Abstract
Objectives: Salvianolic acid B (Sal B) is the main effective water-soluble component of Salvia miltiorrhiza. In this study, the anti-inflammatory effect of Sal B was explored in high-fat-diet (HFD)-induced LDLR-/- mice and oxidized low-density-lipoprotein (ox-LDL)-induced or lipopolysaccharide (LPS)-induced RAW264.7 cells. Methods: The LDLR-/- mice were randomly divided into four groups after 12 weeks of high-fat diet. Then, the mice were administrated with 0.9% saline or Sal B (25 mg/kg) or Atorvastatin (1.3 mg/kg) for 12 weeks. RAW 264.7 cells were induced with ox-LDL/LPS, or ox-LDL/LPS plus different concentrations of Sal B (1.25 μg/mL, 2.5 μg/mL, 5 μg/mL), or ox-LDL plus Sal B plus MAPKs activators. ELISA was used for detecting serum lipid profiles and inflammatory cytokines, RT-qPCR used for gene expression, Oil Red O used for plaque sizes, and immunofluorescence staining used for NF-κB p65 and TNF-α production. Inflammation-related proteins and MAPKs pathways were detected by Western Blot. Results: The results showed that Sal B decreased the levels of serum lipids (TC, TG, and LDL-C), attenuated inflammatory cytokines, and improved lipid accumulation in the aorta. Sal B also attenuated the elevation of inflammatory cytokines induced by ox-LDL or LPS in RAW264.7 cells, and the phosphorylation of MAPKs/NF-κB pathways in the aorta and RAW264.7 cells, resulting in a significant decrease in the contents of p-JNK, p-ERK 1/2, p-P38, p-IκB, and p-NF-κB p65. Conclusions: Sal B could exert anti-inflammatory effects on atherosclerosis via MAPKs/NF-κB signaling pathways in vivo and in vitro.
Keywords: salvianolic acid B, atherosclerosis, inflammation, MAPKs/ NF-κB signal pathway, LDLR-/- mice
Introduction
Cardiovascular diseases (CVDs) are an array of chronic diseases in the heart and circulatory system 1 and a leading cause of premature mortality and disability across the world. 2 The culprit of CVDs is atherosclerosis (AS). 3 In the case of AS, lipids deposit in the intima of large and mid-sized arteries, leading to the formation of atherosclerotic plaques. 4
AS also involves lipid-driven inflammatory processes. 5 Lipid accumulation induces inflammation and subsequent atherosclerotic changes. 4 Studies have shown that dyslipidemia leads to the accumulation of LDL that can be oxidized into ox-LDL. 6 Ox-LDL is immunogenic and activates endothelial cells, monocytes/macrophages, and T cells. 3 Ox-LDL activates Toll-like receptors (TLRs) and inflammasomes to induce the release of proinflammatory factors (such as interleukin-1 (IL-1), IL-6, TNF-α, and MCP-1), thereby initiating/enhancing an inflammatory response. 7 Therefore, inhibiting lipid accumulation and related inflammation may serve as a strategy to prevent AS.
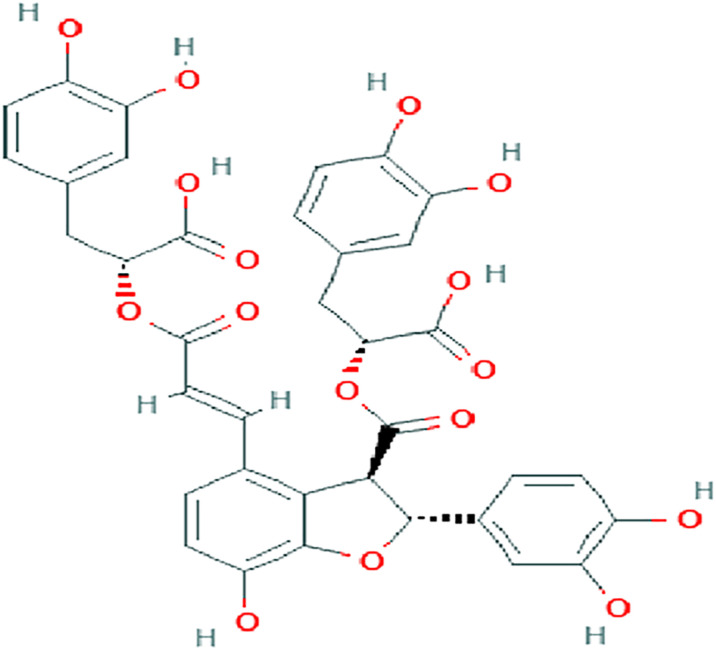
Salvianolic acid B (Sal B) is an active ingredient of Salvia miltiorrhiza, with antioxidative effects 8 (Figure 1). Previous studies have shownthe pharmacological effects of Sal B, such as inhibiting inflammation, apoptosis and fibrosis, and promoting stem cell proliferation and differentiation.9-11 It also protects against cardiovascular and cerebrovascular diseases, aging, and liver fibrosis.12-15 Sal B inhibits TNF-α/NF-κB and TLR4/NF-κB signaling pathways to repress the expression of proinflammatory factors IL-1β and TNF-α.16,17 Sal B also reduces the production of ox-LDL and its uptake by macrophages. 18 However, the anti-inflammatory effects of Sal B on AS have been rarely reported. This study was designed to clarify these effects using ox-LDL/LPS-induced RAW264.7 cells and HFD-induced LDLR-/- mice.
Figure 1.
The chemical structure of Salvianolic acid B.
Materials and methods
Materials
Salvianolic acid B was extracted from PP (purity: 98.28%, available at: http://www.cdmust.com) by Mansite Biological Company (Chengdu, China). The high-fat diet (HFD, 78.85% normal diet+21% lard +0.15% cholesterol) was bought from SYSE Biotechnology Co., Ltd. (Changzhou, China); atorvastatin calcium tablets from Pharmacy of Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine; the Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) from Gibco (Gibco-BRL, Gaithersburg, MD, USA); ox-LDL from Yeasen Biotech Co., Ltd. (Shanghai, China); enzyme immunosorbent assay (ELISA) kits for mouse IL-1β, IL-6, and TNF-α from Shanghai XiTang Biological Technology Co., Ltd. (Shanghai, China); oil red O staining kit from Jiancheng Bioengineering Institute of Nanjing (Nanjing, China); primary antibodies against ERK, p-ERK, P38 MAPK, p-P38 MAPK, JNK, p-JNK, IκB, p-IκB, NF-κB, p-NF-κB, and GAPDH from Cell Signaling Technology Inc. (Beverly, Massachusetts, USA). ECL Star kit was from Beyotime Biotechnology (Shanghai, China); primers for IL-1β, IL-6, and TNF-α from Sangon Biotech Co., Ltd. (Shanghai, China); primeScript RT Reagent Kit for RT-PCR amplification and TB Green Premix EX Taq from TAKARA Biomedical Technology Co., Ltd. (Beijing, China); RNA Purification Kit from EZBioscience (Shanghai, China); ERK activator LM22B-10, JNK activator anisomycin, and p38MAPK activator asiatic acid from Sellect (Shanghai, China); lipopolysaccharide (LPS), 4′,6-diamindino-2-phenylindole (DAPI), Triton X-100, radio immunoprecipitation assay (RIPA), and BCA protein assay kit from Beyotime Biotechnology (Shanghai, China).
Animals and treatment
Six-week-old LDLR-/- male mice (18–22g) were obtained from the GemPharmatech Co., Ltd. (Nanjing, Jiangsu, http://www.gempharmatech.com) (SCXK 2018-0008). All mice were housed in the Animal Center of Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine. After 1 week of environmental adaptation, LDLR-/- mice were randomly assigned to four groups: control group (CON), model group (MOD), Salvianolic acid B group (Sal B, 25 mg/kg), and Atorvastatin group (Ato, 1.3 mg/kg). Studies have found that atorvastatin has significant effects of lowering lipids, anti-inflammation, and increasing plaque stability.19,20 Therefore, this study chose atorvastatin as the positive control drug. The mice in CON group were fed with normal diet for 24 weeks, and other groups with HDF. After the mice were fed for 12 weeks, the LDLR-/- mice in Sal B group were intraperitoneally injected with Sal B, and Ato group received oral atorvastatin for 12 weeks. CON and MOD groups were injected with the same volume of 0.9% saline. After a 12-h fasting, all the mice were sacrificed via carbon dioxide asphyxiation. The Ethics Committee of Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine approved the animal welfare application (No. 2019-N002, shown in supplementary information).
Cells and culture
RAW264.7 cells (Cat. No. SCSP-5036) were purchased from Shanghai Cell Bank Type Culture Collection Committee (Shanghai, China), and cultured in DMEM with 10% FBS. The cells were divided into five groups: control group (CON), ox-LDL (100 μg/mL) group, and ox-LDL plus Sal B (1.25 μg/mL, 2.5 μg/mL, and 5 μg/mL) groups (Sal B-L, Sal B-M, and Sal B-H). The cells were also induced by LPS and divided into five groups: control group (CON), LPS (100 μg/mL) group, and LPS plus Sal B (1.25 μg/mL, 2.5 μg/mL, and 5 μg/mL) groups (Sal B-L, Sal B-M, and Sal B-H). The cells were seeded into 6-well plates. At 70% confluence, Sal B groups were treated with various concentrations of Sal B for 0.5 h. After that, MOD and Sal B groups were stimulated with ox-LDL/LPS for 24 h, and control group was given fresh DMEM. To explore the effect of Sal B on MAPKs signaling pathway, the activators (LM22B-10, anisomycin, and asiatic acid) were used in the cell culture. The cells were divided into six groups: control group, ox-LDL (100 μg/mL) group, ox-LDL plus Sal B (5 μg/mL) group, ox-LDL plus Sal B (5 μg/mL) plus LM22B-10 group, ox-LDL plus Sal B (5 μg/mL) plus anisomycin group, and ox-LDL plus Sal B (5 μg/mL) plus asiatic acid group. The activators were treated at 0.5 h before the addition of ox-LDL. Supernatant was harvested for ELISA. Cells were collected for protein and mRNA detection. In vitro experiment was repeated 3 times.
Determination of serum lipids
Total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C) levels in the blood serum were analyzed in the Clinical Laboratory Department of Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine.
Determination of cytokine
The concentrations of IL-1β, IL-6, and TNF-α in the serum and RAW264.7 supernatant were detected by ELISA kits according to the manufacturer’s instructions. The final concentrations of IL-1β, IL-6, and TNF-α were calculated in accordance with corresponding standard curves.
Histopathological analysis
The aortic roots were fixed in 4% paraformaldehyde for 48 h. The aortic roots were embedded in the optimal cutting temperature (OCT) compound and cut into circular sections (10 μm) with a microtome. The sections of aortic root were stained with Oil Red O solution. The lipid-rich plaque areas were observed under the microscope and analyzed by image J software.
Western blotting
The thoracic aorta was placed in liquid nitrogen till it becomes brittle and grounded into pieces. Then, the thoracic aorta or cultured cells were lysed in RIPA lysis solution for 30 min. The supernatant was collected after centrifugation for 10 min. The total protein was quantified using BCA kit, separated on 10% SDS-PAGE and transferred onto the PVDF membranes. The PVDF membranes were immersed in 5% BSA in Tris-buffered saline with Tween-20 (TBST) for 1 h. The blocked PVDF membranes were incubated with primary antibodies (ERK, p-ERK, P38 MAPK, p-P38 MAPK, JNK, p-JNK, IκB, p-IκB, NF-κB, p-NF-κB, and GAPDH) diluted with 5% BSA in TBST at 4°C overnight. Having been washed with TBST, the membranes were immersed in secondary antibodies for 1 h. The contents of proteins were imaged using ECL reagent and photographed using the ChemiScope 6000. Finally, the density of protein bands was analyzed with image J software. The obtained images were converted to 8-bit format, background value subtracted, and optical density value detected. 21
Real-Time Quantitative PCR (RT-qPCR) assay
Total RNA was extracted from the aorta tissue and cells using RNA Purification Kit, and reverse-transcribed into cDNA using PrimeScript RT Reagent Kit following the standard protocol. The Real-Time Quantitative PCR (RT-qPCR) assay was conducted using TB Green PremixEX Taq with the Applied Biosystems 7500 Real-Time PCR System. The amplification parameters were set at 95°C for 1 min, followed by 40 cycles of 95°C for 5 s and 58°C for 15 s, 72°C for 30 s, and 95°C for 15 s. The relative expression of mRNA was normalized to that of β-actin. The following primers were used for PCR analysis: ββ-Actin forward, 5‘-GAGACCTTCAACACCCCAGC-3’ and reverse, 5‘-ATGTCACGCACGATTTCCC-3’; IL-1β forward, 5‘-GCTTCAGGCAGGCAGTATCA-3’ and reverse, 5‘-TGCAGTTGCTAATGGGAACG -3’; IL-6 forward, 5‘-AAAGCAGCAAAGAGGCACTG-3’ and reverse, 5‘-TACCTCAAACTCCAAAAGACCAG-3’; TNF-α forward, 5‘-CCCTCCAGAAAAGACACCATG-3’ and reverse, 5‘-CACCCCGAAGTTCAGTAGACAG-3’.
Immunofluorescence study
Frozen slices of aortic sinus were made as mentioned before. RAW264.7 cells were cultured and treated as indicated. Having been fixed with 4% paraformaldehyde for 15 min and permeabilized by 0.1% Triton X-100 for 10 min, the cells were blocked with PBS containing 5% BSA for 1 h. Then, the cells or frozen slices were incubated with anti-TNF-α or anti-NF-κB p65 at 4°C overnight. On the following day, the cells were washed with PBS and incubated with goat anti-rabbit IgG H&L for 1 h. Finally, the cells and frozen slices were treated with DAPI. All images were captured with a fluorescence microscope.
Statistical analyses
All experimental results were shown as the mean ± standard deviation (SD). The statistical analyses were accomplished with GraphPad Prism 7.0. software and SPSS 21.0. First, the data were tested for normality and homogeneity of variance. If the data met the above conditions, one-way ANOVA was used; if the data did not meet the above conditions, the nonparametric statistical tests were used. p < 0.05 was considered statistically significant.
Results
Effect of Sal B on serum lipid profiles and atherosclerotic lesions in the aortic sinus of mice
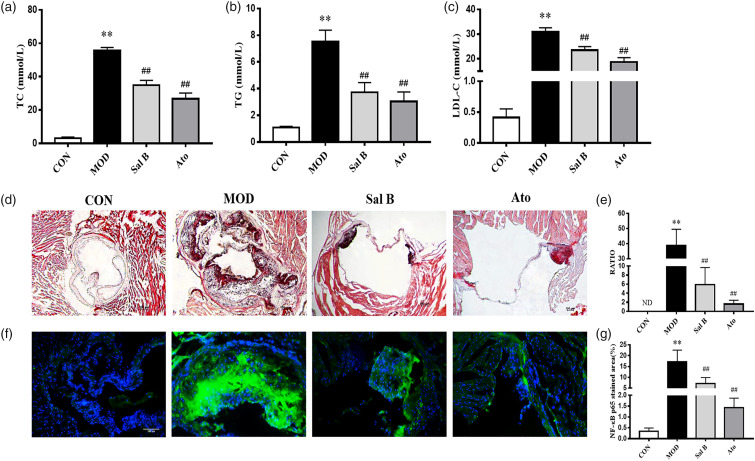
To explore the effect of Sal B on blood lipids in AS mice, the serum levels of TC, TG, and LDL-C were detected. As shown in Figures 2(a)–(c), TC, TG, and LDL-C serum levels were lower in CON group. The TC, TG, and LDL-C serum levels were higher in MOD group than in CON group (p < 0.01); while after 12 weeks’ intervention of Sal B or Ato, all the three indexes were lower, compared to those in MOD group (p < 0.01). The above results showed that Sal B could reduce serum TC, TG, and LDL-C levels and improve lipid metabolism in AS mice.
Figure 2.
Effect of Sal B on serum lipid profiles and atherosclerotic lesion area in aortic sinus of mice. (a)–(c) TC, TG, and LDL levels of serum were detected, n = 8. (d) Oil Red O staining was used to detect the progress of lipid-rich plaques, magnification ×40. (e) The areas of lesion were calculated, respectively, by ImageJ analysis software, n = 8. RATIO = plaque area/aortic sinus lumen area ×100%. (f) Immunofluorescence staining of NF-κB p65 at the aortic roots, magnification ×200. (g) The areas of NF-κB p65 stained lesion were calculated, respectively, by ImageJ analysis software, n = 3. CON, control group; MOD, model group; Sal B, Salvianolic acid B group; Ato, Atorvastatin group. TC, total cholesterol; TG, triglyceride; LDL-C, low density lipoprotein cholesterol; ND, not detected. Data are expressed as mean ± SD. **p < 0.01 vs CON group; ##p < 0.01 vs MOD group.
Lipid plaques in the aorta were observed by Oil Red O staining. As shown in Figures.2(d)–(e), the lipid accumulation in the aortic roots was more significant in MOD group, but not detected in CON group (p < 0.01). In contrast, the atherosclerotic lesion reduced in Sal B-treated and Ato-treated groups (p < 0.01), compared to MOD group. The above results suggested that Sal B could reduce AS plaques in HFD-induced-LDLR-/- mice.
Effect of Sal B on inflammatory cytokine expression in the mice
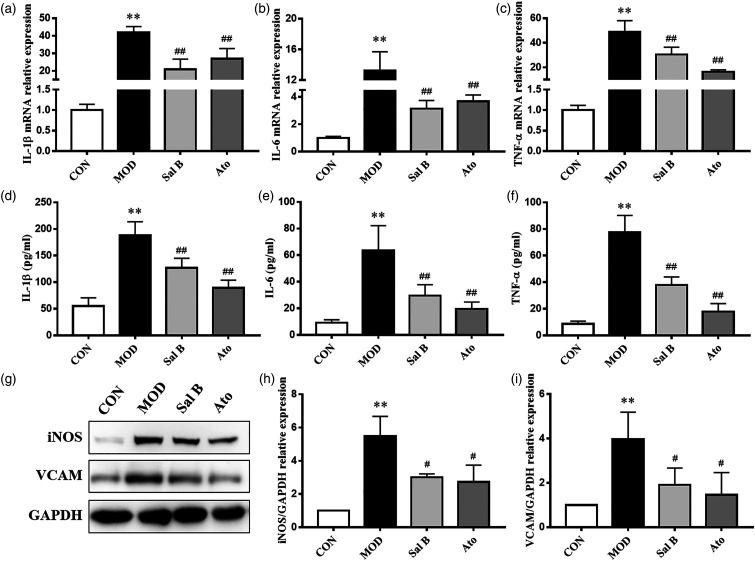
In order to explore the effect of Sal B on the inflammation of atherosclerotic plaques, immunofluorescence staining was performed. As shown in Figures 2(f) and (g), the expression level of NF-κB p65 was significantly increased in atherosclerotic lesions of MOD group. Sal B and Ato inhibited the increase of NF-κB p65. Then, the mRNA levels of inflammatory cytokines, IL-1β, IL-6, and TNF-α in the aorta were detected by RT-qPCR. As shown in Figures 3(a)–(c), the mRNA levels of IL-1β, IL-6, and TNF-α in MOD group increased compared with CON group (p < 0.01). After 12 weeks’ intervention with Sal B or Ato, the levels of IL-1β, IL-6, and TNF-α mRNA expression significantly decreased (p < 0.01). These results were consistent with the changes in the serum levels of inflammatory cytokines, IL-1β, IL-6, and TNF-α (p < 0.01), as revealed by ELISA results (Figures 3(d)–(f)). The protein levels of iNOS and VCAM in the aorta were also detected by Western Blot assays. As shown in Figures 3(g)–(i), the protein levels of iNOS and VCAM expression significantly increased in MOD group, compared to CON group (p < 0.01). These changes were reversed by Sal B or Ato (p < 0.05). The above results showed that Sal B could inhibit the inflammatory response in AS through inflammatory factors.
Figure 3.
Effect of Sal B on inflammatory cytokine expression in mice. (a)–(c) Aorta gene expression of IL-1β, IL-6, and TNF-α was detected by RT-qPCR method, n = 8. (d)–(f) Production of IL-1β, IL-6, and TNF-α in plasma was measured by ELISA kit, n = 8. (g) Protein was extracted from mice aorta. Then iNOS and VCAM were tested by Western Blotting assay. (h)–(i) The quantitative results were calculated, respectively, by ImageJ analysis software, n=3. CON, control group; MOD, model group; Sal B, Salvianolic acid B group; Ato, Atorvastatin group. Data are expressed as mean ± SD. **p < 0.01 vs CON group; #p < 0.05, ##p < 0.01 vs MOD group.
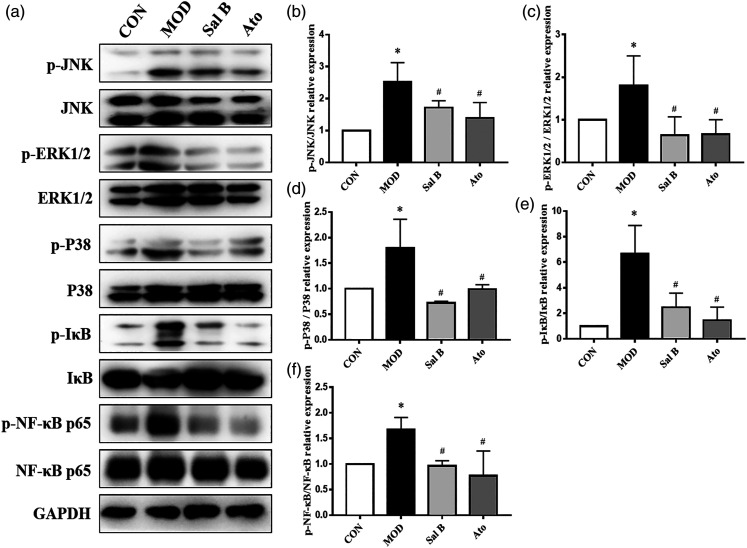
Effect of Sal B on MAPKs/NF-κB pathways in the mice
To testify whether the increased inflammation was related to MAPKs/NF-κB pathways, phosphorylation of JNK, ERK1/2, p38, IκB, and NF-κB protein in the aorta was examined by Western Blot assays (Figure 4). Compared with that in CON group, MAPKs/NF-κB pathways were activated. The levels of phosphorylated JNK, ERK1/2, p38, IκB, and NF-κB proteins were higher in MOD group (p < 0.05), but lower than those in Sal B and Ato groups (p < 0.05). The results showed that Sal B could mitigate the activation of MAPKs/NF-κB and AS damage in the mice.
Figure 4.
Effect of Sal B on MAPKs/NF-κB pathway expression in mice. (a) Protein was extracted from mice aorta. Then the total and phosphorylated levels of JNK, ERK1/2, p38, IκB, and NF-κB were determined by Western Blotting assay. (b)–(f) The quantitative results were calculated, respectively, by ImageJ analysis software, n = 3. CON, control group; MOD, model group; Sal B, Salvianolic acid B group; Ato, Atorvastatin group. Data are expressed as mean ± SD. *p < 0.05 vs CON group; #p < 0.05 vs MOD group.
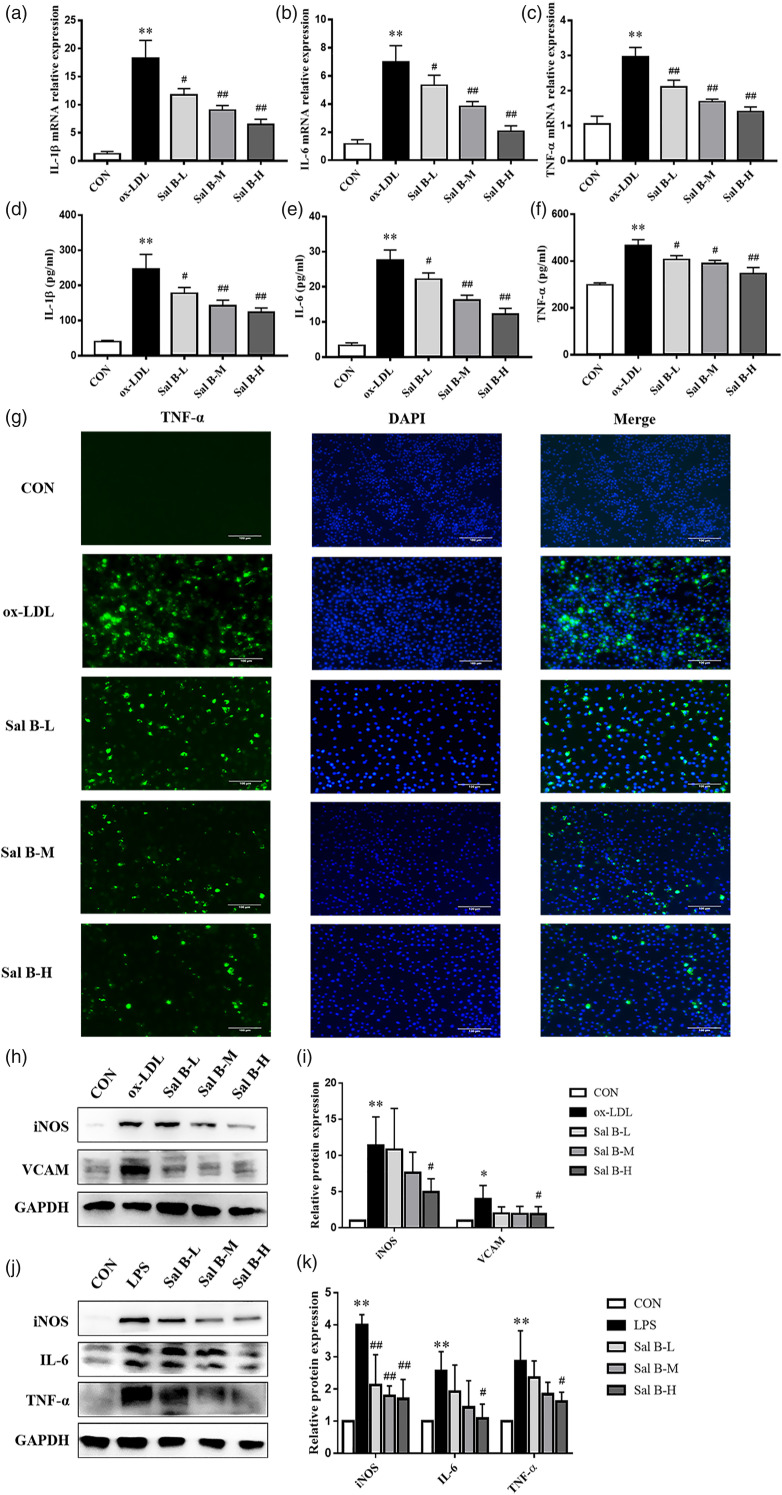
Effect of Sal B on inflammatory cytokine expression in RAW264.7 cells
The mRNA levels of inflammatory factors in RAW264.7 cells were detected (Figures 5(a)–(c)). Compared with CON group, ox-LDL significantly increased the mRNA levels of IL-1β, IL-6, and TNF-α expression (p < 0.01). After pretreatment with Sal B (1.25 μg/mL, 2.5 μg/mL, and 5 μg/mL), the mRNA levels of IL-1β, IL-6, and TNF-α expression were obviously inhibited (p < 0.05). These results were consistent with the changes in the protein levels of IL-1β, IL-6, and TNF-α in RAW264.7 cell supernatant (p < 0.05) (Figures 5(d)–(f)). Moreover, immunofluorescence staining revealed that TNF-α protein level increased in ox-LDL group, compared to CON group (Figure 5(g)). After Sal B intervention, TNF-α level decreased, suggesting that Sal B had anti-inflammatory effects. We also detected the protein levels of iNOS and VCAM by Western Blot assays. As showed in Figures 5(h) and (i), the protein levels of iNOS and VCAM expression increased in ox-LDL group, compared to CON group (p < 0.05). And Sal B intervention decreased the expression levels of those proteins (p < 0.05). The results showed that Sal B could reduce the mRNA levels of IL-1β, IL-6, and TNF-α and the protein levels of iNOS and VCAM. To further illustrate the anti-inflammatory effect of Sal B, which did not depend on lipid-lowering effect, LPS was used to induce RAW264.7 cells. As showed in Figures 5(j) and (k), the protein levels of iNOS, IL-6, and TNF-α expression increased in LPS group, compared to CON group (p < 0.05). Sal B intervention decreased the expression levels of those proteins (p < 0.05).
Figure 5.
Effect of Sal B on inflammatory cytokine expression in RAW264.7 cells. (a)–(c) Gene expression of IL-1β, IL-6, and TNF-α was measured by RT-qPCR method, n = 3. (d)–(f) And production of these three cytokines was explored by ELISA kits, n = 3. (g) Immunofluorescence staining method was used to show TNF-α, magnification ×200. (h) Protein was extracted from RAW264.7 cells induced by ox-LDL. Then iNOS and VCAM were tested by Western Blotting assay. (i) The quantitative results were calculated, respectively, by ImageJ analysis software, n = 3. (j) Protein was extracted from RAW264.7 cells induced by LPS. Then iNOS, IL-6, and TNF-α were tested by Western Blotting assay. (k) The quantitative results were calculated, respectively, by ImageJ analysis software, n=3. CON, control group; ox-LDL, ox-LDL-stimulated group; LPS, LPS-stimulated group; Sal B-L, Sal B at 1.25 μg/ml concentration; Sal B-M, Sal B at 2.5 μg/ml concentration; Sal B-H, Sal B at 5 μg/ml concentration. Data are expressed as mean ± SD. *p < 0.05, **p < 0.01 vs CON group; #p < 0.05, ##p < 0.01 vs ox-LDL group or LPS group.
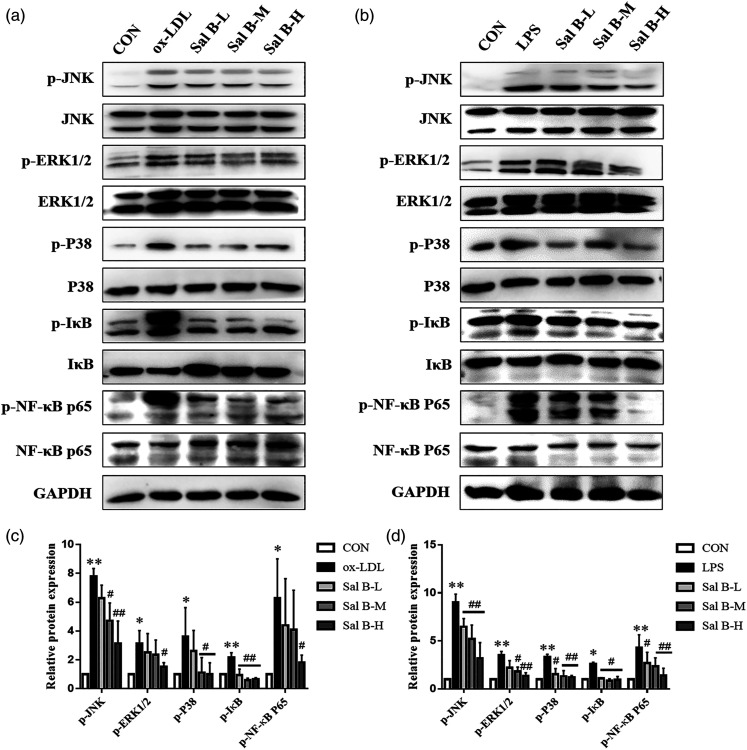
Effect of Sal B on MAPKs/NF-κB pathways in RAW264.7 cells
The MAPKs/NF-κB pathways can be activated by a variety of inflammatory factors. To testify the effect of Sal B on MAPKs/NF-κB pathways, the expression levels of key proteins in MAPKs/NF-κB pathways in RAW264.7 cells were detected (Figure 6). The levels of p-JNK, p-ERK1/2, p-P38, p-IκB, and p-NF-κB in RAW264.7 cells increased significantly after ox-LDL or LPS stimulation (p < 0.05). The phosphorylation levels of these proteins were reduced after Sal B challenge (p < 0.05). These results indicated that Sal B could inhibit the activation of MAPKs/NF-κB pathways induced by ox-LDL or LPS.
Figure 6.
Effect of Sal B on MAPKs/NF-κB pathway expression in RAW264.7 cells. (a) Protein was extracted from RAW264.7 cells induced by ox-LDL. (b) Protein was extracted from RAW264.7 cells induced by LPS. Then the total and phosphorylated levels of JNK, ERK1/2, p38, IκB, and NF-κB were determined by Western Blotting assay. (c)–(d) The quantitative results were calculated, respectively, by ImageJ analysis software, n = 3. CON, control group; ox-LDL, ox-LDL-stimulated group; LPS, LPS-stimulated group; Sal B-L, Sal B at 1.25 μg/ml concentration; Sal B-M, Sal B at 2.5 μg/ml concentration; Sal B-H, Sal B at 5 μg/ml concentration. Data are expressed as mean ± SD. *p < 0.05, **p < 0.01 vs CON group; #p < 0.05, ##p < 0.01 vs ox-LDL group or LPS group.
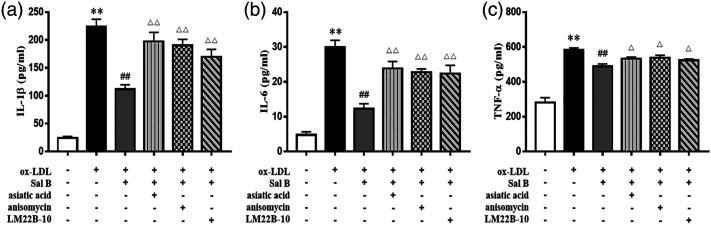
Effect of Sal B on inflammatory cytokine levels through the MAPKs signaling pathway in RAW264.7 cells
To investigate the role of MAPKs in the anti-inflammatory performance of Sal B, three specific activators (asiatic acid, a p38 activator; anisomycin, a JNK activator; LM22B-10, an ERK1/2 activator) were pretreated. As shown in Figure 7, after treatment with ox-LDL, activators, and Sal B in RAW264.7 cells, the concentrations of cytokines (IL-1β, IL-6, and TNF-α) were obviously up-regulated, compared to those in ox-LDL plus Sal B group (p < 0.05). These results suggested that the MAPKs pathway may be one of the mechanisms of Sal B regulating the expression of inflammatory factors in ox-LDL-induced RAW264.7 cells.
Figure 7.
Effect of Sal B on inflammatory cytokine levels through the MAPKs signaling pathway in RAW264.7 cells. Cells were pretreated with 10 μM asiatic acid, 10 μM anisomycin, or 10 μM LM22B-10 for 0.5 h, then treated with ox-LDL for 24 h. (a)–(c) The production of IL-1β, IL-6, and TNF-α was measured by ELISA kits, n = 3. Data are expressed as mean ± SD. **p < 0.01 vs the control group; ##p < 0.01 vs ox-LDL group; △p < 0.05, △△p < 0.01 vs ox-LDL plus Sal B group.
Discussion
AS has been considered as a chronic, lipid-driven inflammatory disease of the arterial wall.22,23 Total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C) concentrations associate with the risk of atherosclerotic events.24,25 Consistent exposure to LDL-C remains a principal trigger of AS.26,27 LDL can be oxidized into ox-LDL. 28 Ox-LDL can bind to pattern recognition receptors, such as scavenger receptors and TLRs, contributing to foam cell formation and inflammatory cytokine secretion.29,30 Therefore, lipid-lowering therapy remains the cornerstone in the management of atherosclerotic disease. In recent decades, statins have been used to treat AS, with many side effects, such as myopathy, rhabdomyolysis, and acute renal failure. 31 Thus, more effective methods are needed for treating AS. The results of in vivo experiments showed that serum TC, TG, and LDL-C levels in MOD group were significantly different from those in CON group. Meanwhile, in MOD group, the aortic roots showed evident oil red O stains. In contrast, in Sal B groups, serum lipid levels and plaque accumulation in the aortic roots were significantly decreased.
Lipid metabolism interacts with inflammation in various diseases. 32 In AS, monocytes/macrophages, smooth muscle cells, and lymphocytes cluster within the arterial wall. Monocytes and macrophages are key factors involved in the inflammatory response that drives atherogenesis. 33 In early-stage lesions, macrophages clear away lipoprotein particles and apoptotic cells. Lipid uptake quickens the differentiation of macrophages into foam cells, producing excess ROS and pro-inflammatory cytokines.34,35 In progressive lesions, macrophages fail to engulf all dead cells. The uncleared apoptotic cells then aggravate inflammatory response; thus, accelerating the progression of atherosclerosis. 36 Therefore, anti-inflammatory management would enhance the treatment of atherosclerosis. Our experiments demonstrated a reduction in the levels of inflammatory cytokines in Sal B-treated LDLR-/- mice. Additionally, in ox-LDL-stimulated RAW264.7 cells, Sal B effectively suppressed the levels of pro-inflammatory factors, IL-1β, IL-6, and TNF-α. Studies have found that Sal B could reduce the expression of inflammation-related molecules induced by LPS.37,38 In order to better illustrate that the anti-inflammatory effect of Sal B is independent of the lipid-lowering effect, we used LPS to induce RAW264.7 cells, and the results showed that Sal B could reduce the expression of iNOS, IL-6, and TNF-α.
MAPKs signaling pathway is a key regulator in AS. 39 MAPKs are activated by various inducers, including oxidative stress, cytokines, and growth factors, all of which are abundant in atherosclerotic and aortic valve sclerotic lesions. 40 Several studies have investigated that MAPKs activation induces the expression of pro-inflammatory cytokines incubated with ox-LDL or LPS.41-43 Repressing MAPKs pathway may attenuate inflammation and AS.44,45 We thus investigated the effects of Sal B on MAPKs phosphorylation. As the results showed, the expression levels of phosphorylated ERK1/2, JNK, and p38 in the aorta of MOD group and ox-LDL-induced or LPS-induced RAW264.7 cells were higher than those in CON group. After Sal B intervention, the phosphorylated expression of these proteins was significantly reduced. Interestingly, the results showed that three MAPK activators (asiatic acid, anisomycin, and LM22B-10) weakened the anti-inflammatory effect of Sal B, suggesting that Sal B plays an anti-inflammatory and anti-atherosclerotic role via inhibiting the activation of MAPKs signaling pathway in vivo and in vitro.
NF-κB, a transcription factor regulating inflammatory responses, can be activated to promote atherosclerosis and thrombosis.29,46 Upon inflammation and oxidative damage, IκB is activated to promote the phosphorylation of NF-κB. 47 Activated NF-κB translocated into the nucleus serves as a transcription factor for inflammatory genes. 48 NF-κB activation increases inducible nitric oxide synthase (iNOS) and vascular cell adhesion molecule (VCAM), consequently activating atherosclerotic development.49,50 The activation of iNOS promotes inflammatory processes within the vascular wall and atherosclerotic progresses. 51 VCAM is considered as an activator of early atherosclerosis. 52 Thus, the inhibition on IκB/NF-κB pathway is also a treatment option of AS. Our data showed that Sal B suppressed the phosphorylated expression of IκB and NF-κB, and the expression of iNOS and VCAM, suggesting that Sal B plays an anti-inflammatory role by countering IκB/NF-κB signaling pathway.
There also have some limitations in this study. First, the sample size of mice was limited. A more in-depth study of the mechanism of Sal B requires a larger sample size of mice. And in vivo and primary cell experiments are needed to confirm the underlying mechanism. In addition, we did not explore the effect of Sal B on the transcription of some growth factors through MAPK signaling pathways. New explorations can help to further clarify the mechanism of Sal B in atherosclerosis.
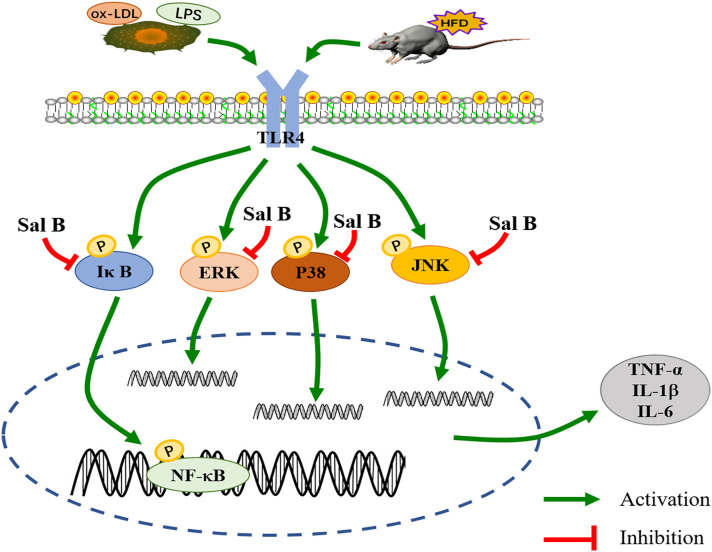
Conclusion
Sal B showed anti-inflammatory effect in HFD-induced LDLR-/- mice and ox-LDL or LPS-induced RAW264.7 cells. In the mice, Sal B decreased the levels of TC, TG, and LDL-C and the expression of IL-1β, IL-6, and TNF-α to alleviate inflammation. In the cells, Sal B also reduced the expression of IL-1β, IL-6, and TNF-α. More importantly, Sal B inhibited the activation of MAPKs/NF-κB pathways in both aorta and RAW264.7 cells, which might be used to explain the anti-atherosclerotic effect of Sal B (Figure 8).
Figure 8.
Schematic diagram of the protective mechanism of Salvianolic acid B against atherosclerosis.
Supplemental Material
Supplemental Material, sj-pdf-1-iji-10.1177_03946320221079468 for Salvianolic acid B attenuates the inflammatory response in atherosclerosis by regulating MAPKs/ NF-κB signaling pathways in LDLR-/- mice and RAW264.7 cells by Yifan Zhang, Xiaoteng Feng, Min Du, Jie Ding and Ping Liu in International Journal of Immunopathology and Pharmacology
Acknowledgments
We thank the Central Laboratory of Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine for offering the laboratory equipment.
Author contributions: YZ and PL conceived and devised the study. YZ and JD performed the experiments. YZ, XF, and MD analyzed the results. YZ was a major contributor in writing the manuscript. YZ and PL drafted the manuscript. PL reviewed and edited the final manuscript. All authors contributed to the article and approved the submitted version
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Nos. 81873117 and 82074200).
Ethical approval and consent to participate: This study was approved by the Laboratory Animal Management and Use (Animal Welfare) Committee, Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
References
- 1.Beaglehole R, Saracci R, Panico S. (2001) Editorial -- Cardiovascular diseases: causes, surveillance and prevention. International Journal of Epidemiology 30(Suppl 1): S1–S4. 2002/01/05. DOI: 10.1093/ije/30.suppl_1.s1. [DOI] [PubMed] [Google Scholar]
- 2.Group, WCRCW (2019) World health organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. The Lancet. Global Health 7: e1332–e1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frostegård J. (2013) Immunity, atherosclerosis and cardiovascular disease. BMC Medicine 11. DOI: 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaftenaar F, Frodermann V, Kuiper J, et al. (2016) Atherosclerosis. Current Opinion in Lipidology 27: 209–215. 2016/04/01. DOI: 10.1097/mol.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 5.Viola J, Soehnlein O. (2015) Atherosclerosis - A matter of unresolved inflammation. Seminars in Immunology 27: 184–193. 2015/04/14. DOI: 10.1016/j.smim.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Sun S-W, Zu X-Y, Tuo Q-H, et al. (2010) Caveolae and caveolin-1 mediate endocytosis and transcytosis of oxidized low density lipoprotein in endothelial cells. Acta Pharmacologica Sinica 31: 1336–1342. 2010/09/14. DOI: 10.1038/aps.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tall AR, Yvan-Charvet L. (2015) Cholesterol, inflammation and innate immunity. Nature Reviews Immunology 15: 104–116. 2015/01/24. DOI: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho JH, Hong CY. (2011) Salvianolic acids: small compounds with multiple mechanisms for cardiovascular protection. Journal of Biomedical Science 18: 30. DOI: 10.1186/1423-0127-18-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao Z, Liu W, Mu Y-P, et al. (2020) Pharmacological effects of salvianolic acid b against oxidative damage. Frontiers in Pharmacology 11: 572373. 2020/12/22. DOI: 10.3389/fphar.2020.572373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou P, Hua F, Wang X, et al. (2020) Therapeutic potential of IKK-β inhibitors from natural phenolics for inflammation in cardiovascular diseases. Inflammopharmacology 28: 19–37. 2020/01/03. DOI: 10.1007/s10787-019-00680-8. [DOI] [PubMed] [Google Scholar]
- 11.Guo G, Li B, Wang Y, et al. (2010) Effects of salvianolic acid B on proliferation, neurite outgrowth and differentiation of neural stem cells derived from the cerebral cortex of embryonic mice. Science China Life Sciences 53: 653–662. DOI: 10.1007/s11427-010-3106-5. [DOI] [PubMed] [Google Scholar]
- 12.Cao W, Guo X-W, Zheng H-Z, et al. (2012) Current progress of research on pharmacologic actions of salvianolic acid B. Chinese Journal of Integrative Medicine 18: 316–320. 2012/03/30. DOI: 10.1007/s11655-012-1052-8. [DOI] [PubMed] [Google Scholar]
- 13.Shen C-Y, Jiang J-G, Yang L, et al. (2017) Anti-ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: pharmacological mechanisms and implications for drug discovery. British Journal of Pharmacology 174: 1395–1425. 2016/10/30. DOI: 10.1111/bph.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu LJ, Zhang KJ, Zhu JZ, et al. (2017) Salvianolic acid exerts cardioprotection through promoting angiogenesis in animal models of acute myocardial infarction: preclinical evidence. Oxidative Medicine and Cellular Longevity. DOI: 10.1155/2017/8192383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu C, Chen W, Ding H, et al. (2019) Salvianolic acid B exerts anti-liver fibrosis effects via inhibition of MAPK-mediated phospho-Smad2/3 at linker regions in vivo and in vitro. Life Sciences 239: 116881. 2019/11/05. DOI: 10.1016/j.lfs.2019.116881. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Chen G, Yu X, et al. (2016) Salvianolic acid b ameliorates cerebral ischemia/reperfusion injury through inhibiting TLR4/MyD88 signaling pathway. Inflammation 39: 1503–1513. 2016/06/04. DOI: 10.1007/s10753-016-0384-5. [DOI] [PubMed] [Google Scholar]
- 17.Fan Y, Luo Q, Wei J, et al. (2018) Mechanism of salvianolic acid B neuroprotection against ischemia/reperfusion induced cerebral injury. Brain Research 1679: 125–133. 2017/11/29. DOI: 10.1016/j.brainres.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Sun A, Liu H, Wang S, et al. (2011) Salvianolic acid B suppresses maturation of human monocyte-derived dendritic cells by activating PPARγ. British Journal of Pharmacology 164: 2042–2053. 2011/06/09. DOI: 10.1111/j.1476-5381.2011.01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F, Tan Y, Chen XH, et al. (2018) Atorvastatin improves plaque stability in diabetic atherosclerosis through the RAGE pathway. European Review for Medical and Pharmacological Sciences 22: 1142–1149. 2018/03/07. DOI: 10.26355/eurrev_201802_14403. [DOI] [PubMed] [Google Scholar]
- 20.Guo X, Wang L, Xia X, et al. (2019) Effects of atorvastatin and/or probucol on recovery of atherosclerosis in high-fat-diet-fed apolipoprotein E-deficient mice. Biomedicine & Pharmacotherapy 109: 1445–1453. 2018/12/16. DOI: 10.1016/j.biopha.2018.10.184. [DOI] [PubMed] [Google Scholar]
- 21.Gallo-Oller G, Ordoñez R, Dotor J. (2018) A new background subtraction method for Western blot densitometry band quantification through image analysis software. Journal of Immunological Methods 457: 1–5. 2018/03/10. DOI: 10.1016/j.jim.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Seneviratne A, Monaco C. (2015) Role of inflammatory cells and toll-like receptors in atherosclerosis. Current Vascular Pharmacology 13: 146–160. 2013/11/06. DOI: 10.2174/15701611113116660160. [DOI] [PubMed] [Google Scholar]
- 23.Poznyak A, Grechko AV, Poggio P, et al. (2020) The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. International Journal of Molecular Sciences 21: 1835. 2020/03/12. DOI: 10.3390/ijms21051835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musunuru K, Kathiresan S. (2016) Surprises from genetic analyses of lipid risk factors for atherosclerosis. Circulation Research 118: 579–585. 2016/02/20. DOI: 10.1161/circresaha.115.306398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ference BA, Graham I, Tokgozoglu L, et al. (2018) Impact of lipids on cardiovascular health. Journal of the American College of Cardiology 72: 1141–1156. 2018/09/01. DOI: 10.1016/j.jacc.2018.06.046. [DOI] [PubMed] [Google Scholar]
- 26.Ference BA, Ginsberg HN, Graham I, et al. (2017) Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the european atherosclerosis society consensus panel. European Heart Journal 38: 2459–2472. 2017/04/27. DOI: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahotupa M. (2017) Oxidized lipoprotein lipids and atherosclerosis. Free Radical Research 51: 439–447. 2017/04/18. DOI: 10.1080/10715762.2017.1319944. [DOI] [PubMed] [Google Scholar]
- 28.Miller YI, Choi S-H, Wiesner P, et al. (2011) Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ ulation Research 108: 235–248. 2011/01/22. DOI: 10.1161/circresaha.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chávez-Sánchez L, Madrid-Miller A, Chávez-Rueda K, et al. (2010) Activation of TLR2 and TLR4 by minimally modified low-density lipoprotein in human macrophages and monocytes triggers the inflammatory response. Human Immunology 71: 737–744. DOI: 10.1016/j.humimm.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Gisterå A, Hansson GK. (2017) The immunology of atherosclerosis. Nature Reviews Nephrology 13: 368–380. 2017/04/11. DOI: 10.1038/nrneph.2017.51. [DOI] [PubMed] [Google Scholar]
- 31.Simic I, Reiner Z. (2015) Adverse effects of statins - myths and reality. Current Pharmaceutical Design 21: 1220–1226. 2014/10/15. DOI: 10.2174/1381612820666141013134447. [DOI] [PubMed] [Google Scholar]
- 32.Bougarne N, Weyers B, Desmet SJ, et al. (2018) Molecular actions of PPARα in lipid metabolism and inflammation. Endocrine Reviews 39: 760–802. 2018/07/19. DOI: 10.1210/er.2018-00064. [DOI] [PubMed] [Google Scholar]
- 33.Hilgendorf I, Swirski FK, Robbins CS. (2015) Monocyte fate in atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology 35: 272–279. 2014/12/30. DOI: 10.1161/atvbaha.114.303565. [DOI] [PubMed] [Google Scholar]
- 34.Poupel L, Combadière C. (2010) Athérosclérose : sur la piste des chimiokines. Biologie aujourd'hui 204: 285–293. 2011/01/11. DOI: 10.1051/jbio/2010026. [DOI] [PubMed] [Google Scholar]
- 35.Marchio P, Guerra-Ojeda S, Vila JM, et al. (2019) Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxid Med Cell Longev 2019: 8563845. DOI: 10.1155/2019/8563845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinet W, Schrijvers DM, De Meyer GRY. (2011) Necrotic cell death in atherosclerosis. Basic Research in Cardiology 106: 749–760. 2011/05/26. DOI: 10.1007/s00395-011-0192-x. [DOI] [PubMed] [Google Scholar]
- 37.Zhao M, Li F, Jian Y, et al. (2020) Salvianolic acid B regulates macrophage polarization in ischemic/reperfused hearts by inhibiting mTORC1-induced glycolysis. European Journal of Pharmacology 871: 172916. 2020/01/14. DOI: 10.1016/j.ejphar.2020.172916. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Zhang Y, Guo L-L, et al. (2011) Salvianolic acid B inhibits the TLR4-NFκB-TNFα pathway and attenuates neonatal rat cardiomyocyte injury induced by lipopolysaccharide. Chinese Journal of Integrative Medicine 17: 775–779. 2011/11/22. DOI: 10.1007/s11655-011-0877-x. [DOI] [PubMed] [Google Scholar]
- 39.Feng M, Kong S-Z, Wang Z-X, et al. (2017) The protective effect of coptisine on experimental atherosclerosis ApoE−/− mice is mediated by MAPK/NF-κB-dependent pathway. Biomedicine & Pharmacotherapy 93: 721–729. 2017/07/13. DOI: 10.1016/j.biopha.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Reustle A, Torzewski M. (2018) Role of p38 MAPK in atherosclerosis and aortic valve sclerosis. International Journal of Molecular Sciences 19: 3761. DOI: 10.3390/ijms19123761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S, Zhou H, Feng T, et al. (2014) β-Glucan attenuates inflammatory responses in oxidized LDL-induced THP-1 cells via the p38 MAPK pathway. Nutrition, Metabolism and Cardiovascular Diseases 24: 248–255. 2014/01/15. DOI: 10.1016/j.numecd.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Hakala JK, Lindstedt KA, Kovanen PT, et al. (2006) Low-density lipoprotein modified by macrophage-derived lysosomal hydrolases induces expression and secretion of IL-8 Via p38 MAPK and NF-κB by human monocyte-derived macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology 26: 2504–2509. DOI: 10.1161/01.ATV.0000245796.97133.ad.. [DOI] [PubMed] [Google Scholar]
- 43.Dong N, Li X, Xue C, et al. (2020) Astragalus polysaccharides alleviates LPS‐induced inflammation via the NF‐κB/MAPK signaling pathway. Journal of Cellular Physiology 235: 5525–5540. DOI: 10.1002/jcp.29452. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Jia Q, Zhang Y, et al. (2020) Amygdalin attenuates atherosclerosis and plays an anti-inflammatory role in apoE knock-out mice and bone marrow-derived macrophages. Frontiers in Pharmacology 11: 590929. 2020/11/17. DOI: 10.3389/fphar.2020.590929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Jia Q, Zhang Y, et al. (2020) Taoren honghua drug attenuates atherosclerosis and plays an anti-inflammatory role in ApoE knock-out mice and RAW264.7 cells. Frontiers in Pharmacology 11: 1070. DOI: 10.3389/fphar.2020.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim S-J, Jeong H-J, Moon P-D, et al. (2005) Anti-inflammatory activity of gumiganghwaltang through the inhibition of nuclear factor-κB activation in peritoneal macrophages. Biological & Pharmaceutical Bulletin 28: 233–237. 2005/02/03. DOI: 10.1248/bpb.28.233. [DOI] [PubMed] [Google Scholar]
- 47.Baker RG, Hayden MS, Ghosh S. (2011) NF-κB, inflammation, and metabolic disease. Cell Metabolism 13: 11–22. 2011/01/05. DOI: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawrence T. (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspectives in Biology 1: a001651. DOI: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braverman J, Stanley SA. (2017). Nitric Oxide Modulates Macrophage Responses to Mycobacterium tuberculosis Infection through Activation of HIF-1α and Repression of NF-κB. The Journal of Immunology (Baltimore, Md:1950) 199: 1805–1816. DOI: 10.4049/jimmunol.1700515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao C, Zhu Y, Chen W, et al. (2013) IKKε knockout prevents high fat diet induced arterial atherosclerosis and NF-κB Signaling in mice. PLoS One 8: e64930. 2013/06/07. DOI: 10.1371/journal.pone.0064930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gliozzi M, Scicchitano M, Bosco F, et al. (2019) Modulation of nitric oxide synthases by oxidized LDLs: role in vascular inflammation and atherosclerosis development. International Journal of Molecular Sciences 20: 3294. 2019/07/07. DOI: 10.3390/ijms20133294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krieglstein CF, Granger DN. (2001) Adhesion molecules and their role in vascular disease. American Journal of Hypertension 14: 44s–54s. 2001/06/20. DOI: 10.1016/s0895-7061(01)02069-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-iji-10.1177_03946320221079468 for Salvianolic acid B attenuates the inflammatory response in atherosclerosis by regulating MAPKs/ NF-κB signaling pathways in LDLR-/- mice and RAW264.7 cells by Yifan Zhang, Xiaoteng Feng, Min Du, Jie Ding and Ping Liu in International Journal of Immunopathology and Pharmacology