SUMMARY
The p53-induced long noncoding RNA (lncRNA) lincRNA-p21 is proposed to act in cis to promote p53-dependent expression of the neighboring cell cycle gene, Cdkn1a/p21. The molecular mechanism through which the transcribed lincRNA-p21 regulatory locus activates p21 expression remains poorly understood. To elucidate the functional elements of cis-regulation, we generate a series of genetic models that disrupt DNA regulatory elements, the transcription of lincRNA-p21, or the accumulation of mature lincRNA-p21. Unexpectedly, we determine that full-length transcription, splicing, and accumulation of lincRNA-p21 are dispensable for the chromatin organization of the locus and for cis-regulation. Instead, we find that production of lincRNA-p21 through conserved regions in exon 1 of lincRNA-p21 promotes cis-activation. These findings demonstrate that the activation of nascent transcription from this lncRNA locus, but not the generation or accumulation of a mature lncRNA transcript, is necessary to enact local gene expression control.
Graphical Abstract
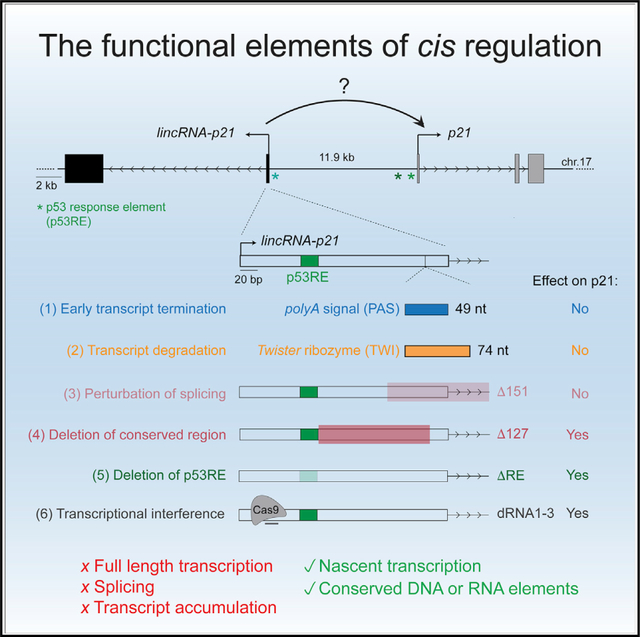
In brief
Winkler et al. analyze a series of genetic models to show that full-length production, splicing, and transcript accumulation of the long noncoding RNA lincRNA-p21 are dispensable for its role as a transcriptional activator of the neighboring gene p21. Instead, nascent transcription through conserved regions of lincRNA-p21 is sufficient for cis-activation.
INTRODUCTION
The lncRNA lincRNA-p21, also known as Trp53cor1 (tumor protein p53 pathway co-repressor 1), is a 3.1-kb spliced, noncoding transcript that is induced by the central tumor suppressor p53, and it has been implicated in a range of processes, including apoptosis, cell cycle control, and the hypoxia response (Dimitrova et al., 2014; Guttman et al., 2009; Huarte et al., 2010; Yang et al., 2014). Despite its potential importance in mediating p53 function, the roles and mechanisms of lincRNA-p21 remain incompletely understood. On the one hand, a large number of studies, using tools based on RNAi and exogenous overexpression, have ascribed lincRNA-p21 trans-regulatory functions throughout the nucleus and in the cytoplasm via interactions with various RNA-binding proteins, mRNAs, and microRNAs (Ao et al., 2019; Huarte et al., 2010; Sun et al., 2019; Yang et al., 2014; Yoon et al., 2012), and efforts have been dedicated to determining how the lincRNA-p21 structure promotes these activities (Chillón and Pyle, 2016). On the other hand, two independent genetic deletion studies have revealed a more restricted role for lincRNA-p21 as a local transcriptional co-activator of its neighboring gene, p21, also known as Cdkn1a (cyclin-dependent kinase inhibitor 1a) (Dimitrova et al., 2014; Groff et al., 2016). Consistent with its role as a cis-regulator, lincRNA-p21 has been shown to accumulate at its site of transcription and to be expressed at low copy number (Dimitrova et al., 2014). However, it has remained unclear whether the RNA, its transcription, or enhancer-like DNA elements in the locus are the primary mediators of lincRNA-p21 cis-regulatory activities (Allen et al., 2014; Groff et al., 2016; Korkmaz et al., 2016). Indeed, while several lncRNAs have emerged as functional modulators of local epigenetic state (Blank-Giwojna et al., 2019; Kotzin et al., 2016; Nagano et al., 2008; Strehle and Guttman, 2020), other transcribed cis-regulatory loci have been shown to act through overlapping transcription or enhancer elements rather than regulatory lncRNAs (Alexanian et al., 2017; Anderson et al., 2016; Engreitz et al., 2016; Isoda et al., 2017; Latos et al., 2012; Paralkar et al., 2016).
Here, we develop a series of genetic tools to dissociate the lincRNA-p21 transcript and transcriptional process from the underlying DNA regulatory sequences and to determine the key elements required for its cis-regulatory activity. Our data highlight the power of complementary genetic approaches to resolve the functional elements in lncRNA loci and reveal a role for transcription of nascent lincRNA-p21 from conserved DNA elements in enacting activation of local gene expression.
RESULTS
p53 cooperatively regulates lincRNA-p21 and p21 through proximal and distal p53 response elements
As previously shown, lincRNA-p21 and p21 are divergent transcripts expressed approximately 12 kb apart and co-activated by the transcription factor p53. In response to stress, such as upon passaging of primary mouse embryonic fibroblasts (MEFs) or following treatment with the DNA damage-inducing agent doxorubicin (Doxo), p53 binds to conserved p53 response elements (p53REs) located in the promoters of lincRNA-p21 (RE) and p21 (RE1 and RE2) (Figure 1A). As expected, CRISPR-Cas9 mutagenesis of RE led to a 75% decrease in lincRNA-p21 levels, confirming its direct regulation by p53 (Figure 1B) (Huarte et al., 2010). Consistent with a previously proposed role for lincRNA-p21 in promoting p21 expression (Dimitrova et al., 2014), RE mutagenesis correlated with an approximately 25%–40% decrease in p21 RNA and protein levels (Figures 1B and 1C). To further characterize the interplay between the two genes, we performed chromosome conformation capture (3C) analysis of the lincRNA-p21/p21 locus. We observed a strong interaction between the regions containing the lincRNA-p21 and p21-associated p53REs, suggesting that the three p53REs may act cooperatively to regulate p21 expression (Figure 1A). Interestingly, the chromatin looping appeared to be constitutive and independent of p53 status or the stress response (Figures 1A and S1). Consistent with a cooperative interaction between the p53REs, CRISPR-Cas9 mutagenesis of RE1 and RE2 also affected the expression of both p21 and lincRNA-p21 (Figures 1D and 1E). The spatial proximity and functional cooperativity between the three p53REs raised the question of whether lincRNA-p21 transcription and/or transcript accumulation contribute to p21 regulation or are nonfunctional by-products of p53 transcriptional activity.
Figure 1. Reciprocal regulation of lincRNA-p21 and p21 by proximal and distal p53 response elements.
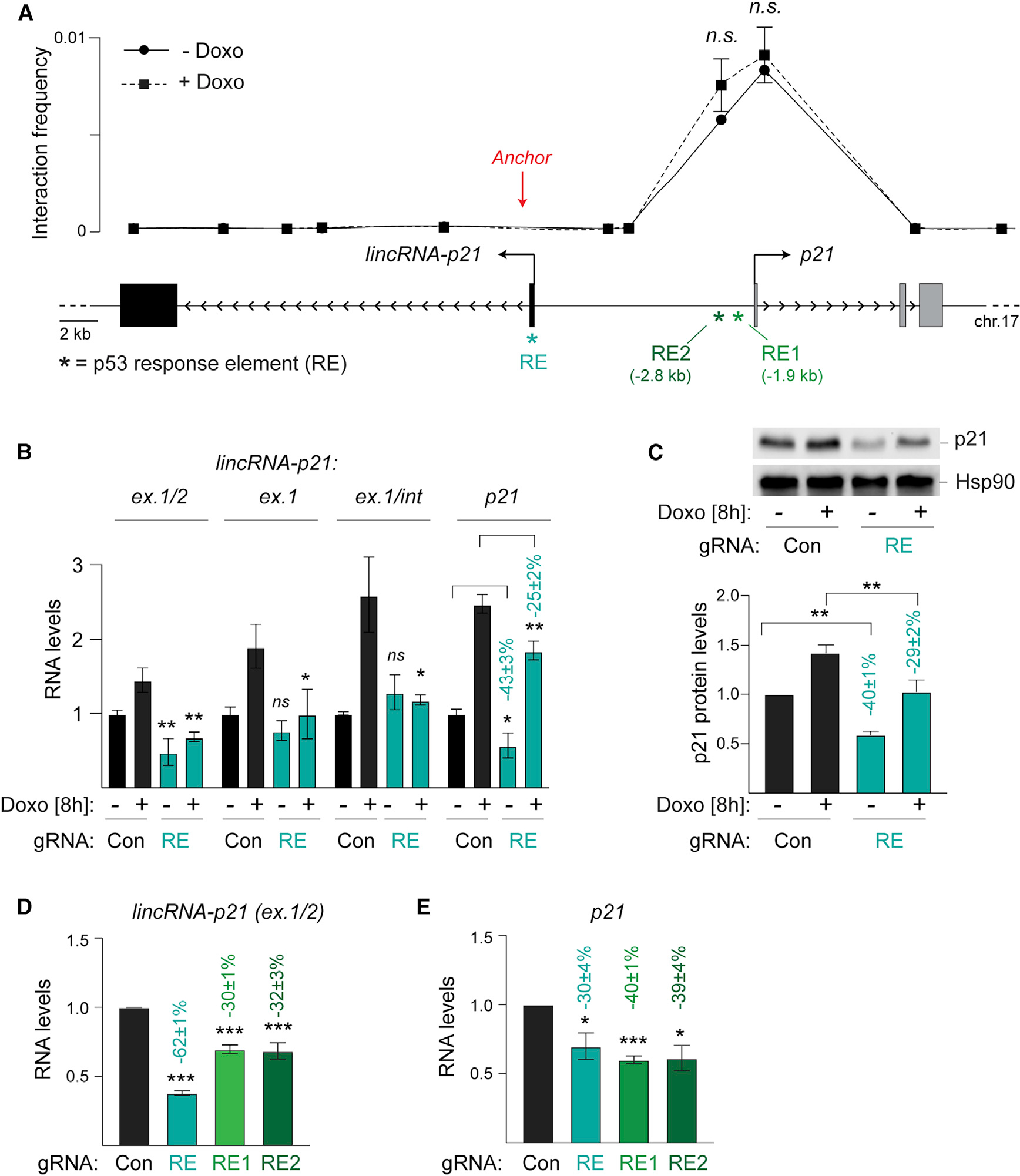
(A) Top, 3C analysis showing interaction frequency relative to genomic position, n = 3; Bottom, schematic of the lincRNA-p21/p21 locus highlighting the locations of the p53REs (*) and 3C anchor position (red arrow).
(B) qRT-PCR of normalized spliced (ex.1/2) and nascent (ex.1 and ex.1/int) lincRNA-p21 and p21 levels in RNA from wild-type MEFs with indicated treatments, n = 9.
(C) Top, representative immunoblot of p21 protein levels in whole cell lysates from MEFs in (B). Hsp90, loading control. Bottom, quantification of normalized p21 protein levels, n = 3.
(D and E) qRT-PCR of normalized lincRNA-p21 (D) and p21 (E) levels in RNA from MEFs, expressing Con or p53RE-targeting gRNAs, n = 3.
Data are represented as mean ± SEM of indicated biological replicates. Numbers indicate the percentage change relative to control samples with corresponding treatment; n.s. not significant, **p < 0.01, ***p < 0.001; paired t test.
Development of genetic models to query the role of lincRNA-p21 transcription and transcript accumulation
To investigate RNA-dependent contributions of the lincRNA-p21 locus to p21 regulation, we generated two independent genetic models. To determine whether transcription through the lincRNA-p21 locus is required for p21 activation, we used CRISPR-Cas9-mediated genetic engineering to insert the 49-nucleotide synthetic polyadenylation signal (PAS) (Levitt et al., 1989) into exon 1 of the endogenous lincRNA-p21 locus in murine blastocysts (Figure 2A). In parallel, to determine the contribution of the lincRNA-p21 RNA to p21 regulation, we introduced the 74-nucleotide Twister (TWI) self-cleaving ribozyme (Roth et al., 2014) at the same site in exon 1 of endogenous murine lincRNA-p21 (Figure 2A). We anticipated that while the PAS element would lead to premature termination, TWI would allow transcription of lincRNA-p21 but lead to transcript cleavage and degradation. LincRNA-p21PAS and lincRNA-p21TWI founder animals were crossed to C57BL/6J mice to obtain germline transmission (Figures S2A and S2B). Next, heterozygous crosses revealed that mice harboring homozygous PAS and TWI lincRNA-p21 alleles are viable, born at Mendelian ratios, and do not display any apparent abnormalities, consistent with previous lincRNA-p21 knockout models (Figure S2C) (Dimitrova et al., 2014; Sauvageau et al., 2013).
Figure 2. Development of genetic tools to probe the contribution of lincRNA-p21 transcription and accumulation to p21 regulation.
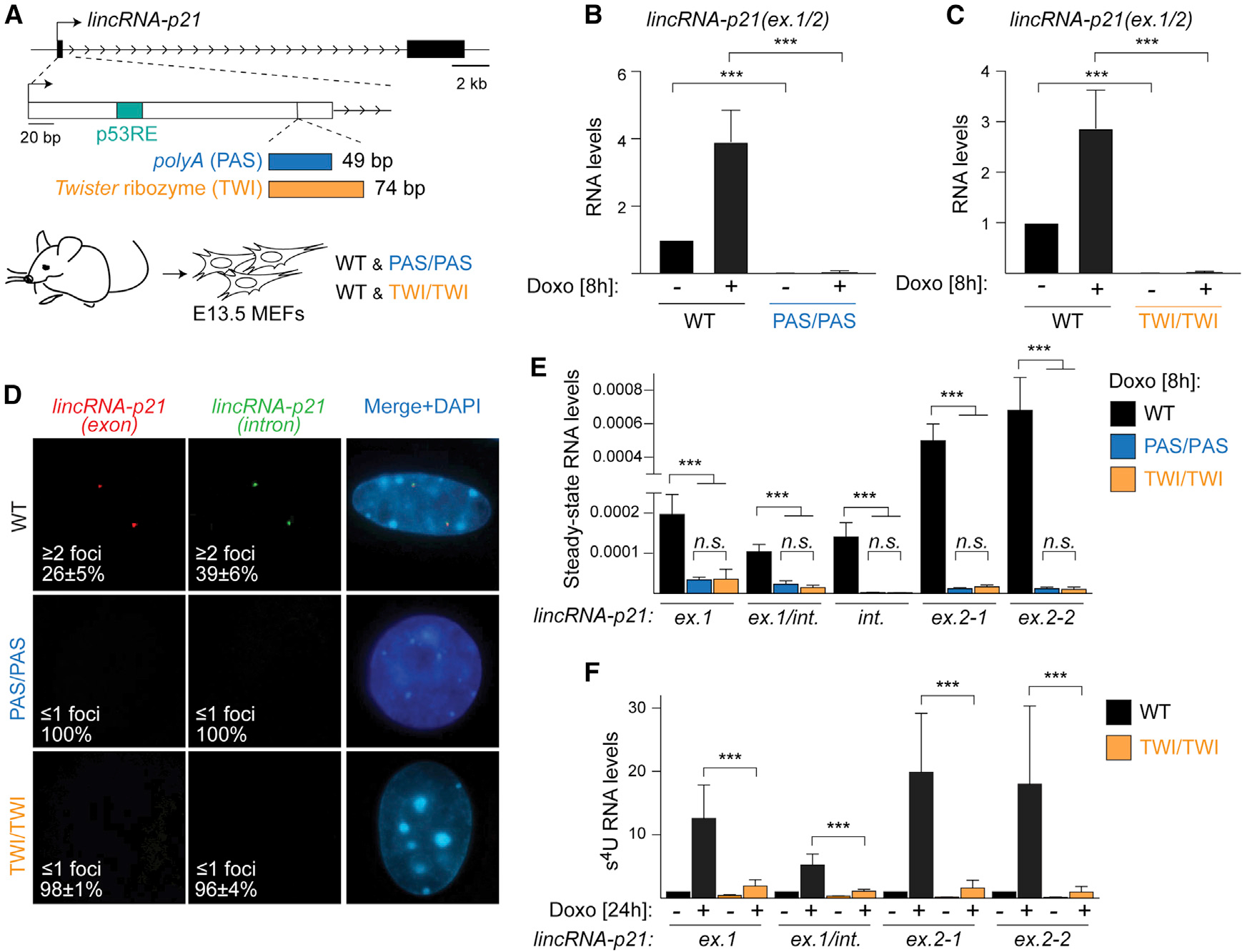
(A) Top, schematic of the lincRNA-p21 locus highlighting the PAS and TWI insertion site in exon 1. Bottom, littermate mutant and wild-type (WT) MEFs were isolated at E13.5.
(B and C) qRT-PCR showing normalized spliced lincRNA-p21 levels in RNA from WT and lincRNA-p21PAS/PAS (B, n = 6) and lincRNA-p21TWI/TWI (C, n = 7) MEFs, treated as indicated.
(D) smRNA-FISH images and quantification of signals using probes detecting lincRNA-p21 exons (red) and intron (green) in etoposide-treated MEFs. DNAcounterstained with DAPI in merged images.
(E) qRT-PCR of relative steady-state lincRNA-p21 levels in total DNAse-treated RNA from Doxo-treated MEFs, n = 4.
(F) Transient transcriptome (TT) qRT-PCR analysis of normalized lincRNA-p21 levels in s4U-labeled RNA from Doxo-treated MEFs.
Data are represented as mean ± SEM of indicated biological replicates; n.s. not significant, ***p < 0.001; paired t test.
To determine the effects of the PAS and TWI insertion on lincRNA-p21 expression, we first analyzed the levels of mature, spliced lincRNA-p21 in total RNA isolated from E13.5 MEFs in the absence or presence of Doxo. While wild-type MEFs displayed the expected 3- to 5-fold induction of lincRNA-p21 upon Doxo treatment, lincRNA-p21 was undetectable in lincRNA-p21PAS/PAS and lincRNA-p21TWI/TWI cells isolated from littermate embryos (Figures 2B and 2C). Consistently, while control cells exhibited the typical 2- to 4-dot pattern of overlapping lincRNA-p21 exon- and intron-specific signals by single molecule RNA-FISH (smRNA-FISH), lincRNA-p21-specific signals were largely absent from mutant cells (Figure 2D).
Next, we evaluated the extent to which PAS and TWI insertions affected total lincRNA-p21 in steady-state RNA isolated from Doxo-treated MEFs. Using primers specific to regions both upstream and downstream of the insertion site in exon 1, we observed significant depletion of lincRNA-p21 in lincRNA-p21PAS/PAS and lincRNA-p21TWI/TWI cells compared to wild-type controls (Figure 2E). Notably, the PAS and TWI mutations led to a comparable reduction of lincRNA-p21 levels (Figure 2E). To distinguish whether the TWI insertion led to post-transcriptional degradation of lincRNA-p21 or affected lincRNA-p21 biogenesis, we performed transient transcriptional me (TT) analysis of s4U-labeled RNA. Primers located in exon 1 both upstream and downstream of the TWI insertion site revealed that TWI led to the degradation of 60%–80% of newly transcribed lincRNA-p21, while primers located in exon 2 approximately 20 kb downstream of the TWI insertion pointed to a 90% reduction in transcription near the transcription termination site (Figure 2F). These results indicated that the PAS led to efficient transcription termination, while TWI mediated co-transcriptional transcript degradation and only allowed approximately 10% of full-length transcript production.
Mature lincRNA-p21 production and accumulation are dispensable for p21 regulation
With these validated tools in hand, we next queried how inhibition of lincRNA-p21 transcription and transcript accumulation affect p21 expression. In contrast to previous lincRNA-p21 promoter and locus genetic deletion models (Dimitrova et al., 2014; Groff et al., 2016), we found that the levels of p21 in lincRNA-p21PAS/PAS and lincRNA-p21TWI/TWI cells did not differ significantly from wild-type MEFs in either the absence or presence of DNA damage (Figures 3A and 3B). Moreover, there was no difference in p21 protein levels (Figures 3C and 3D), and lincRNA-p21PAS/PAS and lincRNA-p21TWI/TWI cells proliferated at comparable rates as littermate controls, indicating normal cell cycle progression (Figures 3E and 3F). We concluded that the production and accumulation of mature lincRNA-p21 are dispensable for the regulation of p21.
Figure 3. lincRNA-p21 transcription and accumulation are dispensable for p21 regulation.
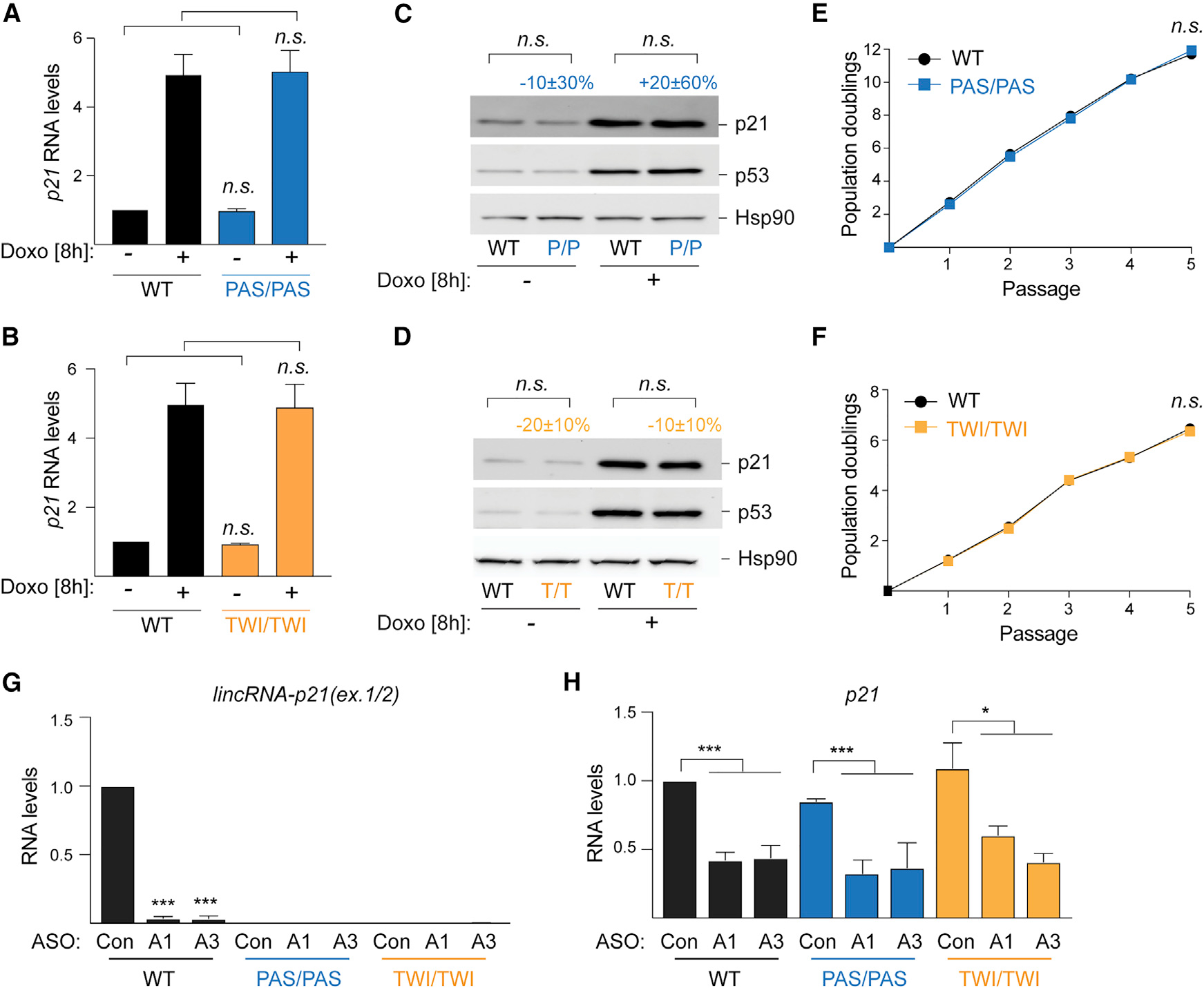
(A and B) qRT-PCR of normalized p21 levels in RNA from indicated MEFs, n = 12 (A); n = 7 (B).
(C and D) Representative immunoblot images of p21 and p53 protein levelsin whole cell lysates fromMEFs in (A) and (B); Hsp90, loading control.n = 7 (C) and n = 8 (D).
(E and F) Growth curve showing population doublings of indicated MEFs over passages, n = 3.
(G and H) qRT-PCR of normalized lincRNA-p21 (H) and p21 (I) levels in RNA from indicated MEFs transfected with control (Con) or lincRNA-p21-targeting ASOs (ASO1 and ASO3), n = 3.
Data are represented as mean ± SEM of indicated biological replicates; n.s. not significant, *p < 0.05, **p < 0.01, ***p < 0.001; paired t test. Numbers indicate percentage change relative to controls with corresponding treatment.
These findings conflicted with previous data, in which antisense oligonucleotide (ASO)-mediated knockdown lincRNA-p21 led to a reduction of p21 expression and implicated lincRNA-p21 production or transcript in cis-regulation (Dimitrova et al., 2014). To address the inconsistency, we introduced the published (Dimitrova et al., 2014) lincRNA-p21-targeting ASO1 (A1) and ASO3 (A3) in wild-type MEFs as well as in littermate lincRNA-p21PAS/PAS and lincRNA-p21TWI/TWI cells. Both A1 and A3 led to greater than 90% knockdown of lincRNA-p21 in wild-type MEFs, whereas lincRNA-p21 levels were undetectable in both control (Con)-, A1-, and A3-treated PAS and TWI cells (Figure 3G). Strikingly, we observed that introduction of A1 and A3 led to comparable reductions of p21 levels regardless of genotype, indicating that the ASOs were not acting through knockdown of lincRNA-p21 (Figure 3H). We concluded that the lincRNA-p21-targeting ASOs affected p21 expression levels through a lincRNA-p21-independent mechanism.
A conserved element in exon 1 of lincRNA-p21 is required for p21 activation
We were intrigued that p53-dependent production of lincRNA-p21 may be entirely dispensable for cis-regulation. To search for functional elements in the lincRNA-p21 locus beyond RE, we engineered two mouse strains with deletions in exon 1 of lincRNA-p21 (Figures S2D and S2E). Δ127 comprises a 127-nucleotide deletion, which includes a highly conserved region of exon 1 but leaves the p53RE intact (Figures 4A and S2F). Δ151 targets a downstream region of exon 1 and includes the 5′-splice site of the lincRNA-p21 intron (Figures 4A and S2F). Analysis of RNA from primary MEFs isolated from lincRNA-p21Δ127/Δ127, lincRNA-p21Δ151/Δ151, and corresponding wild-type littermate embryos revealed that both deletions led to a complete loss of spliced lincRNA-p21 (Figure 4B). Interestingly, while the Δ151 mutation did not affect p21 expression, indicating that correct splicing is not required for cis-regulation, Δ127 led to a significant decrease in p21 expression in both the absence and in the presence of Doxo-induced DNA damage (Figure 4C). We also observed diminished p21 protein levels in lincRNA-p21Δ127/Δ127 MEFs compared to controls (Figure 4D). Consequently, lincRNA-p21Δ127/Δ127 cells proliferated faster than wild-type cells, consistent with a role for p21 in inhibiting cell cycle progression (Figure 4E). The changes in p21 levels were not due to altered chromatin organization in the locus or diminished p53 binding in the p21 promoter (Figures S3A–S3C). These findings pointed to a p53RE-independent element in the lincRNA-p21 locus, which contributes to p21 regulation.
Figure 4. Nascent transcription of lincRNA-p21 promotes p21 expression.
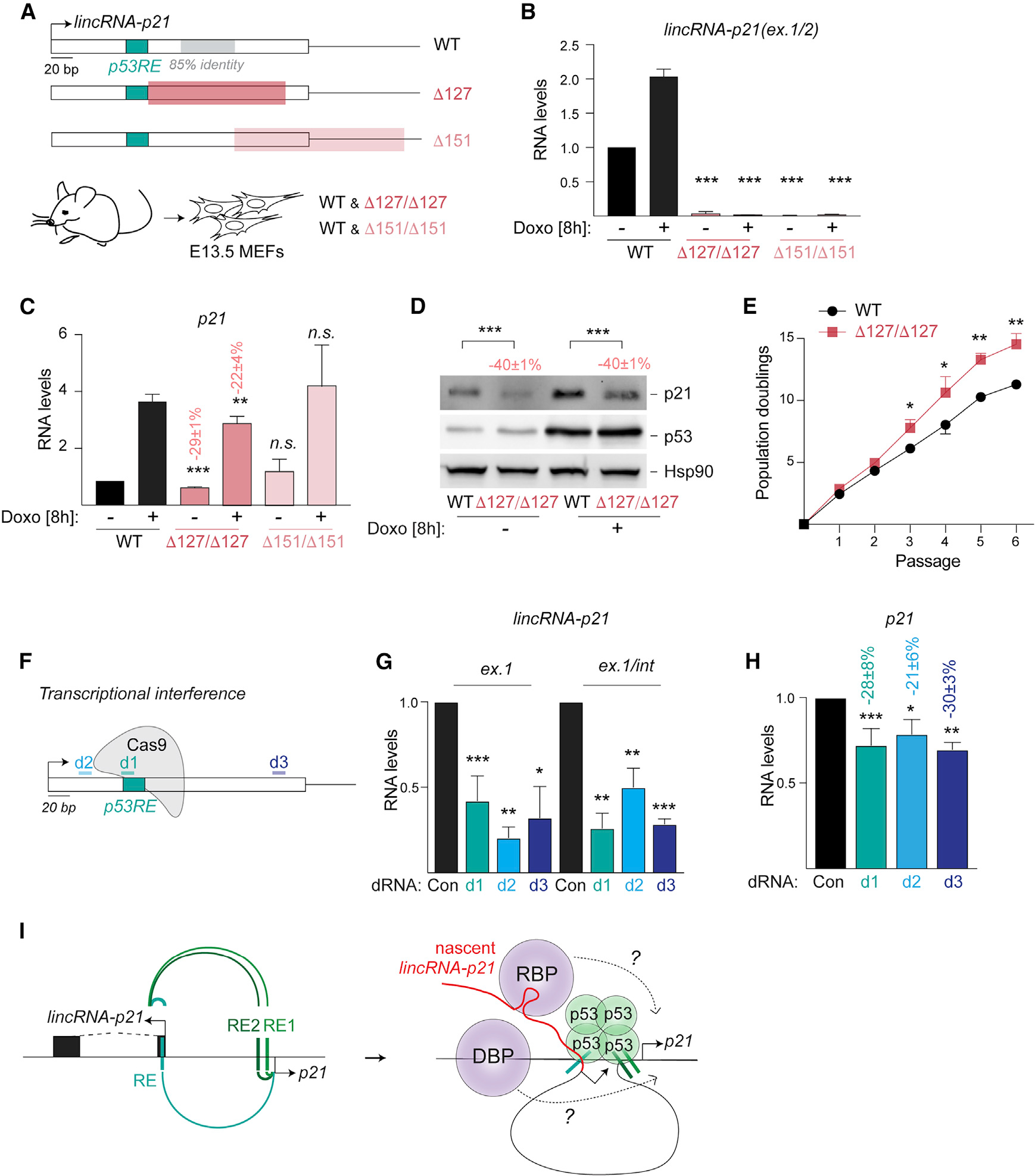
(A) Top, schematic highlighting the regions of D127 (red) and D151 (light red) deletions in lincRNA-p21. Bottom, littermate mutant and wild-type (WT) MEFs were isolated at E13.5.
(B and C) qRT-PCR of normalized lincRNA-p21 (B) and p21 (C) levels in RNA from indicated MEFs, n = 3–8.
(D) Representative immunoblot image of p21 and p53 protein levels in whole cell lysates from MEFs in (C); Hsp90, loading control; n = 6.
(E) Growth curve showing population doublings of indicated MEFs over passages, n = 3.
(F) Schematic showing the location of dRNAs for transcriptional interference.
(G and H) qRT-PCR of normalized nascent lincRNA-p21 (G) and p21 (H) RNA levels in total DNAse-treated RNA isolated from WT MEFs expressing indicated dRNAs.
(I) Left, model depicting the cooperative regulation of lincRNA-p21 and p21 expression by proximal and distal p53REs. Right, models for the proposed contribution of nascent lincRNA-p21 in the recruitment of RNA-binding proteins (RBP) or DNA-binding proteins (DBP), which may directly regulate p21 expression due to looped chromatin architecture of the lincRNA-p21/p21 locus.
Data are represented as mean ± SEM of indicated biological replicates; n.s. not significant, *p < 0.05, **p < 0.01, ***p < 0.001, n = 3, paired t test. Numbers indicate the percentage change relative to controls with corresponding treatment.
Nascent lincRNA-p21 transcription promotes p21 expression
To determine whether transcription through the novel element played a role, we performed transcriptional interference using CRISPR-Cas9. 15-nucleotide “dead” RNA (dRNAs) have been shown to effectively recruit Cas9 and cause transcriptional interference without supporting Cas9 endonuclease activity (Dahlman et al., 2015). We designed two dRNAs, d2 and d3, located upstream and downstream of RE, respectively, to interfere with transcription of lincRNA-p21 (Figure 4F). As a positive control, we introduced a dRNA (d1), targeting the p53RE designed to obstruct p53 binding through steric hindrance (Figure 4F). As a negative control, we introduced a non-targeting dRNA (dCon). We confirmed that the introduction of d1, d2, and d3 in wild-type MEFs led to efficient downregulation of nascent lincRNA-p21 compared to dCon (Figure 4G). Furthermore, we observed that all three dRNAs led to significant decreases in p21 levels (reduction by 28% ± 8%, 21% ± 6%, and 30% ± 2% by d1, d2, and d3, respectively, compared to dCon; Figure 4H). Importantly, the effects of dRNA-mediated transcriptional interference on p21 levels were comparable to the p21 decreases observed with RE (reduction by 30% ± 4%; Figure 1D) and Δ127 (reduction by 29% ± 4%; Figure 4C) mutations. These findings indicated that transcription from the lincRNA-p21 locus is important for cis-activation of p21. We concluded that cooperative activity of proximal and distal p53REs combined with p53-dependent activation of lincRNA-p21 transcription are both required for full activation of p21 expression (Figure 4I).
DISCUSSION
Despite growing knowledge of the context-specific expression patterns and roles of lncRNAs in widespread homeostatic and disease processes, the functional elements within lncRNAs remain poorly understood (Kopp and Mendell, 2018). In this study, we engineered a series of genetic tools to dissect the functional elements of the well-characterized, cis-regulatory lincRNA-p21 locus. Strikingly, we found that transcription, processing, and accumulation of full-length lincRNA-p21 are dispensable for its role in promoting p21 expression. Given these conclusions, previous functional and structural studies of the full-length lincRNA-p21 transcript should be re-evaluated, and findings should be replicated in the context of these novel genetic models. Our findings support the conclusions from previous studies, which observed a lack of correlation between mature lincRNA-p21 and p21 expression levels and identified an enhancer-like regulatory element in the lincRNA-p21 promoter (Allen et al., 2014; Groff et al., 2016; Korkmaz et al., 2016). Notably, our observations are at odds with previous work, which used lincRNA-p21-targeting ASOs to establish a role for lincRNA-p21 production in p21 regulation (Dimitrova et al., 2014). Our findings of off-target effects caution against the use of ASOs as a sole method for determining a role for a lncRNA transcriptional process or transcript (Lai et al., 2020; Lee and Mendell, 2020).
Here, we establish that p53 acts cooperatively through lincRNA-p21- and p21-associated proximal and distal p53REs to regulate the expression of the two neighboring genes during the cellular response to stress (Figure 4I, left). This is aided by the constitutively looped chromatin architecture of the locus, which brings the p53REs within spatial proximity. Surprisingly, we find that full-length lincRNA-p21 production, processing, and transcript accumulation are dispensable for cis-regulation, suggesting that lincRNA-p21 may be a nonfunctional by-product of p53 transcriptional activity. However, we found evidence that nascent lincRNA-p21 transcription reinforces p21 expression independently of p53 binding. We envision two possible functions for lincRNA-p21 transcripts (Figure 4I, right). On the one hand, nascent lincRNA-p21 may serve to directly recruit RNA-binding proteins, such as hnRNP-K, which has previously been found to interact with lincRNA-p21 and to play a role in stimulating p21 expression (Dimitrova et al., 2014; Huarte et al., 2010). Since nascent transcripts are physically tethered to the locus, they are therefore perfectly positioned to act as local recruitment platforms, even at low copy number (Davidovich and Cech, 2015; Sigova et al., 2015). On the other hand, transcription has been suggested to play a role in epigenetic remodeling and “licensing” of distal enhancer elements (Anderson et al., 2016; Engreitz et al., 2016; Kaikkonen et al., 2013; Scruggs et al., 2015). It is therefore conceivable that transcription through DNA elements in the lincRNA-p21 locus may stimulate the recruitment of DNA-binding proteins and transcription factors, such as CEBPα/β and Sp1, which may in turn directly affect p21 expression given the looped threedimensional architecture of the locus. Importantly, our findings determine that nascent transcripts, and not full-length spliced RNA molecules, are the functional elements of cis-regulation in the locus.
Several previous studies have employed genetic approaches, such as PAS and polyadenylation cassette insertions, to interrogate the importance of mature lncRNA molecules (Engreitz et al., 2016; Isoda et al., 2017; Latos et al., 2012; Paralkar et al., 2016). Many of these studies identified DNA elements in lncRNA loci or various aspects of lncRNA biogenesis, such as splicing or the act of transcription through the loci as responsible for cis-activation. Our work identifies nascent transcription as an additional functional player in cis-regulation and highlights the need to use multiple complementary genetic approaches to dissect the functional elements of regulatory lncRNA loci.
Limitations of the study
The present study does not provide insights into the functional features of nascent lincRNA-p21 transcripts, such as their length, stability, and PolII association. Moreover, the importance of the nascent RNA transcripts themselves versus the process of nascent transcript production remains to be elucidated.
STAR★METHODS
Detailed methods are provided in the online version of this paper and include the following:
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead contact, Nadya Dimitrova (nadya.dimitrova@yale.edu).
Materials availability
Mouse lines and reagents generated in this study are available from the Lead contact with a completed Materials Transfer Agreement.
Data and code availability
All data reported in this paper will be shared by the Lead contact upon request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the Lead contactupon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mouse strains
LincRNA-p21 edited mice were generated using CRISPR/Cas9-mediated engineering in C57BL/6J blastocysts at the Jackson Laboratory for Genomic Medicine. Briefly, embryos were electroporated with Cas9, a guide RNA targeting lincRNA-p21 and PAS and TWI HDR templates, described in Table S1. Founders were crossed to wild-type C57BL/6J mice. Germline transmission was identified by PCR genotyping using primers described in Table S1. Correct alleles were confirmed by Sanger sequencing. The two deletion alleles (Δ127 and Δ 151) were fortuitously identified in the founder population and confirmed by Sanger sequencing. To determine viability and normal development, mice with equal sex distribution were aged up to six months. All studies and procedures were conducted with the approval of the Yale University Institutional Animal Care and Use Committee.
Cell culture and drug treatments
Primary MEFs were isolated from E13.5 embryos resulting from timed matings between 3–6 month-old male and female mice heterozygous for the lincRNA-p21 allele of interest. All experiments were performed with littermate wild-type (WT) and mutant MEFs between passages 2 and 8. Primary MEFs were maintained in DMEM (Gibco) supplemented with 15% fetal bovine serum (FBS), 50 U mL−1 penicillin-streptomycin, 2 mM L-glutamine, 0.1 mM non-essential amino acids, and 0.055 mM β-mercaptoethanol. All cells were maintained at 37°C in a humidified incubator with 5% CO2. To induce DNA damage, MEFs were treated with 0.5 μM doxorubicin (Doxo, Sigma-Aldrich) for RNA, protein, and chromatin analyses or 25 μM etoposide (Sigma-Aldrich) for smRNA-FISH studies. ASO studies were performed as described in (Dimitrova et al., 2014).
Constructs
p53RE mutagenesis (RE, RE1, and RE2) and transcriptional interference (d1, d2, and d3) were performed using gRNAs or dRNAs cloned downstream of a U6 promoter in the spCas9-expressing BRD001 (lentiCRISPRv1) lentiviral construct (a gift from Feng Zhang, Broad Institute, MIT). Control gRNA or dRNA targeting dTomato served as a negative control. All gRNA and dRNA sequences are listed in Table S1.
METHOD DETAILS
Lentiviral infection
To generate lentivirus, HEK293 cells were co-transfected with pCMV-Δ8.2 (Addgene 8455) and pCMV-VSV-G (Addgene 8454), and the indicated lentiviral construct. Virus-containing media, harvested at 48, 72, and 96 hours post-transfection and supplemented with 4 μg mL−1 polybrene, was directly applied to MEFs. Following infection, MEFs were selected with 2 μg mL−1 puromycin for 72 hours.
Total RNA isolation and qPCR
Total RNA was isolated using RNeasy Mini (Qiagen) protocol with or without DNAse I digestion (Qiagen) and reverse-transcribed using the High Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems). For qPCR, SYBR Green Mastermix was used with primers listed in Table S1. Expression levels were calculated relative to Gapdh and normalized to corresponding control samples.
Metabolically-labeled RNA isolation and analysis
MEFs, cultured in the absence or presence of Doxo, were treated with 1 mM 4-thiouridine (s4U, Alfa Aesar) for 5 minutes. Samples were placed on ice, rinsed with PBS, harvested by scraping, suspended in TRIzol, and frozen at −80°C. Total RNA was isolated using chloroform extraction and isopropanol precipitation and treated with TURBO DNase to deplete genomic DNA. Purified RNA was then extracted using phenol-chloroform-isoamyl alcohol followed by ethanol precipitation. 50 μg of total RNA was biotinylated using MTSEA biotin-XX (Biotium) and enriched using streptavidin as previously described (Schofield et al., 2018). The resulting nascent RNA was reverse-transcribed using the SuperScript VILO cDNA Synthesis Kit (Thermo Fisher Scientific) and analyzed via qPCR using iTaq Universal SYBR Green Supermix (Bio-Rad) with primers listed in Table S1. Levels of nascent transcripts were calculated relative to Gapdh and normalized to WT, untreated sample.
Western blotting
Whole cell lysates were prepared by resuspending cell pellets in 2x Laemmli buffer (100 mM Tris-HCl pH 6.8, 200 mM DTT, 3% SDS, 20% glycerol) at a concentration of 104 cells/μL. Samples were boiled at 95°C for 7 minutes and passed through an insulin syringe. Protein samples from 105 cells were separated by electrophoresis on a SDS-polyacrylamide gel and transferred to a 0.2 μm nitrocellulose membrane. Immunoblotting was performed using the following antibodies: p21 (clone F-5, sc-6246, Santa Cruz Biotechnology), p53 (clone CM5, NCL-L-p53-CM5p, Leica), and loading control Hsp90 (clone C45G5, #4877S, Cell Signaling Technology).
Single molecule RNA fluorescence in situ hybridization (smRNA-FISH)
smRNA-FISH was performed as previously described (Dimitrova et al., 2014). Briefly, MEFs were seeded on coverslips and cultured for 24 hours in the presence or absence of etoposide before being fixed in 4% methanol-free formaldehyde in DEPC-treated water for 10 minutes at room temperature. After being washed twice in PBS, cells were dehydrated overnight in 70% ethanol at 4°C and stored for up to a week. Dehydrated coverslips were transferred to a hybridization chamber and equilibrated in Wash Buffer A for 5 minutes (LGC, Biosearch Technologies). Samples were incubated overnight at 30°C in hybridization buffer containing a 1:50 dilution of Stellaris probes conjugated to Quasar 570 or Quasar 670 dye. The following day, samples were washed twice with Wash Buffer A for 30 minutes at 30C, washed once with Wash Buffer B for 5 minutes at room temperature, and mounted in Vectashield Antifade Mounting Medium with DAPI (Vector Laboratories). Samples were imaged using the Axio Imager 2 microscope system (Zeiss) with a PlanApo 633×1.4 oil DIC objective lens (Zeiss).
Chromosome conformation capture (3C)
3C was performed as previously described with slight modifications (Hagege et al., 2007; Naumova et al., 2012). Briefly, 5–10 × 3 106 cells were harvested by trypsinization, rinsed once in PBS, and resuspended in 1% methanol-free formaldehyde in PBS. Samples were rotated end-over-end for 10 minutes at room temperature and quenched with ice-cold 2.5 M glycine. Following incubation for 5 minutes at room temperature and on ice for 15 minutes, samples were spun down at 225 g for 8 minutes at 4°C and washed once in ice-cold PBS. Samples were flash frozen and stored for up to six months at −80°C. For cell lysis, frozen samples were thawed on ice and resuspended in 5 mL ice-cold Lysis buffer (20 mM Tris HCl pH 8.0, 85 mM KCl, 0.5% NP-40, and Mini Complete Protease Inhibitor Cocktail Tablet (Roche)) for two hours. Samples were spun down at 400 g for 5 minutes at 4°C and resuspended in 0.5 mL 1.2X NEB Buffer 3.1 with 0.3% SDS. Next, samples were rotated end-over-end for 1 hour at 37°C, quenched with 2% Triton X-100, and again rotated end-over-end for 1 hour at 37°C. For chromatin digestion, samples were treated with 400 U BglII (NEB) and rotated end-over-end overnight at 37°C. To inactivate the restriction enzyme, the SDS concentration was increased to 1.6% and samples were shaken at 900 rpm for 25 minutes at 65°C. Next, samples were diluted by adding 6.125 mL 1.15X ligation buffer (10X ligation buffer: 600 mM Tris HCl pH 7.5, 50 mM DTT, 50 mM MgCl2, 10 mM ATP) and Triton X-100 to a final concentration of 1%. After incubating for 1 hour at 37C with gentle shaking, 100 U of T4 DNA ligase (NEB) was added to each sample. All samples were incubated for 4 hours at 16°C followed by 30 minutes at room temperature. To reverse the crosslinking, samples were treated with 300 μg Proteinase K and incubated overnight at 65°C with end-over-end rotation, followed by treatment with 300 μg RNAse A (Qiagen) for 45 minutes at 37°C. DNA was isolated using phenol-chloroform followed by ethanol precipitation. Purified DNA was resuspended in 10 mM Tris pH 7.5. Digestion efficiency was determined by reversing the cross-linking of chromatin samples taken before and after BglII digestion, followed by Proteinase K and RNAse-treatments, phenol-chloroform DNA purification, and ethanol precipitation. Purified DNA was subjected to qPCR analysis using primer sets spanning BglII sites. Next, qPCR was used to determine the concentration of each 3C library relative to a standard curve obtained by running serial dilutions of a control template. Libraries were diluted to a final concentration of 50 ng μL−1. Interaction frequencies relative to a control region in the lincRNA-p21 locus were measured by performing TaqMan qPCR on 50 ng 3C library using unidirectional primers in tandem with an anchor primer and fluorescent TaqMan probe targeting the lincRNA-p21 promoter (Table S1).
Chromatin immunoprecipitation (ChIP)
To detect p53 and H3K27ac enrichment at p21 promoter, 5–10 × 3 106 MEFs were fixed in 1% methanol-free formaldehyde for 10 minutes at room temperature, quenched with 100 mM ice-cold glycine for five minutes on ice, washed twice in PBS, flash frozen, and stored at −80°C. Nuclei were isolated by incubating the thawed cell pellet in Cell lysis buffer (20 mM Tris-HCl, pH 8.0, 85 mM KCl, 0.5% NP-40), supplemented with protease inhibitors (1 mM PMSF and Mini Complete Protease Inhibitor Cocktail Tablet (Roche)) on ice for 10 min. After centrifugation, the supernatant was removed and the nuclei were resuspended in Nuclei lysis buffer (50 mM Tris-HCl, pH 8.0, 10 mM EDTA, 1% SDS supplemented with protease inhibitors) and incubated for 10 min on ice. Next, chromatin was sonicated to 300–500 bp fragment size for 5 cycles (15″ ON, 90″ OFF) at 4°C using a Bioruptor sonicator (Diagenode). Sonicated lysates were centrifuged at 13, 000 rpm for 20 minutes and diluted in ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.1 mM EDTA, 20 mM Tris-HCl, pH 8.0, 167 mM NaCl, supplemented with protease inhibitors). Input aliquots were saved at this point. Chromatin immunoprecipitations were set up with a p53 antibody (clone CM5, NCL-L-p53-CM5p, Leica), a H3K27ac antibody (ab4729, Abcam), or control IgG (ab46540, Abcam), pre-conjugated to PureProteome Protein G Magnetic Beads (Millipore), blocked with 0.5% BSA in PBS and supplemented with 20 μg salmon sperm DNA (Invitrogen). The immunoprecipitations were incubated overnight at 4°C on a rotator. The next day, beads were washed once in each of the following washes for 5 minutes at 4°C on the rotator: Low salt wash (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl pH 8.0, 150 mM NaCl supplemented with protease inhibitors), High salt wash (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.0, 500 mM NaCl), LiCl wash (0.25 M LiCl, 1% NP-40, 1% Na deoxycholate, 1 mM EDTA, 20 mM Tris-HCl, pH 8.0), and TE wash (10 mM Tris-HCl, pH 8.0, 1 mM EDTA). After completely removing any remaining liquid from the washes, beads were resuspended in Elution buffer (50 mM Tris-HCl, pH 8.0, 10 mM EDTA, pH 8.0, 1% SDS) and incubated at 65°C for 15 min with shaking to prevent settling of beads. After elution, the beads were pelleted, and the supernatant was transferred to a new tube and incubated overnight at 65°C to reverse the crosslinking. The next day, samples were treated with RNaseA for 2 hours at 37°C, followed by a Proteinase K treatment for 30 minutes at 55°C. The DNA was purified by phenol-chloroform extraction and EtOH precipitation. The DNA pellet was air dried, resuspended in 200 μL H2O and used for quantitative PCR analysis (ChIP-qPCR) using primers listed in Table S1.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data in figures are represented as mean ± SEM of biological replicates (n ≥ 3); n.s. not significant, *p < 0.05, **p < 0.01, ***p < 0.001; paired t test.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| Mouse monoclonal anti-p21 (clone F-5) | Santa Cruz Biotechnology | Cat#sc-6246; RRID: AB_628073 |
| Rabbit polyclonal anti-p53 (clone CM5) | Leica Biosystems | Cat# NCL-L-p53-CM5p; RRID: AB_563933 |
| Rabbit monoclonal anti-HSP90 (clone C45G5) | Cell Signaling Technology | Cat#4877; RRID: AB_2233307 |
| Goat Anti-Mouse IgG (H + L)-HRP Conjugate | Bio-Rad | Cat#1706516; RRID: AB_11125547 |
| Peroxidase AffiniPure Donkey Anti-Rabbit IgG (H + L) | Jackson ImmunoResearch | Cat#711-035-152; RRID: AB_10015282 |
| Rabbit Anti-Mouse IgG (H&L) | Abcam | Cat#ab46540; RRID: AB_2614925 |
| Anti-histone H3 (acetyl K27) antibody -ChIP Grade | Abcam | Car#ab4729; RRID: AB_2118291 |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| Doxorubicin hydrochloride | Sigma-Aldrich | Cat#D1515-10MG |
| Etoposide | Millipore Sigma | Cat#E1383-25MG |
| Hexadimethrine bromide (polybrene) | Millipore Sigma | Cat#107689-10G |
| Puromycin dihydrochloride | Sigma-Aldrich | Cat#58-58-2 |
| DMEM, high glucose, pyruvate | GIBCO | Cat#11995065 |
| Fetal bovine serum | Sigma-Aldrich | Cat#F0926-500ML |
| L-glutamine | GIBCO | Cat#25030-081 |
| Pen-strep | GIBCO | Cat#15140-122 |
| NEAA | GIBCO | Cat#11140-050 |
| 2-mercaptoethanol | GIBCO | Cat#21985023 |
| SYBR Fast Master Mix | Kapa Biosystems | Cat#kk4602 |
| TRIzol reagent | Invitrogen | Cat#15-596-018 |
| RNasin Plus RNase inhibitor | Promega | Cat#N2615 |
| QuantiTect Probe PCR Master Mix | Qiagen | Cat#204343 |
| Nitrocellulose membranes | Bio-Rad | Cat#1620112 |
| ECL Prime Western Blotting detection reagent | GE Healthcare | Cat#RPN2232 |
| Methanol-free formaldehyde | ThermoScientific | Cat#28908 |
| Formamide | Millipore Sigma | Cat#F9037 |
| Stellaris_RNA FISH Hybridization Buffer | LGC Biosciences | Cat#SMF-HB1-10 |
| Stellaris_RNA FISH Wash Buffer A | LGC Biosciences | Cat#SMF-WA1-60 |
| Stellaris_RNA FISH Wash Buffer B | LGC Biosciences | Cat#SMF-WB1-20 |
| VECTASHIELD_Antifade Mounting Medium with DAPI | Vector Laboratories | Cat#H-1200 |
| Stellaris® FISH Probes, Custom Assay with Quasar® 670 Dye | LGC Biosciences | Cat#SMF-1065-5 |
| Stellaris® FISH Probes, Custom Assay with Quasar® 570 Dye | LGC Biosciences | Cat#SMF-1063-5 |
| cOmplete, Mini Protease Inhibitor Cocktail | Roche | Cat#4693124001 |
| PureProteome Protein G Magnetic Beads | Millipore Sigma | Cat#LSKMAGG02 |
| Salmon Sperm DNA | Invitrogen | Cat#15632011 |
| Proteinase K | Roche | Cat#03115879001 |
| NEBuffer™ 3.1 | New England Biolabs | Cat#B7203 |
| BglII | New England Biolabs | Cat#R0144 |
| T4 DNA ligase | New England Biolabs | Cat#M0202L |
| Adenosine 50-Triphosphate (ATP) | New England Biolabs | Cat#P0756L |
| 4-thiouridine (s4U) | Alfa Aesar | Cat#AAJ60679MC |
| RNAse-free DNAse | Qiagen | Cat#79254 |
| MTSEA biotin-XX | Biotium | Cat#900661 |
|
| ||
| Critical commercial assays | ||
|
| ||
| RNeasy Mini Kit | Qiagen | Cat#Q74106 |
| High Capacity cDNA Reverse Transcription Kit | Applied Biosystems | Cat#Q74106 |
| Zero Blunt TOPO PCR Cloning Kit | Invitrogen | Cat#450245 |
|
| ||
| Experimental models: Cell lines | ||
|
| ||
| Mouse: primary WT embryonic Fibroblasts (MEFs) | This study | N/A |
|
| ||
| Experimental models: Organisms/strains | ||
|
| ||
| Mouse: Wild-type (WT) C57BL/6J | Jackson Laboratories | Cat#000664 |
| Mouse: lincRNA-p21PAS | This study | N/A |
| Mouse: lincRNA-p21TWI | This study | N/A |
| Mouse: lincRNA-p21Δ127 | This study | N/A |
| Mouse: lincRNA-p21Δ151 | This study | N/A |
|
| ||
| Oligonucleotides | ||
|
| ||
| qRT-PCR primers, see Table S1 | This study | N/A |
| PCR primers, see Table S1 | This study | N/A |
| smRNA FISH probes | Dimitrova et al., 2014 | N/A |
| 3C qPCR primers, see Table S1 | This study | N/A |
| 3C Taqman probe, see Table S1 | This study | N/A |
| ChIP qPCR primers, see Table S1 | This study | N/A |
| gRNA sequences, see Table S1 | This study | N/A |
| dRNA sequences, see Table S1 | This study | N/A |
| HDR templates, see Table S1 | This study | N/A |
| ASOs | Dimitrova et al., 2014 | N/A |
|
| ||
| Recombinant DNA | ||
|
| ||
| pCMV-dR8.2 dvpr | Addgene | Addgene #8455; RRID: Addgene_8455 |
| pCMV-VSV-G | Addgene | Addgene #8454; RRID:Addgene_8454 |
| BRD001 (lentiCRISPRv1) | Gift from Feng Zhang | Addgene #52963; RRID: Addgene_52963. |
|
| ||
| Software and algorithms | ||
|
| ||
| GraphPad Prism, version 7.01 for Windows | N/A | https://www.graphpad.com |
| Biorender | N/A | https://www.biorender.com |
Highlights.
p53 regulates the neighboring p21 and lincRNA-p21 cooperatively
Production of nascent lincRNA-p21 promotes p21 expression in cis
Conserved elements in nascent lincRNA-p21 contribute to cis-activation
Transcription, processing, and accumulation of full-length lincRNA-p21 are dispensable
ACKNOWLEDGMENTS
We thank Elena Martinez, Emily Dangelmaier, and Zara Malik for helpful comments. We are grateful to Christiane Olivero for TWI design and Rick Maser from Jackson Laboratories for lincRNA-p21 mutant founders generation. This work was supported in part by LCRF (N.D.), IRG-ACS 58-012-58 (N.D.), The V Foundation (N.D.), the Pew-Stewart Foundation (N.D.), NIH R37CA230580 grant (N.D.), NIH R01GM137117 (M.D.S.), and by an SPORE in Lung Cancer (Yale University, NIH P50CA196530). L.W. was supported by NIH T32GM007223.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2022.110687.
REFERENCES
- Alexanian M, Maric D, Jenkinson SP, Mina M, Friedman CE, Ting C-C, Micheletti R, Plaisance I, Nemir M, Maison D, et al. (2017). A transcribed enhancer dictates mesendoderm specification in pluripotency. Nat. Commun. 8, 1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MA, Andrysik Z, Dengler VL, Mellert HS, Guarnieri A, Freeman JA, Sullivan KD, Galbraith MD, Luo X, Kraus WL, et al. (2014). Global analysis of p53-regulated transcription identifies its direct targets and unexpected regulatory mechanisms. Elife 3, e02200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KM, Anderson DM, McAnally JR, Shelton JM, Bassel-Duby R, and Olson EN (2016). Transcription of the non-coding RNA upperhand controls Hand2 expression and heart development. Nature 539, 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao X, Jiang M, Zhou J, Liang H, Xia H, and Chen G (2019). lincRNA-p21 inhibits the progression of non-small cell lung cancer via targeting miR-17-5p. Oncol. Rep. 41, 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank-Giwojna A, Postepska-Igielska A, and Grummt I (2019). lncRNA KHPS1 activates a poised enhancer by triplex-dependent recruitment of epigenomic regulators. Cell Rep. 26, 2904–2915.e4. [DOI] [PubMed] [Google Scholar]
- Chilló n I, and Pyle AM (2016). Inverted repeat Alu elements in the human lincRNA-p21 adopt a conserved secondary structure that regulates RNA function. Nucleic Acids Res. 44, 9462–9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlman JE, Abudayyeh OO, Joung J, Gootenberg JS, Zhang F, and Konermann S (2015). Orthogonal gene knockout and activation with a catalytically active Cas9 nuclease. Nat. Biotechnol. 33, 1159–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovich C, and Cech TR (2015). The recruitment of chromatin modifiers by long noncoding RNAs: lessons from PRC2. RNA 21, 2007–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova N, Zamudio JR, Jong RM, Soukup D, Resnick R, Sarma K, Ward AJ, Raj A, Lee JT, Sharp PA, and Jacks T (2014). LincRNA-p21 activates p21 in cis to promote Polycomb target gene expression and to enforce the G1/S checkpoint. Mol. Cell 54, 777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engreitz JM, Haines JE, Perez EM, Munson G, Chen J, Kane M, McDonel PE, Guttman M, and Lander ES (2016). Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 539, 452–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groff AF, Sanchez-Gomez DB, Soruco MM, Gerhardinger C, Barutcu AR, Li E, Elcavage L, Plana O, Sanchez LV, Lee JC, et al. (2016). In vivo characterization of linc-p21 reveals functional cis-regulatory DNA elements. Cell Rep. 16, 2178–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al. (2009). Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458, 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagege H, Klous P, Braem C, Splinter E, Dekker J, Cathala G, de Laat W, and Forne T (2007). Quantitative analysis of chromosome conformation capture assays (3C-qPCR). Nat. Protoc. 2, 1722–1733. [DOI] [PubMed] [Google Scholar]
- Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, et al. (2010). A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 142, 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda T, Moore AJ, He Z, Chandra V, Aida M, Denholtz M, Piet van Hamburg J, Fisch KM, Chang AN, Fahl SP, et al. (2017). Non-coding transcription instructs chromatin folding and compartmentalization to dictate enhancer-promoter communication and T cell fate. Cell 171, 103–119.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaikkonen MU, Spann NJ, Heinz S, Romanoski CE, Allison KA, Stender JD, Chun HB, Tough DF, Prinjha RK, Benner C, and Glass CK (2013). Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol. Cell 51, 310–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp F, and Mendell JT (2018). Functional classification and experimental dissection of long noncoding RNAs. Cell 172, 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz G, Lopes R, Ugalde AP, Nevedomskaya E, Han R, Myacheva K, Zwart W, Elkon R, and Agami R (2016). Functional genetic screens for enhancer elements in the human genome using CRISPR-Cas9. Nat. Biotechnol. 34, 192–198. [DOI] [PubMed] [Google Scholar]
- Kotzin JJ, Spencer SP, McCright SJ, Kumar DB, Collet MA, Mowel WK, Elliott EN, Uyar A, Makiya MA, Dunagin MC, et al. (2016). The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature 537, 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Damle SS, Ling KK, and Rigo F (2020). Directed RNase H cleavage of nascent transcripts causes transcription termination. Mol. Cell 77, 1032–1043.e4. [DOI] [PubMed] [Google Scholar]
- Latos PA, Pauler FM, Koerner MV, Senergin HB, Hudson QJ, Stocsits RR, Allhoff W, Stricker SH, Klement RM, Warczok KE, et al. (2012). Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science 338, 1469–1472. [DOI] [PubMed] [Google Scholar]
- Lee JS, and Mendell JT (2020). Antisense-mediated transcript knockdown triggers premature transcription termination. Mol. Cell 77, 1044–1054.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt N, Briggs D, Gil A, and Proudfoot NJ (1989). Definition of an efficient synthetic poly(A) site. Genes Dev. 3, 1019–1025. [DOI] [PubMed] [Google Scholar]
- Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, Feil R, and Fraser P (2008). The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 322, 1717–1720. [DOI] [PubMed] [Google Scholar]
- Naumova N, Smith EM, Zhan Y, and Dekker J (2012). Analysis of longrange chromatin interactions using chromosome conformation capture. Methods 58, 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paralkar VR, Taborda CC, Huang P, Yao Y, Kossenkov AV, Prasad R, Luan J, Davies JO, Hughes JR, Hardison RC, et al. (2016). Unlinking an lncRNA from its associated cis element. Mol. Cell 62, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A, Weinberg Z, Chen AG, Kim PB, Ames TD, and Breaker RR (2014). A widespread self-cleaving ribozyme class is revealed by bioinformatics. Nat. Chem. Biol. 10, 56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageau M, Goff LA, Lodato S, Bonev B, Groff AF, Gerhardinger C, Sanchez-Gomez DB, Hacisuleyman E, Li E, Spence M, et al. (2013). Multiple knockout mouse models reveal lincRNAs are required for life and brain development. Elife 2, e01749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield JA, Duffy EE, Kiefer L, Sullivan MC, and Simon MD (2018). TimeLapse-seq: adding a temporal dimension to RNA sequencing through nucleoside recoding. Nat. Methods 15, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs BS, Gilchrist DA, Nechaev S, Muse GW, Burkholder A, Fargo DC, and Adelman K (2015). Upstream anti-sense promoters are hubs of transcription factor binding and active histone modifications. Mol. Cell 58, 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigova AA, Abraham BJ, Ji X, Molinie B, Hannett NM, Guo YE, Jangi M, Giallourakis CC, Sharp PA, and Young RA (2015). Transcription factor trapping by RNA in gene regulatory elements. Science 350, 978–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehle M, and Guttman M (2020). Xist drives spatial compartmentalization of DNA and protein to orchestrate initiation and maintenance of X inactivation. Curr. Opin. Cell Biol. 64, 139–147. [DOI] [PubMed] [Google Scholar]
- Sun Q, Zeng Q-C, Chen Y-Q, Zhang M, Wei L-L, and Chen P (2019). Long intergenic noncoding RNA p21 suppresses the apoptosis of hippocampus neurons in streptozotocin-diabetic mice by sponging microRNA-221 through upregulation of FOS. J. Cell Physiol. 234, 21113–21125. [DOI] [PubMed] [Google Scholar]
- Yang F, Zhang H, Mei Y, and Wu M (2014). Reciprocal regulation of HIF-1alpha and lincRNA-p21 modulates the Warburg effect. Mol. Cell 53, 88–100. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Abdelmohsen K, Srikantan S, Yang X, Martindale JL, De S, Huarte M, Zhan M, Becker KG, and Gorospe M (2012). LincRNA-p21 suppresses target mRNA translation. Mol. Cell 47, 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the Lead contact upon request. This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the Lead contactupon request.


