Abstract
Objectives
COVID-19 can result in persistent symptoms leaving potential rehabilitation needs unmet. This study aims to describe persistent symptoms and health status of individuals hospitalised for COVID-19 according to the International Classification of Functioning, Disability and Health domains of impairments, limitations in activity, and participation restrictions.
Design
Cross-sectional study consisting in a telephone interview 3 months after hospital discharge.
Setting
This study was conducted during the first peak of the COVID-19 pandemic by the Local Health Authority of Reggio Emilia (Italy).
Participants
Adult individuals discharged from hospital between April and June 2020 after COVID-19. Exclusion criteria: hospitalisation for reasons other than COVID-19, inability to participate in the study, concomitant acute or chronic conditions causing disability.
Primary and secondary outcome measures
We assessed: dyspnoea (Medical Research Council), fatigue (Fatigue Severity Scale), mood disturbances (Hospital Anxiety and Depression Scale), limitations in activity (Barthel Index) and participation restrictions (Reintegration to Normal Living Index). We also collected data on sociodemographic characteristics, health status prior to COVID-19, COVID-related clinical manifestations and hospital care pathway up to discharge, rehabilitation interventions, accidental falls and emergency room access.
Results
149 participants (men, 62%; average age 62 (±11) years) were enrolled, 35 of which (23%) were admitted to the intensive care unit (ICU) while hospitalised. Three months after hospital discharge, nearly half of the participants still suffered from dyspnoea (44%) or fatigue (39%). Almost all individuals (91.2%) recovered a good level of independence in activity of daily living, but 76% still suffered participation restrictions. Female sex was significantly associated with worse outcomes for all symptoms.
Conclusions
Individuals who had moderate or severe COVID-19 may perceive persistent symptoms which may result in reduced social participation. Sex differences should be monitored, as women may recover more slowly than men.
Trial registration number
Keywords: COVID-19, rehabilitation medicine, respiratory infections
Strengths and limitations of this study.
This cross-sectional study investigated the long-term impact of COVID-19 on functional status of patients after hospital discharge.
The telephone interviews collected data of patients discharged from the hospitals of the Local Health Authority of the Province of Reggio Emilia (Italy) only.
To catch postacute sequelae of SARS-COV2 infection, individuals with acute or chronic concomitant conditions causing disability and with previous complete dependence in activities of daily living were excluded.
Eligible individuals were contacted by a letter of invitation and, if necessary, also by phone.
Sociodemographic characteristics, health status prior to COVID-19, data regarding COVID-19-related hospital care and long-term health outcomes were collected.
Introduction
Background
The onset of the COVID-19 pandemic in early 2020 had a tremendous impact on the world population and on healthcare systems, with over 273 million cases worldwide as of 19 December 2021.1 Early reports about surveillance were promptly released, and a tremendous effort was made to increase knowledge of diffusion patterns and prevention strategies. The presenting features of SARS-COV-2 infection have been well described, with a widely accepted categorisation of acute COVID-19 published by the WHO2 and updated regularly. According to the WHO classification of COVID-19, which includes asymptomatic, mild, moderate, severe and critical disease,2 14%–15% of cases have been severe and 5% critical.3 However, for the first months of the pandemic, the long-term impact of the disease remained underexplored.
COVID-19 patients admitted to hospital experience fever, cough, dyspnoea, muscle soreness and/or acute respiratory distress syndrome, but also fatigue, gastrointestinal symptoms and headache.4 While most patients recover quickly, a growing number of studies have highlighted that several survivors of COVID-19 experience a multisystem condition termed postacute sequelae of SARS-CoV-2 infection (PASC) characterised by fatigue, dyspnoea, brain fog, headache, mood disturbances and atypical chest pain.5 These symptoms can last several weeks after the acute phase of the disease and may worsen functioning and quality of life and hinder participation.6–13 Furthermore, in the presence of comorbidities, they may lead to deconditioning, fatigue and social isolation.14
The International Classification of Functioning, Disability and Health (ICF) is a classification of health and health-related domains which measures health and disability at both the individual and population levels.15 To our knowledge, no clinical trial has comprehensively assessed the persistent impact of COVID-19 according to the ICF,15 although this assessment has been recommended to explore the long-term impairments but also limitations in activity and participation restrictions caused by SARS-CoV-2 infection.6 This study aimed to verify whether individuals who had been hospitalised for COVID-19 had unmet rehabilitation needs lasting long beyond recovery.
Objective
This study describes the persistent symptoms and impairments, limitations in activity and restrictions in participation in social activities of those individuals who required hospitalisation for COVID-19. It investigated the associations between sociodemographic characteristics, health status prior to COVID-19, COVID-19-related clinical manifestations and symptoms, and hospital care pathway up to discharge and health outcomes assessed 3 months after hospital discharge.
Methods
Study design and population
This cross-sectional study is reported according to the Strengthening The Reporting of OBservational studies in Epidemiology guidelines.16 The study consisted in a telephone interview of patients hospitalised for COVID-19 during the first peak of the pandemic to collect current and retrospective data. All adult symptomatic individuals, discharged from the hospitals of the Local Health Authority (LHA) of the Province of Reggio Emilia (Italy) between April and June 2020, were screened for eligibility by medical documentation. We excluded individuals who (1) were hospitalised for reasons other than COVID-19; (2) were unable to participate in the study procedures (eg, dementia, psychiatric disorders, linguistic barriers); (3) had acute or chronic concomitant conditions causing disability (eg, recent stroke, surgical interventions, heart failure); (4) had previous complete dependence in activities of daily living (ADLs). We also excluded pregnant women to avoid a confounding effect of pregnancy on symptoms like fatigue or dyspnoea. Due to the concomitant pandemic, it was not possible to involve patients or the public in the design, conduction, reporting or dissemination of this study.
All eligible individuals were sent a letter of invitation to participate in this study, written information about the study, a consent form and the principal investigator’s request for permission for a researcher affiliated with the study to contact the individual by phone. Two weeks after the letter was sent, the potentially eligible individuals were contacted by a researcher, who gave them any further information, and asked that they return the written informed consent to participate in the interview. Individuals who did not answer the phone after three attempts and those who explicitly stated they did not intend to participate in the study were deleted from the list.
We retrospectively collected the following data of each participant:
Sociodemographic characteristics (age, sex, household composition).
Health status prior to COVID-19 (comorbidities, use of aids and level of independence prior to hospitalisation).
Data regarding COVID-19-related hospital care.
Symptoms and clinical manifestation of COVID-19 (eg, cough, fever, diarrhoea, asthenia, localization of pneumonia, respiratory failure).
Admission to the ICU and its duration.
Any rehabilitation treatment during hospitalisation (eg, mobilisation, chest physiotherapy).
Length of stay (LOS).
Three months from hospital discharge, participants were interviewed by telephone to collect data on the persistency of the following symptoms and limitations:
Dyspnoea, assessed by the Medical Research Council.17
Fatigue, assessed by the Fatigue Severity Scale.18
Mood disturbances, assessed by the Hospital Anxiety and Depression Scale (HADS).19
Limitations in basic-ADL (B-ADL), assessed by the Barthel Index.20
Restrictions in participation, assessed by the Reintegration to Normal Living Index (RNLI)21 (Italian version).
Data on any rehabilitation intervention implemented after hospital discharge (type, duration, frequency) and on any accidental falls and related consequences, emergency room access, or any further hospital admissions after hospital discharge were also collected.
Statistical analysis
In absence of an a priori hypothesis, given the exploratory nature of the study, no formal sample size calculation was performed; all eligible individuals who agreed to participate in the study were recruited. Sociodemographic characteristics, health status prior to COVID-19, COVID-19-related clinical manifestations and symptoms, and hospital care pathway up to discharge are reported, as are the data on long-term outcomes of COVID-19. Data are reported as frequency and percentage for categorical variables, mean and SD for symmetric quantitative variables, and median and IQR for skewed variables.
Proportions between groups were compared using the chi-square test or the Fisher’s exact test. Associations between potential exposures and long-term outcomes were investigated using logistic regression models. Similarly, associations between the presence of long-term outcomes of COVID-19 and rehabilitation interventions, accidental falls/fractures, emergency room accesses and/ or any hospital admission in the 3 months following hospital discharge were investigated. Unless otherwise specified, CIs are two tailed and calculated at the 0.95 confidence level. Tests were considered statistically significant when the p value was <0.05. Statistical analysis was performed using R V.3.5.2 R Core Team 2020.22
Patient and public involvement
Due to the concomitant pandemic, it was not possible to involve patients or the public in the design, conduction, reporting or dissemination of this study.
Results
Participants
Between April and June 2020, 784 patients were discharged from the hospitals of the LHA of Reggio Emilia (Italy), which serves a population of 533 158 residents, after being healed from the acute phase of COVID-19. Overall, 446 individuals were excluded for the reasons listed in figure 1; 338 invitations to participate in the study were mailed to potentially eligible individuals, who were contacted by telephone 2 weeks later. Overall, 150 individuals consented to participate, and a telephone appointment for the interview was set up. One individual could not be reached for the interview, and his data were excluded from the analysis. Thus, 149 participants were interviewed between June and September 2020, at an average of 104 days (±18.5) from hospital discharge. Figure 1 reports the flow diagram of the study participants.
Figure 1.
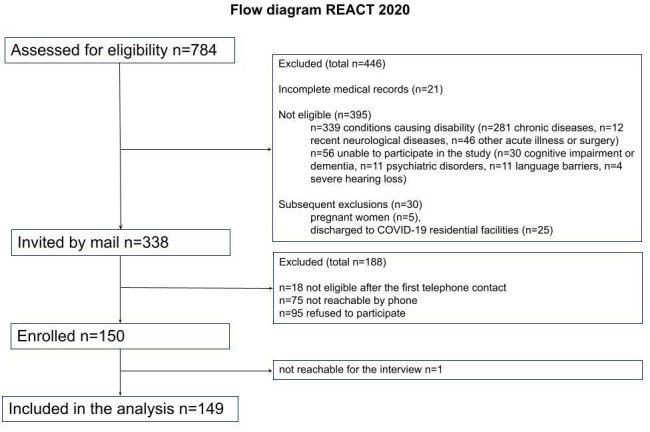
Flow diagram of the study participants.
Descriptive data
The sociodemographic characteristics and health status of study participants are reported in table 1. The average age of the study cohort was 62 (±11) years. Males accounted for 62.4% of the sample, and 51% were employed. Most participants lived with family members (89.3%) and had one or more comorbidities (82.6%), the most frequent being cardiovascular diseases (34.6%), metabolic diseases (15.6%), diabetes (8.7%) and obesity (8%). Before hospitalisation for COVID-19, all but one participant were independent in B-ADL, and only 6% used walking aids for mobility.
Table 1.
Sociodemographic characteristics and health status of the cohort
| Sociodemographic characteristics and health status | Total (N=149) |
| Age, mean (SD) | 62 (±11.5) |
| Sex, N (%) | |
| Male | 93 (62.4) |
| Female | 56 (37.6) |
| Household conditions, N (%) | |
| Alone | 15 (10.0) |
| With others | 133 (89.3) |
| Data missing | 1 (0.7) |
| Occupation, N (%) | |
| Employed | 76 (51.0) |
| Retired | 66 (44.3) |
| Unemployed | 7 (4.7) |
| Smoker, N (%) | |
| Yes | 11 (7.4) |
| No | 92 (61.7) |
| Ex-smoker | 46 (30.9) |
| Comorbidities, N (%) | |
| No | 26 (17.4) |
| Yes | 123 (82.6) |
| N of comorbidities per patient, N (%) | |
| 0 | 26 (17.4) |
| 1 | 43 (28.9) |
| 2 | 39 (26.2) |
| 3 | 23 (15.4) |
| >3 | 18 (12.1) |
| Type of comorbidities, N (%), (Total N=263) | |
| Cardiovascular diseases | 91 (34.6) |
| Metabolic diseases (dyslipidaemia, gout, fatty liver disease, etc) | 41 (15.6) |
| Diabetes | 23 (8.7) |
| Obesity (BMI ≥30) | 21 (8.0) |
| Digestive system diseases | 16 (6.1) |
| Respiratory diseases | 10 (3.8) |
| Haematological diseases | 8 (3.0) |
| Rheumatological diseases | 8 (3.0) |
| Others | 45 (17.1) |
| Independence before hospital admission, N (%) | |
| Yes | 148 (99.3) |
| Minimal assistance for ADL | 1 (0.7) |
| Use of aids before hospital admission, N (%) | |
| Yes | 9 (6.0) |
| No | 140 (94.0) |
ADL, activities of daily living; BMI, body mass index; N, Number.
Table 2 reports data regarding the hospital care of participants, showing ICU admissions and sex-disaggregated data. Thirty-five individuals (23.5%) were admitted to the ICU. Overall, the average LOS was 18 (±14) days, with a higher average LOS for individuals admitted to the ICU (33±20 days). Most participants experienced respiratory failure (83.9%), with 12.1% having documented bilateral pneumonia.
Table 2.
Hospital care of participants and postdischarge period
| Information about patients' hospital care and postdischarge | Sex-disaggregated data | ||||
| Total | ICU | Not-ICU | Male (N=93) | Female (N=56) | |
| Hospital care, N (%) | 149 (100%) | 35 (23.5%) | 114 (76.5%) | ICU 26 (28.0) Not-ICU 67 (72.0) |
ICU 9 (16.1) Not-ICU 47 (83.9) |
| Total LOS, mean (SD) | 18 (±14) | 33 (±20) | 14 (±8) | 18.7 (±13.9) | 17.4 (±15.4) |
| LOS in ICU, mean (SD) | 14 (±11) | 13.2 (±10.8) | 16.1 (±13.8) | ||
| Symptoms at admission, N (%) | |||||
| Respiratory failure | 125 (83.9) | 35 (100) | 90 (78.9) | 80 (86.0) | 46 (82.1) |
| Bilateral pneumonia | 18 (12.1) | 0 (0) | 18 (15.8) | 11 (11.8) | 7 (12.5) |
| Mild symptoms | 4 (2.7) | 0 (0) | 4 (3.5) | 2 (2.2) | 2 (3.6) |
| Other (pulmonary embolism) | 2 (1.3) | 0 (0) | 2 (1.8) | 0 (0) | 1 (1.8) |
| Clinical Category of COVID-19 and type of oxygen support, N (%) | |||||
| Critical COVID-19 (CPAP-NIV-intubation) |
56 (37.6) | 35 (100) | 21 (18.4) | 43 (46.2) | 13 (23.2) |
| Severe COVID-19 (HF oxygen devices) |
61 (40.9) | 0 (0) | 61 (53.6) | 33 (35.5) | 28 (50.0) |
| Moderate COVID-19 (LF oxygen devices) |
16 (10.7) | 0 (0) | 16 (14.0) | 9 (9.7) | 7 (12.5) |
| Mild COVID-19 (no oxygen support) |
16 (10.7) | 0 (0) | 16 (14.0) | 8 (8.6) | 8 (14.3) |
| Rehabilitation during hospitalisation, N (%) | |||||
| No | 128 (85.9) | 17 (48.6) | 111 (97.4) | 81 (87.1) | 47 (83.9) |
| Yes | 21 (14.1) | 18 (51.4) | 3 (2.6) | 12 (12.9) | 9 (16.1) |
| Rehabilitation after discharge, N (%) | |||||
| No | 128 (85.9) | 21 (60.0) | 107 (93.9) | 80 (86.0) | 48 (85.7) |
| Yes | 21 (14.1) | 14 (40.0) | 7 (6.1) | 13 (14.0) | 8 (14.3) |
| Use of aids after discharge, N (%) | |||||
| No | 132 (88.6) | 26 (74.3) | 106 (93.0) | 85 (91.4) | 47 (83.9) |
| Yes | 17 (11.4) | 9 (25.7) | 8 (7.0) | 8 (8.6) | 9 (16.1) |
| Accidental falls after discharge, N (%) | |||||
| No | 139 (93.3) | 32 (91.4) | 107 (93.9) | 88 (94.6) | 51 (91.1) |
| Yes | 10 (6,7) | 3 (8.6) | 7 (6.1) | 5 (5.4) | 5 (8.9) |
CPAP, continuous positive airway pressure; HF, high flow; ICU, intensive care unit; LF, low flow; LOS, length of stay; NIV, non-invasive ventilation.
Inpatient rehabilitation was delivered to 21 individuals, corresponding to 14.1% of the total sample and to 51.4% of participants admitted to the ICU. Early mobilisation was offered to patients in the ICU and to patients hospitalised in acute wards, if they presented severe risk of functional limitations due to frailty or mobility limitations. Inpatient rehabilitation was performed 6 days per week and included pulmonary rehabilitation, mobilisation, exercises and counselling. Also, as soon as patients could self-manage a programme of simple exercise, the physiotherapist gave them instructions and written information to guide them in the execution of breathing exercises, active range of motion exercises and strength training while lying supine or sitting.
Outpatient rehabilitation after hospital discharge was attended by 21 individuals (14.1%), several of whom had been admitted to the ICU (40.0%). Outpatient rehabilitation was provided three times per week at the physical therapy department and consisted in comprehensive pulmonary rehabilitation to improve persistent fatigue, exercise capacity and breathlessness. It included breathing techniques such as pursed lip breathing, positive expiratory pressure-bottle exercises and incentive spirometer. Patients were advised to continue the exercises at home, with individualised home sessions based on their needs (repeating breathing techniques, performing aerobic exercise, balance exercises or resistance training).
Seventeen participants (11.4%) reported using a walking aid for mobility after hospital discharge (wheelchair, walker, stick, crutches). Moreover, accidental falls after hospital discharge were reported by 6.7% of participants, but only one resulted in emergency room access.
Outcome data
Table 3 describes the persistent symptoms, limitations in activity and restrictions in participation 3 months after hospital discharge. Fatigue and dyspnoea were the most prevalent persistent symptoms in the cohort investigated: 87.9% of participants experienced fatigue and 43% suffered from mild to severe dyspnoea. Clinically relevant anxiety and depression scores (HADS ≥8) were detected in 24.8% and 16.1% of participants, respectively.
Table 3.
Persistent symptoms, limitations in activity and restrictions in participation 3 months after hospital discharge
| Outcome | Male (=93) | Female (=56) | Total (=149) |
| Dyspnoea, N (%) | |||
| Absent (MRC=0) | 59 (63.4) | 24 (42.9) | 83 (55.7) |
| Mild (MRC=1) | 26 (28.0) | 17 (30.3) | 43 (28.9) |
| Moderate (MRC 2–3) | 6 (6.4) | 13 (23.2) | 19 (12.8) |
| Severe (MRC=4) | 1 (1.1) | 1 (1.8) | 2 (1.3) |
| Data missing* | 1 (1.1) | 1 (1.8) | 2 (1.3) |
| Fatigue, n (%) | |||
| Absent (FSS=9) | 13 (14.0) | 3 (5.4) | 16 (10.7) |
| Mild-moderate (FSS 10–36) | 54 (58.0) | 19 (33.9) | 73 (49.0) |
| Severe (FSS >36) | 25 (26.9) | 33 (58.9) | 58 (38.9) |
| Data missing* | 1 (1.1) | 1 (1.8) | 2 (1.3) |
| Anxiety, N (%) | |||
| No (HADS-a <8) | 76 (81.7) | 35 (62,5) | 111 (74.5) |
| Yes (HADS-a ≥8) | 16 (17.2) | 21 (37.5) | 37 (24.8) |
| Data missing* | 1 (1.1) | 0 (0.0) | 1 (0.7) |
| Depression, N (%) | |||
| No (HADS-d <8) | 84 (90.3) | 40 (71.4) | 124 (83.2) |
| Yes (HADS-d ≥8) | 8 (8.6) | 16 (28.6) | 24 (16.1) |
| Data missing* | 1 (1.1) | 0 (0.0) | 1 (0.7) |
| Limitation in B-ADL, N (%) | |||
| Independent (BI=100) | 88 (94.6) | 48 (85.7) | 136 (91.3) |
| Mild dependence (BI 91–99) | 2 (2.2) | 5 (8.9) | 7 (4.7) |
| Moderate dependence (BI 61–90) | 2 (2.2) | 2 (3.6) | 4 (2.7) |
| Severe dependence (BI 21–60) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Complete dependence (BI 0–20) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Data missing* | 1 (1.1) | 1 (1.8) | 2 (1.3) |
| Participation, N (%) | |||
| Complete reintegration (RNLI=100) | 32 (34.4) | 4 (7.1) | 36 (24.2) |
| Reduced reintegration (RNLI 60–99) | 55 (59.1) | 45 (80.4) | 100 (67.1) |
| Poor reintegration (RNLI <60) | 5 (5.4) | 7 (12.5) | 12 (8.0) |
| Data missing* | 1 (1.1) | 0 (0.0) | 1 (0.7) |
*Impossibility of administering the assessments due to difficulties in understanding the questions during the phone call on behalf of the participant.
B-ADL, basic activities of daily living; BI, Barthel Index; FSS, Fatigue Severity Scale; HADS-a, Hospital Anxiety and Depression Scale-anxiety; HADS-d, Hospital Anxiety and Depression Scale-depression; MRC, Medical Research Council; RNLI, Reintegration to Normal Living Index.
Most of the sample (91.3%) was completely independent, with only a few individuals (11) reporting need for assistance in B-ADL. Nevertheless, 3 months after discharge, only 24.2% of participants were completely reintegrated, while 75.1% reported moderate (RNLI 60–99) or even severe (RNLI <60) restrictions in participation (67.1% and 8.0%, respectively).
Table 4 shows the ORs of the associations between potential exposures and outcomes 3 months after discharge. Increasing age seemed to be associated with less anxiety (OR 0.94, p=0.006), as each year of age seemed to reduce the risk by about 5%. Similar results were detected for depression (OR 0.95, p=0.036).
Table 4.
Associations between potential exposures and outcomes 3 months after discharge
| Risk factors | Dyspnoea OR (CI) (p value) |
Fatigue OR (CI) (p value) |
Anxiety OR (CI) (p value) |
Depression OR (CI) (p value) |
Dependence in B-ADL OR (CI) (p value) |
Reintegration OR (CI) (p value) |
| Age | 1.00 (0.96 to 1.05) p=0.806 | 0.97 (0.93 to 1.00) p=0.087 | 0.94 (0.90 to 0.98) p=0.006* | 0.95 (0.90 to 0.99) p=0.036* | 1.05 (0.99 to 1.12) p=(0.119) | 0.95 (0.88 to 1.00) p=0.102 |
| Female sex | 3.61 (1.26 to 11.26) p=0.019* | 3.75 (1.75 to 8.26) p<0.001* | 3.26 (1.40 to 7.81) p=0.007* | 3.71 (1.39 to 10.69) p=0.011* | 3.18 (0.90 to 12.79) p=0.078 | 2.59 (0.70 to 10.66) p=0.157 |
| Several comorbidities (>3) | 1.03 (0.20 to 4.26) p=0.970 | 0.92 (0.29 to 1.47) p=0.883 | 1.26 (0.34 to 4.34) p=0.709 | 0.30 (0.01 to 1.89) p=0.281 | 0.57 (0.02 to 4.24) p=0.630 | 2.66 (0.45 to 15.85) p=0.260 |
| Diabetes | 1.57 (0.40 to 5.09) p=0.471 | 0.98 (0.37 to 2.48) p=0.965 | 0.88 (0.26 to 2.49) p=0.823 | 0.45 (0.06 to 1.76) p=0.317 | 3.12(0-75-11.57)p=0.094 | 0.48 (0.02 to 2.77) p=0.499 |
| Cardiovascular diseases | 1.80 (0.54 to 8.23) p=0.380 | 0.73 (0.32 to 1.66) p=0.458 | 0.62 (0.25 to 1.58) p=0.311 | 0.79 (0.28 to 2.44) p=0.675 | 0.60 (0.16 to 2.42) p=0.438 | 1.46 (0.34 to 10.06) p=0.642 |
| Obesity (BMI ≥30) | 1.57 (0.40 to 5.09) p=0.471 | 1.36 (0.52 to 3.53) p=0.520 | 0.67 (0.18 to 2.03) p=0.519 | 0.82 (1.17 to 2.78) p=0.775 | 1.06 (0.15 to 4.55) p=0.940 | 2.23 (0.45 to 8.91) p=0.274 |
| Critical or severe COVID-19 | 0.80 (0.26 to 2.28) p=0.691 | 0.70 (0.32 to 1.48) p=0.360 | 0.29 (0.10 to 0.75) p=0.016* | 0.33 (0.90 to 0.97) p=0.062 | 1.29 (0.35 to 4.56) p=0.681 | 1.03 (0.25 to 3.81) p=0.965 |
| Use of walking aids after discharge | 3.52 (0.97 to 11.62) p=0.042* | 2.38 (0.79 to 7.56) p=0.124 | 2.05 (0.64 to 6.12) p=0.205 | 0.69 (0.10 to 2.79) p=0.653 | 2.79 (0.56 to 11.14) p=0.164 | 0.71 (0.03 to 4.24) p=0.762 |
| Accidental falls after discharge | 5.02 (1.16 to 20.10) p=0.023* | 2.28 (0.61 to 9.37) p=0.220 | 3.48 (0.90 to 13.46) p=0.063 | 2.39 (0.48 to 9.58) p=0.237 | 5.51 (1.04 to 24.56) p=0.029* | 1.27 (0.06 to 8.00) p=0.829 |
| Rehabilitation during hospitalisation | 3.01 (0.59 to 13.69) p=0.158 |
3.40 (0.97 to 13.89) p=0.064 | 0.43 (0.07 to 1.86) p=0.298 | 0.64 (0.07 to 3.40) p=0.639 | 4.12 (0.64 to 22.75) p=0.114 | 0.66 (0.02 to 5.60) p=0.751 |
*Statistically significant.
B-ADL, basic activities of daily living; BMI, body mass index.
Being female was associated with persistent symptoms after COVID-19: 3 months after hospital discharge, 25% of females vs 7.5% of males suffered from dyspnoea (OR 3.61, p=0.019), 59% of females vs 27% of males suffered from fatigue (OR 3.75, p<0.001), 37.5% of females vs 17% of males suffered from anxiety (OR 3.26, p=0.007), and 28.5% of females vs 8.6% of males suffered from depression (3.71, p=0.011); although not significantly, limitations in B-ADL were also more reported in females (14% vs 5.3%; OR 3.18, p=0.078).
Surprisingly, comorbidities were not associated with worse outcomes.
Dyspnoea was more frequently reported by participants who used walking aids for mobility after discharge (OR 3.52, p=0.042) and by those who experienced an accidental fall (OR 5.02, p=0.023).
Moreover, having had critical or severe COVID-19 was associated with a 70% reduction in the risk of anxiety (OR 0.29, p=0.016) and in the risk of depression, bordering on significance (OR 0.33, p=0.062).
Finally, accidental falls occurring after hospital discharge were associated with a fivefold increase in the risk of dyspnoea (OR 5.02, p=0.032) and dependence in B-ADL (OR 5.51, p=0.029).
Discussion
Statement of principal findings
This study focused on the medium-term impact of COVID-19 on functional status of those individuals who were severely affected by this disease. Three months after hospital discharge for COVID-19, individuals still reported moderate to severe fatigue (88%) and dyspnoea (44%). They recovered a good level of independence in basic ADL, but 76% still suffered participation restrictions. Females showed higher levels of fatigue, dyspnoea, anxiety and depression. Thus, these results confirm that individuals hospitalised experience persistent symptoms, adding insight into the impact of COVID-19 on limitations in activities and participation.
As millions of individuals are recovering from the infection, it may be appropriate to recognise those in need of rehabilitation, to help them to recover complete function and previous levels of participation.
Accordingly, the WHO recommends screening COVID-19 patients before hospital discharge to detect any rehabilitation needs they may have.2 Reasonably, in the first few months after the outbreak of the pandemic, the very few studies published on the rehabilitation of patients with COVID-19 focused on treatment during the acute phase23 24 or on the implications for healthcare organisations.14 25 In December 2020, a rapid guideline on the management of the long-term outcomes of COVID-19 was published by the National Institute for Health and Care Excellence, which recommended a careful evaluation of symptoms, but also an overall assessment of the impact of the disease on daily life, including B-ADL, occupations and social activities.26 Recently, the WHO has published a new version of a living clinical guidance,2 updating both the symptoms persisting after COVID-19 and the recommendations for rehabilitation needs assessment.27 Moreover, in October 2021, the WHO coined the definition of ‘post COVID-19 condition’ to describe the condition of ‘individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19, with symptoms lasting for at least 2 months, which cannot be explained by an alternative diagnosis. Common symptoms include fatigue, shortness of breath, cognitive dysfunction among other and generally have an impact on everyday functioning’.28
Our study explored all the dimensions of health status by means of valid tools to assess symptoms, independence in B-ADL and reintegration to normal living. The data collected seem to confirm that the likelihood of developing PASC is not linked to the severity of disease, and also confirm that fatigue and dyspnoea are among the most frequent and persistent symptoms, as reported by some authors in the last months of 2020,8 9 but also by more recent studies.29–35
Moreover, in the cohort investigated, clinically relevant anxiety and depression characterised 25% and 16% of participants, respectively, which are proportions very close to those reported in a similar French cohort12 and in a German cross-sectional study by Lemhöfer et al.13 Certainly, mood disorders can also be caused by the extraordinary nature of the pandemic, which has literally affected the entire planet. In fact, a study conducted on the healthy population living in the same area as the cohort investigated showed that, during the first peak of pandemic, mood disturbances were present in 13.6%–54.5% of individuals.30 Thus, regardless of their triggers, the prevalence of anxiety and depression during the pandemic seems higher than the usual estimate (10%–11%).31
Interestingly, despite the large number of patients who claimed complete postdischarge independence in B-ADL (91.3%), 76% did not recover full social participation 3 months after hospital discharge. Although data were collected during the summer, when the SARS-CoV-2 contagion was low and the restrictions imposed were minimal, we cannot exclude that at least part of those limitations in social participation may have been due to the remaining restrictions or to the fear of contracting the disease again. Whatever the cause or the mix of causes, this finding should not be underestimated, given that social participation is a domain of health and an indicator of successful ageing. In fact, where post-COVID-19 clinics have been activated, the accurate assessment of limitations in B-ADL and social participation is considered important by clinicians.32
Social participation is one of the goals of rehabilitation interventions. However, during the first pandemic peak, rehabilitation was delivered to a limited number of COVID-19 patients, and, in our cohort, daily inpatient rehabilitation was mainly provided to patients admitted to an ICU; outpatient rehabilitation was offered to a small number of individuals. Focusing inpatient rehabilitation mainly on ICU patients was reasonable during the first wave of the pandemic, given that the long-term impact of COVID-19 was not known at the time, and directing all resources to the care of individuals struggling with severe or critical COVID-19 seemed appropriate, in the attempt to prevent the onset of postintensive care syndromes, which affect up to 50% of ICU patients.36
This may explain why our data do not show a significant association between rehabilitation interventions and any of the health outcomes assessed 3 months after hospital discharge. Rehabilitation was delivered to more severe patients, supporting them in recovering a level of activity and participation similar to that of individuals with mild or moderate COVID-19, who were generally not referred to rehabilitation. Moreover, outpatient rehabilitation was offered three times per week only to patients with severe persistent dyspnoea or fatigue, as rearranging health pathways during the early months of the pandemic in Italy was extremely complex.14
Taking into account the growing number of people affected by long-lasting consequences of COVID-19, outpatient rehabilitation is likely to represent a key element to support their recovery, as reported in a recent German survey,37 and it is extremely important to expand outpatient therapeutic options to alleviate PASC and to hasten the return to normal life and working capacity.
The most interesting finding of this study is that it seems that the long-term impact of COVID-19 is worse on women. Since the very first months of the pandemic, the need for sex-disaggregated data was advocated by researchers,38–40 and the role of sex in the early immune response after SARS-CoV-2 infection and in mortality has been highlighted.41 42 While mortality rate for COVID-19 seems higher in men with comorbidities,43 our results, consistent with those of other research studies,33 44suggest that women may be more affected by COVID-19 sequelae several weeks after hospital discharge. Although no clear pathophysiology can explain this phenomenon, it has been hypothesised that the higher representation of women in autoimmune diseases may explain the sex differences in the immunological response to the acute and postacute manifestations of COVID-19.35 45
Strengths and weaknesses of the study
The results of this cross-sectional study should be interpreted with caution, since they originate from a single Italian province. Recruitment bias cannot be ruled out, as several individuals who were invited to participate did not adhere to the study (23% of those eligible) or could never be reached by phone (29%). Thus, it may be that individuals who were asymptomatic or those who still felt too unwell declined to participate. Moreover, for feasibility reasons, we chose to investigate only the most frequent persistent symptoms associated with PASC (dyspnoea and fatigue). Nevertheless, several others, including musculoskeletal pain, mood disturbances and cognitive deficits, among others, may also lead to the need for rehabilitation. Since this study was uncontrolled, we cannot exclude that some of the persistent symptoms and manifestations may have been due to the prolonged hospitalisation or to post-ICU syndrome, or that they might also affect the general population (eg, anxiety, participation restrictions) due to the containment measures imposed by the Italian government. Causal inferencing and generalisation of the conclusions are therefore challenging.
One strength of this study is that the ICF framework was used to guide data collection, and the assessment of health status extended beyond impairment. Moreover, a valid assessment of outcomes allowed us to confirm differences between the sexes in PASC, and, although further exploration is required, these data suggest that female COVID-19 survivors may need specific follow-up to ensure appropriate interventions34 and equity in access to care.
Unanswered questions and future research
After hospital discharge, differences between the sexes emerged in the long-term impact of COVID-19 in this Italian study. These differences should be searched and considered in future research. Future studies should investigate if tailored rehabilitation is offered and if equity is warranted in access to care.
Conclusions
Examining the long-term impact of COVID-19 is essential, given that the number of recovering individuals is growing daily. Healthcare services must implement the best-practice standards of care for individuals with PASC. The results of this study indicate that women may recover more slowly than men. If confirmed, this information may prevent gender inequalities in accessing health services and facilitate appropriate referral to tailored rehabilitation.
Supplementary Material
Acknowledgments
The authors thank Jacqueline M. Costa for the English language editing.
Footnotes
Contributors: As dictated by the Authorship guidelines of the International Committee of Medical Journal Editors, SF, MD, CM, MAA, GB, DG, AB, OE, CG, MS, LB and SC gave substantial contributions to the conception or design of the work or to the acquisition, analysis, or interpretation of data for the work; and gave substantial contributions to the drafting the work or to its critical revision for important intellectual content; and approved the final version to be published. All the authors agreed to be accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This manuscript was completely written by its authors and reviewed in kind contribution for English language by an editor. The authors did not make use of medical writers. SF, as the guarantor, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. LB conducted and is responsible for the data analysis. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. Reasonable requests for all of the individual participant data collected during the trial, after deidentification, should be made to the corresponding author and will be considered by the REACT lead author. The presented data are anonymised and risk of identification is low.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This independent study was approved by Provincial Ethics Committee of Reggio Emilia on 21/04/2020 (ID 2020/0133).
References
- 1.World Health Organisation . Weekly epidemiological update on COVID-19 – 21 December 2021, 2021. Available: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-21-december-2021 [Accessed Dec 27, 2021].
- 2.World Health Organisation . COVID-19 clinical management: living guidance, 2021. Available: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 [Accessed Jan 9, 2022].
- 3.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 4.Hatmi ZN. A Systematic Review of Systematic Reviews on the COVID-19 Pandemic [published online ahead of print, 2021 Jan 26]. SN Compr Clin Med 2021:1–18. 10.1007/s42399-021-00749-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins FS. NIH launches new initiative to study “Long COVID.” National Institutes of Health, 2021. Available: https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid [Accessed March 30, 2021].
- 6.Maxwell E. Living with Covid19: a dynamic review of the evidence around ongoing Covid19 symptoms (often called long Covid). National Institute for health research, 2020. Available: https://evidence.nihr.ac.uk/themedreview/living-with-covid19/ [Accessed March 30, 2021].
- 7.Garrigues E, Janvier P, Kherabi Y, et al. Post-Discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 2020;81:e4–6. 10.1016/j.jinf.2020.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goërtz YMJ, Van Herck M, Delbressine JM, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res 2020;6:00542–2020. 10.1183/23120541.00542-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Borst B, Peters JB, Brink M. Comprehensive health assessment three months after recovery from acute COVID-19 [published online ahead of print, 2020 Nov 21]. Clin Infect Dis 2020:ciaa1750. 10.1093/cid/ciaa1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Townsend L, Dyer AH, Jones K, et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One 2020;15:e0240784. 10.1371/journal.pone.0240784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carfì A, Bernabei R, Landi F. Gemelli against COVID-19 post-acute care Study Group. persistent symptoms in patients after acute COVID-19. JAMA 2020;324:603–5. 10.1001/jama.2020.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Writing Committee for the COMEBAC Study Group, Morin L, Savale L, et al. Four-Month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 2021;325:1525–34. 10.1001/jama.2021.3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemhöfer C, Sturm C, Loudovici-Krug D, et al. The impact of Post-COVID-Syndrome on functioning - results from a community survey in patients after mild and moderate SARS-CoV-2-infections in Germany. J Occup Med Toxicol 2021;16:45. 10.1186/s12995-021-00337-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boldrini P, Bernetti A, Fiore P, et al. Impact of COVID-19 outbreak on rehabilitation services and physical and rehabilitation medicine physicians' activities in Italy. An official document of the Italian PRM Society (SIMFER). Eur J Phys Rehabil Med 2020;56:316–8. 10.23736/S1973-9087.20.06256-5 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organisation . International classification of functioning, disability and health (ICF), 2001. Available: https://www.who.int/standards/classifications/international-classification-of-functioning-disability-and-health [Accessed March 30, 2021].
- 16.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 17.Bestall JC, Paul EA, Garrod R, et al. Usefulness of the medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581–6. 10.1136/thx.54.7.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krupp LB, LaRocca NG, Muir-Nash J, et al. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989;46:1121–3. 10.1001/archneur.1989.00520460115022 [DOI] [PubMed] [Google Scholar]
- 19.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 20.Galeoto G, Lauta A, Palumbo A. The Barthel index: Italian translation, adaptation and validation. Int J Neurol Neurother 2015;2:2378–3001. [Google Scholar]
- 21.Paltrinieri S. Cross-Cultural validation of the reintegration to normal living index (RNLI) in Italian: translation and pilot study. Italy: MSc Thesis in Rehabilitation Science. University of Firenze, 2021. [Google Scholar]
- 22.R Core Team (2020) . R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. Available: https://www.R-project.org/ [Accessed April 14, 2020].
- 23.Chinese Association of Rehabilitation Medicine, Respiratory Rehabilitation Committee of Chinese Association of Rehabilitation Medicine, Cardiopulmonary Rehabilitation Group of Chinese Society of Physical Medicine and Rehabilitation . [Recommendations for respiratory rehabilitation of coronavirus disease 2019 in adult]. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:308–14. Chinese. 10.3760/cma.j.cn112147-20200228-00206 [DOI] [PubMed] [Google Scholar]
- 24.Thomas P, Baldwin C, Bissett B, et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother 2020;66:73–82. 10.1016/j.jphys.2020.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeary L, Maltser S, Verduzco-Gutierrez M. Covid-19) in Physiatry: a can report for inpatient rehabilitation facilities. Pm R 20192020;12:512–5. 10.1002/pmrj.12369 [DOI] [PubMed] [Google Scholar]
- 26.COVID-19 rapid guideline: managing the long-term effects of COVID-19 (NG188): Evidenceevidence review 5: interventions. London:: National Institute for Health and Care Excellence (UK); 2020. [PubMed] [Google Scholar]
- 27.World Health Organisation, . Rehabilitation needs of people recovering from COVID-19 scientific brief, 2021. Available: https://www.who.int/publications/m/item/WHO-2019-nCoV-Sci_Brief-Rehabilitation-2021.1 [Accessed Jan 9,2022].
- 28.World Health Organisation, . A clinical case definition of post COVID-19 condition by a Delphi consensus, 2021. Available: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 [Accessed Jan 9, 2022].
- 29.Shah W, Hillman T, Playford ED, et al. Managing the long term effects of covid-19: summary of NICE, sign, and RCGP rapid guideline. BMJ 2021;2021:n136. 10.1136/bmj.n136 [DOI] [PubMed] [Google Scholar]
- 30.Costi S, Paltrinieri S, Bressi B, et al. Poor sleep during the first peak of the SARS-CoV-2 pandemic: a cross-sectional study. Int J Environ Res Public Health 2021;18:306. 10.3390/ijerph18010306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Girolamo G, Polidori G, Morosini P, et al. Prevalence of common mental disorders in Italy: results from the European study of the epidemiology of mental disorders (ESEMeD). Soc Psychiatry Psychiatr Epidemiol 2006;41:853–61. 10.1007/s00127-006-0097-4 [DOI] [PubMed] [Google Scholar]
- 32.JAMA medical news audio: an inside look at a Post–COVID-19 clinic. Available: https://edhub.ama-assn.org/jn-learning/audio-player/18608245 [Accessed May 7, 2021].
- 33.Huang C, Huang L, Wang Y, et al. 6-Month consequences of COVID-19 in patients discharged from Hospital: a cohort study. Lancet 2021;397:220–32. 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iqbal A, Iqbal K, Arshad Ali S, et al. The COVID-19 sequelae: a cross-sectional evaluation of Post-recovery symptoms and the need for rehabilitation of COVID-19 survivors. Cureus 2021;13:e13080. 10.7759/cureus.13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tleyjeh IM, Saddik B, AlSwaidan N, et al. Prevalence and predictors of post-acute COVID-19 syndrome (PACS) after hospital discharge: a cohort study with 4 months median follow-up. PLoS One 2021;16:e0260568. 10.1371/journal.pone.0260568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaffri A, Jaffri UA. Post-Intensive care syndrome and COVID-19: crisis after a crisis? Heart Lung 2020;49:883–4. 10.1016/j.hrtlng.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemhöfer C, Best N, Bökel A. Satisfaction of COVID-19 sufferers with actors of the health care system and rehabilitative therapy care using the COVID-19-Rehabilitation needs questionnaire (C19-RehabNeQ) in Bavaria. Physikalische Medizin, Rehabilitationsmedizin 2021. 10.1055/a-1528-1667 [DOI] [Google Scholar]
- 38.Wenham C, Smith J, Morgan R. And COVID-19 Working Group. COVID-19: the gendered impacts of the outbreak. Lancet 2020;395:846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purdie A, Hawkes S, Buse K, et al . Sex, gender and COVID-19: disaggregated data and health disparities. Available: https://blogs.bmj.com/bmjgh/2020/03/24/sex-gender-and-covid-19-disaggregated-data-and-health-disparities/ [Accessed March 24, 2020].
- 40.Spagnolo PA, Manson JE, Joffe H. Sex and gender differences in health: what the COVID-19 pandemic can teach us. Ann Intern Med 2020;173:385–6. 10.7326/M20-1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelada M, Anto A, Dave K, et al. The role of sex in the risk of mortality from COVID-19 amongst adult patients: a systematic review. Cureus 2020;12:e10114. 10.7759/cureus.10114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raparelli V, Palmieri L, Canevelli M, et al. Sex differences in clinical phenotype and transitions of care among individuals dying of COVID-19 in Italy. Biol Sex Differ 2020;11:57. 10.1186/s13293-020-00334-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marconi M. Gender differences in Covid-19: the importance of sex-disaggregated data. Ital J Gender-Specific Med 2021;7:4–6. [Google Scholar]
- 44.Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021;27:626–31. 10.1038/s41591-021-01292-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol 2014;35:347–69. 10.1016/j.yfrne.2014.04.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as online supplemental information. Reasonable requests for all of the individual participant data collected during the trial, after deidentification, should be made to the corresponding author and will be considered by the REACT lead author. The presented data are anonymised and risk of identification is low.


