Abstract
Mycoplasma synoviae (MS) is an important avian pathogen that has brought substantial economic losses to the global poultry industry. Fast and accurate diagnosis is one of the critical factors for the control of MS infection. This study established a simple, rapid and visual detection method for MS using a recombinase-aided amplification (RAA) combined with a lateral flow dipstick (LFD). The reaction temperature and time of the RAA-LFD assay were optimized after selecting the primers and probe, and the specificity and sensitivity rates were analyzed. The results showed that RAA could amplify the target gene in 20 min at a constant temperature of 38°C, and the amplification products could be visualized by LFD within 5 min. There was no cross-reaction with Mycoplasma gallisepticum (MG), Pasteurella multocida (P. multocida), Escherichia coli (E. coli), Newcastle disease virus (NDV), infectious bursal disease virus (IBDV), infectious bronchitis virus (IBV), and avian reovirus (ARV). Furthermore, the RAA-LFD assay exhibited high sensitivity with a detection limit of 10 copies/μL. A total of 128 clinical samples with suspected infection of MS were tested by RAA-LFD, PCR, and real-time fluorescence quantitative PCR (RFQ-PCR). The coincidence rate of the detection results was 95.3% between RAA-LFD and PCR, and 98.4% between RAA-LFD and RFQ-PCR. These results suggested that the RAA-LFD method established in the present study was easy to use and was associated with strong specificity and high sensitivity. This method was very suitable for the rapid detection of MS in clinical practice.
Key words: Mycoplasma synoviae, recombinase-aided amplification, lateral flow dipstick, PCR, real-time fluorescence quantitative PCR
INTRODUCTION
Mycoplasma synoviae (MS) is a major avian pathogen that causes synovitis, airsacculitis and respiratory disease in chickens and turkeys, resulting in stunted growth, downgrading at slaughter, and reduced egg production (Dufour-Gesbert et al., 2006; Felice et al., 2020). Moreover, it may present as a mixed infection with other avian pathogens such as Mycoplasma gallisepticum (MG), avian reovirus (ARV), Escherichia coli (E. coli), and infectious bronchitis virus (IBV), thereby causing more severe diseases (Huang et al., 2015; Derksen et al., 2018; Abdelaziz et al., 2019). Since it was first reported in America in the 1950s, MS has been one of the main pathogens in commercial poultry worldwide and has led to severe economic losses (Sun et al., 2017). Given that MS is widely prevalent in native chickens in China, effective measures should be implemented to control its spread (Xue et al., 2017).
The rapid and accurate detection of MS is essential for the prevention and control of the disease. At present, common methods for MS detection include bacteriological culture, serological tests such as ELISA, and molecular approaches, including conventional PCR and real-time fluorescence quantitative PCR (RFQ-PCR) (Moreira et al., 2015; Dijkman et al., 2017; Kuo et al., 2017). Although reliable and accurate, these methods are complex, time-consuming, and rely on specialized equipment and professional technicians, indicating that they are unsuitable for under-equipped laboratories or fields. To overcome these drawbacks, isothermal nucleic acid amplification methods have been developed for MS detection, such as polymerase spiral reaction (PSR) and loop-mediated isothermal amplification (LAMP) (Kursa et al., 2015; Wu et al., 2019). The amplified products of PSR and LAMP can be observed by the naked eye without any instrument. However, PSR and LAMP still require 40 to 60 min to complete the amplification, and LAMP needs 6 complementary primers which are difficult to design.
Recombinase-aided amplification (RAA) is a novel isothermal nucleic acid amplification technique that is simple, fast, and accurate (He et al., 2021; Wang et al., 2021). RAA assay contains three significant proteins: recombinase, single-strand DNA binding protein (SSB) and DNA polymerase. During the RAA process, recombinase in the reaction system combines with the primers to form a complex and search for the homologous sequence in the DNA template. Once the homologous sequence is located, a chain exchange reaction occurs and the SSB binds to the single strand DNA, then the DNA amplification is initiated by DNA polymerase (Tu et al., 2021). RAA can complete the gene amplification at a constant temperature (37–42°C) in a short time (20–30 min), and if a specific probe is added into the reaction solution, the product can be easily visualized by a lateral flow dipstick (LFD) (Zheng et al., 2021). In recent years, RAA-LFD has been successfully used to detect numerous human and animal pathogens (Fan et al., 2019; Wang et al., 2020). In the present study, a rapid RAA-LFD assay with stong specificity and high sensitivity for the visual detection of MS was established, providing a new approach for clinical diagnosis or field evaluation of MS.
MATERIALS AND METHODS
Bacterias,Virus and Clinical Samples
MS strain GX11-T and MG strain NB72 were purchased from the China Veterinary Culture Collection Center. Three MS clinical isolated strains, YC03, XS05, and XS12, were provided by Yancheng Engineering Research Center of Animal Biologics. Pasteurella multocida (P. multocida) strain BZ and E. coli strain YT were purchased from Huahong Biological Technology Co., Ltd., Shandong, China. Newcastle disease virus (NDV) strain LaSota, infectious bursal disease virus (IBDV) strain B87 and IBV strain H120 were purchased from Harbin Pharmaceutical Group Bio-vaccine Co., Ltd., China. ARV strain 1733 was purchased from Shandong Sinder Technology Co., Ltd., China. A total of 128 throat samples were taken from MS-suspected infection chickens with sterile swabs and placed in PBS buffer, then transported back to the laboratory immediately. All the materials were stored at −80°C.
Extraction of Nucleic Acid
The DNA templates of MS, MG, P. multocida, E.coli and clinical samples were extracted following the instructions of the DNA extraction kit (Beijing Tiangen Biotech Co., Ltd., China). The RNA of NDV, IBDV, IBV, and ARV were extracted by viral RNA extraction kit (Takara Biotech Co., Ltd., Beijing, China), and reversely transcribed into cDNA by reverse transcription kit (Takara Biotech Co., Ltd., Beijing, China). All the DNA/cDNA samples were stored at −20°C.
Design and Screening of Primers
Three primer pairs (Table 1) were designed based on the conserved sequence of the VlhA gene of MS (GenBank accession number: MH679867.1), in accordance with the instructions of RAA (Basic) Kit (Weifang Amp-Future Biotech Co., Ltd., Shandong, China). The RAA reaction system (50 μL) was prepared with buffer A, 29.4 μL; forward (10 μM) and reverse (10 μM) primers, 2 μL each; DNA template, 1 μL; sterile water, 13.1 μL; buffer B, 2.5 μL. The DNA extracted from MS strain GX11-T was used as the amplification template. For negative controls, DNA template was replaced by 1 μL sterile water. After 30 min incubation in a water bath at 38°C, the RAA product was purified with phenol-chloroform and detected by 2% agarose gel electrophoresis. The optimal primer pair was selected for the RAA-LFD assay.
Table 1.
Primers and probes used in this work.
| Primers/probes | Sequences (5’-3’) | Position | Product size (bp) |
|---|---|---|---|
| MS F1 | TGTTATAGCAATTTCATGTGGTGATCAAAC | 60–89 | 171 |
| MS R1 | ACTGTACCACCTCCTGGGTTTCCTGGATTT | 201–230 | |
| MS F2 | ACCTGAACCAACACCTGGAAACCCAAATAC | 96–125 | 151 |
| MS R2 | AGCCTCTACAGGGTCAACTGTACCACCTCC | 217–246 | |
| MS F3 | ATTACTATTAGCAGCTAGTGCAGTGGCCAT | 21–50 | 160 |
| MS R3 | ACCTGGATTTCCTGGAGTACCTGGATTTCC | 151–180 | |
| MS R3b | Biotin-ACCTGGATTTCCTGGAGTACCTGGATTTCC | 151–180 | |
| Probe 1 | FAM-TCATGTGGTGATCAAACTCCAGCACCTGAAC-THF-AA CACCTGGAAACCC-C3 spacer |
73–119 | |
| Probe 2 | FAM-TTATAGCAATTTCATGTGGTGATCAAACTCC-THF-GCA CCTGAACCAACA-C3 spacer |
62–108 |
Design and Screening of Probe
The optimal primer pair screened above was MS F3/MS R3, with which the RAA product exhibited a clear and bright band of the expected size in the agarose gel. Then the 5’ end of reverse primer was labeled with biotin and named MS R3b, and two probes (Table 1) were designed based on the instructions of the RAA (LFD) Kit (Weifang Amp-Future Biotech Co., Ltd., Shandong, China). For each probe, the 5’ end was labeled with a FAM fluorophore, and a tetrahydrofuran (THF) site was put in the middle of the probe, while the 3’ end was blocked by a C3 phosphorylation spacer. To test the potential false-positive signals on the dipstick produced by primer-probe complex, each probe was validated by RAA-LFD assay without adding MS DNA template in the RAA reaction.
Establishment of RAA-LFD Assay
In accordance with instructions of the RAA (LFD) Kit (Weifang Amp-Future Biotech Co., Ltd., Shandong, China), the RAA reaction system (50 μL) was prepared with buffer A, 29.4 μL; forward primer (10 μM) 2 μL; reverse primer (10 μM) 2 μL; probe (10 μM), 0.6 μL; DNA template, 1 μL; sterile water, 12.5 μL; buffer B, 2.5 μL. The DNA extracted from MS strain GX11-T was used as the positive control. For negative controls, DNA template was replaced by 1 μL sterile water. After 20 min of incubation at 38°C, 10 μL of the amplification product was used for LFD (Milenia Biotech, Co., Ltd., Giessen, Germany) detection. The amplification product was added to a 90 μL running buffer in the tube, and the dipstick was inserted into the diluted solution for 5 min before observation. The dipstick showed a positive result when the control band and test band were both visible. If only the control band were visible, the result was regarded as negative.
Optimization of RAA-LFD Reaction Conditions
The DNA extracted from MS strain GX11-T was used as the positive control for the RAA-LFD assay. The reaction temperature of the RAA assay was maintained at 26, 30, 34, 38, and 42°C for 15 min, respectively. The reaction time of the RAA assay was set to 10, 15, 20, 25, and 30 min at the optimal temperature. All the RAA products were detected with LFD as described above.
Specificity Test
Under the abovementioned optimized conditions, the DNA/cDNA of MS, MG, P. multocida, E. coli, NDV, IBDV, IBV, and ARV were used as the templates for the RAA-LFD assay, and sterile water was instead of the template as a negative control.
Construction of Standard Plasmid
The lengths of the optimal primer pair MS F3/MS R3 were modified according to the requirements of PCR, F:5’-ATTACTATTAGCAGCTAGTGCA-3’, R:5’-ACCTGGATTTCCTGGAGTACCTGG-3’. The modified primers were used to amplify the DNA fragment by a PCR kit (Vazyme Biotech Co., Ltd., Nanjing, China) with the following reaction system (50 μL): 2 × Rapid Taq Master Mix, 25 μL; forward (10 μM) and reverse (10 μM) primers, 2 μL each; DNA template, 1 μL; sterile water, 20 μL. The DNA extracted from MS strain GX11-T was used as the positive control. The PCR procedure consisted of a pre-denaturation at 95°C for 3 min; 30 cycles of denaturation at 95°C for 15 s, annealing at 58°C for 15 s and extension at 72°C for 10 s; then a final extension at 72° for 5 min. The amplified product was purified with the DNA fragments purification kit (Beyotime Biotechnology Co., Ltd., Shanghai, China) and ligated to pUCm-T Vector (Beyotime Biotechnology Co., Ltd., Shanghai, China). The resulting plasmid pUCm-VlhA was quantified with a spectrophotometer at 260 nm, and its copy number was calculated as the following formula: plasmid copy number (copies/μL) = [plasmid concentration (g/μL) × 6.02 × 1023/plasmid length (bp) × 660 g/mol]. Ten-fold dilutions of the plasmid pUCm-VlhA with the gradient ranging from 107 to 100 copies/μL were prepared and stored at −20°C.
Sensitivity Tests of RAA-LFD, PCR, and RFQ-PCR
The serial dilutions of plasmid pUCm-VlhA ranging from 107 to 100 copies/μL were used as the templates to test the sensitivity of RAA-LFD, PCR, and RFQ-PCR. The RAA-LFD assay was performed according to the optimal conditions. For PCR, the primers, reaction system and procedure were the same as constructing the standard plasmid. The RFQ-PCR assay was carried out by a TB Green qPCR kit (Takara Biotech Co., Ltd., Beijing, China) using the same primers as PCR. The RFQ-PCR reaction system (25 μL) consisted of the following: qPCR Mix, 12.5 μL; forward (10 μM) and reverse (10 μM) primers, 1 μL each; template, 1 μL; sterile water, 9.5 μL. The reaction solution was predenaturalized at 95°C for 30 s, then denaturalized at 95°C for 15 s and annealed at 58°C for 5 s, a total of 40 cycles. The sensitivity tests of these three methods were repeated for 3 times.
Detection of Clinical Samples
A total of 128 laryngeal swabs were collected from chickens with suspected MS infection, which showed symptoms of joint enlargement, movement disorder, breathing difficulty, stunted growth or decreased egg production. The chickens were from 6 layer farms in Jiangsu Province, China, and had not been injected with any MS or MG vaccine. All these samples were detected by RAA-LFD, PCR, and RFQ-PCR.
RESULTS
Screening of Primers and Probe
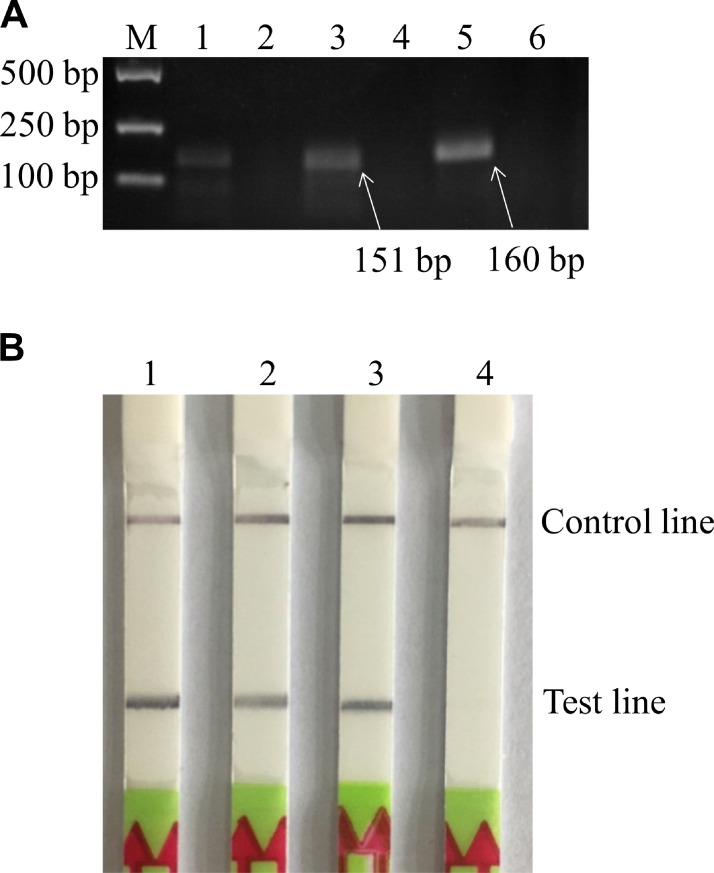
Three pairs of primers targeting MS VlhA gene were designed for the RAA assay. The RAA products were purified and visualized by 2% agarose gel electrophoresis. The results showed that MS F2/MS R2 and MS F3/MS R3 both generated a prominent band of the expected size; however, the band from MS F3/MS R3 was brighter and clearer (Figure 1A). Thus, MS F3/MS R3 was selected as the optimal primer pair for the following experiments. Then, 2 probes were designed and tested by the RAA-LFD assay with MS F3/MS R3b to avoid the potential false-positive signals on LFD. The results revealed that both primer-probe sets showed test bands on dipsticks in positive reactions; however, in no-template negative controls, the test line position from MS F3/MS R3b/Probe 1 showed a false-positive band, while there was no test band for the MS F3/MS R3b/Probe 2 (Figure 1B). Therefore, the primer-probe set MS F3/MS R3b/Probe 2 was used for the subsequent experiments.
Figure 1.
Screening of primers and probe for MS detection. (A) Agarose gel electrophoresis results of RAA products with different primer pairs. M: DNA marker DL2000. 1, 3, 5: Positive reactions using primer pairs No.1-No.3, respectively; 2, 4, 6: No-template controls of primer pairs No.1-No.3, respectively. The primer pair of No.3 (MS F3/MS R3) was selected for subsequent experiments. (B) Screening of probe by RAA-LFD with the optimal primer pair MS F3/MS R3b. 1: Positive reaction using the primer-probe set MS F3/MS R3b/Probe 1; 2: No-template control of MS F3/MS R3b/Probe 1; 3: Positive reaction using the primer-probe set MS F3/MS R3b/Probe 2; 4: No-template control of MS F3/MS R3b/Probe 2. Finally, Probe 2 was selected as the optimal probe for the RAA-LFD assay. Abbreviations: MS, Mycoplasma synoviae; RAA, recombinase-aided amplification; RAA-LFD, recombinase-aided amplification-lateral flow dipstick.
Optimization of RAA-LFD Reaction Temperature and Time
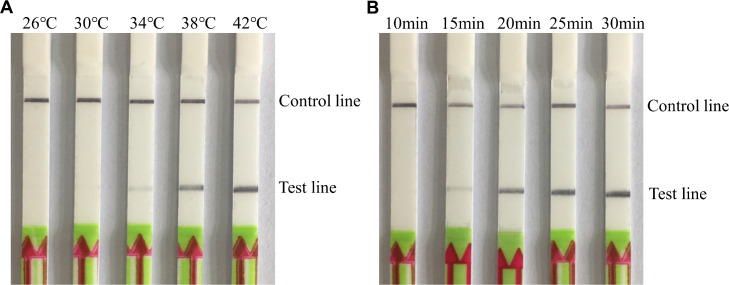
The optimal reaction temperature for the RAA-LFD assay was determined. A clear test band could be observed within a temperature range of 34 to 42°C, and the band was brighter at 38 to 42°C (Figure 2A). Thus, the lower temperature (38°C) was used for the subsequent RAA-LFD assay. Next, we sought to determine the optimal reaction time. Results revealed that a distinct test band could be seen between 15 and 30 min, and the band was brighter at 20 to 30 min (Figure 2B). Consequently, the shorter time (20 min) was selected for the follow-up experiments.
Figure 2.
Optimization of reaction conditions for MS detection by RAA-LFD. (A) RAA-LFD results under different temperatures. Of the range of tested temperatures, 38°C was seleted as the optimal reaction temperature. (B) RAA-LFD results with different reaction times. Of the range of tested times, 20 min was chosen as the optimal reaction time. Abbreviations: MS, Mycoplasma synoviae; RAA-LFD, recombinase-aided amplification-lateral flow dipstick.
Specificity Test
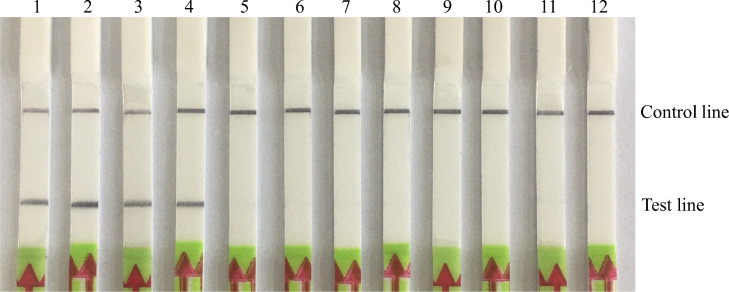
The nucleic acid of MS and other chicken pathogens were used as the templates for the specific detection of the RAA-LFD assay. Results showed that DNA from all MS strains yielded a test band on the dipstick, while DNA/cDNA from other pathogens showed negative results (Figure 3), indicating that the RAA-LFD assay for MS detection has a good specificity with no cross-reaction with other common chicken pathogens.
Figure 3.
Specificity test of RAA-LFD for MS detection. 1-4: RAA-LFD detection results of MS strains GX11-T, YC03, XS05, and XS12, respectively; 5-11: RAA-LFD detection results of MG, P. multocida, E.coli, NDV, IBDV, IBV, and ARV, respectively; 12: Negative control. Abbreviations: RAA-LFD, recombinase-aided amplification-lateral flow dipstick; MS, Mycoplasma synoviae; MG, Mycoplasma gallisepticum; P. multocida, Pasteurella multocida; E.coli, Escherichia coli; NDV, Newcastle disease virus; IBDV, infectious bursal disease virus; IBV, infectious bronchitis virus; ARV, avian reovirus.
Comparison of the Sensitivity of RAA-LFD, PCR and RFQ-PCR
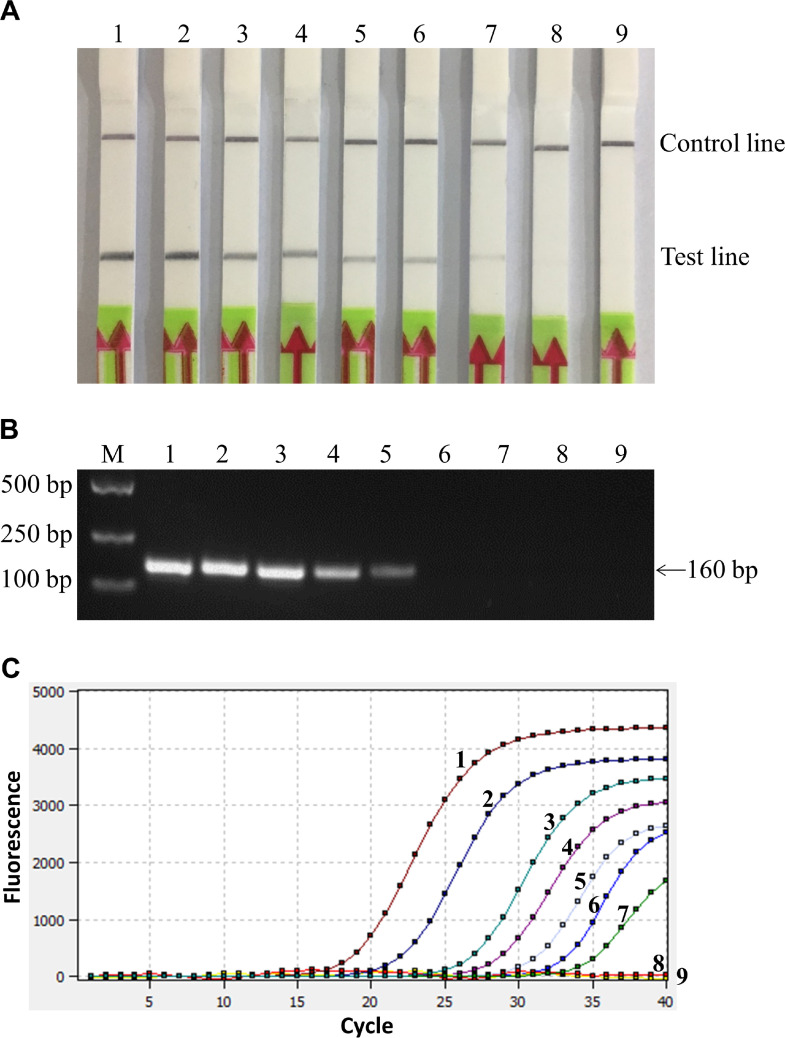
The sensitivity of RAA-LFD, PCR, and RFQ-PCR were tested and compared. Results showed that the lowest detection limit of the three methods was 101 copies/μL, 103 copies/μL and 101 copies/μL, respectively (Figure 4), and the repeated tests showed the same results, suggesting that the sensitivity of the RAA-LFD assay was comparable to RFQ-PCR, which was 100 times higher than for PCR.
Figure 4.
Sensitivity of different methods for MS detection. (A) Sensitivity test of RAA-LFD. 1-8: RAA-LFD detection results with MS DNA template of 107–100 copies/μL, respectively; 9: Negative control. The lowest detection limit of RAA-LFD was 101 copies/μL. (B) Sensitivity test of PCR. 1-8: PCR detection results with MS DNA template of 107–100 copies/μL, respectively; 9: Negative control. The lowest detection limit of PCR was 103 copies/μL. (C) Sensitivity test of RFQ-PCR. 1–8: RFQ-PCR detection results with MS DNA template 107–10° copies/μL, respectively; 9: Negative control. The lowest detection limit of RFQ-PCR was 101 copies/μL. Abbreviations: MS, Mycoplasma synoviae; RAA-LFD, recombinase-aided amplification-lateral flow dipstick; RFQ-PCR, real-time fluorescence quantitative PCR.
Detection of Clinical Samples
A total of 128 suspected clinical samples were detected for MS by RAA-LFD, PCR, and RFQ-PCR simultaneously. Results showed that the positive rate of RAA-LFD was 43.8% (56/128), while that of PCR and RFQ-PCR were 39.1% (50/128) and 45.3% (58/128), respectively. The detection coincidence rate was 95.3% between RAA-LFD and PCR, and 98.4% between RAA-LFD and RFQ-PCR (Table 2). In addition, all of the 50 positive samples identified by PCR also showed positive results by RAA-LFD, resulting in a sensitivity (co-positivity) of 100%. Of the 78 negative samples in PCR, 72 showed negative results with RAA-LFD, gernarating a specificity (co-negativity) of 92.3%. Compared with the PCR method, the kappa value of RAA-LFD was 0.904 (Table 3). These results indicated that the RAA-LFD assay established in this study could be used for MS detection of clinical samples.
Table 2.
Detection results of clinical samples by different methods (n = 128).
| Methods | Positive cases | Negative cases | Positive rate (%) | Coincidence rate (%) |
|---|---|---|---|---|
| RAA-LFD | 56 | 72 | 43.8 | - |
| PCR | 50 | 78 | 39.1 | 95.3 |
| RFQ-PCR | 58 | 70 | 45.3 | 98.4 |
RAA-LFD: recombinase-aided amplification-lateral flow dipstick; RFQ-PCR: real-time fluorescence quantitative PCR.
Table 3.
Performance of RAA-LFD assay compared to PCR method, for dectecing MS in clinical samples (n = 128).
| PCR |
Performance characteristics |
|||||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Sensitivity (%) | Specitivity (%) | PPV (%) | NPV (%) | Kappa | ||
| RAA-LFD | Positive | 50 | 6 | 100 | 92.3 | 89.3 | 100 | 0.904 |
| Negative | 0 | 72 | ||||||
RAA-LFD: recombinase-aided amplification-lateral flow dipstick; PPV: positive predictive value; NPV: negative predictive value.
DISCUSSION
As one of the important avian pathogens in poultry husbandry, MS has brought significant economic losses to the poultry industry worldwide (Wu et al., 2019). A rapid, accurate and convenient detection method is critical for early clinical diagnosis and control of MS infection. Molecular biological diagnostic methods have been successfully used to identify different pathogens in veterinary microbiology. With the advantages of simple, fast and low cost, isothermal nucleic acid amplification technologies have rapidly developed, especially RAA and recombinase polymerase amplification (RPA) (Fan et al., 2020). The principle and process of RAA and RPA are similar; however, the recombinase of RPA is uvsX from T4 bacteriophage, while the RAA recombinase comes from bacteria or fungi, which has a more extensive source and a lower cost (Chen et al., 2021). In recent years, the RAA technique has been successfully used for detection of many animal pathogens, such as African swine fever virus (Fan et al., 2020), avian infectious laryngotracheitis virus (Wang et al., 2021), and Newcastle disease virus (Wang et al., 2020).
In the present study, a novel and simple method for MS detection was proposed based on RAA and LFD. The primers and probe are key factors of the RAA-LFD assay. To achieve maximum amplification specificity and efficiency, the lengths of primers and probe for RAA should be 30 to 35 bp and 46 to 52 bp, respectively (Li et al., 2021). However, these longer primers and probe may generate secondary structures; in addition, the reverse primer and the probe both contain chemical labelings, thus resulting in false-positive signals on LFD (Wang et al., 2019; Wu et al., 2020). Therefore, primers and probe screening are necessary for the RAA-LFD assay. In our experiment, the optimal primer-probe set MS F3/MS R3b/Probe 2 was selected for MS detection, with which no false-positive signal was detected. Using the optimal primer-probe set, the RAA reaction could be completed at 38°C in 20 minutes, and the amplification products could be detected by LFD within 5 minutes. According to the previous studies, other isothermal nucleic acid amplification methods have also been developed for MS detection, such as loop-mediated isothermal amplification (LAMP) and polymerase spiral reaction (PSR) (Kursa et al., 2015; Wu et al., 2019). However, the LAMP and PSR reactions require higher temperatures of 63°C and 62°C, and longer times of 60 min and 40 min.
Currently, PCR and RFQ-PCR are the main techniques used for molecular detection of MS in most laboratories (Shahid et al., 2013; Fujisawa et al., 2019). To validate the performance of the RAA-LFD assay for MS detection, PCR and RFQ-PCR methods designed by our laboratory were used as comparisons. The lowest detection limit of PCR and RFQ-PCR for MS detection were 103 copies/μL and 101 copies/μL, which were consistent with the relevant literature (Huang et al., 2015), indicating that the two methods designed by ourselves could be used as the comparator assays for RAA-LFD. Also, we found that the sensitivity of RAA-LFD was comparable to RFQ-PCR and was 100 times higher than that of PCR. However, PCR and RFQ-PCR both require expensive thermal-cycling instruments and experienced technicians. In contrast, the RAA reaction in our study could be simply conducted with a water bath, and the results could be visually observed by LFD without any instrument. Considering this, the RAA-LFD assay is also suitable for the diagnosis of MS infection at the grassroots level.
CONCLUSION
The RAA-LFD method for MS detection established in this study could be performed at an isothermal temperature of 38°C in 20 min with high specificity. The results could be observed on the dipstick with the naked eye in 5 min without any instrument. It was as sensitive as RFQ-PCR with a detection limit of 10 copies/μL, which was 100 times higher than PCR (103 copies/μL). In conclusion, the RAA-LFD method provides a new option for rapid and visual detection of MS, which is suitable for clinical practice.
ACKNOWLEDGMENTS
This work was supported by Yancheng Demonstration Promotion Base (JATS[2021]233) from Jiangsu Modern Agricultural (Laying Hens) Industry Technology System, Jiangsu Province, China; Innovation and Entrepreneurship Training Program for College Students (202110324112H) in Jiangsu Province, China, and Startup Fund for Scientific Research (YCTU201801) of Yancheng Teachers’ University, Yancheng City, China.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Abdelaziz A.M., Mohamed M.H.A., Fayez M.M., Al-Marri T., Qasim I., Al-Amer A.A. Molecular survey and interaction of common respiratory pathogens in chicken flocks (field perspective) Vet. World. 2019;12:1975–1986. doi: 10.14202/vetworld.2019.1975-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Fan J., Li Z., Zhang Y., Qin Y., Wu K., Li X., Li Y., Fan S., Zhao M. Development of recombinase aided amplification combined with disposable nucleic acid test strip for rapid detection of porcine circovirus Type 2. front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.676294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen T., Lampron R., Hauck R., Pitesky M., Gallardo R.A. Biosecurity assessment and seroprevalence of respiratory diseases in backyard poultry flocks located close to and far from commercial premises. Avian Dis. 2018;62:1–5. doi: 10.1637/11672-050917-Reg.1. [DOI] [PubMed] [Google Scholar]
- Dijkman R., Feberwee A., Landman W.J.M. Development, validation and field evaluation of a quantitative real-time PCR able to differentiate between field Mycoplasma synoviae and the MS-H-live vaccine strain. Avian Pathol. 2017;46:403–415. doi: 10.1080/03079457.2017.1296105. [DOI] [PubMed] [Google Scholar]
- Dufour-Gesbert F., Dheilly A., Marois C., Kempf I. Epidemiological study on Mycoplasma synoviae infection in layers. Vet. Microbiol. 2006;114:148–154. doi: 10.1016/j.vetmic.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Fan G.H., Shen X.X., Li F., Li X.N., Bai X.D., Zhang R.Q., Wang R.H., Lei W.W., Wang H.Y., Ma X.J., Wu G.Z. Development of an internally controlled reverse transcription recombinase-aided amplification assay for the rapid and visual detection of west nile virus. Biomed. Environ. Sci. 2019;32:926–929. doi: 10.3967/bes2019.116. [DOI] [PubMed] [Google Scholar]
- Fan X., Li L., Zhao Y., Liu Y., Liu C., Wang Q., Dong Y., Wang S., Chi T., Song F., Sun C., Wang Y., Ha D., Zhao Y., Bao J., Wu X., Wang Z. Clinical validation of two recombinase-based isothermal amplification assays (RPA/RAA) for the rapid detection of African swine fever virus. Front. Microbiol. 2020;11:1696. doi: 10.3389/fmicb.2020.01696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felice V., Lupini C., Mescolini G., Silveira F., Guerrini A., Catelli E., Di Francesco A. Molecular detection and characterization of Mycoplasma gallisepticum and Mycoplasma synoviae strains in backyard poultry in Italy. Poult. Sci. 2020;99:719–724. doi: 10.1016/j.psj.2019.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa S., Murata S., Takehara M., Katakura K., Hmoon M.M., Win S.Y., Ohashi K. Molecular detection and genetic characterization of Mycoplasma gallisepticum, Mycoplama synoviae, and infectious bronchitis virus in poultry in Myanmar. BMC Vet. Res. 2019;15:261. doi: 10.1186/s12917-019-2018-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Chen W., Fan J., Fan S., Ding H., Chen J., Yi L. Recombinase-aided amplification coupled with lateral flow dipstick for efficient and accurate detection of Porcine Parvovirus. Life (Basel) 2021;11:762. doi: 10.3390/life11080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Xie Z., Xie L., Deng X., Xie Z., Luo S., Huang J., Zeng T., Feng J. A duplex real-time PCR assay for the detection and quantification of avian reovirus and Mycoplasma synoviae. Virol. J. 2015;12:22. doi: 10.1186/s12985-015-0255-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo H.C., Lo D.Y., Chen C.L., Tsai Y.L., Ping J.F., Lee C.H., Lee P.A., Chang H.G. Rapid and sensitive detection of Mycoplasma synoviae by an insulated isothermal polymerase chain reaction-based assay on a field-deployable device. Poult. Sci. 2017;96:35–41. doi: 10.3382/ps/pew228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursa O., Wozniakowski G., Tomczyk G., Sawicka A., Minta Z. Rapid detection of Mycoplasma synoviae by loop-mediated isothermal amplification. Arch. Microbiol. 2015;197:319–325. doi: 10.1007/s00203-014-1063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang C., Wang W., Zhang Z., Zhang Z., Wang C., Zhang T. Research Note: development of rapid isothermal amplification assay for detection of duck circovirus. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira F.A., Cardoso L., Coelho A.C. Epidemiological survey on Mycoplasma synoviae infection in Portuguese broiler breeder flocks. Vet. Ital. 2015;51:93–98. doi: 10.12834/VetIt.116.329.3. [DOI] [PubMed] [Google Scholar]
- Shahid M.A., Ghorashi S.A., Agnew-Crumpton R., Markham P.F., Marenda M.S., Noormohammadi A.H. Combination of differential growth at two different temperatures with a quantitative real-time polymerase chain reaction to determine temperature-sensitive phenotype of Mycoplasma synoviae. Avian Pathol. 2013;42:185–191. doi: 10.1080/03079457.2013.779363. [DOI] [PubMed] [Google Scholar]
- Sun S.K., Lin X., Chen F., Wang D.A., Lu J.P., Qin J.P., Luo T.R. Epidemiological investigation of Mycoplasma Synoviae in native chicken breeds in China. BMC Vet. Res. 2017;13:115. doi: 10.1186/s12917-017-1029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu F., Yang X., Xu S., Chen D., Zhou L., Ge X., Han J., Zhang Y., Guo X., Yang H. Development of a fluorescent probe-based real-time reverse transcription recombinase-aided amplification assay for the rapid detection of classical swine fever virus. Transbound. Emerg. Dis. 2021;68:2017–2027. doi: 10.1111/tbed.13849. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhao P., Si X., Li J., Dai X., Zhang K., Gao S., Dong J. Rapid and specific detection of listeria monocytogenes with an isothermal amplification and lateral flow strip combined method that eliminates false-positive signals from primer-dimers. Front. Microbiol. 2019;10:2959. doi: 10.3389/fmicb.2019.02959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang C., Bai Y., Zhang P., Yao S., Liu J., Zhang T. Establishment of reverse transcription recombinase-aided amplification-lateral-flow dipstick and real-time fluorescence-based reverse transcription recombinase-aided amplification methods for detection of the Newcastle disease virus in chickens. Poult. Sci. 2020;99:3393–3401. doi: 10.1016/j.psj.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang C., Zhang Z., Zhang P., Zhai X., Li X., Zhang T. Recombinase-aided amplification-lateral flow dipstick assay-a specific and sensitive method for visual detection of avian infectious laryngotracheitis virus. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Zhao P., Yang X., Li J., Zhang J., Zhang X., Zeng Z., Dong J., Gao S., Lu C. A recombinase polymerase amplification and lateral flow strip combined method that detects salmonella enterica serotype typhimurium with no worry of primer-dependent artifacts. Front. Microbiol. 2020;11:1015. doi: 10.3389/fmicb.2020.01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Xu X., Chen Q., Zuo K., Zhou Y., Zhang Z., Kan Y., Yao L., Ji J., Bi Y., Xie Q. Rapid and visible detection of Mycoplasma synoviae using a novel polymerase spiral reaction assay. Poult. Sci. 2019;98:5355–5360. doi: 10.3382/ps/pez356. [DOI] [PubMed] [Google Scholar]
- Xue J., Xu M.Y., Ma Z.J., Zhao J., Jin N., Zhang G.Z. Serological investigation of Mycoplasma synoviae infection in China from 2010 to 2015. Poult. Sci. 2017;96:3109–3112. doi: 10.3382/ps/pex134. [DOI] [PubMed] [Google Scholar]
- Zheng Y.Z., Chen J.T., Li J., Wu X.J., Wen J.Z., Liu X.Z., Lin L.Y., Liang X.Y., Huang H.Y., Zha G.C., Yang P.K., Li L.J., Zhong T.Y., Liu L., Cheng W.J., Song X.N., Lin M. Reverse transcription recombinase-aided amplification assay with lateral flow dipstick assay for rapid detection of 2019 novel coronavirus. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.613304. [DOI] [PMC free article] [PubMed] [Google Scholar]