poor diet, etc.), disrupted circadian rhythm/disturbed sleep patterns (McEwen 2016), and depletion of cognitive self-regulatory resources (Arndt et al. 2014) can amplify the effects of stress. Hermelink et al. (2015) found that the presence of posttraumatic stress symptoms (related to either the cancer diagnosis or previous traumatic events) mediated the relationship between breast cancer diagnosis and cognitive performance on a go/no-go task prior to initiation of adjuvant treatment. Additional research in this area is clearly needed.
Racial/Ethnic Diversity and Socioeconomic Status
A significant gap in the field is that most study samples have been made up of affluent, highly educated, Caucasian populations. However, there is ample evidence from other areas of research demonstrating differences in cognitive function, brain structure and function, and vulnerability to cognitive decline based on race, ethnicity, culturally based cognitive preferences, and educational and socioeconomic factors (Zahodne et al. 2015). Genetic variability across racial/ethnic groups may be important; for example, the APOE4 allele does not confer the same risk for Alzheimer’s disease across racial/ethnic populations (Ward et al. 2012), and it was not associated with measures of neuropsychological performance in an African American community–based sample (Borenstein et al. 2006). Cultural differences have been shown to influence preferred cognitive style (e.g., analytic versus holistic), which translates into differences in attentional control and categorical memory errors, as assessed by performance on cognitive tasks and activation patterns evaluated with functional MRI (fMRI) (Jung & Cimprich 2014, Schwartz et al. 2014). Further, the physical and psychological stresses associated with poverty are a pervasive cause of vulnerability to increased allostatic load (the accumulation of dysregulation across multiple biological systems: immune, endocrine, cardiovascular, HPA axis, etc.) and, potentially, vulnerability to the cognitive side effects of cancer treatments (Chattarji et al. 2015, McEwen 2016). Finally, education and cognitive reserve have been shown to be powerful determinants of cognitive performance and cognitive aging, and low socioeconomic status is associated with less education and poorer educational environments (Chattarji et al. 2015). Based on these considerations, it is possible that we have underestimated the cognitive effects of cancer treatments, because research has not included the most vulnerable groups, and the data relevant to posttreatment cognitive decline are not necessarily generalizable to other populations. Therefore, a serious gap in the field is an examination of treatment-related cognitive decline across racial/ethnic/cultural groups and socioeconomic levels.
INTERVENTIONS
Cognitive Rehabilitation
Cognitive rehabilitation involves either specific training and/or teaching survivors to manage/compensate for their cognitive deficits. Cognitive training involves regular practice of skills in an attempt to restore attention, psychomotor speed, memory, and/or executive functioning (increas ingly done with computer software). Managing cognitive deficits includes teaching survivors to (a) pace themselves during cognitive activities to contend with cognitive fatigue; (b) avoid or decrease distractions; (c) plan and organize time; and (d) utilize strategies such as calendars, day planners, and mnemonics. Education about brain functioning, cognitive deficits, and their implications for the instrumental activities of daily living is also an essential part of cognitive rehabilita tion. Manualized individual interventions (Ferguson et al. 2012) and group interventions (Ercoli et al. 2015) have shown promise in improving scores on neuropsychological tests of memory and executive function and on self-reports of cognitive problems and interference with daily activities. Computer-based cognitive rehabilitation programs are being increasingly evaluated. Survivors randomized to use speed of processing training software (Brain HQ by Posit Science) improved their processing speed and memory, whereas those who had exclusively memory training exhibited only improved memory (Von Ah et al. 2012). Using Lumosity software, breast cancer survivors improved on neuropsychological tasks of cognitive flexibility, verbal fluency, and processing speed, as well as on their self-reported ratings of executive functioning skills (planning, organizing, and task monitoring) in daily life (Kesler et al. 2013a). These findings are in contrast to those reported for breast cancer patients with cognitive impairment who were randomized to individual computer-based training from various software vendors or compensatory skills rehabilitation, where no significant intervention effect was found (Poppelreuter et al. 2009).
Pharmacological Treatment
There are no pharmacological treatments specifically targeted for CACD. The National Comprehensive Cancer Network (NCCN) recommends pharmacologic interventions as a last line of therapy (Denlinger et al. 2014). Clinical trials have primarily studied psychostimulant medications, although emerging studies have examined the efficacy of medications like memantine and estradiol (prostate cancer).
Treatments with methylphenidate and, more recently, modafinil have been studied with CACD. Methylphenidate has been approved for the treatment of attentional symptoms and attention deficit disorder through its action as a mild CNS stimulant. Methylphenidate improves neural activation by stimulating predominantly the prefrontal cortex and cortico-striatal regions (Berridge & Devilbiss 2011) and by acting as a dopamine and norepinephrine agonist (Hannestad et al. 2010). Studies of methylphenidate have demonstrated positive outcomes on cognition, QOL, and fatigue in breast cancer patients undergoing adjuvant chemotherapy (Mar Fan et al. 2008), in breast and ovarian cancer survivors (Escalante et al. 2014, Lower et al. 2005), and in melanoma patients treated with interferon (Schwartz et al. 2002).
Modafinil has been approved for treating narcolepsy, shift work disorder (SWD), and the excessive sleepiness in obstructive sleep apnea. It affects dopamine and norepinephrine, but it activates more selectively than methylphenidate, optimizing function in the locus ceruleus and prefrontal cortex and having selective action on hypothalamic sleep-wake centers (Young & Geyer 2010). In a study, breast cancer survivors completed an open-label phase of modafinil for four weeks (phase 1) and a randomization phase that involved modafinil treatment or placebo (phase 2) (Kohli et al. 2009). The results revealed that 200 mg/day of modafinil during phase 1 significantly increased participants’ ability to store, retain, and retrieve verbal and nonverbal information. For participants receiving continued treatment with modafinil in phase 2, greater improvement in memory speed and quality as well as increased attention were found. Evidence for the efficacy of modafinil was also reported in a double-blind, randomized, crossover, single-dose trial in a sample of patients with multiple advanced cancers (Lundorff et al. 2009).
Other pharmacological treatments for CACD include memantine and estradiol. Memantine blocks N-methyl-D-aspartate (NMDA) receptors—a glutamate subfamily broadly involved in brain functioning. Memantine (20 mg/day) yielded modest effects as a pharmaceutical treatment for cognitive deficits in patients receiving whole brain radiotherapy, including survivors of breast, lung, or colon cancer with brain metastases randomized to memantine or placebo (Brown et al. 2013). Memantine-treated patients exhibited a significantly longer time until cognitive decline and had better delayed memory at 24 weeks; however, this difference was not significant. Estradiol treatment in prostate cancer patients receiving either neoadjuvant chemotherapy or luteinizing hormone-releasing hormone agonist has shown mixed results (Taxel et al. 2004).
METHODOLOGICAL ISSUES
Although significant advances have been made in understanding CACD, there are methodological issues that may explain inconsistencies in the pattern of results across studies in terms of which domains are affected by treatment and whether there is evidence of posttreatment cognitive decline. Some scholars have offered methodological and study design differences as potential explanations for these discrepancies (Wefel et al. 2011). However, others have raised concerns about the adequacy of traditional neuropsychological measures to detect subtle changes in cognitive function and the capacity of traditional statistical methods to elucidate associations between treatment exposures and cognitive change. These concerns have led to interest in using measurement approaches based on cognitive neuroscience and to the development of new statistical methods, which are necessary for future progress in the field (Andreotti et al. 2016, Li et al. 2016b).
Limitations of Traditional Neuropsychological Testing
The standardized neuropsychological measures commonly used were developed originally to determine lesion location and impairment in patients with overt neurological injuries and illnesses, such as traumatic brain injury or dementing conditions with moderate to severe dysfunction. The cognitive impact of treatment in survivors, in contrast, is relatively subtle, and measurement error in traditional objective measures alone could obscure true changes. We recently examined to what extent poor sensitivity might be due to measurement error and low test-retest reliability in two control samples collected as part of research projects in two different labs (in the United States and the Netherlands) over six-month and one-year intervals (Andreotti et al. 2016). The results indicated attenuated test-retest reliability at longer intervals compared to the reliability values reported by test developers at shorter intervals (i.e., 1–3 weeks). Reliability values generally fell below r = 0.8, with a subset of measures exhibiting reliability values as low as r = 0.23–0.35. The range of random variation in our healthy control samples between time 1 and time 2, during which no change should be evident, represents medium to large effect sizes, in contrast to much smaller expected changes in survivors. As a result, the inherent noise of measurement error, here represented by low test-retest reliability, obscures the signal of true treatment-related change.
Memory Versus Attention
A major source of confusion relates to the fact that most cancer survivors describe memory deficits but tend to score in the normal range on neuropsychological tests of memory. Findings from our lab have investigated specific learning and memory processes that might contribute to greater reports of memory dysfunction. The subjective experience of forgetting can be due to failures to retain information, to retrieve information, or to acquire information at the time of learning. In our clinical experience, we have routinely identified primary registration and encoding difficulties with some consistency. This may suggest that patient-reported memory complaints are driven by initial learning difficulties that are misidentified as actual forgetting by patients in daily activities. We confirmed this in two separate analyses of clinically referred survivors (Root et al. 2015) and of a research data set of survivors (Root et al. 2016) in which serial list learning measures were administered. Serial learning measures include multiple trials for the acquisition of information, and they allow for the decomposition of single trial learning, multiple trial learning, and retention and recall of information, so that specific areas of weakness can be identified. In both studies, survivors exhibited lower initial learning of information (Trial 1), compensation through repetition (Trial 5), and normal recall of this information following a delay (Long-Delay Free Recall). True-forgetting rates in each study were equivalent to normative and healthy control performance. Significantly, in the second analysis (Root et al. 2016), both lower- and higher-performing breast cancer survivors (stratified by performance on nonmemory measures) exhibited the same pattern of weaker initial learning, with the lower-performing group exhibiting decreased learning even after multiple trials, but both groups showed intact retention and recall of successfully learned information following a delay.
These findings suggest that initial attention, registration, and encoding of information may be altered in survivors, and indicate the need for a greater emphasis on attentional processes and subprocesses. Consistent with other initial research on these topics, we have found increased variability of attention across longer go/no-go tasks, and increasing variability in the latter portions of the task (E. Ryan, T.A. Ahles & J.C. Root, manuscript in preparation). This suggests that survivors tended to lose focus throughout the task, particularly in relatively unstimulating conditions as well as in later phases of the task. Similar findings have been reported by other groups in patients both before (Yao et al. 2016) and following treatment (Bernstein et al. 2014). Finally, one study has focused on identifying inefficiencies in attention networks using the Attention Network Test (ANT). Chen et al. (2014) administered the ANT and traditional neuropsychological measures to breast cancer survivors treated with chemotherapy, surgery only, and healthy controls; they found differences in the alerting and executive networks, but not the orienting network, in chemotherapy-treated individuals compared to either surgery only or healthy controls.
At this point, the precise mechanism(s) for learning difficulties have not been defined; however, we and others have proposed that changes in attentional processes both preattentive (see discussion of EEG studies above) and volitional (orienting, shifting, disengaging, and inhibiting attention) interfere with efficient and effective encoding of information in memory. Therefore, survivors’ perception of memory problems is accurate, but it is related to deficits in earlier stages of information processing related to attention rather than to memory per se.
Leveraging Cognitive Neuroscience
The limitations of traditional neuropsychological measures and the potential importance of attentional processes, including preattentive processes underlying cognitive decline, suggest the need for a different approach to the assessment of cognitive function in cancer survivors. Similar issues have arisen in other clinical areas, leading to the development of cognitive-experimental measures to better assess cognition associated with clinical syndromes (NIH EXAMINER; see Kramer et al. 2014). The National Cancer Institute has also encouraged researchers to leverage cognitive neuroscience measures to improve assessment of cancer and cancer treatment–related cognitive impairment (https://grants.nih.gov/grants/guide/pa-files/PAR-16-212.html).
Treatment Implications
The considerations made so far also have treatment implications. As described above, most cognitive rehabilitation approaches have focused on strategies to enhance memory and compensation. However, researchers from other areas have focused on experimental methods designed to enhance the ability to focus on relevant information and filter out irrelevant information in order to improve memory processes. For example, perceptual training designed to improve signal-to-noise discrimination produced generalized improvement in working memory performance. Further, improvement in working memory was correlated with EEG recordings (N1 amplitude) demonstrating more efficient encoding of stimuli (Berry et al. 2010). Approaches like perceptual training have yet to be tested in the treatment of CACD.
Transcranial direct current stimulation (tDCS), used in other disorders, may also enhance the impact of approaches like perceptual training. tDCS delivers minimal electric current by means of electrodes placed on the scalp and exerts its effect by lowering the threshold at which action potentials are generated (Zhao et al. 2017). As such, combining tDCS with cognitive training may “open windows of neuroplasticity” (McEwen 2016, p. 56) in affected areas that are supportive of a given cognitive task, e.g., attentional function. We have recently begun a clinical trial to test the feasibility and efficacy of this combined treatment in breast cancer survivors (https://clinicaltrials.gov/show/NCT02726763).
STATISTICAL ISSUES
Self-Report Versus Objective Cognitive Measures
Similar to research in other areas, studies of the association of self-reported cognitive dysfunction in cancer survivors with objectively tested performance on traditional neuropsychological measures suggest a weak-to-nonexistent relationship. Although we cannot assume that individuals can make perfectly accurate assessments of their own abilities, one potential contributor to subjective/objective disagreement may be the limitations of conventional statistical methods that rely on aggregating item responses into total scores or subdomains and on submitting these to traditional correlation-based analyses. We have identified significant associations between self-reported dysfunction and traditional neuropsychological measures using latent regression Rasch modeling (Li et al. 2016b). Advantages of the latent Rasch approach include (a) direct modeling of individual, item-level, and cognitive symptom ratings, whereas the conventional approach aggregates over symptom ratings to form subscale or global scores, obscuring specific patterns of symptoms; and (a) weighting the endorsement of rare symptoms more highly than commonly reported symptoms, whereas the conventional approach normally weights all symptoms identically. Using the Rasch approach, we found that changes in objective performance from pretreatment to posttreatment predicted self-report of cognitive problems, whereas traditional correlations were low or non-significant (Li et al. 2016b). Consistent with the proposed role of attention, self-reported memory problems correlated with performance on measures of attention and processing speed rather than measures of memory itself.
Subgroups of Impairment and Trajectories of Change
Another problem in the field is that patients tend to be categorized as impaired or not impaired, with no consideration of the potential for different patterns of impairment at pretreatment and various trajectories of change over time. Using Bayesian latent class analysis, we identified three different patterns of pretreatment performance, which included normal performance across measures, impairment in processing speed, and impairment in memory but not processing speed (Li et al. 2016a). Further, there was a significant interaction between treatment and subgroup, and the patients in the impaired processing speed group who were exposed to chemotherapy demonstrated the worst posttreatment outcomes compared to healthy controls (Li et al. 2016a).
CONCEPTUAL ISSUES AND NEW DIRECTIONS
The Interface of Cancer, Cancer Treatments, and Aging/Cognitive Aging
Recently there has been increasing interest in the intersection of chronic diseases (e.g., HIV, diabetes, and cancer) and the biology of aging (Hodes et al. 2016). Cancer, cancer treatments, and aging are linked through a variety of biological changes, including increased cell senescence, DNA damage, oxidative stress, inflammation, and decreased telomere length (telomerase activity) (Ahles & Saykin 2007, Campisi et al. 2011). Cancer and aging are linked, although the molecular mechanisms responsible for the increasing risk of cancer with increasing age are not completely understood. Systemic cancer treatments, particularly chemotherapy, have been shown to affect each of these systems in both tumor and healthy cells, leading to the hypothesis that cancer treatments may accelerate the aging process (Ahles et al. 2012). Chemotherapy has been associated with increased DNA damage, oxidative stress, inflammation, and shortened telomeres (Ahles & Saykin 2007). Further, research has suggested that the targets for certain cancer treatments have a reciprocal impact on biological markers of aging—e.g., increases in tumor suppressor mechanisms through the P53 pathway are associated with increased cell senescence systemically (Campisi et al. 2011). Tamoxifen has also been shown to be genotoxic, and other endocrine therapies may be associated with increased DNA damage because of the role of estrogen in antioxidant pathways (Wozniak et al. 2007). Finally, as described by Ahles & Saykin (2007), all of the above processes have been implicated in cognitive decline and the development of neurodegenerative diseases.
Recent studies (Sanoff et al. 2014) have demonstrated that breast cancer chemotherapy (anthracycline-based regimens) affects biomarkers of aging (p16INK4a and ARF) in a way that investigators suggest equated to 10.4 years of chronological aging. Animal studies have also demonstrated that the administration of cyclophosphamide and doxorubicin to rats increases the activation of markers of aging and stress (Erk1/2 and AKT) (Salas-Ramirez et al. 2015). Consequently, researchers have speculated that cancer treatments may affect specific brain regions (see the discussion of imaging and animal model studies above) and the biology of aging, including cognitive aging (Ahles et al. 2012, Mandelblatt et al. 2014a). Therefore, as our population ages, a critical research question is whether the diagnosis of cancer and exposure to cancer treatments has an initial posttreatment effect on certain domains of cognitive function and regions of the brain and whether the following age-associated cognitive decline parallels that of older adults with no cancer history (phase shift hypothesis) or follows a steeper slope (accelerated aging hypothesis). These hypotheses are not mutually exclusive, in that one subgroup of cancer survivors may follow the phase shift pattern, whereas another subgroup with multiple risk factors may follow the accelerated aging pattern. Figure 2 illustrates that even if a certain cancer treatment has the same impact on brain resources across patients, the impact on performance on cognitive tests can vary depending on age and risk factors like cognitive reserve, so that older individuals with high cognitive reserve will demonstrate fewer performance deficits compared to similarly aged patients with low cognitive reserve. The change in brain resources in younger patients translates into minimal change in cognitive performance compared to older patients.
Figure 2.
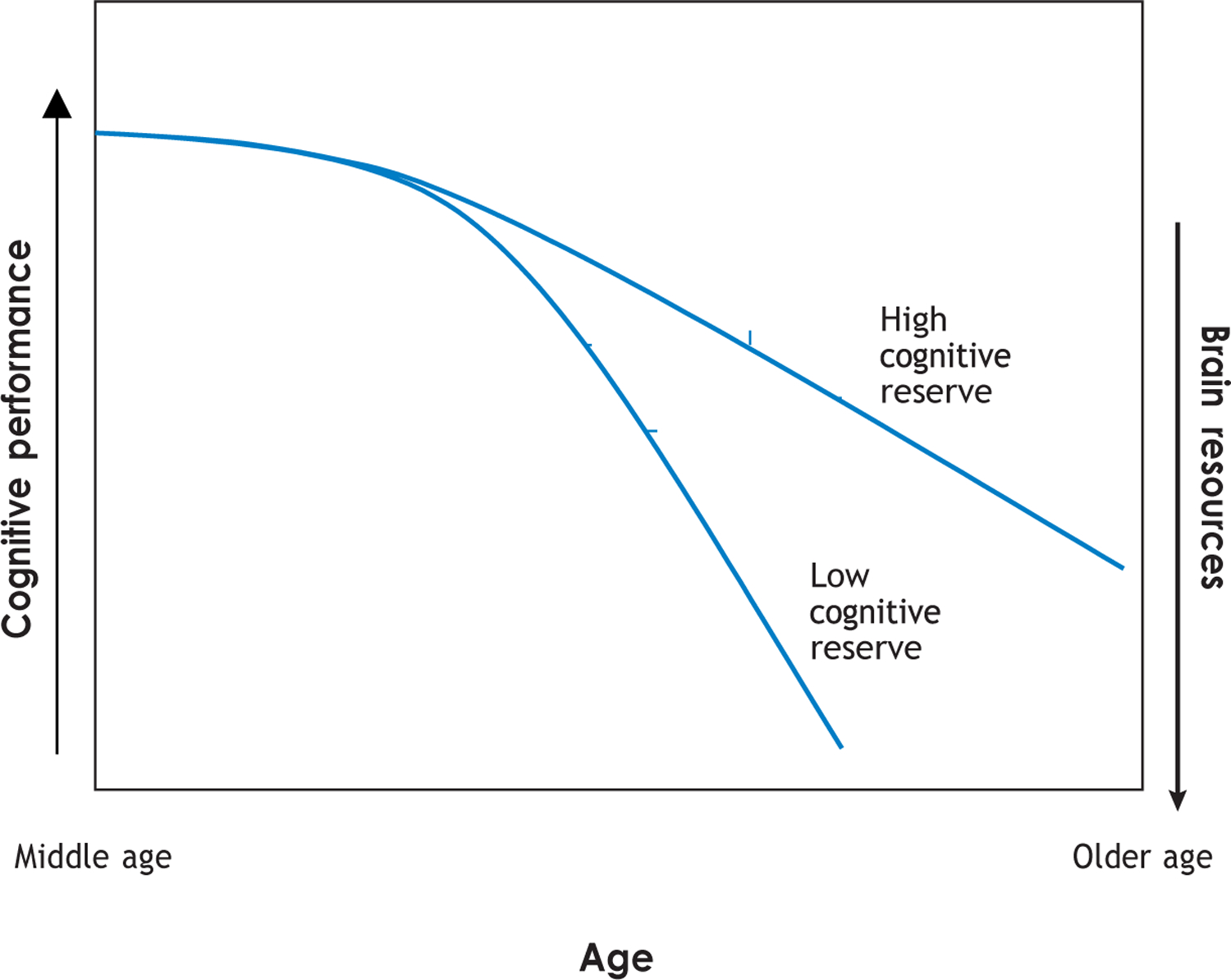
A cancer treatment can cause an identical change in brain resources; however, the impact on cognitive performance will be lower at a younger age and will increase as the individual moves along the continuum of age-related cognitive changes. Further, the impact on cognitive performance will be lower in an individual with high cognitive reserve and higher in an individual with low cognitive reserve.
Intersection of Theories of Aging, Allostatic Load, and Frailty
Given the complex set of factors that are involved in the development of CACD and aging, it is useful to look to theories of aging of complex systems in relationship to the concepts of allostatic load and frailty. The reliability theory of aging (Gavrilov & Gavrilova 2004), developed from systems engineering to explain the failure rates of complex machines, is an example of a model of aging that is consistent with a systems biology perspective. Reliability theory proposes that complex biological systems have developed a high level of redundancy to support survival. In a highly redundant system, failure of system components may not be problematic if other components are available to support a specific function. Due to this redundancy, however, these systems are tolerant of damage accumulation as subcomponents fail, which can lead to increasing energy consumption to maintain a less robust system (Mao et al. 2010). Therefore, aging is determined by the failure rate of systems (loss of redundancy with subsequent damage accumulation), which in turn is influenced by the initial extent of system redundancy, the systems repair potential, and factors that increase failure rate, such as poor health care, lifestyle risk factors, stress/trauma, poverty, and/or exposure to environmental toxins. Someone with a low failure rate and/or high repair potential will show fewer signs of biological aging as they age chronologically, whereas someone with a high failure rate and/or low repair potential will age more rapidly—as evidenced by disease onset due to either a specific set of failures in a given system or isolated failures across multiple systems (see below). One implication of reliability theory is that there are multiple potential combinations of systems failures that contribute to aging rather than specific biological aging pathways.
The imperfectness model of aging is a related theory that posits that aging is related to damage accumulation, which is inevitable due to the imperfectness of biological systems (Gladyshev 2013). Even if biological systems (e.g., enzymatic reactions) are 99% efficient, there is a 1% error rate that causes damage to the system. Evolution has created processes for correcting major sources of damage (e.g., multiple DNA repair mechanisms); however, minor damage types remain because repair mechanisms do not exist and/or because it is impossible to deal with all sources of damage.
Allostatic load refers to cumulative physiological dysregulation related to a lifetime of adapting to exposure to physiological and psychological demands (McEwen 2015, 2016). Accumulation of allostatic load occurs when adaptive responses to challenges chronically fall outside the normal operating range, resulting in wear and tear on the multiple components of the regulatory system. Consistent with reliability theory, these physiological and psychological challenges can increase failure accumulation across biological systems, resulting in decreased resiliency. Measurement of allostatic load is operationalized as the assessment of biological parameters related to risk of disease across several biological systems, including HPA axis, sympathetic nervous system, immune system, cardiovascular system, and metabolic processes. To date, numerous studies have demonstrated that high levels of allostatic load are associated with risk for mortality, development of specific diseases and frailty, and cognitive decline associated with aging (Karlamangla et al. 2014). The concept of frailty, developed by geriatricians, is consistent with the suggestions of reliability theory and the notion of allostatic load. A broad definition of frailty is a diminished biologic reserve and reduced resistance to stressors that are caused by cumulative declines across physiologic systems, leading to vulnerability and adverse outcomes (Fried et al. 2001). Individuals who are pre-frail or frail are at known risk of subsequent functional decline, hospitalization, falls, in stitutionalization, and mortality, and recent evidence suggests that frailty may be a better marker of aging than comorbidities (Fried et al. 2001). A relationship between frailty and cognitive im pairment has been reported in the geriatric literature, with frail individuals having an increased prevalence of cognitive impairment (Avila-Funes et al. 2009) and an increased risk of longitudinal decline in cognitive function. Pre-frail and frail phenotypes typically emerge gradually with aging; however, the biological underpinning (damage accumulation/increased allostatic load) likely develops over years and is measurable prior to the identification of the clinical syndrome.
Consistent with the integration of these three lines of thought, researchers utilizing the reliability theory of aging have demonstrated that the rate of multisystem deficit accumulation (allostatic load) correlates with the slope of age-associated frailty (Rockwood et al. 2010). One implication of these constructs is that vulnerability to posttreatment cognitive change does not necessarily depend upon a given treatment’s affecting a specific biological pathway. Rather, different patterns of failure rate (redundancy loss) across various biological systems may confer more or less vulnerability to specific treatments to each individual. Therefore, one patient may be vulnerable to the DNA damaging effects of a chemotherapy regimen, whereas another patient may be vulnerable to the impact on the hormonal milieu of endocrine treatments. This vulnerability may be strongly influ enced by the pattern of systems failure prior to cancer diagnosis related to smoking, diet, exercise, toxic environmental exposures, stress/trauma, poor neonatal care, inadequate health care, etc.
Inverse Association with Cancer and Neurodegenerative Diseases
As a counterpoint to the discussion on the interaction of cancer treatments and aging, several population studies have suggested an inverse relationship between cancer and various neurodegenerative diseases, including Alzheimer’s disease. Although there are limitations to these studies, there is increasing speculation of plausible biological mechanisms that may explain the inverse relationship, including biological processes that increase the tendency toward cellular proliferation versus aggregation (see Snyder et al. 2017 for review). On the other hand, as discussed above, there is increasing evidence that cancer treatments accelerate the aging process on a biological level. Therefore, there may be an overall inverse relationship between cancer and Alzheimer’s disease; however, there may be individuals with certain vulnerability factors for Alzheimer’s disease (APOE4) whose risk is increased if exposed to certain types of cancer treatments. Additional research is clearly necessary to sort out these complicated relationships. However, from a clinical point of view, answers to these questions are important, given that cancer survivors with a family history of dementia frequently ask whether exposure to chemotherapy will increase their risk for dementia.
Even if future research verifies the inverse association between cancer and neurodegenerative disease, having one disorder does not completely protect from the other. As our population ages, increasing numbers of cancer patients present with significant cognitive problems at diagnosis that may or may not be related to their cancer. Given the increasing complexity of cancer treatments and the need for high patient compliance, the presence of cognitive difficulties can present challenges in treatment planning and care for older adults with cancer. Geriatric oncology is an emerging field that is helping to define appropriate care for older cancer patients with multiple comorbidities/frailty through the development of geriatric assessment tools (Magnuson et al. 2016). However, additional research examining the impact of cognitive dysfunction on treatment decision making and the supportive services (e.g., family, visiting nurses, etc.) needed to ensure patient safety is clearly necessary.
Tipping Point
Although an expansion of our conceptual models may be needed to portray the multiple factors that can lead to the experience of cognitive decline in cancer survivors, this necessity makes research in the area much more complicated. If posttreatment cognitive deficits are determined by a complex interaction of specific impacts of cancer treatments on brain structure and function, innate (e.g., genetic) and accumulated risk factors, and aging, the determination of the specific mechanisms of CACD becomes a significant challenge. However, research related to the concept of tipping points in complex systems may be relevant (Scheffer et al. 2009, van de Leemput et al. 2014).
Many complex systems, ranging from climate change to financial markets to social networks, have tipping points that mark an abrupt change from one state to another. Prediction of these transitions is difficult because of the complexity of the system and because the system may show little evidence of change prior to the transition. However, research has identified early warning signs for critical transitions that relate to the phenomenon known in dynamic systems theory as critical slowing down (Scheffer et al. 2009, van de Leemput et al. 2014). Characteristics of critical slowing down include (a) overall slowing of the system and either (b) increased autocorrelation (i.e., the rate of change decreases because of slowing of the system, and therefore the state of the system at any given time is more similar to past states) or (c) increased variability. Examination of cognitive performance seen in cancer patients has demonstrated (a) slowing of processing speed (Ahles et al. 2010), (b) decreased ability to benefit from practice (performance from time 1 to time 2 remains similar, which may be a sign of higher autocorrelation) (Tager et al. 2010), and (a) increased intra-individual variability on reaction time tasks both before (Yao et al. 2016) and following treatment (Bernstein et al. 2014). At least three questions for future research emerge from this conceptualization. First, does critical slowing prior to treatment—represented by slowed processing speed, inability to benefit from practice, and increased variability in reaction time—predict vulnerability to posttreatment cognitive decline? Second, neuropsychology researchers commonly dichotomize survivors into impaired or not impaired. However, another hypothesis is that all patients are affected at some level (see Figure 2); is it the case that only a subgroup reaches a tipping point where the cognitive system shifts to a new state that is no longer sufficient to maintain pre-diagnosis task performance, and where cognitive deficits are measurable? Finally, are biological markers of systems disruption (e.g., allostatic load) and/or symptoms of frailty associated with critical slowing and movement toward the tipping point?
SUMMARY
As the cancer survivor population continues to increase and our population continues to age, the importance of understanding the clinical significance of the impact of cancer and cancer treatments on cognitive function will continue to grow. Research has evolved from asking whether cognitive dysfunction occurs in cancer survivors, to investigating what specific treatments might be implicated, to examining predisposing risk factors and interactions with biological and genetic variables. Over the years, the field has progressed from viewing this problem from a pharmacotoxicology perspective to understanding CACD from a multidimensional perspective that includes the interaction of risk factors, cancer treatments, cancer biology, and aging. From a measurement perspective, the field has begun to move toward the inclusion of measures developed by cognitive neuroscientists that assess subcomponents of attention, including preattentive processes. The identification of risk factors and mechanisms as well as refinements in assessment techniques will be critical to the development of targeted approaches to the treatment and, hopefully, the prevention of CACD.
SUMMARY POINTS.
The field has evolved from viewing CACD from a pharmacotoxicology (i.e., chemobrain) perspective to a multidimensional model that examines the contribution of multiple cancer treatments, the biology of cancer, and factors that confer risk for posttreatment cognitive decline.
Evidence from self-report, neuropsychological, and imaging studies has demonstrated persistent cognitive change in a vulnerable subgroup of cancer survivors up to 20 years after treatment.
Changes in attentional processes may decrease efficient registration and storage of infor mation and explain the common pattern of self-reported memory deficits in the context of normal performance on neuropsychological tests of memory.
The impact of cancer and cancer treatments on the biology of aging is an emerging area of research; therefore, cancer treatments may have specific effects on brain structure and function that are superimposed on accelerating aging, including cognitive aging.
FUTURE ISSUES.
Concerns about the adequacy of traditional neuropsychological tests to assess relatively subtle changes in cognitive function have led to the recommendation to leverage cognitive neuroscience theory and measures designed to evaluate cognitive subprocesses; however, the optimal assessment battery has not yet been defined.
Despite growing evidence from imaging, genetic, biomarker, and animal studies, the mechanism(s) for CACD have not been defined.
Although intervention research is growing, optimal treatments for CACD have not emerged.
The apparent inverse relationship between cancer and neurodegenerative disorders requires additional research.
Understanding whether the concepts of tipping point and critical slowing down are useful in the context of understanding CACD remains to be determined.
ACKNOWLEDGMENTS
Preparation of the manuscript was supported by grants from the National Cancer Institute (R01 CA172119, R01 CA129769, U54CA137788) and a Cancer Center Core grant (P30 CA008748).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Ahles TA, Li Y, Mcdonald BC, Schwartz GN, Kaufman PA, et al. 2014. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: the impact of APOE and smoking. Psycho-Oncology 23:1382–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Root JC, Ryan EL. 2012. Cancer- and cancer treatment–associated cognitive change: an update on the state of the science. J. Clin. Oncol 30:3675–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. 2007. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat. Rev. Cancer 7:192–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, et al. 2002. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J. Clin. Oncol 20:485–93 [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Mcdonald BC, Furstenberg CT, Cole BF, et al. 2008. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res. Treat 110:143–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Mcdonald BC, Li Y, Furstenberg CT, et al. 2010. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J. Clin. Oncol 28:4434–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, et al. 2003. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psycho-Oncology 12:612–19 [DOI] [PubMed] [Google Scholar]
- Am. Cancer Soc. 2017. Cancer Facts and Figures 2017. Atlanta, GA: Am. Cancer Soc. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html [Google Scholar]
- Amidi A, Agerbaek M, Wu LM, Pedersen AD, Mehlsen M, et al. 2017. Changes in cognitive functions and cerebral grey matter and their associations with inflammatory markers, endocrine markers, and APOE genotypes in testicular cancer patients undergoing treatment. Brain Imaging Behav. 11:769–83 [DOI] [PubMed] [Google Scholar]
- Amidi A, Wu LM, Agerbaek M, Larsen PL, Pedersen AD, et al. 2015. Cognitive impairment and potential biological and psychological correlates of neuropsychological performance in recently orchiectomized testicular cancer patients. Psycho-Oncology 24:1174–80 [DOI] [PubMed] [Google Scholar]
- Andreotti C, Root J, Ahles T, Mcewen B, Compas B. 2015. Cancer, coping, and cognition: a model for the role of stress reactivity in cancer-related cognitive decline. Psycho-Oncology 24(6):617–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreotti C, Root JC, Schagen SB, Mcdonald BC, Saykin AJ, et al. 2016. Reliable change in neuropsychological assessment of breast cancer survivors. Psycho-Oncology 25(1):43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apple AC, Ryals AJ, Alpert KI, Wagner LI, Shih PA, et al. 2017. Subtle hippocampal deformities in breast cancer survivors with reduced episodic memory and self-reported cognitive concerns. Neuroimage Clin. 14:685–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt J, Das E, Schagen SB, Reid-Arndt SA, Cameron LD, Ahles TA. 2014. Broadening the cancer and cognition landscape: the role of self-regulatory challenges. Psycho-Oncology 23:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Funes JA, Amieva H, Barberger-Gateau P, Le Goff M, Raoux N, et al. 2009. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J. Am. Geriatr. Soc 57:453–61 [DOI] [PubMed] [Google Scholar]
- Barulli D, Stern Y. 2013. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn. Sci 17:502–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos P, Gomes T, Ribeiro L. 2017. Catechol-O-methyltransferase (COMT): an update on its role in cancer, neurological and cardiovascular diseases. Rev. Physiol. Biochem. Pharmacol 173:1–39 [DOI] [PubMed] [Google Scholar]
- Bender CM, Sereika SM, Berga SL, Vogel VG, Brufsky AM, et al. 2006. Cognitive impairment associated with adjuvant therapy in breast cancer. Psycho-Oncology 15:422–30 [DOI] [PubMed] [Google Scholar]
- Bernstein LJ, Catton PA, Tannock IF. 2014. Intra-individual variability in women with breast cancer. J. Int. Neuropsychol. Soc 20:380–90 [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM. 2011. Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder. Biol. Psychiatry 69:e101–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Clapp WC, Hardy JL, Delahunt PB, et al. 2010. The influence of perceptual training on working memory in older adults. PLOS ONE 5:e11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biglia N, Bounous VE, Malabaila A, Palmisano D, Torta DM, et al. 2012. Objective and self-reported cognitive dysfunction in breast cancer women treated with chemotherapy: a prospective study. Eur. J. Cancer Care 21:485–92 [DOI] [PubMed] [Google Scholar]
- Borenstein AR, Mortimer JA, Wu Y, Jureidini-Webb FM, Fallin MD, et al. 2006. Apolipoprotein E and cognition in community-based samples of African Americans and Caucasians. Ethn. Dis 16:9–15 [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N. 2005. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom. Med 67:277–80 [DOI] [PubMed] [Google Scholar]
- Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, et al. 2013. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro-Oncology 15:1429–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Andersen JK, Kapahi P, Melov S. 2011. Cellular senescence: a link between cancer and age-related degenerative disease? Semin. Cancer Biol 21:354–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. 2004. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J. Clin. Exp. Neuropsychol 26:955–69 [DOI] [PubMed] [Google Scholar]
- Chae JW, Ng T, Yeo HL, Shwe M, Gan YX, et al. 2016. Impact of TNF-α (rs1800629) and IL-6 (rs1800795) polymorphisms on cognitive impairment in Asian breast cancer patients. PLOS ONE 11:e0164204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattarji S, Tomar A, Suvrathan A, Ghosh S, Rahman MM. 2015. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat. Neurosci 18:1364–75 [DOI] [PubMed] [Google Scholar]
- Chen X, Li J, Ren J, Hu X, Zhu C, et al. 2014. Selective impairment of attention networks in breast cancer patients receiving chemotherapy treatment. Psycho-Oncology 23:1165–71 [DOI] [PubMed] [Google Scholar]
- Cheung YT, Krull KR. 2015. Neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: a systematic review. Neurosci. Biobehav. Rev 53:108–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PD, Finkelstein Y, Stevenson KE, Blonquist TM, Vijayanathan V, et al. 2015. Polymorphisms in genes related to oxidative stress are associated with inferior cognitive function after therapy for childhood acute lymphoblastic leukemia. J. Clin. Oncol 33:2205–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. 2009. Cognitive effects of chemotherapy in postmenopausal breast cancer patients 1 year after treatment. Psycho-Oncology 18:134–43 [DOI] [PubMed] [Google Scholar]
- Conroy SK, Mcdonald BC, Smith DJ, Moser LR, West JD, et al. 2013. Alterations in brain structure and function in breast cancer survivors: effect of post-chemotherapy interval and relation to oxidative DNA damage. Breast Cancer Res. Treat 137:493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DD. 2010. Neurocognitive function in brain tumors. Curr. Neurol. Neurosci. Rep 10:232–39 [DOI] [PubMed] [Google Scholar]
- Correa DD, Root JC, Baser R, Moore D, Peck KK, et al. 2013. A prospective evaluation of changes in brain structure and cognitive functions in adult stem cell transplant recipients. Brain Imaging Behav. 7(4):478–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DD, Satagopan J, Baser RE, Cheung K, Richards E, et al. 2014. APOE polymorphisms and cognitive functions in patients with brain tumors. Neurology 83:320–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DD, Wang Y, West JD, Peck KK, Root JC, et al. 2016. Prospective assessment of white matter integrity in adult stem cell transplant recipients. Brain Imaging Behav. 10(2):486–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull A, Hay C, Love SB, Mackie M, Smets E, Stewart M. 1996. What do cancer patients mean when they complain of concentration and memory problems? Br. J. Cancer 74:1674–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull A, Stewart M, Altman DG. 1995. Assessment of and intervention for psychosocial problems in routine oncology practice. Br. J. Cancer 72:229–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FS, et al. 2011. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum. Brain Mapp 32:1206–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debess J, Riis JO, Engebjerg MC, Ewertz M. 2010. Cognitive function after adjuvant treatment for early breast cancer: a population-based longitudinal study. Breast Cancer Res. Treat 121:91–100 [DOI] [PubMed] [Google Scholar]
- Denlinger CS, Ligibel JA, Are M, Baker KS, Demark-Wahnefried W, et al. 2014. Survivorship: cognitive function, version 1. J. Natl. Compr. Cancer Netw 12:976–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deprez S, Amant F, Yigit R, Porke K, Verhoeven J, et al. 2011. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum. Brain Mapp 32(3):480–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercoli LM, Petersen L, Hunter AM, Castellon SA, Kwan L, et al. 2015. Cognitive rehabilitation group intervention for breast cancer survivors: results of a randomized clinical trial. Psycho-Oncology 24:1360–67 [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, et al. 2010. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J. Neurosci 30:5368–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante CP, Meyers C, Reuben JM, Wang X, Qiao W, et al. 2014. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J. 20:8–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HG, Houede-Tchen N, Yi QL, Chemerynsky I, Downie FP, et al. 2005. Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J. Clin. Oncol 23:8025–32 [DOI] [PubMed] [Google Scholar]
- Ferguson RJ, Mcdonald BC, Rocque MA, Furstenberg CT, Horrigan S, et al. 2012. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psycho-Oncology 21:176–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson RJ, Mcdonald BC, Saykin AJ, Ahles TA. 2007. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J. Clin. Oncol 25:3866–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, et al. 2001. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci 56:M146–56 [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Ehrlichman RS, Rudnick ND, Siegel SJ. 2008. A novel electrophysiological model of chemotherapy-induced cognitive impairments in mice. Neuroscience 157:95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DH, et al. 2013. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain Behav. Immun 30(Suppl.):S99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilov LA, Gavrilova NS. 2004. The reliability-engineering approach to the problem of biological aging. Ann. N. Y. Acad. Sci 1019:509–12 [DOI] [PubMed] [Google Scholar]
- Gladyshev VN. 2013. The origin of aging: imperfectness-driven non-random damage defines the aging process and control of lifespan. Trends Genet. 29:506–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, Gallezot JD, Planeta-Wilson B, Lin SF, Williams WA, et al. 2010. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol. Psychiatry 68:854–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedayati E, Alinaghizadeh H, Schedin A, Nyman H, Albertsson M. 2012. Effects of adjuvant treatment on cognitive function in women with early breast cancer. Eur. J. Oncol. Nurs 16:315–22 [DOI] [PubMed] [Google Scholar]
- Hedayati E, Schedin A, Nyman H, Alinaghizadeh H, Albertsson M. 2011. The effects of breast cancer diagnosis and surgery on cognitive functions. Acta Oncol. 50:1027–36 [DOI] [PubMed] [Google Scholar]
- Hermelink K, Henschel V, Untch M, Bauerfeind I, Lux MP, Munzel K. 2008. Short-term effects of treatment-induced hormonal changes on cognitive function in breast cancer patients: results of a multicenter, prospective, longitudinal study. Cancer 113:2431–39 [DOI] [PubMed] [Google Scholar]
- Hermelink K, Untch M, Lux MP, Kreienberg R, Beck T, et al. 2007. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer 109:1905–13 [DOI] [PubMed] [Google Scholar]
- Hermelink K, Voigt V, Kaste J, Neufeld F, Wuerstlein R, et al. 2015. Elucidating pretreatment cognitive impairment in breast cancer patients: the impact of cancer-related post-traumatic stress. J. Natl. Cancer Inst 107(7):djv099. [DOI] [PubMed] [Google Scholar]
- Hodes RJ, Sierra F, Austad SN, Epel E, Neigh GN, et al. 2016. Disease drivers of aging. Ann. N. Y. Acad. Sci 1386:45–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurria A, Rosen C, Hudis C, Zuckerman E, Panageas KS, et al. 2006. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study. J. Am. Geriatr. Soc 54:925–31 [DOI] [PubMed] [Google Scholar]
- Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, et al. 2007. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer 109:146–56 [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Heckler CE, Peppone LJ, Kamen C, Mustian KM, et al. 2017. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J. Clin. Oncol 35(5):506–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Heckler CE, Peppone LJ, Mohile SG, Mustian KM, et al. 2017. Longitudinal assessment of cancer-related cognitive impairment (CRCI) up to six-months post-chemotherapy with multiple cognitive testing methods in 943 breast cancer (BC) patients and controls. J. Clin. Oncol 35(15 Suppl.):10014 [Google Scholar]
- Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, et al. 2006. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br. J. Cancer 94:828–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MS, Cimprich B. 2014. Cognitive deficits in Korean women treated with chemotherapy for breast cancer. Cancer Nurs. 37:E31–42 [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Lachman ME, Tun PA, Koretz BK, Seeman TE. 2014. Biological correlates of adult cognition: Midlife in the United States (MIDUS). Neurobiol. Aging 35:387–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S, Hadi Hosseini SM, Heckler C, Janelsins M, Palesh O, et al. 2013a. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin. Breast Cancer 13:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, et al. 2013b. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav. Immun 30(Suppl.):S109–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Bennett FC, Mahaffey ML, Spiegel D. 2009. Regional brain activation during verbal declarative memory in metastatic breast cancer. Clin. Cancer Res 15:6665–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli S, Fisher SG, Tra Y, Adams MJ, Mapstone ME, et al. 2009. The effect of modafinil on cognitive function in breast cancer survivors. Cancer 115:2605–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleck TA, Bender CM, Sereika SM, Ahrendt G, Jankowitz RC, et al. 2014. Apolipoprotein E genotype and cognitive function in postmenopausal women with early-stage breast cancer. Oncol. Nurs. Forum 41:E313–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleck TA, Bender CM, Sereika SM, Ryan CM, Ghotkar P, et al. 2017. Associations between pathologic tumor features and preadjuvant therapy cognitive performance in women diagnosed with breast cancer. Cancer Med. 6:339–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. 2012. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J. Clin. Oncol 30:1080–86 [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Possin KL, Rankin KP, Boxer AL, et al. 2014. NIH EXAMINER: conceptualization and development of an executive function battery. J. Int. Neuropsychol. Soc 20:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreukels BP, Hamburger HL, de Ruiter MB, van Dam FS, Ridderinkhof KR, et al. 2008. ERP amplitude and latency in breast cancer survivors treated with adjuvant chemotherapy. Clin. Neurophysiol 119:533–41 [DOI] [PubMed] [Google Scholar]
- Kreukels BP, Schagen SB, Ridderinkhof KR, Boogerd W, Hamburger HL, et al. 2006. Effects of high-dose and conventional-dose adjuvant chemotherapy on long-term cognitive sequelae in patients with breast cancer: an electrophysiologic study. Clin. Breast Cancer 7:67–78 [DOI] [PubMed] [Google Scholar]
- Krynetskiy E, Krynetskaia N, Rihawi D, Wieczerzak K, Ciummo V, Walker E. 2013. Establishing a model for assessing DNA damage in murine brain cells as a molecular marker of chemotherapy-associated cognitive impairment. Life Sci. 93:605–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M, Heutte N, Rigal O, Noal S, Kurtz JE, et al. 2017. Decline in cognitive function in older adults with early-stage breast cancer after adjuvant treatment. Oncologist. In press. 10.1634/theoncologist.2016-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Strat Y 2012. Cognitive trajectories after postoperative delirium. N. Engl. J. Med 367(12):1164. Author reply. 2012. N. Engl. J. Med. 367(12):1164–65 [DOI] [PubMed] [Google Scholar]
- Lengacher CA, Reich RR, Kip KE, Paterson CL, Park HY, et al. 2015. Moderating effects of genetic polymorphisms on improvements in cognitive impairment in breast cancer survivors participating in a 6-week mindfulness-based stress reduction program. Biol. Res. Nurs 17:393–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Atkinson TM, Root JC, Ahles TA. 2016a. Latent classes of neurocognitive impairment before cancer treatment and how they affect post-treatment cognitive deficits. Presented at Int. Soc. Qual. Life Res. 23rd Annu. Conf., Oct. 19–22, Copenhagen, Den. [Google Scholar]
- Li Y, Root JC, Atkinson TM, Ahles TA. 2016b. Examining the association between patient-reported symptoms of attention and memory dysfunction with objective cognitive performance: a latent regression Rasch model approach. Arch. Clin. Neuropsychol 31:365–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lower E, Fleishman S, Cooper A, Zeldis J, Faleck H, Manning D. 2005. A phase III, randomized placebo-controlled trial of the safety and efficacy of d-MPH as new treatment of fatigue and “chemobrain” in adult cancer patients. J. Clin. Oncol 23(16 Suppl.):8000 [Google Scholar]
- Lundorff LE, Jonsson BH, Sjogren P. 2009. Modafinil for attentional and psychomotor dysfunction in advanced cancer: a double-blind, randomised, cross-over trial. Palliat. Med 23:731–38 [DOI] [PubMed] [Google Scholar]
- Magnuson A, Allore H, Cohen HJ, Mohile SG, Williams GR, et al. 2016. Geriatric assessment with management in cancer care: current evidence and potential mechanisms for future research. J. Geriatr. Oncol 7:242–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblatt JS, Jacobsen PB, Ahles T. 2014a. Cognitive effects of cancer systemic therapy: implications for the care of older patients and survivors. J. Clin. Oncol 32:2617–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelblatt JS, Stern RA, Luta G, Mcguckin M, Clapp JD, et al. 2014b. Cognitive impairment in older patients with breast cancer before systemic therapy: Is there an interaction between cancer and comorbidity? J. Clin. Oncol 32:1909–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Romer I, Nebrich G, Klein O, Koppelstatter A, et al. 2010. Aging in mouse brain is a cell/tissue-level phenomenon exacerbated by proteasome loss. J. Proteome Res 9:3551–60 [DOI] [PubMed] [Google Scholar]
- Mar Fan HG, Clemons M, Xu W, Chemerynsky I, Breunis H, et al. 2008. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer 16:577–83 [DOI] [PubMed] [Google Scholar]
- Mcdonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. 2010. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res. Treat 123(3):819–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. 2012. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J. Clin. Oncol 30:2500–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald BC, Saykin AJ. 2013. Alterations in brain structure related to breast cancer and its treatment: chemotherapy and other considerations. Brain Imaging Behav. 7:374–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcewen BS. 2015. Biomarkers for assessing population and individual health and disease related to stress and adaptation. Metabolism 64:S2–10 [DOI] [PubMed] [Google Scholar]
- Mcewen BS. 2016. In pursuit of resilience: stress, epigenetics, and brain plasticity. Ann. N. Y. Acad. Sci 1373:56–64 [DOI] [PubMed] [Google Scholar]
- Mehlsen M, Pedersen AD, Jensen AB, Zachariae R. 2009. No indications of cognitive side-effects in a prospective study of breast cancer patients receiving adjuvant chemotherapy. Psycho-Oncology 18:248–57 [DOI] [PubMed] [Google Scholar]
- Mehnert A, Scherwath A, Schirmer L, Schleimer B, Petersen C, et al. 2007. The association between neuropsychological impairment, self-perceived cognitive deficits, fatigue and health related quality of life in breast cancer survivors following standard adjuvant versus high-dose chemotherapy. Patient Educ. Couns 66:108–18 [DOI] [PubMed] [Google Scholar]
- Menning S, de Ruiter MB, Veltman DJ, Koppelmans V, Kirschbaum C, et al. 2015. Multimodal MRI and cognitive function in patients with breast cancer prior to adjuvant treatment—the role of fatigue. Neuroimage Clin. 7:547–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriman JD, Sereika SM, Brufsky AM, Mcauliffe PF, Mcguire KP, et al. 2017. Trajectories of self-reported cognitive function in postmenopausal women during adjuvant systemic therapy for breast cancer. Psycho-Oncology 26:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T, Teo SM, Yeo HL, Shwe M, Gan YX, et al. 2015. Brain-derived neurotrophic factor genetic polymorphism (rs6265) is protective against chemotherapy-associated cognitive impairment in patients with early-stage breast cancer. Neuro-Oncology 18(2):244–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman KN, Wang Y, Mcdonald BC, Conroy SK, Smith DJ, et al. 2014. Altered cerebral blood flow one month after systemic chemotherapy for breast cancer: a prospective study using pulsed arterial spin labeling MRI perfusion. PLOS ONE 9:e96713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KM, Pinilla-Ibarz J, Sotomayor E, Lee MR, Jim HSL, et al. 2013. Quality of life outcomes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a controlled comparison. Support Care Cancer 21:1097–103 [DOI] [PubMed] [Google Scholar]
- Pomykala KL, Ganz PA, Bower JE, Kwan L, Castellon SA, et al. 2013. The association between proinflammatory cytokines, regional cerebral metabolism, and cognitive complaints following adjuvant chemotherapy for breast cancer. Brain Imaging Behav. 7:511–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppelreuter M, Weis J, Bartsch HH. 2009. Effects of specific neuropsychological training programs for breast cancer patients after adjuvant chemotherapy. J. Psychosoc. Oncol 27:274–96 [DOI] [PubMed] [Google Scholar]
- Poppelreuter M, Weis J, Kulz AK, Tucha O, Lange KW, Bartsch HH. 2004. Cognitive dysfunction and subjective complaints of cancer patients: a cross-sectional study in a cancer rehabilitation centre. Eur. J. Cancer 40:43–49 [DOI] [PubMed] [Google Scholar]
- Quesnel C, Savard J, Ivers H. 2009. Cognitive impairments associated with breast cancer treatments: results from a longitudinal study. Breast Cancer Res. Treat 116:113–23 [DOI] [PubMed] [Google Scholar]
- Rockwood K, Rockwood MR, Mitnitski A. 2010. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J. Am. Geriatr. Soc 58:318–23 [DOI] [PubMed] [Google Scholar]
- Root JC, Andreotti C, Tsu L, Ellmore TM, Ahles TA. 2016. Learning and memory performance in breast cancer survivors 2 to 6 years post-treatment: the role of encoding versus forgetting. J. Cancer Surviv 10:593–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root JC, Ryan E, Barnett G, Andreotti C, Bolutayo K, Ahles T. 2015. Learning and memory performance in a cohort of clinically referred breast cancer survivors: the role of attention versus forgetting in patient-reported memory complaints. Psycho-Oncology 24(5):548–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Ramirez KY, Bagnall C, Frias L, Abdali SA, Ahles TA, Hubbard K. 2015. Doxorubicin and cyclophosphamide induce cognitive dysfunction and activate the ERK and AKT signaling pathways. Behav. Brain Res 292:133–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanoff HK, Deal AM, Krishnamurthy J, Torrice C, Dillon P, et al. 2014. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J. Natl. Cancer Inst 106:dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagen SB, Boogerd W, Muller MJ, Huinink WT, Moonen L, et al. 2008. Cognitive complaints and cognitive impairment following BEP chemotherapy in patients with testicular cancer. Acta Oncol. 47:63–70 [DOI] [PubMed] [Google Scholar]
- Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FS. 2006. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. J. Natl. Cancer Inst 98:1742–45 [DOI] [PubMed] [Google Scholar]
- Schagen SB, van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. 1999. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer 85:640–50 [DOI] [PubMed] [Google Scholar]
- Scheffer M, Bascompte J, Brock WA, Brovkin V, Carpenter SR, et al. 2009. Early-warning signals for critical transitions. Nature 461:53–59 [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, Harris SE, Gow AJ, Pattie A, Brett CE, et al. 2012. APOE E4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Mol. Psychiatry 17:315–24 [DOI] [PubMed] [Google Scholar]
- Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, et al. 2010a. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J. Clin. Oncol 28:1294–300 [DOI] [PubMed] [Google Scholar]
- Schilder CM, Seynaeve C, Linn SC, Boogerd W, Beex LV, et al. 2010b. Cognitive functioning of postmenopausal breast cancer patients before adjuvant systemic therapy, and its association with medical and psychological factors. Crit. Rev. Oncol. Hematol 76:133–41 [DOI] [PubMed] [Google Scholar]
- Schwartz AJ, Boduroglu A, Gutchess AH. 2014. Cross-cultural differences in categorical memory errors. Cogn. Sci 38:997–1007 [DOI] [PubMed] [Google Scholar]
- Schwartz AL, Thompson JA, Masood N. 2002. Interferon-induced fatigue in patients with melanoma: a pilot study of exercise and methylphenidate. Oncol. Nurs. Forum 29:E85–90 [DOI] [PubMed] [Google Scholar]
- Seigers R, Schagen SB, Beerling W, Boogerd W, van Tellingen O, et al. 2008. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav. Brain Res 186:168–75 [DOI] [PubMed] [Google Scholar]
- Seigers R, Schagen SB, van Tellingen O, Dietrich J. 2013. Chemotherapy-related cognitive dysfunction: current animal studies and future directions. Brain Imaging Behav. 7:453–59 [DOI] [PubMed] [Google Scholar]
- Selamat MH, Loh SY, Mackenzie L, Vardy J. 2014. Chemobrain experienced by breast cancer survivors: a meta-ethnography study investigating research and care implications. PLOS ONE 9:e108002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama O, Yoshiuchi K, Inagaki M, Matsuoka Y, Yoshikawa E, et al. 2014. Association between adjuvant regional radiotherapy and cognitive function in breast cancer patients treated with conservation therapy. Cancer Med. 3:702–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilling V, Jenkins V, Morris R, Deutsch G, Bloomfield D. 2005. The effects of adjuvant chemotherapy on cognition in women with breast cancer—preliminary results of an observational longitudinal study. Breast 14:142–50 [DOI] [PubMed] [Google Scholar]
- Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, et al. 2007. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res. Treat 103:303–11 [DOI] [PubMed] [Google Scholar]
- Small BJ, Rawson KS, Walsh E, Jim HS, Hughes TF, et al. 2011. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer 117:1369–76 [DOI] [PubMed] [Google Scholar]
- Snyder HM, Ahles T, Calderwood S, Carrillo MC, Chen H, et al. 2017. Exploring the nexus of Alzheimer’s disease and related dementias with cancer and cancer therapies: a convening of the Alzheimer’s Association & Alzheimer’s Drug Discovery Foundation. Alzheimer’s Dement. 13:267–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Collins B, Mackenzie J, Tomiak E, Verma S, Bielajew C. 2008. The cognitive effects of adjuvant chemotherapy in early stage breast cancer: a prospective study. Psycho-Oncology 17:122–30 [DOI] [PubMed] [Google Scholar]
- Tager FA, Mckinley PS, Schnabel FR, el-Tamer M, Cheung YK, et al. 2010. The cognitive effects of chemotherapy in post-menopausal breast cancer patients: a controlled longitudinal study. Breast Cancer Res. Treat 123:25–34 [DOI] [PubMed] [Google Scholar]
- Taxel P, Stevens MC, Trahiotis M, Zimmerman J, Kaplan RF. 2004. The effect of short-term estradiol therapy on cognitive function in older men receiving hormonal suppression therapy for prostate cancer. J. Am. Geriatr. Soc 52:269–73 [DOI] [PubMed] [Google Scholar]
- van Dam FS, Schagen SB, Muller MJ, Boogerd W, Vd Wall E, et al. 1998. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J. Natl. Cancer Inst 90:210–18 [DOI] [PubMed] [Google Scholar]
- van de Leemput IA, Wichers M, Cramer AO, Borsboom D, Tuerlinckx F, et al. 2014. Critical slowing down as early warning for the onset and termination of depression. PNAS 111:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier WM, Schoonenboom SN, Pijnenburg YA, Fox NC, Scheltens P. 2006. The effect of APOE genotype on clinical phenotype in Alzheimer disease. Neurology 67:526–27 [DOI] [PubMed] [Google Scholar]
- Vardy J, Dhillon HM, Pond GR, Rourke SB, Xu W, et al. 2014. Cognitive function and fatigue after diagnosis of colorectal cancer. Ann. Oncol 25:2404–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy JL, Dhillon HM, Pond GR, Rourke SB, Bekele T, et al. 2015. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: a prospective, longitudinal, controlled study. J. Clin. Oncol 33:4085–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vearncombe KJ, Rolfe M, Wright M, Pachana NA, Andrew B, Beadle G. 2009. Predictors of cognitive decline after chemotherapy in breast cancer patients. J. Int. Neuropsychol. Soc 15:951–62 [DOI] [PubMed] [Google Scholar]
- von Ah D, Carpenter JS, Saykin A, Monahan P, Wu J, et al. 2012. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Res. Treat 135:799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ah D, Habermann B, Carpenter JS, Schneider BL. 2013. Impact of perceived cognitive impairment in breast cancer survivors. Eur. J. Oncol. Nurs 17:236–41 [DOI] [PubMed] [Google Scholar]
- Wang L, Apple AC, Schroeder MP, Ryals AJ, Voss JL, et al. 2016. Reduced prefrontal activation during working and long-term memory tasks and impaired patient-reported cognition among cancer survivors postchemotherapy compared with healthy controls. Cancer 122:258–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A, Crean S, Mercaldi CJ, Collins JM, Boyd D, et al. 2012. Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer’s disease: a systematic review and meta-analysis. Neuroepidemiology 38:1–17 [DOI] [PubMed] [Google Scholar]
- Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. 2004. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer 100:2292–99 [DOI] [PubMed] [Google Scholar]
- Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. 2010. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer 116:3348–56 [DOI] [PubMed] [Google Scholar]
- Wefel JS, Vardy J, Ahles T, Schagen SB. 2011. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 12:703–8 [DOI] [PubMed] [Google Scholar]
- Winick N 2011. Neurocognitive outcome in survivors of pediatric cancer. Curr. Opin. Pediatr 23:27–33 [DOI] [PubMed] [Google Scholar]
- Winocur G, Henkelman M, Wojtowicz JM, Zhang H, Binns MA, Tannock IF. 2012. The effects of chemotherapy on cognitive function in a mouse model: a prospective study. Clin. Cancer Res 18:3112–21 [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Huang J, Tannock IF. 2014. Physical exercise prevents suppression of hippocampal neurogenesis and reduces cognitive impairment in chemotherapy-treated rats. Psychopharmacology (Berl.) 231:2311–20 [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Tannock IF. 2015. Memory loss in chemotherapy-treated rats is exacerbated in high-interference conditions and related to suppression of hippocampal neurogenesis. Behav. Brain Res 281:239–44 [DOI] [PubMed] [Google Scholar]
- Wouters H, Baars JW, Schagen SB. 2016. Neurocognitive function of lymphoma patients after treatment with chemotherapy. Acta Oncol. 55:1121–25 [DOI] [PubMed] [Google Scholar]
- Wozniak K, Kolacinska A, Blasinska-Morawiec M, Morawiec-Bajda A, Morawiec Z, et al. 2007. The DNA-damaging potential of tamoxifen in breast cancer and normal cells. Arch. Toxicol 81:519–27 [DOI] [PubMed] [Google Scholar]
- Yamada TH, Denburg NL, Beglinger LJ, Schultz SK. 2010. Neuropsychological outcomes of older breast cancer survivors: cognitive features ten or more years after chemotherapy. J. Neuropsychiatry Clin. Neurosci 22:48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Rich JB, Tannock IF, Seruga B, Tirona K, Bernstein LJ. 2016. Pretreatment differences in intrain-dividual variability in reaction time between women diagnosed with breast cancer and healthy controls. J. Int. Neuropsychol. Soc 22:530–39 [DOI] [PubMed] [Google Scholar]
- Young JW, Geyer MA. 2010. Action of modafinil-increased motivation via the dopamine transporter inhibition and D1 receptors? Biol. Psychiatry 67:784–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Manly JJ, Narkhede A, Griffith EY, Decarli C, et al. 2015. Structural MRI predictors of late-life cognition differ across African Americans, Hispanics, and Whites. Curr. Alzheimer Res 12:632–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Qiao L, Fan D, Zhang S, Turel O, et al. 2017. Modulation of brain activity with noninvasive transcranial direct current stimulation (tDCS): clinical applications and safety concerns. Front. Psychol 8:685. [DOI] [PMC free article] [PubMed] [Google Scholar]


