Abstract

In the present work, for the first time, the in situ formation of blue emissive carbon dots (bCDs) and encapsulation into the pores of chromium-based metal–organic frameworks (Cr-MOFs) are described. The luminescent bCDs via in situ process are formed and entrapped inside the pores of Cr-MOFs to form a nanocomposite of bCDs@Cr-MOFs. The bCDs@Cr-MOFs showed a strong broad blue emission at 420 nm (excited at 310 nm), which corresponds to both, the ligand (2-aminoterephthalic acid) in the Cr-MOF and the entrapped bCDs. This is assigned for the entrapping of bCDs in the pores of the MOFs. Additionally, transmission electron microscopy (TEM) images showed two types of particles, 150 rod-like shapes for Cr-MOF and 5–10 nm spherical shapes assigned for the presence of bCDs. The bCDs alone (without Cr-MOF) showed no selectivity, and their emission was quenched by different biomolecules and ions, such as ascorbic acid, uric acid, Fe3+, Cu2+, and Hg2+. The selectivity of bCDs toward uric acid was increased dramatically when they were encapsulated in the Cr-MOF. The linear range for uric acid was 20–50 μM, and the LOD was measured as 1.3 μM. Spike recoveries for the detection of uric acid in serum samples were between 94 and 108%. The relative standard deviation (RSD, n = 3) at each concentration value was less than 2%. The results showed high ruggedness and robustness of the assay due to its high shelf-life stability of probe (four weeks), water stability, and long working pH range. Validation experiments showed that the established MOF-based sensing system is appropriate for uric acid detection in real samples.
1. Introduction
Uric acid (UA) is a waste product in the blood and the end product of purine catabolism that is excreted from the kidney into the urine.1,2 In healthy adults, uric acid in the body is about 1.1 g, with 15% of that in the blood.3 Excessive uric acid production or uric acid excretion malfunction will result in a large amount of uric acid being retained in the body. A blood uric acid content of more than (3.4–7.0) mg dL–1 would disrupt human cell activity and cause symptoms such as gout. Severe renal impairment and lead poisoning can also cause an abnormally high level of uric acid in the blood.4 Increased uric acid in the blood is also a sign of disorders such as pneumonia, multiple myeloma, polycythemia, and leukemia.5,6 Therefore, it is of significant importance to develop an efficient and rapid method for uric acid detection in biofluid samples. Different methods and techniques are reported in the literature for the determination of UA in biological samples, such as enzymatic methods,7 high-performance liquid chromatography,8 chemiluminescence,9 surface-enhanced Raman spectroscopy analysis,10 potentiometry,11 and electrochemical approach.5 To date, various micro- and nanomaterials with different characteristics have been employed for the monitoring and detection of uric acid, including nanocarbon materials,2 metals and metal oxides,12,13 semiconducting materials,14,15 quantum dots,16 polymers,17,18 and metal–organic frameworks (MOFs).19,20
Metal–organic frameworks (MOFs) are porous materials resulting from the combination of organic ligands and metal ions.21−23 The coordination between metal centers and organic ligands (linkers) gives rise to complex assemblies, which can develop from one-dimensional to three-dimensional structure coordination polymers. These materials are usually porous with well-defined channels or pores.24 Depending on the combination of the metal and organic components, selectivity toward different classes of gases or biomolecules (guests) can be achieved, which is the aim of this research.25 Analogous to the reaction specificity achieved in conventional enzyme pockets, MOFs are also powerful platforms for regulating the selectivity via engineering their microenvironments, such as metal node alternation, ligand functionalization, pore decoration, topology variation, and others.26
To improve the functionality of MOFs toward various target molecules, different luminescent materials are encapsulated into their pores, such as molecular fluorophores,31 quantum dots,32,33 carbon dots,34 dyes,35 and metallic nanoparticles.36 Various strategies have been reported in the literature for the encapsulation of luminescent materials into MOF pores. Integrating MOFs with functional materials can be achieved through (1) encapsulating guest functional materials in the pores, matrices, or layers of MOFs or (2) encapsulating/coating MOFs in/with functional supports/layers.37 In this case, MOFs act as porous supports to accommodate functional materials (such as metal NPs, QDs, POMs), preventing the leaching and aggregation of functional materials while allowing the free diffusion of substrates and products. In some cases, functional materials (such as silica and polymers) can act as shelters to enhance the chemical stability and mechanical strength of MOFs and facilitate catalyst formulation for catalytic applications.38
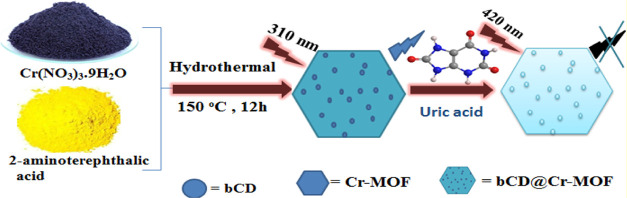
However, to the best of our knowledge, there is no report showing encapsulation of luminescent carbon dots into MOF using the same ligand as a linker and a precursor for the carbon dots. To fill this gap, here, we are aiming at the in situ preparation of MOFs and encapsulation of luminescent carbon nanomaterials. Blue emissive carbon dots (bCDs) encapsulated in Cr-MOFs have been prepared using a one-pot hydrothermal process as shown in Figure 1. The bCD@Cr-MOFs and CD fluorescence in the blue wavelength, even excitation at 310 nm, for both nanocomposites are quenched after the addition of uric acid.
Figure 1.
Scheme showing in situ preparation and encapsulation of carbon dots into the MOF to form bCD@Cr-MOF.
2. Experimental Section
2.1. Materials
All reagents used throughout this work were of analytical grade and purchased from commercial suppliers and used without further purification. Chromium nitrate hydrate (Cr(NO3)3·9H2O), uric acid, 2-aminoterephthalic acid (NH2-H2BDC) (99%), Cu(NO3)2·6H2O (98%), mercury nitrate (Hg(NO3)2), sodium hydroxide (NaOH) (98%), N,N-dimethylformamide (DMF), and absolute methanol were purchased from Sigma-Aldrich. Urea, lactose, sucrose, glucose, ascorbic acid, l-argnine, glycine, aluminum(III) nitrate nonahydrate Al(NO3)3·9H2O, iron(III) nitrate nonahydrate Fe(NO3)3·9H2O, Cd(NO3)2, Co(NO3)2, and Ni(NO3)2 were purchased from Merck (Darmstadt, Germany). The distilled water was used throughout the experiments.
2.2. Instrumentation
A transmission electron microscope (TEM) (Zeiss-EM1OC-100 KV, Germany) was used to take TEM images to determine the morphological features and particle size of the prepared bCDs@Cr-MOFs. A FE-SEM (field emission-scanning electron microscope (TESCAN Mira 3, USA)) was used to take SEM images to determine the shape of the crystal. Subsequently, a Cary 60 Spectrophotometer (Agilent Technologies, USA) was used to obtain the UV–vis absorption spectra. Fluorescence spectra were recorded via a Cary Eclipse fluorescence spectrophotometer (Agilent Technologies, USA), and both the emission and excitation slits were set at 5.0 nm. Empyrean X-ray diffractometer (PANalytical, Netherland) was used to collect the X-ray diffraction patterns XRD. The functional groups of the CDs were detected by a Fourier transform infrared spectrometer FTIR Nicolet iS50 (Thermo Scientific, USA) through a diamond ATR attachment.
2.3. Synthesis of bCDs@Cr-MOFs
The bCDs@Cr-MOFs were synthesized via the hydrothermal method according to a previous procedure39 with modifications. Typically, 0.8 g of Cr(NO3)3·9H2O was added into 30 mL of distilled water containing 0.2 g of NaOH and sonicated for 30 min. NH2-H2BDC (0.36 g) was then dissolved in the above mixture solution and sonicated for another 30 min. The mixed solution was transferred to a 50 mL Teflon-lined stainless steel autoclave and was maintained at 150 °C for 12 h. After cooling at room temperature, the obtained mixture was collected by filtration and washed three times with water, DMF, and methanol, successively. The green product was dried under vacuum at 80 °C overnight.
2.4. Synthesis of Blue Emissive Carbon Dots (bCDs)
bCDs were synthesized through a one-pot hydrothermal carbonization method as reported in the literature.29 In a typical synthetic procedure, 0.4 g of 2-aminoterephthalic acid and 0.2 g of NaOH were dissolved in 30 mL water at 60 °C under stirring. Subsequently, the solution was transferred into a 50 mL Teflon-lined stainless steel autoclave and maintained at 150 °C for 12 h. After the autoclave was cooled down, the resulting light yellow solution was dialyzed in a dialysis bag (500 Da) for 24 h to remove NaOH and unreacted 2-aminoterephthalic acid by refreshing the outside water of the bag periodically. Finally, the obtained bCD solution in the dialysis bag was collected for further experiments.
2.5. Fluorometric Assay for Uric Acid
bCD@Cr-MOFs (50 mg) were dissolved in 1 L of deionized water, and then the prepared probe was mixed with 2 mL of different concentrations of uric acid solutions. The pH of each solution was adjusted to 7.0 with 1 mL of phosphate-buffered saline (PBS). The fluorescence spectra were recorded for 15 min after mixing. Each measurement was repeated three times for the sake of repeatability.
2.6. Analysis of Uric Acid in Real Blood Samples
Serum fluid samples were taken from patients in Shahid Shawkat Hospital in SaidSadiq town, Kurdistan, Iraq. The serum samples were then diluted 10 times with deionized water to form a diluted serum solution. The bCDs@Cr-MOFs solution (1.0 mL) was mixed with 2.0 mL of the diluted serum solution after the addition of 1.0 mL of PBS (pH 7.0). Each sample solution was spiked with standard solutions of the target molecule.
3. Results and Discussion
3.1. Preparation and Characterizations
Common microscopic and spectroscopic techniques were used to characterize the chemical structure, morphology, and composition of MOF and entrapped bCDs. According to the literature, there are three methods to entrap (encapsulate) a nanomaterial into the pores of MOF, such as: (1) prepared carbon dots are mixed with MOF precursors;27 (2) prepared carbon dots and MOF are mixed together;28 and (3) different precursors for MOF and carbon dots are mixed together. However, in the present work, the ligand is the precursor of the linker (for the MOF) and the bCDs as well. Based on the literature, 2-aminoterephthalic acid can be converted to carbon dots (CD or CQD) when heated hydrothermally at 150 °C.29 On the other hand, it can be converted to MIL-101 when mixed with chromium nitrate under the same condition.30 Thus, the ligand has a dual function here, as we will prove these functions below.
3.2. Size and Morphology
SEM and TEM were used to determine the size and morphology bCD@MOFs.40−42 The average diameter of CD@Cr-MOFs can be estimated by counting the particle size on the TEM images. Figure 2 shows the TEM images, size distribution, and SEM image of the CD@Cr-MOFs (Figures S1 and S2, Supporting Information). One can notice from the TEM images that two kinds of nanoparticles are observed, 10 nm spherical and 120 nm rodlike shape. The 10 nm particles can be attributed to bCDs, while the 120 nm rodlike particles are attributed to MOFs. Based on this (also based on the selectivity study in the next section), we conclude that at 150 °C, bCDs are formed in parallel to the formation of Cr-MOFs. Thus, it is for the first time we show the in situ synthesis of carbon dots and MOFs accompanied by encapsulation with the nanostructure of MOFs.
Figure 2.
(A, B) TEM image of bCD@Cr-MOFs, (C) size distribution histogram, and (D) SEM image.
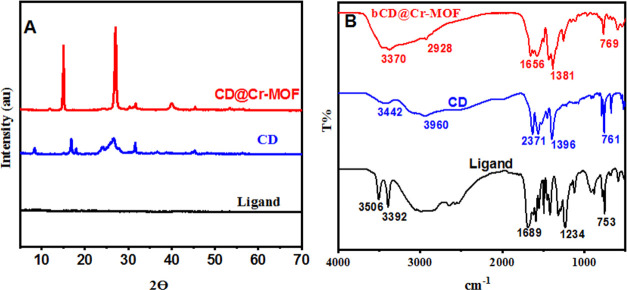
The XRD analysis was carried out to explore the crystal structures of the precursors, the ligand, bCD, and the bCDs@Cr-MOFs. As illustrated in Figure 3A, the XRD patterns of the obtained bCDs@Cr-MOFs, bCD, and the ligand 2-aminoterephthalic acid indicate that the well-defined crystal structure was formed and Cr-MOFs were successfully prepared. The XRD pattern of the ligand (2-aminoterephthalic acid) showed an amorphous structure as shown in Figure 3A. The XRD spectra of the CD@Cr-MOF shown in Figure 3A displayed sharp peaks centered at 15 and 26°, which was attributed to highly ordered atoms in bCD@Cr-MOF. The decrease in the peak intensity and the increase of the full width at half-maximum in bCD (blue line) were due to the small size and amorphous nature of the resultant bCDs. According to the XRD results of bCD@Cr-MOF samples synthesized with different molar ratios of H2O/H2BDC, the results showed that the amount of water in the composition of reactants has a considerable effect on the structure of bCD@Cr-MOF. All of the samples with an increase in molar ratios of H2O/H2BDC have characteristic diffractions of MIL-101. In addition, at a high water content in the reactant mixtures, a lower peak intensity was observed. Therefore, the water content in the reactant mixtures not only affected the structure of materials but also reduced their crystallinity.43,44
Figure 3.
(A) XRD patterns of bCDs@Cr-MOF, bCDs, and the ligand. (B) FTIR spectra of bCDs@Cr-MOF, CD, and the ligand, respectively.
To explore the possible functional groups on the surface of the ligand, bCDs, and bCD@Cr-MOF, FTIR spectra were recorded. As displayed in Figure 3B, the N–H peaks of ligand 2-aminoterephthalic acid (black line) at 3506, 3392, and 1689 cm–1 were weakened to varying degrees after combined with chromium ions to form bCD@Cr-MOF (red line), which suggests the excellent and strong coordination between amino groups and Cr3+.4545 The FTIR absorbance peak of pure CDs (blue line) rules out the presence of free and unbound CDs in the sample; in contrast, it confirms the encapsulation of CDs in the MOF cavities.46,47
The optical properties of the bCDs@Cr-MOF were assessed using UV–vis absorption and fluorescence emission spectra (Figure 4A,B). As shown in the UV–vis spectra (Figure 4A), bCD@Cr-MOF solution showed an absorption spectrum centered at 307 nm, which could be attributed to the n–π* transition of C = O band of Cr-MOF, and also showed an absorption spectrum of bCD (red line) and ligand (black line).47 Photoluminescence spectra and excitation spectra of the bCD@Cr-MOFs solution were recorded as shown in Figure 4B. The emission spectra of bCD, the ligand, and bCD@Cr-MOF are displayed in Figure 4C, all having different emission spectra when they are excited at the same wavelength as shown in Figure 4C. The fluorescence emission of the ligand centered at 405 nm, while the bCDs were broad, i.e., around 420–430 nm, and it was in good agreement with that reported around 420 nm.29 The emission spectra of CD@Cr-MOF are broad, and they are more like a combination of both emission spectra of CDs and the ligand. This is another evidence that the product contains entrapped CDs inside the pore of Cr-MOFs. It is worth mentioning that, during the preparation step, the product was washed many times with water and methanol to remove carbon dots in the solution and even on the surface of the MOF.
Figure 4.
(A) UV–vis absorption spectra of Cr-MOF, CD, and ligand; (B) excitation and emission spectra of Cr-MOF and absorption spectrum of uric acid (green one); (C) emission spectra of bCD@Cr-MOF, CD, and ligand excited at 310 nm; (D) excitation-dependent emission spectra of the probe bCD@Cr-MOF; (E) fluorescence intensities quenching of the CDs@Cr-MOF suspension upon adding different concentrations (0–250 μM) of uric acid at 310 nm excitation; and (F) the corresponding Stern–Volmer fitting curves of CDs@Cr-MOF toward uric acid (the inset represents the linear response range).
Excitation-dependent fluorescence spectra were recorded as shown in Figure 4D. It is obvious that the product is excitation-dependent. It is another evidence that the prepared Cr-MOFs contained carbon dots, as carbon dots are well known to show excitation-dependent emission.48,49
The emission of bCDs@Cr-MOFs was quenched selectively after the addition of uric acid (Figure 4E). The possible uric acid detection mechanism was explored by collecting UV–vis spectra of uric acid and fluorescence spectra of the bCDs@Cr-MOF. As shown in Figure 4B, the absorption band of uric acid is overlapped with the excitation band of bCDs@Cr-MOF, which suggests that the fluorescence quenching process could take place via the inner filter effect (IFE).50,51 These results suggested that the (IFE) may dominate the fluorescence quenching process for the quantification of uric acid.52
In the fluorescence quenching process of uric acid detection, the strong fluorescence intensities of bCDs@Cr-MOF are a prerequisite for their superior detection capability. Therefore, the intrinsic fluorescence intensities of bCDs@Cr-MOF were measured at the specific concentration. The result presented in Figure 4E implied that the prepared bCDs@Cr-MOF possesses a strong fluorescence intensity that can be used for the quantification of uric acid, which may be determined by the different degrees of the inner filter effect.53,54 As shown in Figure 4F, the bCD@Cr-MOF probe was quenched by a micromolar concentration range of uric acid, which can be used for the quantification of uric acid as a target molecule selectively, in a manner that exhibited good linearity from 20 to 45 μM, 1.29 μM as LOD and 3.8 μM as LOQ (Figure 4F). Table 1 shows a comparison between commonly reported articles in the literature and our proposed assay.
Table 1. Comparison of Other Analytical Methods or Probes for Uric Acid Detection.
| analytical method | probe or sensor | real sample | linear range (μM) | LOD (μM) | refs |
|---|---|---|---|---|---|
| fluorescence | N-CQDs | urine | 0.1–45 | 0.05 | (3) |
| enzymatic | CuInS/ZnS | serum | 0.25–4.0 | 0.05 | (55) |
| fluorescence | UiO-66-NH2 MOF | serum | 0.01–400 | 0.0023 | (19) |
| fluorescence | Au/Ag core–shell NPs | urine | 0.5–10 | 0.4 | (56) |
| enzymatic | graphene/HfO2 | serum | 1–1000 | 1 | (14) |
| electrochemical (DPV) | g-C3N4 NS | urine | 100–1000 | 4.45 | (15) |
| electrochemical (CV) | ZnO QDs | serum | 1000–10 000 | 22.97 | (16) |
| electrochemical (CV) | MOF-71 | serum | 50–1000 | 15.61 | (20) |
| fluorescence | Cr-MOF | serum | 20–45 | 1.29 | this work |
3.3. Selectivity of bCD@Cr-MOF for Uric Acid Detection
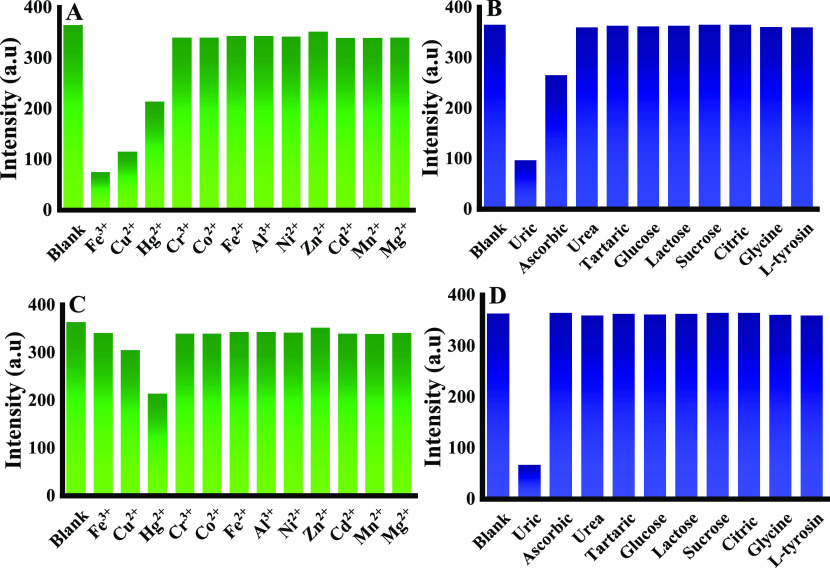
Considering the complexity and feasibility of the practical application environment, the specific identification of analytes and excellent measuring ability are also necessary criteria to ensure that the material becomes an ideal sensor. Thus, the uric acid detection selectivity of bCDs@Cr-MOF was studied by monitoring the effects of different metal ions such as Fe2+, Cd2+, Cr3+, Al3+, Ni2+, Co2+, Fe3+, Hg2+, Cu2+, Zn2+, Mn2+, and Mg2+. Other biomolecules were tested, such as ascorbic acid, urea, tartaric acid, citric acid, l-arginine, glycine, glucose, lactose, sucrose, aspartic acid, and l-tyrosine, as shown in Figure 5a,c,d, respectively. The uric acid molecule exhibited optimal fluorescence quenching ability and resulted in a final fluorescence intensity that is about two-tenths of the initial fluorescence intensity, as shown in Figure 5D, and uric acid quenched the emission intensity of the probe from 350 to 70 nm (i.e., reduced 80% of the initial emission intensity). Furthermore, most of the interfering ions showed a negligible effect on the fluorescence intensity of bCDs@Cr-MOF (Figure 5A–D), suggesting its qualified selectivity for uric acid detection. Meanwhile, the competition experiments were performed by adding various interference ions and biomolecules at the concentration of 0.01 M to the bCDs@Cr-MOF solution to explore their quenching ability, and we found that the fluorescence intensity of the prepared bCDs@Cr-MOF was not affected significantly in the presence of various interfering species, further suggesting that the as-prepared bCDs@Cr-MOF sensor possesses excellent selectivity toward uric acid detection. While the fluorescence intensity of the CD alone will be quenched by uric acid, ascorbic acid, Cu2+, Fe3+, and Hg2+ (i.e., CDs alone without MOFs are not selective toward uric acid), as revealed in Figure 5A,B, bCDs@Cr-MOF has good response to uric acid and low response to Hg2+; therefore, it can be used selectively for the quantification of uric acid in the sample as shown in Figure 5C,D.
Figure 5.
Selectivity of bCDs@Cr-MOF and CD for uric acid detection in the presence of different metal ions and molecules (10.0 equiv) and uric acid (1.0 equiv): (A) bCD with metal ions; (B) bCD with molecules; (C) bCDs@Cr-MOF with metal ions; and (D) bCDs@Cr-MOF with molecules.
The reasons behind improving the selectivity of bCD@Cr-MOF over CDs alone are a bit complicated and not well established. Definitely, the size and shape selectivity afforded by MOFs originates from the sieving effect of their uniform pores of MOF.
The pH effect on the luminescence performance of bCDs@Cr-MOF was also investigated by monitoring the fluorescence intensity change under different pHs (1.0–12.0). The results presented in Figure 6A showed an almost unchanged and good fluorescence intensity in the pH range of 6.0–10.0, which suggested that the bCDs@Cr-MOF possesses excellent pH-independent fluorescence characteristics in the range of pH = 6.0–10.0.57,58 In this work, the bCDs@Cr-MOF can well meet the uric acid detection requirements, and the results are not affected by the pH environment. Furthermore, the excellent fluorescence stability of bCD@Cr-MOF was demonstrated by the fluorescence intensity without significant change despite being placed in the solution for 3 weeks (Figure 6B). These remarkable properties motivate us to explore its application in the field of fluorescence detection.
Figure 6.
(A) Effect of pH on the fluorescence intensity of bCD@Cr-MOF. (B) Fluorescence stability of the bCDs@Cr-MOF.
4. Applications
To evaluate the practical application of the sensor, the sensing behavior of uric acid in samples was further studied. The collected blood serum sample was pretreated through serum from the blood before the experiment and then spiked with different concentrations of uric acid. The fluorescence spectra were recorded for each unspiked and spiked sample, and the F°/F value is measured and the content of uric acid in the real samples was determined based on the obtained working calibration curve. The feasibility of determining the concentration of uric acid in blood serum by bCDs@Cr-MOF was further discussed. As shown in Table 2, the recoveries of uric acid in serum samples were 94 and 108% and the relative standard deviations (RSD) were 1.51 and 1.14%, respectively. The results showed that the bCDs@Cr-MOF as a fluorescence sensor could be successfully applied for the determination of uric acid in serum samples. To evaluate the suitability of the designed sensor in real sample analysis, the standard addition method was applied for three serum samples. The serum was diluted 10 times in 0.1 M phosphate-buffered saline (PBS), and certain amounts of uric acid were added into the sample. The amount of the analyte was estimated by comparing the peak current with the calibration curves (Figure 4F).
Table 2. Detection Results of Uric Acid in Serum Sample Using bCDs@Cr-MOF Probe.
| sample | uric acid detection (μM) | added (μM) | found (μM) | recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 1 | 15.11 | 5 | 20.41 | 108 | 1.51 |
| 1 | 15.11 | 10 | 25.18 | 102 | 1.42 |
| 1 | 15.11 | 20 | 34.3 | 96 | 1.07 |
| 2 | 20.14 | 5 | 24.81 | 98 | 1.01 |
| 2 | 20.14 | 10 | 29.36 | 94 | 1.14 |
| 2 | 20.14 | 15 | 33.52 | 91 | 1.67 |
5. Conclusions
In summary, in situ encapsulation, for the first time, of blue carbon dots into Cr-MOF was achieved and selectively used for the detection of uric acid in serum samples. Spectroscopic and microscopic results showed that the product contains bCDs inside MOFs. This outcome is most likely to obtain in procedures containing hydrothermal treatment of carbonaceous ligands. The encapsulation enhanced the selectivity toward the detection of uric acid. Compared with the pure bCD, all bCDs entrapped in Cr-MOF nanocomposites exhibited remarkable selectivity for the quantification of uric acid. Moreover, the nanocomposite probe was photostable, and working in long pH ranges makes it a robust and rugged probe. The above collective features prove that the resulting bCD@Cr-MOF is a promising platform for the synchronous detection and quantification of uric acid in biological and environmental samples, providing a new perspective on monitoring uric acid in different samples. The detection of uric acid in real serum fluid was also examined and showed satisfactory recovery.
Acknowledgments
This research was supported by the University of Sulaimani. The authors thank the College of Science and Department of Chemistry of Sulaimani University for support. O.B.A.S. thanks Ministry of Education for the study leave.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00790.
SEM and TEM data of the prepared probe (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Riches P. L.; Wright A. F.; Ralston S. H. Recent insights into the pathogenesis of hyperuricaemia and gout. Hum. Mol. Genet. 2009, 18, R177–R184. 10.1093/hmg/ddp369. [DOI] [PubMed] [Google Scholar]
- Álvarez-Lario B.; Macarrón-Vicente J. Uric acid and evolution. Rheumatology 2010, 49, 2010–2015. 10.1093/rheumatology/keq204. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Yang Y.; Liu W.; Ding F.; Zhao Q.; Zou P.; Wang X.; Rao H. Colorimetric and fluorometric determination of uric acid based on the use of nitrogen- doped carbon quantum dots and silver triangular nanoprisms. Microchim. Acta 2018, 185, 281 10.1007/s00604-018-2814-6. [DOI] [PubMed] [Google Scholar]
- Burini O. High Plasma Uric Acid Concentration: Causes and Consequences. Diabetol. Metab. Syndr. 2012, 4, 12 10.1186/1758-5996-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y. C.; Do J. S.; Liu C. C. An amperometric uric acid biosensor based on modified Ir-C electrode. Biosens. Bioelectron. 2006, 22, 482–488. 10.1016/j.bios.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Kutzing M. K.; Firestein B. L. Altered uric acid levels and disease states. J. Pharmacol. Exp. Ther. 2008, 324, 1–7. 10.1124/jpet.107.129031. [DOI] [PubMed] [Google Scholar]
- Erden P. E.; Kiliç E. A review of enzymatic uric acid biosensors based on amperometric detection. Talanta 2013, 107, 312–323. 10.1016/j.talanta.2013.01.043. [DOI] [PubMed] [Google Scholar]
- Dai X.; Fang X.; Zhang C.; Xu R.; Xu B. Determination of serum uric acid using high-performance liquid chromatography (HPLC)/isotope dilution mass spectrometry (ID-MS) as a candidate reference method. J. Chromatogr. B 2007, 857, 287–295. 10.1016/j.jchromb.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Galbán J.; Galba J.; Andreu Y.; Almenara M. J.; Marcos S. De. Direct determination of uric acid in serum by a fluorometric-enzymatic method based on uricase fluorometric-enzymatic method based on uricase. Talanta 2001, 54, 847–854. 10.1016/s0039-9140(01)00335-6. [DOI] [PubMed] [Google Scholar]
- Wang F.; Cao S.; Yan R.; Wang Z.; Wang D.; Yang H. Selectivity/specificity improvement strategies in surface-enhanced raman spectroscopy analysis. Sensors 2017, 17, 2689 10.3390/s17112689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. M. U.; Ul N. H.; Alvi H.; Ibupoto Z. H.; Nur O. Linköping University Post Print Selective potentiometric determination of uric acid with uricase immobilized on ZnO nanowires Selective Potentiometric Determination of Uric Acid with Uricase Immobilized on ZnO Nanowires. Science 2011, 152, 241–247. 10.1016/j.snb.2010.12.015. [DOI] [Google Scholar]
- Alam M. M.; Asiri A. M.; Uddin M. T.; Islam M. A.; Awualc M. R.; Rahman M. M. Detection of uric acid based on doped ZnO/Ag2O/Co3O4 nanoparticle loaded glassy carbon electrode. New J. Chem. 2019, 43, 8651–8659. 10.1039/C9NJ01287G. [DOI] [Google Scholar]
- Minta D.; Moyseowicz A.; Gryglewicz S.; Gryglewicz G. A Promising Electrochemical Platform for Dopamine and Uric Acid Detection Based on a Polyaniline/Iron Oxide-Tin Oxide/Reduced Graphene Oxide Ternary Composite. Molecules 2020, 25, 5869 10.3390/molecules25245869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Zhao X.; Diao L.; Li H.; Tong Z.; Gu Z.; Miao B.; Xu Z.; Zhang H.; Wu Y.; Li J. Highly Sensitive Uric Acid Detection Based on a Graphene Chemoresistor and Magnetic Beads. Biosensors 2021, 304 10.3390/bios11090304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan N.; Chan-Park M. B.; Sundramoorthy A. K. Electrochemical Detection of Uric Acid on Exfoliated Nanosheets of Graphitic-Like Carbon Nitride (g-C 3 N 4) Based Sensor. J. Electrochem. Soc. 2019, 166, B3163–B3170. 10.1149/2.0261909jes. [DOI] [Google Scholar]
- Ali M.; Shah I.; Kim S. W.; Sajid M.; Lim J. H.; Choi K. H. Quantitative detection of uric acid through ZnO quantum dots based highly sensitive electrochemical biosensor. Sens. Actuators, A. 2018, 283, 282–290. 10.1016/j.sna.2018.10.009. [DOI] [Google Scholar]
- Revin S. B.; John S. A. Highly sensitive determination of uric acid in the presence of major interferents using a conducting polymer film modified electrode. Bioelectrochemistry 2012, 88, 22–29. 10.1016/j.bioelechem.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Ratautaite V.; Samukaite-Bubniene U.; Plausinaitis D.; Boguzaite R.; Balciunas D.; Ramanaviciene A.; Neunert G.; Ramanavicius A. Molecular imprinting technology for determination of uric acid. Int. J. Mol. Sci. 2021, 22, 5032 10.3390/ijms22095032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu S.; Li Z.; Jia Q. Detection of Purine Metabolite Uric Acid with Picolinic-Acid-Functionalized Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2019, 11, 34196–34202. 10.1021/acsami.9b07442. [DOI] [PubMed] [Google Scholar]
- Abrori S. A.; Septiani N. L. W.; Hakim F. N.; Maulana A.; Suyatman S.; Nugraha S.; Anshori I.; Yuliarto B. Non-Enzymatic Electrochemical Detection for Uric Acid Based on a Glassy Carbon Electrode Modified with MOF-71. IEEE Sens. J. 2021, 21, 170–177. [Google Scholar]
- Lee Y.; Lee J.-S.; Kao C.-C.; Yoon J.-H.; Vogt T.; Lee Y. Role of cation-water disorder during cation exchange in small-pore zeolite sodium natrolite. J. Phys. Chem. C 2013, 117, 16119–16126. 10.1021/jp405360s. [DOI] [Google Scholar]
- Wang J.; Fan Y.; Lee H. W.; Yi C.; Cheng C.; Zhao X.; Yang M. Ultrasmall metal-organic framework zn-mof-74 nanodots: Size-controlled synthesis and application for highly selective colorimetric sensing of iron(III) in Aqueous Solution. ACS Appl. Nano Mater. 2018, 1, 3747–3753. 10.1021/acsanm.8b01083. [DOI] [Google Scholar]
- Luo L.; Huang L.; Liu X.; Zhang W.; Yao X.; Dou L.; Zhang X.; Nian Y.; Sun J.; Wang J. Mixed-Valence Ce-BPyDC Metal-Organic Framework with Dual Enzyme-like Activities for Colorimetric Biosensing. Inorg. Chem. 2019, 58, 11382–11388. 10.1021/acs.inorgchem.9b00661. [DOI] [PubMed] [Google Scholar]
- Miller S. E.; Teplensky M. H.; Moghadam P. Z.; Fairen-Jimenez D. Metal-organic frameworks as biosensors for luminescence-based detection and imaging. Interface Focus 2016, 6, 20160027 10.1098/rsfs.2016.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Da.; Huang L. R.; Wu J. X.; Gu Z. Y. Enhancing selectivity through decrypting the uncoordinated zirconium sites in MOF electrocatalysts. Chem. Commun. 2021, 57, 5191–5194. 10.1039/D1CC01362A. [DOI] [PubMed] [Google Scholar]
- Guo J.; Qin Y.; Zhu Y.; Zhang X.; Long C.; Zhao M.; Tang Z. Metal-organic frameworks as catalytic selectivity regulators for organic transformations. Chem. Soc. Rev. 2021, 50, 5366–5396. 10.1039/D0CS01538E. [DOI] [PubMed] [Google Scholar]
- Li B.; Suo T.; Xie S.; Xia A.; Ma Y. j.; Huang H.; Zhang X.; Hu Q. Rational design, synthesis, and applications of carbon dots@metal–organic frameworks (CD@MOF) based sensors. TrAC, Trends Anal. Chem. 2021, 135, 116163 10.1016/j.trac.2020.116163. [DOI] [Google Scholar]
- Xiang W.; Zhang Y.; Lin H.; Liu C. J. Nanoparticle/metal-organic framework composites for catalytic applications: Current status and perspective. Molecules 2017, 22, 2103 10.3390/molecules22122103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y.; Li G.; Wang X.; Wang S.; Cheng S.; Zuo D.; Xu J.; Zhang H. A facile synthesis of self-doped carbon dots from 2-aminoterephthalic acid and their applications. Mater. Today Commun. 2019, 20, 100599 10.1016/j.mtcomm.2019.100599. [DOI] [Google Scholar]
- Zhang L.; Wang J.; Du T.; Zhang W.; Zhu W.; Yang C.; Yue T.; Sun J.; Li T.; Wang J. NH2-MIL-53(Al) Metal-Organic Framework as the Smart Platform for Simultaneous High-Performance Detection and Removal of Hg2+. Inorg. Chem. 2019, 58, 12573–12581. 10.1021/acs.inorgchem.9b01242. [DOI] [PubMed] [Google Scholar]
- Collet G.; Hrvat A.; Eliseeva S. V.; Besnard C.; Kovalenko A.; Petoud S. A near-infrared emitting MOF: controlled encapsulation of a fluorescein sensitizer at the time of crystal growth. Chem. Commun. 2021, 57, 3351–3354. 10.1039/D0CC08234A. [DOI] [PubMed] [Google Scholar]
- Son Y. R.; Kwak M.; Lee S.; Kim H. S. Strategy for encapsulation of CdS quantum dots into zeolitic imidazole frameworks for photocatalytic activity. Nanomaterials 2020, 10, 2498 10.3390/nano10122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. S.; Yoon K. B. Preparation and characterization of CdS and PbS quantum dots in zeolite Y and their applications for nonlinear optical materials and solar cell. Coord. Chem. Rev. 2014, 263–264, 239–256. 10.1016/j.ccr.2013.12.001. [DOI] [Google Scholar]
- Wang Y.; Wang M.; Wang X.; Ma W.; Liu J.; Li J. Designed synthesis of CD@Cu-ZIF-8 composites as excellent peroxidase mimics for assaying glutathione. Mater. Chem. Front. 2021, 5, 6125–6132. 10.1039/D1QM00702E. [DOI] [Google Scholar]
- Sun Z.; Khurshid A.; Sohail M.; Qiu W.; Cao D.; Su S.-J. Encapsulation of dyes in luminescent metal-organic frameworks for white light emitting diodes. Nanomaterials 2021, 11, 2761 10.3390/nano11102761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Yuqun.; Xu Haitao.; Wang Jiajia.; Luo Xikuo.; Xu Zhen-liang; Wang Kefu; Wang Wenzhong Nanorattle Au@PtAg encapsulated in ZIF-8 for enhancing CO2 photoreduction to CO. Nano Res. 2019, 12, 625–630. 10.1007/s12274-018-2269-4. [DOI] [Google Scholar]
- Chen L.; Xu Q. Metal-Organic Framework Composites for Catalysis. Matter 2019, 1, 57–89. 10.1016/j.matt.2019.05.018. [DOI] [Google Scholar]
- Gascon J.; Kapteijn F.; Ramos-fernandez E. V.; Garcia-domingos M.; Juan-alca J. MOFs meet monoliths: Hierarchical structuring metal organic framework catalysts. Appl. Catal., A 2011, 391, 261–267. 10.1016/j.apcata.2010.05.019. [DOI] [Google Scholar]
- Abdpour S.; Kowsari E.; Bazri B.; Moghaddam M. R. A.; Tafreshi S. S.; de Leeuw N. H.; Simon I.; Schmolke L.; Dietrich D.; Ramakrishna S.; Janiak C. Amino-functionalized MIL-101(Cr) photodegradation enhancement by sulfur-enriched copper sulfide nanoparticles: An experimental and DFT study. J. Mol. Liq. 2020, 319, 114341 10.1016/j.molliq.2020.114341. [DOI] [Google Scholar]
- Chowdhuri A. R.; Singh T.; Ghosh S. K.; Sahu S. K. Carbon Dots Embedded Magnetic Nanoparticles @Chitosan @Metal Organic Framework as a Nanoprobe for pH Sensitive Targeted Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 16573–16583. 10.1021/acsami.6b03988. [DOI] [PubMed] [Google Scholar]
- Liu X.; Zhou Z.; Wang T.; Deng P.; Yan Y. Carbon dots incorporated metal–organic framework for enhancing fluorescence detection performance. J. Mater. Sci. 2020, 55, 14153–14165. 10.1007/s10853-020-05027-1. [DOI] [Google Scholar]
- Xu L.; Fang G.; Liu J.; Pan M.; Wang R.; Wang S.. One-pot synthesis of nanoscale carbon dots-embedded metal-organic frameworks at room temperature for enhanced. J. Mater. Chem. A 4, 15880–15887.. [Google Scholar]
- Du P. D.; Thanh H. T. M.; To T. C.; Thang H. S.; Tinh M. X.; Tuyen T. N.; Hoa T. T.; Khieu D. Q. Metal-organic framework MIL-101: Synthesis and photocatalytic degradation of remazol black B dye. J. Nanomater. 2019, 2019, 6061275 10.1155/2019/6061275. [DOI] [Google Scholar]
- Zhao T.; Dong M.; Yang L.; Liu Y. Synthesis of stable hierarchical MIL-101(Cr) with enhanced catalytic activity in the oxidation of indene. Catalysts 2018, 8, 394 10.3390/catal8090394. [DOI] [Google Scholar]
- Babaee S.; Zarei M.; Sepehrmansourie H.; Zolfigol M. A.; Rostamnia S. Synthesis of Metal-Organic Frameworks MIL-101(Cr)-NH2Containing Phosphorous Acid Functional Groups: Application for the Synthesis of N-Amino-2-pyridone and Pyrano [2,3- c]pyrazole Derivatives via a Cooperative Vinylogous Anomeric-Based Oxidation. ACS Omega 2020, 5, 6240–6249. 10.1021/acsomega.9b02133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miri B.; Motakef-Kazemi N.; Shojaosadati S. A.; Morsali A. Application of a nanoporous metal organic framework based on iron carboxylate as drug delivery system. Iran. J. Pharm. Res. 2018, 17, 1164–1171. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Cui P.; Zhang F.; Feng X.; Wang Y.; Yang Y.; Liu X. Fluorescent probes for “off-on” highly sensitive detection of Hg2+ and L-cysteine based on nitrogen-doped carbon dots. Talanta 2016, 152, 288–300. 10.1016/j.talanta.2016.02.018. [DOI] [PubMed] [Google Scholar]
- Chun Li; Liu W.; Sun X.; et al. Excitation dependent emission combined with different quenching manners supports carbon dots to achieve multi-mode sensing. Sens. Actuators, B 2018, 263, 1–9. 10.1016/j.snb.2018.02.050. [DOI] [Google Scholar]
- van Dam B.; Nie H.; Ju B.; Marino E.; Paulusse J. M. J.; Schall P.; Li M.; Dohnalová K. Excitation-Dependent Photoluminescence from Single-Carbon Dots. Small 2017, 13, 1702098 10.1002/smll.201702098. [DOI] [PubMed] [Google Scholar]
- Malik A. H.; Iyer P. K. Conjugated Polyelectrolyte Based Sensitive Detection and Removal of Antibiotics Tetracycline from Water. ACS Appl. Mater. Interfaces 2017, 9, 4433–4439. 10.1021/acsami.6b13949. [DOI] [PubMed] [Google Scholar]
- Kent C. A.; Liu D.; Ma L.; Papanikolas J. M.; Meyer T. J.; Lin W. Light harvesting in microscale metal-organic frameworks by energy migration and interfacial electron transfer quenching. J. Am. Chem. Soc. 2011, 133, 12940–12943. 10.1021/ja204214t. [DOI] [PubMed] [Google Scholar]
- Kabel J.; Sharma S.; Acharya A.; Zhang D.; Yap Y. K. Molybdenum Disulfide Quantum Dots: Properties, Synthesis, and Applications. Journal of Carbon Research 2021, 7 (2), 45. 10.3390/c7020045. [DOI] [Google Scholar]
- Shi L.; Wang T.; Zhang H.; Chang K.; Meng X.; Liu H.; Ye J. An Amine-Functionalized Iron(III) Metal–Organic Framework as Efficient Visible-Light Photocatalyst for Cr(VI) Reduction. Adv. Sci. 2015, 2, 1500006 10.1002/advs.201500006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. M.; Warnan J. Molecular dye-sensitized photocatalysis with metal-organic framework and metal oxide colloids for fuel production. Energies 2021, 14, 4260 10.3390/en14144260. [DOI] [Google Scholar]
- Zhang F.; Ma P.; Deng X.; Sun Y.; Wang X.; Song D. Enzymatic determination of uric acid using water-soluble CuInS/ZnS quantum dots as a fluorescent probe. Microchim. Acta 2018, 185, 499 10.1007/s00604-018-3030-0. [DOI] [PubMed] [Google Scholar]
- Hallaj T.; Azizi N.; Amjadi M. A dual-mode colorimetric and fluorometric nanosensor for detection of uric acid based on N, P co-doped carbon dots and in-situ formation of Au/Ag core-shell nanoparticles. Microchem. J. 2021, 162, 105865 10.1016/j.microc.2020.105865. [DOI] [Google Scholar]
- Lu T.; Zhang L.; Sun M.; Deng D.; Su Y.; Lv Y. Amino-Functionalized Metal-Organic Frameworks Nanoplates-Based Energy Transfer Probe for Highly Selective Fluorescence Detection of Free Chlorine. Anal. Chem. 2016, 88, 3413–3420. 10.1021/acs.analchem.6b00253. [DOI] [PubMed] [Google Scholar]
- Wei Y.; Xia Y. A dual emission metal-organic framework for rapid ratiometric fluorescence detection of CO32-in seawater. RSC Adv. 2020, 10, 24764–24771. 10.1039/D0RA02581J. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.