Abstract
Heat stress is one of the major environmental stressors challenging the global poultry industry. Identifying the genes responsible for heat tolerance is fundamentally important for direct breeding programs. To uncover the genetic basis underlying the ambient temperature adaptation of chickens, we analyzed a total of 59 whole genomes from indigenous chickens that inhabit South Asian tropical regions and temperate regions from Northern China. We applied FST and π-ratio to scan selective sweeps and identified 34 genes with a signature of positive selection in chickens from tropical regions. Several of these genes are functionally implicated in metabolism (FABP2, RAMP3, SUGCT, and TSHR) and vascular smooth muscle contractility (CAMK2), and they may be associated with adaptation to tropical regions. In particular, we found a missense mutation in thyroid-stimulating hormone receptor (41020238:G>A) that shows significant differences in allele frequency between the chicken populations of the two regions. To evaluate whether the missense mutation in TSHR could enhance the heat tolerance of chickens, we constructed segregated chicken populations and conducted heat stress experiments using homozygous mutations (AA) and wild-type (GG) chickens. We found that GG chickens exhibited significantly higher concentrations of alanine aminotransferase, lactate dehydrogenase, and creatine kinase than AA chickens under heat stress (35 ± 1°C) conditions (P < 0.05). These results suggest that TSHR (41020238:G>A) can facilitate heat tolerance and adaptation to higher ambient temperature conditions in tropical climates. Overall, our results provide potential candidate genes for molecular breeding of heat-tolerant chickens.
Key words: chicken, heat tolerance, TSHR, adaptation, genome sequencing
INTRODUCTION
With the development of society and the increase in the human population, the need for protein resources and food is increasing. Domestic chickens are one of the most economically important poultry species, providing the primary protein resource for humans in most areas of the world (Liu et al., 2020). However, chickens are sensitive to high ambient temperatures due to their feather coverage and the lack of functional sweat glands (Loyau et al., 2013). Under an environmental temperature of 18 to 21°C, chickens have optimal production performance (Kumari and Nath, 2019), but above 25°C, chickens may suffer from heat stress, which could lead to death in severe heat stress situations (Donkoh, 1989). Exposure of chickens to heat stress results in a series of physiological dyshomeostatic processes, including systemic immune dysregulation, endocrine disorders, respiratory alkalosis, and electrolyte imbalance, which affect health and performance (Liu et al., 2020). Heat stress reduces feed intake and weight gain (Ma et al., 2021), resulting in impairment of growth performance (Beckford et al., 2020). Heat stress also decreases egg production by inducing apoptosis of follicular cells (Li et al., 2020) and reduces meat quality by changing aerobic metabolism and glycolysis (Zaboli et al., 2019), which results in pale meat color, a decrease in muscle pH, reduced water-binding capacity, and increased cook and drip losses (Zaboli et al., 2019). In addition, heat stress affects carcass traits and animal well-being (Cândido et al., 2020; Huang et al., 2020) and impairs the function of the digestive (Quinteiro-Filho et al., 2012) and immune systems (Sugiharto et al., 2017). With the rising severity of global warming, heat stress triggered by high ambient temperature is the main environmental factor causing huge enormous economic losses (St-Pierre et al., 2003), challenging the poultry industry, especially in tropical and subtropical regions (Gregory, 2010). Thus, uncovering the genetic basis underlying the heat stress response has both theoretical importance and practical applications in facilitating breeding programs to address global warming.
Since domestication, chickens have been translocated across the globe and developed into thousands of breeds with remarkable phenotypic characteristics in morphology, physiology, and behavior (Guo et al., 2016; Ulfah et al., 2016; Wang et al., 2020). These chicken breeds have adaptations to diverse environmental conditions spanning torrid and subtropic environments (Wang et al., 2015; Ulfah et al., 2016), providing excellent models with which to study the genetic mechanisms underlying the temperature adaptations. Recently, using a comparative genomic strategy, Tian et al. (2020) suggested that 12 genes found to be under selection are involved in adaptations to both tropical desert and tropical monsoon island climates of Saudi Arabian and Sri Lankan indigenous chickens. Additionally, based on 600k chicken genotyping array data of Sri Lankan, Brazilian, and Egyptian chickens, Walugembe et al. (2019) identified several genes, including TRMT1L, SOCS2, and NFKB1, with a signal of positive selection and concluded that these genes may be involved in the survival of chickens in hot conditions. However, the aforementioned studies were restricted to a single chicken breed or small sample size, and the results, particularly the selective sweeps analysis, may be biased by genetic drift. Despite the many efforts that have been made, our understanding of the genetic basis adaptation of chickens to high ambient temperatures remains limited.
In the present study, we analyzed 59 genomes of indigenous chickens from the tropical and temperate zones to evaluate the potential genomic footprints responsible for environmental heat adaptation. Our results add to the growing knowledge on the genetic mechanisms of temperature adaptation and may benefit future breeding design.
MATERIALS AND METHODS
Ethical Approval of the Study
The study protocol was approved by the Animal Care and Use Committee of Anhui Agricultural University (approval no: SYXK2016-007).
Samples, Read Mapping, and SNP Calling
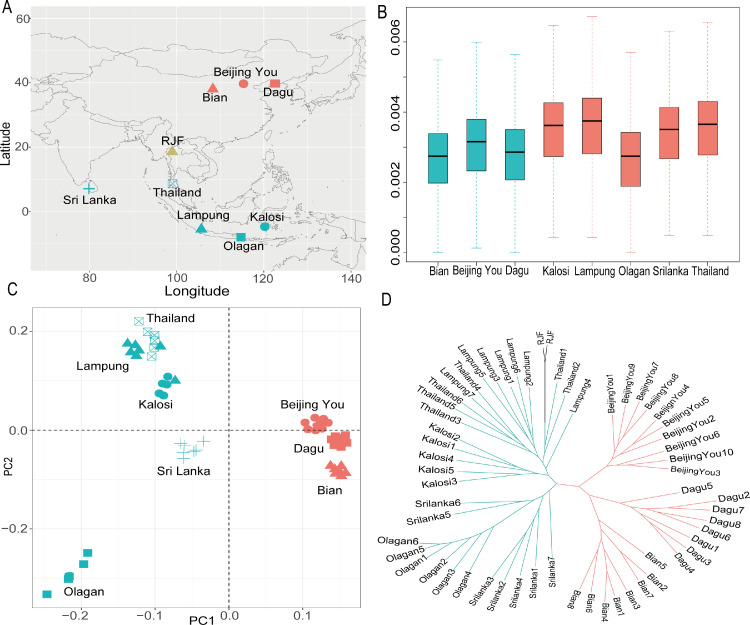
Fifty-nine genomes from our previous studies (Wang et al., 2020), including 2 Red Jungle Fowls, 26 village chickens from northern China, 18 indigenous Indonesian chickens, 7 individuals from Sri Lanka, and 6 indigenous chickens from Thailand, were analyzed in the present study. The geographic distribution of these samples is provided in Figure 1A, and the sample information is available in Supplementary Table S1.
Figure 1.
Geographic distribution, population relationships, and genetic diversity of chickens. (A) Geographic distribution of samples used in this study. (B) Population nucleotide diversity. (C) Principal component analysis. (D) ML tree constructed based on autosomal data.
We removed low-quality bases using Btrim (version 0.2.0) (Kong, 2011) with parameres ‘-s -a 20 -q’. The high-quality sequence reads were mapped onto the chicken reference genome (GRCg6a) using BWA-MEM (Li, 2014) with default parameters. The BAM file produced for each individual was sorted using the ‘SortSam’ program in the Picard package (version 1.119) (http://picard.sourceforge.net). PCR duplicates were identified and removed using the ‘MarkDuplicates’ program of Picard (version 1.119). We then used the function index from Samtools (version 1.9) (Li et al., 2009) to create index files and used the ‘UnifiedGenotyper’ function implemented in Genome Analysis Toolkit (GATK v3.5) to call SNPs with default parameters (McKenna et al., 2010). SNPs were filtered with VariantFiltration tools in GATK (version 3.5) with the same parameters used previously (Wang et al., 2017;) (“QUAL < 40.0 MQ < 25.0 MQ0 >= 4 & ((MQ0/(1.0*DP)) > 0.1) -cluster 3 -window 10”).
Population Structure, Diversity, and Relationships
For the estimation of population structure, PCA was performed using GCTA (Yang et al., 2011). Our analysis was based on a SNP dataset that was pruned with PLINK with the settings “–indep-pairwise 50 10 0.1”. First, we calculated the genetic relationship matrix with the “–make-grm” option and then estimated the three principal components with the “–pca 3” option. The genome-wide nucleotide diversity (π) of each population was calculated using VCFtools (Danecek et al., 2011) with a nonoverlapping window size of 50 kb. To reveal the phylogenetic relationships of chickens, neighbor-joining (NJ) and maximum-likelihood (ML) trees were constructed using MEGA7 (Kumar et al., 2016) and FastTree (Price et al., 2010), respectively. To reduce potential bias arising from putatively missing and/or unreliable genotypes, the SNP dataset used for phylogenetic reconstruction was filtered using VCFtools (version 0.1.16) (Danecek et al., 2011), with the parameters “–maf 0.01 –max-missing 0.5”.
Screening for Selective Sweeps
The allele frequency of variable sites was used to identify regions with signatures of positive selection. We calculated the population fixation statistics (FST) and π-ratio (Temperate/Tropical) across the chicken genome using a genome-wide sliding window strategy (50-kb windows with 25-kb steps). The putatively positively selected genes (PSGs) with the top 1% of both the π-ratio and FST values were extracted within the windows. A gene enrichment analysis was performed with g:profiler (Reimand et al., 2016). Benjamini–Hochberg FDR (false discovery rate) was used for correcting the P values. The enrichments for the Gene Ontology categories “molecular function”, “biological process” and “cellular component” and human phenotype (HP) categories were analyzed.
To survey advantageous alleles for chickens living in the tropical region, we further extracted allele counts for the non-synonymous variants of the PSGs among the 57 domestic chickens and used the chi-squared test to filter variants that showed significant differences in allele frequencies between the tropical and temperate populations. After filtration, 15 variants located within chromosomes 2 to 5 were retained (Supplementary Table S2).
Prediction of gga-mir-7468 Target Genes and Functional Annotation
The target genes of gga-mir-7468 were predicted using TargetScan (Agarwal et al., 2015) and miRDB (Chen and Wang, 2020). Based on the combination of the methods, only those supported by 2 programs were selected for further study. Enrichment analyses of target genes were performed with g:profiler (Reimand et al., 2016).
Sequence Retrieval and Structural Analysis
The protein sequences of thyroid-stimulating hormone receptor (TSHR) and carboxypeptidase Z (CPZ) were downloaded from Ensembl (https://asia.ensembl.org/index.html). Their protein architecture (i.e., domain organization) was examined using the Pfam sequence search engine (Mistry et al., 2021).
SNP Validation
A total of 142 individuals from 18 chicken breeds, including 7 breeds (Hetian chicken, Shouguang chicken, Luxi gamefowl, Gushi chicken, Nanjiang gamefowl, Hetian black chicken, and Tulufan gamefowl) from temperate regions and 11 breeds (Wenchang chicken, Huiyang chicken, Black Java chicken, Black Sumatra chicken, Kedu Hitam chicken, indigenous Sumatran breed, indigenous Yunnan breed, Merawang, indigenous Guangxi breed, indigenous Vietnamese breed, and Balinese fighting chicken) from tropical regions, were used to measure the allele frequency of 15 SNPs. Detailed information is listed in Supplementary Table S3. We only mapped sequencing reads to chicken chromosome 2 to chromosome 5 and called SNPs using the aforementioned methods and extracted allele counts of the 15 SNPs in each chicken population. A chi-squared test was used to measure the differences in allele frequencies between the tropical and temperate populations.
TSHR (41020238: G/A) Segregation Resource Population
The TSHR (41020238: G/A) segregation population was constructed by crossing between the 20 heterozygous Huainan roosters and 100 heterozygous Huainan hens (a Chinese indigenous breed with dual purpose). In total, 600 eggs were collected and incubated. After hatching, the chickens were wing-banded. Water and commercial chicken feed were supplied ad libitum. The diet contained 12.59 MJ/kg of metabolizable energy (ME) and 20.5% crude protein (CP) at 1 to 3 wk of age, 12.98 MJ/kg ME and 18.5% CP at 4 to 7 wk of age, and 11.75 MJ/kg ME and 17.0% CP thereafter. At 9 wk of age, blood samples from each individual were collected via the wing vein. Genomic DNA was extracted from blood using the phenol–chloroform method. The DNA concentration and quality were examined using gel electrophoresis and a NanoDrop 2000 spectrophotometer (Thermo Scientific, Wilmington, DE). Genotyping was performed using the PCR-Sanger sequencing method. Individual PCR amplification was performed with the primer F: 5’-TGGACCTCTACACCAGGTCA-3’&&R: 5’ACAGCCATCCTCTTGGCAAT-3’. The PCR conditions were as follows: 5 min at 94°C for one cycle and 30 s at 94°C, 30 s at 60°C, and 30 s at 72°C for 35 cycles. The PCR products were sequenced on an ABI 3730 sequencer (Applied Biosystems, Forster City, CA). From the PCR results we found 64 homozygous mutations (AA), 128 heterozygous mutations (AG), and 54 wild-type (GG) male chickens.
Thermal Stress of Chickens
At 140 d of age, 30 males Huainan chickens with similar body weights were selected from the AA (1796 ± 123 g) and GG groups (1,789 ± 130 g). Fifteen birds for each genotype were assigned to the control group and placed under normal temperature (20 ± 1°C). Fifteen birds from the 2 groups were assigned to the heat stress group and exposed to high temperatures (35 ± 1°C) for 12 h.
Serum Parameters
Blood samples of 60 birds were collected via the wing vein before and after the heat stress experiment and centrifuged at 4,000 × g for 15 min to separate the serum. The serum samples were stored at −20°C until analysis. The levels of serum total protein (TP), albumins (ALB), alanine aminotransferase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and creatine kinase (CK) were measured by the methods of biuret, bromocresol green, alanine substrate, 2-amino-2-methyl-1-propanol (AMP) buffer, lactic acid substrate, and N-acetylcysteine, respectively, using a Toshiba 120 automated biochemical analyzer (Toshiba, Tokyo, Japan).
Statistical Analysis
Serum biochemical parameters were analyzed by two-way ANOVA using R software (version 4.04) with stress temperature and genotype as the main effects, and their interactions were also analyzed. The Tukey method was used for comparisons among multiple means, and P < 0.05 was considered statistically significant.
RESULTS
Genomic Variants and Diversity
A total of 59 genomes were analyzed in this study (Supplementary Table S1). After quality filtering and mapping the reads to the chicken reference genome, we counted the genome sequencing depth for each sample, which ranged from 4.7 to 12.0 × . We identified ∼12.99 million biallelic SNPs. A total of 93.04% of the SNPs were located in intronic regions and intergenic regions (Supplementary Table S4), and 3.59% were located within regions upstream or downstream of the transcription start or end sites. Only 1.84% was assigned to exonic regions (Supplementary Table S4). To measure the genetic diversity for each population, we estimated the nucleotide diversity. We found that the chickens from the tropical regions (except Olagan chicken) had higher levels of nuclear diversity than the chickens from the temperate regions (Figure 1B).
Population Relationship and Structure
To investigate the genetic structure of these chicken populations, we performed a principal component analysis (PCA). The first principal component (PC1) explained more than 30.3% of the total variance and clearly distinguished tropical chickens from temperate chickens (Figure 1C). This analysis suggests genetic differentiation between the two groups of chickens. The genetic clustering analysis using ADMIXTURE also corroborates the pattern found with PCA (Supplementary Figure S1). When K = 2, we found a division between the tropical and temperate chickens, although tropical chickens appear to harbor a component that is found predominantly in temperate chickens (Supplementary Figure S1). To infer the phylogenetic relationships of these chickens, we further used autosomal SNPs to construct ML and NJ phylogenetic trees. Both the ML and NJ trees showed that chickens from the temperate region clustered together, and chickens from the tropical regions grouped separately (Figure 1D and Supplementary Figure S2).
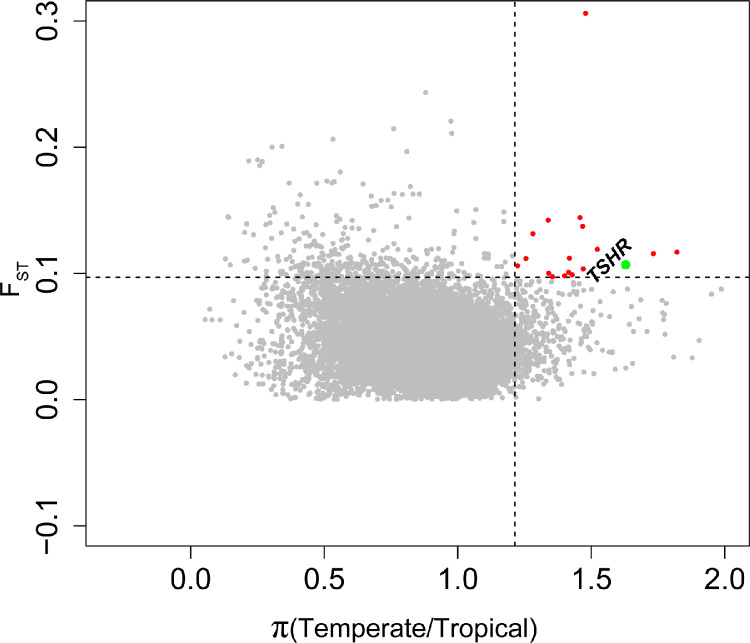
PSGs in Tropical Chickens
The above analysis showed a clear genetic differentiation between the tropical region and temperate chickens, which might be partially driven by location environments. To identify potential mechanisms for the adaptation to tropical temperature, we used FST and π-ratio to scan for footprints of positive selection in tropical region chickens, and a total of 301 and 389 PSGs were identified, respectively (Supplementary Tables S5 and S6). Thirty-four genes, including one minRNA (gga-mir-7468) and 6 long noncoding RNAs (lncRNAs), were detected by both statistics (Figure 2 and Supplementary Table S7). The functional enrichment analysis using protein-coding PSGs showed that these genes were associated with ‘sphingosine N-acyltransferase activity’ and ‘thyroid-stimulating hormone receptor activity’ (Supplementary Table S8). Among these PSGs, several genes (FABP2, RAMP3, SUGCT, CAMK2, and TSHR) are functionally related to metabolism and vascular smooth muscle contractility and could potentially be involved in the adaptation of chickens to hot environments.
Figure 2.
The putative selected genomic regions in tropical populations were identified using both fixation statistics (FST) and π-ratio (Temperate/Tropical) approaches with a sliding window strategy (50-kb windows with 25-kb steps).
We further predicted target genes of gga-mir-7468. A total of 319 genes were identified (Supplementary Table S9). There were no overlapping genes between the 319 target genes and 34 PSGs. The enrichment analyses showed that these target genes were involved in ‘metabolic process’ and ‘regulation of signal transduction’ (Supplementary Table S10).
Potential Causative Mutations in PSGs
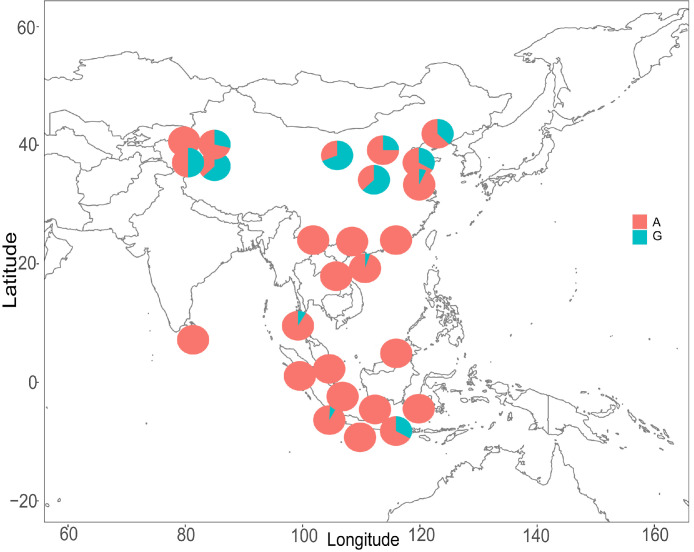
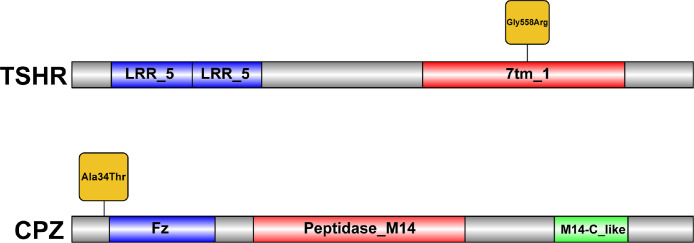
We screened for nonsynonymous variants among the PSGs across the tropical and temperate populations. After filtration, 15 variants remained, and they had significant differences in allele frequencies between the 2 populations (Supplementary Table S2). We further genotyped the 15 SNPs in 142 chickens from 18 breeds and found that 2 variants, TSHR (41020238:G>A, resulting in a Gly558Arg mutation) and CPZ (81175728:G > A, resulting in an Ala34Thr mutation), showed significant differences in allele frequencies between the tropical and temperate populations (Figure 3 and Supplementary Table S11). The protein domain prediction analysis revealed that the missense mutation in the TSHR gene c.41020238:G>A (p. Gly558Arg) is located in the 7tm_1 domain (Figure 4).
Figure 3.
Allele frequency distribution of TSHR (41020238:G>A) in domestic chickens from temperate (10 breeds) and tropical regions (16 breeds).
Figure 4.
Schematic representation of the thyroid stimulating hormone receptor (TSHR) and carboxypeptidase Z (CPZ) domain organization. LRR_5: leucine-rich repeat domain; 7tm_1: seven-(pass)-transmembrane domain receptors, also known as G protein-coupled receptors (GPCRs); Fz: cysteine-rich domain of CPZ; Peptidase_M14:peptidase M14-like domain of CPZ; M14-C_like: CPZ regulatory-like domain.
Blood Chemistry Parameters
We examined components of serum from both AA and GG group chickens that are both under normal temperature (20 ± 1°C) and high temperatures (35 ± 1°C). The effects of heat stress on the blood biochemistry parameters of AA and GG chickens are presented in Table 1. Neither heat stress nor genotype had an effect on the TP, ALB, or ALP concentrations (P > 0.05). Genotype had no effects on the ALT, LDH, or CK concentrations. However, heat stress significantly elevated the ALT, LDH, and CK concentrations in each group (P < 0.01). Moreover, after heat stress, wild-type chickens exhibited significantly higher concentrations of ALT, LDH, and CK than homozygous chickens (P < 0.05 or P < 0.01).
Table 1.
Effects of heat stress on serum biochemical parameters of homozygous mutations of TSHR and wild-type chickens.
| Parameter | AA1 |
GG1 |
Pvalue |
||||
|---|---|---|---|---|---|---|---|
| Standard2 | HS2 | Standard2 | HS2 | G2 | T2 | G*T | |
| TP (g/L) | 48.32 ± 3.94 | 47.00 ± 4.77 | 48.07 ± 4.67 | 48.30 ± 6.65 | 0.30 | 0.22 | 0.20 |
| ALB (g/L) | 21.10 ± 1.23 | 20.22 ± 1.54 | 21.87 ± 1.26 | 22.12 ± 2.25 | 0.19 | 0.17 | 0.87 |
| ALT (U/L) | 3.20 ± 0.72c | 7.10 ± 1.89b | 3.65 ± 0.65c | 12.20 ± 2.44a | 0.42 | 0.006 | 0.007 |
| ALP (U/L) | 798.44 ± 110.13 | 814.89 ± 134.94 | 811.56 ± 148.95 | 815.89 ± 134.83 | 0.27 | 0.30 | 0.41 |
| LDH (U/L) | 1,800.56 ± 259.5c | 2,043.22 ± 234.94b | 1,894.22 ± 248.94c | 2,832.56 ± 248.32a | 0.07 | 0.01 | 0.02 |
| CK (U/L) | 1,550.00 ± 261.7c | 2,379.22 ± 257.12b | 1,373.56 ± 142.75c | 2,968.33 ± 100.12a | 0.15 | 0.005 | 0.001 |
abcValues of each parameter in a row with different superscripts indicate significance.
Abbreviations: ALB, albumin; ALT, alanine transaminase; ALP, alkaline phosphatase; CK, creatine kinase; LDH, lactate dehydrogenase; TP, total protein.
AA: chickens with the TSHR (41020238:A/A) genotype; GG: chickens with the TSHR (41020238:G/G) genotype.
Standard: ambient temperature was 20 ± 1°C; HS: ambient temperature was 35 ± 1°C; G: genotype; T: temperature.
DISCUSSION
With rising global temperatures, heat stress triggered by high ambient temperature is the crucial factor challenging the development of poultry production. Genetic selection for heat-tolerant chicken breeds is an effective strategy for alleviating heat stress in the poultry industry. Since domestication, chickens have been translocated across the world and have adapted to a variety of local environments, such as hot and temperate conditions. To study the tropical adaptive mechanism of chickens, 3 indigenous Chinese chicken breeds from temperate zones and 5 indigenous chicken breeds from tropical regions were analyzed. We calculated π-ratio (Temperate/Tropical) and FST to identify the PSGs in chickens from tropical climates.
We found that CAMK2D (calcium/calmodulin-dependent protein kinase II delta) showed a signal of positive selection in tropical chickens. CAMK2 is expressed as 4 different isoforms (CAMK2α, CAMK2β, CAMK2d, and CAMK2γ) (Marganski et al., 2005; Saddouk et al., 2017) and plays an important role in the control of vascular smooth muscle contractility (Rokolya and Singer, 2000). The balance between vascular contraction and relaxation plays a pivotal role in body temperature homeostasis (Ai et al., 2015; Tian et al., 2020). Vasoconstriction has been linked to a reduction in peripheral blood flow leading to an increase in internal body temperature and vasodilatation. Increased cutaneous blood flow has been reported to increase heat loss (Klotz et al., 2016). The positively selected CAMK2D gene suggested that dissipating excessive heat through regulation of blood pressure to maintain the core body temperature is a crucial way for chickens from tropical regions to respond to heat stress.
We found several genes, including FABP2 (fatty acid-binding protein 2), RAMP3 (associated receptor-activity modifying protein 3), SUGCT (succinyl-CoA: glutarate-CoA transferase), and TSHR, which are functionally implicated in metabolism and energy production, to be potentially involved in adaptation to hot environments. This result is in agreement with a previous study (Tian et al., 2020) and in other animals (Ai et al., 2015; Kim et al., 2016). For example, energy metabolism-related genes (MYH1, MYH3, MYH8, MYH10, and MYH13) showed a signal of selection in sheep that may play an important role in their adaptation to hot arid environments (Kim et al., 2016). Similarly, Ramírez-Ayala et al. (2021) reported that positively selected genes involved in thermogenesis (ATP9A, GABBR1, PGR, PTPN1, and UCP1) may have crucial roles for French Charolais cattle in adaptation to Cuban tropical conditions. FABP2 is highly abundant in intestinal enterocytes (Agellon et al., 2002), which play a major role in the intracellular transportation of long-chain fatty acids and whole-body energy homeostasis (Gajda et al., 2013). SUGCT encodes an enzyme that synthesizes glutaryl-CoA from glutarate in tryptophan and lysine catabolism. A previous study showed that SUGCT-null mice exhibited imbalanced lipid and acylcarnitine metabolism (Niska-Blakie et al., 2020). RAMP3 is a critical member of the amylin receptor (Christopoulos et al., 1999) that plays an important role in glucose and energy homeostasis (Coester et al., 2020). TSHR belongs to the G protein-coupled receptor (GPCR) superfamily. Usually, TSH combines with TSH receptors to regulate growth and proliferation and plays essential roles in thyroid development and function (Zhou et al., 2018). Recently, many studies have demonstrated that TSHR may be involved in regulating energy balance, metabolism, and thermoregulation (Warner et al., 2013; Jiang et al., 2015; Lundbäck et al., 2020; Zhang et al., 2020). In particular, Wang et al. (2021) constructed chicken TSHR (Gly558Arg) knock-in mice and found that TSHR (Gly558Arg) homozygous mice had significantly lower energy expenditure and lower metabolism rates than wild-type mice, especially when fed under high-temperature conditions. Thus, we hypothesize that these energy metabolism-related genes may contribute to the chicken response and adaptation to hot temperature environments. Moreover, other mechanisms, such as epigenetic regulation, may also be involved in the tropical adaptation of chickens. A miRNA (gga-mir-7468) showed a signal of positive selection in tropical chickens. We further predicted target genes of gga-mir-7468 by using TargetScan (Agarwal et al., 2015) and miRDB (Chen and Wang, 2020) and identified 319 genes. There were no overlapping genes between the 319 target genes and 34 PSGs. Enrichment analyses showed that these target genes were involved in the “metabolic process”, suggesting that gga-mir-7468 is potentially involved in chicken adaptation to hot environments.
LncRNAs can be not only cis-acting lncRNAs that regulate the expression of target genes that are located at or near the same genomic locus but also trans-acting lncRNAs that can either inhibit or activate gene transcription at independent chromosomal loci (Fatica and Bozzoni, 2014). Among the 6 lncRNAs under selection in chickens from tropical climates, the location of ENSGALG00000047413 overlaps with TSHR. However, the function of both gga-mir-7468 and 6 lncRNAs is still unknown, and the role of these noncoding RNAs needs to be studied in the future.
We further searched for nonsynonymous variants among the PSGs found in the 57 sequenced chicken genomes. After filtration, 15 missense mutations in 6 genes remained. We next calculated the allele frequency in another 142 chickens representing both temperate and tropical populations and found only 2 variants with significant differences in allele frequency between the temperate and tropical populations: a missense mutation in TSHR (41020238:G>A, resulting in a Gly558Arg mutation) and a missense mutation in CPZ (81175728:G>A, resulting in an Ala34Thr mutation). CPZ is a member of the carboxypeptidase E subfamily of metallocarboxypeptidases that functions as a regulator in the development of skeletal elements in chickens (Moeller et al., 2003). CPZ was also reported to be associated with the aggressive behavior of gamecock chickens (Luo et al., 2020).
Based on the function of these candidate genes, we decided to evaluate whether the missense mutation in TSHR (41020238:G>A) is associated with heat tolerance in chickens because the gene has been well established to have biological significance in TSHR signaling for metabolism and thermoregulation (Warner et al., 2013; Lundbäck et al., 2020, Wang et al., 2021). We constructed TSHR (41020238:G/A)-segregating chicken populations and used AA and GG chickens to conduct heat stress experiments. Under thermoneutral conditions, there was no significant difference in blood biochemistry parameters between the 2 groups. However, when chickens were exposed to high temperature, GG chickens exhibited significantly higher concentrations of ALT, LDH, and CK than AA chickens. ALT enzymes were normally expressed in the liver. When the liver is damaged, ALT enzymes are released into the blood (Kim et al., 2015); therefore, the ALT level in the serum is used as a reliable and specific marker of liver damage (Chang et al., 2020). Heat stress is known to induce liver damage (Zeng et al., 2014) and increase serum ALT levels (Chang et al., 2020; Roushdy et al., 2020). Similarly, under normal conditions, LDH and CK were mainly present inside cardiomyocytes but could be released when the cardiomyocytes were damaged (Yin et al., 2020); thus, the contents of serum can be used as important indicators for myocardial damage (Tang et al., 2018; Xu et al., 2019). Previous studies have revealed that heat stress increases the activities of serum CK and LDH (Xie et al., 2015; Luo et al., 2018). In the present study, the ALT, LDH, and CK levels in chickens with homozygous TSHR mutations were lower than those in the wild-type group, indicating less cell damage in homozygous chickens under exposure to heat stress conditions.
CONCLUSIONS
In conclusion, our results suggest that the TSHR (41020238:G>A) variation might regulate the metabolic rate of chickens to enhance heat tolerance and contribute to chicken adaptation to high ambient temperature conditions in tropical climates. However, further studies are necessary to uncover the molecular basis and networks of TSHR (41020238:G>A) in the regulation of the heat stress response of chickens. Overall, our study provides insight into the tropical climates of chickens and will contribute to the molecular breeding of heat-tolerant chicken breeds.
ACKNOWLEDGMENTS
This study was supported by the Natural Science Foundation of Anhui Province (grant no. 1908085QC143), the National Natural Science Foundation of China (31801054 and 31771415), the Natural Science Foundation for Young Scholars of Anhui Agricultural University (yj2018-51) and the China Agriculture Research System of MOF and MARA (CARS-41).
DISCLOSURES
The authors declare that they have no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.101821.
Appendix. Supplementary materials
REFERENCES
- Agarwal V., Bell G.W., Nam J.W., Bartel D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4:e05005. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agellon L.B., Toth M.J., Thomson A.B.R. Intracellular lipid binding proteins of the small intestine. Mol. Cell. Biochem. 2002;239:79–82. [PubMed] [Google Scholar]
- Ai H.S., Fang X.D., Yang B., Huang Z.Y., Chen H., Mao L.K., Zhang F., Zhang L., Cui L.L., He W.M., Yang J., Yao X.M., Zhou L.S., J.Han L., Li J., Sun S.L., Xie X.H., Lai B.X, Su Y., Lu Y., Yang H., Huang T., Deng W.J, Nielsen R., Ren J., Huang L.S. Adaptation and possible ancient interspecies introgression in pigs identified by whole-genome sequencing. Nat. Genet. 2015;47:217–225. doi: 10.1038/ng.3199. [DOI] [PubMed] [Google Scholar]
- Beckford R.C., Ellestad L.E., Proszkowiec-Weglarz M., Farley L., Brady K., Angel R., Liu H.C., Porter T.E. Effects of heat stress on performance, blood chemistry, and hypothalamic and pituitary mRNA expression in broiler chickens. Poult. Sci. 2020;99:6317–6325. doi: 10.1016/j.psj.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cândido M.G.L., Tinôco I.F.F., Albino L.F.T., Freitas L.C.S.R., Santos T.C., Cecon P.R., Gates R.S. Effects of heat stress on pullet cloacal and body temperature. Poult. Sci. 2020;99:2469–2477. doi: 10.1016/j.psj.2019.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q.Q., Lu Y.Q., Lan R.X. Chitosan oligosaccharide as an effective feed additive to maintain growth performance, meat quality, muscle glycolytic metabolism, and oxidative status in yellow-feather broilers under heat stress. Poult. Sci. 2020;99:4824–4831. doi: 10.1016/j.psj.2020.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.H., Wang X.W. miRDB: an online database for prediction of functional microRNA targets. Nucleic. Acids. Res. 2020;48:D127–D131. doi: 10.1093/nar/gkz757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos G., Perry K.J., Morfis M., Tilakaratne N., Gao Y., Fraser N.J., Main M.J., Foord S.M., Sexton P.M. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol. Pharmacol. 1999;56:235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- Coester B., Pence S.W., Arrigoni S., Boyle C.N., Foll C.Le, Lutz T.A. RAMP1 and RAMP3 differentially control Amylin's effects on food intake, glucose and energy balance in male and female mice. Neuroscience. 2020;447:74–93. doi: 10.1016/j.neuroscience.2019.11.036. [DOI] [PubMed] [Google Scholar]
- Danecek P., Auton A., Abecasis G., Albers C.A., Banks E., DePristo M.A., Handsaker R.E., Lunter G., Marth G.T., Sherry S.T., McVean G., Durbin R. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkoh A. Ambient temperature: a factor affecting performance and physiological response of broiler chickens. Int. J. Biometeorol. 1989;33:259–265. doi: 10.1007/BF01051087. [DOI] [PubMed] [Google Scholar]
- Fatica A., Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat. Rev. Genet. 2014;15:7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- Gajda A.M., Zhou Y.X., Agellon L.B., Fried S.K., Kodukula S., Fortson W., Patel K., Storch J. Direct comparison of mice null for liver or intestinal fatty acid-binding proteins reveals highly divergent phenotypic responses to high fat feeding. J. Biol. Chem. 2013;288:30330–30344. doi: 10.1074/jbc.M113.501676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory N.G. How climatic changes could affect meat quality. Food. Res. Int. 2010;43:1866–1873. [Google Scholar]
- Guo X., Fang Q., Ma C.D., Zhou B.Y., Wan Y., Jiang R.S. Whole-genome resequencing of Xishuangbanna fighting chicken to identify signatures of selection. Genet. Sel. Evol. 2016;48:62. doi: 10.1186/s12711-016-0239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.L., Luo Q.H., Xiao F., Lin X., Spears J.W. Research note: responses of growth performance, immune traits, and small intestinal morphology to dietary supplementation of chromium propionate in heat-stressed broilers. Poult. Sci. 2020;99:5070–5073. doi: 10.1016/j.psj.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D.Q., Ma S.Z., Jing F., Xu C., Yan F., Wang A.H., Zhao J.J. Thyroid-stimulating hormone inhibits adipose triglyceride lipase in 3T3-L1 adipocytes through the PKA pathway. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.W., Mushtaq M.M.H., Parvin R., Kang H.K., Kim J.H., Na J.C., Hwangbo J., Kim J.D., Yang C.B., Park B.J., Choi C.H. Various levels and forms of dietary alpha-lipoic acid in broiler chickens: impact on blood biochemistry, stress response, liver enzymes, and antibody titers. Poult Sci. 2015;94:226–231. doi: 10.3382/ps/peu056. [DOI] [PubMed] [Google Scholar]
- Kim E.S., Elbeltagy A.R., Aboul-Naga A.M., Rischkowsky B., Sayre B., Mwacharo J.M., Rothschild M.F. Multiple genomic signatures of selection in goats and sheep indigenous to a hot arid environment. Heredity. 2016;116:255–264. doi: 10.1038/hdy.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J.L., Aiken G.E., Bussard J.R., Foote A.P., Harmon D.L., Goff B.M., Schrick F.N., Strickland J.R. Vasoactivity and vasoconstriction changes in cattle related to time off toxic endophyte-infected tall fescue. Toxins. 2016;8:27. doi: 10.3390/toxins8100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y. Btrim: a fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics. 2011;98:152–153. doi: 10.1016/j.ygeno.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari K.N.R, Nath D.N. Ameliorative measures to counter heat stress in poultry. Worlds. Poult. Sci. J. 2019;74:117–130. [Google Scholar]
- Li G.M., Liu L.P., Yin B., Liu Y.Y., Dong W.W., Gong S., Zhang J., Tan J.H. Heat stress decreases egg production of laying hens by inducing apoptosis of follicular cells via activating the FasL/Fas and TNF-alpha systems. Poult. Sci. 2020;99:6084–6093. doi: 10.1016/j.psj.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Toward better understanding of artifacts in variant calling from high-coverage samples. Bioinformatics. 2014;30:2843–2851. doi: 10.1093/bioinformatics/btu356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.L., Ren M.Y., Ren K., Jin Y.C., Yan M.L. Heat stress impacts on broiler performance: a systematic review and meta-analysis. Poult. Sci. 2020;99:6205–6211. doi: 10.1016/j.psj.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyau T., Berri C., Bedrani L., M´etayer-Coustard S., Praud C., Duclos M.J., Tesseraud S., Rideau N., Everaert N., Yahav S., Mignon-Grasteau S., Collin A. Thermal manipulation of the embryo modifies the physiology and body composition of broiler chickens reared in floor pens without affecting breast meat processing quality. J. Anim. Sci. 2013;91:3674–3685. doi: 10.2527/jas.2013-6445. [DOI] [PubMed] [Google Scholar]
- Lundbäck V., Kulyté A., Dahlman I., Marcus C. Adipose-specific inactivation of thyroid stimulating hormone receptors in mice modifies body weight, temperature and gene expression in adipocytes. Physiol. Rep. 2020;8:e14538. doi: 10.14814/phy2.14538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J.X., Song J., Liu L.Z., Xue B., Tian G.M., Yang Y. Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poult. Sci. 2018;97:599–606. doi: 10.3382/ps/pex353. [DOI] [PubMed] [Google Scholar]
- Luo W., Luo C.L., Wang M., Guo L.J., Chen X.L., Li Z.H., Zheng M., Folaniyi B.S., Luo W., Shu D.M., Song L.L., Fang M.X., Zhang X.Q., Qu H., Nie Q.H. Genome diversity of Chinese indigenous chicken and the selective signatures in chinese gamecock chicken. Sci. Rep. 2020;10:14532. doi: 10.1038/s41598-020-71421-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B.B., Zhang L., Li J.L., Xing T., Jiang Y., Gao F. Heat stress alters muscle protein and amino acid metabolism and accelerates liver gluconeogenesis for energy supply in broilers. Poult. Sci. 2021;100:215–223. doi: 10.1016/j.psj.2020.09.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marganski W.A., Gangopadhyay S.S., Je H.D., Gallant C., Morgan K.G. Targeting of a novel Ca2/Calmodulin-dependent protein kinase II is essential for extracellular signal-regulated kinase–mediated signaling in differentiated smooth muscle cells. Circul. Res. 2005;97:541–549. doi: 10.1161/01.RES.0000182630.29093.0d. [DOI] [PubMed] [Google Scholar]
- McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., DePristo M.A. The Genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome. Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry J., Chuguransky S., Williams L., Qureshi M., Salazar G.A., Sonnhammer E.L.L., Tosatto S.C.E., Paladin L., Raj S., Richardson L.J., Finn R.D., Bateman A. Pfam: the protein families database in 2021. Nucleic. Acids. Res. 2021;49:D412–D419. doi: 10.1093/nar/gkaa913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller C., Swindell E.C., Kispert A., Eichele G. Carboxypeptidase Z (CPZ) modulates Wnt signaling and regulates the development of skeletal elements in the chicken. Development. 2003;130:5103–5111. doi: 10.1242/dev.00686. [DOI] [PubMed] [Google Scholar]
- NiskaBlakie1 J., Gopinathan L., Low K.N., Kien Y.L., Goh C.M.F., Caldez M.J., Pfeiffenberger E., Jones O.S., Ong C.B., Kurochkin I.V., Coppola V., Tessarollo L., Choi H., Kanagasundaram Y., Eisenhaber F., MaurerStroh S., Kaldis P. Knockout of the nonessential gene SUGCT creates dietlinked,agerelated microbiome disbalance with a diabeteslike metabolic syndrome phenotype. Cell. Molec. Life. Sci. 2020;77:3423–3439. doi: 10.1007/s00018-019-03359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M.N., Dehal P.S., Arkin A.P. FastTree 2-approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinteiro-Filho W.M., Rodrigues M.V., Ribeiro A., Ferraz-dePaula V., Pinheiro M.L., Sa L.R.M., Ferreira A.J.P., Palermo-Neto J. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: role of acute hypothalamic-pituitary-adrenal axis activation. J. Anim. Sci. 2012;90:1986–1994. doi: 10.2527/jas.2011-3949. [DOI] [PubMed] [Google Scholar]
- RamírezAyala L.C, Rocha D., RamosOnsins S.E., LenoColorado J., Charles M., Bouchez O., RodríguezValera Y., PérezEnciso M., RamayoCaldas Y. Wholegenome sequencing reveals insights into the adaptation of French charolais cattle to Cuban tropical conditions. Genet. Sel. Evol. 2021;53:3. doi: 10.1186/s12711-020-00597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimand J., Arak T., Adler P., Kolberg L., Reisberg S., Peterson H., Vilo J. g:Profiler-a web server for functional interpretation of gene lists (2016 update) Nucleic Acids Res. 2016;44:W8. doi: 10.1093/nar/gkw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokolya A., Singer H.A. Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. Am. J. Physiol.Cell. Physiol. 2000;278:C537–C545. doi: 10.1152/ajpcell.2000.278.3.C537. [DOI] [PubMed] [Google Scholar]
- Roushdy E.M., Zaglool A.W., Hassan F.A.M. Thermal stress consequences on growth performance, immunological response, antioxidant status, and profitability of finishing broilers: transcriptomic profile change of stress-related genes. Trop. Anim. Health. Prod. 2020;52:3685–3696. doi: 10.1007/s11250-020-02405-4. [DOI] [PubMed] [Google Scholar]
- Saddouk F.Z., Ginnan R., Singer H.A. Ca2+/Calmodulin-dependent protein kinase II in vascular smooth muscle. Adv. Pharmacol. 2017;78:171–202. doi: 10.1016/bs.apha.2016.08.003. [DOI] [PubMed] [Google Scholar]
- St-Pierre N.R., Cobanov B., Schnitkey G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003;86:E52–E77. [Google Scholar]
- Sugiharto S., Yudiarti T., Isroli I., Widiastuti E., Kusumanti E. Dietary supplementation of probiotics in poultry exposed to heat stress–a review. Ann. Anim. Sci. 2017;17:591–604. [Google Scholar]
- Tang S., Yin B., Xu J., Bao E. Rosemary reduces heat stress by inducing CRYAB and HSP70 expression in broiler chickens. Oxid. Med. Cell. Longev. 2018. 2018 doi: 10.1155/2018/7014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S.L., Zhou X.M., Phuntsok T., Zhao N., Zhang D.J., Ning C.Y., Li D.Y., Zhao H.B. Genomic analyses reveal genetic adaptations to tropical climates in chickens. Iscience. 2020;23 doi: 10.1016/j.isci.2020.101644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfah M., Kawahara-Miki R., Farajalllah A., Muladno M., Dorshorst B., Martin A., Kono T. Genetic features of red and green junglefowls and relationship with Indonesian native chickens Sumatera and Kedu Hitam. BMC Genomics. 2016;17:320. doi: 10.1186/s12864-016-2652-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walugembe M., Bertolini F., Dematawewa C.M.B., Reis M.P., Elbeltagy A.R., Schmidt C.J., Lamont S.J., Rothschild M.F. Detection of selection signatures among Brazilian, Sri Lankan, and Egyptian chicken populations under different environmental conditions. Front. Genet. 2019;9:737. doi: 10.3389/fgene.2018.00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.S., Li Y., Peng M.S., Zhong L., Wang Z.J., Li Q.Y., Tu X.L., Dong Y., Zhu C.L., Wang L., Yang M.M., Wu S.F., Miao Y.W., Liu J.P., Irwin D.M., Wang W., Wu D.D., Zhang Y.P. Genomic analyses reveal potential independent adaptation to high altitude in tibetan chickens. Mol. Biol. Evol. 2015;32:1880–1889. doi: 10.1093/molbev/msv071. [DOI] [PubMed] [Google Scholar]
- Wang M.S., Otecko N.O., Wang S., Wu D.D., Yang M.M., Xu Y.L., Murphy R.W., Peng M.S., Zhang Y.P. An evolutionary genomic perspective on the breeding of dwarf chickens. Mol. Biol. Evol. 2017;34:3081–3088. doi: 10.1093/molbev/msx227. [DOI] [PubMed] [Google Scholar]
- Wang M.S., Thakur M., Peng M.S., Jiang Y., Frantz L.A.F., Li M., Zhang J.J., Wang S., Peters J., Otecko N.O., Suwannapoom C., Guo X., Zheng Z.Q., Esmailizadeh A., Hirimuthugoda N.Y., Ashari H., Suladari S., Zein M.S.A., Kusza S., Sohrabi S., Kharrati-Koopaee H., Shen Q.K., Zeng L., Yang M.M., Wu Y.J., Yang X.Y., Lu X.M., Jia X.Z., Nie Q.H., Lamont S.J., Lasagna E., Ceccobelli S., Gunwardana H., Senasige T.M., Feng S.H., Si J.F., Zhang H., Jin J.Q., Li M.L., Liu Y.H., Chen H.M., Ma C., Dai S.S., Bhuiyan A., Khan M.S., Silva G., Le T.T., Mwai O.A., Ibrahim M.N.M., Supple M., Shapiro B., Hanotte O., Zhang G., Larson G., Han J.L., Wu D.D., Zhang Y.P. 863 genomes reveal the origin and domestication of chicken. Cell. Res. 2020;30:693–701. doi: 10.1038/s41422-020-0349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.S., Zhang J.J., Guo X., Li M., Meyer R., Ashari H., Zheng Z.Q., Wang S., Peng M.S., Jiang Y., Thakur M., Suwannapoom C., Esmailizadeh A., Hirimuthugoda N.Y., Zein M.S.A., Kusza S., Kharrati-Koopaee H., Zeng L., Wang Y.M., Yin T.T., Yang M.M., Li M.L., Lu X.M., Lasagna E., Ceccobelli S., Gunwardana H.G.T.N., Senasig T.M., Feng S.H., Zhang H., Bhuiyan A.K.F.H., Khan M.S., Silva G.L.L.P., Thuy L.T., Mwai O.A., Ibrahim M.N.M., Zhang G.J., Qu K.X., Hanotte O., Shapiro B., Bosse M., Wu D.D., Han J.L., Zhang Y.P. Large-scale genomic analysis reveals the genetic cost of chicken domestication. BMC. Biol. 2021;19:118. doi: 10.1186/s12915-021-01052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner A., Rahman A., Solsjo P., Gottschling K., Davis B., Vennstrom B., Arner A., Mittag J. Inappropriate heat dissipation ignites brown fat thermogenesis in mice with a mutant thyroid hormone receptor α1. Proc. Natl. Acad. Sci. U. S. A. 2013;110:16241–16246. doi: 10.1073/pnas.1310300110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J.J., Tang L., Lu L., Zhang L.Y., Lin X., Liu H.C., Odle L., Luo X.G. Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders. Poult. Sci. 2015;94:1635–1644. doi: 10.3382/ps/pev105. [DOI] [PubMed] [Google Scholar]
- Xu J., Yin B., Huang B., Tang S., Zhang X., Sun J., Bao E. Co-enzyme Q10 protects chicken hearts from in vivo heat stress via inducing HSF1 binding activity and Hsp70 expression. Poult. Sci. 2019;98:1002–1011. doi: 10.3382/ps/pey498. [DOI] [PubMed] [Google Scholar]
- Yang J.A.S., Lee H., Goddard M.E., Visscher P.M. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin B., Di L.J., Tang S., Bao E.D. Vitamin C-Na enhances the antioxidant ability of chicken myocardium cells and induces heat shock proteins to relieve heat stress injury. Res. Vet. Sci. 2020;133:124–130. doi: 10.1016/j.rvsc.2020.09.008. [DOI] [PubMed] [Google Scholar]
- Zaboli G., Huang X., Feng X., Ahn D.U. How can heat stress affect chicken meat quality? -a review. Poult. Sci. 2019;98:1551–1556. doi: 10.3382/ps/pey399. [DOI] [PubMed] [Google Scholar]
- Zeng T., Li J.J., Wang D.Q., Li G.Q., Wang G.L., Lu L.Z. Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of muscovy and pekin ducks: evidence for differential thermal sensitivities. Cell. Stress. Chaperones. 2014;19:895–901. doi: 10.1007/s12192-014-0514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.M., Wu H.X., Ma S.Z., Gao L., Yu C.X., Jing F., Zhao J.J. TSH promotes adiposity by inhibiting the browning of white fat. Adipocyte. 2020;9:264–278. doi: 10.1080/21623945.2020.1783101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L.Y., Wu K.P., Zhang L.Y., Gao L., Chen S.H. Liver-specific deletion of TSHR inhibits hepatic lipid accumulation in mice. Biochem. Biophys. Res. Commun. 2018;497:39–45. doi: 10.1016/j.bbrc.2018.01.187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.