Abstract
Hip and knee joint replacements are common and largely successful procedures that utilise implants to restore mobility and relieve pain for patients suffering from e.g. osteoarthritis. However, metallic ions and particles released from both the bearing surfaces and non-articulating interfaces, as in modular components, can cause hypersensitivity and local tissue necrosis, while particles originating from a polymer component have been associated with aseptic loosening and osteolysis. Implant coatings have the potential to improve properties compared to both bulk metal and ceramic alternatives. Ceramic coatings have the potential to increase scratch resistance, enhance wettability and reduce wear of the articulating surfaces compared to the metallic substrate, whilst maintaining overall toughness of the implant ensuring a lower risk of catastrophic failure of the device compared to use of a bulk ceramic. Coatings can also act as barriers to inhibit ion release from the underlying material caused by corrosion. This review aims to provide a comprehensive overview of wear-resistant coatings for joint replacements – both those that are in current clinical use as well as those under investigation for future use. While the majority of coatings belong predominantly in the latter group, a few coated implants have been successfully marketed and are available for clinical use in specific applications. Commercially available coatings for implants include titanium nitride (TiN), titanium niobium nitride (TiNbN), oxidized zirconium (OxZr) and zirconium nitride (ZrN) based coatings, whereas current research is focused not only on these, but also on diamond-like-carbon (DLC), silicon nitride (SiN), chromium nitride (CrN) and tantalum-based coatings (TaN and TaO). The coating materials referred to above that are still at the research stage have been shown to be non-cytotoxic and to reduce wear in a laboratory setting. However, the adhesion of implant coatings remains a main area of concern, as poor adhesion can cause delamination and excessive wear. In clinical applications zirconium implant surfaces treated to achieve a zirconium oxide film and TiNbN coated implants have however been proven comparable to traditional cobalt chromium implants with regards to revision numbers. In addition, the chromium ion levels measured in the plasma of patients were lower and allergy symptoms were relieved. Therefore, coated implants could be considered an alternative to uncoated metal implants, in particular for patients with metal hypersensitivity. There have also been unsuccessful introductions to the market, such as DLC coated implants, and therefore this review also attempts to summarize the lessons learnt.
Keywords: Joint implants, Coatings, Surface layers, Ceramics, Biomaterials, Wear resistance
Graphical abstract
1. Introduction
A damaged or diseased joint can cause severe pain and limited mobility, which impairs the quality of life of the afflicted individual. To treat this condition, it might be necessary to replace the joint with an implant in a surgical procedure. Two of these types of implants, namely hip and knee implants, will be the focus of this review. The number of primary, or first time, total hip surgeries performed every year has steadily increased and is predicted to continue to rise [1] as the ageing population increases, and indications are that younger patients can also benefit from these procedures. While hip joint implants have a generally high survival rate of approximately 95% or more at 10 years for all currently used material combinations, metal-on-polyethylene (MoP) being the most common, [[2], [3], [4], [5]]. However, the increasingly active, ageing and younger populations require longer lasting implants and a focus of current research is on prolonging the lifespan of implants to spare patients from further pain and revision surgeries. Similarly, the revision rates for knee implants are reported to range from 4.3% [5] to 5.3% [4] at 10 years.
The primary cause of revision surgery for metal or ceramic hip replacement components paired with polyethylene (PE) is aseptic loosening of the implant (28–51% [[2], [3], [4], [5], [6], [7], [8], [9], [10]]). In these, one of the main causes for this is believed to be the presence of wear debris from the articulating surfaces, mainly polymeric particles. However, it should be noted that PE wear has decreased dramatically since the introduction of highly crosslinked polyethylene (XLPE) 20 years ago [11]. Necrosis, pseudo tumours and pain have also been found to be the cause of revision surgeries particularly in alternative metal on metal bearing systems. These complications are believed to be caused by metallic particulate and soluble (ionic) debris originating from the articulating and non-articulating surfaces of the implant [[12], [13], [14]]. While the particulate debris may originate from any surface of the implant, it is primarily formed at the articulating interface.
The most common materials currently implanted in this context are cobalt chromium alloy (CoCr) and highly crosslinked polyethylene (XLPE) in MoP implants [3,4,9,10], together with a zirconia/alumina ceramic combined with PE (CoP) [4,10]. In these cases, the dominant particulate debris is that of the polymer, which can range between 10 nm and 1 mm in size. Particles in the size range 0.1–1.0 μm are believed to play an important role in the activation of macrophages [15], which might initiate a cascade of reactions that eventually cause wear-induced osteolysis (loss of bone), possibly resulting in implant loosening [[15], [16], [17], [18], [19]]. Metal-on-metal (MoM) hip re-surfacing implants were considered an alternative for young patients, however, the implants experienced high short-term failure rates and an increased rate of revision surgeries [20]. Due to these catastrophic outcomes MoM hip replacements are now rarely used [2,4,5,8]. Debris from MoM implants is generally in the nanoscale range (<100 nm) and there is evidence to suggest that metal particles in this size range and ions can cause a variety of biological effects such as hypersensitivity, pseudo-tumours [12,14,[21], [22], [23], [24], [25]] and aseptic lymphocytic vasculitis-associated lesions [13]. In addition high concentrations of cobalt and chromium ions are believed to cause e.g. acute visual and auditory impairment, peripheral neuropathy and cardiomyopathy [26]. It should be noted that the latter are extreme cases, and the incidence of adverse reactions to metal debris has been found to be less than 1.2% for MoM hip implants [27]. The revision per 1000 prosthesis years caused by adverse reactions to particulate debris for MoM was found to be 9.90, which can be compared to 0.19 for MoP [5].
Several different strategies have been explored for reducing wear and the negative biological effects of metal ions and PE wear debris. Among the most noteworthy of these are the improvement of polymer wear properties [11,28] and the development of e.g. textured surfaces [29]. However, this review will focus on the use of ceramic coatings or surface modifications, such as nitriding, to reduce wear and ion release. These aim to combine the advantages of a wear resistant ceramic with the ductility and toughness of a metal. The ceramic coating can also act as a barrier to, and decrease, ion release from the underlying metal [30].
Previous reviews [31,32] have focused on tantalum, graphite-like carbon (GLC) [33,34], diamond-like carbon (DLC) [[35], [36], [37], [38]], titanium nitride based coatings [[39], [40], [41], [42], [43]] and chromium nitride (CrN) [44,45]. However, a number of novel experimental coatings have been reported (e.g. silicon nitride (SiN) [[46], [47], [48], [49], [50], [51], [52], [53]], multilayer structured coatings [42,44,54]) as well as commercially available coatings (oxidized zirconium (OxZr) [55] and titanium nitride (TiN) [56,57]), which are utilised in interfaces of joint prostheses, and currently, there is no comprehensive review available. This review aims to provide readers with a comprehensive overview of coatings that are (i) commercially available and (ii) currently being researched, and to summarize the current status and future potential of such coatings for hip and knee joint implants. We focus on coatings and treatments for articulating surfaces, and as such, other types of coatings, e.g. those for improved bone ingrowth and fixation lie beyond the scope of this review.
2. Methodology
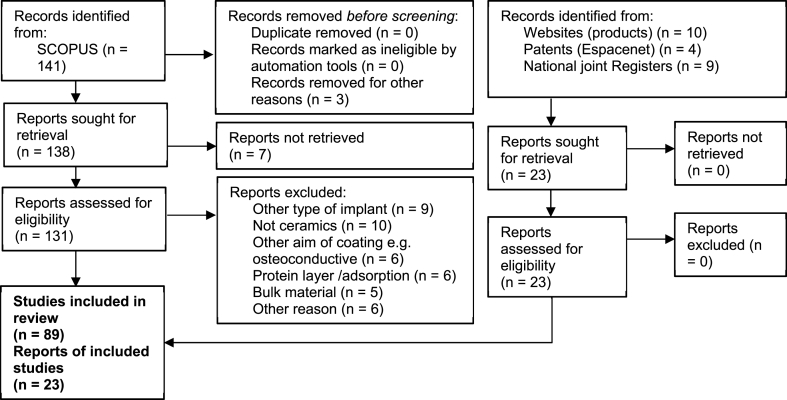
The material in this review is based on published research articles, patents and product information in order to cover all phases from early stage research to products in clinical use. The research articles were found using the database SCOPUS with the primary search terms “wear” AND “coatings OR film” AND “joint implant” for coatings and the search terms “wear” AND “surface modification” AND “joint implant” for surface modifications. An additional search with the terms “biocompatibility” AND “coatings OR film” AND “joint implant” was conducted to include studies regarding the biocompatibility and finally, through references, an additional 6 papers were identified. The patents were found by using the same search terms on Espacenet, and the products were found using Google (with search terms “joint replacement” “products” “coating”). Research papers, patents and products concerning dental implants, osteoinductive/conductive coatings, protein and other biological coatings as well as bulk materials were excluded and the joint types were limited to hip and knee. This left a total number of 89 research papers (27 of them being case reports or revision studies) and 4 patents. Information such as wear rates and surface roughness was compiled in a table that may be found in the supplementary information. Therefore, some of the references are not included in the main paper but can be found in the supplementary information. Fig. 1 shows a flow diagram of the procedures followed in selecting the reports and associated data for inclusion in this review.
Fig. 1.
A flow chart showing how the data was collected and treated. The supplementary information contains additional references such as ISO and ASTM standards, studies on background such as biological reactions to debris, and information regarding deposition techniques.
3. Substrate material and deposition methods
A coating used for joint implants should be hard, wear resistant, corrosion resistant, biocompatible, not release particulates and any particulates that are generated should be biocompatible. In addition, the coating should have a low surface roughness and a good adhesion to the substrate material. Some of these properties are specified in standards such as ISO 7206-2 while others, such as wear resistance, have no specified target value. The important properties and what to consider are specified in Table 1. It should be noted that the table distinguishes between wear resistance and corrosion resistance, however theses are processes that occur synergistically. Typically, these properties are however reported separately.
Table 1.
The important properties of coatings for joint implants, their evaluation methods and their target profile.
| Property | Target profile | Typical method for evaluation and standards |
|---|---|---|
| Hardness | A high hardness will help mitigate wear. However, the stiffness should also be considered as the ratio of hardness to Young's modulus gives a measure of the elastic limit in the contact and hence provides an indicator of wear performance. | Nano- or microindentation. Values are given in Pa or Vickers hardness number (HV). |
| • ASTM E2546-07 [58] | ||
| • ISO 14577-4 [59] | ||
| Wear resistance | A low wear rate is desirable, but actual values will depend on the specific tribological situation and are therefore not specified here. Attention should be paid to the generated wear debris, the size, shape and volume will likely influence the immune response in the final application. | Tribological set-up ranging for pin-on-disc to joint simulators. The resulting wear is measured as specific wear rate (mm3/Nm) or mass loss per million cycles (mg/Mc). |
| • EN 1071-12 [60] | ||
| • EN 1071-13 [61] | ||
| • ASTM F732 [62] | ||
| • ISO 20808 [63] | ||
| Corrosion resistance | A coating should protect the underlying metal from corrosion as well as have a low rate of degradation. However, it is also important to consider the character of the particles and ions that inevitably are released. | Measuring open circuit potential (V) and corrosion current (μA). |
| • ASTM G5 [64] | ||
| • ISO 16429 [65] | ||
| • ISO 16773 [66] | ||
| Toxicity | Ultimately the coating, and more importantly the ions and wear debris, should not elicit an adverse immune response. The toxicity will depend on the volume of debris or ions, i.e. a dose dependency, and the volume will depend on the wear properties of the coating. | In vitro studies using cell lines. Results are given as cell viability. |
| • ISO 10993 [67] | ||
| Surface roughness | A smooth surface is necessary to reduce PE wear. An Ra value of ≤20 nm has been specified for ceramics in ISO 7206-2. | Optical or stylus methods for surface characterization. The most common parameter to report is the average surface roughness, Ra, (nm). |
| • ISO 4287 [68,69] | ||
| • ISO 4288 [70] | ||
| • ISO 25178-604 [71] | ||
| Adhesion | A coating that adheres well to the substrate is of utmost importance as delamination of the coating could cause excessive wear through the release of abrasive debris. It is important to consider factors such as time and corrosive environments when evaluating the adhesion. | Most common methods are scratch tests, from which critical loads are obtained (N), or Rockwell indentations that are categorized according to a standard. |
| • ISO 26443 [72] | ||
| • ISO 20502 [73] |
These properties are covered in more detail in the supplementary information section.
Ceramic coatings are typically deposited onto a metal implant using a wide range of techniques [[74], [75], [76]]. Possible substrate materials include the metals commonly used as bulk materials for joint implants i.e. CoCr and stainless steel but also titanium. Titanium is not used in articulating surfaces due to its poor wear properties [[77], [78], [79]], but this becomes possible with a coating or surface treatment that improves such properties [75]. The use of titanium as a substrate material could even be advantageous in terms of coating adhesion [80]. It is however important to keep in mind that the coating should be compared with the material it aims to substitute, e.g. in the case of articulating surfaces typically CoCr.
The choice of deposition method or surface treatment will have a strong influence on the above-mentioned parameters. The most common deposition techniques used for coatings for articulating surfaces can be divided into either physical vapor deposition (PVD) methods [81] or chemical vapor deposition (CVD) methods [82], the former group is more commonly used than the latter in the studies found in this review. These deposition techniques, as well as their advantages and challenges, have been covered in detail in previously published articles [[82], [83], [84], [85], [86]]. PVD techniques include, amongst others, vacuum evaporation, magnetron sputtering (MS), reactive magnetron sputtering (rMS), pulsed laser deposition (PLD) and high-power impulse magnetron sputtering (HiPIMS). They utilise metallic sources that are either evaporated, typically using an electron beam, or sputtered with the help of a plasma to produce a flux of metal atoms/ions which is deposited onto the surface to be coated in the presence of one or more reactive gases to form a layer of ceramic material. Coatings deposited using PVD techniques typically have a low surface roughness, are hard and wear resistant. There are however limitations due to the technique being line-of-sight but these are usually mitigated by manipulation of the substrate during deposition. The second group of methods, CVD, entail exposing the substrate to a mixture of gases that react at high temperature (typically >600 °C) to form a ceramic compound, though it is possible to use a plasma to enhance the reactivity of the gas precursors (plasma-enhanced CVD (PECVD)) to reduce the deposition temperature. Using CVD techniques it is possible to grow uniform, well adhering coatings on complicated substrates. However, there are limitations related to e.g. heat resistance of the substrate. Another approach is to treat the surface by e.g. heat or laser in the presence of gases such as nitrogen [87]. This often yields an increased hardness and wear resistance, however the surface roughness is often increased and the techniques are limited to materials that can form suitable ceramic surface layers such as zirconium oxide and titanium nitride.
Another family of surface deposition techniques is thermal spraying in which heated or melted material is sprayed onto a surface [88]. The coating material is either in the form of powder or a filament that is typically melted by flame or plasma [75].
A harder surface can also be achieved by surface treatment processes such as plasma electrolytic oxidation where metals are oxidized through a process similar to anodization but with higher potentials [89].
A technique with future potential is additive manufacturing where it is theoretically possible to manufacture implants with a surface layer with different properties compared to the underlying material. However, the manufactured materials currently require extensive post processing to achieve smooth surfaces suitable for wear-resistant applications [90].
The most important properties relevant to coatings for joint implants, as well as the methods used in their evaluation, are discussed in the supplementary information section. It should be noted however that somewhat different requirements exist depending on joint application, e.g. hip joints may experience more complex movement patterns, as well as more severe edge loading [91] than knee joints [92], and different standards are available to evaluate their performance.
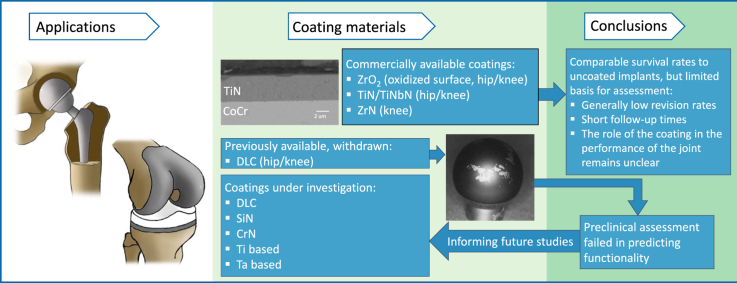
4. Commercial coatings
There are currently three types of commercially available coated or surface treated hip and knee joint implants for clinical applications, namely:
- •
-
•Zirconium nitride (ZrN):
- Aesculap (B Braun) [98].
-
•Oxidized zirconium (OxZr)
- Smith and Nephew [99].
TiN coated implants are available with either a Ti6Al4V [100] or a CoCr [101] substrate and the coated surface is usually paired with PE in the articulating surfaces. These implants are aimed for young, active patients, in particular patients with metal sensitivity [102] and studies have shown them to be stable over time [103].
As TiN, ZrN and OxZr are commercially available it is possible to study patient outcomes (Table 2). Since their introduction, long-term follow up studies have found that OxZr implants perform well, with low revision rates [55], comparable to uncoated CoCr implants [104,105] (Table 2). The implant has performed better, with lower rates of corrosion and fretting wear, in comparison to CoCr, as seen in retrieved implants [106]. Taking all these results together Oxinium implants show great promise as a viable option for joint implants, not just for patients suffering from or at risk of developing metal hypersensitivity but also for otherwise healthy patients.
Table 2.
Revised implants and follow-up studies of coated implants. In the case of revision-retrieved samples one must be aware of the fact that they are failures and may depart from the general performance of the cohort.
| Coating | Implant | Product | Number of coated implants | Average time of implantation/follow-up [months] | Revison rate | Reason for revision/retrieval | Key findings | Reference |
|---|---|---|---|---|---|---|---|---|
| TiNbN | Hip | – | 1 | 53 | n.a. | Aseptic loosening | No signs of metallosis. Coating failure due to insufficient adhesion, corrosion, and involvement of third bodies. | Łapaj et al., 2016 [112] |
| TiN | Knee | Implantcast ACS and Corin Uniglide | 5 | 16 | Retrieval study (100%) | Aseptic loosening: 4 | TiN coatings of knee replacements undergo wear and degradation related to presence of third bodies and microscopic defects on their surface. | Łapaj et al., 2020 [113] |
| Periprosthetic inflammation: 1 | ||||||||
| TiNbN | Knee | – | 59 | 36 | 0 | n.a. | Chromium concentrations in patient plasma increased from 0.25 to 0.75 μg/l in the coated TKA group compared with of 0.25–1.30 μg/l in the standard TKA group. | Postler et al., 2018 [114] |
| TiN | Knee | ACS® MB system, Implantcast | 25 | 30.7 | Infection: 11 Septic loosening: 4 Ligament instability: 2 Pain: 2 Aseptic loosening: 1 Dislocation: 3 Fracture: 1 Recurrent effusion: 1 |
TiN provides low wear rates and little surface damage | Fabry et al., 2017 [115] | |
| Retrieval study (100%) | ||||||||
| TiN | Knee | B-P™ knee system | 1031 | 46 | 2.2% | Malpositioning of tibial component: 6 Traumatic event: 5 Pain (Isolated patellofemoral, instability and arthrofibrosis): 11 Infection: 2 |
TiN coated total knee replacements perform up to par with conventional implants, but does not solve the problem with residual pain. | Breugem et al., 2017 [116] |
| TiN | Knee | ACS® Basic, Implantcast | 51 | 62 | 5.8% | Aseptic loosening: 2 Layer spinout: 1 |
No difference between coated and conventional implants. | van Hove et al., 2015 [117] |
| TiN | Knee | B-P™ knee system | 61 | 33 | n.a. | n.a. | TiN coated implants showed a high degree of satisfaction and less intraoperative bone mass removal compared to NexGen-LPS implants. | Moon et al., 2012 [118] |
| TiN | Hip | B-P™ Integrated Hip system, Endotec | 1 | 12 | n.a. | Unrelated causes | Well-functioning implant, close future monitoring needed | Harman et al., 1997 [111] |
| OxZr | Hip | Oxinium, Smith & Nephew | 3 | 7 | Retrieval study (100%) | Dislocation | The Zr substrate may deform in the case of dislocation because of its low hardness | Kop et al., 2007 [110] |
| OxZr | Hip | Oxinium, Smith & Nephew | 1 | 0.5 | Retrieval study (100%) | Dislocation | Damage to the ZrO2 coating and exposed Zr substrate after dislocation | Evangelista et al., 2007 [107] |
| OxZr | Hip | Oxinium, Smith & Nephew | 56 | 30 | n.a. | Not revised | 2D wear analysis of radiographs show reduced wear of oxinium femoral heads compared to CoCr | Garvin et al., 2009 [55] |
| OxZr | Knee | Genesis II, Smith & Nephew | 98 | 74.4 | 0% | Not revised | Survivorship of 98.7% at 7 years | Innocenti et al., 2010 [119] |
| OxZr | Knee | Oxinium, Smith & Nephew | 11 | 18.5 | Retrieval study (100%) | Stiffness: 7 Infection: 1 Instability: 1 Mal-positioning: 2 Loosening: 1 |
Lower damage of both the OxZr femoral component and PE tibial component for Oxinium compared to CoCr. | Heyse et al., 2011 [120] |
| OxZr | Hip | Oxinium, Smith & Nephew | 1 | 48 h | Retrieval study (100%) | Correction of leg length discrepancy | Extensive PE wear, loss of the ZrOx layer and Ti transfer from the acetabular shell. | McCalden et al., 2011 [108] |
| OxZr | Hip | Oxinium, Smith & Nephew | 60 | 24 | n.a. | Not revised | Further follow-up needed to be able to discern differences between CoCr and Oxinium | Kadar et al., 2011 [105] |
| OxZr | Knee | Oxinium, Smith & Nephew | 16 | 16.4 | Retrieval study (100%) | Stiffness, infection, instability and dislocation | Wear comparable to conventional MoP implants | Heyse et al., 2011 [121] |
| OxZr | Knee | Oxinium, Smith & Nephew | 109 | 70.8 | n.a. | Not revised | OxZr is an attractive option for patients with metal sensitivity and patients in risk of high rates of wear (due to young age or high activity levels). | Hofer et al., 2014 [122] |
| OxZr | Knee | Oxinium, Smith & Nephew | 98 | 135.6 | 2.3% | Loosening. | Survival rate of OxZr of 97.8% at 10 years. | Innocenti et al., 2014 [123] |
| OxZr | Knee | Oxinium, Smith & Nephew | 71 | 62 | n.a. | No revision for loosening. | OxZr comparable to the standard knee prosthesis but further follow up needed. | Park et al., 2014 [124] |
| OxZr | Hip | Oxinium, Smith & Nephew | 60 | 60 | n.a. | Not revised. | Radiostereometric analysis was used to determine OxZr was comparable to, but not better than, CoCr. | Jonsson et al., 2015 [104] |
| OxZr | Hip | Oxinium, Smith & Nephew | 11 | 8.64∗ | Retrieval study (100%) | Aseptic loosening: 14∗ PE wear/osteolysis: 17∗ Fracture: 1∗ Instability: 5∗ Infection: 10∗ Malposition: 4∗ Multiple reasons: 1∗ |
No difference between OxZr and CoCr femoral heads with regards to fretting and corrosion, however bulk ceramic performed better than both OxZr and CoCr. | Tan et al., 2016 [106] |
| ∗ Both bulk ceramic and OxZr | ∗ Both bulk ceramic and OxZr | |||||||
| OxZr | Hip | Oxinium, Smith & Nephew | 3 | 57.3 | Retrieval study (100%) | Pain, hip squeak and limited movement. | Misuse of Oxinium heads (pairing Oxinium femoral heads with alumina liners) caused damage to the coated surface and high wear rates. | Ozden et el. 2017 [109] |
| OxZr | Knee | Oxinium, Smith & Nephew | 5969 | 144 | 7.7% | Infection, loosening or lysis, patellofemoral pain, pain and instability the most common reasons for revision. | The cumulative revision risk was higher for Oxinium than CoCr (7.7% and 4.8% respectively). Loosening/lysis was the reason for revision in 1.1% of cases. | Vertullo et al., 2017 [125] |
| OxZr | Knee | Oxinium, Smith & Nephew | 10,477 | 156 | 0.46% | Infection (the only reason investigated) | Overall same risk of infection for OxZr as CoCr. | Vertullo et al., 2018 [126] |
| ZrN | Knee | Aesculap | 1 | 18 | Not revised | n.a. | The wound healed without complications and the patients eczema as well as the knee pain had disappeared at the last follow up of 18 months. | Thomsen et al., 2011 [127] |
| ZrN, TiN and TiNbN | Knee | Implantcast, AlphaNorm (now aquired by Corin), Mathys, Link and Aesculap | 28 | TiN(CoCrMo): 42 TiNbN(CoCrMo): 40.8 ZrN(CoCrMo): 7.8 TiN(Ti6Al4V): 114 TiNbN(Ti6Al4V): 12 |
Infection: 12 Aseptic loosening: 10 Instability/Luxation: 3 Arthrofibrosis: 1 Periprosthetic fracture: 1 Movement deficit: 1 |
Herbster et al., 2020 [80] | ||
| DLC | Hip | Adamante®, Biomecanique | 101 | 110.4 | 25.8% | Aseptic loosening: 41 Ossification: 1 Pain: 2 Infection: 1 Implant failure: 1 |
54% survival for DLC/PE implants at 8.5 years compared to 88.2% for Al2O3/PE implants. Delamination of the coating caused aggravated wear of the PE liner. | Taeger et al., 2003 [128] |
However, there have been cases with both TiN and OxZr in which retrieved implants were reported to exhibit surface damage, including an exposed substrate [[100], [101], [102], [103],108]. While the femoral heads in these studies have been paired with a PE liner the oxidized surface has shown damage and metallic transfer likely caused by contact with the metallic shell following dislocations. The authors concluded that Oxinium femoral heads should not be used in patients at risk of joint instability.
5. Potential candidate wear-resistant coatings
Several coatings are being investigated for their potential to reduce wear in joint implants. The following section will examine the published research, categorized into six groups according to coating composition: diamond-like carbon, silicon nitride, chromium nitride, zirconium based, titanium based and tantalum based. A summary of the properties can be found in Table 3 (hardness, Young's modulus and adhesion) and Table 1 in supplementary information (surface roughness and wear properties). The properties vary, with hardness ranging from 8 to 44 GPa and Young's modulus from 100 to 466 GPa. The tribological properties such as wear rates were obtained using different set-ups, with differences in contact pressure and counter surface, leading to large inherent variation between samples, making results difficult to compare.
Table 3.
Hardness and Young's modulus, as obtained with nanoindentation, and adhesion test values for the reviewed coatings.
| Coating (substrate) | Deposition technique | H [GPa] | E [GPa] | Adhesion from scratch Test [N]a | Reference |
|---|---|---|---|---|---|
| DLC (CoCr) | Unbalanced MS | 13 | 100 | Guo et al., 2015 [129] | |
| DLC (CoCr) | PECVD | 24 | Thorwarth et al., 2010 [130] | ||
| DLC (cemented carbide) | Enhanced cathodic arc MS | 16.7 | 166 | Wang et al., 2015 [131] | |
| F-FLC (Si) | PECVD | 16.41 | 132.65 | Wang et al., 2020 [132] | |
| SiNx (CoCr) | HiPIMS | 12–26 | 173–293 | Skjöldebrand et al., 2017 [47] | |
| SiNx and SiNxCy (CoCr and Si) | HiPIMS | 18 | 200 | Pettersson et al., 2013 [49] | |
| SiNx (CoCr and Si) | RF MS | 18–24 | 0–7 | Olofsson et al., 2012 [53] | |
| SiNO and F:SiCN (CoCr) | Unbalanced MS | 15 | 236 | Shi et al., 2012 [50] | |
| SiNx (CoCr) | HiPIMS | 14–88 | Filho et al., 2019 [52] | ||
| SiNx, SiCN, SiCrN and SiNbN (CoCr) | HiPIMS | 13–25 | 148–286 | Filho et al., 2019 [51] | |
| SiNx (CoCr) | HiPIMS | Filho et al., 2020 [46] | |||
| TiCN (Ti6Al4V) | Cathodic arc deposition | 8–10 | Sáenz de Viteri et al., 2015 [41] | ||
| TiN (CoCr) | MS | 21–23 | 45–70 | Gallegos-Cantú et al., 2015 [42] | |
| Multilayered TiN/CrN (CoCr) | MS | 8.0–13.5 | 50–70 | Gallegos-Cantú et al., 2015 [42] | |
| TiN (cemented carbide) | Enhanced cathodic arc MS | 23.6 | 397 | Wang et al., 2015 [131] | |
| TiAlN (cemented carbide) | Enhanced cathodic arc MS | 27.3 | 466 | Wang et al., 2015 [131] | |
| Multilayered TiAlN (Ti6Al4V) | Closed field unbalanced magnetron sputter ion plating | 18.8–44.1 | 302.6–516.5 | 17-7-47.7 | Yi et al., 2016 [54] |
| TiN (Ti or Ti6Al4V) | Laser nitriding | 997-1099 HV | Chan et al., 2017 [43] | ||
| Nitrated TNZT | Laser nitriding | 14 | 171 (Er) | Chan et al., 2016 [133] | |
| TiC (steel) | PECVD | 829-1500 HV | 40–70 | Vitu et al., 2008 [134] | |
| CrN/NbN (CoCr) | HiPIMS | 34 | 447 | 50–100 | Hovsepian et al., 2016 |
| CrN (cemented carbide) | Enhanced cathodic arc MS | 17.9 | 422 | Wang et al., 2015 [131] | |
| CrN (CoCr) | Plasma nitriding | 12–19 | Liu et al., 2013 [135] | ||
| CrCN (CoCr) | Plasma carbonitriding | 16–18 | Liu et al., 2013 [135] | ||
| CrN and Cr2N (CoCr) | Plasma nitriding | 660-900 HV | Wang et al., 2010 [136] | ||
| CrN/NbN (Stainless steel 304) | MS | 28 | 390 | 0.02 | Huang et al., 2017 [45] |
| CrAlTiN (Stainless steel 304) | MS | 33 | 450 | 30.4 | Huang et al., 2017 [45] |
| Multilayer TaC and Ta2C (CoCr) | Thermal treatment in molten salts |
24–37 | 250–316 | LC3: 11-48 | Balagna et al., 2012 [36] |
| TaN(CoCr) | RF sputtering | 15–28 | 255–319 | Corona-Gomez 2021 |
LC2 according to ISO 20502 unless otherwise indicated.
5.1. Diamond-like carbon and nano-crystalline diamond
The term diamond-like carbon (DLC) covers a range of hard carbon-based materials with a wide range of properties such as hardness and wear resistance. The variety of structures and consequently properties for carbon is explained by its ability to exist in three hybridizations (sp1, sp2 and sp3). DLC coatings have a significant fraction of sp3 bonds [137]. However, it is important to keep in mind that DLC coatings do not consist solely of amorphous carbon (a-C) but also hydrogenated amorphous carbon (a-C:H) alloys and the structure of the coating will largely depend on the deposition method. For example sputtered coatings typically can extend from sp2 to sp3 and plasma-enhanced CVD produced coatings have a higher fraction of hydrogenated carbons [137].
DLC coatings have been considered for joint implants because of the promise of a chemically inert, hard, wear resistant surface and significant research has been conducted on DLC-coated implants industrially; e.g. in 2001 the company Implant Design AG put forward a DLC-coated knee implant but had to withdraw it the same year after it was banned by the Swiss Federal Office of Public Health (SFOPH). The implants failed due to excessive wear caused by partial delamination of the coating, which led to early revisions [128,138]. Since then research has focused on understanding the mechanism behind the failures as well as improving the coating adhesion. Falub et al. observed an interlayer measuring approximately 5 nm in thickness that occurred gradually between the CoCr implant and DLC coating. This interlayer consisted mainly of carbides with an overall stoichiometry close to Me2C and delamination is believed to be caused by the instability of Co carbides in this interlayer (Fig. 1) [139]. The mechanism is believed to be stress-corrosion cracking, i.e. delayed failure due to environmentally induced crack propagation [140].
Attempts to improve the adhesion by using interlayers was made by Thorwarth et al. [37] in a study whereby tantalum (Ta) was used as an interlayer for a DLC coating. The results were promising, with little noticeable wear for DLC coatings with Ta interlayers in the case of low concentrations of oxygen impurities. However, it was hypothesised that oxygen contamination would lead to an increased occurrence of the β-phase in the Ta interlayer, which could lead to mechanical failure due to its brittleness. Wang et al. on the other hand proposed the introduction of a fullerene-like structure and incorporating fluoride (F-FLC) to obtain long-term stability in in vitro environments [132]. The results revealed coatings with lower coefficient of friction and wear compared with DLC as well as promising in vitro results with e.g. cell adhesion of rabbit bone marrow mesenchymal stem cells. In addition Cr has been used to in an attempt to improve adhesion and these coatings exhibited improved corrosion resistance compared with CoCr [141].
The closely related nanocrystalline diamond coatings (NCD) consist of nano-sized diamond crystals in the range of 3–15 nm with a large fraction of amorphous carbon at the grain boundaries [142]. These coatings have similar wear properties to DLC coatings and have been shown to reduce the wear compared to uncoated Ti6Al4V components [143]. However, as previously mentioned CoCr is the common choice for articulating surfaces due to its superior wear properties, and a comparison to this material is lacking. Cell studies using primary human bone marrow cells have shown cell attachment, spreading and proliferation on the coatings, i.e. non-cytotoxicity. The available biocompatibility studies however seem to focus on osteoblasts, which may be more suitable for coatings aimed at bone ingrowth [[144], [145], [146]].
After investigating and addressing the risk of delamination in DLC coatings the use of interlayers could make them a viable option for joint implants as observed from the low wear rates (Table 1 in supplementary information section) [38,129,147].
5.2. Silicon nitride
Bulk silicon nitride, Si3N4, and related materials have been used in applications such as combustion engines due to high temperature and wear resistance [148], and components made of Si3N4 have been used in several applications subjected to wear, e.g. bearings [[149], [150], [151], [152]]. It is also used in biomedical applications such as spinal implants [153].
The main advantage of silicon nitride, SiNx, as a coating for joint implants has been shown to be, not only its ability to reduce wear, but also to minimize the adverse immune response to released ions and particles. SiNx dissolves in aqueous solutions into only biocompatible elements [154], which could mean that the generated wear particles would dissolve in the body without triggering the immune system response that would eventually lead to bone resorption. It should be noted however that ammonia is formed during the dissolution of SiNx, which may result in an elevated pH. However, this has been found to be beneficial in terms of antibacterial properties [155,156]. Further, there needs to be a compromise between the dissolution of the wear particles and the requirement of a coating that provides sufficient performance for the intended period of use i.e. significantly greater than 20–25 years.
SiNx coatings have been manufactured both through PVD and CVD methods with a wide range of hardness (12–24 GPa) [47,49,50,53] and Young's moduli (173–293 GPa) [47,49,50]. Specific wear rate measurements through pin-on-disc setups have found reduced wear compared to CoCr [49,53]. The generated wear debris was investigated in a study where a ball-on-disc set-up was used to produce the same, which was found to lie in the range of 0.01–0.05 μm [157]. It was noticed that the debris agglomerated and that these agglomerates were in the range of 0.15–1.96 μm, while the individual particles measured between 0.01 and 0.05 μm in size. It was also found that the pH increased from 7.45 to around 8 after 20 days, likely due to the formation of ammonia [158,159], as mentioned above.
Biocompatibility studies have mainly been focused on simulated wear particles, often commercially available alternatives. The particles, both micron-scale and nanoscale, did not give rise to any significant release of proinflammatory cytokines in a study with primary human peripheral blood mononuclear cells [160]. Bulk Si3N4 has also been found to show no cytotoxic effects [161,162] but instead antiviral properties [163].
The aforementioned dissolution behaviour of the coatings was investigated in simulated body fluid (25 vol% foetal bovine serum diluted in phosphate buffer saline solution) for up to 60 days (Fig. 2). The coatings successfully reduced the metal ion release by two orders of magnitude, as compared to uncoated CoCr references. The dissolution rates of the coatings were lower or comparable to the CoCr (0.2–1.2 nm/day for SiNx coatings compared to 0.7–1.2 nm/day for CoCr) [48]. It should be noted however, that the dissolution rate will depend on factors such as composition and density of the coating - an increased nitrogen content has e.g. found to yield lower dissolution rates [48]. Recent studies have also found that alloying the SiN coating with Fe and C or Cr and Nb could give reduced dissolution rates [164].
Fig. 2.
Transmission electron microscopy (TEM) investigations of the interface of a DLC coating on a CoCr substrate. Both the TEM image (a), high resolution TEM image (b) reveal the presence of a metal carbide, which could lead to delamination when exposed to the environment of the body [139]. Reprinted by permission from Acta Materialia, Elsevier.
5.3. Chromium nitride
Similar to other ceramic coatings CrN coatings have been shown to increase hardness and reduce wear compared to CoCr [44,165], as well as reducing the release of metal ions. The added advantage of CrN coatings is the possibility to achieve a CrN surface layer through e.g. plasma nitriding. Because of its potential for strong adhesion CrN is also sometimes used as an interlayer between the substrate and a top coating [46,52].
In a study comparing the performance of CrN with TiN, TiAlN and DLC, CrN was found to exhibit superior corrosion and wear resistance [131]. CrN coatings have been subject to additional investigation whereby coated CoCr femoral heads were paired with PE cups and tested in a hip simulator for 5 million cycles. The CrN coatings were shown to produce similar amounts of PE wear under standard conditions compared to adverse conditions (9.5 mm3/mc and 12 mm3/mc for standard and adverse conditions – for the latter alumina particles were introduced to simulate third body abrasive wear), while the uncoated CoCr head showed a large increase in wear for the adverse conditions (9.2 mm3/mc and 469 mm3/mc under standard and adverse conditions, respectively) [166]. Another study comparing TiN, CrN, CrCN and DLC coatings in a hip simulator showed a 36-fold reduction in wear rate for self-mating CrN and CrCN coatings compared with uncoated CoCrMo MoM implants. In addition, the ion release was dramatically reduced [165].
Another method of creating a CrN rich surface is through incorporation of nitrogen into the surface of CoCr using reactive plasma. This was reported by Wang et al. [136], who exposed CoCr substrates to a plasma of NH3 at a constant pressure of 500 Pa for 9 h. The formation of a CrN and Cr2N layer was confirmed by X-ray diffraction (XRD), and evaluation showed that the nitrided surface was harder and had lower wear rates compared to the untreated surface when in contact with a cemented carbide (WC/Co) ball during ball-on-disc wear tests. By using different plasma gases it is possible to obtain both nitrided and e.g. carbonitrided surface layers [135]. This was investigated by Liu et al. [135], who found both plasma nitriding and plasma carbonitriding of CoCr increased the hardness and wear resistance as compared to untreated CoCr. In addition, corrosion resistance was improved for both treatments, and the carbonitrided surface showed a better corrosion resistance compared to the nitrided surface.
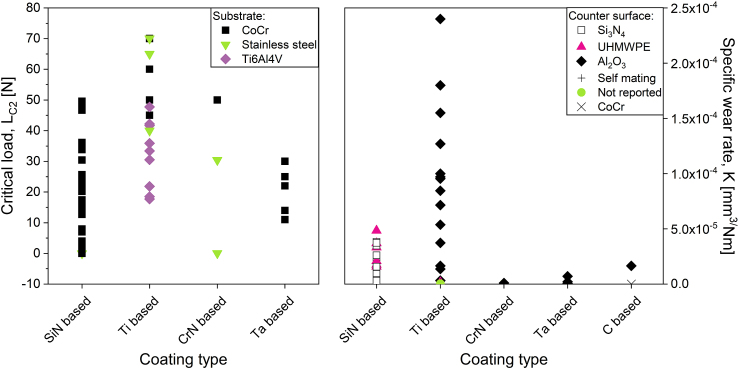
Reported wear rate values of CrN based coatings have been consistently low (1∙10−6 to 8.8∙10−7 mm3/Nm) [44,167] and adhesion tests have given high critical load values (LC2) - up to 50 N for CrN/NbN deposited on CoCr substrates [44,45]. Overall the low wear rates and contingency of good adhesion make CrN-based coatings potentially suitable for joint implants.
5.4. Zirconia and yttria-stabilized zirconia
Zirconia and yttria-stabilized zirconia have been investigated as potential candidate coatings for orthopaedic implants because of their ability to reduce wear. The number of studies available on these materials for this application are still limited but the results are comparable to other investigated coatings [168,169]. These coatings were deposited onto a substrate such as titanium and should not be confused with the surface treated Oxinium implants.
The wear properties of yttria-stabilized zirconium dioxide (YSZ)-coated titanium balls paired with UHMWPE have been evaluated in a ball-on-disc set-up using different lubricants. The coatings were found to decrease the wear of the PE under dry conditions and when lubricated with a NaCl solution. However, when lubricated with a serum solution i.e. under conditions more closely emulating those of a natural joint, the results were similar to those obtained for the uncoated Ti spheres [169] (3–13∙10−4 mm3/Nm for dry conditions and 3–8∙10−4 mm3/Nm for serum solution as a lubricant; the root mean square surface roughness of the coatings ranged from 28 ± 1 nm to 60 ± 5 nm). The coatings did not exhibit any cytotoxicity when tested with mesenchymal stem cells and pre-osteoblast cell lines [168], indicating that they are biocompatible and could be useful as coatings for orthopaedic implants.
One way of achieving a surface oxide layer is through thermal oxidation, this was conducted by Luo et al. who oxidized a ZrNb alloy. An increased treatment temperature (of 700 °C compared to 500 °C) improved both the hardness and wear resistance [170].
The hydrolytic long-term stability of zirconia-based material remains a concern however [171,172], and would have to be thoroughly investigated before being an option for biomedical implants.
5.5. Alloyed and structured titanium nitride and carbide
While titanium nitride has successfully made it to the market (Implantcast, Cellumed, Link medical technology and Link orthopaedics) there is still ongoing research aimed to improving these coatings. One such strategy is to alloy the TiN coating with one or more elements and another is using a multi-layer structure. These strategies have the potential to further reduce the wear and/or improve adhesion.
Although alloying TiN with elements such as aluminium has been found to increase the hardness and Young's modulus, and decrease the wear rate compared to Ti6Al4V, however it should again be noted that CoCr is more commonly used in articulating surfaces and should be the material used for comparison [54]. Other investigated alloying elements include niobium and carbon, which have been shown to be non-cytotoxic [39]. Titanium carbide has also been shown to reduce wear compared to uncoated Ti6Al4V when tested in a reciprocating pin-on-disc set-up [173].
Another strategy for the improvement of wear properties is to deposit multilayers. The mechanical and tribological properties of such a coating (TiN/CrN) has been investigated and compared to a TiN monolayer. The multilayer structures reduced the friction coefficient, however, the wear rate was not reported [42].
Another option is to create a ceramic surface layer, by e.g. heat-accelerated diffusion. There are several techniques available for alloying the surface of a material by exposure to elevated temperatures in a controlled environment. Another example is the powder immersion reaction assisted coating (PIRAC). Yet another process whereby nitrogen is incorporated into the surface is laser nitriding, where the substrate is irradiated with a laser in a chamber with nitrogen gas. The laser-illuminated area of the surface will melt and create a plasma above it. Subsequently the nitrogen, which is now ionized, will be absorbed by the melted surface. All methods result in increased hardness as well as increased wear resistance compared with Ti6Al4V. The PIRAC method and laser melting did however yield an increased surface roughness compared to an untreated reference [43,133,[174], [175], [176]].
While TiN based coatings offer a possibility to improve the wear properties of Ti6Al4V, they are in many of the published current studies compared only to uncoated Ti6Al4V, which makes it challenging to assess the efficacy of these proposed coatings.
5.6. Tantalum carbide, tantalum oxide and tantalum nitride
Porous tantalum is used in orthopaedic applications such as cranioplasty plates and hip implant fixation due to its osseintegrative properties [177]. Proven to be non-cytotoxic, tantalum-rich coatings, such as TaN and TaO, have been proposed for application on the articulating surfaces of joint implants due to their corrosion resistance, promising results in vitro [177] and favourable mechanical properties.
Tantalum carbide coatings deposited on CoCr by thermal treatment in molten salts have been investigated with regards to their adhesion, mechanical and tribological properties [36,178]. The coatings were found to have a TaC or Ta2C-TaC structure depending on the carbon content, manufacturing process of the CoCr substrate and temperature during coating growth. In addition, the coatings were deposited in a multilayer structure with layers comprising different carbon contents. The thickness of the coatings varied depending on structure and ranged from 300 to 1000 nm. The structure of the coating proved important for adhesion, where a multilayer structure (TaC-Ta2C-Ta) yielded higher critical loads during scratch tests (delamination at 30 N compared with delamination at 14 N for a single layer TaC) [36]. When evaluating hardness and Young's modulus by nanoindentation (with a maximum load of 10 mN), the hardness was significantly increased (27 GPa for a multi-layer structure and 23 GPa for a single layer coating compared to 12 GPa for the uncoated substrate) [178]. The wear volume obtained in a pin-on-disc set-up with 25 vol% bovine serum diluted in distilled water as lubricant was similar for the different multi-layer structures. The wear was reduced compared to the uncoated substrate, however there were no discernible differences between coatings in terms of wear performance. Noteworthy is that during wear testing, third body abrasion was the most prominent wear mechanism due to pull-out of carbides that acted as third body abrasive particles.
Investigation of the corrosion and wear resistance of tantalum oxide (TaO2) deposited onto Ti6Al4V has shown improved corrosion properties with an increased corrosion potential, reduced anodic current and reduced Ti ion release (Icorr of 6.770∙10−8 A/cm2 for TaO compared to an Icorr of 2.560∙10−7 A/cm2 for Ti6Al4V). The wear volume of the coated samples was reduced compared to Ti6Al4V (2.22 mm3/Nm compared to 7.78 mm3/Nm for uncoated Ti6Al4V) [179]. Again, a limitation of the study was the comparison only to Ti6Al4V. Another study where TaN was deposited on CoCr by RF sputtering the coatings were shown to have comparable or lower wear rates of a PE counter surface to uncoated CoCr [180].
In summary, tantalum carbide and oxide coatings have been shown to be biocompatible, but their wear performance in the application requires further investigations.
5.7. Alumina based coatings
In addition to the previously discussed coatings alumina based coatings have been proposed as an option. These coatings include monolithic micron alumina (IDA), micron alumina yttria-stabilized zirconia (YSZ) composite coating (IDAZ), and nanostructured alumina titania/YSZ (IDZAT) deposited on Ti-6Al-4V alloy and have been shown to have better wear and corrosion compared to Ti6Al4V [181].
6. Discussion
This review has provided a comprehensive overview of both commercially available coated implants, as well as coatings currently being researched for potential application in joint implants. The coated implants currently available in the market are ZrO2 coated Zr (Smith and Nephew), TiN coated CoCr or Ti6Al4V (Implantcast), Link orthopaedic), TiNbN coated CoCr (OHST medical technology) and TiN coated Ti6Al4V (Endotec). Reported follow up studies as well as case studies of retrieved implants have revealed revision rates comparable to traditional CoCr implants [104,105,[124], [125], [126]]. The potential coating materials currently being investigated include SiNx, CrN, TaO, TaC, DLC, TiN, TiCN, ZrO2 and ZrN. Looking at total knee replacements the use of coatings is more widespread and the retrieval data reveals the coated implants to be comparable to CoCr but there is not enough evidence to support a lower amount of osteolysis-caused failures [80,113,[116], [117], [118], [119], [120], [121], [122], [123], [124], [125]].
When comparing the specific wear rates of coatings currently being researched (Fig. 3b) it is evident that all coating types (silicon nitride based, titanium based, chromium nitride based, tantalum based and DLC) have the potential for low wear rates. However, it is difficult to distinguish between them since i) different set-ups have been used to assess the wear performance, and ii) different counter surfaces have been used in the tests, i.e. hard-on-hard (e.g. alumina) or hard-on-soft (a polymer). The available standards cover procedures for both types of material combinations, including suitable testing conditions. However, even when the testing is performed in accordance with the standards it might not reflect the full range of loading scenarios the implant will be exposed to. To better predict clinical outcome the current standardized approaches require further development. Important considerations for such an approach are of course the choice of material combinations, i.e. hard-on-hard versus hard-on-soft as well as the effect of activities other than steady state gait. Common activities such as standing up from a chair and climbing stairs put a higher demand on the implant and will affect the implant performance.
Fig. 3.
A comparison of the LC2 values [36,45,[51], [52], [53], [54],134] (a) and specific wear rates from ball/pin-on-disc evaluations [46,49,[51], [52], [53], [54],129,131,[134], [134],173,179,183] (b) found for coatings currently being researched. The reported values are divided into groups based on the main constituents of the coating.
The previous introduction to and subsequent removal from the market of DLC coated implants illustrates the need for more rigorous testing before clinical use. DLC coated implants were removed from the market after the revelation that exposure to synovial fluid over time led to a delamination of the coatings due to the instability of metal carbides in the interlayer, which in turn caused early revisions. Scratch and Rockwell tests typically evaluate the adhesion of as-deposited coatings, but typically do not consider corrosion or changes to the coating over time. To better predict the adhesion of the coating, the scratch or Rockwell tests can e.g. be performed on coatings exposed to liquid at different soaking times [52]. In this review, DLC coatings were included as materials that are not currently in the market but show potential for implant applications. Having been introduced to the market and suffered from early implant revisions, DLC coatings are often not considered an option. However, the mechanisms causing the delamination have since been investigated and are now better understood. By using interlayers, DLC coatings could be stable long-term, reduce wear and potentially increase implant longevity.
In summary, several of the investigated coatings show potential because of their ability to reduce wear and ion release. Different coatings carry different advantages, e.g. CrN on CoCr and TiN on Ti6Al4V can give enhanced adhesion. One material that has the potential to provide additional biological advantages is silicon nitride, which has demonstrated antibacterial and antiviral properties.
Revision and follow up studies of commercially available coatings have found revision rates to be comparable to, but not better than, conventional CoCr implants. This could be due to low revision rates or not long enough follow-up studies (Table 2) [55,80,96,[104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128]]. The signs of wear and damage of the PE countersurface has been found to be lower for oxidized zirconium knee implants compared with CoCr [120], which is promising as it speaks to the potential for reduced generation of PE debris. Since this debris is believed to be a major cause of revision its reduction could lead to a longer implant lifetime. Assuming the coating adheres well to the substrate it is possible to have a biocompatible surface that generates low amounts of wear debris and ion release, which would be ideal for articulating surfaces.
Whilst much of the coating work has focussed on the application of coatings to bearing surfaces to reduce wear and corrosion, there is now also a growing interest in translating such technologies to other interfaces, such as hip modular-tapers, where fretting-corrosion processes may dominate. The ability to develop novel multi-material systems also paves the way for a new generation of multi-functional coating technologies to combat the aforementioned issues as well as the emerging grand challenges within the area of orthopaedics (e.g. infection and treatment of metastatic cancers).
7. Conclusions
In conclusion,
-
•
Coated implants are available in the marked. These implants include TiN, TiNbN, ZrN coatings and surface treated Zr resulting in an oxidized surface layer.
-
•
Several candidate coating materials such as carbon-based, silicon nitride, chromium nitride, Ti-based, Zr-based, Ta-based and alumina-based are being researched.
-
•
Coated implants exhibit comparable survival rates to uncoated implants, however the basis for assessment is limited due to generally low revision rates and short follow-up times.
-
•
Coatings could be relevant for other surfaces such as modular interfaces.
Data availability
The data in this review consists of information found in published research papers.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Funding from the European Union's Seventh Framework Program (FP7/2007–2013), grant agreement GA-310477 (Life-Long Joints) and the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 812765 (NU-SPINE).
Susan Peacock is gratefully acknowledged for her helpful comments and invaluable discussions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mtbio.2022.100270.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 2.Norwegian Arthroplasty Register . 2019. Annual Report.http://nrlweb.ihelse.net/eng/ [Google Scholar]
- 3.Swedish Hip Arthroplasty Register . 2018. Annual Report.https://shpr.registercentrum.se/om-registret-1/arsrapporter/p/Hys5hJaLg [Google Scholar]
- 4.Australian Orthopaedic Association National Joint Replacement Registry . 2019. Annual Report.https://aoanjrr.sahmri.com/annual-reports-2019 [Google Scholar]
- 5.N.I. and the I. of M. National Joint Registry for England, Wales . 2019. Annual Report. [Google Scholar]
- 6.Canadian Joint Replacement Registry . 2018. Annual Report.https://www.cihi.ca/en/canadian-joint-replacement-registry-cjrr [Google Scholar]
- 7.The New Zealand Joint Registry . 2018. Annual Report.https://nzoa.org.nz/nzoa-joint-registry [Google Scholar]
- 8.Swedish Knee Arthroplasty Register . 2019. Annual Report.http://www.myknee.se/en/publications/annual-reports [Google Scholar]
- 9.Danish Hip Arthroplasty Register . 2019. Annual Report.http://danskhoftealloplastikregister.dk/en/publications/annual-reports/ [Google Scholar]
- 10.American Joint Replacement Registry . 2019. Annual Report.https://www.aaos.org/registries/publications/ajrr-annual-report/ [Google Scholar]
- 11.Chakrabarty G., Vashishtha M., Leeder D. Polyethylene in knee arthroplasty: a review. J. Clin. Orthop. Trauma. 2015;6:108–112. doi: 10.1016/j.jcot.2015.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matharu G.S., Pandit H.G., Murray D.W., Judge A. Adverse reactions to metal debris occur with all types of hip replacement not just metal-on-metal hips: a retrospective observational study of 3340 revisions for adverse reactions to metal debris from the National Joint Registry for England, Wales, Northe. BMC Muscoskel. Disord. 2016;17:1–12. doi: 10.1186/s12891-016-1329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nawabi D.H., Gold S., Lyman S., Fields K., Padgett D.E., Potter H.G. MRI predicts ALVAL and tissue damage in metal-on-metal hip arthroplasty. Clin. Orthop. Relat. Res. 2014;472:471–481. doi: 10.1007/s11999-013-2788-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korovessis P., Petsinis G., Repanti M., Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty. J. Bone Jt. Surg. 2006;88:1183–1191. doi: 10.2106/JBJS.D.02916. [DOI] [PubMed] [Google Scholar]
- 15.Ingham E., Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–1286. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Hallab N.J., Jacobs J.J. Biologic effects of implant debris. Bull. NYU Hosp. Jt. Dis. 2009;67:182–188. [PubMed] [Google Scholar]
- 17.Amstutz H.C., Campbell P., Kossovsky N., Clarke I. Mechanism and clinical significance of wear debris induced osteolysis. Clin. Orthop. Relat. Res. 1992:7–18. [PubMed] [Google Scholar]
- 18.Dumbleton J.H., Manley M.T., Edidin A.A. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J. Arthroplasty. 2002;17:649–661. doi: 10.1054/arth.2002.33664. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs J.J., Roebuck K.A., Archibeck M., Hallab N.J., Glant T.T. Osteolysis: basic science. Clin. Orthop. Relat. Res. 2001;71–7 doi: 10.1097/00003086-200112000-00008. http://www.ncbi.nlm.nih.gov/pubmed/11764373 [DOI] [PubMed] [Google Scholar]
- 20.Smith A.J., Dieppe P., Howard P.W., Blom A.W. Failure rates of metal-on-metal hip resurfacings: analysis of data from the national joint registry for England and Wales. Lancet. 2012;380:1759–1766. doi: 10.1016/S0140-6736(12)60989-1. [DOI] [PubMed] [Google Scholar]
- 21.Doorn P.F., Campbell P.A., Worrall J., Benya P.D., McKellop H.A., Amstutz H.C. Metal wear particle characterization from metal on metal total hip replacements: Transmission electron microscopy study of periprosthetic tissues and isolated particles. J. Biomed. Mater. Res. 1998;42:103–111. doi: 10.1002/(SICI)1097-4636(199810)42:1<103::AID-JBM13>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 22.Granchi D., Ciapetti G., Stea S., Savarino L., Filippini F., Sudanese a, Zinghi G., Montanaro L. Cytokine release in mononuclear cells of patients with Co-Cr hip prosthesis. Biomaterials. 1999;20:1079–1086. doi: 10.1016/s0142-9612(99)00004-6. [DOI] [PubMed] [Google Scholar]
- 23.Keegan G.M., Learmonth I.D., Case C.P. Orthopaedic metals and their potential toxicity in the arthroplasty patient: a review OF current knowledge and future strategies. J. Bone Jt. Surg. - Br. 2007;89-B:567–573. doi: 10.1302/0301-620X.89B5.18903. [DOI] [PubMed] [Google Scholar]
- 24.Huber M., Reinisch G., Trettenhahn G., Zweymüller K., Lintner F. Presence of corrosion products and hypersensitivity-associated reactions in periprosthetic tissue after aseptic loosening of total hip replacements with metal bearing surfaces. Acta Biomater. 2009;5:172–180. doi: 10.1016/j.actbio.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 25.Fleury C., Petit A., Mwale F., Antoniou J., Zukor D.J., Tabrizian M., Huk O.L. Effect of cobalt and chromium ions on human MG-63 osteoblasts in vitro: morphology, cytotoxicity, and oxidative stress. Biomaterials. 2006;27:3351–3360. doi: 10.1016/j.biomaterials.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 26.Campbell J.R., Estey M.P. Metal release from hip prostheses: cobalt and chromium toxicity and the role of the clinical laboratory. Clin. Chem. Lab. Med. 2013;51:213–220. doi: 10.1515/cclm-2012-0492. [DOI] [PubMed] [Google Scholar]
- 27.Sugano N., Iida H., Akiyama H., Takatori Y., Nagoya S., Hasegawa M., Kabata T., Hachiya Y., Yasunaga Y. Nationwide investigation into adverse tissue reactions to metal debris after metal-on-metal total hip arthroplasty in Japan. J. Orthop. Sci. 2014;19:85–89. doi: 10.1007/s00776-013-0490-2. [DOI] [PubMed] [Google Scholar]
- 28.Kurtz S.M., Gawel H.A., Patel J.D. History and systematic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene. Clin. Orthop. Relat. Res. 2011;469:2262–2277. doi: 10.1007/s11999-011-1872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen Q., Raeymaekers B. Surface texturing of prosthetic hip implant bearing surfaces: a review. J. Tribol. 2021;143 doi: 10.1115/1.4048409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lappalainen R., Santavirta S.S. Potential of coatings in total hip replacement. Clin. Orthop. Relat. Res. 2005:72–79. doi: 10.1097/01.blo.0000150000.75660.ff. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh S., Abanteriba S. Status of surface modification techniques for artificial hip implants. Sci. Technol. Adv. Mater. 2016;17:715–735. doi: 10.1080/14686996.2016.1240575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ching H.A., Choudhury D., Nine M.J., Abu Osman N.A. Effects of surface coating on reducing friction and wear of orthopaedic implants. Sci. Technol. Adv. Mater. 2014;15 doi: 10.1088/1468-6996/15/1/014402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y., Li H., Ji L., Liu X., Wu Y., Lv Y., Fu Y., Zhou H., Chen J. Synthesis and characterization of titanium-containing graphite-like carbon films with low internal stress and superior tribological properties. J. Phys. D Appl. Phys. 2012;45 doi: 10.1088/0022-3727/45/29/295301. [DOI] [Google Scholar]
- 34.Chen J., Wang Y., Li H., Ji L., Wu Y., Lv Y., Liu X., Fu Y., Zhou H. Microstructure, morphology and properties of titanium containing graphite-like carbon films deposited by unbalanced magnetron sputtering. Tribol. Lett. 2013;49:47–59. doi: 10.1007/s11249-012-0041-6. [DOI] [Google Scholar]
- 35.Bisht A., Chockalingam S., Tripathi R.K., Dwivedi N., Dayal S., Kumar S., Panwar O.S., Chand J., Singh S., Kesarwani A. Improved surface properties of β-SiAlON by diamond-like carbon coatings. Diam. Relat. Mater. 2013;36:44–50. doi: 10.1016/j.diamond.2013.04.004. [DOI] [Google Scholar]
- 36.Balagna C., Faga M.G., Spriano S. Tantalum-based multilayer coating on cobalt alloys in total hip and knee replacement. Mater. Sci. Eng. C. 2012;32:887–895. doi: 10.1016/j.msec.2012.02.007. [DOI] [Google Scholar]
- 37.Thorwarth K., Thorwarth G., Figi R., Weisse B., Stiefel M., Hauert R. On interlayer stability and high-cycle simulator performance of diamond-like carbon layers for articulating joint replacements. Int. J. Mol. Sci. 2014 doi: 10.3390/ijms150610527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oñate J.I., Comin M., Braceras I., Garcia A., Viviente J.L., Brizuela M., Garagorri N., Peris J.L., Alava J.I. Wear reduction effect on ultra-high-molecular-weight polyethylene by application of hard coatings and ion implanation on cobalt chromium ally, as measured in a knee wear simulation machine. Surf. Coating. Technol. 2001;142–144:1056–1062. doi: 10.1016/S0257-8972(01)01074-X. [DOI] [Google Scholar]
- 39.Serro A.P., Completo C., Colaço R., dos Santos F., da Silva C.L., Cabral J.M.S., Araújo H., Pires E., Saramago B. A comparative study of titanium nitrides, TiN, TiNbN and TiCN, as coatings for biomedical applications. Surf. Coating. Technol. 2009;203:3701–3707. doi: 10.1016/j.surfcoat.2009.06.010. [DOI] [Google Scholar]
- 40.Ruiz de Gopegui U., Bayón R., Zubizarreta C., Sáenz de Viteri V., Fernández X. Carbon containing titanium coating for Ti6Al4V implants. ECS Trans. 2010;25:17–32. doi: 10.1149/1.3298946. [DOI] [Google Scholar]
- 41.Sáenz de Viteri V., Barandika G., Bayón R., Fernández X., Ciarsolo I., Igartua A., Pérez Tanoira R., Moreno J.E., Peremarch C.P.J. Development of Ti-C-N coatings with improved tribological behavior and antibacterial properties. J. Mech. Behav. Biomed. Mater. 2015;55:75–86. doi: 10.1016/j.jmbbm.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Gallegos-Cantú S., Hernandez-Rodriguez M.A.L., Garcia-Sanchez E., Juarez-Hernandez A., Hernandez-Sandoval J., Cue-Sampedro R. Tribological study of TiN monolayer and TiN/CrN (multilayer and superlattice) on Co–Cr alloy. Wear. 2015;330–331:439–447. doi: 10.1016/j.wear.2015.02.010. [DOI] [Google Scholar]
- 43.Chan C.W., Lee S., Smith G.C., Donaghy C. Fibre laser nitriding of titanium and its alloy in open atmosphere for orthopaedic implant applications: investigations on surface quality, microstructure and tribological properties. Surf. Coating. Technol. 2017;309:628–640. doi: 10.1016/j.surfcoat.2016.12.036. [DOI] [Google Scholar]
- 44.Hovsepian P.E., Ehiasarian A.P., Purandare Y., Sugumaran A.A., Marriott T., Khan I. Development of superlattice CrN/NbN coatings for joint replacements deposited by high power impulse magnetron sputtering. J. Mater. Sci. Mater. Med. 2016;27 doi: 10.1007/s10856-016-5751-0. [DOI] [PubMed] [Google Scholar]
- 45.Huang W., Zalnezhad E., Musharavati F., Jahanshahi P. Investigation of the tribological and biomechanical properties of CrAlTiN and CrN/NbN coatings on SST 304. Ceram. Int. 2017;43:7992–8003. doi: 10.1016/j.ceramint.2017.03.081. [DOI] [Google Scholar]
- 46.Filho L., Schmidt S., Leifer K., Engqvist H., Högberg H., Persson C. Towards functional silicon nitride coatings for joint replacements. Coatings. 2019;9:1–10. doi: 10.3390/COATINGS9020073. [DOI] [Google Scholar]
- 47.Skjöldebrand C., Schmidt S., Vuong V., Pettersson M., Grandfield K., Högberg H., Engqvist H., Persson C. Influence of substrate heating and nitrogen flow on the composition, morphological and mechanical properties of SiNx coatings aimed for joint replacements. Materials. 2017;10:1–11. doi: 10.3390/ma10020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pettersson M., Bryant M., Schmidt S., Engqvist H., Hall R.M., Neville A., Persson C. Dissolution behaviour of silicon nitride coatings for joint replacements. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016;62:497–505. doi: 10.1016/j.msec.2016.01.049. [DOI] [PubMed] [Google Scholar]
- 49.Pettersson M., Tkachenko S., Schmidt S., Berlind T., Jacobson S., Hultman L., Engqvist H., Persson C. Mechanical and tribological behavior of silicon nitride and silicon carbon nitride coatings for total joint replacements. J. Mech. Behav. Biomed. Mater. 2013;25:41–47. doi: 10.1016/j.jmbbm.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Shi Z., Wang Y., Du C., Huang N., Wang L., Ning C. Silicon nitride films for the protective functional coating: blood compatibility and biomechanical property study. J. Mech. Behav. Biomed. Mater. 2012;16:9–20. doi: 10.1016/j.jmbbm.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 51.Filho L.C., Schmidt S., Goyenola C., Skjöldebrand C., Engqvist H., Högberg H., Toble M., Persson C. The effect of N, C, Cr, and Nb content on silicon nitride coatings for joint applications. Materials. 2020;13 doi: 10.3390/MA13081896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Filho L.C., Schmidt S., López A., Cogrel M., Leifer K., Engqvist H., Högberg H., Persson C. The effect of coating density on functional properties of SiNx coated implants. Materials. 2019;12:11–14. doi: 10.3390/ma12203370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olofsson J., Pettersson M., Teuscher N., Heilmann A., Larsson K., Grandfield K., Persson C., Jacobson S., Engqvist H. Fabrication and evaluation of SixNy coatings for total joint replacements. J. Mater. Sci. Mater. Med. 2012;23:1879–1889. doi: 10.1007/s10856-012-4625-3. [DOI] [PubMed] [Google Scholar]
- 54.Yi P., Peng L., Huang J. Multilayered TiAlN films on Ti6Al4V alloy for biomedical applications by closed field unbalanced magnetron sputter ion plating process. Mater. Sci. Eng. C. 2016;59:669–676. doi: 10.1016/j.msec.2015.10.071. [DOI] [PubMed] [Google Scholar]
- 55.Garvin K.L., Hartman C.W., Bs N.M., Martell J.M. Wear analysis in THA utilizing oxidized zirconium and crosslinked polyethylene. Clin. Orthop. Relat. Res. 2009:141–145. doi: 10.1007/s11999-008-0544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Implantcast, ic-head, (n.d.). https://www.implantcast.de/en/for-medical-professionals/products/standard-tumour-prosthetics/pelvis-and-hip-endoprosthetics/primary-endoprosthetics-femoral-heads-acetabulum/ic-head/%0A.
- 57.Endotec, B-PTM Integrated Hip System, (n.d.). http://www.endotec.com/main/sub01_02_01.php.
- 58.ASTM E2546-07 . 2007. Standard Practice for Instrumented Indentation Testing. [DOI] [Google Scholar]
- 59.ISO 14577-4 . 2016. Metallic Materials — Instrumented Indentation Test for Hardness and Materials Parameters — Part 4: Test Method for Metallic and Non-metallic Coatings. [Google Scholar]
- 60.EN 1071-12 . 2001. Advanced Technical Ceramics – Methods of Test for Ceramic Coatings – Part 12: Reciprocating Wear Test. [Google Scholar]
- 61.EN 1071-13 . 2010. Advanced technical ceramics. Methods of test for ceramic coatings - Part 13: Determination of Wear Rate by the Pin-on-disk Method. [Google Scholar]
- 62.ASTM F732 . 2000. Standard Test Method for Wear Testing of Polymeric Materials Used in Total Joint. [DOI] [Google Scholar]
- 63.ISO 20808 . 2016. Fine Ceramics (advanced Ceramics, advanced Technical Ceramics) - Determination of Friction and Wear Characteristics of Monolithic Ceramics by Ball-On-Disc Method. [Google Scholar]
- 64.ASTM G5, Standard Reference Test Method for Making Potentiodynamic Anodic Polarization Measurements, (n.d.). http://www.astm.org/cgi-bin/resolver.cgi?G5-14e1.
- 65.ISO 16429 . 2004. Implants for Surgery — Measurements of Open-circuit Potential to Assess Corrosion Behaviour of Metallic Implantable Materials and Medical Devices Over Extended Time Periods. [Google Scholar]
- 66.ISO 16773-1 . 2012. Electrochemical Impedance Spectroscopy (EIS) on Coated and Uncoated Metallic Specimens.www.sis.se 08. [Google Scholar]
- 67.ISO 10993 . 2009. Biological Evaluation of Medical Devices.http://library1.nida.ac.th/termpaper6/sd/2554/19755.pdf [Google Scholar]
- 68.ISO 4287 . 2013. Geometrical Product Specifications (GPS) — Surface Texture: Profile Method — Terms, Definitions and Surface Texture Parameters Amendment 1: Peak Count Number. [Google Scholar]
- 69.ISO 4287 . 1997. Geometrical Product Specifications (GPS) - Surface Texture: Profile Method - Terms, Definitions and Surface Texture Parameters. [Google Scholar]
- 70.ISO 4288 . 1996. Geometrical Product Specifica- tions (GPS) – Surface Texture: Profile Method – Rules and Proce- dures for the Assessment of Surface Texture. [Google Scholar]
- 71.ISO 25178-604:2013 . 2013. Geometrical Product Specifications (GPS) - Surface Texture: Areal - Part 604: Nominal Characteristics of Non-contact (Coherence Scanning Interferometry) Instruments; pp. 1–4. [Google Scholar]
- 72.ISO 26443 . 2008. Internation Standard: Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics) - Rockwell Indentation Test for Evaluation of Adhesion of Ceramic Coatings. [Google Scholar]
- 73.ISO 20502 . 2015. Fine Ceramics (advanced Ceramics, advanced Technical Ceramics) - Determination of adhesion of Ceramic Coatings by Scratch Testing. [Google Scholar]
- 74.Kelly P.J., Arnell R.D. Magnetron sputtering: a review of recent developments and applications. Vacuum. 2000;56:159–172. doi: 10.1016/S0042-207X(99)00189-X. [DOI] [Google Scholar]
- 75.Santecchia E., Hamouda A.M.S., Musharavati F., Zalnezhad E., Cabibbo M., Spigarelli S. Wear resistance investigation of titanium nitride-based coatings. Ceram. Int. 2015;41:10349–10379. doi: 10.1016/j.ceramint.2015.04.152. [DOI] [Google Scholar]
- 76.Heimann R.B., Lehmann L.D. Wiley VCH; 2015. Bioceramic Coatings for Medical Implants - Trends and Techniques. [Google Scholar]
- 77.Geetha M., Singh A.K., Asokamani R., Gogia A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants - a review. Prog. Mater. Sci. 2009;54:397–425. doi: 10.1016/j.pmatsci.2008.06.004. [DOI] [Google Scholar]
- 78.Hussein M.A., Mohammed A.S., Al-Aqeeli N. Wear characteristics of metallic biomaterials: a review. Materials. 2015;8:2749–2768. doi: 10.3390/ma8052749. [DOI] [Google Scholar]
- 79.Chen Q., Thouas G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Reports. 2015;87:1–57. doi: 10.1016/j.mser.2014.10.001. [DOI] [Google Scholar]
- 80.Herbster M., Döring J., Nohava J., Lohmann C.H., Halle T., Bertrand J. Retrieval study of commercially available knee implant coatings TiN, TiNbN and ZrN on TiAl6V4 and CoCr28Mo6. J. Mech. Behav. Biomed. Mater. 2020;112 doi: 10.1016/j.jmbbm.2020.104034. [DOI] [PubMed] [Google Scholar]
- 81.Mattox D.M. 2010. Handbook of Physical Vapor Deposition (PVD) Processing. [Google Scholar]
- 82.Choy K.L. Chemical vapour deposition of coatings. Prog. Mater. Sci. 2003;48:57–170. doi: 10.1016/S0079-6425(01)00009-3. [DOI] [Google Scholar]
- 83.Abegunde O.O., Akinlabi E.T., Oladijo O.P., Akinlabi S., Ude A.U. Overview of thin film deposition techniques. AIMS Mater. Sci. 2019;6:174–199. doi: 10.3934/MATERSCI.2019.2.174. [DOI] [Google Scholar]
- 84.Baptista A., Silva F., Porteiro J., Míguez J., Pinto G. Sputtering physical vapour deposition (PVD) coatings: a critical review on process improvement and market trend demands. Coatings. 2018;8 doi: 10.3390/COATINGS8110402. [DOI] [Google Scholar]
- 85.Bobzin K. High-performance coatings for cutting tools. CIRP J. Manuf. Sci. Technol. 2017;18:1–9. doi: 10.1016/j.cirpj.2016.11.004. [DOI] [Google Scholar]
- 86.Fotovvati B., Namdari N., Dehghanghadikolaei A. On coating techniques for surface protection: a review. J. Manuf. Mater. Process. 2019;3 doi: 10.3390/jmmp3010028. [DOI] [Google Scholar]
- 87.Schaaf P. Laser nitriding in materials. Prog. Mater. Sci. 2002;47:1–161. [Google Scholar]
- 88.Chagnon P., Fauchais P. Thermal spraying of ceramics. Ceram. Int. 1984;10:119–131. doi: 10.1016/0272-8842(84)90001-4. [DOI] [Google Scholar]
- 89.Lu X., Mohedano M., Blawert C., Matykina E., Arrabal R., Kainer K.U., Zheludkevich M.L. Plasma electrolytic oxidation coatings with particle additions – a review. Surf. Coating. Technol. 2016;307:1165–1182. doi: 10.1016/j.surfcoat.2016.08.055. [DOI] [Google Scholar]
- 90.Aksa H.C., Hacısalihoğlu İ., Yıldız F., Varol T., Güler O., Kaya G., Akçay S.B. Effects of fabrication parameters and post-processing treatments on the mechanical and tribological behavior of surface-enhanced copper based materials by selective laser melting. J. Mater. Process. Technol. 2022;304 doi: 10.1016/j.jmatprotec.2022.117564. [DOI] [Google Scholar]
- 91.Morlock M.M., Bishop N., Zustin J., Hahn M., Rüther W., Amling M. Modes of implant failure after hip resurfacing: morphological and wear analysis of 267 retrieval specimens. J. Bone Joint Surg. Am. 2008;90(Suppl 3):89–95. doi: 10.2106/JBJS.H.00621. [DOI] [PubMed] [Google Scholar]
- 92.Carr B.C., Goswami T. Knee implants - review of models and biomechanics. Mater. Des. 2009;30:398–413. doi: 10.1016/j.matdes.2008.03.032. [DOI] [Google Scholar]
- 93.Cellumed, Luminus Flex Total Knee System, (n.d.). http://www.cellumed.co.kr/wb_board/view.php?&bbs_code=1561093526&bd_num=694 (accessed May 5, 2021).
- 94.OHST medical technology, Zen Knee mobile bearing ZEN, (n.d.) 0–1.
- 95.Link, Gemini Sl Total Knee Replacement, (n.d.). https://www.linkorthopaedics.com/us/for-the-physician/products/knee-prostheses#prod32 (accessed February 2, 2021).
- 96.Corin, UniglideTM Unicompartmental Knee Replacement, (n.d.). https://www.coringroup.com/legacy/medical_professionals/products/knees/uniglide/index.html (accessed August 21, 2018).
- 97.Corin, AMC MkIITM Mobile Knee System, (n.d.). https://www.coringroup.com/legacy/medical_professionals/products/knees/amc_mkii/index.html.
- 98.B Braun, AS Advanced Surface - 7 Layers to protect you, (n.d.). https://www.bbraun.com/en/products/b0/as-advanced-surface.html%0A (accessed July 11, 2018).
- 99.Smith & Nephew, OXINIUM◊, (n.d.). http://www.smith-nephew.com/key-products/orthopaedic-reconstruction/oxinium-oxidized-zirconium/%0A%0A.
- 100.ISO 5832-3:2016 . 2016. Implants for Surgery – Metallic Materials – Part 3: Wrought Titanium 6-Aluminium 4-Vanadium Alloy.https://www.iso.org/standard/66637.html [Google Scholar]
- 101.ISO 5832-4:2014 . 2014. Implants for Surgery - Metallic Materials - Part 4: Cobalt-chromium-molybdenum Casting Alloy.https://www.iso.org/ics/11.040.40/x/ [Google Scholar]
- 102.ACS® MB SC, (n.d.). https://www.implantcast.de/en/for-medical-professionals/products/standard-tumour-prosthetics/knee-endoprosthetics/revision-tumour-endoprosthetics-knee/acsr-mb-sc/.
- 103.Fabry C., Zietz C., Baumann A., Ehall R., Bader R. High wear resistance of femoral components coated with titanium nitride: a retrieval analysis, Knee Surgery. Sport. Traumatol. Arthrosc. 2018;26:2630–2639. doi: 10.1007/s00167-017-4578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jonsson B.A., Kadar T., Havelin L.I., Espehaug B., Indrekvam K., Furnes O., Hallan G. vol. 97. 2015. pp. 1463–1469. (Oxinium Modular Femoral Heads do not Reduce Polyethylene Wear in Cemented Total Hip Arthroplasty at Five Years). [DOI] [PubMed] [Google Scholar]
- 105.Kadar T., Hallan G., Aamodt A., Indrekvam K., Badawy M., Skredderstuen A., Havelin L.I., Stokke T., Haugan K., Espehaug B., Furnes O. 2011. Wear and Migration of Highly Cross-linked and Conventional Cemented Polyethylene Cups with Cobalt Chrome or Oxinium Femoral Heads: A Randomized Radiostereometric Study of 150 Patients; pp. 1222–1229. [DOI] [PubMed] [Google Scholar]
- 106.Tan S.C., Edin F., Lau A.C.K., Edin F., Del Balso C., Howard J.L., Lanting B.A., Teeter M.G. Tribocorrosion : ceramic and oxidized zirconium vs cobalt-chromium heads in total hip arthroplasty. J. Arthroplasty. 2016;31:2064–2071. doi: 10.1016/j.arth.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 107.Evangelista G.T., Fulkerson E., Kummer F., Di Cesare P.E. vol. 89. 2007. pp. 535–537. (Case Report Surface Damage to an Oxinium Femoral Head Prosthesis After Dislocation). [DOI] [PubMed] [Google Scholar]
- 108.McCalden R.W., Charron K.D., Davidson R.D., Teeter M.G., Holdsworth D.W. Damage of an Oxinium femoral head and polyethylene liner following “routine” total hip replacement. J. Bone Jt. Surg. - Ser. B. 2011;93 B:409–413. doi: 10.1302/0301-620X.93B3.25327. [DOI] [PubMed] [Google Scholar]
- 109.Ozden V.E., Saglam N., Dikmen G., Tozun I.R. Oxidized zirconium on ceramic; Catastrophic coupling. Orthop. Traumatol. Surg. Res. 2017;103:137–140. doi: 10.1016/j.otsr.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 110.Kop A.M., Dip P.G., Whitewood C., Johnston D.J.L. Case report damage of Oxinium femoral heads subsequent to hip arthroplasty dislocation three. Retrieval Case Studies. 2007;22:775–779. doi: 10.1016/j.arth.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 111.Harman M.K., Banks S.A., Andrew Hodge W. Wear analysis of a retrieved hip implant with titanium nitride coating. J. Arthroplasty. 1997;12:938–945. doi: 10.1016/S0883-5403(97)90164-9. [DOI] [PubMed] [Google Scholar]
- 112.Łapaj Ł., Markuszewski J., Wendland J., Mróz A., Wierusz-Kozłowska M. Massive failure of TiNbN coating in surface engineered metal-on-metal hip arthroplasty: retrieval analysis. J. Biomed. Mater. Res. B Appl. Biomater. 2016;104:1043–1049. doi: 10.1002/jbm.b.33421. [DOI] [PubMed] [Google Scholar]
- 113.Łapaj Ł., Rozwalka J. Retrieval analysis of TiN (titanium nitride) coated knee replacements: coating wear and degradation in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2020;108:1251–1261. doi: 10.1002/jbm.b.34473. [DOI] [PubMed] [Google Scholar]
- 114.Postler A., Beyer F., Lützner C., Tille E., Lützner J. Similar outcome during short-term follow-up after coated and uncoated total knee arthroplasty: a randomized controlled study, Knee Surgery, Sport. Traumatol. Arthrosc. 2018;26:3459–3467. doi: 10.1007/s00167-018-4928-0. [DOI] [PubMed] [Google Scholar]
- 115.Fabry C., Zietz C., Baumann A., Ehall R., Bader R. High wear resistance of femoral components coated with titanium nitride: a retrieval analysis, Knee Surgery. Sport. Traumatol. Arthrosc. 2017:1–10. doi: 10.1007/s00167-017-4578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Breugem S.J.M., Linnartz J., Sierevelt I., Bruijn J.D., Driessen M.J.M., Breugem S.J.M., Linnartz J., Bruijn J.D., Bruijn J.D. vol. 8. 2017. pp. 922–928. (Evaluation of 1031 Primary Titanium Nitride Coated Mobile Bearing Total Knee Arthroplasties in an Orthopedic Clinic). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hove R.P., Brohet R.M., Royen B.J., Nolte P.A. No clinical benefit of titanium nitride coating in cementless mobile-bearing total knee arthroplasty, Knee Surgery, Sport. Traumatol. Arthrosc. 2015;23:1833–1840. doi: 10.1007/s00167-014-3359-9. [DOI] [PubMed] [Google Scholar]
- 118.Moon K.H., Hong S.H., Hong T.H. Total knee replacement arthroplasty with Buechel and Pappas knee: minimum 2-year follow-up. CiOS Clin. Orthop. Surg. 2015;7:62–68. doi: 10.4055/cios.2015.7.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Innocenti M., Civinini R. 2010. The 5-year Results of an Oxidized Zirconium Femoral Component for TKA; pp. 1258–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heyse T.J., Chen D.X., Kelly N., Boettner F., Wright T.M., Haas S.B. Matched-pair total knee arthroplasty retrieval analysis: oxidized zirconium vs. CoCrMo, Knee. 2011;18:448–452. doi: 10.1016/j.knee.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 121.Heyse T.J., Davis J., Haas S.B., Chen D.X., Wright T.M., Laskin R.S. Retrieval analysis of femoral zirconium components in total knee arthroplasty. Preliminary results. J. Arthroplasty. 2011;26:445–450. doi: 10.1016/j.arth.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 122.Hofer J.K., Ezzet K.A. The Knee A minimum 5-year follow-up of an oxidized zirconium femoral prosthesis used for total knee arthroplasty. Knee. 2014;21:168–171. doi: 10.1016/j.knee.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 123.Innocenti M., Matassi F., Carulli C., Nistri L., Civinini R. Oxidized zirconium femoral component for TKA: a follow-up note of a previous report at a minimum of 10 years. Knee. 2014;21:858–861. doi: 10.1016/j.knee.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 124.Park D.H., Leong J., Palmer S.J. vol. 22. 2014. pp. 75–79. (Total Knee Arthroplasty with an Oxidised Zirconium Femoral Component : a 5-year Follow-up Study). [DOI] [PubMed] [Google Scholar]
- 125.Vertullo C.J., Lewis P.L., Graves S., Kelly L., Lorimer M., Myers P. 2017. Twelve-Year Outcomes of an Oxinium Total Knee Replacement Compared with the Same Cobalt-Chromium Design; pp. 275–283. [DOI] [PubMed] [Google Scholar]
- 126.Vertullo C.J., Lewis P.L., Peng Y., Graves S.E., de Steiger R.N. The effect of alternative bearing surfaces on the risk of revision due to infection in minimally stabilized total knee replacement. J. Bone Jt. Surg. 2018;100:115–123. doi: 10.2106/jbjs.17.00269. [DOI] [PubMed] [Google Scholar]
- 127.Thomsen M., Rozak M., Thomas P. Pain in a chromium-allergic patient with total knee arthroplasty: disappearance of symptoms after revision with a special surface-coated TKA--a case report. Acta Orthop. 2011;82:386–388. doi: 10.3109/17453674.2011.579521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Taeger G., Podleska L.E., Schmidt B., Ziegler M., Nast-Kolb D. Comparison of diamond-like-carbon and alumina-oxide articulating with polyethylene in total hip arthroplasty, materwiss. Werksttech. 2003;34:1094–1100. doi: 10.1002/mawe.200300717. [DOI] [Google Scholar]
- 129.Guo F., Zhou Z., Hua M., Dong G. Effect of aqueous solution and load on the formation of DLC transfer layer against Co-Cr-Mo for joint prosthesis. J. Mech. Behav. Biomed. Mater. 2015;49:12–22. doi: 10.1016/j.jmbbm.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 130.Thorwarth G., Falub C.V., Müller U., Weisse B., Voisard C., Tobler M., Hauert R. Tribological behavior of DLC-coated articulating joint implants. Acta Biomater. 2010;6:2335–2341. doi: 10.1016/j.actbio.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 131.Wang Q., Zhou F., Wang C., Yuen M.F., Wang M., Qian T., Matsumoto M., Yan J. Comparison of tribological and electrochemical properties of TiN, CrN, TiAlN and a-C:H coatings in simulated body fluid. Mater. Chem. Phys. 2015;158:74–81. doi: 10.1016/j.matchemphys.2015.03.039. [DOI] [Google Scholar]
- 132.Wang J., Qing Y.A., Xiao L.G., Wang Y.G., Bao X.F., Qin Y.G., Zhang J.Y., Zhang K. Design of new-type F-FLC artificial joint coatings via fluorine incorporation and fullerene-like structure construction. Surf. Coating. Technol. 2020;385 doi: 10.1016/j.surfcoat.2020.125419. [DOI] [Google Scholar]
- 133.Chan C.W., Lee S., Smith G., Sarri G., Ng C.H., Sharba A., Man H.C. Enhancement of wear and corrosion resistance of beta titanium alloy by laser gas alloying with nitrogen. Appl. Surf. Sci. 2016;367:80–90. doi: 10.1016/j.apsusc.2016.01.091. [DOI] [Google Scholar]
- 134.Vitu T., Polcar T., Cvrcek L., Novak R., Macak J., Vyskocil J., Cavaleiro A. Surface & coatings technology structure and tribology of biocompatible Ti – C : H coatings. Surf. Coating. Technol. 2008;202:5790–5793. doi: 10.1016/j.surfcoat.2008.06.040. [DOI] [Google Scholar]
- 135.Liu R., Li X., Hu X., Dong H. Surface modification of a medical grade Co-Cr-Mo alloy by low-temperature plasma surface alloying with nitrogen and carbon. Surf. Coating. Technol. 2013;232:906–911. doi: 10.1016/j.surfcoat.2013.06.122. [DOI] [Google Scholar]
- 136.Wang Q., Zhang L., Dong J. Effects of plasma nitriding on microstructure and tribological properties of CoCrMo alloy implant materials. J. Bionic Eng. 2010;7:337–344. doi: 10.1016/S1672-6529(10)60265-X. [DOI] [Google Scholar]
- 137.Robertson J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002;37:129–281. doi: 10.1016/s0927-796x(02)00005-0. [DOI] [Google Scholar]
- 138.Hauert R. A review of modified DLC coatings for biological applications. Diam. Relat. Mater. 2003;12:583–589. doi: 10.1016/S0925-9635(03)00081-5. [DOI] [Google Scholar]
- 139.Falub C.V., Müller U., Thorwarth G., Parlinska-Wojtan M., Voisard C., Hauert R. In vitro studies of the adhesion of diamond-like carbon thin films on CoCrMo biomedical implant alloy. Acta Mater. 2011;59:4678–4689. doi: 10.1016/j.actamat.2011.04.014. [DOI] [Google Scholar]
- 140.Falub C.V., Thorwarth G., Affolter C., Müller U., Voisard C., Hauert R. A quantitative in vitro method to predict the adhesion lifetime of diamond-like carbon thin films on biomedical implants. Acta Biomater. 2009;5:3086–3097. doi: 10.1016/j.actbio.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 141.Xiang D.D., Sui X.D., Tan X.P., Hao J.Y., Wang Z.W., Liao Z.H., Liu W.Q., Tor S.B. Improving biotribological properties and corrosion resistance of CoCrMo alloy via a Cr-GLC nanocomposite film in simulated body fluids. Surf. Coating. Technol. 2019;378 doi: 10.1016/j.surfcoat.2019.07.064. [DOI] [Google Scholar]
- 142.Gruen D.M. Nanocrystalline diamond films. Annu. Rev. Mater. Sci. 1999;29:211–259. [Google Scholar]
- 143.Sokołowska A., Rudnicki J., Niedzielski P., Boczkowska A., Bogusławski G., Wierzchoń T., Mitura S. TiN-NCD composite coating produced on the Ti6Al4V alloy for medical applications. Surf. Coating. Technol. 2005;200:87–89. https://www.scopus.com/inward/record.uri?eid=2-s2.0-24644500020&doi=10.1016%2Fj.surfcoat.2005.02.037&partnerID=40&md5=42701a4069b1fae11035fc9d87385f9d [Google Scholar]
- 144.Amaral M., Dias A.G., Gomes P.S., Lopes M.A., Silva R.F., Santos J.D., Fernandes M.H. Nanocrystalline diamond: in vitro biocompatibility assessment by MG63 and human bone marrow cells cultures. J. Biomed. Mater. Res. 2008;87:91–99. doi: 10.1002/jbm.a.31742. [DOI] [PubMed] [Google Scholar]
- 145.Amaral M., Gomes P.S., Lopes M.A., Santos J.D., Silva R.F., Fernandes M.H. Cytotoxicity evaluation of nanocrystalline diamond coatings by fibroblast cell cultures. Acta Biomater. 2009;5:755–763. doi: 10.1016/j.actbio.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 146.Amaral M., Gomes P.S., Lopes M.A., Santos J.D., Silva R.F., Fernandes M.H. Nanocrystalline diamond as a coating for joint implants: cytotoxicity and biocompatibility assessment. J. Nanomater. 2008;2008 doi: 10.1155/2008/894352. [DOI] [Google Scholar]
- 147.Maru M.M., Amaral M., Rodrigues S.P., Santos R., Gouvea C.P., Archanjo B.S., Trommer R.M., Oliveira F.J., Silva R.F., Achete C.A. The High performance of nanocrystalline CVD diamond coated hip joints in wear simulator test. J. Mech. Behav. Biomed. Mater. 2015;49:175–185. doi: 10.1016/j.jmbbm.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 148.Riley F.L. Silicon nitride and related materials. J. Am. Ceram. Soc. 2000;83:245–265. doi: 10.1111/j.1151-2916.2000.tb01182.x. [DOI] [Google Scholar]
- 149.Ceratec . 2021. Ceramic Materials.https://ceratec-ceramic-bearings.com/materials.html#sic_si3n4_al203_zro2_en [Google Scholar]
- 150.SMB . 2021. Ceramic Bearings.https://www.smbbearings.com/products/si3n4-ceramic-bearings.html [Google Scholar]
- 151.3m . 2021. Silicon Nitride Ball Bearing Components.https://www.3m.com/3M/en_US/company-us/all-3m-products/˜/3M-Silicon-Nitride-Ball-Bearing-Components/?N=5002385+3290920186&rt=rud [Google Scholar]
- 152.Wang L., Snidle R.W., Gu L. Rolling contact silicon nitride bearing technology: a review of recent research. Wear. 2000;246:159–173. doi: 10.1016/S0043-1648(00)00504-4. [DOI] [Google Scholar]
- 153.C. Amedica, No Title, (n.d.). https://www.ctlamedica.com/portfolio.
- 154.Laarz E., Zhmud B.V., Bergström L. Dissolution and deagglomeration of silicon nitride in aqueous medium. J. Am. Ceram. Soc. 2004;83:2394–2400. doi: 10.1111/j.1151-2916.2000.tb01567.x. [DOI] [Google Scholar]
- 155.Boschetto F., Toyama N., Horiguchi S., Bock R.M. In vitro antibacterial activity of oxide and non-oxide bioceramics for arthroplastic devices: II. Fourier transform infrared spectroscopy. Analyst. 2018:2128–2140. doi: 10.1039/c8an00234g. [DOI] [PubMed] [Google Scholar]
- 156.Pezzotti G., Bock R.M., McEntire B.J., Adachi T., Marin E., Boschetto F., Zhu W., Mazdad O., Bal S.B. In vitro antibacterial activity of oxide and non- oxide bioceramics for arthroplastic devices: I. In situ time-lapse Raman spectroscopy. R. Soc. Chem. 2018;143:3708–3721. doi: 10.1039/c8an00233a. [DOI] [PubMed] [Google Scholar]
- 157.Pettersson M., Skjoldebrand C., Filho L., Engqvist H., Persson C. Morphology and dissolution rate of wear debris from silicon nitride coatings. ACS Biomater. Sci. Eng. 2016:1–28. doi: 10.1021/acsbiomaterials.6b00133. [DOI] [PubMed] [Google Scholar]
- 158.Fischer T.E., Mullins W.M. Chemical aspects of tribology. J. Phys. Chem. 1992;96:5690–5701. [Google Scholar]
- 159.Bock R.M., McEntire B.J., Bal B.S., Rahaman M.N., Boffelli M., Pezzotti G. Surface modulation of silicon nitride ceramics for orthopaedic applications. Acta Biomater. 2015;26:318–330. doi: 10.1016/j.actbio.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 160.Lal S., Caseley E.A., Hall R.M., Tipper J.L. Biological impact of silicon nitride for orthopaedic applications: role of particle size, surface composition and donor variation. Sci. Rep. 2018;8:1–12. doi: 10.1038/s41598-018-27494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Neumann A., Reske T., Held M., Jahnke K., Ragoß C., Maier H.R. Comparative investigation of the biocompatibility of various silicon nitride ceramic qualities in vitro. J. Mater. Sci. Mater. Med. 2004;15:1135–1140. doi: 10.1023/B:JMSM.0000046396.14073.92. [DOI] [PubMed] [Google Scholar]
- 162.Kue R., Sohrabi A., Nagle D., Frondoza C., Hungerford D. Enhanced proliferation and osteocalcin production by human osteoblast-like MG63 cells on silicon nitride ceramic discs. Biomaterials. 1999;20:1195–1201. doi: 10.1016/S0142-9612(99)00007-1. [DOI] [PubMed] [Google Scholar]
- 163.Pezzotti G., Boschetto F., Ohgitani E., Fujita Y., Zhu W., Marin E., McEntire B.J., Bal B.S., Mazda O. Silicon nitride: a potent solid-state bioceramic inactivator of ssRNA viruses. Sci. Rep. 2021;11:1–18. doi: 10.1038/s41598-021-82608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Echeverri Correa C., Skjöldebrand Estefania, Hulsart-Billström G., Persson C. Tailoring the dissolution rate and in vitro cell response of silicon nitride coatings through combinatorial sputtering with chromium and niobium. in: 31st Annu. Conf. Eur. Soc. Biomater. 2021 doi: 10.1039/d1bm01978c. [DOI] [PubMed] [Google Scholar]
- 165.Fisher J., Hu X.Q., Stewart T.D., Williams S., Tipper J.L., Ingham E., Stone M.H., Davies C., Hatto P., Bolton J., Riley M., Hardaker C., Isaac G.H., Berry G. Wear of surface engineered metal-on-metal hip prostheses. J. Mater. Sci. Mater. Med. 2004;15:225–235. doi: 10.1023/B:JMSM.0000015482.24542.76. [DOI] [PubMed] [Google Scholar]
- 166.de Villiers D., Traynor A., Collins S.N., Banfield S., Housden J., Shelton J.C. Chromium nitride coating for large diameter metal-on-polyethylene hip bearings under extreme adverse hip simulator conditions. Wear. 2015;328–329:363–368. doi: 10.1016/j.wear.2015.02.060. [DOI] [Google Scholar]
- 167.Shukla K., Sugumaran A.A., Khan I., Ehiasarian A.P., Hovsepian P.E. Low pressure plasma nitrided CoCrMo alloy utilising HIPIMS discharge for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020;111 doi: 10.1016/j.jmbbm.2020.104004. [DOI] [PubMed] [Google Scholar]
- 168.Bianchi M., Gambardella A., Berni M., Panseri S., Montesi M., Lopomo N., Tampieri A., Marcacci M., Russo A. Surface morphology, tribological properties and in vitro biocompatibility of nanostructured zirconia thin films. J. Mater. Sci. Mater. Med. 2016;27:1–10. doi: 10.1007/s10856-016-5707-4. [DOI] [PubMed] [Google Scholar]
- 169.Berni M., Marchiori G., Gambardella A., Boi M., Bianchi M., Russo A., Visani A., Marcacci M., Pavan P.G., Lopomo N.F. Effects of working gas pressure on zirconium dioxide thin film prepared by pulsed plasma deposition: roughness, wettability, friction and wear characteristics. J. Mech. Behav. Biomed. Mater. 2017;72:200–208. doi: 10.1016/j.jmbbm.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 170.Luo Y., Yang T., Rao X., Zhu J., Li M. Thermal oxidation of Zr-2.5Nb alloy and its biotribological properties. Int. J. Adv. Manuf. Technol. 2018;96:1539–1543. doi: 10.1007/s00170-017-0607-4. [DOI] [Google Scholar]
- 171.Hummer C.D., Rothman R.H., Hozack W.J. Catastrophic failure of modular Zirconia-Ceramic femoral head components after total hip arthroplasty. J. Arthroplasty. 1995;10:848–850. doi: 10.1016/S0883-5403(05)80085-3. [DOI] [PubMed] [Google Scholar]
- 172.Porporati A.A., Gremillard L., Chevalier J., Pitto R., Deluca M. Is surface metastability of today's ceramic bearings a clinical issue? J. Compos. Sci. 2021;5 doi: 10.3390/jcs5100273. [DOI] [Google Scholar]
- 173.Feng F., Zhou Y., Yun H., Rocha A., Polcar T., Cvrcek L., Liang H. Potential application of a Ti--C: H coating in implants. J. Am. Ceram. Soc. 2012;95:2741–2745. doi: 10.1111/j.1551-2916.2011.05000.x. [DOI] [Google Scholar]
- 174.Bonello T., Avelar-Batista Wilson J.C., Housden J., Gutmanas E.Y., Gotman I., Matthews A., Leyland A., Cassar G. Evaluating the effects of PIRAC nitrogen-diffusion treatments on the mechanical performance of Ti-6Al-4V alloy. Mater. Sci. Eng. 2014;619:300–311. doi: 10.1016/j.msea.2014.09.055. [DOI] [Google Scholar]
- 175.Shenhar A., Gotman I., Gutmanas E., Ducheyne P. Surface modification of titanium alloy orthopaedic implants via novel powder immersion reaction assisted coating nitriding method. Mater. Sci. Eng. 1999;268:40–46. doi: 10.1016/S0921-5093(99)00111-2. [DOI] [Google Scholar]
- 176.Watari F., Tamura Y., Yokoyama A., Uo M., Kawasaki T. Mechanical properties and biocompatibility of surface-nitrided titanium for abrasion resistant implant. Key Eng. Mater. 2004;254–256:873–876. doi: 10.4012/dmj.21.355. doi:10.4028/www.scientific.net/KEM.254-256.873. [DOI] [PubMed] [Google Scholar]
- 177.Levine B.R., Sporer S., Poggie R.A., Della Valle C.J., Jacobs J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials. 2006;27:4671–4681. doi: 10.1016/j.biomaterials.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 178.Balagna C., Faga M.G., Spriano S. Tribological behavior of a Ta-based coating on a Co-Cr-Mo alloy. Surf. Coating. Technol. 2014;258:1159–1170. doi: 10.1016/j.surfcoat.2014.07.016. [DOI] [Google Scholar]
- 179.Rahmati B., Sarhan A.A.D., Basirun W.J., Abas W.A.B.W. Ceramic tantalum oxide thin film coating to enhance the corrosion and wear characteristics of Ti-6Al-4V alloy. J. Alloys Compd. 2016 doi: 10.1016/j.jallcom.2016.03.188. [DOI] [Google Scholar]
- 180.Corona-Gomez J., Jack T.A., Feng R., Yang Q. Wear and corrosion characteristics of nano-crystalline tantalum nitride coatings deposited on CoCrMo alloy for hip joint applications. Mater. Char. 2021;182 doi: 10.1016/j.matchar.2021.111516. [DOI] [Google Scholar]
- 181.Cheng K.Y., Gopal V., McNallan M., Manivasagam G., Mathew M.T. Enhanced tribocorrosion resistance of hard ceramic coated Ti-6Al-4V alloy for hip implant application: in-vitro simulation study. ACS Biomater. Sci. Eng. 2019;5:4817–4824. doi: 10.1021/acsbiomaterials.9b00609. [DOI] [PubMed] [Google Scholar]
- 183.Balagna C., Faga M.G., Spriano S. Tribological behavior of a Ta-based coating on a Co–Cr–Mo alloy. Surf. Coating. Technol. 2014 doi: 10.1016/j.surfcoat.2014.07.016. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this review consists of information found in published research papers.