Abstract
The flavonoids, baicalin, and its aglycone baicalein possess multi-fold therapeutic properties and are mainly found in the roots of Oroxylum indicum (L.) Kurz and Scutellaria baicalensis Georgi. These flavonoids have been reported to possess various pharmacological properties, including antibacterial, antiviral, anticancer, anticonvulsant, anti-oxidant, hepatoprotective, and neuroprotective effects. The pharmacological properties of baicalin and baicalein are due to their abilities to scavenge reactive oxygen species (ROS) and interaction with various signaling molecules associated with apoptosis, inflammation, autophagy, cell cycle, mitochondrial dynamics, and cytoprotection. In this review, we summarized the molecular mechanisms underlying the chemopreventive and chemotherapeutic applications of baicalin and baicalein in the treatment of cancer and inflammatory diseases. In addition, the preventive effects of baicalin and baicalein on mitochondrial dynamics and functions were highlighted with a particular emphasis on their anti-oxidative and cytoprotective properties. The current review highlights could be useful for future prospective studies to further improve the pharmacological applications of baicalein and baicalin. These studies should define the threshold for optimal drug exposure, dose optimization and focus on therapeutic drug monitoring, objective disease markers, and baicalin/baicalein drug levels.
Key Words: Baicalein, Baicalin, Cancer, Inflammatory diseases, Mitochondrial functions
Introduction
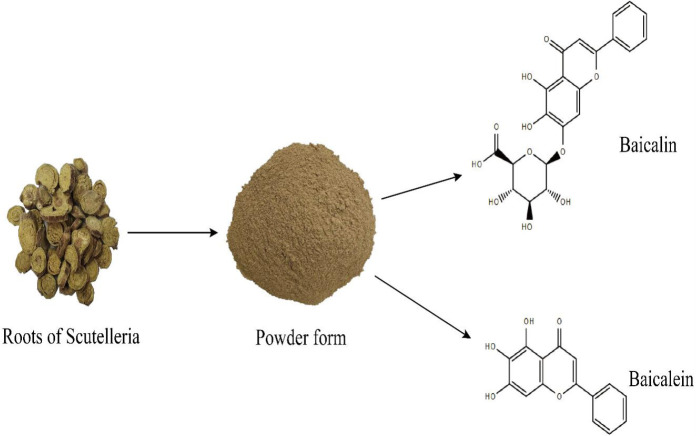
The flavonoids, baicalin, and its aglycone, baicalein, were extracted from S. baicalensis Georgi (SBG), Scutellaria galericulata, Scutellaria rivularia Wall, and Scutellaria lateflora L. as well as in O. indicum (L.) Kurz (OI, Bignoniaceae) (1-6). O. indicum is mostly found in India, Sri Lanka, Pakistan, Bangladesh, Cambodia, China, Thailand, and other south Asian countries (7). Whereas, plant species of Scutellaria are grown in eastern Russia, Japan, China, Siberia, Mongolia, and Korea (8). The chemical structures of baicalin and baicalein are represented in Figure 1. Previous studies reported that the roots, seeds, and stem-bark of O. indicum have been applied for the treatment of diseases in China, India, and several other countries for the cure of dysentery, rheumatic pain, diarrhea, pharyngitis, coughs, and other respiratory diseases such as bronchitis, etc. (9). Besides, Scutellaria radix is used for the cure of dysentery, atherosclerosis, hyperlipidemia, hypertension, and respiratory ailments in traditional Chinese medicines (10). Baicalin and baicalein have been receiving much interest from cosmetic, food, and pharmaceutical industries due to their excellent antioxidant, anti-inflammatory, anticancer, antidiabetic, anti-ulcerative colitis, antithrombotic, antiviral, eye-protective, cardioprotective, neuroprotective, and hepatoprotective properties (11, 12). In addition, baicalin and baicalein exhibited anti-cancer and favorable against various anti-inflammatory disorders targeting relevant signaling pathways (13). Previous studies on baicalin and baicalein have summarized the pharmacological activities including anti-inflammatory, anti-tumor, cardioprotective, neuroprotective, anti-ocular disorders, and mitochondrial functions (14-16). However, limited information is available about the clinical uses and dose optimization of both compounds. In this review, we highlighted the signaling pathways of baicalin and baicalein in the treatment and chemoprevention anti-cancer, anti-inflammatory, antioxidative and cytoprotective properties as well as baicalin-baicalein’s interactions. This study could be useful in exploring the therapeutic drug targets, defining the threshold for optimal drug exposure, and dose-optimization that could lay a foundation to further enhance the biological activities of both compounds in curing various health disorders.
Figure 1.
Roots of Scutellaria baicalensis, powder form, and chemical structure of baicalin and baicalein
Protective effects of baicalin and baicalein against inflammatory disorders
Inflammation is a protective reaction in a localized area and characterized by symptoms such are pain, redness, heat, loss of function, and swelling. Stimulation of IKKβ and IKKα and translocation of NF-κB to the nucleus leads to increased pro-inflammatory, anti-inflammatory cytokines, and chemokines to defend against shock or trauma (17). Chronic inflammation is often related to increased expression of NF-κB by the invaded microbes and injured tissues, leading to several diseases including asthma, cancer, inflammatory bowel disease, cardiovascular diseases, sepsis, psoriasis, atherosclerosis, rheumatoid arthritis, acquired immunodeficiency disorder syndrome (AIDS), gastritis, CNS depression, multiple sclerosis (MS), etc. (18-20). In the last couple of years, new genome-wide association studies have been carried out on common inflammation-related diseases; for instance, diabetes, asthma, rheumatoid arthritis, atherosclerosis, colorectal cancer, Crohn’s disease, and MS to determine alleles for these ailments (21-23), which drew the interest of researchers, because significant economic losses occurred due to these diseases (24). The effects of flavonoids for alleviation of inflammation-related ailments through inflammatory cytokines are discussed in this review. The mechanisms of baicalein and baicalin are depicted in Figure 2.
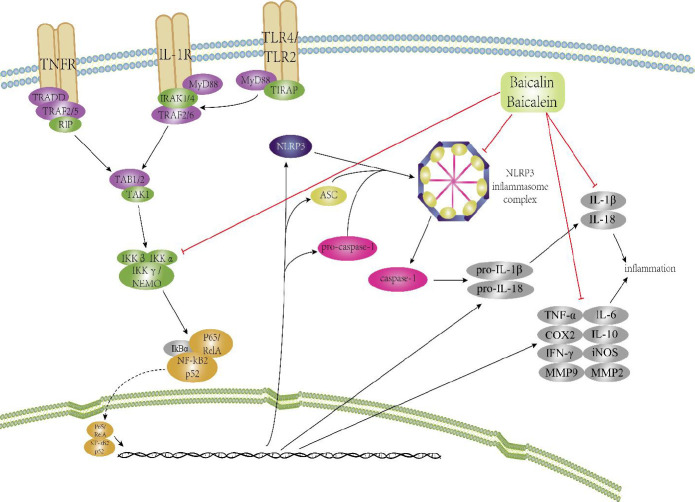
Figure 2.
Effect and mechanism of baicalin and baicalein for inflammation-related ailments via inflammation-related signaling pathways are shown. Baicalin/baicalein effectively inhibited inflammation through NF-κB and inflammasome pathway
Anti-inflammatory effects in respiratory ailments
Recently, it has been demonstrated that baicalein given orally at doses of 50 and 100 mg/kg/d for 28 days significantly ameliorated pulmonary fibrosis in rats. The results indicated that baicalein significantly reduced the expressions of smad2/3 and TGFβ1 and markedly reduced miR-21 expression and expression of alpha-smooth muscle actin (α-SMA) and hydroxyproline content. It has been noted that baicalein exerts protective effects through the TGF-β/smad signaling pathway (25). Besides, baicalin at a dose of 120 mg/kg/d (IP) treatment for 28 days protected mice by decreasing collagen deposition, lung coefficient, and hydroxyproline levels through the ERK1/2 pathway. Thus, these outcomes unveiled that baicalin exhibited an antifibrotic effect possibly via adenosine A2a receptor, which is involved in tissue repair and inflammation process (26, 27). Baicalin pretreatment (30 mg/kg, IP) ameliorated heart dysfunction and pulmonary artery hypertension (PAH) by attenuating the elevated expression of p38 MAPK in tissue homogenates and reducing matrix metalloproteinase-9 expression in lung arterioles. Moreover, baicalin inhibited the elevated levels of cytokines via the p38 MAPK signaling pathway in the lung tissues (28). The inhibition of smooth muscle cells in the pulmonary artery offers treatment for PAH (29). Baicalin administration at doses of 10 and 20 mM/L significantly down-regulated p-Akt and HIF-1α protein and mRNA levels and elevated p27 proteins in rat PASMCs during hypoxia. In another in vivo study, baicalin (100 mg/kg, IP) significantly alleviated the right/left ventricle plus septum ratio and right ventricular systolic pressure (RVSP) via up-regulating p27 protein and suppressing the expression of mRNA expressions of p-Akt and HIF-1α via attenuating the increased Akt protein level (30). A previous study demonstrated that the human mast cells played a crucial role in respiratory disorders and were deposited at inflammatory sites in asthmatic (31) and allergic rhinitis patients (32). HMCs activate innate immune responses through secretion of IL-6, MCP-1, and IL-8 (33). In previous experiments, baicalein was reported to exhibit potential therapeutic effects in allergic and asthmatic disorders and inhibited IL-8, IL-6, and MCP-1 in the culture of the HMCs through degradation of IkBα and inhibition of both IkBα phosphorylation and NF-κB activation (34).
Anti-inflammatory effects in arthritis
Inflammation of the joints, which results in joint destruction and bone and cartilage erosion is known as rheumatoid arthritis (RA) (35). Baicalin acts as a potential remedy for RA; in an in vivo study, baicalin (100 mg/kg/d, IP for 7 days) markedly reduced ankle swelling and inhibited splenic Th17 cell population in murine arthritic mice 14 days postimmunization. In vitro results revealed that baicalin (dose of 20 mM for 24 hr) inhibited IL-17-induced inflammatory cascade, blocked lymphocytes’ attachment to synovial cells, and decreased the expression of IL-6, TNF-α, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) (36). Baicalein (20 mg/kg orally) exhibited preventive effects against food allergy in mice by alleviating the signs of anaphylaxis, diarrhea, and rectal temperature and through activation of B cells and reducing serum IgE levels, as well as by promoting the function of intestinal barrier via regulating tight junctions in Caco-2 epithelial cells. Thus, baicalein may be used as a healing agent for the cure of inflammatory bowel diseases (IBD) and food allergies (17, 37). Baicalin alleviated ulcerative colitis in mice which is a form of IBD. The underlying mechanism of prevention involves modulation of polarization of macrophages via suppression of protein known as interferon regulatory factor 5 (IRF5) and enhanced the expression of IRF4 expression in dextran sodium sulfate-induced colitis in mice. In in vitro experiments, baicalin modulated M1 macrophage polarization in LPS-stimulated murine macrophages and reduced the expression of IL-23, IRF5, and TNF-α protein expressions (38). A previous study reported the clinical efficacy of baicalin in patients with RA and coronary artery disease. The lipid profiles of coronary artery disease were reduced with 20 mg baicalin after 12-week oral administration, further showing the effectiveness of baicalin in curing coronary artery disease (39). Recently, Isola, et al. compared and scrutinized the effectiveness of a phytotherapeutic drug composed of herbal extracts including baicalin on postsurgical discomfort, and it was noticed that the herbal extract composed of baicalin decreased the severity of postoperative pain compared with ibuprofen and placebo (40).
Anti-inflammatory effects in type-2 diabetes and obesity
Baicalin could be used for the treatment of obesity in humans. Baicalin at a dose of 80 mg/kg/d (IP) significantly reduced the bodyweight of high-fat diet (HFD) rats. In addition, baicalin decreased the elevated level of free fatty acids (FFA), TNF-α, and serum cholesterol through stimulation of acetyl CoA-carboxylate and AMP-activated protein kinase (AMPK). In HepG2 cells, baicalin-treatment (5 and 10 mM/L) for 24 hr decreased lipid accumulation through activation of AMPK (41). Previous reports demonstrated that baicalein at doses of 0.25 and 0.5 g/kg/d for 5 weeks proved effective in type 2 diabetes (T2D) mice, and showed improved glucose tolerance, blood insulin levels, and hyperglycemia. It has also been noted that baicalein (5 mM) in human islets culture cells and insulin-secreting pancreatic INS382/13 cells significantly promoted viability and enhanced glucose-stimulated insulin secretion (GSIS) (42). Besides, a recent study reported that baicalin and baicalein enhanced mitochondrial function and viability via a cAMP-dependent pathway (43). In HFD-fed mice, baicalein (400 mg/kg/d) supplementation alleviated inflammation, obesity, hyperglycemia, insulin resistance, and hyperlipidemia in diabetic mice through activation of AMPK, which in turn suppressed the synthesis of fatty acids and cholesterol by reducing the transcription of fatty acid synthase and SREBP-1c and elevated the level of PPAR-α and its downstream genes that are involved in fatty acid oxidation. In another study, 90 mg/ml baicalein pretreatment of hepatocytes culture and 25 mM solution of baicalein in AMPKα2-stimulated mice in glucose significantly down-regulated ERK and p38 phosphorylation, which results in inhibition of the MAPKs signaling pathway (44, 45).
Anti-inflammatory effects in cardiovascular ailments
Researchers have demonstrated that baicalein could be used for treatment of cardiovascular ailments. Researchers reported that baicalein at 200 mg/kg/d (IP) treatment for 12 weeks ameliorated fibrosis of heart tissues in rats via suppression of p-ERK, 12-lipoxygenase, and matrix-metalloproteinase-9 (MMP-9) and alleviated the intraventricular septum thickness (46). Atherosclerosis, where arteries become hardened and narrowed, results in stroke, coronary thrombosis, myocardial infarction, and other cardiovascular-related ailments (47, 48). Baicalin/baicalein can be a promising agent for alleviating cardiovascular-related diseases. A previous report explained that baicalin and baicalein (5 and 10 mM) reduced vascular inflammation through suppression of disruption of endothelial barrier function and cellular adhesion molecules, and up-regulation of NF-κB in cultured human umbilical vein endothelial cells (HUVECs) (49). In vascular smooth muscle cells (VSMCs) of rats, baicalin (20, 40, and 60 mM) for 24 hr significantly reduced the migration and proliferation of VSMCs by enhancing the expression of the p27 protein and reducing the level of E-CDK2 protein. Moreover, treatment with baicalin at a dose of 70 mg/kg/d in rats significantly reduced the thickness of the left carotid artery by inhibiting neointimal hyperplasia through suppression of the protein cyclin E (PCNA) and up-regulation of p27 proteins level (50). Baicalin administration (20 mg/kg, IV) markedly restored vascular function through suppression of the NF-κB pathway and plasma superoxide anions, iNOS, NO, and TNF-α by improving blood pressure in LPS-induced septic rats (51). Studies indicated that baicalein suppressed NF-κB, which in turn inhibited thioredoxin reductase (TrxR) activity in lymphocytes (52). Similarly, another researcher reported that baicalein at a dose of 10 mg/kg (IV) reduced myocardial inflammatory responses, apoptosis, and oxidative stress through suppression of iNOS, MCP-1, cardiac superoxide anion, phospho-p65, and phospho-IkBα proteins in LPS-induced septic rats (53, 54). In experimental autoimmune encephalomyelitis (EAE) mice, baicalin at doses of (5 and 10)-mg/kg/d, (IP) significantly reduced the severity of the disease according to scoring (2.2+0.3), induced IL-4 expression, suppressed IFN-γ, and inhibited mononuclear cell proliferation (55). In an EAE model of mice, baicalin at a dose of 100 mg/kg/d (IP) for 20 days ameliorated the severity of the disease by reducing immune cell infiltration through up-regulating cytokine signaling-3 (SOC-3) protein and inhibited Th-17 and Th-1 cell differentiation (56).
Anti-inflammatory effects in liver diseases
Baicalein acts as an effective therapeutic strategy for autoimmune hepatitis (AIH). Baicalein at a dose of 10- and 20-mM induced apoptosis through mitochondrial pathway in concanavalin A-stimulated CD3+ T cells. Previously, it has been shown that baicalein (100 mg/kg, IV) alleviated liver injury in mice through suppression of IFN-γ and TNF-α (57). Researchers reported that the prolonged use of baicalein at a dose of 40 and 80 mg/kg/d orally for 10 weeks alleviated CCl4-induced liver fibrosis in rats. The mechanisms involved inhibition of hepatic stellate cell activation and reduced ALT, AST, laminin, collagens, and hyaluronic acid in the liver serum (58). Baicalein (80 mg/kg/d, orally) for 4 d significantly ameliorated CCl4-induced acute liver injury in mice through suppression of inflammatory cytokines IL-6 and TNF-α (59). In another animal model, application of baicalin at a dose of 70 mg/kg/d (IP) for 56 days significantly reduced liver index, collagen deposition area, AST, ALT, IL-6, and TNF-α. In contrast, the IL-10 level was up-regulated in CCl4-induced liver fibrotic rats (60).
Anti-inflammatory effects in neurodegenerative diseases
Baicalein proved an effective anti-inflammatory mechanism against neurodegenerative diseases. Baicalein administration (560 and 280 mg/kg/d) protected mice neurotoxicity caused by 1-methyl-4-phenyl- 1,2,3,6- tetrahydropyridine (MPTP) and reduced apoptosis by alleviating mitochondrial dysfunction of dopaminergic neurons (61). Another study also reported that baicalin (200 mg/kg/d) exerted preventive effects in MPTP-induced neurotoxicity in mice treated for a period of one week (62). In human SH-SY5Y cells, baicalein (50 and 100 mM) for 24 hr significantly reduced ROS production, attenuated apoptosis through Bcl-2 and Bax proteins, and inactivated the ERK1/2 pathway (63). Additionally, baicalein (0.5 and 5 mg/ml) for 24 hr attenuated apoptosis and alleviated parkinsonism in 6-hydroxydopamine (6-OHDA)-stimulated SH-SY5Y cells. In a rat 6-OHDA-induced parkinsonism model, baicalein given at a dose of 200 mg/kg/d for 15 days reduced apoptosis and down-regulated ROS of neurons (64). Previous studies demonstrated that baicalein could be used as an anti-inflammatory in chronic kidney diseases. In unilateral ureteral obstruction (UUO)-induced mice model of renal fibrosis, baicalein administered at a dose of 100 and 50 mg/kg/d for a week significantly reduced the accumulation of collagen and fibronectin through suppression of NF-κB expression and its downstream genes including IL-1β, TNF-α, and MCP-1 expression and inactivating the MAPK signaling pathway (65).
Anti-inflammatory effects in microbial infections
Recently, studies reported that baicalin acts as a potential anti-inflammatory agent against bacterial infections. For instance, Mycoplasma gallisepticum (MG) infection causes significant losses in the poultry industry (66, 67). Researchers reported that baicalin (50, 100, and 200 mg/kg BW) suppressed the NF-κB pathway through TLR4 receptor in LPS-induced infection in chicken liver. The underlying mechanism of action involves suppression of inflammatory markers including iNOS, IL-1β, COX-2, TNF-α, and IL-6 expression. The levels of AST, ALT, and NO content were significantly reduced compared with the control group (68). In a model of MG-infection, baicalin at a dose of 450 mg/kg attenuated oxidative stress and apoptosis in chicken spleen through elevation of Nrf2/HO-1 signaling pathway and suppressed the NF-κB pathway (66). Similarly, another study reported that baicalin (450 mg/kg) significantly ameliorated structural damage in the chicken thymus and alleviated MG-induced inflammatory cell infiltrates, reduced oxidative stress and expression of pro-inflammatory cytokines, and attenuated apoptosis (69). In addition, baicalin (450 mg/kg) administration for 7 days significantly reduced inflammation in chicken lungs and trachea during MG infection. The authors reported that baicalin treatment restored energy metabolism in chicken lungs and attenuated apoptosis through the mitochondrial pathway (70, 71). Most of the studies reported the molecular mechanism of both compounds, employed for the cure of diabetes, inflammatory bowel diseases, cardiovascular disorders, rheumatoid arthritis, respiratory ailments, kidney diseases, neurodegenerative diseases, hepatitis, cancers, and autoimmune encephalomyelitis. It is worthy to mention that several aspects of baicalein and baicalin such as bioavailability need to be studied before for the treatment of human ailments in clinical cases (72, 73).
Protective effects of baicalin and baicalein on cancer
Apoptosis is programmed cell death, triggered by two pathways: the extrinsic and intrinsic pathways (74). The intrinsic pathway (mitochondrial pathway) is often activated by viral infections, toxic materials, ionizing radiation, different cytokines, certain types of hormones, or loss of growth factors. The extrinsic pathway (receptor-mediated pathway) is often activated to exterminate unhealthy cells through activation of the death receptor located on the cell membrane (75, 76). Numerous studies reported that baicalin and baicalein induced apoptosis in various types of tumors through both extrinsic and intrinsic pathways (77). The anti-cancer effects of baicalin and baicalein are multi-fold, such as, through induction of apoptosis and triggering of autophagy as shown in Figure 3.
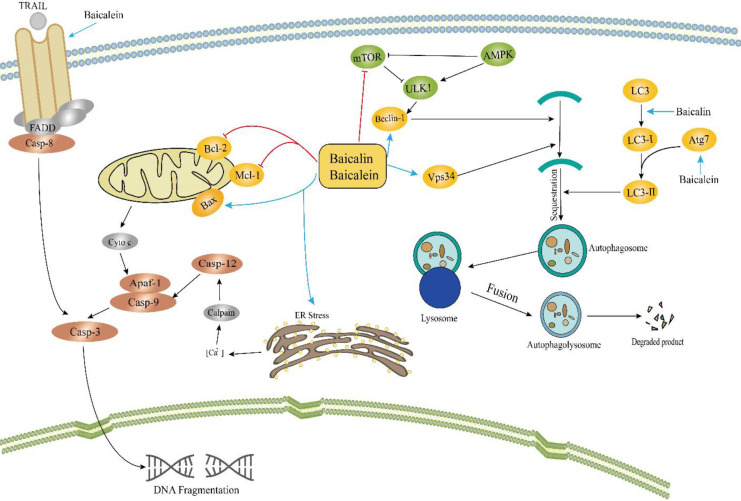
Figure 3.
Baicalin and baicalein triggered programmed cell death in cancer cells via releasing cytochrome-C from mitochondria in the intrinsic pathway. Baicalein activated the TNFR-associated death domain (TRADD) through the extrinsic pathway. In addition, baicalin or baicalein activated autophagy in cancer cells and intervened in the formation of autophagosomes in various steps. The pathways involved the inhibition of AKT/mTOR and activation of AMPK/ULK1
Programmed cell death in cancer cells
Baicalein mainly induced apoptosis through Ca+2 influx via Ca2+ release from the reticulum to cytosol dependent on phospholipase C protein. ROS production is associated with baicalein-induced apoptosis via Ca2+-dependent apoptosis in tongue and breast cancer cells (78, 79). An intracellular calcium chelator BAPTA was applied to inhibit caspase-3 activity to confirm that apoptosis was induced by baicalein in MDA MB-231 cells. The level of Bax/Bcl-2 increased and caspase-3 and -9 were activated following the release of cytochrome C (80). In gastric cancer cells, baicalein mediated apoptosis in a dose-dependent manner through disruption of mitochondrial membrane potential (81). It has been reported that baicalein forms hydrogen bonds with Asp253 and Ser251 at the active site of caspase 3 and interacts through its hydroxyl groups with Asp228 and Ser251 residues in caspase 9, which results in activation of these caspases (82). In pancreatic cancer cells, baicalein induced apoptosis via suppression of the Mcl-1 protein. In contrast, Mcl-1 protein overexpression significantly alleviated baicalein-induced apoptosis (83). Additionally, researchers reported several signaling pathways associated with baicalein/baicalin-induced apoptosis. In HepG2 cells, baicalin-copper induced apoptosis through down-regulation of phosphoinositide-3 kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling pathway (84). Similarly, baicalin down-regulated the level of anti-apoptotic protein Bcl-2 and activated caspase-3 and caspase-9 in breast cancer cells through the ERK/p38 MAPK signaling pathway (85). Studies demonstrated that baicalein treatment suppressed Bad, ERK1/2 phosphorylation, and MEK1 expression both in vitro and in vivo. While baicalein-induced inhibition of human hepatocellular carcinoma was reversed by overexpression of MEK1 (86, 87). Baicalein enhanced the activity of death receptor-5 (DR5) in prostate cancer PC3 cells. It has been noted that DR5 was enhanced both at protein and mRNA levels (88). In herbal medicine, baicalin is the active ingredient that acts as Fas ligand and caused up-regulation of Fas protein (89).
Suppression of metastasis
Baicalin/baicalein not only induced apoptosis in cancer cells but also suppressed metastasis. Tumor metastasis is a multistep process including invasion, survival, arrest, and colonization (90, 91). Previous studies demonstrated that both baicalin and baicalein inhibited epithelial-mesenchymal transition (EMT) through the suppression of TGF-β in breast epithelial cells through the NF-κB pathway (92). In another study, baicalein suppressed metastasis in gastric cancer through inactivation of the Smad4/TGF-β pathway (93). Baicalein down-regulated the Wnt/b-catenin pathway which results in the suppression of metastasis in breast cancer through suppression of EMT (94). Similarly, another study reported the same results: baicalein inhibited SATB in the MDA-MB-231 human breast cancer cell line (95). A number of studies investigated baicalin and baicalein inhibition of the expression level of matrix metalloproteinases (MMP) such as MMP-9 and MMP-2 in liver, breast, lung, ovarian, gastric, and colorectal cancers and glioma (96-103). Baicalein suppressed metastasis in prostate cancer cells via suppression of the caveolin-1/AKT/mTOR pathway (104). Besides, novel G protein-coupled estrogen receptor (GPR30) signaling pathway was inactivated by baicalein and decreased phosphorylation of Akt and ERK as well as tyrosine phosphorylation of epidermal growth factor receptor (EGFR) in breast cancer cells, leading to suppression of migration and invasion of cancer (105). Apart from these results, baicalein (2.5-40 mM) suppressed metastasis through inhibition of phos-Ezrin and total Ezrin protein, which plays a crucial role in tumor development (106). In addition, in gallbladder cancer, baicalein suppressed metastasis through Zinc finger protein, X-linked (ZFX) (107). Moreover, baicalein interferes with platelet aggregation involved in the early stage of metastasis and down-regulated the PI3K kinase activity (108). Baicalin and baicalein both have the potential to control angiogenesis through basic fibroblast growth factor (bFGF) (109, 110). In human umbilical vein endothelial cells (HUVECs), baicalein suppressed angiogenesis via the p53/Rb signaling pathway (111). Baicalin attenuated lung metastasis through inhibition of hypoxia-inducible factor (HIF) (112). Similarly, baicalein reduced the transcription activity of HIF, responsible for activating genes associated with angiogenesis such as vascular endothelial growth factor (VEGF) in MCF-7 cells (113). Baicalein acts as an anticancer agent via inhibiting 12-lipooxygenase (12-LOX), which produces 12(S)-hydroxy eicosatetraenoic acid (HETE), associated with tumor growth, proliferation, and metastasis (87, 114-117).
Triggering of autophagy and cell cycle arrest in cancer cells
It is well documented that autophagy is involved in the degradation of foreign invaders or dysfunctional cytoplasmic organelles by the lysosomes (118, 119). Studies reported that autophagy plays a critical role in the progression and inhibition of cancer (120, 121). Baicalin and baicalein induced autophagic cell death associated with several autophagy-related proteins as shown in Figure 3. In an in vitro assay, baicalin triggered autophagy in a dose and time-dependent manner through Beclin-1 and down-regulated CD147 (122). Similarly, both flavonoid compounds (baicalein and baicalin) caused autophagy in various cancers including cleavage of LC3, autophagic flux, autophagosome formation and activation of Atg5/Atg7, Beclin-1, and vacuolar protein sorting 34, involvement of AKT/mTOR pathway, and activation of RelB/p52 proteins (123-128). Besides apoptosis and autophagy, these compounds also induced cell cycle arrest at certain checkpoints in cells as shown in Figure 4. Baicalin and baicalein arrested the S phase in the cell cycle through suppression of cyclin A in lung cancer A549 cells and decreased the level of cyclin D1 in SK-MES-1 cells (129). In human lung squamous carcinoma CH27 cells, baicalein (50 mM) treatment for 24 hr induced cell cycle arrest at G0/G1 phase through down-regulating the expression of CDK4, cyclin D1, and cyclin B1 (130). Moreover, baicalein in combination with silymarin decreased cell growth in the S-phase along with increase in the G0/G1 phase in HepG2 cells, implicated in down-regulation of CDK4, cyclin D, phosphor-Rb, and cyclin E and up-regulation of p53, Rb, p27 (Kip1), and p21 (Cip1) (131). In HepG2 cells, a dramatic increase has been noticed in G2/M population, whereas in Hep3B cultured cells, a significant increase was observed in sub G1 (hypoploid peak) caused by baicalin and baicalein (132). In another study, G0/G1-phase arrest has been noted 12 hr post-treatment of baicalein and S-phase arrest in MCF-7 cells, showing that baicalein effectively inhibited cancer via cell cycle arrest at different phases (133). In hepatocellular carcinoma cells, baicalein induced G0/G1-phase arrest through inhibition of cyclin D1 and β-catenin pathway (134). Besides, it has been reported that baicalein induced G1 phase arrest and facilitated degradation of cyclin D1 through activation of aryl hydrocarbon receptor (AhR) (135). Therefore baicalin/baicalein could be efficiently used as a potential candidate against a variety of tumors.
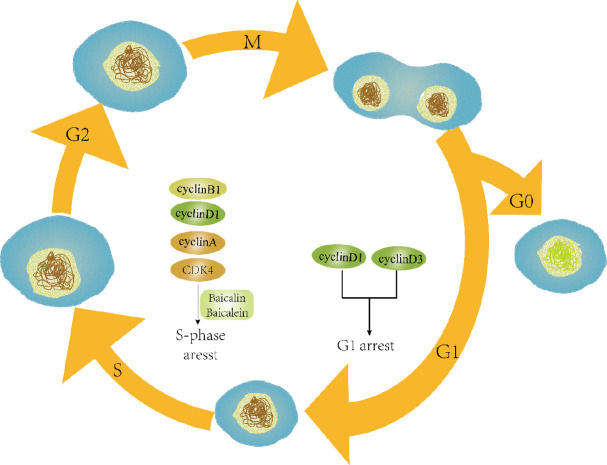
Figure 4.
Baicalin or baicalein arrested the cell cycle at various checkpoints and caused inhibition of cyclin-D3 and cyclin-D1, which results in inhibition of the cell cycle at the G1 phase. The compounds, baicalin and/or baicalein also reduced the expression of cyclin-A, cyclin-B1, cyclin-D1, and CDK-4, leading to the arrest of the S phase in the cell cycle
Protective effects of baicalin and baicalein on mitochondrial function and interactions
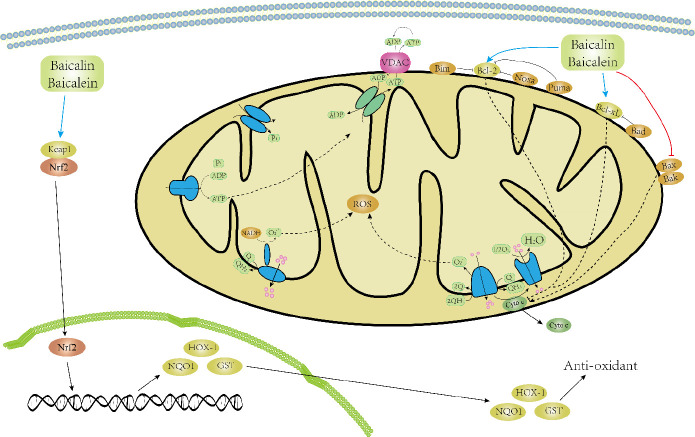
It is well understood that mitochondria are the powerhouses of the cells, which provide energy and play a crucial role in maintaining cell homeostasis and cell death (136-138). Oxygen (O2) is necessary for generation of energy in mitochondrial bioenergetic reactions (136, 138). ROS is produced because of electron transport chains such as superoxide anion and reactive oxygen free radical (139-142). Excessive ROS produced should be catalyzed to avoid damage to biomolecules including RNA, DNA, lipids, and proteins. Antioxidant enzymes such as glutathione (GSH), bioactive molecules, and vitamins protect the cells from free radicals and ROS-induced damage (143, 144). Mitochondria also play a crucial role in cell death during maintenance of tissue homeostasis and development through the intrinsic apoptotic pathway (145, 146). The mechanism of baicalin/baicalein in the protective role of mitochondria is still not completely understood. However, studies demonstrated that baicalin/baicalein protects mitochondrial dysfunction. The protective effects of baicalin/baicalein on mitochondria are shown in Figure 5.
Figure 5.
Baicalin or baicalein prevents mitochondrial dysfunction. Baicalein improves mitochondrial redox-related aspects and enhances mitochondrial activity. Baicalein reduces the changes in mitochondrial dynamics and loss of MMP. Moreover, baicalin/baicalein activates Nrf2, the master regulator of ROS and its downstream antioxidant genes, and therefore balances the redox system, improves mitochondrial function, and exerts cytoprotective effects
Protective effects of baicalein and baicalin on mitochondrial signaling pathways
Baicalein (6.25 and 12.5 µM) improved cell viability and protected mitochondria against 6-hydroxydopamine (6-OHDA)-induced toxicity in SH-SY5Y neuroblastoma cells (147). In addition, baicalein pretreatment (2 hr) protected mitochondria through the redox-dependent mechanism in cellular toxicity caused by N-acetylcysteine (147). In PC12 cells, baicalein at a dose of 5-40 µM prevented MMP imbalance 12 hr post-treatment and reduced apoptosis through the reduction of Bax and increased Bcl2 contents (148). Moreover, in human epidermal melanocyte (PIG1) cells, pretreatment with baicalein (10-40 µM) for 1 hr reduced MMP loss, inhibited the activation of caspase, and reduced apoptosis in PIG1 cells exposed to H2O2. The underlying mechanism involved the stimulation of nuclear factor erythroid 2 [NF-E2]-related factor 2 (Nrf2) and inhibition of cytochrome c release from mitochondria in Chinese hamster lung fibroblast (V79-4) cells (149). A study reported that baicalein administration for 27 days at doses of 30 or 100 mg/kg inhibited the loss of MMP, improved the production of ATP, increased the consumption of ADP and respiration control ratio (RCR) in the rotenone rat model (150). In a model of benzo[a]pyrene-induced carcinogenesis, baicalein treatment (12 mg/kg) for 16 weeks restored the normal function of mitochondria via the suppression of ROS, reduced GSH content, and enzyme activities such as isocitrate dehydrogenase–ICDH, malate dehydrogenase-MDH, α-ketoglutarate dehydrogenase–α-KDH, succinate dehydrogenase-SDH, cytochrome c oxidase, and NADH dehydrogenase. The authors suggested that baicalein treatment contributed to the protection of mitochondrial function through the maintenance of bioenergetic reactions associated with the Krebs cycle (151). Baicalin administration (120 mg/kg) for 30 days attenuated streptozotocin-induced mitochondrial damage in a rat model of diabetes (152). Baicalin at a dose of 200 mg/kg protected mitochondria in a rat model of hepatic ischemia/reperfusion (I/R) (153). In addition, baicalin reduced oxidative stress and decreased inflammation through NF-κB suppression and its downstream genes and prevented mitochondrial swelling (66, 154). Baicalin (50 µg/ml) for 1 hr improved cell viability, decreased superoxide ion, ATP production, and inhibited loss of MMP. The data revealed that baicalin up-regulated mitochondrial biogenesis through peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) by 40% during heart failure and hypertrophy (155). Yan and Liu studied the effect of baicalin (0.8–1.5 mM) on mitochondria isolated from the brain of rats. They found that baicalin decreased state 3 but did not affect MMP and state 4, and also did not prevent mitochondrial changes in hypoxic conditions (156). Numerous studies investigated the effect of baicalin/baicalein on cell signaling pathways associated with mitochondrial functions in the context of mitochondrial physiology maintenance and cell fate (157-163). A previous study demonstrated that the combination of the two flavonoids, baicalin, and baicalein produced a stronger effect on mitochondria in the process of apoptosis by activating caspase 3 and caspase 9 activation and triggering the release of cytochrome c (145, 158). As discussed in this section, baicalin and baicalein may exert an indirect role in preventing mitochondrial dysfunction through blocking mitochondrial ROS production and loss of MMP. Overall, the two flavonoids can increase ATP production in situations of stress and amplify mitochondrial functions.
Baicalin-baicalein’s interaction and signaling pathways
Despite extensive studies in several therapeutic areas owing to the antioxidant, anti-bacterial, anti-cancer, and anti-inflammatory properties of baicalin and baicalein; there is still limited information available about baicalin-baicalein’s interaction and their association with signaling pathways. Previously, Lai et al. demonstrated the pharmacokinetics/metabolism of baicalin and baicalein in rats. Following oral dosing of baicalin, extensive levels of baicalein conjugated with glucuronide and sulfate were observed in the systemic circulation. Besides, absorption of baicalein was negligible after oral administration in rats (164). Another study investigated that baicalin underwent extensive metabolism through conjugation reactions in the intestine of rats and rapidly converted to its aglycone baicalein. In addition, the metabolism of baicalin was affected by the higher loading dose which results in the saturation of metabolism reaction and surpassed the first-pass metabolism (165, 166). Liu et al. explained that the rapid conversion of baicalin conjugates to baicalein could be due to increased intestinal beta-glucuronidase activity due to the diabetic condition in rats (166, 167). Based on the evidence with regard to the metabolism of baicalin, there appears to be an imminent challenge for researchers to study the molecular mechanisms of baicalin and baicalein in association with cell signaling pathways. It is worth mentioning that cytochrome p450 (CYP) enzymes and their downstream signaling pathways are of prime importance in the context of baicalin-baicalein’s interactions. For example, a researcher demonstrated that daily administration of baicalin induced CYP2B6 in human subjects (168). While another study showed that baicalin acts as a hepatoprotective against acetaminophen-induced hepatic injury by inhibiting the expression of CYP2E1 (169). However, it also raises an important challenge if baicalin modulates the expression of CYP enzymes in clinical therapy. This may influence the metabolism of other drugs (170-172). Therefore, baicalin-baicalein’s interaction studies will further provide clarity of the signaling pathways that are associated with the mechanism of action of baicalin and baicalein.
Conclusion
Abundant scientific evidence revealed that flavonoid compounds have protective effects on cancer, inflammation, and act as potential anti-bacterial, anti-viral, and antioxidants (as summarized in Table 1). Among them, baicalin and baicalein received much attention from researchers. In this review, ample evidence has been shown from previous studies that baicalin/baicalein has the potential to mitigate oxidative stress injury, alleviate inflammation, and combat tumors in various experimental models. In addition, baicalin/baicalein improved mitochondrial functions through redox-dependent mechanisms. However, there are still limited clinical studies on baicalin/baicalein. Most of the previous studies worked on the molecular mechanisms of the two compounds (11, 173, 174). Therefore, it is still difficult to undertake a clear decision on the use of the two natural compounds and their clinical impacts. Future studies are needed to enhance the bioavailability of baicalin and baicalein including nano-emulsion, solid-liquid nanoparticles, nano-crystallization, and baicalin/baicalein-loaded liposomes. Researchers should work in different experimental models on non-toxic doses to avoid toxicity, side effects, and adverse reactions. Modern approaches such as transcriptomics, system biology, and metabolomics are needed to perform to find its therapeutic targets, optimize dosage and enhance its bioavailability through different routes. The synergism/antagonism of baicalin/baicalein in combination with other drugs is still not reported in detail. Another important issue is the interaction studies of baicalin and baicalein in modulating several signaling pathways at multiple areas including absorption, distribution, metabolism, and excretion. Hence, the clinical settings of these phytomedicines including their long-term toxicity study, pharmacokinetics, and pharmacodynamics are to be carried out by different routes to obtain maximum efficacy in various ailments.
Table 1.
Summary of the protective effects of baicalin/baicalein in different experimental models
| S. No. | Experimental model | Mechanisms and associated signaling pathways | Dose | Ref. |
|---|---|---|---|---|
| 1 | Rat | Inhibited apoptosis by suppressing via mitochondrial signaling pathway | 100 mg/kg | |
| 2 | Rats | Reduced intracellular calcium level and lactate dehydrogenase release. | 0.35, 3.5, 10 and 35 pM | |
| 3 | Mouse | Protective effects on hepatocarcinogenicity | 50 and 100 mg/kg | |
| 4 | Microglial cells (mice) | Inhibited NO production | 0.1, 1, 10 and 50 pM | |
| 5 | Rat | Decreased mitochondrial swelling, NF-kB activation, and suppressed caspase activation | 200 mg/kg | |
| 6 | Mouse | Protective effects on colon cancer | 50, 100 and 200 mg/kg | |
| 7 | Microglial neurotoxicity | Suppressed iNOS expression, and inhibited the binding activity of transcription factors with DNA | 1, 5, 10, 20 and 25 pM | |
| 8 | Culture of Neuron-glia extracted from the embryos of E-14 rat | Restored [3H] dopamine uptake and loss in tyrosine hydroxylase-immunoreactive neurons. Alleviated the increased expression of superoxide, NO and TNF-α | 1, 5, and, 10 pM | |
| 9 | Mouse | Reducing gallbladder cancer | 15, 30, and 60 mg/kg | |
| 10 | Culture of HT22 cell | Alleviated the iodoacetic acid (IAA)-induced toxicity in cells | 1–10 pM | |
| 11 | Cell line (PC12 cells) | Suppressed ROS production in PC12 cell line | 0.1, 1, and 10 pM | |
| 12 | Brain injury due to trauma | Reduced TNF-a, IL-6 protein, and mRNA expression | 30 mg/kg, IP | |
| 13 | Rat diabetes model | Baicalin protected mitochondrial damage from STZ-induced morphological changes | 120 mg/kg for 30 days | |
| 14 | Endothelial cells of the human brain | Inhibited the degradation of claudin-5 protein and protected endothelial cells | 10 pM | |
| 15 | Mouse | Reducing cervical cancer | 80 mg/kg | |
| 16 | SH-SY5Y and PCI2 cells | Ameliorated cell apoptosis and promoted neurite outgrowth | 0.05, 0.5 and 5 pg/ml | |
| 17 | Mice pulmonary carcinogenesis model | Decreased the mitochondrial ROS production and protected mitochondrial damage | 12.0 mg/kg once a week | |
| 18 | Rat CCR model | Improved mitochondrial integrity by reducing MMP | 30 or 100 mg/kg.day-1 | |
| 19 | Mouse | Inhibited prostate cancer | 10, 20, and 40 mg/kg | |
| 20 | Cell culture of COS-7 cells | Up-regulated TREK-2 protein in a direct/indirect manner | 100 pM | |
| 21 | Culture of CATH.a cells | Up-regulated the intracellular GSH content and inhibited the dopamine quinone formation | 1 pM | |
| 22 | Mouse | Prevent lung cancer | 12 mg/kg | |
| 23 | Rat | Increased phosphorylation of Akt and CREB and inhibited LTP potentiation | 0.1, 1, 10, and 50 pM | |
| 24 | Culture of PC12 cells line | Inhibited apoptosis and stimulated Nrf2/HO-1 pathway | 50, 100, and 200 pM | |
| 25 | Rotenone-induced neurotoxicity in PCI2 cells | Inhibited ROS, apoptosis, and caspase 3/7 activation in PCI2 cells | 10, 20 and 40 pM - PC12 cells.0.5 and 5 pM mitochondria. | |
| 26 | Mouse | Proved effective against pancreatic cancer | 1% S. baicalensis diet | |
| 27 | Rat | Inhibited hepatic cancer | 250 mg/kg | |
| 28 | Culture of PCI2 cells line | Suppressed the A|3-induced cytotoxicity and A|5 aggregation | 0.1, 1, and 10 pM | |
| 29 | Mouse | Reduced prostate cancer | 10, 20, and 40 mg/kg | |
| 30 | Culture of SH-SY5Y cells | Suppressed ROS and NO inside the cells and reduced extracellular NO production | 0.02, 0.2, and 2 pM | |
| 31 | SK-N-MC cells | Modulated caspase-9 and Bax activities and bcl2 proteins | 10, 20, 40, and 50 pM | |
| 32 | Mouse | Protected against bladder cancer | 0.8 mg/mouse | |
| 33 | Mouse | Inhibited mucoepidermoid cancer effects | 50, 100, and 200 | |
| 34 | Culture of rat cortical neurons and astrocytes | Protected neurons through production of VEGF and Epo expression in neurons | 3.5, 10, and 35 pM |
|
| 35 | Culture of CHO cells | Suppressed the production of A|5 and increased APP a-secretase. | 2.5, 5, and 10 pM | |
| 36 | Mouse | Proved effective against skin cancer | 1 mg/cm2 skin area/mouse/100 ml acetone | |
| 37 | Culture of cortical neurons of mice | Protected neurons from cell death | 30 pM | |
| 38 | Culture of cortical neurons of rats | Inhibited dopaminergic neuron loss, suppressed up-regulation of JNK and ERK, and prevented the translocation of NF-kB to the nucleus | 10 pM | |
| 39 | Culture of SK-N-SH and SH-SY5Y cells | Prevented mitochondrial dysfunction and suppressed ROS production. | 10, 40, and 80 pM | |
| 40 | Culture of cortical neurons | Enhanced sodium current and protected ROS | 1 nM-10 pM | |
| 41 | Culture of rat hippocampal cells | Suppressed glutamate release via regulating depolarization | 5-70 pM | |
| 42 31 |
Culture of glial cells (C6) | Suppressed the generation of H2O2 and ROS, and protected mitochondrial integrity | 0, 25, 50 and 100 μM | |
| 43 | Culture of microglial cells | Inhibited iNOS protein expression and NO production through down-regulating TLR4 | 0.1, 1, 10 μM | |
| 44 | Culture of cortical neurons of mice | Inhibited the depolarization caused by Aβ/AMPA/NMDA | 4, 8, and 14 μM | |
| 45 | Mice model | Restored LIMK1, SNCA, and GLRA1 expressions to normal and protected the behavior of mice | 140 and 280 mg/kg | |
| 46 | Rat model | Inhibited p-GSK3|3 protein, up-regulated p-Akt and p-PI3 K. Inhibited apoptosis through reducing the expression of caspase-3 and caspase-9. | 2 and 4 mg/kg | |
| 47 | Rat model | Reduced the expression TLR4 and NF-kB translocation to the nucleus. | 30 and 100 mg/kg | |
| 48 | Collagenase-induced ICH rat model | Increased ZO-1 protein expression and reduced iNOS protein. Inhibited the phosphorylation of JNK and p-38 MAPK and suppressed the NF-kB pathway. | 15 and 30 mg/kg | |
| 49 | MPTP-induced neurotoxicity in Zebrafish | Reversed locomotor deficiency and prevented dopaminergic loss in neurons | 10, 20, and 50 pM | |
| 50 | Mouse | Inhibited pancreatic cancer | 1% in diet | 1 |
Authors’ Contributions
ZH and MI Supervised the research; MI, WH and YG Draft manuscript preparation; ZX Critically revised the paper. ZH, MI, WH, YG, and ZX Read and approved the final version to be published.
Conflicts of Interest
We confirm that none of the authors have any competing interests.
Acknowledgment
This work was supported by the University industrial research innovation fund of science and technology development center of the Ministry of education of China (2020ITA05022), and the Natural Science Foundation of Hubei Province (2021CFB316), and the Preliminary support project of Hubei Social Science Foundation (21ZD137), and the Hundreds of Schools Unite with Hundreds of Counties-University Serving Rural Revitalization Science and Technology Support Action Plan (BXLBX0847). The results presented in this paper were part of a student thesis.
References
- 1.Chen LJ, Games DE, Jones J. Isolation and identification of four flavonoid constituents from the seeds of Oroxylum indicum by high-speed counter-current chromatography. J Chromatogr A. 2003;988:95–105. doi: 10.1016/s0021-9673(02)01954-4. [DOI] [PubMed] [Google Scholar]
- 2.Cui X, Shen YM, Jiang S, Qian DW, Shang EX, Zhu ZH, et al. Comparative analysis of the main active components and hypoglycemic effects after the compatibility of Scutellariae radix and Coptidis rhizoma. J Sep Sci. 2019;42:1520–1527. doi: 10.1002/jssc.201801204. [DOI] [PubMed] [Google Scholar]
- 3.Dinda B, Mohanta BC, Arima S, Sato N, Harigaya Y. Flavonoids from the stem-bark of Oroxylum indicum. Natural Product Sciences. 2007;13:190–194. [Google Scholar]
- 4.Li HB, Jiang Y, Chen F. Separation methods used for Scutellaria baicalensis active components. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:277–290. doi: 10.1016/j.jchromb.2004.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin CC, Shieh DE. The anti-inflammatory activity of Scutellaria rivularis extracts and its active components, baicalin, baicalein and wogonin. Am J Chin Med. 1996;24:31–36. doi: 10.1142/S0192415X96000050. [DOI] [PubMed] [Google Scholar]
- 6.Xie LH, Wang X, Basnet P, Matsunaga N, Yamaji S, Yang DY, et al. Evaluation of variation of acteoside and three major flavonoids in wild and cultivated Scutellaria baicalensis roots by micellar electrokinetic chromatography. Chem Pharm Bull (Tokyo) 2002;50:896–899. doi: 10.1248/cpb.50.896. [DOI] [PubMed] [Google Scholar]
- 7.Biswas K, Ghosh SE. Bharater bonoushodi. 1994; vol 3:858. [Google Scholar]
- 8.Bhandari M, Bhandari A, Prakash R, Bhandari A. Scutellaria baicalensis Georgi : A Rising Paradigm of Herbal Remedies. Webmed Central PHARMACEUTICAL SCIENCES. 2010;1:11. [Google Scholar]
- 9.Kirtikar KR, Basu BD. Indian Medicinal Plants. New Delhi: International Book Distribution; 1996. [Google Scholar]
- 10.Cheng CS, Chen J, Tan HY, Wang N, Chen Z, Feng Y. Scutellaria baicalensis and cancer treatment: Recent progress and perspectives in biomedical and clinical studies. Am J Chin Med. 2018;46:25–54. doi: 10.1142/S0192415X18500027. [DOI] [PubMed] [Google Scholar]
- 11.Dinda B, Dinda S, DasSharma S, Banik R, Chakraborty A, Dinda M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur J Med Chem. 2017;131:68–80. doi: 10.1016/j.ejmech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Gasiorowski K, Lamer-Zarawska E, Leszek J, Parvathaneni K, Yendluri BB, Blach-Olszewska Z, et al. Flavones from root of Scutellaria baicalensis Georgi: drugs of the future in neurodegeneration? CNS Neurol Disord Drug Targets. 2011;10:184–191. doi: 10.2174/187152711794480384. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Gao Y, Wu J, Chen Y, Chen B, Hu J, et al. Exploring therapeutic potentials of baicalin and its aglycone baicalein for hematological malignancies. Cancer Lett. 2014;354:5–11. doi: 10.1016/j.canlet.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang CZ, Calway TD, Wen XD, Smith J, Yu C, Wang Y, et al. Hydrophobic flavonoids from Scutellaria baicalensis induce colorectal cancer cell apoptosis through a mitochondrial-mediated pathway. Int J Oncol. 2013;42:1018–1026. doi: 10.3892/ijo.2013.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu CC, Chen YR, Lu DH, Hsu LH, Yang KC, Sumi S. Evaluation of the post-treatment anti-inflammatory capacity of osteoarthritic chondrocytes: An in vitro study using baicalein. Regen Ther. 2020;14:177–183. doi: 10.1016/j.reth.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Q, Chen XY, Martin C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci Bull (Beijing) 2016;61:1391–1398. doi: 10.1007/s11434-016-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalgleish AG, O’Byrne KJ. Chronic immune activation and inflammation in the pathogenesis of AIDS and cancer. Adv Cancer Res. 2002;84:231–276. doi: 10.1016/s0065-230x(02)84008-8. [DOI] [PubMed] [Google Scholar]
- 19.Rani A, Dasgupta P, Murphy JJ. Prostate Cancer: The role of inflammation and chemokines. Am J Pathol. 2019;189:2119–2137. doi: 10.1016/j.ajpath.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 22.Study C, Houlston RS, Webb E, Broderick P, Pittman AM, Di Bernardo MC, et al. Meta-analysis of genome-wide association data identifies four new susceptibility loci for colorectal cancer. Nat Genet. 2008;40:1426–1435. doi: 10.1038/ng.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeggini E, Scott LJ, Saxena R, Voight BF, Marchini JL, Hu T, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40:638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondylis V, Kumari S, Vlantis K, Pasparakis M. The interplay of IKK, NF-kappaB and RIPK1 signaling in the regulation of cell death, tissue homeostasis and inflammation. Immunol Rev. 2017;277:113–127. doi: 10.1111/imr.12550. [DOI] [PubMed] [Google Scholar]
- 25.Gao Y, Lu J, Zhang Y, Chen Y, Gu Z, Jiang X. Baicalein attenuates bleomycin-induced pulmonary fibrosis in rats through inhibition of miR-21. Pulm Pharmacol Ther. 2013;26:649–654. doi: 10.1016/j.pupt.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Huang X, He Y, Chen Y, Wu P, Gui D, Cai H, et al. Baicalin attenuates bleomycin-induced pulmonary fibrosis via adenosine A2a receptor related TGF-beta1-induced ERK1/2 signaling pathway. BMC Pulm Med. 2016;16 doi: 10.1186/s12890-016-0294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koroskenyi K, Kiss B, Szondy Z. Adenosine A2A receptor signaling attenuates LPS-induced pro-inflammatory cytokine formation of mouse macrophages by inducing the expression of DUSP1. Biochim Biophys Acta. 2016;1863:1461–1471. doi: 10.1016/j.bbamcr.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Yan S, Wang Y, Liu P, Chen A, Chen M, Yao D, et al. Baicalin Attenuates Hypoxia-Induced Pulmonary Arterial Hypertension to improve hypoxic cor pulmonale by reducing the activity of the p38 mapk signaling pathway and MMP-9. Evid Based Complement Alternat Med. 2016;2016:2546402. doi: 10.1155/2016/2546402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourgeois A, Lambert C, Habbout K, Ranchoux B, Paquet-Marceau S, Trinh I, et al. FOXM1 promotes pulmonary artery smooth muscle cell expansion in pulmonary arterial hypertension. J Mol Med (Berl) 2018;96:223–235. doi: 10.1007/s00109-017-1619-0. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Pu Z, Wang J, Zhang Z, Hu D, Wang J. Baicalin inhibits hypoxia-induced pulmonary artery smooth muscle cell proliferation via the AKT/HIF-1alpha/p27-associated pathway. Int J Mol Sci. 2014;15:8153–8168. doi: 10.3390/ijms15058153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradding P, Arthur G. Mast cells in asthma--state of the art. Clin Exp Allergy. 2016;46:194–263. doi: 10.1111/cea.12675. [DOI] [PubMed] [Google Scholar]
- 32.Lin W, Su F, Gautam R, Wang N, Zhang Y, Wang X. Raf kinase inhibitor protein negatively regulates FcepsilonRI-mediated mast cell activation and allergic response. Proc Natl Acad Sci U S A. 2018;115:E9859–E9868. doi: 10.1073/pnas.1805474115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cannon JG, Evans WJ, Hughes VA, Meredith CN, Dinarello CA. Physiological mechanisms contributing to increased interleukin-1 secretion. J Appl Physiol. 1986;61:1869–1874. doi: 10.1152/jappl.1986.61.5.1869. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh CJ, Hall K, Ha T, Li C, Krishnaswamy G, Chi DS. Baicalein inhibits IL-1beta- and TNF-alpha-induced inflammatory cytokine production from human mast cells via regulation of the NF-kappaB pathway. Clin Mol Allergy. 2007;5:5. doi: 10.1186/1476-7961-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18002. doi: 10.1038/nrdp.2018.2. [DOI] [PubMed] [Google Scholar]
- 36.Yang X, Yang J, Zou H. Baicalin inhibits IL-17-mediated joint inflammation in murine adjuvant-induced arthritis. Clin Dev Immunol. 2013;2013:268065. doi: 10.1155/2013/268065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bae MJ, Shin HS, See HJ, Jung SY, Kwon DA, Shon DH. Baicalein induces CD4(+)Foxp3(+) T cells and enhances intestinal barrier function in a mouse model of food allergy. Sci Rep. 2016;6:32225. doi: 10.1038/srep32225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu W, Jin Z, Yu J, Liang J, Yang Q, Li F, et al. Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. Int Immunopharmacol. 2016;35:119–126. doi: 10.1016/j.intimp.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 39.Hang Y, Qin X, Ren T, Cao J. Baicalin reduces blood lipids and inflammation in patients with coronary artery disease and rheumatoid arthritis: a randomized, double-blind, placebo-controlled trial. Lipids Health Dis. 2018;17:146. doi: 10.1186/s12944-018-0797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Isola G, Matarese M, Ramaglia L, Iorio-Siciliano V, Cordasco G, Matarese G. Efficacy of a drug composed of herbal extracts on postoperative discomfort after surgical removal of impacted mandibular third molar: A randomized, triple-blind, controlled clinical trial. Clin Oral Investig. 2019;23:2443–2453. doi: 10.1007/s00784-018-2690-9. [DOI] [PubMed] [Google Scholar]
- 41.Guo HX, Liu DH, Ma Y, Liu JF, Wang Y, Du ZY, et al. Long-term baicalin administration ameliorates metabolic disorders and hepatic steatosis in rats given a high-fat diet. Acta Pharmacol Sin. 2009;30:1505–1512. doi: 10.1038/aps.2009.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu Y, Luo J, Jia Z, Zhen W, Zhou K, Gilbert E, et al. Baicalein Protects against Type 2 Diabetes via Promoting Islet beta-Cell Function in Obese Diabetic Mice. Int J Endocrinol. 2014;2014:846742. doi: 10.1155/2014/846742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang X, Du L, Zhang W, Yang Y, Zhou Q, Du G. Therapeutic effects of baicalein on rotenone-induced Parkinson’s disease through protecting mitochondrial function and biogenesis. Sci Rep. 2017;7:9968. doi: 10.1038/s41598-017-07442-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pu P, Wang XA, Salim M, Zhu LH, Wang L, Chen KJ, et al. Baicalein, a natural product, selectively activating AMPKalpha(2) and ameliorates metabolic disorder in diet-induced mice. Mol Cell Endocrinol. 2012;362:128–138. doi: 10.1016/j.mce.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 46.Kong EK, Yu S, Sanderson JE, Chen KB, Huang Y, Yu CM. A novel anti-fibrotic agent, baicalein, for the treatment of myocardial fibrosis in spontaneously hypertensive rats. Eur J Pharmacol. 2011;658:175–181. doi: 10.1016/j.ejphar.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 47.Kasikara C, Doran AC, Cai B, Tabas I. The role of non-resolving inflammation in atherosclerosis. J Clin Invest. 2018;128:2713–2723. doi: 10.1172/JCI97950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf D, Ley K. Immunity and Inflammation in Atherosclerosis. Circ Res. 2019;124:315–327. doi: 10.1161/CIRCRESAHA.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ku SK, Bae JS. Baicalin, baicalein and wogonin inhibits high glucose-induced vascular inflammation in vitro and in vivo. BMB Rep. 2015;48:519–524. doi: 10.5483/BMBRep.2015.48.9.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong LH, Wen JK, Miao SB, Jia Z, Hu HJ, Sun RH, et al. Baicalin inhibits PDGF-BB-stimulated vascular smooth muscle cell proliferation through suppressing PDGFRbeta-ERK signaling and increase in p27 accumulation and prevents injury-induced neointimal hyperplasia. Cell Res. 2010;20:1252–1262. doi: 10.1038/cr.2010.111. [DOI] [PubMed] [Google Scholar]
- 51.Cheng PY, Lee YM, Wu YS, Chang TW, Jin JS, Yen MH. Protective effect of baicalein against endotoxic shock in rats in vivo and in vitro. Biochem Pharmacol. 2007;73:793–804. doi: 10.1016/j.bcp.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 52.Patwardhan RS, Sharma D, Thoh M, Checker R, Sandur SK. Baicalein exhibits anti-inflammatory effects via inhibition of NF-kappaB transactivation. Biochem Pharmacol. 2016;108:75–89. doi: 10.1016/j.bcp.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Lee YM, Cheng PY, Chim LS, Kung CW, Ka SM, Chung MT, et al. Baicalein, an active component of Scutellaria baicalensis Georgi, improves cardiac contractile function in endotoxaemic rats via induction of heme oxygenase-1 and suppression of inflammatory responses. J Ethnopharmacol. 2011;135:179–185. doi: 10.1016/j.jep.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Shao ZH, Vanden Hoek TL, Qin Y, Becker LB, Schumacker PT, Li CQ, et al. Baicalein attenuates oxidant stress in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2002;282:H999–H1006. doi: 10.1152/ajpheart.00163.2001. [DOI] [PubMed] [Google Scholar]
- 55.Zeng Y, Song C, Ding X, Ji X, Yi L, Zhu K. Baicalin reduces the severity of experimental autoimmune encephalomyelitis. Braz J Med Biol Res. 2007;40:1003–1010. doi: 10.1590/s0100-879x2006005000115. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Li X, Ciric B, Ma CG, Gran B, Rostami A, et al. Therapeutic effect of baicalin on experimental autoimmune encephalomyelitis is mediated by SOCS3 regulatory pathway. Sci Rep. 2015;5:17407. doi: 10.1038/srep17407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Staff PO. Correction: baicalein selectively induces apoptosis in activated lymphocytes and ameliorates concanavalin a-induced hepatitis in mice. PLoS One. 2015;10:e0117635. doi: 10.1371/journal.pone.0117635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun H, Che QM, Zhao X, Pu XP. Antifibrotic effects of chronic baicalein administration in a CCl4 liver fibrosis model in rats. Eur J Pharmacol. 2010;631:53–60. doi: 10.1016/j.ejphar.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Huang HL, Wang YJ, Zhang QY, Liu B, Wang FY, Li JJ, et al. Hepatoprotective effects of baicalein against CCl4-induced acute liver injury in mice. World J Gastroenterol. 2012;18:6605–6613. doi: 10.3748/wjg.v18.i45.6605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng XD, Dai LL, Huang CQ, He CM, Chen LJ. Correlation between anti-fibrotic effect of baicalin and serum cytokines in rat hepatic fibrosis. World J Gastroenterol. 2009;15:4720–4725. doi: 10.3748/wjg.15.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sowndhararajan K, Deepa P, Kim M, Park SJ, Kim S. Baicalein as a potent neuroprotective agent: A review. Biomed Pharmacother. 95:1021–1032. doi: 10.1016/j.biopha.2017.08.135. [DOI] [PubMed] [Google Scholar]
- 62.Cheng Y, He G, Mu X, Zhang T, Li X, Hu J, et al. Neuroprotective effect of baicalein against MPTP neurotoxicity: behavioral, biochemical and immunohistochemical profile. Neurosci Lett. 2008;441:16–20. doi: 10.1016/j.neulet.2008.05.116. [DOI] [PubMed] [Google Scholar]
- 63.Song JX, Choi MY, Wong KC, Chung WW, Sze SC, Ng TB, et al. Baicalein antagonizes rotenone-induced apoptosis in dopaminergic SH-SY5Y cells related to Parkinsonism. Chin Med. 2012;7:1. doi: 10.1186/1749-8546-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mu X, He G, Cheng Y, Li X, Xu B, Du G. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro. Pharmacol Biochem Behav. 2009;92:642–648. doi: 10.1016/j.pbb.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 65.Wang W, Zhou PH, Xu CG, Zhou XJ, Hu W, Zhang J. Baicalein attenuates renal fibrosis by inhibiting inflammation via down-regulating NF-kappaB and MAPK signal pathways. J Mol Histol. 2015;46:283–290. doi: 10.1007/s10735-015-9621-8. [DOI] [PubMed] [Google Scholar]
- 66.Ishfaq M, Zhang W, Hu W, Waqas Ali Shah S, Liu Y, Wang J, et al. Antagonistic Effects of baicalin on Mycoplasma gallisepticum-induced inflammation and apoptosis by restoring energy metabolism in the chicken lungs. Infect Drug Resist. 2019;12:3075–3089. doi: 10.2147/IDR.S223085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bao J, Wu Z, Ishfaq M, Miao Y, Li R, Clifton AC, et al. Comparison of Experimental Infection of Normal and Immunosuppressed Chickens with Mycoplasma gallisepticum. J Comp Pathol. 2020;175:5–12. doi: 10.1016/j.jcpa.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Cheng P, Wang T, Li W, Muhammad I, Wang H, Sun X, et al. Baicalin alleviates lipopolysaccharide-induced liver inflammation in chicken by suppressing TLR4-mediated NF-kappaB pathway. Front Pharmacol. 2017;8:547. doi: 10.3389/fphar.2017.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J, Qiao Z, Hu W, Zhang W, Shah SWA, Ishfaq M. Baicalin mitigated Mycoplasma gallisepticum-induced structural damage and attenuated oxidative stress and apoptosis in chicken thymus through the Nrf2/HO-1 defence pathway. Vet Res. 2019;50 doi: 10.1186/s13567-019-0703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen C, Li J, Zhang W, Shah SWA, Ishfaq M. Mycoplasma gallisepticum triggers immune damage in the chicken thymus by activating the TLR-2/MyD88/NF-kappaB signaling pathway and NLRP3 inflammasome. Vet Res. 2020;51:52. doi: 10.1186/s13567-020-00777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu Z, Chen C, Miao Y, Liu Y, Zhang Q, Li R, et al. Baicalin Attenuates Mycoplasma gallisepticum-Induced Inflammation via Inhibition of the TLR2-NF-kappaB Pathway in Chicken and DF-1 Cells. Infect Drug Resist. 2019;12:3911–3923. doi: 10.2147/IDR.S231908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang T, Liu Y, Zhang C. Pharmacokinetics and Bioavailability Enhancement of Baicalin: A Review. Eur J Drug Metab Pharmacokinet. 2019;44:159–168. doi: 10.1007/s13318-018-0509-3. [DOI] [PubMed] [Google Scholar]
- 73.Luo J, Kong H, Zhang M, Cheng J, Sun Z, Xiong W, et al. Novel Carbon Dots-Derived from Radix Puerariae Carbonisata Significantly Improve the Solubility and Bioavailability of Baicalin. J Biomed Nanotechnol. 2019;15:151–161. doi: 10.1166/jbn.2019.2675. [DOI] [PubMed] [Google Scholar]
- 74.Cavalcante GC, Schaan AP, Cabral GF, Santana-da-Silva MN, Pinto P, Vidal AF, et al. A Cell’s fate: An overview of the molecular biology and genetics of apoptosis. Int J Mol Sci. 2019:20. doi: 10.3390/ijms20174133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kiraz Y, Adan A, Kartal Yandim M, Baran Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol. 2016;37:8471–8486. doi: 10.1007/s13277-016-5035-9. [DOI] [PubMed] [Google Scholar]
- 76.Liu G, Pei F, Yang F, Li L, Amin AD, Liu S, et al. Role of autophagy and apoptosis in non-small-cell lung cancer. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gong WY, Zhao ZX, Liu BJ, Lu LW, Dong JC. Exploring the chemopreventive properties and perspectives of baicalin and its aglycone baicalein in solid tumors. Eur J Med Chem. 2017;126:844–852. doi: 10.1016/j.ejmech.2016.11.058. [DOI] [PubMed] [Google Scholar]
- 78.Chang HT, Chou CT, Kuo DH, Shieh P, Jan CR, Liang WZ. The mechanism of Ca2+ movement in the involvement of baicalein-induced cytotoxicity in ZR-75-1 human breast cancer cells. J Nat Prod. 2015;78:1624–1634. doi: 10.1021/acs.jnatprod.5b00173. [DOI] [PubMed] [Google Scholar]
- 79.Lin YT, Yang JS, Lin HJ, Tan TW, Tang NY, Chaing JH, et al. Baicalein induces apoptosis in SCC-4 human tongue cancer cells via a Ca2+-dependent mitochondrial pathway. In Vivo. 2007;21:1053–1058. [PubMed] [Google Scholar]
- 80.Lee JH, Li YC, Ip SW, Hsu SC, Chang NW, Tang NY, et al. The role of Ca2+ in baicalein-induced apoptosis in human breast MDA-MB-231 cancer cells through mitochondria- and caspase-3-dependent pathway. Anticancer Res. 2008;28:1701–1711. [PubMed] [Google Scholar]
- 81.Mu J, Liu T, Jiang L, Wu X, Cao Y, Li M, et al. The traditional chinese medicine baicalein potently inhibits gastric cancer cells. J Cancer. 2016;7:453–461. doi: 10.7150/jca.13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang CZ, Zhang CF, Chen L, Anderson S, Lu F, Yuan CS. Colon cancer chemopreventive effects of baicalein, an active enteric microbiome metabolite from baicalin. Int J Oncol. 2015;47:1749–1758. doi: 10.3892/ijo.2015.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takahashi H, Chen MC, Pham H, Angst E, King JC, Park J, et al. Baicalein, a component of Scutellaria baicalensis, induces apoptosis by Mcl-1 down-regulation in human pancreatic cancer cells. Biochim Biophys Acta. 2011;1813:1465–1474. doi: 10.1016/j.bbamcr.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li X, Zou K, Gou J, Du Q, Li D, He X, et al. Effect of baicalin-copper on the induction of apoptosis in human hepatoblastoma cancer HepG2 cells. Med Oncol. 2015;32 doi: 10.1007/s12032-015-0527-9. [DOI] [PubMed] [Google Scholar]
- 85.Zhou QM, Wang S, Zhang H, Lu YY, Wang XF, Motoo Y, et al. The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol Sin. 2009;30:1648–1658. doi: 10.1038/aps.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang RR, Zhang S, Qi JA, Wang ZD, Li J, Liu PJ, et al. Preferential inhibition of hepatocellular carcinoma by the flavonoid Baicalein through blocking MEK-ERK signaling. Int J Oncol. 2012;41:969–978. doi: 10.3892/ijo.2012.1510. [DOI] [PubMed] [Google Scholar]
- 87.Xu XM, Yuan GJ, Deng JJ, Guo HT, Xiang M, Yang F, et al. Inhibition of 12-lipoxygenase reduces proliferation and induces apoptosis of hepatocellular carcinoma cells in vitro and in vivo. Hepatobiliary Pancreat Dis Int. 2012;11:193–202. doi: 10.1016/s1499-3872(12)60147-7. [DOI] [PubMed] [Google Scholar]
- 88.Taniguchi H, Yoshida T, Horinaka M, Yasuda T, Goda AE, Konishi M, et al. Baicalein overcomes tumor necrosis factor-related apoptosis-inducing ligand resistance via two different cell-specific pathways in cancer cells but not in normal cells. Cancer Res. 2008;68:8918–8927. doi: 10.1158/0008-5472.CAN-08-1120. [DOI] [PubMed] [Google Scholar]
- 89.Ren X, Zhang Z, Tian J, Wang H, Song G, Guo Q, et al. The down-regulation of c-Myc and its target gene hTERT is associated with the antiproliferative effects of baicalin on HL-60 cells. Oncol Lett. 2017;14:6833–6840. doi: 10.3892/ol.2017.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Massague J, Batlle E, Gomis RR. Understanding the molecular mechanisms driving metastasis. Mol Oncol. 2017;11:3–4. doi: 10.1002/1878-0261.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chung H, Choi HS, Seo EK, Kang DH, Oh ES. Baicalin and baicalein inhibit transforming growth factor-beta1-mediated epithelial-mesenchymal transition in human breast epithelial cells. Biochem Biophys Res Commun. 2015;458:707–713. doi: 10.1016/j.bbrc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 93.Chen F, Zhuang M, Peng J, Wang X, Huang T, Li S, et al. Baicalein inhibits migration and invasion of gastric cancer cells through suppression of the TGF-beta signaling pathway. Mol Med Rep. 2014;10:1999–2003. doi: 10.3892/mmr.2014.2452. [DOI] [PubMed] [Google Scholar]
- 94.Ma X, Yan W, Dai Z, Gao X, Ma Y, Xu Q, et al. Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via down-regulation of SATB1 and Wnt/beta-catenin pathway. Drug Des Devel Ther. 2016;10:1419–1441. doi: 10.2147/DDDT.S102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao XY, Xue XH, Ma YN, Zhang SQ. Effect of baicalein on the expression of SATB1 in human breast cancer cells. Exp Ther Med. 2015;9:1665–1669. doi: 10.3892/etm.2015.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen K, Zhang S, Ji Y, Li J, An P, Ren H, et al. Baicalein inhibits the invasion and metastatic capabilities of hepatocellular carcinoma cells via down-regulation of the ERK pathway. PLoS One. 2013;8:e72927. doi: 10.1371/journal.pone.0072927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chiu YW, Lin TH, Huang WS, Teng CY, Liou YS, Kuo WH, et al. Baicalein inhibits the migration and invasive properties of human hepatoma cells. Toxicol Appl Pharmacol. 2011;255:316–326. doi: 10.1016/j.taap.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 98.Gong WY, Wu JF, Liu BJ, Zhang HY, Cao YX, Sun J, et al. Flavonoid components in Scutellaria baicalensis inhibit nicotine-induced proliferation, metastasis and lung cancer-associated inflammation in vitro. Int J Oncol. 2014;44:1561–1570. doi: 10.3892/ijo.2014.2320. [DOI] [PubMed] [Google Scholar]
- 99.Rui X, Yan XI, Zhang K. Baicalein inhibits the migration and invasion of colorectal cancer cells via suppression of the AKT signaling pathway. Oncol Lett. 2016;11:685–688. doi: 10.3892/ol.2015.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang L, Ling Y, Chen Y, Li CL, Feng F, You QD, et al. Flavonoid baicalein suppresses adhesion, migration and invasion of MDA-MB-231 human breast cancer cells. Cancer Lett. 2010;297:42–48. doi: 10.1016/j.canlet.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 101.Wang XF, Zhou QM, Du J, Zhang H, Lu YY, Su SB. Baicalin suppresses migration, invasion and metastasis of breast cancer via p38MAPK signaling pathway. Anticancer Agents Med Chem. 2013;13:923–931. doi: 10.2174/18715206113139990143. [DOI] [PubMed] [Google Scholar]
- 102.Yan H, Xin S, Wang H, Ma J, Zhang H, Wei H. Baicalein inhibits MMP-2 expression in human ovarian cancer cells by suppressing the p38 MAPK-dependent NF-kappaB signaling pathway. Anticancer Drugs. 2015;26:649–656. doi: 10.1097/CAD.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 103.Yan X, Rui X, Zhang K. Baicalein inhibits the invasion of gastric cancer cells by suppressing the activity of the p38 signaling pathway. Oncol Rep. 2015;33:737–743. doi: 10.3892/or.2014.3669. [DOI] [PubMed] [Google Scholar]
- 104.Guo Z, Hu X, Xing Z, Xing R, Lv R, Cheng X, et al. Baicalein inhibits prostate cancer cell growth and metastasis via the caveolin-1/AKT/mTOR pathway. Mol Cell Biochem. 2015;406:111–119. doi: 10.1007/s11010-015-2429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shang D, Li Z, Zhu Z, Chen H, Zhao L, Wang X, et al. Baicalein suppresses 17-beta-estradiol-induced migration, adhesion and invasion of breast cancer cells via the G protein-coupled receptor 30 signaling pathway. Oncol Rep. 2015;33:2077–2085. doi: 10.3892/or.2015.3786. [DOI] [PubMed] [Google Scholar]
- 106.Wu B, Li J, Huang D, Wang W, Chen Y, Liao Y, et al. Baicalein mediates inhibition of migration and invasiveness of skin carcinoma through Ezrin in A431 cells. BMC Cancer. 2011;11:527. doi: 10.1186/1471-2407-11-527. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 107.Liu TY, Gong W, Tan ZJ, Lu W, Wu XS, Weng H, et al. Baicalein inhibits progression of gallbladder cancer cells by down-regulating ZFX. PLoS One. 2015;10:e0114851. doi: 10.1371/journal.pone.0114851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim SD, Lee YJ, Baik JS, Han JY, Lee CG, Heo K, et al. Baicalein inhibits agonist- and tumor cell-induced platelet aggregation while suppressing pulmonary tumor metastasis via cAMP-mediated VASP phosphorylation along with impaired MAPKs and PI3K-Akt activation. Biochem Pharmacol. 2014;92:251–265. doi: 10.1016/j.bcp.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 109.Liu JJ, Huang TS, Cheng WF, Lu FJ. Baicalein and baicalin are potent inhibitors of angiogenesis: Inhibition of endothelial cell proliferation, migration and differentiation. Int J Cancer. 2003;106:559–565. doi: 10.1002/ijc.11267. [DOI] [PubMed] [Google Scholar]
- 110.Miocinovic R, McCabe NP, Keck RW, Jankun J, Hampton JA, Selman SH. In vivo and in vitro effect of baicalein on human prostate cancer cells. Int J Oncol. 2005;26:241–246. [PubMed] [Google Scholar]
- 111.Nie D, Krishnamoorthy S, Jin R, Tang K, Chen Y, Qiao Y, et al. Mechanisms regulating tumor angiogenesis by 12-lipoxygenase in prostate cancer cells. J Biol Chem. 2006;281:18601–18609. doi: 10.1074/jbc.M601887200. [DOI] [PubMed] [Google Scholar]
- 112.Du G, Han G, Zhang S, Lin H, Wu X, Wang M, et al. Baicalin suppresses lung carcinoma and lung metastasis by SOD mimic and HIF-1alpha inhibition. Eur J Pharmacol. 2010;630:121–130. doi: 10.1016/j.ejphar.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 113.Gade S, Gandhi NM. Baicalein inhibits MCF-7 cell proliferation in vitro, induces radiosensitivity, and inhibits hypoxia inducible factor. J Environ Pathol Toxicol Oncol. 2015;34:299–308. doi: 10.1615/jenvironpatholtoxicoloncol.2015013806. [DOI] [PubMed] [Google Scholar]
- 114.Agarwal S, Achari C, Praveen D, Roy KR, Reddy GV, Reddanna P. Inhibition of 12-LOX and COX-2 reduces the proliferation of human epidermoid carcinoma cells (A431) by modulating the ERK and PI3K-Akt signalling pathways. Exp Dermatol. 2009;18:939–946. doi: 10.1111/j.1600-0625.2009.00874.x. [DOI] [PubMed] [Google Scholar]
- 115.Bednar W, Holzmann K, Marian B. Assessing 12(S)-lipoxygenase inhibitory activity using colorectal cancer cells overexpressing the enzyme. Food Chem Toxicol. 2007;45:508–514. doi: 10.1016/j.fct.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 116.Cathcart MC, Useckaite Z, Drakeford C, Semik V, Lysaght J, Gately K, et al. Anti-cancer effects of baicalein in non-small cell lung cancer in-vitro and in-vivo. BMC Cancer. 2016;16:707. doi: 10.1186/s12885-016-2740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leung HW, Yang WH, Lai MY, Lin CJ, Lee HZ. Inhibition of 12-lipoxygenase during baicalein-induced human lung nonsmall carcinoma H460 cell apoptosis. Food Chem Toxicol. 2007;45:403–411. doi: 10.1016/j.fct.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 118.Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye WC, et al. Autophagy and multidrug resistance in cancer. Chin J Cancer. 2017;36:52. doi: 10.1186/s40880-017-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu L, Chen Y, Tooze SA. Autophagy pathway: Cellular and molecular mechanisms. Autophagy. 2018;14:207–215. doi: 10.1080/15548627.2017.1378838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Amaravadi R, Kimmelman AC, White E. Recent insights into the function of autophagy in cancer. Genes Dev. 2016;30:1913–1930. doi: 10.1101/gad.287524.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Poillet-Perez L, White E. Role of tumor and host autophagy in cancer metabolism. Genes Dev. 2019;33:610–619. doi: 10.1101/gad.325514.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang X, Tang X, Liu H, Li L, Hou Q, Gao J. Autophagy induced by baicalin involves down-regulation of CD147 in SMMC-7721 cells in vitro. Oncol Rep. 2012;27:1128–1134. doi: 10.3892/or.2011.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aryal P, Kim K, Park PH, Ham S, Cho J, Song K. Baicalein induces autophagic cell death through AMPK/ULK1 activation and down-regulation of mTORC1 complex components in human cancer cells. FEBS J. 2014;281:4644–4658. doi: 10.1111/febs.12969. [DOI] [PubMed] [Google Scholar]
- 124.Deng X, Liu J, Liu L, Sun X, Huang J, Dong J. Drp1-mediated mitochondrial fission contributes to baicalein-induced apoptosis and autophagy in lung cancer via activation of AMPK signaling pathway. Int J Biol Sci. 2020;16:1403–1416. doi: 10.7150/ijbs.41768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin C, Tsai SC, Tseng MT, Peng SF, Kuo SC, Lin MW, et al. AKT serine/threonine protein kinase modulates baicalin-triggered autophagy in human bladder cancer T24 cells. Int J Oncol. 2013;42:993–1000. doi: 10.3892/ijo.2013.1791. [DOI] [PubMed] [Google Scholar]
- 126.Tan HY, Wang N, Man K, Tsao SW, Che CM, Feng Y. Autophagy-induced RelB/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin. Cell Death Dis. 2015;6:e1942. doi: 10.1038/cddis.2015.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang YF, Li T, Tang ZH, Chang LL, Zhu H, Chen XP, et al. Baicalein triggers autophagy and inhibits the protein kinase B/mammalian target of rapamycin pathway in hepatocellular carcinoma HepG2 cells. Phytother Res. 2015;29:674–679. doi: 10.1002/ptr.5298. [DOI] [PubMed] [Google Scholar]
- 128.Wang Z, Jiang C, Chen W, Zhang G, Luo D, Cao Y, et al. Baicalein induces apoptosis and autophagy via endoplasmic reticulum stress in hepatocellular carcinoma cells. Biomed Res Int. 2014;2014:732516. doi: 10.1155/2014/732516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gao J, Morgan WA, Sanchez-Medina A, Corcoran O. The ethanol extract of Scutellaria baicalensis and the active compounds induce cell cycle arrest and apoptosis including up-regulation of p53 and Bax in human lung cancer cells. Toxicol Appl Pharmacol. 2011;254:221–228. doi: 10.1016/j.taap.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 130.Lee HZ, Leung HW, Lai MY, Wu CH. Baicalein induced cell cycle arrest and apoptosis in human lung squamous carcinoma CH27 cells. Anticancer Res. 2005;25:959–964. [PubMed] [Google Scholar]
- 131.Chen CH, Huang TS, Wong CH, Hong CL, Tsai YH, Liang CC, et al. Synergistic anti-cancer effect of baicalein and silymarin on human hepatoma HepG2 Cells. Food Chem Toxicol. 2009;47:638–644. doi: 10.1016/j.fct.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 132.Chang WH, Chen CH, Lu FJ. Different effects of baicalein, baicalin and wogonin on mitochondrial function, glutathione content and cell cycle progression in human hepatoma cell lines. Planta Med. 2002;68:128–132. doi: 10.1055/s-2002-20246. [DOI] [PubMed] [Google Scholar]
- 133.Wang N, Ren D, Deng S, Yang X. Differential effects of baicalein and its sulfated derivatives in inhibiting proliferation of human breast cancer MCF-7 cells. Chem Biol Interact. 2014;221:99–108. doi: 10.1016/j.cbi.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 134.Zheng YH, Yin LH, Grahn TH, Ye AF, Zhao YR, Zhang QY. Anticancer effects of baicalein on hepatocellular carcinoma cells. Phytother Res. 2014;28:1342–1348. doi: 10.1002/ptr.5135. [DOI] [PubMed] [Google Scholar]
- 135.Cheng YH, Li LA, Lin P, Cheng LC, Hung CH, Chang NW, et al. Baicalein induces G1 arrest in oral cancer cells by enhancing the degradation of cyclin D1 and activating AhR to decrease Rb phosphorylation. Toxicol Appl Pharmacol. 2012;263:360–367. doi: 10.1016/j.taap.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 136.Cogliati S, Lorenzi I, Rigoni G, Caicci F, Soriano ME. Regulation of Mitochondrial Electron Transport Chain Assembly. J Mol Biol. 2018;430:4849–4873. doi: 10.1016/j.jmb.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 137.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Role of cardiolipin in mitochondrial function and dynamics in health and disease: Molecular and Pharmacological Aspects. Cells. 2019:8. doi: 10.3390/cells8070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Signes A, Fernandez-Vizarra E. Assembly of mammalian oxidative phosphorylation complexes I-V and supercomplexes. Essays Biochem. 2018;62:255–270. doi: 10.1042/EBC20170098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Angelova PR, Abramov AY. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic Biol Med. 2016;100:81–85. doi: 10.1016/j.freeradbiomed.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 140.Berry BJ, Trewin AJ, Amitrano AM, Kim M, Wojtovich AP. Use the protonmotive force: mitochondrial uncoupling and reactive oxygen species. J Mol Biol. 2018;430:3873–3891. doi: 10.1016/j.jmb.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta. 2006;1757:553–561. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 142.Raimondi V, Ciccarese F, Ciminale V. Oncogenic pathways and the electron transport chain: a dangeROS liaison. Br J Cancer. 2020;122:168–181. doi: 10.1038/s41416-019-0651-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 144.Singh R, Karakoti AS, Self W, Seal S, Singh S. Redox-sensitive cerium oxide nanoparticles protect human keratinocytes from oxidative stress induced by glutathione depletion. Langmuir. 2016;32:12202–12211. doi: 10.1021/acs.langmuir.6b03022. [DOI] [PubMed] [Google Scholar]
- 145.Green DR, Galluzzi L, Kroemer G. Cell biology. Metabolic control of cell death. Science. 2014;345:1250256. doi: 10.1126/science.1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vakifahmetoglu-Norberg H, Ouchida AT, Norberg E. The role of mitochondria in metabolism and cell death. Biochem Biophys Res Commun. 2017;482:426–431. doi: 10.1016/j.bbrc.2016.11.088. [DOI] [PubMed] [Google Scholar]
- 147.Lee HJ, Noh YH, Lee DY, Kim YS, Kim KY, Chung YH, et al. Baicalein attenuates 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. Eur J Cell Biol. 2005;84:897–905. doi: 10.1016/j.ejcb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 148.Zhang S, Ye J, Dong G. Neuroprotective effect of baicalein on hydrogen peroxide-mediated oxidative stress and mitochondrial dysfunction in PC12 cells. J Mol Neurosci. 2010;40:311–320. doi: 10.1007/s12031-009-9285-5. [DOI] [PubMed] [Google Scholar]
- 149.Liu B, Jian Z, Li Q, Li K, Wang Z, Liu L, et al. Baicalein protects human melanocytes from H2O2-induced apoptosis via inhibiting mitochondria-dependent caspase activation and the p38 MAPK pathway. Free Radic Biol Med. 2012;53:183–193. doi: 10.1016/j.freeradbiomed.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 150.Li XX, He GR, Mu X, Xu B, Tian S, Yu X, et al. Protective effects of baicalein against rotenone-induced neurotoxicity in PC12 cells and isolated rat brain mitochondria. Eur J Pharmacol. 2012;674:227–233. doi: 10.1016/j.ejphar.2011.09.181. [DOI] [PubMed] [Google Scholar]
- 151.Naveenkumar C, Raghunandhakumar S, Asokkumar S, Devaki T. Baicalein abrogates reactive oxygen species (ROS)-mediated mitochondrial dysfunction during experimental pulmonary carcinogenesis in vivo. Basic Clin Pharmacol Toxicol. 2013;112:270–281. doi: 10.1111/bcpt.12025. [DOI] [PubMed] [Google Scholar]
- 152.Waisundara VY, Hsu A, Tan BK, Huang D. Baicalin reduces mitochondrial damage in streptozotocin-induced diabetic Wistar rats. Diabetes Metab Res Rev. 2009;25:671–677. doi: 10.1002/dmrr.1005. [DOI] [PubMed] [Google Scholar]
- 153.Kim SJ, Moon YJ, Lee SM. Protective effects of baicalin against ischemia/reperfusion injury in rat liver. J Nat Prod. 2010;73:2003–2008. doi: 10.1021/np100389z. [DOI] [PubMed] [Google Scholar]
- 154.Lin M, Li L, Li L, Pokhrel G, Qi G, Rong R, et al. The protective effect of baicalin against renal ischemia-reperfusion injury through inhibition of inflammation and apoptosis. BMC Complement Altern Med. 2014;14 doi: 10.1186/1472-6882-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 156.Yan W, Liu J. Effects of Chinese herbal monomers on oxidative phosphorylation and membrane potential in cerebral mitochondria isolated from hypoxia-exposed rats in vitro. Neural Regen Res. 2012;7:2099–2106. doi: 10.3969/j.issn.1673-5374.2012.27.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cui L, Zhang X, Yang R, Liu L, Wang L, Li M, et al. Baicalein is neuroprotective in rat MCAO model: role of 12/15-lipoxygenase, mitogen-activated protein kinase and cytosolic phospholipase A2. Pharmacol Biochem Behav. 2010;96:469–475. doi: 10.1016/j.pbb.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 158.de Oliveira MR, Nabavi SF, Habtemariam S, Erdogan Orhan I, Daglia M, Nabavi SM. The effects of baicalein and baicalin on mitochondrial function and dynamics: A review. Pharmacol Res. 2015;100:296–308. doi: 10.1016/j.phrs.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 159.Liang W, Huang X, Chen W. The effects of baicalin and baicalein on cerebral ischemia: A review. Aging Dis. 2017;8:850–867. doi: 10.14336/AD.2017.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu C, Wu J, Xu K, Cai F, Gu J, Ma L, et al. Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. J Neurochem. 2010;112:1500–1512. doi: 10.1111/j.1471-4159.2009.06561.x. [DOI] [PubMed] [Google Scholar]
- 161.Pallast S, Arai K, Pekcec A, Yigitkanli K, Yu Z, Wang X, et al. Increased nuclear apoptosis-inducing factor after transient focal ischemia: a 12/15-lipoxygenase-dependent organelle damage pathway. J Cereb Blood Flow Metab. 2010;30:1157–1167. doi: 10.1038/jcbfm.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qi Z, Xu Y, Liang Z, Li S, Wang J, Wei Y, et al. Baicalein alters PI3K/Akt/GSK3beta signaling pathway in rats with diabetes-associated cognitive deficits. Int J Clin Exp Med. 2015;8:1993–2000. [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang Z, Cui W, Li G, Yuan S, Xu D, Hoi MP, et al. Baicalein protects against 6-OHDA-induced neurotoxicity through activation of Keap1/Nrf2/HO-1 and involving PKCalpha and PI3K/AKT signaling pathways. J Agric Food Chem. 2012;60:8171–8182. doi: 10.1021/jf301511m. [DOI] [PubMed] [Google Scholar]
- 164.Lai MY, Hsiu SL, Chen CC, Hou YC, Chao PD. Urinary pharmacokinetics of baicalein, wogonin and their glycosides after oral administration of Scutellariae rdix in humans. Biol Pharm Bull. 2003;26:79–83. doi: 10.1248/bpb.26.79. [DOI] [PubMed] [Google Scholar]
- 165.Srinivas NR. Baicalin, an emerging multi-therapeutic agent: pharmacodynamics, pharmacokinetics, and considerations from drug development perspectives. Xenobiotica. 2010;40:357–367. doi: 10.3109/00498251003663724. [DOI] [PubMed] [Google Scholar]
- 166.Morisaki T, Hou XL, Takahashi K, Takahashi K. Baicalin pharmacokinetic profile of absorption process using novel in-vitro model: Cytochrome P450 3A4-induced Caco-2 cell monolayers combined with rat intestinal rinse fluids. J Pharm Pharmacol. 2013;65:1526–1535. doi: 10.1111/jphp.12127. [DOI] [PubMed] [Google Scholar]
- 167.Liu L, Deng YX, Liang Y, Pang XY, Liu XD, Liu YW, et al. Increased oral AUC of baicalin in streptozotocin-induced diabetic rats due to the increased activity of intestinal beta-glucuronidase. Planta Med. 2010;76:70–75. doi: 10.1055/s-0029-1185946. [DOI] [PubMed] [Google Scholar]
- 168.Fan L, Wang JC, Jiang F, Tan ZR, Chen Y, Li Q, et al. Induction of cytochrome P450 2B6 activity by the herbal medicine baicalin as measured by bupropion hydroxylation. Eur J Clin Pharmacol. 2009;65:403–409. doi: 10.1007/s00228-008-0594-3. [DOI] [PubMed] [Google Scholar]
- 169.Jang SI, Kim HJ, Hwang KM, Jekal SJ, Pae HO, Choi BM, et al. Hepatoprotective effect of baicalin, a major flavone from Scutellaria radix, on acetaminophen-induced liver injury in mice. Immunopharmacol Immunotoxicol. 2003;25:585–594. doi: 10.1081/iph-120026443. [DOI] [PubMed] [Google Scholar]
- 170.Ernstgard L, Johanson G, Karlsson AS, Warholm M. Phenotyping of cytochrome P450 2E1 in vitro and in vivo. Curr Drug Metab. 2007;8:493–498. doi: 10.2174/138920007780866843. [DOI] [PubMed] [Google Scholar]
- 171.Mo SL, Liu WF, Chen Y, Luo HB, Sun LB, Chen XW, et al. Ligand- and protein-based modeling studies of the inhibitors of human cytochrome P450 2D6 and a virtual screening for potential inhibitors from the Chinese herbal medicine, Scutellaria baicalensis (Huangqin,Baikal Skullcap) Comb Chem High Throughput Screen. 2012;15:36–80. doi: 10.2174/138620712798280826. [DOI] [PubMed] [Google Scholar]
- 172.Tian X, Cheng ZY, He J, Jia LJ, Qiao HL. Concentration-dependent inhibitory effects of baicalin on the metabolism of dextromethorphan, a dual probe of CYP2D and CYP3A, in rats. Chem Biol Interact. 2013;203:522–529. doi: 10.1016/j.cbi.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 173.Ming J, Zhuoneng L, Guangxun Z. Protective role of flavonoid baicalin from Scutellaria baicalensis in periodontal disease pathogenesis: A literature review. Complement Ther Med. 2018;38:11–18. doi: 10.1016/j.ctim.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 174.Yan WJ, Ma XC, Gao XY, Xue XH, Zhang SQ. Latest research progress in the correlation between baicalein and breast cancer invasion and metastasis. Mol Clin Oncol. 2016;4:472–476. doi: 10.3892/mco.2016.750. [DOI] [PMC free article] [PubMed] [Google Scholar]