Abstract
Purpose: The first clinical biology-guided radiation therapy (BgRT) system—RefleXionTM X1—was installed and commissioned for clinical use at our institution. This study aimed at evaluating the treatment plan quality and delivery efficiency for IMRT/SBRT cases without PET guidance. Methods: A total of 42 patient plans across 6 cancer sites (conventionally fractionated lung, head, and neck, anus, prostate, brain, and lung SBRT) planned with the EclipseTM treatment planning system (TPS) and treated with either a TrueBeam® or Trilogy® were selected for this retrospective study. For each Eclipse VMAT plan, 2 corresponding plans were generated on the X1 TPS with 10 mm jaws (X1-10mm) and 20 mm jaws (X1-20mm) using our institutional planning constraints. All clinically relevant metrics in this study, including PTV D95%, PTV D2%, Conformity Index (CI), R50, organs-at-risk (OAR) constraints, and beam-on time were analyzed and compared between 126 VMAT and RefleXion plans using paired t-tests. Results: All but 3 planning metrics were either equivalent or superior for the X1-10mm plans as compared to the Eclipse VMAT plans across all planning sites investigated. The Eclipse VMAT and X1-10mm plans generally achieved superior plan quality and sharper dose fall-off superior/inferior to targets as compared to the X1-20mm plans, however, the X1-20mm plans were still considered acceptable for treatment. On average, the required beam-on time increased by a factor of 1.6 across all sites for X1-10mm compared to X1-20mm plans. Conclusions: Clinically acceptable IMRT/SBRT treatment plans were generated with the X1 TPS for both the 10 mm and 20 mm jaw settings.
Keywords: Dosimetry, stereotactic body radiation therapy, radiation dosimetry, stereotactic radiotherapy, stereotactic radiosurgery
Introduction
The RefleXionTM X1 (RefleXion Medical, Inc., Hayward, CA) is a novel radiotherapy delivery system that consists of a 6 MV flattening-filter-free (FFF) linear accelerator mounted on a ring gantry equipped with near-diagnostic-quality on-board kilovoltage fan-beam CT for IGRT and on-board PET imaging to facilitate real-time beamlet delivery guidance based on the tumor uptake of PET tracers (ie, the so-called “biology-guided radiotherapy or BgRT”) 1 . Incorporating PET imaging with radiotherapy delivery systems represents the next evolutionary step in radiation therapy where the PET-avid tumor becomes its own fiducial for real-time guidance during radiotherapy delivery 2 . In addition to (18)F-fluorodeoxyglucose (FDG), there is an increasing number of novel, tumor-specific radiotracers that have a potential to increase the therapeutic ratio when treating with BgRT3–5. While the X1 system provides the tools (ie, hardware and software) for BgRT, it is still under investigation and not FDA approved for clinical use. However, the RefleXion X1 has received FDA clearance for Intensity Modulated Radiation Therapy (IMRT) and Stereotactic Body Radiation Therapy (SBRT).
The first clinical RefleXion X1 system was recently installed at our institution, commissioned6–8, and is currently in clinical use for IMRT and SBRT since May 2021. This work is the first treatment planning study investigating the capabilities of the clinical X1 TPS to determine the achievable plan quality for various common treatment sites including conventionally fractionated lung, head-and-neck, prostate, anus, brain, and lung SBRT. The achieved plan quality on the X1 TPS was compared to the achieved plan quality for the same patients, but planned on the Varian EclipseTM TPS and treated with Varian TrueBeam® or Trilogy® linear accelerators (Varian Medical Systems, Palo Alto, CA). This comparison, including plan quality and delivery efficiency, provided insight into which planning sites would be well-suited for IMRT/SBRT treatment on the X1. As this is the first treatment planning study for a clinical installation of the RefleXion X1, it could be used as a reference for other institutions considering the X1 system.
Methods
RefleXion X1 System
The X1 system consists of a 6 MV FFF linac mounted on a fast-rotating, slip-ring gantry (60 RPM), and fast transitioning MLC leaves (7 ms/transition) to deliver modulated beamlets at 50 gantry angles with multiple full gantry rotations per beam station (ie, a particular couch position) 2 . The beam is delivered at a nominal dose-rate of 850 cGy/min and is shaped by split-jaws (field size of 10 mm or 20 mm in the IEC-Y direction) that sandwich 64 binary MLCs. The width of each MLC is 6.25 mm, which provides a total maximum field width of 40 cm in IEC-X at 85 cm source-to-axes distance (SAD). Treatments can be delivered in one of 3 modes: IMRT (single pass), SBRT (4 passes), and BgRT (4 passes). In each pass, the couch moves at a discrete interval of 2.1 mm in the IEC-Y (ie, superior–inferior) direction.
Patient Aata Collection
A total of 42 patients were selected for planning analysis in this study. The clinical sites were selected based on suitability for treatment on the X1 delivery system. Specifically, this inclusion criteria consisted of no target volume longer than 50 cm in cranio-caudal direction, no target smaller than 15 mm in diameter, no target with off-axis distance greater than 15 cm, and no target requiring gating or breath-hold, and no simulation CT scan in non-Head-First-Supine (HFS) orientation. Six treatment plan types chosen for this analysis were conventionally fractionated lung, head and neck, prostate, anus, brain, and lung SBRT. For each treatment site, the most recently treated patients were selected. All patients were simulated head-first-supine either on a multi-slice Siemens BiographTM (Siemens Healthcare, Germany) or a GE DiscoveryTM (GE Healthcare, USA) PET-CT scanner where the images were reconstructed with a uniform slice thickness ranging between 1 mm and 2.5 mm. For thoracic targets, an additional 4D planning CT scan was acquired to derive a motion-inclusive target for treatment. All data was anonymized for this study.
Eclipse Treatment Planning
Table 1 shows the characteristics of the Eclipse plans selected for this study. VMAT plans were generated in Eclipse v15.6 using clinical models of either a Truebeam® or Trilogy® linear accelerators equipped with either the high-definition (minimum leaf width of 2.5 mm at 100 cm SAD) or MilleniumTM (minimum leaf width of 5.0 mm at 100 cm SAD) MLCs (Varian Medical Systems, Palo Alto, CA). Treatment plans were optimized using our institutional planning constraints based on RTOG/NRG protocols (NRG HN005, RTOG0126, RTOG0623, RTOG0822, RTOG-0529) and AAPM TG-101 9 and normalized such that 95% of the highest dose PTV was covered by the prescription dose. The beam geometry was dependent on the target location. In general, the beam arrangement consisted of 1 to 4 full arcs for central or bilateral targets and 1 to 3 partial arcs for ipsilateral targets. Beam energy varied between 6 and 15 MV and was site dependent. The Photon Optimizer v15.6 algorithm was used for optimization and either AAATM or Acuros® AXB v15.6 algorithms were used for dose calculation with a 1.25 mm or 2.5 mm calculation grid.
Table 1.
The Characteristics of the Eclipse VMAT Plans for Each Treatment Site Investigated.
| Site | Prostate | Lung | Head and neck | Brain | Anus | Lung SBRT |
|---|---|---|---|---|---|---|
| No plans | N = 8 | N = 9 | N = 8 | N = 5 | N = 5 | N = 7 |
| Energy | 6 MV/15 MV | 6 MV | 6 MV | 6 MV | 10 MV/15 MV | 10 MV FFF |
| Beams | 2 full arcs | 2 partial arcs | 2-4 partial arcs (ipsilateral) | 2 full or partial arcs | 3-4 full arcs | 1-2 partial arcs |
| or 3-4 full arcs (bilateral) | ||||||
| MLC type | HD | HD/Millenium | HD/Millenium | HD/Millenium | Millenium | HD |
| Algorithm | AAA | AXB | AXB | AXB | AAA | AXB |
| Dose grid | 1.5-2.5 mm | 1.5-2.5 mm | 1.5-2.5 mm | 2-2.5 mm | 2.5 mm | 1.2-1.5 mm |
| Total dose | 80 Gy | 50-66 Gy | 60-70 Gy | 60 Gy | 45-54 Gy | 50 Gy |
| Fractions | 40 | 25-30 | 30-33 | 30 | 25 | 4 |
RefleXion Treatment Planning
The planning CTs and associated structure sets used to generate the Eclipse VMAT plans were used to generate plans on the X1 TPS. In addition to the original VMAT planning structures, a RefleXion X1 couch structure was created in Eclipse using an in-house developed Eclipse Scripting API (ESAPI) script. For ipsilateral targets, an optimization structure covering the contralateral side of the patient was created. This optimization structure was used in the X1 TPS as a low-dose modifier to mimic the dosimetry of a partial arc-type delivery. Two plans were generated for each patient in the X1 TPS: one using the 10 mm jaw setting (X1-10mm) and one using the 20 mm jaw setting (X1-20mm). In the X1 TPS, the smoothing parameter is an important optimization parameter, which is used to limit the number of MLC transitions in the optimized plan. A default smoothing parameter of 10 was used for all treatment sites except anus, where a smoothing factor up to 50 had to be used for large DICOM-X and -Y size anal cancer targets to stay within deliverable tolerances for average and maximum MLC transitions of 200 and 400, respectively.
Following our institutional planning constraints, the IMRT and SBRT plans were created in the X1 TPS v1.0.46 using accelerated proximal gradient descent based on the fast iterative shrinkage-thresholding algorithm (FISTA)10,11 for optimization and collapsed-cone convolution-superposition (CCC)12,13 for dose calculation with a grid size of 2.1 mm. At the time of planning, the X1 TPS did not allow plan dose normalization. Therefore, the optimization parameters were defined such that the prescription dose(s) covered, as close as possible, 95% of the PTV(s) to match the normalization used in the analogous VMAT plans. The priorities for the optimization objectives emphasized coverage to the target(s), homogeneity, conformity, reduction of dose to organs at risk (OARs) and delivery efficiency. The optimization objectives for the X1-10mm and X1-20mm plans were fine-tuned to produce the clinically acceptable plans which met our institutional constraints to organs at risk, prescription dose to cover 95% of PTV and plan homogeneity within 110% of the prescription dose. The majority of the developed treatment plans were used for treatment planning system commissioning. The quality assurance for all tested plans passed gamma 3%/2 mm criteria as described in a related X1 TPS commissioning manuscript. 6
Data Analysis
The dose covering 95% of all PTVs (ie, PTV D95%) and dose covering 2% of the highest-prescription PTV (ie, PTV D2%) were collected for all plans. The maximum ( ), mean ( ), dose (D), volume (V), and volume spared (VS) to organs-at-risk were collected from each plan where clinically appropriate. For lung SBRT and brain plans, conformality index (CI) was also calculated as the ratio between the 100% isodose volume and the PTV volume. For lung SBRT plans, the intermediate dose spill metric, R50, was calculated as the ratio of V50% of the prescription dose to the PTV volume. Beam-on times for the X1 plans were obtained from the plan report generated by the X1 TPS. To analyze treatment time relative to PTV length and volume across all groups, the TPS-reported treatment times were re-normalized based on a dose prescription of 2 Gy/fraction. For each plan, the dose fall-off in the superior–inferior direction was recorded at distances ranging from 5 to 20 mm from the PTV edge in 5 mm increments and compared between X1-10mm, X1-20mm, and Eclipse VMAT plans.
All clinically relevant metrics in this study, including plan PTV D95%, PTV D2%, CI, R50, organs at risk constraints, and estimated treatment delivery duration were analyzed and compared between the VMAT, X1-10mm, and X1-20mm plans using paired t-tests. A difference with a two-tailed P-value .05 was considered statistically significant.
Results
Plan Quality
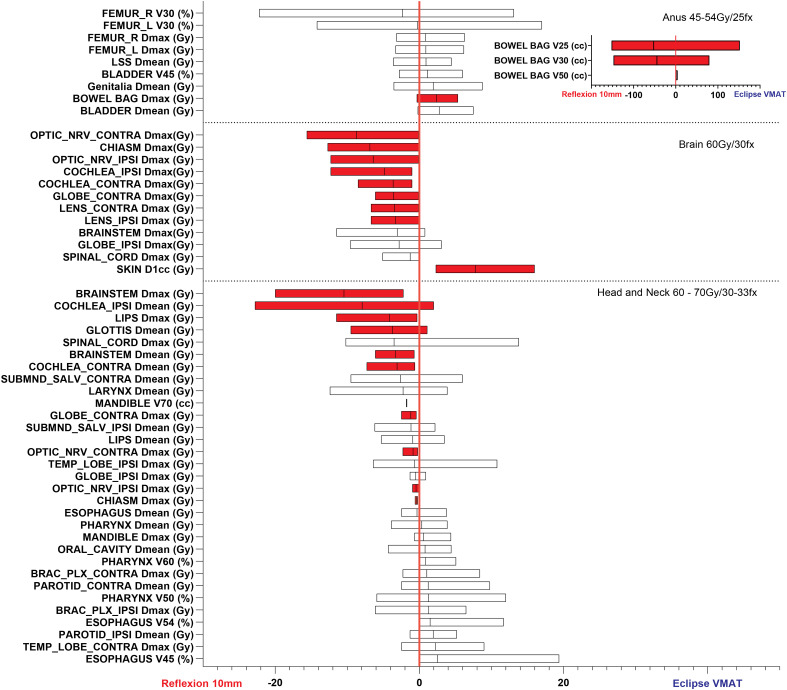
Clinically acceptable treatment plans were generated with the X1 TPS for both the 10 and 20 mm jaw settings in both the IMRT and SBRT modes. Box and whisker plots of the differences in achieved plan metrics between the X1-10mm and Eclipse VMAT plans are shown for the various treatment sites in Figures 1 and 2. Differences in PTV coverage and dose heterogeneity between the X1-10mm and Eclipse plans were not significantly across all treatment sites investigated. Out of 103 OAR dosimetric parameters analyzed, 27 (26.2%) were found to be statistically significant (highlighted in red), with 24 (23.3%) favoring the X1-10mm plans and 3 (2.9%) favoring the Eclipse VMAT plans.
Figure 1.
Differences in achieved plan quality metrics between the X1-10mm and Eclipse VMAT plans for anus, brain, and head-and-neck treatment sites. Parameters plotted in the left part of the graph (ie, difference less than zero) show X1-10mm plan superiority. Red shading denotes statistically significant differences (ie, P 0.05). The differences in PTV coverage and plan heterogeneity were not statistically significant for these sites. Due to the large differences in bowel V25Gy, V30Gy, and V50Gy between the X1 and Eclipse VMAT plans, these metrics are shown in a separate inset plot for the anus site.
Figure 2.
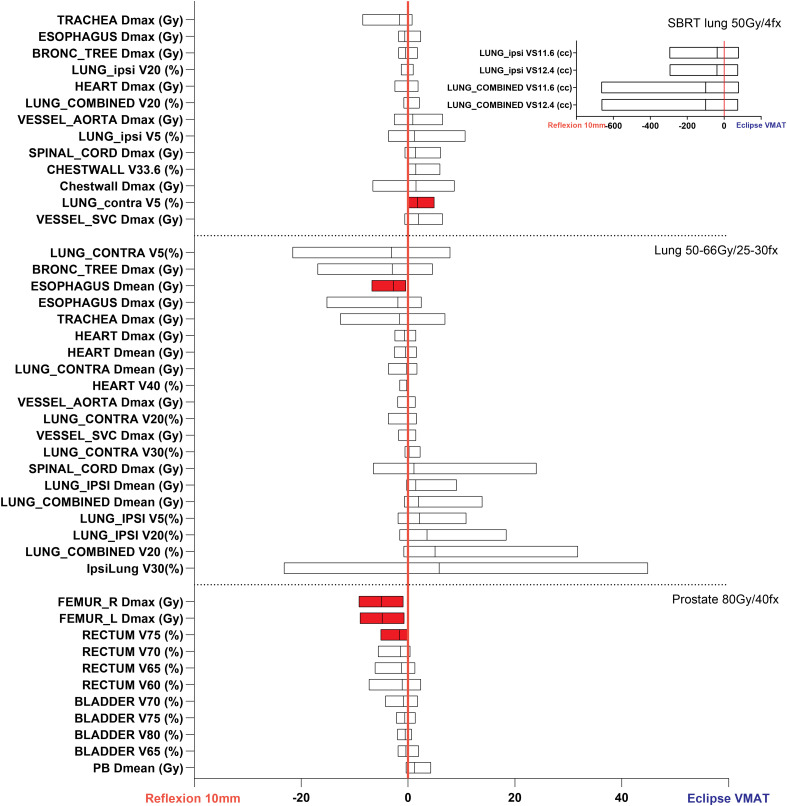
Differences in achieved plan quality metrics between the X1-10mm and Eclipse VMAT plans for lung SBRT, lung, and prostate treatment sites. Parameters plotted in the left part of the graph show X1-10mm plan superiority. Red shading denotes statistically significant differences. The differences in PTV coverage and plan heterogeneity were not statistically significant for these treatment sites. Due to the large differences in ipsilateral and combined lung VS11.6Gy and VS12.4Gy, these metrics are shown in a separate inset plot for the lung SBRT site.
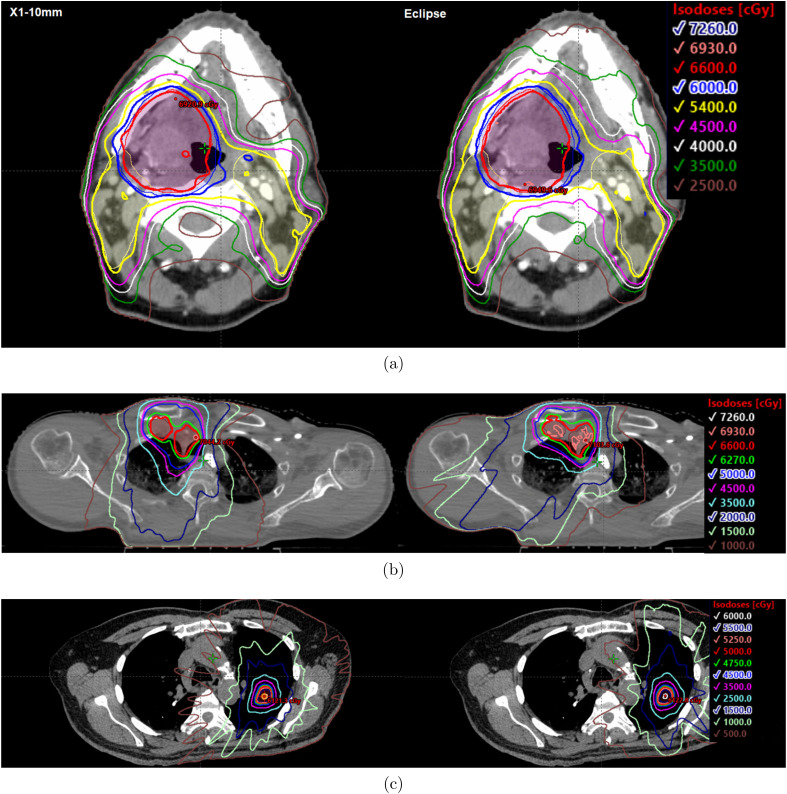
The fraction of individual OAR parameters that resulted in statistically significant differences favoring the X1-10mm plans, favoring the Eclipse plans, and favoring neither plan were as follows: brain (66.7%, 8.3%, and 25%, respectively), head and neck (32.3%, 0%, and 67.7%), prostate (27.3%, 0%, and 72.7%), anus (16.7%, 8.3%, and 75.0%), conventionally fractionated lung (5.0%, 0%, and 95.0%), and lung SBRT (0%, 5.8%, and 94.2%). On average, the largest statistically significant gains in normal tissue sparing achieved by the X1-10mm plans relative to the Eclipse plans were brainstem D (10.4 Gy) and ipsilateral cochlea D (7.9 Gy) for the head and neck plans, contralateral and ipsilateral optic nerves D (8.7 Gy and 6.4 Gy) and chiasm D (6.9 Gy) for brain plans, right and left femur D (5.0 Gy and 4.8 Gy) for prostate plans, and bowel bag V25 and V30 (52.3 cc and 44.6 cc) for anus plans. Examples of the achieved dose distributions for the X1-10mm and Eclipse VMAT plans for head and neck, conventional lung, and SBRT lung sites are shown in Figure 3. Example videos of the axial dose distribution over the extent of the target for each site and plan type are provided in the Supplemental Material of this work.
Figure 3.
Representative dose distributions for the RefleXion X1 10 mm jaw (left) and Eclipse VMAT (right) plans for the (a) head and neck, (b) conventionally fractionated lung, and (c) lung SBRT. The prescription doses were 66 Gy in (a) and (b) and 50 Gy in (c). Videos showing the dose distribution over the extent of the target for each of the investigated treatment sites are provided in the Supplemental Material.
Tables 2 and 3 show a subset of the achieved plan dosimetric parameters for the X1-10mm, X1-20mm, and Eclipse plans with the associated P-values of their differences. Significant differences are indicated by the bold text. In general, the X1 plans that utilized the 10 mm jaw were comparable or superior to the Eclipse VMAT plans.
Table 2.
Achieved Average Dosimetric Indices, Beam-on Times and the Associated P-Values for the X1 10 mm Jaw (X1-10), X1 20 mm Jaw (X1-20), and Eclipse VMAT Plans for Head and Neck, Prostate, and Lung SBRT Sites. A P-Value 0.05 was Considered Significant (Bold Text).
| Site | Dosimetric constraint/parameter | X1-10 | X1-20 | Eclipse | P (X1-10 vs Eclipse) | P (X1-20 vs Eclipse) | P (X1-10 vs X1-20) |
|---|---|---|---|---|---|---|---|
| Head and neck | PTV_high D95% 100% | 100.7 | 100.8 | 100.0 | .06 | .06 | .45 |
| PTV_intermed D95% 100% | 102.3 | 102.0 | 101.4 | .10 | .06 | .27 | |
| PTV_low D95% 100% | 100.5 | 101.0 | 99.8 | .09 | .06 | .25 | |
| PTV_high D2%<106% | 104.7 | 105.7 | 104.4 | .35 | .03 | .07 | |
| BrainStem Dmax<54 Gy | 17.7 | 21.5 | 28.1 | .003 | .03 | .07 | |
| Cochlea_ipsi Dmean<45 Gy | 18.3 | 18.8 | 26.2 | .02 | .02 | .36 | |
| Glottis Dmean<25 Gy | 9.4 | 10.4 | 13.1 | .04 | .08 | .02 | |
| Lips Dmax<30 Gy | 20.0 | 20.3 | 24.2 | .01 | .004 | .22 | |
| Parotid_contra Dmean<26 Gy | 12.3 | 13.6 | 11.1 | .22 | .06 | .01 | |
| Pharynx Dmean<45 Gy | 25.1 | 26.5 | 24.7 | .39 | .10 | .09 | |
| SpinalCord Dmax<45 Gy | 18.8 | 20.3 | 22.3 | .12 | .31 | .25 | |
| Submand_contra Dmean<39 Gy | 20.8 | 19.3 | 23.4 | .09 | .05 | .15 | |
| TempLobe_contra Dmax<60 Gy | 9.6 | 11.0 | 7.4 | .15 | .08 | .04 | |
| Beam-on Time, min | 11.9 | 8.3 | 2.0 | <.001 | <.001 | <.001 | |
| Prostate | PTV_80 D95% 80 Gy | 80.1 | 80.5 | 80.0 | .10 | .06 | .11 |
| PTV_80 D2%<106% | 105.7 | 105.3 | 105.1 | .08 | .34 | .24 | |
| Bladder V80<15% | 7.5 | 8.2 | 8.0 | .05 | .36 | .10 | |
| Bladder V65<50% | 13.9 | 16.4 | 14.2 | .21 | .05 | .03 | |
| Femur_L Dmax<50 Gy | 35.5 | 36.3 | 40.3 | .003 | .01 | .23 | |
| Femur_R Dmax<50 Gy | 35.9 | 36.4 | 41.0 | .001 | .01 | .31 | |
| PenileBulb, Dmean<52.5 Gy | 27.1 | 37.8 | 25.9 | .06 | .01 | .01 | |
| Rectum V75<15% | 9.7 | 11.0 | 11.3 | .01 | .38 | .04 | |
| Rectum V60<35% | 19.9 | 22.0 | 21.0 | .18 | .29 | .05 | |
| Beam-on Time, min | 8.6 | 5.3 | 2.0 | <.001 | <.001 | <.001 | |
| Lung SBRT | PTV_50 D95% 50 Gy | 50.8 | 50.5 | 50.0 | .08 | .06 | .10 |
| PTV_50 D2%<135% | 124.8 | 121.7 | 123.9 | .37 | .10 | .08 | |
| CI<1.2 | 1.0 | 1.0 | 1.0 | .16 | .17 | .44 | |
| R50 | 5.7 | 6.5 | 5.2 | .20 | .03 | .02 | |
| Chestwall Dmax<52.5 Gy | 43.4 | 41.3 | 41.9 | .31 | .41 | .04 | |
| Lungs VS12.4>1000cc | 2967.8 | 2930.3 | 2867.4 | .17 | .26 | .001 | |
| Lungs VS11.6>1500cc | 2950.0 | 2909.5 | 2850.1 | .17 | .28 | .001 | |
| Lungs V20<10% | 3.2 | 3.7 | 3.2 | .47 | .10 | .002 | |
| SpinalCord Dmax<14 Gy | 8.3 | 8.2 | 7.0 | .09 | .09 | .33 | |
| Beam-on Time, min | 22.7 | 14.4 | 1.3 | <.001 | <.001 | <.001 |
Table 3.
Achieved Average Dosimetric Indices, Beam-on Times and the Associated P-Values for the X1 10 mm Jaw (X1-10), X1 20 mm Jaw (X1-20), and Eclipse VMAT Plans for Lung, Anus, and Brain Sites. A P-Value .05 was Considered Significant (Bold Text).
| Site | Dosimetric constraint/parameter | X1-10 | X1-20 | Eclipse | P (X1-10 vs Eclipse) | P (X1-20 vs Eclipse) | P (X1-10 vs X1-20) |
|---|---|---|---|---|---|---|---|
| Lung | PTV_high D95% 100% | 100.2 | 100.5 | 100.0 | .10 | .06 | .09 |
| PTV_high D2%<106% | 104.9 | 106.3 | 105.0 | .39 | .01 | .01 | |
| Esophagus Dmean<34Gy | 12.5 | 13.4 | 15.2 | .003 | .08 | .19 | |
| Heart V40<35% | 1.7 | 2.2 | 2.0 | .10 | .14 | .04 | |
| Lungs Dmean<15Gy | 13.1 | 14.5 | 10.9 | .12 | .07 | .02 | |
| Lungs V20<30% | 23.2 | 25.4 | 17.5 | .09 | .06 | .03 | |
| Lung_contra Dmean<15Gy | 5.0 | 6.1 | 5.3 | .31 | .09 | .05 | |
| Lung_ipsi Dmean<15Gy | 19.0 | 19.9 | 17.4 | .09 | .004 | .15 | |
| SpinalCord Dmax<45Gy | 26.7 | 25.9 | 25.6 | .36 | .46 | .13 | |
| Vessel_Aorta Dmax<70Gy | 64.3 | 65.1 | 64.4 | .47 | .05 | .02 | |
| Vessel_SVC Dmax<70Gy | 65.7 | 66.5 | 65.7 | .49 | .01 | .03 | |
| Beam-on Time, min | 9.0 | 5.5 | 1.0 | <.001 | <.001 | <.001 | |
| Anus | PTV_high D95% 100% | 100.3 | 101.0 | 100.1 | .06 | .06 | .06 |
| PTV_intermed D95% 100% | 102.9 | 101.2 | 100.0 | .26 | .08 | .33 | |
| PTV_low D95% 100% | 100.2 | 100.9 | 100.0 | .22 | .13 | .13 | |
| PTV_high D2%<106% | 105.3 | 106.3 | 105.0 | .40 | .16 | .22 | |
| Bladder Dmean<24Gy | 34.2 | 33.5 | 31.4 | .06 | .02 | .10 | |
| Bladder V45<50% | 29.9 | 31.7 | 28.8 | .24 | .01 | .29 | |
| Bowel Dmax<54Gy | 49.5 | 48.7 | 47.2 | .04 | .11 | .01 | |
| Bowel V25<300cc | 280.8 | 275.7 | 333.1 | .04 | .22 | .15 | |
| Bowel V30<250cc | 212.2 | 210.8 | 256.8 | .04 | .17 | .16 | |
| Femur_L V30<50% | 11.3 | 13.9 | 11.6 | .48 | .21 | .23 | |
| Femur_R V30<50% | 10.9 | 12.1 | 13.2 | .35 | .40 | .36 | |
| Beam-on Time, min | 17.0 | 10.1 | 3.0 | <.001 | <.001 | <.001 | |
| Brain | PTV_60 D95% 60Gy | 60.0 | 60.1 | 60.1 | .41 | .29 | .19 |
| PTV_60 D2%<106% | 105.1 | 106.2 | 104.2 | .24 | .05 | .01 | |
| CI<1.2 | 0.99 | 1.02 | 1.01 | .14 | .24 | .12 | |
| BrainStem Dmax<45Gy | 33.7 | 35.8 | 36.7 | .13 | .42 | .26 | |
| Chiasm Dmax<45Gy | 21.2 | 22.3 | 28.1 | .03 | .05 | .08 | |
| Cochlea_contra Dmax<30Gy | 2.3 | 2.6 | 5.9 | .03 | .06 | .14 | |
| Cochlea_ipsi Dmax<30Gy | 7.7 | 9.1 | 12.6 | .05 | .07 | .07 | |
| Optic_nrv_contra Dmax<45Gy | 11.2 | 11.5 | 19.9 | .01 | .02 | .11 | |
| Optic_nrv_ipsi Dmax<45Gy | 17.2 | 18.0 | 23.6 | .03 | .04 | .12 | |
| Skin D1cc<45Gy | 43.1 | 44.2 | 35.3 | .02 | .01 | .14 | |
| Beam-on Time, min | 6.8 | 4.8 | 1.7 | <.001 | <.001 | <.001 |
When comparing X1-20mm and Eclipse plans, target coverage was comparable, whereas plan heterogeneity (PTV_high D2%) was inferior with the X1-20mm plans for the head and neck, brain, and lung plans. The performance of the X1-20mm plans relative to the Eclipse VMAT plans varied based on the treatment site. The fraction of individual OAR parameters that resulted in statistically significant differences favoring the X1-20mm plans, favoring the Eclipse plans, and favoring neither plan were as follows: brain (50.0%, 8.3%, and 41.7%, respectively), head and neck (16.1%, 3.2%, and 80.6%), prostate (18.2%, 9.1%, and 63.6%), anus (0%, 16.7%, and 83.3%), conventionally fractionated lung (0%, 26.3%, and 73.7%), and lung SBRT (0%, 5.9%, and 94.1%). For lung SBRT, the significant differences were observed in R50 between the X1-10mm and X1-20mm plans, indicating slower intermediate dose fall-off for the 20 mm jaw plans compared to the 10 mm jaw plans (6.5 vs 5.7). CI was comparable between the X1-10mm, X1-20mm, and VMAT lung SBRT plans. The significant differences in the required beam-on time were observed for all sites with the X1-10mm plans requiring the most time (Tables 2 and 3).
Beam-on Time and Dose Fall-off
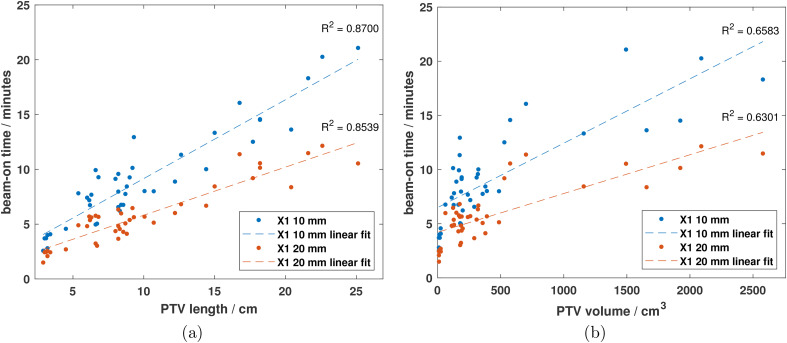
The beam-on times for the X1 treatment plans are shown in Figure 4a and b as functions of PTV length (superior–inferior direction) and volume, respectively. Strong correlation was observed between beam-on time and PTV length for both the X1-10mm ( ) and X1-20mm ( ) plans whereas moderate correlation was observed as a function of PTV volume ( for X1-10mm and for X1-20mm). As shown in Figure 4, using the 10 mm jaw results in a significant time penalty compared to the 20 mm jaw for planning. On average, the beam-on time increased by a factor of 1.6 across all PTV sizes when using the 10 mm jaw as compared to the 20 mm jaw.
Figure 4.
Treatment beam-on time versus PTV (a) length in cranio-caudal direction and (b) volume for all treatment sites as reported by the X1 TPS for the 10 and 20 mm jaw plans. Linear fits (dashed lines) were applied to the data sets to evaluate any correlation between beam-on time and PTV length and volume. The beam-on time for all plans were scaled such that the prescription dose was equal to 2 Gy/fraction. For 2 Gy/fx prescription, the equations for the linear fits in (a) were and for the 10 and 20 mm jaw plans, respectively.
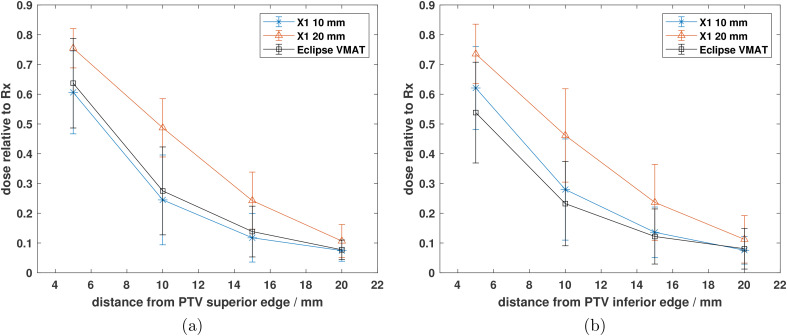
The average dose fall-off from the superior and inferior edges of the PTV for the X1 and Eclipse VMAT plans across all treatment sites investigated are shown in Figures 5a and b, respectively. The average relative dose at 5, 10, 15, and 20 mm superior to the PTV was 61%, 24%, 12%, and 7%, respectively, for the X1-10mm plans; 64%, 28%, 14%, and 8% for the Eclipse VMAT plans; and 75%, 49%, 24%, and 11% for the X1-20mm plans. Similarly, the relative dose at 5, 10, 15, and 20 mm inferior to the PTV was 62%, 28%, 14%, and 8%, respectively, for the X1-10mm plans; 54%, 23%, 12%, and 8% for the Eclipse VMAT plans; and 74%, 46%, 24%, and 11% for the X1-20mm plans. The dose at all distances from the PTV was on average 12.3 7.1% lower for the 10 mm jaw plans as compared to the 20 mm jaw plans. Within 2 cm from the PTV, the dose fall-off for the X1-10mm plans was on average 0.6 4.1% greater than the achieved dose fall-off from the Eclipse VMAT plans.
Figure 5.
Average dose fall-off relative to the prescription dose from the (a) superior and (b) inferior edges of the PTV for the Eclipse, X1 10 mm jaw, and X1 20 mm jaw plans. Each data point in (a) and (b) represents the mean dose relative to the prescription dose for each plan type. The error bars represent one standard deviation of the mean value.
Discussion
This is the first treatment planning study investigating the capabilities of the clinical RefleXionTM X1 TPS for various cancer sites. Clinically acceptable treatment plans were generated with the X1 TPS for both the 10 and 20 mm jaw settings in IMRT and SBRT modes. Comparable or superior plan quality was achieved between the X1-10mm plans and the Eclipse VMAT plans for most of the treatment sites investigated in this work. From Figures 1 and 2, only 3 out of 103 normal tissue sparing metrics had significant differences that were in favor of the Eclipse VMAT plans. The rest of the metrics were either not statistically significant or had significant differences that favored the X1-10mm plans. The X1-10mm plans generally achieved better plan quality as compared to the 20 mm jaw plans (Tables 2 and 3) at the expense of beam-on time which was on average 1.6 times greater for X-10mm plans compared to X1-20mm plans (Figure 4). While dose fall-off 2 cm superior/inferior of PTV was comparable between X1-10mm and Eclipse plans, it was on average 12.3 7.1% lower for the 10 mm jaw plans as compared to the 20 mm jaw plans (Figure 5).
In certain cases, additional beam-on time may be warranted where high dose conformity is required (eg, SBRT/SRS or prioritizing sparing a critical organ located superior/inferior to PTV). Further investigation is needed to determine if the tighter planned dose conformity from the 10 mm jaw translates to a more conformal delivered dose distribution as it may be challenging to maintain accurate PTV positioning for 22 min for a 12.5 Gy/fx lung SBRT. However, longer beam-on times with X1-10mm plans compared to X1-20mm plans may be acceptable for other treatment sites that do not exhibit significant intrafraction motion14,15: prostate (8.6 min vs 5.3 min), anus (17.0 min vs 10.1 min), brain (6.8 min vs 4.8 min), and head and neck (11.9 min vs 8.3 min). Compared to the linac-based VMAT plans, the dosimetric gains observed with RefleXion X1 plans could be explained by several factors. In the lung and ipsilateral head and neck setting, linac-based VMAT plans were generated with partial arcs. On the RefleXion X1 system, gantry-firing positions span a full 360 rotation thus allowing a greater degree of beam entry points. Combined with the ability to perform multiple gantry passes at a single couch position, this provides a unique opportunity for the X1 system to greatly modulate the dose around organs-at-risk.
Strong correlation was observed between the TPS-calculated treatment time and PTV length in the superior–inferior direction. This finding implies the length of the PTV in the cranial-caudal direction provides a good indication of the required treatment delivery time. The average treatment time for the Eclipse VMAT plans ranged from 1 to 3 min (Tables 2 and 3). While the beam-on time is largely influenced by the total number of arcs and dose per fraction (Table 1), the degree of modulation within a plan can also influence the required delivery time. At our institution, Eclipse lung SBRT plans are generated with a single partial arc, maximum dose rate (2400 MU/min), and minimal modulation to shorten beam-on time (1.3 min). While Eclipse TPS allows MU objectives during optimization, the X1 TPS utilizes a hard-coded (ie, not user adjustable) treatment time penalty with the weight of 1. Thus, increasing the weights for other optimization constraints in the plan decreases the prioritization of the time penalty. Another parameter that can impact the required beam-on time is the smoothing parameter, which dictates the number of allowed MLC transitions within the plan. While this parameter was fixed for this work for most of the plans except the anus site, additional studies are needed to investigate the trade-off between plan modulation, beam-on time, and plan quality for the X1 TPS.
To date, there is limited peer-reviewed literature on the performance of the X1 TPS for non-BgRT plans. Furthermore, these studies used un-validated, prototype X1 planning systems, whereas the results reported in this work are from an X1 system that has been commissioned for clinical use. Han et al 1 compared the plan quality between prototype X1 IMRT plans, Helical Tomotherapy (HT) plans, and VMAT plans for ten nasopharyngeal carcinoma patients. The prototype X1 and the HT plans used jaw settings of 20 and 25 mm, respectively. They observed comparable dose coverage, mean dose, and dose homogeneity to the primary PTV between the 3 modalities, while the prototype X1 plans had greater dose heterogeneity to the non-primary PTVs (average range: 1.28-1.50). Statistically significant dose reductions to multiple OARs were observed with the prototype X1 plans, including, but not limited to, cochlea, lenses, the TM joint, and parotids, which were also observed in the present study, albeit to a lesser degree. In this work, significant dose reductions were observed for the brainstem, cochlea, lips, and contralateral submandibular gland for the X1-20mm head and neck treatment plans compared to the VMAT plans (Table 2). The improved OAR sparing with the prototype X1 plans compared to the results of this work might be due to relaxed target heterogeneity requirements during optimization. Han et al also found no significant difference in maximum dose to OARs overlapping or in proximity to the PTVs. In this study, out of 3 statistically significant differences in normal tissue sparing metrics between the X1-10mm and Eclipse plans favoring the Eclipse plans, 2 were for OARs overlapping with targets (bowel bag D for anal cancer plans and skin D1cc for brain cancer plans).
While comparisons between the X1 and Eclipse VMAT plans were performed for multiple treatment sites in this work, there were also several limitations to the present study. First, as highlighted in Table 1, there were a limited number of subjects for each planning site (maximum of 9 and minimum of 5), which could impact the results of this work due to any differences in planner technique or style. Second, while several planning metrics reported in Tables 2 and 3 had statistically significant differences from the other plans, the clinical relevance of these differences should also be considered. For instance, there was a statistically significant difference in the heart V40 between the X1-10mm and 20 mm jaw plans ( ) for the conventional lung site. However, the mean volume difference (in percent) between these plans was 0.5%, which may not be clinically relevant. The data analysis techniqiue utilized in this work only represents one method for evaluating plan quality. Other treatment planning studies or planning grand challenges often combine or collapse plan quality metrics into an overall plan score or single plan quality index (PQI), as suggested by Giglioli et al 16 However, multi-parameter score comparisons were not used in this work to avoid subjectivity in choosing the weights and penalty points for the quality parameters. The goal of this study was to determine if clinically acceptable treatment plans could be produced with the RefleXion X1 TPS and compare the resulting plans to comparable VMAT plans. Future work includes increasing the number of patient plans to each treatment site to improve the power of the statistical analysis. In addition, treatment plans will also be created for the novel BgRT mode for each site and will be compared to the plans generated with the SBRT mode.
Conclusions
This treatment planning study evaluated the plan quality and treatment delivery efficiency for several cancer sites using the first clinical installation of the RefleXion X1 TPS and Eclipse VMAT for standard c-arm linear accelerators. The X1-10mm plans were equivalent or superior to the Eclipse VMAT plans for all treatment sites for all but 3 planning metrics. The X1-20mm plans generally resulted in greater normal tissue dose and slower cranio-caudal dose fall-off as compared to the X1-10mm plans and Eclipse VMAT plans (Tables 2 and 3), but also decreased the beam-on time by 62% compared to the X1-10mm plans. However, this increase in treatment time may be warranted in cases where sharp dose gradients are required (eg, SBRT or prioritizing critical organ sparing superior/inferior to the PTV) as the 10 mm jaw plans had tighter conformity in the superior–inferior direction as compared to the 20 mm jaw plans. The required beam-on time for the X1 plans was significantly greater than the time required for the Eclipse VMAT plans. In conclusion, this work has shown that clinically acceptable treatment plans can be generated with the novel RefleXion X1 system and are comparable or superior to VMAT plans generated with the Eclipse TPS.
Acknowledgements
The authors wish to thank the whole radiation oncology team at our clinic that helped make this study possible
Abbreviations
- BgRT
biology-guided radiation therapy
- IMRT
intensity-modulated radiation therapy
- SBRT
stereotactic body radiation therapy
Footnotes
Ethics Statement: Our study did not require an ethical board approval because it did not contain human or animal trials. All patients signed a general research prior to treatment at our institution and all patient data was anonymized.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Eric Simiele https://orcid.org/0000-0002-1721-2224
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Han C, Da Silva A, Liang J, Wohlers C, Huntzinger C, Neylon J, Du D, Wong J, Liu A. Comparative evaluation of treatment plan quality for a prototype biology-guided radiotherapy system in the treatment of nasopharyngeal carcinoma. Med Dosim. 2021; 46: 171‐178. [DOI] [PubMed] [Google Scholar]
- 2.Oderinde O, Shirvani S, Olcott P, Kuduvalli G, Mazin S, Larkin D. The technical design and concept of a PET/CT Linac for biology-guided radiotherapy. Clin Transl Radiat Oncol. 2021; 17: 106‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fendler W, Calais J, Eiber M, Flavell R, Mishoe A, Feng F, Nguyen H, Reiter R, Rettig M, Okamoto S, Emmett H, Zacho L, Ilhan H, Wetter A, Rischpler C, Schoder H, Burger IA, Gartmann J, Smith R, Small EJ, Hope T. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019; 5: 856‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SH, Roytman M, Kamen E, Skafida M, Strauss S, Lin E, Kutler D, Zan E, Ivanidze J. [68Ga]-DOTATATE PET/MRI in the diagnosis and management of recurrent head and neck paraganglioma with spinal metastasis. Clin Imaging. 2021; 79: 314‐318. [DOI] [PubMed] [Google Scholar]
- 5.Wiedenmann N, Bunea H, Rischke HC, Bunea A, Majerus L, Bielak L, Protopopov A, Ludwig U, Bchert M, Stoykow C, Nicolay NH, Weber WA, Mix M, Meyer PT, Hennig J, Bock M, Grosu AL. Effect of radiochemotherapy on T2* MRI in HNSCC and its relation to FMISO PET derived hypoxia and FDG PET. Radiat Oncol. 2018; 13: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simiele E, Capaldi D, Breitkreutz D, Bin H, Yeung T, White J, Owens M, Vitzthum L, Chang D, Xing L, Surucu M, Kovalchuk N. Treatment planning system commissioning results for the first clinical biology-guided radiotherapy machine (in press). J Appl Clin Med Phys. 2022. [DOI] [PMC free article] [PubMed]
- 7. Han B, Capaldi D, Kovalchuk N, Simiele E, White J, Zaks D, Xing L, Surucu M. Beam commissioning of the first clinical biology-guided radiotherapy system. J Appli Clin Med Phys. 2022. [DOI] [PMC free article] [PubMed]
- 8.Hu Z, Bieniosek M, Ferri V, Iagaru A, Kovalchuk N, Han B, Xing L, Vitzthum L, Olcott P, Narayanan M, Laurence T, Ren Y, Oderinde O, Shirvani S, Chang D, Surucu M. Characterization of PET subsystem for biology-guided radiotherapy (BgRT) (under review), British J Radiol. 2022. [DOI] [PMC free article] [PubMed]
- 9.Benedict S, Yenice K, Followill D. et al. Stereotactic body radiation therapy: The report of AAPM task group 101. Med Phys. 2010; 37: 4078‐4101. [DOI] [PubMed] [Google Scholar]
- 10.Beck A, Teboulle M. A fast iterative shrinkage-thresholding algorithm for linear inverse problems. Med Phys. 2009; 2: 183‐202. [Google Scholar]
- 11.Beck A, Teboulle M. A fast iterative shrinkage-thresholding algorithm for linear inverse problems. SIAM J Imaging Sci. 2009; 2: 183‐202. [Google Scholar]
- 12.Lu W, Olivera GH, Chen M-L, Reckwerdt PJ, Mackie TR. Accurate convolution/superposition for multi-resolution dose calculation using cumulative tabulated kernels. Physics in Medicine & Biology. 2005; 50: 655‐680. [DOI] [PubMed] [Google Scholar]
- 13.Ahnesj A. Collapsed cone convolution of radiant energy for photon dose calculation in heterogeneous media. Med Phys. 1989; 16: 577‐592. [DOI] [PubMed] [Google Scholar]
- 14.Thomas S, Ashburner M, Tudor G, Treeby J, Dean J, Routsis D, Rimmer Y, Russell S, Burnet N. Intra-fraction motion of the prostate during treatment with helical tomotherapy. Radiother Oncol. 2013; 109: 482‐486. [DOI] [PubMed] [Google Scholar]
- 15.Bruijnen T, Stemkens B, Terhaard C, Lagendijk J, Raaijmakers C, Tijssen R. Intrafraction motion quantification and planning target volume margin determination of head-and-neck tumors using cine magnetic resonance imaging. Radiother Oncol. 2019; 130: 82‐88. [DOI] [PubMed] [Google Scholar]
- 16.Giglioli F, Garibaldi C, Blanck O, Villaggi E, Russo S, Esposito M, Marino C, Stasi M, Mancosu P. Dosimetric multicenter planning comparison studies for stereotactic body radiation therapy: Methodology and future perspectives. Int J Radiat Oncol Biol Phys. 2020; 106: 403‐412. [DOI] [PubMed] [Google Scholar]