Abstract
Previous studies indicate sex differences in incidence and severity of bloodstream infections (BSI). We examined the effect of sex on risk of BSI, BSI mortality, and BSI caused by the most common infecting bacteria. Using causal mediation analyses, we assessed if this effect is mediated by health behaviours (smoking, alcohol consumption), education, cardiovascular risk factors (systolic blood pressure, non-HDL cholesterol, body mass index) and selected comorbidities. This prospective study included 64,040 participants (46.8% men) in the population-based HUNT2 Survey (1995–1997) linked with hospital records in incident BSI. During median follow-up of 15.2 years, 1840 (2.9%) participants (51.3% men) experienced a BSI and 396 (0.6%) died (56.6% men). Men had 41% higher risk of first-time BSI (95% confidence interval (CI), 28–54%) than women. Together, health behaviours, education, cardiovascular risk factors and comorbidities mediated 34% of the excess risk of BSI observed in men. The HR of BSI mortality was 1.87 (95% CI 1.53–2.28), for BSI due to S. aureus 2.09 (1.28–2.54), S. pneumoniae 1.36 (1.05–1.76), E. coli 0.97 (0.84–1.13) in men vs women. This study shows that men have higher risk of BSI and BSI mortality than women. One-third of this effect was mediated by potential modifiable risk factors for incident BSI.
Subject terms: Infectious diseases, Immunology, Microbiology
Introduction
Bloodstream infection (BSI) is a major global burden and may lead to sepsis which constitutes up to 60% of the global mortality burden1,2. The risk of acquiring BSI depends on the bacterial virulence, host characteristics, geographical location, and biological factors1,3–8. Epidemiological studies indicate a male predominance in BSI and sepsis. Nevertheless former studies on sex differences in incidence and mortality of BSI and sepsis have given conflicting results with increased risk in women1, increased risk in men6,9, increased mortality in women10, or increased mortality in men1,11,12. Importantly, disparities in immune function between sexes may arise from differences in biological characteristics such as anatomy and hormonal status, medical conditions, health behaviours, lifestyle, and exposure to different pathogens13,14. Most previous studies on sex differences in BSI and sepsis have been performed in small and selected cohorts, mainly from the intensive care unit (ICU)6,10,11, and there are limited population-based studies9,15,16 which better account for selection bias17. Studies on severe infections and sepsis tend to adjust for sex in their analyses18 but the mechanisms behind the observed sex differences are unexplored19. Little is known whether conditions that are known to increase BSI risk, like health behaviours4 cardiovascular disease risk factors or comorbidity20,21, contribute to the observed difference in risk of BSI between men and women. Such knowledge may help identify targets for intervention to reduce BSI and sepsis risk.
To assess the impact of sex as a risk factor for first-time BSI, BSI mortality, and BSI caused by the most common infecting bacteria, Staphylococcus (S.) aureus, Streptococcus (S.) pneumoniae and Escherichia (E.) coli we used data from the Norwegian HUNT study linked with prospectively recorded BSI episodes. Further, we examined if sex differences in health behaviours and education attainment, cardiovascular risk factors and selected comorbidities, which reflect known risk factors for BSI4,20,21 may explain the observed sex difference in risk of first-time BSI. We applied sequential mediation analysis22 using inverse-odds weighting23 to explore their potential mediating effect on the associations between sex and BSI.
Methods
Study population
The HUNT Study is a population-based health study conducted in the Nord-Trøndelag region in Norway and consists of four consecutive surveys inviting the total adult population approximately every 10th year. The second survey (HUNT2, 1995–1997), invited all adult inhabitants ≥ 20 years (n = 93,898) to a clinical examination and a comprehensive self-report of health-related topics. Of these, 65,237 (69%) chose to participate. The HUNT study database is regularly updated with information on date of migration and death from the National Registry. More details on the HUNT study are published elsewhere24. For the purpose of the present study, we excluded 47 (0.07%) participants who had a prior positive blood culture and 1150 (1.8%) who migrated or died before start of follow-up. A total of 64,040 participants were eligible for analyses (Supplemental Fig. S1).
Measures
The exposure is sex as registered in the National Registry. The two main outcomes were first-time BSI and BSI mortality. The participants were followed for incident BSI by linkage to the Nord-Trøndelag Hospital Trust (HNT HF) Sepsis Registry using the personal identification number of Norwegian citizens25. All BSIs were confirmed at the microbiology laboratories at Levanger Hospital which provided all microbiology services in the Nord-Trøndelag region or at St. Olavs Hospital. Details about HNT HF sepsis registry are included in the supplemental material. We defined BSI mortality as death occurring within 30 days after detection of any BSI. In secondary analyses we assessed first-time BSI caused by the most common bacteria E. coli, S. aureus and S. pneumonia, and performed age-stratified analyses.
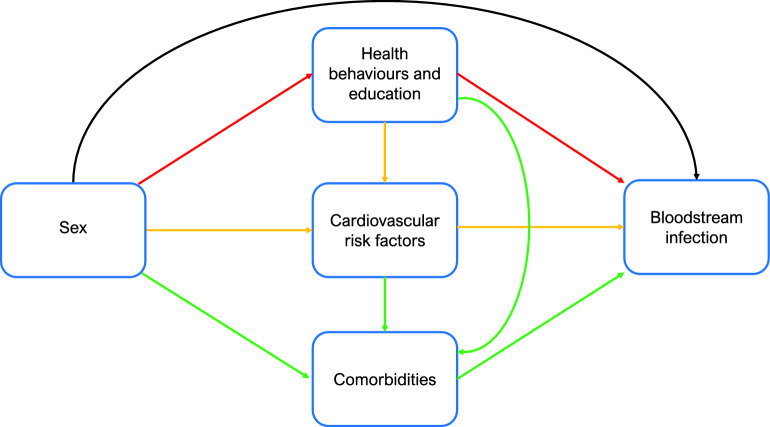
Mediators are variables that are causally located between exposure and outcome variables, and that partly explain the effect of the exposure on outcome22,26. Mediation analysis can assess indirect and direct effects and estimate the proportion of the total effect that works through the mediator of interest (i.e. proportion mediated)27. We used three distinct sets of mediators measured at inclusion to HUNT2; (1) health behaviours (smoking and alcohol use) and educational attainment; (2) cardiovascular risk factors (body mass index (BMI, kg/m2), systolic blood pressure (mmHg) and non-high-density lipoprotein cholesterol (non-HDL cholesterol, mmol/L); (3) comorbidities defined by self-report of cardiovascular disease (history of myocardial infarction, angina pectoris, and/or stroke), diabetes, cancer history, lung disease (asthma or chronic obstructive pulmonary disease) and standardised measurements of kidney function (estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2). The three sets of mediators reflect known risk factors for BSI4,20,21. For some of the included mediators there are reports of sex differences in prevalence, pathophysiology and outcomes28,29. The proposed diagram for the relationship between sex and risk of BSI is shown in Fig. 1. The aim of the analysis was to examine to which extent sex differences in risk of BSI may be related to these mediating factors. Details about the measurements and categorisation of mediators are included in the Supplemental Material.
Figure 1.
Mediation analysis. Diagram of the direct and indirect (i.e., mediated) effects of sex on bloodstream infection. The black arrow represents the natural direct effect of the association. Red arrows represent model 1, proportion mediated by health behaviours and education attainment. Yellow arrows represent model 2, proportion mediated jointly by health behaviours, education, and cardiovascular risk factors. Green arrows represent model 3, proportion mediated jointly by health behaviours, education, cardiovascular risk factors and comorbidities. Model 1) Smoking, alcohol use and educational attainment. Model 2) Systolic blood pressure, non-high-density lipoprotein cholesterol and Body Mass Index. Model 3) Cardiovascular disease, chronic kidney disease, diabetes, history of cancer, and chronic lung disease.
Statistical analyses
We used Cox proportional hazard regression to estimate the hazard ratios (HRs) with 95% confidence intervals (95% CI) of a first-time BSI and of BSI mortality in men compared to women. Attained age was used as the time scale. Start of follow-up was defined by the availability of data in the sepsis registry. For patients referred to St. Olavs hospital, the tertiary referral centre, BSI information was included depending on their primary hospital. Participants contributed person-years from inclusion date in HUNT2 except for participants having Namsos as their primary hospital, they contributed from 1 September 1999.
In the analysis of BSI risk, participants were followed until their first BSI. For BSI-mortality participants were followed until death within 30 days of any BSI episode. For both analyses participants were censored at time of migration out of Nord-Trøndelag, death of all causes, or end of follow-up set to 31 December 2011, whichever occurred first. The proportional hazards assumption was examined by visual inspection of log–log plots and tests of Schoenfeld residuals. Using Stata stcompadj, we estimated cumulative incidence and mortality from start of follow-up to first-time BSI and BSI mortality, accounting for death by all causes as a competing risk and we provide cumulative incidence and cumulative mortality curves to illustrate changes during follow-up. As supplemental analyses we assessed subhazard ratios taking death as a competing event into account for first-time BSI and BSI mortality.
In secondary analyses we estimated hazard ratios and cumulative incidence of first-time BSI caused by the most common infecting bacteria. Further, we conducted age-stratified analyses on risk of first-time BSI; < 50, 50 to < 65, 65 to 79, and ≥ 80 years to address whether menopause affects women’s risk of BSI, and to assess sex differences in BSI risk with advancing age. To examine the associations between the mediators included in Model 2 and risk of first-time BSI, we conducted sensitivity analyses using Cox regression for BMI, systolic blood pressure and non-HDL cholesterol.
For the mediation analysis we used an inverse odds weighting (IOW) procedure23. IOW is a counterfactual method that enables a decomposition of the total effect of the exposure (sex) on the outcome (first-time BSI) into a natural direct effect (NDE) from exposure on outcome, and a natural indirect effect (NIE) through multiple mediators22,23. The method accommodates multiple mediators simultaneously and is robust to unmeasured common causes of the mediators22.
The inverse odds weights were obtained by regressing the exposure on all mediators of interest with age as a covariate. In our analysis, the total effect is interpreted as the total association between sex and first-time BSI, the NIE is the proportion of excess BSI risk in men mediated by the risk factors, whereas the NDE is the proportion of excess BSI risk in men not associated with these factors. The proportion mediated is the percent of the total association that is mediated through the risk factors. We did not estimate the NIE of individual mediators separately as it may not be appropriate when the mediators affect each other or when single mediator-outcome confounders may be affected by exposure22,30. Instead, we estimated the NIE with a sequential approach using three models. In model 1, we assessed education attainment and health behaviours (smoking and alcohol use); in model 2, we added the cardiovascular risk factors (BMI, systolic blood pressure and non-HDL cholesterol) to address potential preclinical disease; and in model 3, the selected comorbidities (cardiovascular, diabetes, cancer history, lung, and kidney disease) were included to the complete set of mediators. This approach assumes that the cardiovascular risk factors and further the comorbidities are causal descendants of the health behaviours and educational attainment. The sequential approach further implies that model 3 reflects the best interpretation of the mediation analyses as all mediators and age are included22.
We performed bootstrapping based on 1000 replications to derive percentile-based CIs for all mediation parameters31, and the NDE and NIE are presented as HRs with 95% CIs. The proportion mediated on the log scale was calculated using the formula (lnHRNIE /lnHRTOTAL)23. All statistical analyses were performed using Stata version 17.0. A detailed description of the IOW analyses is included in Supplemental Table S1.
Ethical approval
The study was approved by the Regional Committee for Medical and Health Research Ethics of Central Norway (REK no 2012/153 and REK no 94135), and by the HUNT data access committee. Participation in HUNT 2 was voluntary, and informed written consent to data collection and linking their data to other registers was obtained from all participants. All methods were performed in accordance with the Declaration of Helsinki.
Results
Population characteristics
During a median follow-up of 15.2 years (IQR 12.3–15.5 years), among 64,040 participants (46.8% men), 1840 (2.9%) experienced a BSI and 396 (0.6%) died within 30 days after a BSI episode. The median age at inclusion was similar for both sexes. Both men and women who experienced a BSI were older (median age at inclusion 67.4 and 68.0 respectively), and they had a higher comorbidity burden than participants who did not have a BSI during follow-up (Table 1).
Table 1.
Baseline characteristics of the study population at inclusion in HUNT2, n = 64,040.
| Men | Women | |
|---|---|---|
| Total population n (%) | 29,962 (46.8) | 34,087 (53.2) |
| First-time BSI n (%)1 | 943 (51.3) | 897 (48.7) |
| BSI mortality n (%)2 | 224 (56.6) | 172 (43.4) |
| Age (mean, IQR) | 48.6 (36.5–62.9) | 48.7 (36.2–64.2) |
| Smoking | ||
| Current (%) | 8334 (27.8) | 9726 (28.5) |
| Prior (%) | 9422 (31.4) | 6516 (19.1) |
| Never (%) | 10,668 (35.6) | 15,230 (44.7) |
| Alcohol use | ||
| < 1 unit/2 weeks (%) | 8448 (28.2) | 16,069 (47.3) |
| 1–7 units/2 weeks (%) | 14,258 (47.6) | 14,484 (42.6) |
| 8–14 units/2 weeks (%) | 4602 (15.4) | 1861 (5.5) |
| ≥ 15 units/2 weeks (%) | 1643 (5.5) | 272 (0.8) |
| Education | ||
| < 10 years (%) | 20,625 (68.9) | 22,259 (65.3) |
| 10–12 years (%) | 2302 (7.7) | 3436 (10.1) |
| > 12 years (%) | 5650 (18.9) | 6412 (18.8) |
| BMI (kg/m2) | ||
| < 18.5 (%) | 118 (3.9) | 349 (1.0) |
| 18.5–24.9 (%) | 10,498 (35.0) | 14,736 (43.2) |
| 25–29.9 (%) | 14,757 (49.3) | 12,345 (36.2) |
| 30–34.9 (%) | 3674 (12.3) | 4640 (13.6) |
| 35–39.9 (%) | 496 (1.7) | 1236 (3.6) |
| ≥ 40 (%) | 74 (0.3) | 344 (1.0) |
| Systolic blood pressure (mmHg) median (IQR) | 137 (127–150) | 131 (118–149) |
| Non-HDL cholesterol (mmol/L) median (IQR) | 4.5 (3.7–5.3) | 4.3 (3.5–5.3) |
| Comorbidities | ||
| Cardiovascular disease3 (%) | 2918 (9.7) | 2014 (5.9) |
| Chronic kidney disease (%) | 979 (3.3) | 1802 (5.3) |
| Diabetes (%) | 895 (3.1) | 970 (2.9) |
| Cancer history (%) | 878 (2.8) | 1413 (4.1) |
| Chronic lung disease4 (%) | 1183 (4.0) | 1011 (3.0) |
BSI bloodstream infection, n numbers, IQR interquartile range, BMI body mass index, HDL high-density lipoprotein.
1Percentage of total first-time BSI in both sexes.
2BSI mortality was defined as all-cause mortality within 30 days after a BSI. Percentage of BSI mortality on both sexes.
3History of myocardial infarction, angina pectoris and/or stroke.
4History of chronic obstructive pulmonary disease or asthma.
Risk of BSI and BSI mortality
Men were more likely to experience a first-time BSI and to die from a BSI compared to women. Men had 1.41 (HR, 95% CI 1.28–1.54) times the risk of first-time BSI, and had 1.87 (HR, 95% CI 1.53–2.28) times the risk of dying from a BSI (Table 2) compared with women. In analyses by the most common infecting bacteria, men had a 2.09-fold (HR, 95% CI 1.28–2.54) risk of BSI caused by S. aureus, and 1.36 (HR, 95% CI 1.05–1.76) increased risk of S. pneumonia. The corresponding result for E. coli did not show higher risk in men with HR of 0.97 (95% CI 0.84–1.13) (Table 3).
Table 2.
Associations of sex with risk of bloodstream infection and BSI mortality.
| Risk of first-time BSI adjusted for age1 | BSI mortality2 adjusted for age1 | |||||||
|---|---|---|---|---|---|---|---|---|
| Years at risk | No. BSI | HR | 95% CI | Years at risk | No. BSI deaths | HR | 95% CI | |
| Women | 436,758 | 897 | 1.0 | Reference | 472,012 | 172 | 1.0 | Reference |
| Men | 373,915 | 943 | 1.41 | 1.28–1.54 | 404,723 | 224 | 1.87 | 1.53–2.28 |
BSI bloodstream infection, HRs hazard ratios, 95% CI 95% confidence intervals, No. numbers.
1Cox regression analyses were adjusted with age as the underlying scale.
2BSI mortality was defined as all-cause mortality within 30 days after a bloodstream infection.
Table 3.
Associations of sex with risk of bloodstream infections caused by the most common bacteria.
| Years at risk | Risk of S. aureus BSI1 | Risk of S. pneumoniae BSI1 | Risk of E. coli BSI1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. BSI | HR | 95% CI | No. BSI | HR | 95% CI | No. BSI | HR | 95% CI | ||
| Women | 436,758 | 83 | 1.0 | Reference | 113 | 1.0 | Reference | 399 | 1.0 | Reference |
| Men | 373,915 | 129 | 2.09 | 1.28–2.54 | 119 | 1.36 | 1.05–1.76 | 285 | 0.97 | 0.84–1.13 |
BSI bloodstream infection, HRs hazard ratios, 95% CI 95% confidence intervals.
1Cox regression analyses were adjusted for age as the underlying scale.
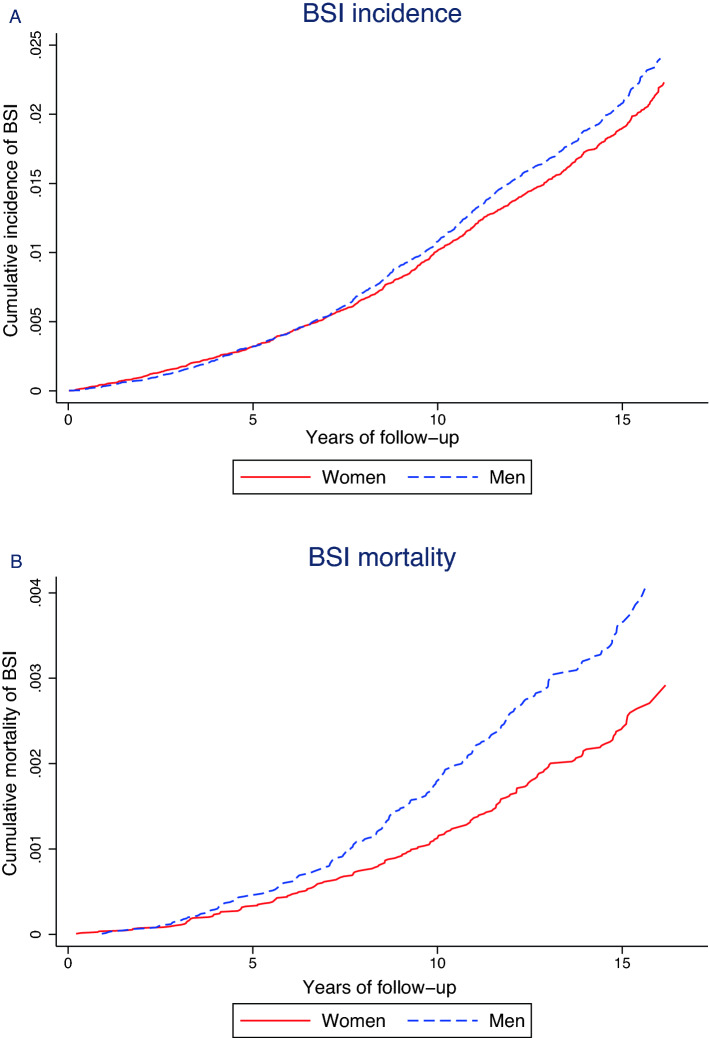
The above findings are illustrated by graphing the age-adjusted cumulative incidence of first-time BSI and cumulative BSI mortality in Fig. 2. The cumulative incidence of BSI was higher among men than women after the first five years of follow-up. For BSI mortality the sex difference was apparent after the first 2.5 years of follow-up and during follow-up the sex differences in mortality increased. The subhazards obtained for first-time BSI and BSI mortality, provide the same direction of the associations as the Cox regression analyses (Supplemental Table S2). We additionally present cumulative incidence curves for the three most common infecting bacteria in Fig. 3A–C. Men had higher cumulative incidence of S. aureus, especially after the first seven years of follow-up, whereas E. coli had higher cumulative incidence among women.
Figure 2.
Sex differences in cumulative incidence and mortality of BSI. Age-adjusted sex difference in cumulative incidence of BSI (A), and in cumulative mortality (B), estimated for age 49.99 (the mean age of the total population). Note: due to the variation in incidence of different outcomes the scale of the Y-axis is not uniform across the panels.
Figure 3.
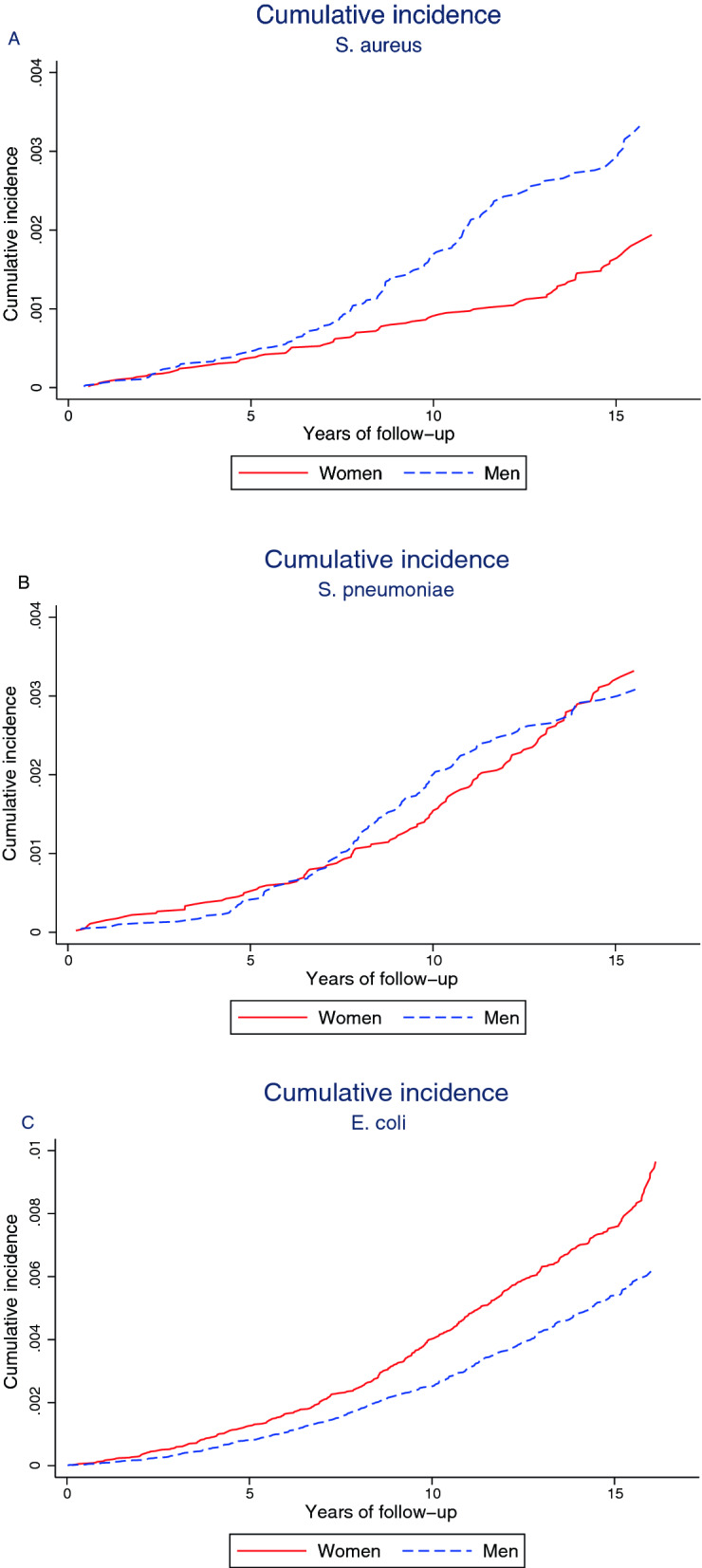
Sex differences in cumulative incidence of BSI caused by the most common bacteria. Age-adjusted sex difference in cumulative incidence of S. aureus (A), S. pneumoniae (B), and E. coli (C), estimated for age 49.99 (the mean age of the total population). Note: due to the variation in incidence of different bacteria the scale of the Y-axis is not uniform across the panels.
The age-stratified analyses at estimated menopause (< 50 years) did not reveal any clear sex difference in risk of BSI before menopause. However, the youngest age-group had few episodes of BSI. In the older age-groups we observed an increased incidence of BSI in both men and women, where men show a substantially increased risk of BSI as they age compared to women (Supplemental Table S3).
Mediation analyses
In Table 4 we present the total effect, the natural direct and indirect effects of sex on risk of first-time BSI. Compared with women men had an estimated HR of 1.40 (95% CI 1.24–1.55) for first-time BSI. Behavioural risk factors and education mediated 10% (model 1), after adding the cardiovascular risk factors the proportion mediated was reduced to 5% (model 2), whereas the whole set of mediators, including comorbidities, jointly mediated 34% of the total effect (model 3).
Table 4.
Mediation of the associations between sex and BSI by behavioural risk factors, educational attainment, cardiovascular risk factors and comorbidities.
| Mediation by behavioural risk factors1 and education | Risk of first-time BSI |
|---|---|
| HRs (95% CIa)b | |
| Model 1 | |
| Total effect | 1.40 (1.24–1.55) |
| Natural direct effect (NDE) | 1.36 (1.18–1.57) |
| Natural indirect effect (NIE) | 1.04 (0.97–1.07) |
| Proportion mediatedc | 10% |
| Mediation by behavioural risk factors1, education, and cardiovascular risk factors2 | HRs (95% CIa)b |
|---|---|
| Model 2 | |
| Total effect | 1.40 (1.24–1.55) |
| Natural direct effect (NDE) | 1.38 (1.19–1.58) |
| Natural indirect effect (NIE) | 1.02 (0.92–1.07) |
| Proportion mediatedc | 5% |
| Mediation by behavioural risk factors1, education, cardiovascular risk factors2 and comorbidity risk factors3 | HRs (95% CIa)b |
|---|---|
| Model 3 | |
| Total effect | 1.40 (1.24–1.55) |
| Natural direct effect (NDE) | 1.25 (1.05–1.47) |
| Natural indirect effect (NIE) | 1.12 (1.02–1.17) |
| Proportion mediatedc | 34% |
BSI bloodstream infection, HRs hazard ratios, 95% CI 95% confidence intervals.
1Smoking, alcohol use and educational attainment at baseline.
2Systolic blood pressure, non-high-density lipoprotein cholesterol and Body Mass Index.
3Cardiovascular disease, chronic kidney disease, diabetes, history of cancer, or chronic lung disease.
aPercentile-based bootstrap CIs are reported.
bEstimates are adjusted for age as a covariate.
cProportion mediated: (ln HRNIE/ln HRTOTAL).
To examine the reduction in proportion mediated from 10 to 5% in model 2, in sensitivity analyses, we observed an increased risk of BSI in persons with low BMI (< 18.5) and in persons with increasing BMI compared to the normal BMI group (18.5–24.4). For systolic blood pressure we did not observe any risk difference, and for non-HDL cholesterol the HR suggested a protective effect but with imprecise estimates (Supplemental Table S4).
Discussion
In this large Norwegian population-based study with a follow-up of more than 15 years, male sex was associated with 41% higher risk of BSI and 87% higher risk of dying from a BSI. An estimated 34% of the increased risk of BSI in men was mediated by known BSI risk factors. We additionally found that men had 2.09 times the risk of BSI caused by S. aureus compared to women. These findings add weight to the observed male preponderance seen in severe infections and point out modifiable BSI risk factors that are targets for preventive measures to reduce the burden of BSI.
There are few population-based studies on sex differences in the epidemiology of BSI and to our knowledge, no previous studies have performed mediation analysis to explain the sex differences of BSI. We used the IOW method which is known to be robust using multiple mediators en bloc and the rich baseline information from HUNT2 allowed us to implement mediation analyses in a time-to-event context22,23. The sequential approach enabled us to examine if the observed excess risk in men was mediated through different known risk factors for BSI. For many medical conditions men and women differ regarding incidence, the underlying pathophysiology and responses to therapy28,29. For health behaviours, more men reported smoking, and they reported higher alcohol use. We also observed higher prevalence of obesity among women in HUNT2. Adding cardiovascular risk factors to health behaviours and education, the proportion mediated lowered from 10 to 5%, which indicates some interactions or common pathways for these mediators22. This result might be due to some of the mediators included in model 2 being more frequent or harmful in women, or the mediators might reduce the risk of BSI. The complete model with all mediators included accommodates the assumptions required, and most likely reflects the best modelling of the associations22,30 explaining 34% of the excess BSI risk in men. Interventions to reduce modifiable risk factors in the population will likely reduce the burden of BSI, particularly in older men with high burden of known BSI risk factors.
The population-based design ensures that all BSI occurring in residents of a defined geographical area are included, which is an advantage over ICU cohorts17. Our results are supported by one study including 1051 patients, showing that men had higher risk of BSI. Like our study they described that BSI incidence increases by age, and men had twice the rate of S. aureus BSI15. Another population-based study comprising 9266 patients with BSI admitted to ICU found that male sex is a risk factor for BSI9. A recent study restricted to persons aged ≥ 65, found that men were at increased risk of BSI compared to females (incidence rate ratio 1.44, 95% CI 1.32–1.59) and the sex difference was most pronounced in the oldest patients, similar to our results16. On the other hand, the Global Burden of Disease Study found that age-standardised sepsis incidence was higher among women, while sepsis-related mortality was higher among men1. This study included results from 195 countries and comprised all age groups. They found higher sepsis incidence in low-income countries, and the pattern of sepsis incidence and mortality varied according to location, which is not directly comparable to our study population.
We identified higher BSI mortality in men which is in line with a recent study of infection related death in UK Biobank12. Conversely, some ICU studies report higher sepsis-related mortality in women10,29. A recent meta-analysis evaluating the associations between sex and mortality in critically ill adults showed inconclusive results30. This conflicting evidence concerning sex differences in mortality is most likely due to the heterogeneity of BSI and sepsis depending on the aetiology and the cohort studied17, but may also be affected by sex differences in immune responses13,14,32 and differences in treatment10,18.
The second most common infecting agent in our study was S. aureus which was far more common in men. S. aureus is associated with superficial infections of soft tissues with the potential for invasive infections and is the most important cause of BSI-associated death33. Previous studies indicate higher probability of nasal colonization in men, which is a risk factor for invasive S. aureus infections34. Other studies show that testosterone levels and use of hormone contraceptives among females alter nasal colonization, indicating that sex hormones affect the immune response to S. aureus35,36. The higher prevalence of S. aureus colonization in men is of particular interest, as preventive measures like eradication or temporary suppression could lower the risk of invasive infections which is especially important in hospitalized patients37.
In a sensitivity analysis we found that the sex differences in BSI risk are evident after predicted age of menopause indicating that alterations in both innate and adaptive immune functions with age may be sex specific. Aging is associated with chronic inflammation and a generally reduced immune function. Sex hormone levels in men and women change with age. Women face an abrupt decline during menopause, whereas men have a steady decline from second decade of life38. As in former studies, our study points out that advancing age is a risk factor for developing and dying from BSI and elderly men are at particular risk9,15,16.
Major strengths of our study include its large size, the population-based design, long-term follow-up and linkage to microbiological records which represent the gold standard to identify BSI within a population17. Our definition of BSI as a laboratory verified positive blood culture, excluding blood cultures solely with microorganisms associated with skin contamination, ascertains the accuracy of the outcome studied. In addition, reviews of medical records of patients with S. aureus and S. pneumoniae BSI in this cohort showed that ~ 98% met the 2001 sepsis criteria32,39. We were able to study BSI incidence and BSI mortality in a large population without the potential referral bias seen in single institution studies of BSI. The complete ascertainment of all BSI, together with the rich baseline measurements of known BSI risk factors in HUNT2, allows for an accurate estimation of incidence and mortality in the population, with the potential of risk factor identification and mediation analyses.
There are some limitations of our study that merit attention. First, we lack information on immunosuppressive medication which are known risk factors for BSI and BSI mortality40. We did not have information on the clinical course after detection of a BSI. Therefore, we did not perform mediation analyses on risk of BSI mortality as in-hospital factors such as correct and timely antibiotics and resuscitation measures strongly influence mortality41. Second, investigating BSI is dependent on clinician’s suspicion and decision to submit blood cultures for testing, with the chance of some undetected cases. Further, we cannot rule out if the clinical presentation of infections is different in men and women, and that this could result in disproportionate blood culture sampling depending on sex. Another concern would be if the clinical presentation of infection led to disproportionate and sex dependent hospital admissions. Third, the mediators were only measured once at inclusion to HUNT2 and could have changed during the 16 years of follow-up. This potential misclassification would most likely lead to underestimation of the mediating effect. Forth, the subjective assessment of some mediators, and dichotomised mediators, are more prone to possible mediator misclassification. This could lead to underestimation of the indirect effect and overestimation of the direct effect22. Fifth, we were not able to assess the mediators individually, as this could have violated the model assumptions22,30.
Despite these limitations, our study provides a foundational observation of the existing sex differences in BSI epidemiology and adds important information for clinicians, researchers and policymakers concerning BSIs. Our results suggest that sex disparities in BSI cannot be explained fully by the mediating factors investigated. Sex affects the shape of immune responses attributed to genetic, hormonal, and environmental factors13,14,32. The human X-chromosome encodes a number of critical genes involved in the regulation of immune functions32. It is clear that sex extensively influences the host immune responses, but this sexual dimorphism is underappreciated, and sex bias is a major challenge in clinical trials18. Sex hormones act as important modulators of immune functions and responses; testosterone and progesterone are immunosuppressive, while oestradiol is immunoenhancing14. Few human studies have investigated sex hormones’ effects in severe infections and sepsis. Interestingly, in covid-19 where men are more prone to a severe course, the use of antiandrogens in men have shown promising results on severity42. Furthermore, in a review of health records in post-menopausal women with regular use of oestradiol, the fatality risk of covid-19 is reduced by more than 50%43.
Future perspectives of our results include the need for targeted research on how these sex differences could be addressed to achieve a longer and healthier life for both men and women. Additional work should focus on how health behaviours, education level, cardiovascular risk factors and comorbidities play a role in the sex disparities seen in severe infectious diseases. Knowledge of mediating factors together with recognition of sex differences in severe infections are important for public health leaders, researchers, and clinicians as it can inform preventive actions and identify individuals especially at risk19.
Conclusion
Our study has shown that men have an increased risk of BSI and BSI mortality. Using mediation analyses we estimated that 34% of the increased risk of BSI is mediated through known BSI risk factors. As BSI represents an important global burden of disease, our study serves as a catalyst for additional investigations by establishing the presence of sex differences and mediating risk factors. This will potentially lead to targeted management strategies to prevent BSI and sepsis in both men and women.
Supplementary Information
Acknowledgements
The Trøndelag Health Study (the HUNT study) is a collaboration of the HUNT Research Centre (Faculty of Medicine and Health Sciences, NTNU, Norwegian University of Science and Technology), Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health. The authors sincerely thank Arne Mehl for his extensive and thorough work with the HNT HF sepsis registry, the microbiology departments of Levanger, Namsos and St. Olavs hospitals for providing microbial data, and the Department for Research at Nord-Trøndelag Hospital Trust for assistance with data linkage.
Author contributions
R.M.M., L.T.G., M.K.M., K.V.L., S.E.Å., T.N., J.K.D. and E.S. conceived and designed the study. T.N., B.O.Å., L.G., A.T.D., T.R., J.K.D. and E.S. supervised the study. R.M.M., B.O.Å., Å.A. and L.T.G. contributed in the acquisition of data for statistical analyses. R.M.M. analyzed the data, prepared tables, and figures. All authors interpreted the results, drafted the manuscript, contributed to the discussions, and revised the manuscript critically for important intellectual content. All authors have read and approved the final version of manuscript and agreed to be accountable for all aspects of the work.
Funding
N/A. This work was supported by a Grant from The Liaison Committee for Education, Research, and Innovation in Central Norway. Open access funding provided by Norwegian University of Science and Technology.
Data availability
Data is available from the authors upon reasonable request and by application to HUNT Research Centre. https://hunt-db.medisin.ntnu.no/hunt-db/.
Code availability
A detailed description of the IOW analyses is included in Supplemental Material.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-12569-8.
References
- 1.Rudd KE, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ. Late mortality after sepsis: Propensity matched cohort study. BMJ. 2016;353:i2375. doi: 10.1136/bmj.i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddick LE, Alto NM. Bacteria fighting back: How pathogens target and subvert the host innate immune system. Mol. Cell. 2014;54:321–328. doi: 10.1016/j.molcel.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulsen J, et al. Associations of obesity and lifestyle with the risk and mortality of bloodstream infection in a general population: A 15-year follow-up of 64 027 individuals in the HUNT study. Int. J. Epidemiol. 2017;46:1573–1581. doi: 10.1093/ije/dyx091. [DOI] [PubMed] [Google Scholar]
- 5.Mohus RM, et al. Association of iron status with the risk of bloodstream infections: Results from the prospective population-based HUNT Study in Norway. Intens. Care Med. 2018;44:1276–1283. doi: 10.1007/s00134-018-5320-8. [DOI] [PubMed] [Google Scholar]
- 6.Esper AM, et al. The role of infection and comorbidity: Factors that influence disparities in sepsis. Crit. Care Med. 2006;34:2576–2582. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Askim A, et al. Anxiety and depression symptoms in a general population and future risk of bloodstream infection: The HUNT study. Psychosom. Med. 2018;80:673–679. doi: 10.1097/PSY.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 8.Moore JX, et al. Community characteristics and regional variations in sepsis. Int. J. Epidemiol. 2017;46:1607–1617. doi: 10.1093/ije/dyx099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laupland KB, et al. Severe bloodstream infections: A population-based assessment. Crit. Care Med. 2004;32:992–997. doi: 10.1097/01.ccm.0000119424.31648.1e. [DOI] [PubMed] [Google Scholar]
- 10.Pietropaoli AP, Glance LG, Oakes D, Fisher SG. Gender differences in mortality in patients with severe sepsis or septic shock. Gender Med. 2010;7:422–437. doi: 10.1016/j.genm.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adrie C, et al. Influence of gender on the outcome of severe sepsis: A reappraisal. Chest. 2007;132:1786–1793. doi: 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- 12.Drozd M, et al. Non-communicable disease, sociodemographic factors, and risk of death from infection: A UK Biobank observational cohort study. Lancet Infect. Dis. 2021;21:1184–1191. doi: 10.1016/S1473-3099(20)30978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vázquez-Martínez ER, García-Gómez E, Camacho-Arroyo I, González-Pedrajo B. Sexual dimorphism in bacterial infections. Biol. Sex Differ. 2018;9:27. doi: 10.1186/s13293-018-0187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein SL, Flanagan KL. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 15.Uslan DZ, et al. Age- and sex-associated trends in bloodstream infection: A population-based study in Olmsted County, Minnesota. Arch. Intern. Med. 2007;167:834–839. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 16.Laupland KB, Pasquill K, Steele L, Parfitt EC. Burden of bloodstream infection in older persons: A population-based study. BMC Geriatr. 2021;21:31. doi: 10.1186/s12877-020-01984-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laupland KB. Defining the epidemiology of bloodstream infections: The 'gold standard' of population-based assessment. Epidemiol. Infect. 2013;141:2149–2157. doi: 10.1017/S0950268812002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauvais-Jarvis F, et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet. 2020;396:565–582. doi: 10.1016/S0140-6736(20)31561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . Addressing Sex and Gender in Epidemic-Prone Infectious Diseases. World Health Organization; 2007. [Google Scholar]
- 20.Laupland KB, et al. Population-based risk factors for community-onset bloodstream infections. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:753–758. doi: 10.1007/s10096-019-03777-8. [DOI] [PubMed] [Google Scholar]
- 21.Lindström A-C, Eriksson M, Mårtensson J, Oldner A, Larsson E. Nationwide case–control study of risk factors and outcomes for community-acquired sepsis. Sci. Rep. 2021 doi: 10.1038/s41598-021-94558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VanderWeele T, Vansteelandt S. Mediation analysis with multiple mediators. Epidemiol. Methods. 2014;2:95–115. doi: 10.1515/em-2012-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen QC, Osypuk TL, Schmidt NM, Glymour MM, Tchetgen Tchetgen EJ. Practical guidance for conducting mediation analysis with multiple mediators using inverse odds ratio weighting. Am. J. Epidemiol. 2015;181:349–356. doi: 10.1093/aje/kwu278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krokstad S, et al. Cohort profile: The HUNT study, Norway. Int. J. Epidemiol. 2012;42:968–977. doi: 10.1093/ije/dys095. [DOI] [PubMed] [Google Scholar]
- 25.Mehl A, et al. Burden of bloodstream infection in an area of Mid-Norway 2002–2013: A prospective population-based observational study. BMC Infect. Dis. 2017;17:205. doi: 10.1186/s12879-017-2291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H, et al. A guideline for reporting mediation analyses of randomized trials and observational studies: The AGReMA statement. JAMA. 2021;326:1045–1056. doi: 10.1001/jama.2021.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H, Herbert RD, McAuley JH. Mediation analysis. JAMA. 2019;321:697–698. doi: 10.1001/jama.2018.21973. [DOI] [PubMed] [Google Scholar]
- 28.Gerdts E, Regitz-Zagrosek V. Sex differences in cardiometabolic disorders. Nat. Med. 2019;25:1657–1666. doi: 10.1038/s41591-019-0643-8. [DOI] [PubMed] [Google Scholar]
- 29.Mauvais-Jarvis F. Epidemiology of gender differences in diabetes and obesity. In: Mauvais-Jarvis F, editor. Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity. Springer; 2017. pp. 3–8. [Google Scholar]
- 30.Antequera, A. et al. Sex as a prognostic factor for mortality in critically ill adults with sepsis: a systematic review and meta-analysis. BMJ Open.11(9), e048982. 10.1136/bmjopen-2021-048982 (2021). [DOI] [PMC free article] [PubMed]
- 31.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 32.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: When a chromosome makes the difference. Nat. Rev. Immunol. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- 33.Paulsen J, et al. Epidemiology and outcome of Staphylococcus aureus bloodstream infection and sepsis in a Norwegian county 1996–2011: An observational study. BMC Infect. Dis. 2015;15:116. doi: 10.1186/s12879-015-0849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakr A, Brégeon F, Mège JL, Rolain JM, Blin O. Staphylococcus aureus nasal colonization: An update on mechanisms, epidemiology, risk factors, and subsequent infections. Front. Microbiol. 2018;9:2419. doi: 10.3389/fmicb.2018.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stensen DB, et al. Circulating sex-steroids and Staphylococcus aureus nasal carriage in a general female population. Eur. J. Endocrinol. 2021;184:337–346. doi: 10.1530/EJE-20-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stensen DB, et al. Hormonal contraceptive use and Staphylococcus aureus nasal and throat carriage in a Norwegian youth population. PLoS ONE. 2019;14:e0218511. doi: 10.1371/journal.pone.0218511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z, et al. Nasal decontamination for the prevention of surgical site infection in Staphylococcus aureus carriers. Cochrane Database Syst. Rev. 2017;5:012462. doi: 10.1002/14651858.CD012462.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309–321. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Askim A, et al. Epidemiology and outcome of sepsis in adult patients with Streptococcus pneumoniae infection in a Norwegian county 1993–2011: An observational study. BMC Infect. Dis. 2016;16:223. doi: 10.1186/s12879-016-1553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tolsma V, et al. Sepsis severe or septic shock: Outcome according to immune status and immunodeficiency profile. Chest. 2014;146:1205–1213. doi: 10.1378/chest.13-2618. [DOI] [PubMed] [Google Scholar]
- 41.Evans L, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intens. Care Med. 2021;47:1181. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cadegiani FA, McCoy J, Gustavo Wambier C, Goren A. Early antiandrogen therapy with dutasteride reduces viral shedding, inflammatory responses, and time-to-remission in males with COVID-19: A randomized, double-blind, placebo-controlled interventional trial (EAT-DUTA AndroCoV Trial—Biochemical) Cureus. 2021;13:e13047. doi: 10.7759/cureus.13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seeland U, et al. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Med. 2020;18:369. doi: 10.1186/s12916-020-01851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available from the authors upon reasonable request and by application to HUNT Research Centre. https://hunt-db.medisin.ntnu.no/hunt-db/.
A detailed description of the IOW analyses is included in Supplemental Material.