Abstract
The relationship between psychosocial factors and cancer has intrigued people for centuries. In the last several decades there has been an expansion of mechanistic research that has revealed insights regarding how stress activates neuroendocrine stress-response systems to impact cancer progression. Here, we review emerging mechanistic findings on key pathways implicated in the effect of stress on cancer progression, including the cellular immune response, inflammation, angiogenesis, and metastasis, with a primary focus on the mediating role of the sympathetic nervous system. We discuss converging findings from preclinical and clinical cancer research that describe these pathways and research that reveals how these stress pathways may be targeted via pharmacological and mind-body based interventions. While further research is required, the body of work reviewed here highlights the need for and feasibility of an integrated approach to target stress pathways in cancer patients to achieve comprehensive cancer treatment.
Keywords: biobehavioral, stress, cancer progression, beta-adrenergic, sympathetic nervous system, mind-body interventions, cortisol, inflammation
Introduction
There has been a long history of fascination regarding a potential relationship between psychological factors and cancer dating back to the ancient Greeks. 1 In the second century A.D. the Greek physician Galen proposed that women with melancholic (depressive) dispositions were more likely to have tumors of the reproductive organs than women with a “sanguine” (optimistic) disposition. This notion was propounded by physicians throughout the Middle Ages and even into the 20th century.1,2 Epidemiologic studies, using more systematic approaches, have further revealed the association between psychological factors in both cancer incidence (emergence of a new cancer in a previously cancer-free individual) and progression of an already existing cancer.
The relationship between psychological factors and cancer incidence remains controversial, as some studies have identified a relationship between cancer incidence and psychological adversity, including traumatic or severe life events, severe distress, or long-term depression,3-9 while other studies have been unable to document such relationships.4,10-12 Interested readers are referred to a recent review addressing the potential molecular mechanisms underlying the role of stress in tumor initiation. 13
In contrast, more consistent associations between psychosocial risk factors and cancer progression have emerged in the epidemiological literature. The majority of studies have identified an association between trauma history, 14 social isolation,15-17 distress, 18 or depression,19-21 with more rapid disease progression or shorter survival, although some studies have not supported such relationships. 22 A recent meta-analysis of over 280 000 patients with breast cancer reported that both depression and anxiety were associated with a higher risk of recurrence and of all-cause mortality, and that depression was additionally associated with greater breast-cancer specific mortality. 23 Other meta-analyses provide further support that depression, 24 stressful life events,25,26 and social isolation 27 are associated with poorer survival in cancer patients.
Over the last several decades there has been an expansion of mechanistic research examining how biobehavioral pathways impact cancer progression. This review will discuss the key stress pathways that have been implicated in tumor progression with a focus on the sympathetic nervous system. We will examine effects of stress on immune cells including effects on cellular immunity and inflammation, noting that investigation of psychoneuro immunology was the primary driver of this field of research in its early stages. We will also describe evidence that has emerged over the last 2 decades on the direct effects of stress on cancer cells and other cells in the tumor microenvironment. Finally, we will discuss how this understanding can be leveraged to improve outcomes for patients, including some of the pharmacological and mind-body based interventions that operate via stress-related pathways.
Neuroendocrine Stress Response Systems
Stress has been defined as a challenge that exceeds the organism’s perceived ability to respond. 28 Stressors can be acute (short-term) or chronic (long-lasting or ongoing). 29 The human organism has a highly orchestrated response to stress. When a stressor is encountered, the individual’s evaluation of the severity of the challenge and their ability to respond results in activation of a variety of pathways in the central nervous system, particularly in the cortical and limbic areas of the brain. The integrated response of the brain is transduced into the body via 2 key systems. The sympathetic nervous system (SNS) mediates the “fight or flight response” with release of neurotransmitters including epinephrine and norepinephrine, and the hypothalamic pituitary adrenal (HPA) axis mediates the “defeat/withdrawal” response with release of cortisol. A variety of neurohormonal mediators such as oxytocin and endorphins are also involved in the stress response. These responses are integrated into a physiological stress response.28,30-32
The SNS serves as a pivotal homeostatic switch that regulates major physiological systems in response to external stress stimuli. 33 Activation of the SNS leads to the release of epinephrine (adrenaline) from the adrenal medulla into the blood circulation and release of norepinephrine (noradrenaline) from sympathetic nerve fibers that are present in most tissues throughout the body 33 including various types of tumor.34-37 The sympathetic nerves and adrenal medulla comprise the sympathoadrenal system. 28 Epinephrine and norepinephrine bind to α- and β-adrenergic receptors (βAR). Both of these neurotransmitters have higher affinity towards αAR than βAR, although βAR subtypes are dominant in the tumor microenvironment. 38 Upon binding with these receptors, neurotransmitters activate a cascade of downstream signaling that regulates gene transcription, protein expression, and cellular functions. 39
The HPA response involves production of corticotrophin releasing factor (CRF) and arginine vasopressin by the hypothalamus, activating the pituitary to secrete adrenocorticotropic hormone (ACTH) which stimulates the adrenal cortex to produce the glucocorticoid hormone cortisol. Cortisol is secreted according to a diurnal rhythm, cresting in the early morning before awakening and decreasing over the course of the day to reach a nadir late at night.40,41 The diurnal cortisol rhythm can become dysregulated through extensive stress, inflammation, or disease. 41 In such cases the slope often becomes flatter; with elevations of evening cortisol or blunting of the rise of morning cortisol. 41 Flattened cortisol slopes have been associated with poorer health in multiple conditions, including cancer as will be described below. 41 Glucocorticoids play a key role in regulating growth and metabolism and also provide endogenous control of inflammation. 32 The SNS and HPA stress response pathways are evolutionarily adaptive in that they prepare the organism to mobilize resources in the face of threat. However, prolonged mobilization of these stress response systems, which is common in many modern-day chronic stressors, can have negative consequences for many body systems.31,42,43 The impact of prolonged activation of the SNS and the HPA includes downregulation of cellular immunity, upregulation of inflammatory responses, metabolic dysregulation, and loss of sensitivity to glucocorticoid feedback which would otherwise downregulate inflammation.31,42,43 Chronic over-activation of the neuroendocrine stress response may result in allostatic overload, which in turn can lead to negative health outcomes, increased risk for cardiovascular and metabolic diseases, and increased vulnerability to infections.28,31,44,45
Certain checks and balances are built into the stress response systems, along with processes that promote restoration. The parasympathetic nervous system, including the vagus nerve and cholinergic mediators, plays an important role in antagonism of SNS signaling and inflammatory control. 28 Oxytocin, a peptide synthesized in the hypothalamus and secreted by the posterior pituitary, attenuates the stress response by decreasing cortisol production, lowering blood pressure, activating the parasympathetic nervous system, and increasing vagal tone. 46 In keeping with its role as an anxiolytic, oxytocin is linked with positive mood states, stimulates affiliative behavior in response to stress, decreases inflammation, and enhances the cellular immune response. 47
While neurohormones from these neuroendocrine systems may communicate with the tumor via peripheral circulation, anatomical evidence suggests direct communication with the tumor may also occur. Nerves have been documented in different types of tumors, including breast,48-50 prostate, 36 pancreatic, 51 head and neck, 34 gastric, 52 ovarian, 53 and salivary cystic carcinoma. 35 Additionally, the presence of nerves in tumors has been linked with more invasive tumors, higher tumor grade, and enhanced regional and distant metastasis.34,37,48,49,51 Examination of the type of nerves found in tumors showed that sympathetic nerves were associated with poor recurrence-free survival, while the presence of parasympathetic nerves was associated with better recurrence-free survival in women with breast cancer. 37 Preclinical studies have confirmed a causal role of sympathetic nerves in cancer progression. Depletion of sympathetic nerves using either a toxin called 6-hydroxydopamine, a viral vector, or a surgical strategy, reduced norepinephrine levels in tumors and decreased tumor mass and metastasis in preclinical models of breast cancer.37,54,55 On the other hand, selective activation of parasympathetic nerves using a viral vector approach similarly decreased tumor mass and metastasis in preclinical models of breast cancer. 37 However, parasympathetic nerves have also been shown to exert pro-tumorigenic effects in stomach cancer, suggesting that the role of different types of nerves in cancer progression may differ across different cancer types. 52 These findings highlight that neuroendocrine systems can interact with the tumor via systemic pathways and local tumor innervation.
In addition to these systemic effects, a variety of cells in the tumor microenvironment also express receptors that are responsive to these neuroendocrine pathways, allowing stress to induce localized changes in the microenvironment that regulate cancer progression. In times of stress, cancer cells, immune cells and other stromal cells in the tumor microenvironment (eg, adipocytes, fibroblasts) respond to neuroendocrine effectors through cell surface receptors including β-adrenergic receptors (βAR)56-59 and glucocorticoid receptors.60,61 A body of preclinical studies has demonstrated that elevation of neuroendocrine signaling by stressors such as chronic restraint or social isolation increased progression of solid tumors in mouse models of breast cancer,56,58,62,63 pancreatic cancer, 64 ovarian cancer, 65 prostate cancer, 66 colorectal cancer,67,68 lung cancer, 69 and hematopoietic tumors including leukemia 70 and lymphoma. 57 On the other hand, paradigms including enriched environment and exercise that elevate catecholamine and endorphins while also activating sensory nerves have been shown to exert anti-tumor effects in animal models.71,72 These seemingly opposing findings highlight the complex interaction between these neuroendocrine pathways and their impact on cancer progression. In the next sections, we will examine preclinical and clinical findings that describe how the SNS and HPA axes mediate the adverse effects of stress on cancer progression.
Stress and the Cellular Immune Response: The Role of Psychoneuroimmunology in the Context of Cancer
The immune system has a critical role in tumor surveillance and elimination. Immune effector cells—including natural killer (NK) and T cells—identify tumor cells in peripheral circulation and target them for destruction, as well as attacking tumor cells in primary and metastatic tumor sites. The impact of stress on the cancer-related immune response is evident in a substantial body of psychoneuroimmunology (PNI) research dating back more than 50 years. Studies in the general population documented that stress and other negative psychological states such as social isolation, depression, bereavement, and marital discord are associated with consistent neuroendocrine alterations and impairments of the cellular immune response, including number and activity of T cells, B cells and related cytokines, and NK cells. 73 Neuroendocrine alterations associated with social support/isolation that are thought to mediate downstream effects on the immune response and on other tumor-related pathways are described in Box 1.
| Box 1. Neuroendocrine Correlates of Social Support |
|---|
| Some of the strongest links between psychosocial factors and cancer come from studies of social support. These studies suggest that in the context of cancer, social support may modulate key neuroendocrine mediators of tumor progression, including norepinephrine, cortisol, and oxytocin. These mediators are thought to underlie the social support-immune relationships and social support-tumor relationships discussed in the review. |
| High social support has been associated with lower mean salivary cortisol in metastatic breast cancer patients 74 and with steeper diurnal cortisol slope in ovarian cancer patients surviving more than 5 years. 75 Social support may also modulate signaling through the sympathetic nervous system. Higher levels of social attachment (emotional social support) were associated with lower levels of both tumor and ascites norepinephrine in ovarian cancer patients. 76 Similar associations have been observed in childhood cancer patients, where social support from friends predicted lower urinary norepinephrine, and self-worth and family support were related to lower urinary epinephrine. 77 Additionally, at the time of surgery, ovarian cancer patients reporting higher levels of the facet of social support involving nurturing of others aspect had higher levels of tumor-associated oxytocin. 78 Taken together these findings indicate more normalized neuroendocrine profiles associated with social support. |
| It may also be important to distinguish between negative aspects of social support (such as criticism or social constraints) and positive aspects of social support, and to consider that they may have differential effects, and that negative social support may be qualitatively different than social isolation. Illustrating this point, one study of 181 breast cancer patients found that high levels of negative social support were associated with a flatter (less healthy) diurnal cortisol slope, but in contrast to findings in other labs, found no relationships of positive social support with cortisol slope. 79 |
| Social support has important implications with respect to clinical prognoses in cancer patients. For example, among epithelial ovarian cancer patients, those with greater social support had an approximately 13% lower risk of death, controlling for clinical covariates. 80 A meta-analysis including 87 studies of cancer patients reported that high levels of perceived social support were associated with a 25% decreased relative risk for mortality, and presence of a larger social network and being married were associated with 20% and 12% decreased relative risk for mortality, respectively. 27 |
| It is not clear what elements of social support are most potent in driving the neuroendocrine-immune-tumor cascade. It is possible that an increased sense of safety, opportunities for emotional expression, feeling understood or supported by others, or a sense of efficacy may reduce threat physiology and be driving some of the neuroendocrine processes underlying these effects. |
Some of the earliest research on stress and cancer in humans focused on NK cells, which perform surveillance for tumor cells and destroy tumor cells independent of the effects of T cells. For example, among early-stage breast cancer patients, poor social support after surgery was associated with decrements in NK cell cytotoxicity both concurrently and 3 months later.81-83 Subsequently, Andersen and colleagues reported that breast cancer patients with higher levels of stress between surgery and chemotherapy showed impairments in NK cell activation and cytotoxicity and reduced T cell proliferation, indicating compromised innate and cellular immunity. 84 Moreover, changes in the immune response paralleled changes in stress levels following breast cancer treatment. Specifically, those patients who reported an early decrease in post-operative stress also had the most rapid recovery of NK cell cytotoxicity following treatment. 85 Another study reported that greater social attachment (emotional social support) was associated with increased numbers of white blood cells in breast cancer patients 3 months after completion of chemotherapy. 86 Social support has also been associated with higher levels of cellular immune functioning (NK and T cell response) in breast and ovarian cancer patients.87,88 Similarly, in post-surgical breast cancer patients, greater anxiety was related to lower production of interleukin-2 (IL-2), a key growth factor for T cell proliferation. In contrast, greater positive affect (often defined as a tendency to experience positive emotions)89,90 was related to higher levels of interleukin-12 (IL-12) and interferon gamma (IFNγ) production, suggesting more robust cell-mediated immunity and potentially better tumor control. 91
In addition to these observations in the peripheral blood, stress-immune relationships impair the local immune response in several cancer types by impacting tumor infiltrating lymphocytes (TIL) in the tumor microenvironment (TME). Among women undergoing surgery for ovarian cancer, those reporting lower levels of social support showed poorer NK cell cytotoxicity in both peripheral blood and in tumor infiltrating immune cells. 88 Additionally, ovarian cancer patients reporting higher levels of distress had poorer NK cell cytotoxicity in TIL and impaired anti-tumor T cell cytokine response in tumors, ascites, and in circulating lymphocytes. 92 Another study examined the effects of stress on the local immune response to tumor in basal cell carcinoma. Tumor biopsies were taken from basal cell carcinoma patients who had experienced early life adversity. Those who had experienced not only early life adversity but also had experienced a recent traumatic event had an impaired local immune response to the tumor as indicated by markers linked to signal transduction, immune cell activation, and migration (CD25, CD3e, ICAM-1, and CD68) as compared to patients with early childhood adversity who had not experienced a recent severe life event. 93 Taken together these findings demonstrate that social factors and psychological stress are associated with changes in immune cells in the tumor microenvironment that can impair cancer control.
The impact of chronic stress on anticancer immunity is mediated at least in part through the activation of the sympathetic nervous system and downstream βAR signaling. Mechanistic studies have shown that activation of sympathetic nerves in lymphoid tissues including the spleen and lymph nodes inhibits trafficking of lymphocytes to these organs58,94 and inhibits production of cytokines including Type 1 interferons that support the cellular immune response. 95 Activation of βAR signaling, in particular β2AR, also stimulates immune cells that downregulate the cellular immune response and promote humoral immunity,96-98 thereby downregulating components of the immune system that are most relevant to tumor control. Similar regulation of βAR signaling in immune cells in the tumor microenvironment has also been reported. Animal studies showed that activation of the SNS by chronic stress (physical restraint) or cold stress (exposure to cold temperatures) increased recruitment of macrophages and myeloid-derived suppressor cells to tumors56,58,97 and reduced numbers of functional cytotoxic CD8+ T cells within the tumor.57,99 Conversely, blocking βAR signaling with the beta-blocker (β-blocker) drug propranolol inhibited the effects of stress on the recruitment of immunosuppressive myeloid cells, restored NK cell cytotoxicity, increased CD8+ T cells in mammary tumors, and consequently slowed cancer progression.56,58,97,99,100 These studies raise the possibility that the SNS may be targeted to enhance anti-cancer immunity within the tumor microenvironment.
Stress and Inflammation in Cancer
Inflammation is described by Hanahan and Weinberg as an “enabling characteristic” that supports the development and progression of cancer.101,102 Negative psychosocial factors such as depression, stress, and social isolation have been associated with higher levels of inflammation across several cancer types. Pro-inflammatory cytokines including IL-6 are activated as part of the stress response, and are also involved in key processes related to tumor metastasis such as angiogenesis.103,104
In women with advanced stage ovarian cancer, greater social isolation was associated with higher levels of IL-6 both in peripheral blood and in ascites (malignant effusions surrounding tumors), highlighting a relationship between psychosocial risk factors and inflammatory processes that could support tumor growth. 105 In women with breast cancer, elevated depressive symptomatology after surgery was associated with higher circulating levels of inflammatory cytokines including IL-6, tumor necrosis factor alpha (TNF-α), and interleukin-1 beta (IL-1β). 106 Similarly, breast cancer patients with higher levels of social isolation had a greater shift from an anti-tumor M1 macrophage phenotype to a pro-tumor M2 macrophage phenotype in their tumor tissue. 107 In breast cancer patients, negative mood and greater serum cortisol levels have been associated with RAGE receptor (Receptor for Advanced Glycation End products) ligand s100A8/A9, a key driver of inflammation, as described in more detail below. 108 Additionally, higher levels of negative affect and lower levels of positive affect in women with breast cancer post-surgery were associated with greater expression of inflammatory genes and their receptors in circulating leukocytes, 109 whereas higher levels of social well-being were associated with lower levels of pro-inflammatory and pro-metastatic leukocyte gene expression. 87 Similar patterns were observed in metastatic renal cell cancer patients, in whom higher levels of depressive symptoms were associated with increased expression of pro-inflammatory and pro-metastatic genes in leukocytes. A subset of these patients showed a similar profile of inflammatory changes in tumor as well. 21
Mechanistically, chronic stress promotes inflammation via activation of the SNS and downstream βAR signaling. For example, neural activation of adrenergic signaling in immune cells including NK cells, monocytes, and macrophages increases production of pro-inflammatory cytokines and chemokines, as well as enzymes that support prostaglandin synthesis, inflammation, and pain including cyclooxygenase-2 (COX2).56,110-112 Stress-induced βAR signaling modulates the pattern of gene expression by macrophages, leading to a wound healing phenotype. 113 These macrophages have increased expression of inflammatory mediators and reduced antigen presentation, leading to impaired anticancer immunity. 113 Similarly, adrenergic signaling also polarizes monocytes released from the bone marrow to an inflammatory phenotype.95,114,115 These myeloid cells modulate both immune cells and tumor cells via the RAGE receptor. 116 RAGE and RAGE ligands are important drivers of inflammation, and when activated are associated with greater lymph node metastasis, distant metastasis, and differentiation of tumor tissue in breast cancer.116,117 A key mediator of RAGE activation is the heterodimer s100A8/A9 ligand which has been associated with more rapid development of tumors and metastasis. 117 Additionally, stress hormones, including norepinephrine and cortisol have been shown to increase production of pro-inflammatory S100A8/A9 proteins by polymorphonuclear leukocytes, which can lead to the reactivation of dormant tumor cells, 118 highlighting an important mechanism whereby stress is implicated in tumor recurrence.
In addition to heightening the inflammatory signature of immune cells, chronic stress also supports a pro-inflammatory tumor microenvironment. Direct activation of βAR signaling in tumor cells elevated the expression of pro-inflammatory genes and has been linked to cancer progression in multiple studies.56,58,62,64 Adrenergic signaling promotes inflammation by increasing the recruitment of macrophages to primary tumors in mouse models of breast cancer56,58 and other cancer types.119,120 Tumor-associated macrophages have a critical role in supporting cancer progression by increasing the blood and lymph vascular network in the primary tumor.56,58 The recruitment of macrophages into the tumor is effectively blocked by propranolol.56,58 Moreover, the gut microbiome has also been implicated in the effects of stress on inflammation. 121 However, whether sympathetic nerves or βAR signaling play a role in the effects of stress on microbiome remains unknown. A recent study reported a critical role of gut microbiome in modulating sympathetic activity in the gut, raising the possibility that strategies that target the gut microbiome could be harnessed to inhibit the adverse effects of sympathetic activity on the progression of solid tumors in the gut. 122 These studies highlight a role for the SNS in mediating the effects of stress on inflammation in cancer, thus pointing to the possibility of targeting this system to improve cancer outcomes.
Pathways of Cancer Progression: Angiogenesis and Lymphangiogenesis
Angiogenesis and lymphangiogenesis refer to the growth of new blood and lymph vessels, respectively, in the tumor microenvironment. 123 These processes contribute to cancer progression: new vessels serve as conduits for nutrient supply, which is critical for exponential tumor growth, and also serve as pathways for tumor cell dissemination. 124 Both processes are regulated by positive and negative signaling from tumor cells and stromal cells in the tumor microenvironment.125,126 Key molecules supporting angiogenesis and lymphangiogenesis include vascular endothelial growth factor (VEGF), IL-6, and interleukin-8 (IL-8).103,104 The impact of psychosocial factors on angiogenesis and lymphangiogenesis is evident in studies that examined these key molecules in cancer patients with specific psychosocial risk factors. Loneliness was associated with greater expression of tumor VEGF at the time of surgery in colon cancer patients, 127 while depression and poor quality of life were associated with higher levels of serum VEGF both before and 6 weeks after surgery in these patients. 128 Conversely, women with ovarian cancer reporting higher levels of social support had lower levels of VEGF in serum pre-surgery 129 as well as in primary tumor, after adjusting for relevant clinical variables. 130
Preclinical research revealed that both angiogenesis and lymphangiogenesis are highly regulated by the SNS, indicating a possible role of the SNS in mediating the effects of psychosocial factors on vessel growth. SNS activation increases angiogenesis and lymphangiogenesis in tumors by upregulating expression of vascular endothelial growth factors (VEGF-A and VEGF-C).56,58,65,131 The expanded vasculature network in the tumor provides new routes of tumor cell dissemination that enhance metastasis progression.56,58 Preclinical studies showed that activation of βAR signaling in tumor cells increases the production of VEGF and IL-6 in different cancer types including melanoma, nasopharyngeal, and ovarian cancer cells.56,65,132-134 Emerging studies have shown that the SNS also interacts with endothelial cells to promote angiogenesis via βAR signaling.59,135 Genetic deletion of βAR in endothelial cells altered endothelial metabolism which inhibited angiogenesis in the tumor and slowed the progression of prostate cancer in a mouse model. 59 However, whether this effect is generalizable to other cancer types is yet to be explored.
Pathways of Cancer Progression: Invasion and Metastasis
In addition to the impact on anti-cancer immunity, inflammation, angiogenesis, and lymphangiogenesis, stress regulates various aspects of tumor cell behavior that drive tumor cell dissemination. During cancer progression, tumor cells switch from an epithelial to a mesenchymal phenotype in a process known as the epithelial mesenchymal transition (EMT). 136 In the switch to a mesenchymal phenotype, tumor cells take on embryonic characteristics and become invasive. In addition to increased invasiveness, EMT polarization is associated with immunosuppression, chemoresistance, and evasion of apoptosis.137,138 Clinical studies show links between stress factors and EMT polarization. In socially isolated breast cancer patients, the primary tumor showed polarization to a pattern of mesenchymal gene expression, a process that appeared to be β-adrenergically mediated. 107 A similar EMT polarization was observed in both primary tumor 139 and exosomes (tumor-derived extracellular vesicles) of socially isolated women with ovarian cancer. 140 In addition to polarizing tumor cells to a more mesenchymal phenotype, stress also affected the release of proteases such as matrix metalloproteases (MMPs), that promote the breakdown and remodeling of the extracellular matrix (ECM), enabling both local and distal tumor spread. 141 In women with ovarian cancer, depression, current life stress, or negative affect were associated with greater expression of pro-metastatic MMP9 in tumor-associated macrophages (CD68+ cells); conversely, higher levels of social support were associated with lower levels of MMP9 in primary tumors. 130 Similarly, depressed patients with renal cell carcinoma showed elevated expression of pro-metastatic MMPs in tumor tissue. 21
Paralleling these findings, in vitro studies revealed that β-adrenergic signaling upregulates expression of MMPs including MMP2 and MMP9 in tumor cells of various cancer types.62,64,133,142 Pharmacological activation of βAR signaling using isoprenaline promoted migration and invasion of breast cancer,62,143-145 ovarian cancer, 142 and pancreatic cancer cell lines. 64 Mechanistic studies showed that βAR signaling enhanced tumor cell invasion by inducing the formation of invadopodia, subcellular structures that degrade the extracellular matrix. 143 Additionally, βAR regulation of actomyosin dynamics reduced the deformability of tumor cells, resulting in stiffer tumor cells with enhanced contractile and invasive properties. 144 Mechanistic studies in mice revealed that the β2AR subtype of the receptor was critical for these effects as downregulation of β2AR in tumor cells using short hairpin RNA inhibited invasion and metastasis following SNS activation. 62
Epithelial cells are anchorage dependent, meaning they normally survive only when adhered to the extracellular matrix (ECM). When epithelial cells detach from the surrounding matrix, they undergo a form of programmed cell death (apoptosis) called anoikis. Tumor cells become resistant to anoikis, enhancing their survival and their metastatic potential.146,147 Resistance to anoikis is increased by βAR signaling in pre-clinical models of ovarian cancer, effects which are abrogated by β-blockade. These effects are mediated by focal adhesion kinase (FAK), a tyrosine kinase that promotes cell cycle progression, survival of tumor cells and migration. In response to stimulation by NE, FAK demonstrated increased activation (pFAKY397). Clinically, primary tumor tissue from ovarian cancer patients with higher levels of depression or higher levels of NE showed elevations in pFAKY397, which was also linked to poorer overall survival in these patients. 148
Taken together, these findings indicate that psychosocial stress factors are linked to many tumor pathways that support invasion and metastasis through SNS activation and βAR signaling. Collectively, these clinical and mechanistic findings converge to show that diverse cellular components of the TME, including immune effector cells, tumor cells and other stromal cells, are sensitive to the regulation by stress, particularly via βAR signaling. Therefore, approaches that target βAR signaling may slow cancer progression by targeting these different cellular components of the tumor microenvironment. These intervention strategies will be discussed shortly.
Effects of Glucocorticoids and Oxytocin on Tumor Growth and Progression
In addition to activating the SNS, stress impacts cancer progression through the actions of glucocorticoids. Stress activates the HPA axis, which controls glucocorticoid release from the adrenal cortex. Glucocorticoids are important in inflammatory control, but at elevated levels have negative effects, including suppression of the cellular immune response, which impairs immunosurveillance of cancer cells.28,32 Glucocorticoids can also act directly on cancer cells to stimulate growth, 149 inhibit apoptosis, 135 promote tumor progression,150-152 and induce chemoresistance. 153 Additionally, glucocorticoids are able to modulate transcriptional activity in tumor-associated fibroblasts and adipocytes to make the tumor microenvironment more favorable for tumor growth and progression. 154 Glucocorticoids have been shown to affect DNA repair in cancer cells, suggesting HPA activation may magnify the accumulation of DNA damage as cancer develops. 150 High levels of the glucocorticoid receptor (GR) in early stage breast cancer patients who are estrogen receptor negative (ER−) have been associated with shorter relapse free survival; moreover, a glucocorticoid activity signature has been identified in ER− breast cancer patients associated with chemotherapy resistance and greater likelihood of relapse. 155 Glucocorticoid treatment was shown to increase GR activation in metastatic sites, modulating expression of genes involved in invasion, and increasing colonization of metastatic target organs by tumor cells, 151 all processes which could promote disease progression. Altered diurnal cortisol rhythms, specifically more flattened cortisol slopes, have been observed in several types of cancer and have been associated with poorer survival in patients with ovarian, breast, lung, and renal cell cancers.21,156-158 Taken together, these findings highlight the importance of glucocorticoid related processes in tumor progression.
Further studies are needed to fully understand how HPA signaling via glucocorticoids impacts cancer progression and what processes are involved in alterations of diurnal rhythms. As synthetic glucocorticoids are often used to offset the side effects of chemotherapy, the findings may have significant implications for their routine use in cancer treatment.151,154 A recent preclinical breast cancer study showed that the effects of synthetic glucocorticoids on cancer progression may change depending on the dose, with lower doses suppressing tumor growth and metastasis and higher doses promoting tumor progression. 159 For specific cancers, glucocorticoid receptor antagonists administered in conjunction with chemotherapy may enhance the effectiveness of chemotherapy. 155
Oxytocin is a neuropeptide that is released from the brain and plays a key role in social bonding. Oxytocin has anti-proliferative, anti-migratory, and anti-invasive effects on a variety of tumor cells, including ovarian cancer cells, both in vitro and in vivo.160-162 Oxytocin levels in the ovarian tumor microenvironment were associated with lower levels of inflammation as measured by IL-6 both in circulating blood and in the tumor microenvironment. In vitro studies also showed that oxytocin blunted IL-6 secretion from multiple ovarian tumor cell lines. Moreover, ascites oxytocin was related to longer survival in ovarian cancer patients. 163
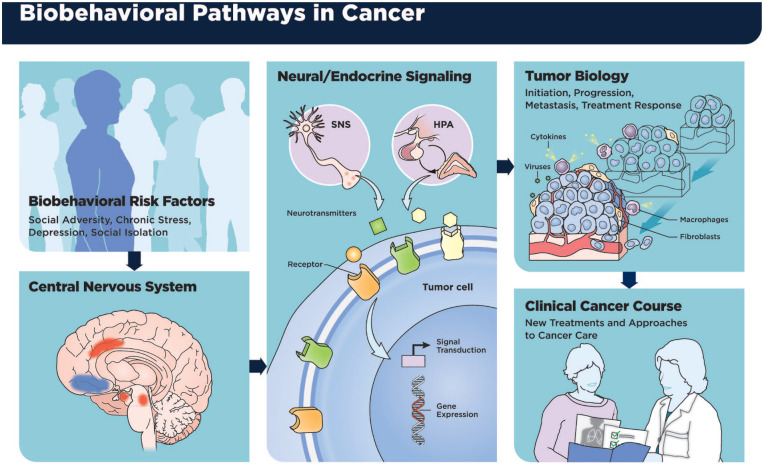
Figure 1 describes the mechanisms outlined above and their relationship to the clinical cancer course. 164
Figure 1.
Effects of stress response processes on evaluation of level of threat, interaction of sympathetic nervous system (SNS), and hypothalamic-pituitary-adrenal (HPA) axis with tumor cells and cells in the tumor microenvironment, and ultimate effects on tumor progression and the clinical course of cancer.
Source: Figure originally published in Green McDonald P, O’Connell M, Lutgendorf SK. Psychoneuroimmunology and cancer: a decade of discovery, paradigm shifts, and methodological innovations. Brain Behav Immun. 2013;30:S1-S9. doi:10.1016/j.bbi.2013.01.003. 164
Psychological Well-Being and Cancer: Physiological Mechanisms
Positive psychological factors are thought to act as resources to buffer the effects of disease on both mental and physical health. 165 Psychological well-being is a multifaceted concept including factors such as benefit finding, eudaimonic well-being, positive affect, social connection, and resilience. 165 These resources are qualitatively different than the mere absence of negative attributes such as stress, depression, and anxiety.166,167 Each of these individual aspects of well-being has been associated with biological outcomes in cancer patients. This section will focus on clinical studies as well-being is more difficult to measure in pre-clinical models.
Benefit Finding
Many cancer patients report finding benefit in the experience of cancer, and a growing body of evidence has demonstrated associations between benefit finding and positive physiological effects in cancer.168,169 Benefit finding has been described through a variety of constructs, including posttraumatic growth (beneficial changes in life perspective following a traumatic life event) and meaning making.170,171 Several studies have examined potential mechanisms that might explain the relationships observed between benefit finding and biological outcomes; a systematic review of this literature proposed that changes in a variety of psychological domains involved in benefit finding could enhance allostatic processes, buffering against negative catabolic stress systems in cancer patients and other chronically ill populations. 168 Benefit finding has been associated with steeper (healthier) diurnal cortisol slopes in men treated for prostate cancer, 172 and increases in benefit finding have been related to reductions in afternoon to evening serum cortisol levels after a stress management intervention in women with early-stage breast cancer, suggesting that this aspect of well-being is associated with better HPA axis regulation in cancer patients. 173 Posttraumatic growth has been associated with steeper diurnal cortisol slopes in women with metastatic breast cancer. 174 As flatter diurnal cortisol slopes have shown prognostic significance in a variety of cancers as noted above,21,156-158 these relationships of benefit finding and post-traumatic growth with cortisol slope may have clinical significance.
In addition to associations with the HPA axis, possible immunomodulatory effects have been explored with respect to benefit finding. Among early-stage breast cancer patients participating in a psychosocial intervention in the months following surgery, increases in benefit-finding were associated with increases in lymphocyte proliferation, 175 indicative of a more robust immune response. In hepatocellular carcinoma patients, assessed before chemotherapy and at 3 and 6-month follow-ups, those reporting greater posttraumatic growth had higher counts of peripheral blood leukocytes at each follow-up, suggesting more rapid recovery from chemotherapy. Moreover, those patients above the median in post-traumatic growth had approximately 6 months longer survival time than those patients below the median. 171 It has also been proposed that benefit finding could mediate positive effects of interventions like yoga. 176 Benefit finding has been shown to be both independent of disease severity 177 and associated with disease severity, 178 depending on the context; this indicates the potential importance of evaluating clinical covariates when examining this construct.
Eudaimonic Well-Being
Eudaimonic well-being involves meaning, fulfillment, and purpose, as opposed to hedonic well-being which focuses on positive emotions, pleasure, and pain-avoidance. 179 One specific correlate that has received substantial attention is the conserved transcriptional response to adversity (CTRA), a shift in gene expression associated with chronic stress or uncertainty that is characterized by up-regulation of pro-inflammatory genes and down-regulation of Type I interferon- and antibody-related genes that, when chronically activated, are associated with inflammation-mediated diseases and poor health outcomes. 180 Eudaimonic well-being has been associated with down-regulation of the CTRA in healthy adults,180-182 as well as in cancer patients. 183 Among ovarian cancer patients, women reporting higher levels of eudaimonic well-being had lower levels of tumor norepinephrine, a stress hormone that, as noted above, has been associated with many pathways supporting tumor growth and progression. Additionally, at the time of surgery, ovarian cancer patients reporting higher levels of purpose in life, positive affect, and nurturing of others had higher levels of tumor-associated oxytocin, 77 which as noted above has anti-inflammatory and anti-stress properties.
Although the construct of hedonic well-being has not been as strongly related to advantageous biological outcomes, positive affect has nevertheless been associated with a variety of beneficial biological outcomes in cancer patients and in community populations. In patients with metastatic renal-cell carcinoma, positive affect was associated with increased hemoglobin and improved survival outcomes. 184 In post-surgical breast cancer patients, a greater ratio of positive-to-negative affect was associated with less pro-inflammatory gene expression in leukocytes, including cytokine, chemokine, and COX2 genes. 109 In early-stage breast cancer patients at treatment completion, high arousal positive affect was associated with lower levels of soluble tumor necrosis factor receptor II (sTNF-RII), thought to be a marker of TNFα pro-inflammatory activity, and predicted stability in those lowered levels over 1 year. 185
Many of the factors described above come together in the concept of resilience, or the capacity to recover quickly from challenges. In one recent large-scale registry study, low levels of stress resilience among men assessed by interview in late adolescence were associated with increased mortality risk among those who subsequently developed cancer, particularly for oropharyngeal, upper respiratory, and prostate cancers and Hodgkin’s lymphoma. 186 Another study of 487 invasive breast cancer patients reported that patients with higher levels of resilience assessed pre-surgery had a 63% lower risk of cancer progression, and an 80% lower risk of both cancer-related mortality and all-cause mortality over the subsequent 10 years as compared to those with less resilience. 187 Although there are a limited number of preclinical studies on these relationships, one potential mechanism for these effects can be seen in murine models, where positive environment stimulation (eustress) such as life in an enriched environment were recently associated with anti-tumor immune function. 71 Taken together, these findings indicate a modest association of positive psychosocial factors with more normalized neuroendocrine profiles, higher levels of cellular immunity, lower inflammation, less expression of tumor transcriptional profiles supporting invasion and metastasis, and to some extent with survival.
Pharmacologic Approaches to Reduce Stress-Related Cancer Progression
Understanding the role of β-adrenergic signaling has provided molecular insight into the translational applicability of integrative care interventions and a molecular target for pharmacologic intervention. As discussed above, pre-clinical studies show that β-blockade mitigates stress-induced cancer progression through βAR signaling. Researchers have now begun to utilize the same β-adrenergic antagonist drugs that were used in pre-clinical studies to block stress effects on tumor progression and metastasis in human trials. This approach is supported by a significant body of retrospective pharmacoepidemiologic studies that demonstrate reduced cancer progression among individuals exposed to β-blockers.188-197 A number of studies have highlighted effects for nonselective β-blockers that target both β1AR and β2AR. The use of β-blockers has been linked to reduced rates of progression for several solid and hematologic malignancies.188-190,197-200 In breast cancer patients these effects include reduced distant metastases and decreased cancer recurrence and cancer-specific mortality.188,189 Decreased tumor progression at 2.5 year follow up was observed in propranolol-treated patients with melanoma compared to a subgroup that did not use beta-blockade, 190 while incidental β-blocker use among patients with metastatic colorectal cancer significantly predicted both progression-free and overall survival. 197 β-blocker use has also been associated with decreased distant metastasis-free survival, disease-free survival, and overall survival among patients with non-small-cell lung cancer 199 and a reduced risk of disease-specific death and overall mortality among hematologic malignancy patients. 200
While there is substantial pharmacoepidemiologic evidence for a potential protective effect of β-blockers on cancer outcomes, other observational studies have failed to identify similar association on cancer progression (breast 201 ), cancer-specific mortality (melanoma 202 and colorectal203,204), or recurrence-free or overall survival in patients with non-small cell lung cancer. 205 There are many plausible reasons for this lack of observed association, including potential variation by tumor subtype, incomplete data, sampling bias, or variation in surgical or other cancer interventions. One plausible issue is that most patients included in those studies were prescribed newer generation β-blockers that are cardio selective (targeting the β1-adrenergic receptor) and thus do not target the β2AR. Experimental model systems have identified the β2-adrenergic pathway to be implicated in the physiological effects of stress on cancer progression.62,97,98,206-209 Epidemiological studies in cancer patients that compared use of cardio selective versus non-selective β-blockade found a favorable effect of non-selective β-blockade.189,200 Another important issue is the potential for confounding in non-randomized observational research where indication for β-blocker use is correlated with many diseases that are likely to adversely impact cancer progression. As such, it is critical to conduct additional experimental studies involving randomization of cancer patients to treatment specifically with antagonists that also target β2AR such as propranolol.
Several small randomized controlled trials (RCTs) have examined the effect of β-blockade on biomarkers of tumor progression, controlling for some of the confounders described above. These studies have been conducted in breast, ovarian, colorectal, and hematopoietic cancers and found promising initial results showing favorable changes in tumor gene expression profiles following β-blocker administration.210-217 Breast cancer patients have been evaluated both with propranolol alone and in combination with COX-2 inhibitors212,217,218; similar favorable changes in tumor biomarkers were observed as in their companion preclinical models (described above). Propranolol administered for 1 week prior to surgery in early-stage breast cancer patients resulted in down-regulated expression of mesenchymal genes within the tumor, an indication of reduced tumor aggressiveness. 212 Results from this RCT of 60 women with early-stage breast cancer support the potential for β-blockade to reduce metastatic capacity. Data from another RCT of 38 women with early-stage breast cancer receiving perioperative treatment with propranolol and the COX-2 inhibitor etodolac have also demonstrated favorable impacts on other tumor transcriptome profiles. 217 Similar impacts of perioperative treatment with propranolol and etodolac on tumor transcriptome profiles were observed in a RCT of 34 patients with colorectal cancer. 210 Finally, in another RCT of breast cancer patients undergoing surgery and receiving propranolol and/or the COX-2 inhibitor parecoxib, propranolol administration, but not parecoxib alone, abrogated the increased T regulatory cell activity and accompanying suppression of CD4+ T cell responses after surgery. 218 Here, the addition of parecoxib to the propranolol regimen did not demonstrate any additional benefit beyond those evident for propranolol alone.
Molecular biomarker patterns have demonstrated similar improvements following propranolol exposure in non-breast cancer populations as well. In one study, patients undergoing hematopoietic stem cell transplantation (HCT) following a multiple myeloma diagnosis were administered peri-transplant propranolol, with gene expression assessed once before transplant and 2 times following HCT. 215 Propranolol-treated patients showed significantly greater decreases in the stress-related ‘conserved transcriptional response to adversity’ (CTRA) gene expression signature from baseline to post-transplant compared to the control group. As noted above, the CTRA involves up-regulated expression of genes involved in inflammation (eg, IL1B, IL8, PTGS2[COX2]) and a complementary down-regulation of genes involved in antiviral responses (eg, IFIT-, OAS-, and MX-family genes). Studies in cellular and animal models have shown that CTRA gene expression is evoked primarily by sympathetic nervous system signaling through β-adrenergic receptors on immune cells.114,115 Further, it has been identified as an indicator of biobehavioral impact on cancer progression.219,220 Hematopoietic stem cell transplantation patients treated with propranolol showed improvement in other pertinent hematological gene transcripts as well. Results also indicated nonsignificant trends toward accelerated platelet and neutrophil engraftment and decreased infections posttransplant in propranolol-treated patients, providing preliminary indications of potential clinical benefit of β-blocker administration. In an RCT of ovarian cancer patients undergoing tumor debulking, peri-surgical propranolol significantly lowered plasma CA-125 levels, a marker of tumor burden, though it was not effective at reducing C-reactive protein, cortisol, or anxiety. 213
Non-randomized treatment trials also suggest a potential positive influence of β-blockade in cancer. In a prospective study of 53 patients with Stage IB to IIIA cutaneous melanoma, patients taking daily propranolol were significantly less likely to experience melanoma recurrence than their non-propranolol counterparts, 221 amounting to an 80% reduction in cancer recurrence risk among propranolol users. This effect persisted even after adjusting for known prognostic factors. Another study of 23 patients with Stage II-IV epithelial ovarian cancer showed that overall QOL, anxiety, and depression improved, while leukocyte expression of pro-inflammatory genes declined significantly after completion of chemotherapy accompanied by propranolol. 216
While findings from these recent Phase II RCTs have yielded promising biomarker results in tumor tissues and circulating immune cells, it is important to note that none of these studies involved sufficient sample size or follow-up duration to detect impact on clinical outcomes. In line with the majority of rigorous preclinical data, these observations underscore the translational need for larger Phase III clinical trials powered to detect the impact of β-blockade on cancer recurrence and survival. There are an increasing number of larger ongoing β-blocker RCTs aimed at assessing clinical cancer outcomes. 222 However, an obstacle to success in these studies is the limitation in recruitment due to competition with other traditional pharma-supported oncology trials that typically prohibit additional treatment with another agent such as propranolol.214,223 As such, several attempts to test the impact of β-blockade in the context of cancer treatment have been terminated prematurely due to poor accrual (NCT02596867, NCT01857817, NCT03323710) or funding obstacles (NCT01988831). One potential solution is to evaluate propranolol as a stratified arm in trials of a traditional antineoplastic agents224,225; however, this approach is still in its nascency.
If these barriers to translation can be overcome, β-blockade may end up being leveraged for particularly vulnerable populations (high distress, low socioeconomic status, depressed, etc.). Preclinical and early clinical data suggest that β-blockers could be used alongside traditional and emerging cancer treatments such as immunotherapy. It will be important for future studies to determine if it is sufficient to target the downstream neurobiological effects of psychosocial stress (eg, using β-blockers), or whether it is also important to target psychosocial distress itself.
Psychosocial Intervention Effects on Stress and Biobehavioral Processes in Cancer
Given the parallel data from pre-clinical experiments and observational clinical studies cited above, indicating strong effects of stress response systems on tumor growth and on the tumor microenvironment, the next step in understanding the role of stress in clinical populations is to experimentally block the stress response and examine downstream effects on cancer-relevant biomarkers and clinical outcomes such as disease progression and survival. Using a similar logic to that underlying the use of β-blockers to block SNS signaling in clinical populations, stress management interventions have been used in cancer patients to modulate stress processes. Stress-management interventions have the potential advantage of working across all stress-response systems and not confining their actions to SNS signaling. Interventions used include cognitive-behavioral therapy (CBT)-based, mindfulness-based and physical-based stress management approaches. The CBT-based approaches teach skills for changing cognitive appraisals of stressful stimuli (cognitive restructuring), improving coping responses to emerging challenges, and teaching interpersonal skills to build social support and reduce social disruption.226,227 Mindfulness approaches work by increasing awareness and developing a non-judgmental attitude toward stressful thoughts. 228 Other interventions work by changing bodily tension and physiological activation through physical approaches such as yoga, Tai-Chi, massage, exercise, acupuncture, and biofield/energy manipulation. 229 Relatively few studies have experimentally demonstrated that interventions can modulate psychological adaptation (eg, lowered distress, negative affect and social disruption, and increased positive affect and benefit finding) in tandem with changes in neuroendocrine (eg, decreased or normalized SNS and HPA activity), and immune system functioning (decreased inflammation and improved cellular/antiviral immunity).73,230 Studies cited below are a selection of some of the strongest evidence available.
Cognitive-Behavioral Approaches
In one of the first studies to show effects of a CBT-based intervention on biobehavioral processes in cancer patients, Fawzy et al 231 showed in patients with Stage I to II malignant melanoma that a 6-week stress management intervention (relaxation techniques and coping skills training) reduced distress and negative mood, increased cell-mediated immune function (NK cell cytotoxicity) at 6 months 232 and increased time to recurrence and greater overall survival at 6 and 10 years.233,234
Another CBT-based group intervention that included relaxation, cognitive restructuring, coping skills training, and health behavior change strategies provided over 12 months in post-surgical Stage II to III breast cancer patients decreased distress, decreased lymphocyte proliferative responses (LPR− indicative of functional capacity of the immune system to respond to a stimulus), increased healthy eating habits, and reduced smoking rates over 4 months compared to treatment as usual (TAU). 226 By 12 months intervention participants had better staff-rated health status. 235 Patients in the intervention also had lower breast cancer specific mortality rates as well as a 45% reduced risk of cancer recurrence at 11.5-year median follow-up versus TAU. 236
Investigators then conducted secondary analyses comparing patients in the trial who had recurred (N = 48) versus those who had not (N = 48) and who were matched on sociodemographic and prognostic factors. 237 During the 12-months following recurrence, those who had previously received the intervention showed decreased negative mood, increased social support, and greater LPR and NK cell cytotoxicity compared to their counterparts who had received TAU. Once women had recurred there was a reduced risk of death over an 80-month follow-up among those who had been previously assigned to the intervention arm (vs control). 238 Thus CBT-based stress management that improves psychological adaptation (decreased distress) may increase cellular immune function (LPR) early in treatment, prevent inflammatory changes during survivorship, decrease the odds of recurrence and produce persisting benefits in psychological and immune functioning and health outcomes after the disease recurs.
Another CBT-based group stress management intervention, cognitive behavioral stress management (CBSM), is a 10-weekprogram that teaches cognitive, behavioral and interpersonal skills through in-session activities, CBT-based homework and daily practice of relaxation exercises (progressive muscle relaxation, diaphragmatic breathing, guided imagery, meditation). 227 CBSM was shown in 2 RCTs of Stage 0 to III breast cancer patients recruited after surgery to improve cancer-specific intrusive thoughts, mood, social disruption, and quality of life,239-241 decrease evening serum cortisol 173,242; and increase LPR and interleukin-2 (IL-2) and interferon-gamma (IFN-γ) production to anti-CD3 stimulation175,243 over the initial 12 months of primary treatment compared to those assigned to a 1-day psychoeducational control. CBSM effects on Th1 cytokine (IL-2 and IFNγ) production may be important for supporting anti-cancer and anti-viral immune signaling.244,245 As noted above, reducing evening cortisol is important since flatter diurnal cortisol slopes (due in part to higher evening output) predict decreased survival in multiple cancers.21,156-158
Women assigned to CBSM (vs psychoeducational control) in this trial also showed down-regulation of leukocyte genes for pro-inflammatory cytokines, inflammatory chemokines and their receptors, COX2, and mediators of tissue remodeling and EMT (MMPs); and upregulation of anti-viral immune and anti-tumor response genes. 109 Bioinformatic analyses inferred that this gene expression pattern reflects decreased nuclear factor kappa beta NF-κB/Rel and the Globin Transcription Factor (GATA) family activity, and increased activity of interferon response factors, which were linked to stress and SNS signaling in prior work. 246 CBSM also increased leukocyte glucocorticoid receptor (GR)-related gene expression including an over-representation of GR response elements in the promoters of CBSM-up-regulated genes. 109 These findings suggest that CBSM may reduce inflammatory activity and mediators of tumor invasion as well as reducing stress-induced desensitization of the glucocorticoid receptor, 247 thus making cells more responsive to the anti-inflammatory effects of glucocorticoids. 248 Taken together, these changes would be consistent with processes that would inhibit disease progression.
Patients assigned to CBSM also showed lower odds of all-cause and breast cancer-specific mortality and recurrence at 11-year median follow-up versus psychoeducation controls, after controlling for demographic and medical covariates. 249 Since inflammation relates to breast cancer progression,58,250 it was plausible that CBSM-related changes in leukocyte transcriptional activities during primary treatment might explain its effects on increased time to recurrence at 8 to 15 years. Using the 53-gene Conserved Transcriptional Response to Adversity (CTRA) composite derived from circulating leukocytes, investigators found that women assigned to the control condition had significantly increased CTRA over 6 to 12 months while those in CBSM had slightly decreased CTRA. Less CTRA increases over 12 months of primary treatment predicted greater 11 year disease free survival (DFS). 220 This may have implications for stress management in other cancers; for example, greater expression of CTRA predicts decreased disease-free survival in recipients of hematopoietic stem cell transplant for acute myelogenous leukemia. 219 Together these CBT-based stress management RCTs suggest it might be possible to improve long-term health outcomes in breast cancer patients by modulating immune cell activities (eg, inflammation and anti-viral immune signaling).
Since these combined approaches, which include relaxation training, CBT, and Health Education226,227 have been shown to improve psychological adaptation, biobehavioral processes, and health outcomes in breast cancer patients, it is important to understand which specific intervention elements are accounting for these effects. One “dismantling” trial compared the effects of 3 group-based interventions—5-week relaxation training versus 5-week CBT versus 5-week health education—in a 3-armed RCT in post-surgical breast cancer patients. Those assigned to either relaxation training or CBT showed improved mood and emotional well-being 251 and reduced inflammatory signaling (circulating s100A8/A9 levels 252 and leukocyte NF-κB DNA binding 253 ) over 12 months compared to those in Health Education. Women showing the greatest increases in perceived stress management skills showed the least s100A8/A9 levels and NF-κB binding over 12 months.252,253 Since all 3 conditions were the same length and group-based the differential effects of CBT and RT versus HE are likely due to stress management skills training rather than attention or the presence of a supportive group. Importantly s100A8/A9 levels have been shown to predict breast cancer metastasis 250 and greater NF-κB nuclear binding may enhance inflammatory gene expression. Hence a brief stress management intervention focused on either CBT or relaxation training may be sufficient to bring about changes in biobehavioral processes relevant for breast cancer disease progression. Even briefer interventions have been associated with immunologic changes in cancer patients. One study showed a 2-session stress management intervention teaching CBT and relaxation training skills offered to men prior to prostate cancer surgery decreased negative mood and increased NK cell cytotoxicity by 48 hours after surgery. 21
In sum, a small number of CBT-based stress management studies have shown effects on biobehavioral processes relevant to cancer progression, and 3 of these have also shown effects on long-term clinical outcomes approximately 10 to 11 years later.233,236,249 Similar CBT-based stress management interventions have not been evaluated for long-term clinical effects in cancers beyond breast cancer and malignant melanoma. Given work reviewed here linking stress and biobehavioral processes to clinical outcomes in ovarian cancer, lung cancer, renal cell carcinoma, and hematological cancers it is important to test whether the effects of CBT-based approaches generalize beyond those established to date.
Mindfulness Based Stress Reduction (MBSR)
MBSR interventions have been tested with a similar goal, to determine if the MBSR-induced reductions in the stress response could ultimately enhance biomarkers indicative of stronger protection against recurrence. MBSR comprises 4 to 8 weeks of training in meditation techniques (awareness-raising and mindful movement), mindfulness and stress didactics, and group support, and is often followed by a weekend retreat. 228 In an early non-randomized intervention, study patients with breast or prostate cancer, Stages 0 to II, who were at least 3 months post-surgery and prior to receipt of chemotherapy or radiation received an 8-week MBSR program showed reductions in salivary cortisol 254 and NK cell production of IL-10 over 12 months. 228 One RCT in Stage 0 to II post-surgical breast cancer patients who did not receive chemotherapy found that 8 weeks of MBSR was associated with greater increases in NK cell cytotoxicity and IFNγ production versus a no-treatment control. 255 In another RCT among 82 Stage 0 to III breast cancer patients recently completing lumpectomy and adjuvant radiation with or without chemotherapy, women receiving 6 weeks of MBSR showed greater LPR to phytohemagglutinin (PHA) and an increased ratio of Th1:Th2 cell numbers versus TAU controls up to 2 weeks after the intervention.255,256 In another RCT, younger breast cancer survivors (<50 years) who had completed cancer treatment 3 months to 10 years prior were randomized to 6-week group-based mindfulness awareness practices (MAP) intervention (N = 39) or wait-list control (N = 32). Those assigned to the MAP intervention showed decreases in pre-post intervention perceived stress, and reduced leukocyte NF-κB and increased GR and IFN Type-I gene expression versus controls, suggestive of decreased inflammation and increased inflammatory control. 257 Although mindfulness has shown salutary biobehavioral effects in cancer patients these are based on small samples and short follow-up periods with unclear relevance for cancer progression. Future work should test the effects of mindfulness-based approaches on biobehavioral processes and longer-term health outcomes in larger samples of cancer patients.
Physical Stress Management Approaches
The National Center for Complementary and Integrative Health (NCCIH) classifies yoga, Tai Chi, massage, acupuncture as physical, or combined physical/psychological integrative medicine approaches. 229 Breast cancer survivors who had completed treatment assigned to 12 weeks of yoga showed decreased inflammatory markers in 2 RCTs.258,259 Kiecolt-Glaser et al 258 reported lower LPS-stimulated production of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α at 3-month follow-up in those assigned to Hatha yoga (N = 100) versus wait-list controls (N = 100). Bower and colleagues showed that breast cancer survivors with persistent fatigue randomized to a 12-week Iyengar yoga intervention (N = 16) demonstrated reduced leukocyte gene expression for N-κB and cAMP response element-binding protein (CREB) family transcription factors and increased GR gene expression versus those assigned to a 12-week health education control (N = 15) over a 3-month follow-up. Those in yoga also showed reduced sTNFR-II pre-post intervention versus controls but had no significant difference in changes in CRP, IL-6, or diurnal salivary cortisol. 259 These results point to lowered propensity for inflammation and greater inflammatory control, which may be related to both fatigue and recurrence.
An RCT examining effects of Tai-Chi showed decreased leukocyte inflammatory gene expression in breast cancer survivors with insomnia. 260 Compared to breast cancer survivors assigned to CBT for insomnia (CBT-I) those in the Tai-Chi condition (both 3 months of weekly sessions) showed greater reductions in monocyte production of IL-6 and TNF; and reduced leukocyte gene expression for pro-inflammatory mediators. Bioinformatics analyses inferred that these transcriptional changes were representative of reduced NF-kB signaling, and increased Type I Interferon anti-viral responding and antibody-making genes—mirroring the CTRA pattern—over 3 months, and that these transcriptional changes were largely accounted for by monocytes. 260
Physical exercise is another physical-based stress management approach that has been used in cancer patients. 73 Physical exercise interventions targeting physical activity, strength, and aerobic fitness have been shown to produce beneficial effects in cancer patients. 261 Findings showing short-term stress appears to enhance anti-tumor immunity 73 raise the possibility that the beneficial effects of exercise or physical activity in the context of cancer262,263 may work by activating short-term stress physiology and its effects on anti-tumor immunity. 73
More “passive” physical approaches involving body manipulations such as massage (breast cancer patients undergoing radiation), 264 acupuncture with warmed needles (moxibustion) (colorectal cancer patients undergoing chemotherapy), 265 and Biofield therapy/healing touch (cervical cancer patients receiving chemoradiation 266 ) showed increased or stabilized NK cell counts or NK cell cytotoxicity in cancer patients. The results of these trials of physical-based stress management interventions, though based on small samples and short follow-ups, are provocative, suggesting improved control over inflammation and enhanced cellular immunity, and encourage future work with larger samples and longer follow-up periods to assess effects on recurrence and survival.
While the RCTs for CBT-based approaches involved the largest samples sizes and are the only trials showing effects of stress management on both biobehavioral processes and long-term clinical outcomes, they may involve a large investment of time in learning techniques, a willingness to disclose in group formats, and therefore may be only feasible in highly motivated populations. Approaches that do not require patients’ exploration of psychological issues (Yoga, Tai-Chi, Biofield therapy) or challenging long-standing cognitive appraisal patterns and coping strategies (mindfulness meditation), may in fact be more preferable to some patients. Comparative effectiveness trials examining effects of different stress management and pharmacological approaches on patient-reported outcomes, biobehavioral processes, and long-term clinical outcomes may be used to address what works best for which patients.
Future Directions
Remotely-Delivered Interventions
Technological innovations make it now possible to offer psychological and mind-body interventions remotely. 267 Little is known about the ability of remotely-delivered empirically validated stress management interventions to affect stress and biobehavioral processes in cancer patients. 268 One trial showed that men with advanced prostate cancer assigned to an on-line CBSM intervention showed greater improvements in perceived stress management skills and quality of life and a steeper diurnal salivary cortisol slope at 6 months 269 but no differences in serum inflammatory cytokines compared to those assigned to an on-line health education control. 270 Other ongoing trials are examining the impact of remotely-delivered CBSM in breast 271 and prostate cancer patients 272 who are at earlier stages of disease. Because chronic stress can dampen the immune response to the influenza vaccine in older populations 273 and since treated cancer patients have 4 times the risk of influenza-related mortality, 274 one ongoing trial examines the effects of a remotely-delivered CBSM intervention on stress, inflammation and immunologic responses to influenza vaccine in distressed older women undergoing primary treatment for Stage 0 to III breast cancer. Another ongoing RCT is testing whether older Hispanic men with prostate cancer assigned to a similar 10-week remotely-delivered CBSM intervention (Spanish translated) show improvements in inflammatory gene expression, physical symptoms, and QoL. 272 These ongoing trials will require long-term follow-up (~10 years) to establish the clinical impact of remotely-delivered interventions.
Addressing Understudied and Underserved Populations
While stress management interventions have been efficacious in reducing self-reported stress and adversity in different patient groups including Black breast cancer survivors 275 and Hispanic men with prostate cancer, 276 effects on biobehavioral processes and clinical outcomes have been poorly characterized if at all. Expansion of stress management and mind-body trials to diverse populations and ethnic, cultural adaptation of interventions as indicated for these populations, and attention to feasibility/acceptability of interventions is important in this regard. Since stress processes contribute to poorer clinical outcomes in a wide range of solid tumors19,21,80,140,158,277 and hematologic cancers,219,278 it is imperative that trials evaluate the effects of stress management interventions in these and other cancers, especially in those conditions known to be characterized by racial/ethnic health disparities.
Considerations for Dissemination of Biobehavioral Research in Clinical Oncology Settings
Contemporary questions in intervention research address when, where, and for whom stress management interventions might be best used in clinical oncology settings (for review see Antoni and Dhabhar 73 ). It may be most fruitful to intervene to modulate biobehavioral processes at the earliest possible point in the cancer experience, for example, at the time of diagnosis or peri-surgically. With the emerging use of neo-adjuvant chemotherapy in the context of breast cancer it is plausible to test whether stress management interventions can improve the effectiveness of these regimens in shrinking tumor size between neoadjuvant initiation and surgical debulking, along with examination of the role of stress management on post-surgical outcomes.
Given the established effects of surgery on stress-related biobehavioral processes, 279 it is arguable that the peri-surgical period is an important point to explore in further biobehavioral intervention trials. 280 This setting has already been exploited in pharmacologic trials targeting biobehavioral stress processes.217,281-283 It will also be important to conduct trials testing the effects of “embedding” stress management interventions into adjuvant chemotherapy therapy settings such as chemotherapy infusion suites and the peri-and post hematopoietic stem cell transplant setting, where interventions could be delivered remotely to test effects on biobehavioral processes during treatment, and lasting clinical benefits between and beyond infusion visits. 73 In light of the strong links between stress processes and the immune response, biobehavioral processes may be important moderators of the effects of immunotherapy and should be investigated in that setting as well. Given research showing that stress-induced activation of neuroendocrine systems may compromise the effects of adjuvant therapies for cancer,284-286 and that some stress management interventions may decrease circulating levels of cortisol 242 and leukocyte glucocorticoid receptor expression 109 in breast cancer patients undergoing primary treatment, this raises the intriguing possibility of using pharmacologic or cognitive-behavioral stress management approaches to optimize the effectiveness of adjuvant chemotherapy regimens.
Future work should also test the effects of evidence-based interventions delivered in the period just after notification of recurrence. This is a very stressful period, possibly more stressful than the initial diagnosis of primary disease, where stress management skills may improve immune functioning and survival. 238 We also know little about the effects of stress management interventions later in the cancer treatment process. Most evidence on the effects of stress management interventions on biobehavioral processes comes from post-surgical patients or cancer “survivors” who have completed primary treatment only months prior. It remains to be determined whether these interventions can modulate biobehavioral processes in patients on longer-term oral endocrine or chemotherapeutic regimens or those with chronic (eg, hematologic cancers) or advanced cancers. For example, one large multisite psychosocial RCT for metastatic breast cancer patients failed to show effects of a supportive expressive group psychotherapy intervention on survival, suggesting that more needs to be understood about the impact of stress management on biologically advanced cancers. 287 It is possible that the effects of modulating stress through psychosocial interventions may be limited to early stages of disease. A meta-analysis of 15 randomized trials for cancer patients that met Cochrane criteria for methodological quality (N = 3000) found that although psychosocial interventions did not provide an overall survival benefit, interventions tested in patients with non-metastatic disease (6 trials; N = 1448) showed a 41% reduced risk of cancer mortality. 288 Beyond considerations of extent of disease and timing within curative and adjuvant treatment it is important to explore psychosocial and biomedical host factors that predict differential effects of one stress management approach over another (eg, mindfulness meditation vs CBT vs β-adrenergic blockade), or a combination of these approaches. Targeting specific stress management approaches for specific cancer patients may become another extension of precision oncology care in the future. 73
Implications for Clinical Practice
As increasing numbers of cancer patients recognize the effects of stress on cancer outcomes and actively pursue integrative and other complementary forms of adjunct cancer treatments, it is increasingly important that healthcare providers understand patients’ expectations and motivations 289 as well as the evidence-based literature supporting such interventions. As many integrative interventions such as meditation, yoga, Tai Chi, and QiGong are known to modulate neuroendocrine stress response systems as well as inflammation, it is likely that such integrative practices engage the systems described above and can impact mechanisms known to impact tumor growth. 290 One of the important lessons from the literature reviewed above is the role of systemic influences on tumor growth and development—the fact that tumor growth is influenced by signaling and metabolites from far outside the tumor microenvironment. This suggests that other integrative approaches that affect balance in the body including manipulative and body-based practices, biofield therapies, and whole medical systems as well as pharmacologic stress management approaches may have significant impacts on the tumor microenvironment.
Directions for Future Research
In summary, some of the key questions for future research address specificity of intervention doses, and integration of stress management approaches with medical care. Future research is needed to specify the extent of practice (or dose) of these interventions that is necessary to have clinically meaningful effects, the mechanisms by which such effects may occur, and windows in treatment when such interventions will be most effective (eg, peri-surgical, during or post treatment, post-recurrence). RCTs are needed to examine whether certain treatments are more well-suited to specific windows in treatment (eg, β-blockers and COX2 inhibitors during a peri-surgical window), 291 and to what extent are the effects of meditation or CBSM equivalent to those of β-blockers for those patients for whom β-blockers would be contraindicated. Many of the biomarker studies are short term and more research needs to address long-term health outcomes and differential efficacy of interventions for long-term outcomes. As noted above, RCTs testing efficacy of these interventions in diverse populations and cultural adaptation of interventions are critically needed. As the emergence of late effects of cancer treatment, including cardio-toxicity 292 and cancer-accelerated aging 293 have become more salient research concerns, testing the effects of stress management interventions and mechanisms of action on these processes presents another challenge for future research. RCTs examining whether stress modulating interventions can enhance efficacy of immunotherapy and decrease chemoresistance are also needed. Other questions for future research include how to provide optimal integration of these modalities with standard cancer care to provide a truly integrative approach.
Conclusions
Given the extensive nature of the evidence supporting a role of stress response systems in tumor growth, it becomes critical to integrate these principles in assessment and treatment of cancer patients who may be at risk, in both traditional and in integrative settings. With increasing research, supportive evidence, and patient interest in integrative care modalities, biobehavioral oncologic interventions that reduce cancer progression by modulating targeted molecular pathways are well poised to become an integral part of comprehensive cancer treatment.
Acknowledgments
We gratefully acknowledge Sharaf Zia for editorial assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MHA is a paid consultant for Blue Note Therapeutics, and Atlantis Healthcare, 2 digital health software companies specializing in developing psychosocial interventions for medical patients. EKS is on the scientific advisory board of Cygnal Therapeutics.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported in part by NIH grants R01CA193249, R01CA246540 (SKL), R01CA238562 (JMK), 1P30CA240139-01, 7R01CA206456-01A1, R01CA206456-03S1, UG3 CA260317, R37 CA255875, R01CA196953, Sylvester Comprehensive Cancer Center Bridge Grant PG013720 (MHA), P30CA086862 (PI G. Weiner), National Breast Cancer Foundation, Australia, IIRS-20-025, National Health and Medical Research Council, Australia grants: 1147498, 2002772, Cancer Council Victoria, Australia, Grant-in-Aid Scheme (EKS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
ORCID iD: Susan K. Lutgendorf  https://orcid.org/0000-0003-3793-5881
https://orcid.org/0000-0003-3793-5881
References
- 1. Karamanou M, Tzavellas E, Laios K, Koutsilieris M, Androutsos G. Melancholy as a risk factor for cancer: a historical overview. J BUON. 2016;21:756-759. [PubMed] [Google Scholar]
- 2. Mravec B, Tibensky M, Horvathova L. Stress and cancer. Part I: mechanisms mediating the effect of stressors on cancer. J Neuroimmunol. 2020;346:577311. [DOI] [PubMed] [Google Scholar]
- 3. Geyer S. Life events prior to manifestation of breast cancer: a limited prospective study covering eight years before diagnosis. J Psychosom Res. 1991;35:355-363. [DOI] [PubMed] [Google Scholar]
- 4. Michael YL, Carlson NE, Chlebowski RT, et al. Influence of stressors on breast cancer incidence in the Women’s Health Initiative. Health Psychol. 2009;28:137-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holman DM, Ports KA, Buchanan ND, et al. The association between adverse childhood experiences and risk of cancer in adulthood: a systematic review of the literature. Pediatrics. 2016;138:S81-S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fuller-Thomson E, Brennenstuhl S. Making a link between childhood physical abuse and cancer: results from a regional representative survey. Cancer. 2009;115:3341-3350. [DOI] [PubMed] [Google Scholar]
- 7. Lillberg K, Verkasalo PK, Kaprio J, Teppo L, Helenius H, Koskenvuo M. Stressful life events and risk of breast cancer in 10,808 women: a cohort study. Am J Epidemiol. 2003;157:415-423. [DOI] [PubMed] [Google Scholar]
- 8. Penninx BW, Guralnik JM, Pahor M, et al. Chronically depressed mood and cancer risk in older persons. J Natl Cancer Inst. 1998;90:1888-1893. [DOI] [PubMed] [Google Scholar]
- 9. Gross AL, Gallo JJ, Eaton WW. Depression and cancer risk: 24 years of follow-up of the Baltimore Epidemiologic Catchment Area sample. Cancer Causes Control. 2010;21:191-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schoemaker MJ, Jones ME, Wright LB, et al. Psychological stress, adverse life events and breast cancer incidence: a cohort investigation in 106,000 women in the United Kingdom. Breast Cancer Res. 2016;18:72-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Surtees PG, Wainwright NW, Luben RN, Khaw K-T, Bingham SA. No evidence that social stress is associated with breast cancer incidence. Breast Cancer Res Treat. 2010;120:169-174. [DOI] [PubMed] [Google Scholar]
- 12. Lillberg K, Verkasalo PK, Kaprio J, Teppo L, Helenius H, Koskenvuo M. Stress of daily activities and risk of breast cancer: a prospective cohort study in Finland. Int J Cancer. 2001;91:888-893. [DOI] [PubMed] [Google Scholar]
- 13. Falcinelli M, Thaker PH, Lutgendorf SK, Conzen SD, Flaherty RL, Flint MS. The role of psychologic stress in cancer initiation: clinical relevance and potential molecular mechanisms. Cancer Res. 2021;81:5131-5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palesh O, Butler LD, Koopman C, Giese-Davis J, Carlson R, Spiegel D. Stress history and breast cancer recurrence. J Psychosom Res. 2007;63:233-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol. 2006;24:1105-1111. [DOI] [PubMed] [Google Scholar]
- 16. Sprehn GC, Chambers JE, Saykin AJ, Konski A, Johnstone PA. Decreased cancer survival in individuals separated at time of diagnosis: critical period for cancer pathophysiology? Cancer. 2009;115:5108-5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kravdal O. The impact of marital status on cancer survival. Soc Sci Med. 2001;52:357-368. [DOI] [PubMed] [Google Scholar]
- 18. Hamer M, Chida Y, Molloy GJ. Psychological distress and cancer mortality. J Psychosom Res. 2009;66:255-258. [DOI] [PubMed] [Google Scholar]
- 19. Steel JL, Geller DA, Gamblin TC, Olek MC, Carr BI. Depression, immunity, and survival in patients with hepatobiliary carcinoma. J Clin Oncol. 2007;25:2397-2405. [DOI] [PubMed] [Google Scholar]
- 20. Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349-5361. [DOI] [PubMed] [Google Scholar]
- 21. Cohen L, Cole SW, Sood AK, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PLoS One. 2012;7:e42324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trudel-Fitzgerald C, Poole EM, Idahl A, et al. The association of work characteristics with ovarian cancer risk and mortality. Psychosom Med. 2017;79:1059-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang X, Wang N, Zhong L, et al. Prognostic value of depression and anxiety on breast cancer recurrence and mortality: a systematic review and meta-analysis of 282,203 patients. Mol Psychiatry. 2020;25:3186-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40:1797-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duijts SF, Zeegers MP, Borne BV. The association between stressful life events and breast cancer risk: a meta-analysis. Int J Cancer. 2003;107:1023-1029. [DOI] [PubMed] [Google Scholar]
- 26. Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466-475. [DOI] [PubMed] [Google Scholar]
- 27. Pinquart M, Duberstein PR. Associations of social networks with cancer mortality: a meta-analysis. Crit Rev Oncol Hematol. 2010;75:122-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weiner H. Perturbing the Organism: The Biology of Stressful Experience. University of Chicago Press; 1992. [Google Scholar]
- 29. Rohleder N. Stress and inflammation – the need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology. 2019;105:164-171. [DOI] [PubMed] [Google Scholar]
- 30. Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25-45. [DOI] [PubMed] [Google Scholar]
- 31. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873-904. [DOI] [PubMed] [Google Scholar]
- 32. Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374-381. [DOI] [PubMed] [Google Scholar]
- 33. Scott-Solomon E, Boehm E, Kuruvilla R. The sympathetic nervous system in development and disease. Nat Rev Neurosci. 2021;22:685-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Amit M, Takahashi H, Dragomir MP, et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature. 2020;578:449-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ma C, Gao T, Ju J, et al. Sympathetic innervation contributes to perineural invasion of salivary adenoid cystic carcinoma via the β2-adrenergic receptor. Onco Targets Ther. 2019;12:1475-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. [DOI] [PubMed] [Google Scholar]
- 37. Kamiya A, Hayama Y, Kato S, et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat Neurosci. 2019;22:1289-1305. [DOI] [PubMed] [Google Scholar]
- 38. Wu Y, Zeng L, Zhao S. Ligands of adrenergic receptors: a structural point of view. Biomolecules. 2021;11. doi: 10.3390/biom11070936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Elenkov IJ, Chrousos GP, Wilder RL. Neuroendocrine regulation of IL-12 and TNF-α/IL-10 balance: clinical implications. Ann N Y Acad Sci. 2006;917:94-105. [DOI] [PubMed] [Google Scholar]
- 40. Focke CMB, Iremonger KJ. Rhythmicity matters: circadian and ultradian patterns of HPA axis activity. Mol Cell Endocrinol. 2020;501:110652. [DOI] [PubMed] [Google Scholar]
- 41. Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE. Diurnal cortisol slopes and mental and physical health outcomes: a systematic review and meta-analysis. Psychoneuroendocrinology. 2017;83:25-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol Psychiatry. 2016;80:23-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eskandari F, Sternberg EM. Neural-immune interactions in health and disease. Ann N Y Acad Sci. 2002;966:20-27. [DOI] [PubMed] [Google Scholar]
- 44. McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921-939. [DOI] [PubMed] [Google Scholar]
- 45. Guidi J, Lucente M, Sonino N, Fava GA. Allostatic load and its impact on health: a systematic review. Psychother Psychosom. 2021;90:11-27. [DOI] [PubMed] [Google Scholar]
- 46. Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol. 2000;12:235-243. [DOI] [PubMed] [Google Scholar]
- 47. Li T, Wang P, Wang SC, Wang Y-F. Approaches mediating oxytocin regulation of the immune system. Front Immunol. 2017;7:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang D, Su S, Cui X, et al. Nerve fibers in breast cancer tissues indicate aggressive tumor progression. Medicine. 2014;93:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pundavela J, Roselli S, Faulkner S, et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol Oncol. 2015;9:1626-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao Q, Yang Y, Liang X, et al. The clinicopathological significance of neurogenesis in breast cancer. BMC Cancer. 2014;14:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferdoushi A, Griffin N, Marsland M, et al. Tumor innervation and clinical outcome in pancreatic cancer. Sci Rep. 2021;11:7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao CM, Hayakawa Y, Kodama Y, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6:250ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Allen JK, Armaiz-Pena GN, Nagaraja AS, et al. Sustained adrenergic signaling promotes intratumoral innervation through BDNF induction. Cancer Res. 2018;78:3233-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kappos EA, Engels PE, Tremp M, et al. Denervation leads to volume regression in breast cancer. J Plast Reconstr Aesthet Surg. 2018;71:833-839. [DOI] [PubMed] [Google Scholar]
- 55. Szpunar MJ, Belcher EK, Dawes RP, Madden KS. Sympathetic innervation, norepinephrine content, and norepinephrine turnover in orthotopic and spontaneous models of breast cancer. Brain Behav Immun. 2016;53:223-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Le CP, Nowell CJ, Kim-Fuchs C, et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nissen MD, Sloan EK, Mattarollo SR. β-adrenergic signaling impairs antitumor CD8+ T-cell responses to B-cell lymphoma immunotherapy. Cancer Immunol Res. 2018;6:98-109. doi: 10.1158/2326-6066.CIR-17-0401 [DOI] [PubMed] [Google Scholar]
- 58. Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042-7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zahalka AH, Arnal-Estapé A, Maryanovich M, et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science. 2017;358:321-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Conzen SD. Recent advances in understanding glucocorticoid receptor function in cancer. Clin Adv Hematol Oncol. 2017;15:338-340. [PMC free article] [PubMed] [Google Scholar]
- 61. Kach J, Conzen SD, Szmulewitz RZ. Targeting the glucocorticoid receptor in breast and prostate cancers. Sci Transl Med. 2015;7:305s19-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chang A, Le CP, Walker AK, et al. β2-adrenoceptors on tumor cells play a critical role in stress-enhanced metastasis in a mouse model of breast cancer. Brain Behav Immun. 2016;57:106-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Walker AK, Martelli D, Ziegler AI, et al. Circulating epinephrine is not required for chronic stress to enhance metastasis. Psychoneuroendocrinology. 2019;99:191-195. [DOI] [PubMed] [Google Scholar]
- 64. Kim-Fuchs C, Le CP, Pimentel MA, et al. Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun. 2014;40:40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939-944. [DOI] [PubMed] [Google Scholar]
- 66. Hassan S, Karpova Y, Baiz D, et al. Behavioral stress accelerates prostate cancer development in mice. J Clin Investig. 2013;123:874-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lin Q, Wang F, Yang R, Zheng X, Gao H, Zhang P. Effect of chronic restraint stress on human colorectal carcinoma growth in mice. PLoS One. 2013;8:e61435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhao L, Xu J, Liang F, Li A, Zhang Y, Sun J. Effect of chronic psychological stress on liver metastasis of colon cancer in mice. PLoS One. 2015;10:e0139978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jang HJ, Boo HJ, Lee HJ, Min HY, Lee HY. Chronic stress facilitates lung tumorigenesis by promoting exocytosis of IGF2 in lung epithelial cells. Cancer Res. 2016;76:6607-6619. [DOI] [PubMed] [Google Scholar]
- 70. Lamkin DM, Sloan EK, Patel AJ, Chiang BS, Pimentel MA, Ma JC, Arevalo JM, Morizono K, Cole SW. Chronic stress enhances progression of acute lymphoblastic leukemia via β-adrenergic signaling. Brain Behav Immun. 2012; 26:635–641. doi:10.1016/j.bbi.2012.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pedersen L, Idorn M, Olofsson GH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23:554-562. [DOI] [PubMed] [Google Scholar]
- 72. Liu C, Yang Y, Chen C, et al. Publisher correction: environmental eustress modulates β-ARs/CCL2 axis to induce anti-tumor immunity and sensitize immunotherapy against liver cancer in mice. Nat Commun. 2021;12:6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Antoni MH, Dhabhar FS. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer. 2019;125:1417-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Turner-Cobb JM, Sephton SE, Koopman C, Blake-Mortimer J, Spiegel D. Social support and salivary cortisol in women with metastatic breast cancer. Psychosom Med. 2000;62:337-345. [DOI] [PubMed] [Google Scholar]
- 75. Cuneo MG, Schrepf A, Slavich GM, et al. Diurnal cortisol rhythms, fatigue and psychosocial factors in five-year survivors of ovarian cancer. Psychoneuroendocrinology. 2017;84:139-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lutgendorf SK, DeGeest K, Sung CY, et al. GoodheartM, LubaroffD, FarleyDM, SoodAK, ColeSW. Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav Immun. 2009;23:176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hockenberry-Eaton M, Kemp V, DiIorio C. Cancer stressors and protective factors: predictors of stress experienced during treatment for childhood cancer. Res Nurs Health. 1994;17:351-361. [DOI] [PubMed] [Google Scholar]
- 78. Cuneo MG, Szeto A, Schrepf A, et al. Positive psychosocial factors and oxytocin in the ovarian tumor microenvironment. Psychosom Med. 2021;83:417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ho RT, Fong TC, Chan CK, Chan CL. The associations between diurnal cortisol patterns, self-perceived social support, and sleep behavior in Chinese breast cancer patients. Psychoneuroendocrinology. 2013;38:2337-2342. [DOI] [PubMed] [Google Scholar]
- 80. Lutgendorf SK, De Geest K, Bender D, et al. Social influences on clinical outcomes of patients with ovarian cancer. J Clin Oncol. 2012;30:2885-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Levy SM, Herberman RB, Whiteside T, Sanzo K, Lee J, Kirkwood J. Perceived social support and tumor estrogen/progesterone receptor status as predictors of natural killer cell activity in breast cancer patients. Psychosom Med. 1990;52:73-85. [DOI] [PubMed] [Google Scholar]
- 82. Levy S, Herberman R, Lippman M, d'Angelo T. Correlation of stress factors with sustained depression of natural killer cell activity and predicted prognosis in patients with breast cancer. J Clin Oncol. 1987;5:348-353. [DOI] [PubMed] [Google Scholar]
- 83. Levy SM, Herberman RB, Maluish AM, Schlien B, Lippman M. Prognostic risk assessment in primary breast cancer by behavioral and immunological parameters. Health Psychol. 1985;4:99-113. [DOI] [PubMed] [Google Scholar]
- 84. Andersen BL, Farrar WB, Golden-Kreutz D, et al. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998;90:30-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Thornton LM, Andersen BL, Crespin TR, Carson WE. Individual trajectories in stress covary with immunity during recovery from cancer diagnosis and treatments. Brain Behav Immun. 2007;21:185-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lekander M, Fürst CJ, Rotstein S, Blomgren H, Fredrikson M. Social support and immune status during and after chemotherapy for breast cancer. Acta Oncol. 1996;35:31-37. [DOI] [PubMed] [Google Scholar]
- 87. Jutagir DR, Blomberg BB, Carver CS, et al. Social well-being is associated with less pro-inflammatory and pro-metastatic leukocyte gene expression in women after surgery for breast cancer. Breast Cancer Res Treat. 2017;165:169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lutgendorf SK, Sood AK, Anderson B, et al. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;23:7105-7113. [DOI] [PubMed] [Google Scholar]
- 89. Figueira JSB, Pacheco LB, Lobo I, et al. “Keep that in mind!” The role of positive affect in working memory for maintaining goal-relevant information. Front Psychol. 2018;9. doi: 10.3389/fpsyg.2018.01228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Watson D, Tellegen A. Toward a consensual structure of mood. Psychol Bull. 1985;98:219-235. [DOI] [PubMed] [Google Scholar]
- 91. Blomberg BB, Alvarez JP, Diaz A, et al. Psychosocial adaptation and cellular immunity in breast cancer patients in the weeks after surgery: an exploratory study. J Psychosom Res. 2009;67:369-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lutgendorf SK, Lamkin DM, DeGeest K, et al. Depressed and anxious mood and T-cell cytokine expressing populations in ovarian cancer patients. Brain Behav Immun. 2008;22:890-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Fagundes CP, Glaser R, Johnson SL, et al. Basal cell carcinoma: stressful life events and the tumor environment. Arch Gen Psychiatry. 2012;69:618-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J Exp Med. 2014;211:2583-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sloan EK, Tarara RP, Capitanio JP, Cole SW. Enhanced replication of simian immunodeficiency virus adjacent to catecholaminergic varicosities in primate lymph nodes. J Virol. 2006;80:4326-4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kohm AP, Sanders VM. Norepinephrine and β2-adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev. 2001;53:487-525. [PubMed] [Google Scholar]
- 97. Mohammadpour H, MacDonald CR, Qiao G, et al. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Investig. 2019;129:5537-5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mohammadpour H, MacDonald CR, McCarthy PL, Abrams SI, Repasky EA. β2-adrenergic receptor signaling regulates metabolic pathways critical to myeloid-derived suppressor cell function within the TME. Cell Rep. 2021;37:109883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kokolus KM, Capitano ML, Lee C-T, et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci USA. 2013;110:20176-20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Benish M, Bartal I, Goldfarb Y, et al. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. 2008;15:2042-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [DOI] [PubMed] [Google Scholar]
- 102. Hagemann T, Balkwill F, Lawrence T. Inflammation and cancer: a double-edged sword. Cancer Cell. 2007;12:300-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Spannuth WA, Sood AK, Coleman RL. Angiogenesis as a strategic target for ovarian cancer therapy. Nat Clin Pract Oncol. 2008;5:194-204. [DOI] [PubMed] [Google Scholar]
- 104. Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65:10794-10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Costanzo ES, Lutgendorf SK, Sood AK, Anderson B, Sorosky J, Lubaroff DM. Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer. 2005;104:305-313. [DOI] [PubMed] [Google Scholar]
- 106. Bouchard LC, Antoni MH, Blomberg BB, et al. Post-surgical depressive symptoms and pro-inflammatory cytokine elevations in women undergoing primary treatment for breast cancer. Psychosom Med. 2016;78:26-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bower JE, Shiao SL, Sullivan P, et al. Prometastatic molecular profiles in breast tumors from socially isolated women. JNCI Cancer Spectr. 2018;2:y029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Taub C, Diaz A, Blomberg BB, et al. Greater serum cortisol relates to self-reported cancer-related distress and RAGE-associated s100A8/A9 levels in women with non-metastatic breast cancer (under review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Antoni MH, Lutgendorf SK, Blomberg B, et al. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol Psychiatry. 2012;71:366-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve—an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595-638. [PubMed] [Google Scholar]
- 112. Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lamkin DM, Ho HY, Ong TH, et al. β-Adrenergic-stimulated macrophages: comprehensive localization in the M1-M2 spectrum. Brain Behav Immun. 2016;57:338-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Powell ND, Sloan EK, Bailey MT, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA. 2013;110:16574-16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Heidt T, Sager HB, Courties G, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Yin C, Li H, Zhang B, et al. RAGE-binding S100A8/A9 promotes the migration and invasion of human breast cancer cells through actin polymerization and epithelial-mesenchymal transition. Breast Cancer Res Treat. 2013;142:297-309. [DOI] [PubMed] [Google Scholar]
- 117. Drews-Elger K, Iorns E, Dias A, et al. Infiltrating S100A8+ myeloid cells promote metastatic spread of human breast cancer and predict poor clinical outcome. Breast Cancer Res Treat. 2014;148:41-59. [DOI] [PubMed] [Google Scholar]
- 118. Perego M, Tyurin VA, Tyurina YY, et al. Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. Sci Transl Med. 2020;12:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Armaiz-Pena GN, Gonzalez-Villasana V, Nagaraja AS, et al. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget. 2015;6:4266-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Jiang W, Li Y, Li ZZ, et al. Chronic restraint stress promotes hepatocellular carcinoma growth by mobilizing splenic myeloid cells through activating β-adrenergic signaling. Brain Behav Immun. 2019;80:825-838. [DOI] [PubMed] [Google Scholar]
- 121. Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25:397-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gut–brain circuit. Nature. 2020;583:441-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Folkman J. Proceedings: tumor angiogenesis factor. Cancer Res. 1974;34:2109-2113. [PubMed] [Google Scholar]
- 124. Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407-424. [DOI] [PubMed] [Google Scholar]
- 125. Folkman J. What is the evidence that tumors are angiogenesis dependant? J Natl Cancer Inst. 1990;82:4-7. [DOI] [PubMed] [Google Scholar]
- 126. Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442-447. [DOI] [PubMed] [Google Scholar]
- 127. Nausheen B, Carr NJ, Peveler RC, et al. Relationship between loneliness and proangiogenic cytokines in newly diagnosed tumors of colon and rectum. Psychosom Med. 2010;72:912-916. [DOI] [PubMed] [Google Scholar]
- 128. Sharma A, Greenman J, Sharp DM, Walker LG, Monson JR. Vascular endothelial growth factor and psychosocial factors in colorectal cancer. Psychooncology. 2008;17:66-73. [DOI] [PubMed] [Google Scholar]
- 129. Lutgendorf SK, Johnsen EL, Cooper B, et al. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95:808-815. [DOI] [PubMed] [Google Scholar]
- 130. Lutgendorf SK, Lamkin DM, Jennings NB, et al. Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin Cancer Res. 2008;14:6839-6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhou J, Liu Z, Zhang L, et al. Activation of β2-adrenergic receptor promotes growth and angiogenesis in breast cancer by down-regulating PPARγ. Cancer Res Treat. 2020;52:830-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yang EV, Kim SJ, Donovan EL, et al. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23:267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yang EV, Sood AK, Chen M, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66:10357-10364. [DOI] [PubMed] [Google Scholar]
- 134. Nilsson MB, Armaiz-Pena G, Takahashi R, et al. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a SRC-dependent mechanism. J Biol Chem. 2007;282:29919-29926. [DOI] [PubMed] [Google Scholar]
- 135. Devi S, Alexandre YO, Loi JK, et al. Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity. 2021;54:1219-1230.e7. [DOI] [PubMed] [Google Scholar]
- 136. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investig. 2009;119:1420-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Loret N, Denys H, Tummers P, Berx G. The role of epithelial-to-mesenchymal plasticity in ovarian cancer progression and therapy resistance. Cancers. 2019;11:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Soundararajan R, Fradette JJ, Konen JM, et al. Targeting the interplay between epithelial-to-mesenchymal-transition and the immune system for effective immunotherapy. Cancers. 2019;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lutgendorf SK, Penedo F, Goodheart MJ, et al. Epithelial-mesenchymal transition polarization in ovarian carcinomas from patients with high social isolation. Cancer. 2020;126:4407-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lutgendorf SK, Thaker PH, Arevalo JM, et al. Biobehavioral modulation of the exosome transcriptome in ovarian carcinoma. Cancer. 2018;124:580-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Hagemann T, Robinson SC, Schulz M, Trümper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25:1543-1549. [DOI] [PubMed] [Google Scholar]
- 142. Sood AK, Bhatty R, Kamat AA, et al. Stress hormone – mediated invasion of ovarian cancer cells. Clin Cancer Res. 2006;12:369-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Creed SJ, Le CP, Hassan M, et al. β2-adrenoceptor signaling regulates invadopodia formation to enhance tumor cell invasion. Breast Cancer Res. 2015;17:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kim TH, Gill NK, Nyberg KD, et al. Cancer cells become less deformable and more invasive with activation of β-adrenergic signaling. J Cell Sci. 2016;129:4563-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pon CK, Lane JR, Sloan EK, Halls ML. The β2-adrenoceptor activates a positive cAMP-calcium feedforward loop to drive breast cancer cell invasion. FASEB J. 2016;30:1144-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yawata A, Adachi M, Okuda H, et al. Prolonged cell survival enhances peritoneal dissemination of gastric cancer cells. Oncogene. 1998;16:2681-2686. [DOI] [PubMed] [Google Scholar]
- 147. Shanmugathasan M, Jothy S. Apoptosis, anoikis and their relevance to the pathobiology of colon cancer. Pathol Int. 2000;50:273-279. [DOI] [PubMed] [Google Scholar]
- 148. Sood AK, Armaiz-Pena GN, Halder J, et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Investig. 2010;120:1515-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Moran TJ, Gray S, Mikosz CA, Conzen SD. The glucocorticoid receptor mediates a survival signal in human mammary epithelial cells. Cancer Res. 2000;60:867-872. [PubMed] [Google Scholar]
- 150. Flaherty RL, Owen M, Fagan-Murphy A, et al. Glucocorticoids induce production of reactive oxygen species/reactive nitrogen species and DNA damage through an iNOS mediated pathway in breast cancer. Breast Cancer Res. 2017;19:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Obradović MMS, Hamelin B, Manevski N, et al. Glucocorticoids promote breast cancer metastasis. Nature. 2019;567:540-544. [DOI] [PubMed] [Google Scholar]
- 152. Pan D, Kocherginsky M, Conzen SD. Activation of the glucocorticoid receptor is associated with poor prognosis in estrogen receptor-negative breast cancer. Cancer Res. 2011;71:6360-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zhang C, Beckermann B, Kallifatidis G, et al. Corticosteroids induce chemotherapy resistance in the majority of tumour cells from bone, brain, breast, cervix, melanoma and neuroblastoma. Internet J Oncol. 2006;29:1295-1301. [PubMed] [Google Scholar]
- 154. Volden PA, Conzen SD. The influence of glucocorticoid signaling on tumor progression. Brain Behav Immun. 2013;30:S26-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. West DC, Kocherginsky M, Tonsing-Carter EY, et al. Discovery of a glucocorticoid receptor (GR) activity signature using selective GR antagonism in ER-negative breast cancer. Clin Cancer Res. 2018;24:3433-3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Schrepf A, Thaker PH, Goodheart MJ, et al. Diurnal cortisol and survival in epithelial ovarian cancer. Psychoneuroendocrinology. 2015;53:256-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sephton S, Sapolsky RM, Kraemer HC, Speigel D. Early mortality in metastatic breast cancer patients with absent of abnormal diurnal cortisol rhythms. J Natl Cancer Inst. 2000;92:994-1000. [DOI] [PubMed] [Google Scholar]
- 158. Sephton SE, Lush E, Dedert EA, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30 Suppl:S163-S170. [DOI] [PubMed] [Google Scholar]
- 159. Pang JM, Huang Y-C, Sun S-P, et al. Effects of synthetic glucocorticoids on breast cancer progression. Steroids. 2020;164:108738. [DOI] [PubMed] [Google Scholar]
- 160. Ji H, Liu N, Yin Y, et al. Oxytocin inhibits ovarian cancer metastasis by repressing the expression of MMP-2 and VEGF. J Cancer. 2018;9:1379-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Morita T, Shibata K, Kikkawa F, Kajiyama H, Ino K, Mizutani S. Oxytocin inhibits the progression of human ovarian carcinoma cells in vitro and in vivo. Int J Cancer. 2004;109:525-532. [DOI] [PubMed] [Google Scholar]
- 162. Mankarious A, Dave F, Pados G, et al. The pro-social neurohormone oxytocin reverses the actions of the stress hormone cortisol in human ovarian carcinoma cells in vitro. Internet J Oncol. 2016;48:1805-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Cuneo MG, Szeto A, Schrepf A, et al. Oxytocin in the tumor microenvironment is associated with lower inflammation and longer survival in advanced epithelial ovarian cancer patients. Psychoneuroendocrinology. 2019;106:244-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Green McDonald P, O’Connell M, Lutgendorf SK. Psychoneuroimmunology and cancer: a decade of discovery, paradigm shifts, and methodological innovations. Brain Behav Immun. 2013;30 Suppl:S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Taylor SE, Kemeny ME, Reed GM, Bower JE, Gruenewald TL. Psychological resources, positive illusions, and health. Am Psychol. 2000;55:99-109. [DOI] [PubMed] [Google Scholar]
- 166. Ryff CD. Psychological well-being revisited: advances in the science and practice of eudaimonia. Psychother Psychosom. 2014;83:10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Boehm JK, Vie LL, Kubzansky LD. The promise of well-being interventions for improving health risk behaviors. Curr Cardiovasc Risk Rep. 2012;6:511-519. [Google Scholar]
- 168. Bower JE, Low CA, Moskowitz JT, Sepah S, Epel E. Benefit finding and physical health: positive psychological changes and enhanced allostasis. Soc Personal Psychol Compass. 2008;2:223-244. [Google Scholar]
- 169. Pascoe L, Edvardsson D. Benefit finding in cancer: a review of influencing factors and health outcomes. Eur J Oncol Nurs. 2013;17:760-766. [DOI] [PubMed] [Google Scholar]
- 170. Park CL, Lechner SC, Antoni MH, Stanton AL. Medical Illness and Positive Life Change: Can Crisis Lead to Personal Transformation? American Psychological Association; 2009. [Google Scholar]
- 171. Dunigan JT, Carr BI, Steel JL. Posttraumatic growth, immunity and survival in patients with hepatoma. Dig Dis Sci. 2007;52:2452-2459. [DOI] [PubMed] [Google Scholar]
- 172. Wang AW, Hoyt MA. Benefit finding and diurnal cortisol after prostate cancer: the mediating role of positive affect. Psychooncology. 2018;27:1200-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Cruess DG, Antoni MH, McGregor BA, et al. Cognitive-behavioral stress management reduces serum cortisol by enhancing benefit finding among women being treated for early stage breast cancer. Psychosom Med. 2000;62:304-308. [DOI] [PubMed] [Google Scholar]
- 174. Diaz M, Aldridge-Gerry A, Spiegel D. Posttraumatic growth and diurnal cortisol slope among women with metastatic breast cancer. Psychoneuroendocrinology. 2014;44:83-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. McGregor BA, Antoni MH, Boyers A, Alferi SM, Blomberg BB, Carver CS. Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. J Psychosom Res. 2004;56:1-8. [DOI] [PubMed] [Google Scholar]
- 176. Ratcliff CG, Milbury K, Chandwani KD, et al. Examining mediators and moderators of yoga for women with breast cancer undergoing radiotherapy. Integr Cancer Ther. 2016;15:250-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Lassmann I, Dinkel A, Marten-Mittag B, et al. Benefit finding in long-term prostate cancer survivors. Support Care Cancer. 2021;29:4451-4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Lechner SC, Zakowski SG, Antoni MH, Greenhawt M, Block K, Block P. Do sociodemographic and disease-related variables influence benefit-finding in cancer patients? Psychooncology. 2003;12:491-499. [DOI] [PubMed] [Google Scholar]
- 179. Ryff CD, Singer BH. Know thyself and become what you are: a eudaimonic approach to psychological well-being.J Happiness Stud. 2008;9:13-39. [Google Scholar]
- 180. Fredrickson BL, Grewen KM, Coffey KA, et al. A functional genomic perspective on human well-being. Proc Natl Acad Sci USA. 2013;110:13684-13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Cole SW, Levine ME, Arevalo JM, Ma J, Weir DR, Crimmins EM. Loneliness, eudaimonia, and the human conserved transcriptional response to adversity. Psychoneuroendocrinology. 2015;62:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Seeman T, Merkin SS, Goldwater D, Cole SW. Intergenerational mentoring, eudaimonic well-being and gene regulation in older adults: a pilot study. Psychoneuroendocrinology. 2020;111:104468. [DOI] [PubMed] [Google Scholar]
- 183. Boyle CC, Cole SW, Dutcher JM, Eisenberger NI, Bower JE. Changes in eudaimonic well-being and the conserved transcriptional response to adversity in younger breast cancer survivors. Psychoneuroendocrinology. 2019;103:173-179. [DOI] [PubMed] [Google Scholar]
- 184. Prinsloo S, Wei Q, Scott SM, et al. Psychological states, serum markers and survival: associations and predictors of survival in patients with renal cell carcinoma. J Behav Med. 2015;38:48-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Moreno PI, Moskowitz AL, Ganz PA, Bower JE. Positive affect and inflammatory activity in breast cancer survivors: examining the role of affective arousal. Psychosom Med. 2016;78:532-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Udumyan R, Montgomery S, Fang F, Valdimarsdottir U, Fall K. Stress resilience in late adolescence and survival among cancer patients: a Swedish register-based cohort study. Cancer Epidemiol Biomarkers Prev. 2019;28:400-408. [DOI] [PubMed] [Google Scholar]
- 187. Seiler A, Jenewein J. Resilience in cancer patients. Front Psychiatry. 2019;10:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Powe DG, Voss MJ, Zänker KS, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29:2635-2644. [DOI] [PubMed] [Google Scholar]
- 190. De Giorgi V, Grazzini M, Gandini S, et al. Treatment with β-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med. 2011;171:779-781. [DOI] [PubMed] [Google Scholar]
- 191. Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645-2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Jansen L, Hoffmeister M, Arndt V, Chang-Claude J, Brenner H. Stage-specific associations between beta blocker use and prognosis after colorectal cancer. Cancer. 2014;120:1178-1186. [DOI] [PubMed] [Google Scholar]
- 193. Choi CH, Song T, Kim TH, et al. Meta-analysis of the effects of beta blocker on survival time in cancer patients. J Cancer Res Clin Oncol. 2014;140:1179-1188. [DOI] [PubMed] [Google Scholar]
- 194. Grytli HH, Fagerland MW, Fosså SD, Taskén KA. Association between use of β-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol. 2014;65:635-641. [DOI] [PubMed] [Google Scholar]
- 195. Watkins JL, Thaker PH, Nick AM, et al. Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer. 2015;121:3444-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Udumyan R, Montgomery S, Fang F, et al. Beta-blocker drug use and survival among patients with pancreatic adenocarcinoma. Cancer Res. 2017;77:3700-3707. [DOI] [PubMed] [Google Scholar]
- 197. Fiala O, Ostasov P, Sorejs O, et al. Incidental use of beta-blockers is associated with outcome of metastatic colorectal cancer patients treated with bevacizumab-based therapy: a single-institution retrospective analysis of 514 patients. Cancers. 2019;11:1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Aydiner A, Ciftci R, Karabulut S, Kilic L. Does beta-blocker therapy improve the survival of patients with metastatic non-small cell lung cancer? Asian Pac J Cancer Prev. 2013;14:6109-6114. [DOI] [PubMed] [Google Scholar]
- 199. Wang HM, Liao ZX, Komaki R, et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol. 2013;24:1312-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Hwa YL, Shi Q, Kumar SK, et al. Beta-blockers improve survival outcomes in patients with multiple myeloma: a retrospective evaluation. Am J Hematol. 2017;92:50-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Cardwell CR, Coleman HG, Murray LJ, Entschladen F, Powe DG. Beta-blocker usage and breast cancer survival: a nested case-control study within a UK clinical practice research datalink cohort. Internet J Epidemiol. 2013;42:1852-1861. [DOI] [PubMed] [Google Scholar]
- 202. McCourt C, Coleman HG, Murray LJ, et al. Beta-blocker usage after malignant melanoma diagnosis and survival: a population-based nested case-control study. Br J Dermatol. 2014;170:930-938. [DOI] [PubMed] [Google Scholar]
- 203. Hicks BM, Murray LJ, Powe DG, Hughes CM, Cardwell CR. β-Blocker usage and colorectal cancer mortality: a nested case-control study in the UK Clinical Practice Research Datalink cohort. Ann Oncol. 2013;24:3100-3106. [DOI] [PubMed] [Google Scholar]
- 204. Jansen L, Weberpals J, Kuiper JG, et al. Pre- and post-diagnostic beta-blocker use and prognosis after colorectal cancer: results from a population-based study. Int J Cancer. 2017;141:62-71. [DOI] [PubMed] [Google Scholar]
- 205. Cata JP, Villarreal J, Keerty D, et al. Perioperative beta-blocker use and survival in lung cancer patients. J Clin Anesth. 2014;26:106-117. [DOI] [PubMed] [Google Scholar]
- 206. Zhang B, Wu C, Chen W, et al. The stress hormone norepinephrine promotes tumor progression through β2-adrenoreceptors in oral cancer. Arch Oral Biol. 2020;113:104712. [DOI] [PubMed] [Google Scholar]
- 207. Zhang M, Wang Q, Sun X, et al. β2-adrenergic receptor signaling drives prostate cancer progression by targeting the Sonic hedgehog-Gli1 signaling activation. Prostate. 2020;80:1328-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Renz BW, Takahashi R, Tanaka T, et al. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;34:863-890.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Lucido CT, Callejas-Valera JL, Colbert PL, et al. β(2)-Adrenergic receptor modulates mitochondrial metabolism and disease progression in recurrent/metastatic HPV(+) HNSCC. Oncogenesis. 2018;7:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Haldar R, Ricon-Becker I, Radin A, et al. Perioperative COX2 andβ-adrenergic blockade improves biomarkers of tumor metastasis, immunity, and inflammation in colorectal cancer: a randomized controlled trial. Cancer. 2020;126:3991-4001. [DOI] [PubMed] [Google Scholar]
- 211. Haldar R, Shaashua L, Lavon H, et al. Perioperative inhibition of β-adrenergic and COX2 signaling in a clinical trial in breast cancer patients improves tumor Ki-67 expression, serum cytokine levels, and PBMCs transcriptome. Brain Behav Immun. 2018;73:294-309. [DOI] [PubMed] [Google Scholar]
- 212. Hiller JG, Cole SW, Crone EM, et al. Preoperative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a phase II randomized trial. Clin Cancer Res. 2020;26:1803-1811. [DOI] [PubMed] [Google Scholar]
- 213. Jang H-I, Lim S-H, Lee Y-Y, et al. Perioperative administration of propranolol to women undergoing ovarian cancer surgery: a pilot study. Obstet Gynecol Sci. 2017;60:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Knight JM, Kerswill SA, Hari P, et al. Repurposing existing medications as cancer therapy: design and feasibility of a randomized pilot investigating propranolol administration in patients receiving hematopoietic cell transplantation. BMC Cancer. 2018;18:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Knight JM, Rizzo JD, Hari P, et al. Propranolol inhibits molecular risk markers in HCT recipients: a phase 2 randomized controlled biomarker trial. Blood Adv. 2020;4:467-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Ramondetta LM, Hu W, Thaker PH, et al. Prospective pilot trial with combination of propranolol with chemotherapy in patients with epithelial ovarian cancer and evaluation on circulating immune cell gene expression. Gynecol Oncol. 2019;154:524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Shaashua L, Shabat-Simon M, Haldar R, et al. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin Cancer Res. 2017;23:4651-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Zhou L, Li Y, Li X, et al. Propranolol attenuates surgical stress-induced elevation of the regulatory T cell response in patients undergoing radical mastectomy. J Immunol. 2016;196:3460-3469. [DOI] [PubMed] [Google Scholar]
- 219. Knight JM, Rizzo JD, Logan BR, et al. Low socioeconomic status, adverse gene expression profiles, and clinical outcomes in hematopoietic stem cell transplant recipients. Clin Cancer Res. 2016;22:69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Antoni MH, Bouchard LC, Jacobs JM, et al. Stress management, leukocyte transcriptional changes and breast cancer recurrence in a randomized trial: an exploratory analysis. Psychoneuroendocrinology. 2016;74:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. De Giorgi V, Grazzini M, Benemei S, et al. Propranolol for off-label treatment of patients with melanoma: results from a cohort study. JAMA Oncol. 2018;4:e172908-e172908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Yamamoto H, Hamasaki T, Onda K, et al. Landiolol, an ultra-short acting beta-1 blocker, for preventing postoperative lung cancer recurrence: study protocol for a phase III, multicenter randomized trial with two parallel groups of patients. Trials. 2019;20:715-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP. Repurposing drugs in your medicine cabinet: untapped opportunities for cancer therapy? Future Oncol. 2015;11:181-184. [DOI] [PubMed] [Google Scholar]
- 224. Gandhi S, Pandey MR, Attwood K, et al. Phase I clinical trial of combination propranolol and pembrolizumab in locally advanced and metastatic melanoma: safety, tolerability, and preliminary evidence of antitumor activity. Clin Cancer Res. 2021;27:87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Hopson MB, Lee S, Accordino M, et al. Phase II study of propranolol feasibility with neoadjuvant chemotherapy in patients with newly diagnosed breast cancer. Breast Cancer Res Treat. 2021;188:427-432. [DOI] [PubMed] [Google Scholar]
- 226. Andersen BL, Farrar WB, Golden-Kreutz DM, et al. Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J Clin Oncol. 2004;22:3570-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Antoni MH. Psychoneuroendocrinology and psychoneuroimmunology of cancer: plausible mechanisms worth pursuing? Brain Behav Immun. 2003;17 Suppl 1:S84-S91. [DOI] [PubMed] [Google Scholar]
- 228. Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom Med. 2003;65:571-581. [DOI] [PubMed] [Google Scholar]
- 229. NCCIH strategic plan FY 2021–2025: mapping the pathway to research on whole person health. https://www.nccih.nih.gov/about/nccih-strategic-plan-2021-2025 (accessed 12 February 2022).
- 230. Eckerling A, Ricon-Becker I, Sorski L, Sandbank E, Ben-Eliyahu S. Stress and cancer: mechanisms, significance and future directions. Nat Rev Cancer. 2021;21:767-785. [DOI] [PubMed] [Google Scholar]
- 231. Fawzy FI, Cousins N, Fawzy NW, Kemeny ME, Elashoff R, Morton D. A structured psychiatric intervention for cancer patients: I. Changes over time in methods of coping and affective disturbance. Arch Gen Psychiatry. 1990;47:720-725. [DOI] [PubMed] [Google Scholar]
- 232. Fawzy FI, Kemeny ME, Fawzy NW, et al. A structured psychiatric intervention for cancer patients: II. Changes over time in immunological measures. Arch Gen Psychiatry. 1990;47:729-735. [DOI] [PubMed] [Google Scholar]
- 233. Fawzy FI, Canada AL, Fawzy NW. Malignant melanoma: effects of a brief, structured psychiatric intervention on survival and recurrence at 10-year follow-up. Arch Gen Psychiatry. 2003;60:100-103. [DOI] [PubMed] [Google Scholar]
- 234. Fawzy FI, Fawzy NW, Hyun CS, et al. Malignant melanoma: effects of an early structured psychiatric intervention, coping, and affective state on recurrence and survival 6 years later. Arch Gen Psychiatry. 1993;50:681-689. [DOI] [PubMed] [Google Scholar]
- 235. Andersen BL, Farrar WB, Golden-Kreutz D, et al. Distress reduction from a psychological intervention contributes to improved health for cancer patients. Brain Behav Immun. 2007;21:953-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: a randomized clinical trial. Cancer. 2008;113:3450-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Thornton LM, Andersen BL, Schuler TA, Carson WE, 3rd. A psychological intervention reduces inflammatory markers by alleviating depressive symptoms: secondary analysis of a randomized controlled trial. Psychosom Med. 2009;71:715-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Andersen BL, Thornton LM, Shapiro CL, et al. Biobehavioral, immune, and health benefits following recurrence for psychological intervention participants. Clin Cancer Res. 2010;16:3270-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Antoni MH, Lechner SC, Kazi A, et al. How stress management improves quality of life after treatment for breast cancer. J Consult Clin Psychol. 2006;74:1143-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Antoni MH, Lehman JM, Kilbourn KM, et al. Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychol. 2001;20:20-32. [DOI] [PubMed] [Google Scholar]
- 241. Antoni MH, Wimberly SR, Lechner SC, et al. Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. Am J Psychiatr. 2006;163:1791-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Phillips KM, Antoni MH, Lechner SC, et al. Stress management intervention reduces serum cortisol and increases relaxation during treatment for nonmetastatic breast cancer. Psychosom Med. 2008;70:1044-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Antoni MH, Lechner S, Diaz A, et al. Cognitive behavioral stress management effects on psychosocial and physiological adaptation in women undergoing treatment for breast cancer. Brain Behav Immun. 2009;23:580-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Ni L, Lu J. Interferon gamma in cancer immunotherapy. Cancer Med. 2018;7:4509-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Mortara L, Balza E, Bruno A, Poggi A, Orecchia P, Carnemolla B. Anti-cancer therapies employing IL-2 cytokine tumor targeting: contribution of innate, adaptive and immunosuppressive cells in the anti-tumor efficacy. Front Immunol. 2018;9:2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistancein macrophages. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1799-R1805. [DOI] [PubMed] [Google Scholar]
- 247. Miller GE, Chen E, Sze J, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Miller GE, Chen E, Fok AK, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci USA. 2009;106:14716-14721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Stagl JM, Lechner SC, Carver CS, et al. A randomized controlled trial of cognitive-behavioral stress management in breast cancer: survival and recurrence at 11-year follow-up. Breast Cancer Res Treat. 2015;154:319-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Kwak T, Drews-Elger K, Ergonul A, et al. Targeting of RAGE-ligand signaling impairs breast cancer cell invasion and metastasis. Oncogene. 2017;36:1559-1572. [DOI] [PubMed] [Google Scholar]
- 251. Gudenkauf LM, Antoni MH, Stagl JM, et al. Brief cognitive-behavioral and relaxation training interventions for breast cancer: a randomized controlled trial. J Consult Clin Psychol. 2015;83:677-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Taub CJ, Lippman ME, Hudson BI, et al. The effects of a randomized trial of brief forms of stress management on RAGE-associated S100A8/A9 in patients with breast cancer undergoing primary treatment. Cancer. 2019;125:1717-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Diaz A, Taub CJ, Lippman ME, Antoni MH, Blomberg BB. Effects of brief stress management interventions on distress and leukocyte nuclear factor kappa B expression during primary treatment for breast cancer: a randomized trial. Psychoneuroendocrinology. 2021;126:105163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21:1038-1049. [DOI] [PubMed] [Google Scholar]
- 255. Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, Mathews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. 2008;22:969-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Lengacher CA, Kip KE, Post-White J, et al. Lymphocyte recovery after breast cancer treatment and mindfulness-based stress reduction (MBSR) therapy. Biol Res Nurs. 2013;15:37-47. [DOI] [PubMed] [Google Scholar]
- 257. Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: a randomized controlled trial. Cancer. 2015;121:1231-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Kiecolt-Glaser JK, Bennett JM, Andridge R, et al. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J Clin Oncol. 2014;32:1040-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Bower JE, Greendale G, Crosswell AD, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology. 2014;43:20-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Irwin MR, Olmstead R, Breen EC, et al. Tai chi, cellular inflammation, and transcriptome dynamics in breast cancer survivors with insomnia: a randomized controlled trial. J Natl Cancer Inst Monographs. 2014;2014:295-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Betof AS, Dewhirst MW, Jones LW. Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain Behav Immun. 2013;30 Suppl:S75-S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87-100. [DOI] [PubMed] [Google Scholar]
- 264. Hernandez-Reif M, Field T, Ironson G, et al. Natural killer cells and lymphocytes increase in women with breast cancer following massage therapy. Int J Neurosci. 2005;115:495-510. [DOI] [PubMed] [Google Scholar]
- 265. Pais I, Correia N, Pimentel I, et al. Effects of acupuncture on leucopenia, neutropenia, NK, and B cells in cancer patients: a randomized pilot study. Evid Based Complement Alternat Med. 2014;2014:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Lutgendorf SK, Mullen-Houser E, Russell D, et al. Preservation of immune function in cervical cancer patients during chemoradiation using a novel integrative approach. Brain Behav Immun. 2010;24:1231-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Banbury A, Nancarrow S, Dart J, Gray L, Parkinson L. Telehealth interventions delivering home-based support group videoconferencing: systematic review. J Med Internet Res. 2018;20:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Penedo FJ, Oswald LB, Kronenfeld JP, Garcia SF, Cella D, Yanez B. The increasing value of eHealth in the delivery of patient-centred cancer care. Lancet Oncol. 2020;21:e240-e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Penedo FJ, Fox RS, Oswald LB, et al. Technology-based psychosocial intervention to improve quality of life and reduce symptom burden in men with advanced prostate cancer: results from a randomized controlled trial. Int J Behav Med. 2020;27:490-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Penedo FJ, Fox RS, Walsh EA, et al. Effects of web-based cognitive behavioral stress management and health promotion interventions on neuroendocrine and inflammatory markers in men with advanced prostate cancer: a randomized controlled trial. Brain Behav Immun. 2021;95:168-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Antoni M, Taub C, Ream M, et al. Video-conferenced stress management and relaxation training (VSMART) for older women undergoing treatment for breast cancer. Int J Behav Med. 2021;28:S71. [Google Scholar]
- 272. Penedo FJ, Antoni MH, Moreno PI, et al. Study design and protocol for a culturally adapted cognitive behavioral stress and self-management intervention for localized prostate cancer: the Encuentros de Salud study. Contemp Clin Trials. 2018;71:173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Kiecolt-Glaser JK, Bennett JM, Derry HM, Gillie BL, Fagundes CP. Chapter 2: resilience and immune function in older adults. Annu Rev Gerontol Geriatr. 2012;32:29-47. [Google Scholar]
- 274. Cooksley CD, Avritscher EB, Bekele BN, Rolston KV, Geraci JM, Elting LS. Epidemiology and outcomes of serious influenza-related infections in the cancer population. Cancer. 2005;104:618-628. [DOI] [PubMed] [Google Scholar]
- 275. Lechner SC, Whitehead NE, Vargas S, et al. Does a community-based stress management intervention affect psychological adaptation among underserved black breast cancer survivors? J Natl Cancer Inst Monographs. 2014;2014:315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Penedo FJ, Traeger L, Dahn J, et al. Cognitive behavioral stress management intervention improves quality of life in Spanish monolingual hispanic men treated for localized prostate cancer: results of a randomized controlled trial. Int J Behav Med. 2007;14:164-172. [DOI] [PubMed] [Google Scholar]
- 277. Dhabhar FS, Saul AN, Holmes TH, et al. High-anxious individuals show increased chronic stress burden, decreased protective immunity, and increased cancer progression in a mouse model of squamous cell carcinoma. PLoS One. 2012;7:e33069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. El-Jawahri A, Chen YB, Brazauskas R, et al. The impact of pre-transplant depression on outcomes of allogeneic and autologous hematopoietic stem cell transplantation. Blood. 2015;126:265-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Shaashua L, Rosenne E, Neeman E, et al. Plasma IL-12 levels are suppressed in vivo by stress and surgery through endogenous release of glucocorticoids and prostaglandins but not catecholamines or opioids. Psychoneuroendocrinology. 2014;42:11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015;12:213-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Glasner A, Avraham R, Rosenne E, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a β-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184:2449-2457. [DOI] [PubMed] [Google Scholar]
- 282. Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a β-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19:114-126. [DOI] [PubMed] [Google Scholar]
- 283. Sorski L, Melamed R, Matzner P, et al. Reducing liver metastases of colon cancer in the context of extensive and minor surgeries through β-adrenoceptors blockade and COX2 inhibition. Brain Behav Immun. 2016;58:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Kang Y, Nagaraja AS, Armaiz-Pena GN, et al. Adrenergic stimulation of DUSP1 impairs chemotherapy response in ovarian cancer. Clin Cancer Res. 2016;22:1713-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Su F, Ouyang N, Zhu P, et al. Psychological stress induces chemoresistance in breast cancer by upregulating MDR1. Biochem Biophys Res Commun. 2005;329:888-897. [DOI] [PubMed] [Google Scholar]
- 286. Yao H, Duan Z, Wang M, Awonuga AO, Rappolee D, Xie Y. Adrenaline induces chemoresistance in HT-29 colon adenocarcinoma cells. Cancer Genet Cytogenet. 2009;190:81-87. [DOI] [PubMed] [Google Scholar]
- 287. Goodwin PJ, Leszcz M, Ennis M, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345:1719-1726. [DOI] [PubMed] [Google Scholar]
- 288. Oh PJ, Shin SR, Ahn HS, Kim HJ. Meta-analysis of psychosocial interventions on survival time in patients with cancer. Psychol Health. 2016;31:396-419. [DOI] [PubMed] [Google Scholar]
- 289. Latte-Naor S. Managing patient expectations: integrative, not alternative. Cancer J. 2019;25:307-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Tsang HW, Fung KM. A review on neurobiological and psychological mechanisms underlying the anti-depressive effect of qigong exercise. J Health Psychol. 2008;13:857-863. [DOI] [PubMed] [Google Scholar]
- 291. Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15:205-218. [DOI] [PubMed] [Google Scholar]
- 292. Mehta LS, Watson KE, Barac A, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137:e30-e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Guida JL, Ahles TA, Belsky D, et al. Measuring aging and identifying aging phenotypes in cancer survivors. J Natl Cancer Inst. 2019;111:1245-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]